Abstract
Aging occurs with enhanced sympathetic nerve activity and endothelial dysfunction; however, little is known of how successive branches of microvascular resistance networks are affected in vivo. We questioned whether vascular reactivity is altered differentially along resistance networks with advanced age. The left gluteus maximus muscle of anesthetized 4-mo-old and 24-mo-old male C57BL/6 mice (Young and Old, respectively) was exposed for intravital microscopy and superfused with physiological salt solution (3 ml/min; pH 7.4, 34°C). Spontaneous vasomotor tone increased progressively from proximal feed arteries (FA) and first-order (1A) arterioles through distal second-order (2A) and third-order (3A) arterioles and was ∼15% greater in 2A and 3A of Old versus Young. Vasoconstriction during elevated superfusion Po2 increased with branch order and to a greater extent in Young. Peak constrictions to phenylephrine [α1 adrenoreceptor (α1AR) agonist] were similar for FA and 1A of both ages and ∼20% greater for 2A and 3A of Young. Across arterioles (but not FA), constrictions to UK 14304 (α2AR agonist) were depressed ∼30% in Old versus Young. Thus advanced age attenuated vasoconstriction to O2 throughout networks while blunting vasoconstriction to α1AR and α2AR activation in arterioles. With ACh, endothelium-dependent dilation (EDD) was ∼20% greater in FA of Young yet was approximately twofold greater for 2A and 3A of Old. Sodium nitroprusside evoked maximal dilations similar to ACh. Thus, with advanced age, EDD was attenuated in FA while robust in distal arterioles having enhanced vasomotor tone. We conclude that advanced age differentially alters reactivity among branches of microvascular resistance networks.
Keywords: adrenoreceptors, arteriole, blood flow control, endothelium-dependent dilation, feed artery, microcirculation, skeletal muscle
advanced age is a major risk factor for cardiovascular disease (25, 36, 37, 44). Although impaired endothelium-dependent dilation (EDD) has been well-characterized in large arteries of older human subjects (9, 12, 17, 25, 44), how aging affects reactivity in the microvessels that govern skeletal muscle perfusion is less well-defined. Early studies in the cremaster muscle of male rats found impaired dilation of first-order (1A) and second-order (2A) arterioles to adenosine (6), whereas constriction to norepinephrine was maintained. However, the cremaster muscle is not a true skeletal muscle because it neither attaches to the skeleton nor is it involved in locomotion. Consistent with reduced blood flow to skeletal muscle with aging in humans (8, 10, 39), the blood flow response to contraction of the plantar flexor muscles was impaired in senescent rats (20). Contributing to the restriction of skeletal muscle blood flow is that 1A isolated from rat soleus and gastrocnemius muscles demonstrated impairment in EDD with aging (2, 35), as did feed arteries (FA) isolated from the soleus muscle (50, 55). In the gluteus maximus muscle (GM) of mice, which also has FA that give rise to branching arteriolar networks (1, 33), impaired dilation and perfusion of 2A in response to muscle contraction was attributed to the enhanced activation of α-adrenoreceptors (αARs) (21). These findings in rodents are consistent with restricted muscle blood flow and enhanced sympathetic nerve activity (SNA) in older humans (5, 8). Whereas studies in humans have shown impaired α-adrenergic vasoconstriction in the forearm (7) and leg (10, 47), the actual site(s) of such responses within the vascular supply remain obscure.
In response to increased oxygen demand, the volume of blood flow entering a muscle is governed by FAs and proximal (1A) arterioles upstream, whereas the distribution of blood flow within a muscle is regulated by the smaller daughter 2A and third-order (3A) arteriolar branches downstream (45). A key feature of resistance networks is their ability to coordinate vasomotor responses among vessel branches. For example, ascending vasodilation of FAs arises from signals originating from arterioles embedded within the muscle fibers (46). The wall of arterioles and their proximal FA is comprises primarily a single layer of smooth muscle cells (SMCs) surrounding the endothelial cell monolayer in contact with the blood. In turn, all branches of the resistance network are surrounded by sympathetic nerve fibers coursing through the adventitia. During blood flow regulation, changes in vessel diameter reflect the interaction between signaling events generated in SMCs, endothelial cells, and perivascular nerves. Sympathetic vasoconstriction is mediated through the activation of αARs on vascular SMCs, of which there are two major subtypes: α1 and α2 (13). Regional variability in the functional distribution of α1ARs and α2ARs has been demonstrated along arteriolar networks in rat and mouse cremaster muscles (33, 38) and the mouse GM (33). Remarkably, little is known of how or where respective adrenergic signaling pathways are affected by advanced age. Whereas the effect of aging on EDD has been evaluated in FAs and 1As extensively in vitro (2, 35, 55, 56), little is known of what happens in vivo, particularly in smaller 2A and 3A branches.
The response of microvessels to changes in oxygen availability can be evaluated by altering Po2 in the superfusion solution bathing the tissue. The role of α1ARs and α2ARs in mediating vasoconstriction and of the endothelium in mediating vasodilation can be evaluated using selective agonists. Thus, α1AR-mediated responses are evoked by phenylephrine (PE), whereas α2AR-mediated responses are evoked by UK 14304 (16, 33). In turn, agonist-mediated EDD is most readily studied with ACh via activation of muscarinic receptors on endothelial cells (2, 12, 35, 55). The mouse GM preparation enables direct visualization of respective microvessel branch orders (e.g., FA, 1A, 2A, and 3A) using intravital microscopy (1, 21, 33). Therefore, evaluating diameter changes of respective branch orders in the GM during changes in Po2, selective activation of AR subtypes and during EDD provides an experimental approach for determining how aging influences the microcirculation of skeletal muscle in vivo. In the present study, we tested the hypothesis that advanced age differentially alters reactivity throughout microvascular resistance networks of skeletal muscle using the mouse GM as an experimental model (1, 21, 33).
METHODS
Animal care and use.
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Missouri (Columbia, MO) and were performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals (2011). Male C57BL/6 mice [4 mo old (Young; n = 5) and 24 mo old (Old; n = 5)] were obtained from National Institute on Aging colonies (Charles River Laboratories, Wilmington, MA). These age groups correspond to humans in their mid-20s and late 60s, respectively (15). Mice were housed in animal care facilities of the University of Missouri maintained at ∼24°C on a 12-h:12-h light/dark cycle with food and water available ad libitum for at least a week before being studied. On the morning of an experiment, a mouse was anesthetized by intraperitoneal injection of pentobarbital sodium (60 mg/kg) with supplemental doses (20 mg/kg) as needed to maintain a stable plane of anesthesia as confirmed by lack of withdrawal to toe pinch. Throughout surgical preparation and the experimental protocol (Fig. 1), esophageal temperature was maintained at ∼37°C by placing the mouse on an aluminum warming plate (5 cm × 11 cm). Upon completion of the experimental protocol (duration, 5 to 6 h), the mouse was euthanized by an overdose of pentobarbital sodium (intraperitoneal injection) followed by cervical dislocation.
Fig. 1.

Experimental protocol for evaluating diameters and reactivity in microvascular resistance networks of mouse gluteus maximus muscle (GM). Once anesthesia was induced, surgery required ∼1 h, followed by 30 min equilibration (E30'). Evaluation of constriction in respective vessel branch orders [feed artery (FA), first-order arteriole (1A), second-order arteriole (2A), third-order arteriole (3A)] during equilibration with 21% oxygen (O2) in the superfusion solution required ∼15 min. The superfusion was then re-equilibrated with 0% O2 for another 20 min before proceeding (E 20′). Cumulative concentration-response relationships (10−9 to 10−5 M) required ∼40 min to evaluate each agonist [phenylephrine (PE), UK 14304, ACh] across vessel branch orders followed by 30 min washout and equilibration (E 30′) with control physiological salt solution to restore spontaneous vasomotor tone. Each preparation experienced all stimuli with the order of agonist treatment and the sequence in which vessel branch orders were studied randomized across preparations. An entire experiment required 5 to 6 h to complete. ID, internal diameter; IDrest, resting ID; IDmax, maximal ID to 10−4 M sodium nitroprusside (SNP) or 10−5 M ACh, whichever is greater; IDO2, ID during 21% O2; IDssr = steady-state response ID.
Intravital microscopy.
The GM was prepared as described (1, 21, 33). Briefly, after the surgical area was carefully shaved, the mouse was placed in the prone position on the warming plate. As it was viewed through a stereomicroscope, the overlying skin was removed and the GM was superfused continuously (3 ml/min) thereafter with bicarbonate-buffered physiological salt solution (PSS; 34°C, pH 7.4) containing (in mM) 131.9 NaCl, 4.7 KCl, 2 CaCl2, 1.17 MgSO4, and 18 NaHCO3 equilibrated with 5% CO2-95% N2. Chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO). To expose the resistance vasculature, the GM was carefully dissected from its insertion (lumbar fascia and iliac crest) and spread over a transparent pedestal (Sylgard 184; Dow Corning, Midland, MI), and the edges were pinned to approximate in situ muscle dimensions. The completed preparation was transferred to the fixed stage of an intravital microscope (Nikon E600FN; Melville, NY) mounted on an X-Y translational stage (Gibraltar; Burleigh Instruments, Fishers, NY). The image was acquired through a Nikon SLWD 20× (numerical aperture = 0.35) objective onto a color camera (KP-D50U CCD; Hitachi-Denshi Japan) and observed at a final magnification of 1,200× on a video monitor; spatial resolution was <1 μm. The internal diameter (ID) of vessels was measured as the widest distance between luminal edges with use of a video caliper (Microcirculation Research Institute; Texas A&M University, College Station, TX) calibrated against a stage micrometer (0.01 × 100 = 1 mm; Graticules, Tonbridge Kent, England). The output of the caliper was sampled at 40 Hz with use of a Powerlab/400 system (ADInstruments; Colorado Springs, CO) coupled to a personal computer.
Branch order.
The branch order of arterioles and FA were defined as follows. The FA is the inferior gluteal artery before it enters the muscle. The 1A is the continuation of the same vessel embedded within the muscle. The 2A is a branch originating from the 1A. The 3A is a branch originating from the 2A. Branch angles were typically ∼120°. One vessel from each branch order was studied in each GM; IDs were measured at the same locations (midway along respective branches) throughout experiments. The order in which respective branch orders were observed was varied across experiments as was the age group studied. Three-dimensional mapping with quantitative analyses have shown that the architecture of arteriolar networks supplying the GM of male C57BL/6 mice is conserved during aging (1, 33), substantiating direct comparisons between respective branch orders between Old and Young.
Experimental protocol.
Observation sites for each network were defined during a 30-min equilibration following surgery (Fig. 1) and maintained throughout the experimental protocol. Extensive preliminary experiments defined our protocol and confirmed that completed GM preparations remained stable with reproducible responses for at least 5 h (33).
Oxygen reactivity.
To evaluate the vasomotor response to a rise in Po2, the O2 content of the superfusion solution was increased by equilibrating the PSS with 21% O2, 5% CO2, and 74% N2 for ∼5 min and ID values were recorded (Fig. 1). The PSS was then re-equilibrated with 5% CO2 and 95% N2 for the remainder of the protocol. Empirically, reactivity to changes in PO2 is a sensitive index of the viability of a preparation for intravital microscopy (11, 23, 33).
Concentration-response relationships.
Respective agonists (PE, UK 14304, ACh) were added (≤500 μl) to the 50-ml chamber containing PSS in a cumulative fashion to achieve final agonist concentrations that started at 10−9 M and increased to 10−5 M in 0.5 log increments; steady state IDs were recorded during minutes 2-8 at each concentration. The order of superfusion with respective agonists was randomized across experiments, with a 30-min equilibration following each agonist to restore resting ID (Fig. 1). At the end of each protocol, the GM preparation was superfused with 10−4 M sodium nitroprusside (SNP) for 5 min to obtain values for maximal internal diameter (IDmax) (33). In some cases (irrespective of age) the maximal diameter in response to 10−5 M ACh was slightly greater than that evoked by SNP (Table 1). Therefore, the IDmax (Fig. 2) was defined as the maximal value for ID obtained with either 10−5 M ACh or 10−4 M SNP.
Table 1.
Maximal internal diameters (in μm) for FA and arterioles in young and old mice
| Young |
Old |
|||
|---|---|---|---|---|
| Vessel Branch | SNP | ACh | SNP | ACh |
| FA | 57 ± 2 | 60 ± 2* | 61 ± 4 | 62 ± 5 |
| 1A | 50 ± 2 | 51 ± 2 | 55 ± 5 | 56 ± 5 |
| 2A | 35 ± 3 | 35 ± 2 | 44 ± 3 | 44 ± 4 |
| 3A | 23 ± 2 | 23 ± 1 | 32 ± 3 | 31 ± 4 |
Values are means ± SE; n = 5 per group. One vessel from each branch order was studied in each gluteus maximus muscle (GM) preparation. Maximal internal diameters were obtained during superfusion with 10−4 M sodium nitroprusside (SNP) and with 10−5 M ACh. Young, 4-mo-old male C57BL/6 mice; Old, 24-mo-old male C57BL/6 mice; FA, feed arteries; 1A, first-order arteriole; 2A, second-order arteriole; 3A, third-order arteriole.
P < 0.05, ACh vs. SNP.
Fig. 2.
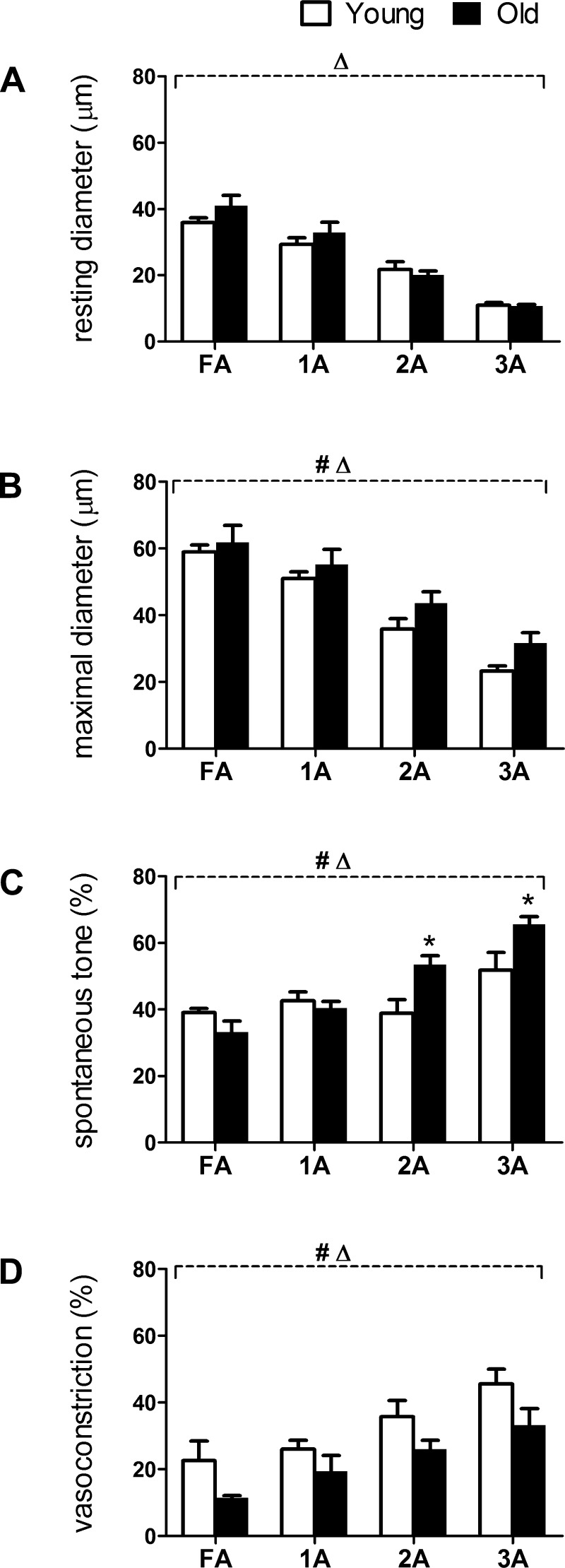
Diameters, vasomotor tone, and O2 response in FA and arterioles of 4-mo-old and 24-mo-old male C57BL/6 mice (Young and Old, respectively). A: resting diameters decreased from proximal to distal vessels as branch order increased from FA to 3A in both age groups. B: maximal diameters were decreased as in A and were slightly but consistently greater in Old versus Young. C: spontaneous vasomotor tone (percentage; calculated as [(IDmax − IDrest)/IDmax] × 100%) of 2A and 3A was greater in Old versus Young. D: vasoconstriction (in percentage) to 21% O2 in the superfusion solution (calculated as [(IDrest − IDO2)/IDrest] × 100%) was reduced throughout network branches in Old compared with Young. Summary data are means ± SE; n = 5 per group. #P < 0.05, main effect of age; ΔP < 0.05, main effect of vessel branch order; *P < 0.05, Old vs. Young.
Data analyses and statistics.
For each vessel branch order, spontaneous vasomotor tone was calculated as the difference between resting and maximal ID and expressed relative to maximal ID. Thus, vasomotor tone (%) = [(IDmax − IDrest)/IDmax] × 100%, where IDrest = resting baseline (control) ID. The response to elevated O2 was calculated as the magnitude of vasoconstriction during equilibration of the superfusion solution with 21% O2 and expressed relative to resting ID. Thus, O2 response (%) = [(IDrest − IDO2)/IDrest] × 100%, where IDO2 = ID during superfusion with 21% O2. To evaluate reductions in ID from control during AR activation, vasoconstriction was expressed relative to respective resting IDs. Thus, vasoconstriction (%) = [(IDrest − IDssr)/IDrest] × 100%, where IDssr = ID of the steady-state response to a given agonist concentration and 100% indicates closure of the vessel lumen.
To evaluate the sensitivity of EDD for respective vessel branches in response to ACh, vasodilation was normalized to the respective maximal change in ID. Thus, “vasodilator capacity” (%) = [(IDssr − IDrest)/(IDmax − IDrest)] × 100%. This definition spans from 0% to 100% for all vessels irrespective of actual diameter values and thereby enables relative differences in sensitivity to be evaluated for a given agonist. Thus EC50 values can be determined for each vessel on the same relative scale. However, this normalization does not account for changes in diameter as they pertain to vascular conductance and blood flow regulation. Therefore to evaluate the functional increase in diameter relative to control conditions at rest, vasodilation was normalized to IDrest. Thus, “functional vasodilation” (%) = [(IDssr − IDrest)/IDrest] × 100%. For example, a 100% increase indicates a doubling of ID from the resting baseline and predicts (according Poiseuille's law) a 16-fold increase in blood flow through the vessel at constant perfusion pressure.
Data were analyzed using two-way ANOVA (GraphPad Prism 5; La Jolla, CA) to evaluate the main effects of age and vessel branch orders. Bonferroni tests were performed for post hoc comparisons. Summary data are expressed as means ± SE. Differences were accepted as statistically significant with P < 0.05.
RESULTS
All GM preparations included in this study exhibited spontaneous vasomotor tone throughout their resistance networks. Each vessel that was studied underwent constriction during superfusion with elevated O2, confirming the viability of all preparations for further study.
Diameters, vasomotor tone, and O2 response.
At rest under control conditions, ID decreased as branch order increased from proximal FA to distal 3A (P < 0.05) with no difference between Old and Young for any branch order (Fig. 2A). During maximal dilation, IDs of Old were slightly but consistently greater than Young across vessel branch orders (P < 0.05; Fig. 2B). Spontaneous vasomotor tone was not different for FA or 1A between age groups but was greater (P < 0.05) in 2A and 3A of Old versus Young (Fig. 2C). Vasoconstriction during elevated Po2 of the superfusion solution increased with branch order from FA to 3A (P < 0.05) but was reduced in Old compared with Young across vessel branches (P < 0.05; Fig. 2D).
Vasoconstriction to PE.
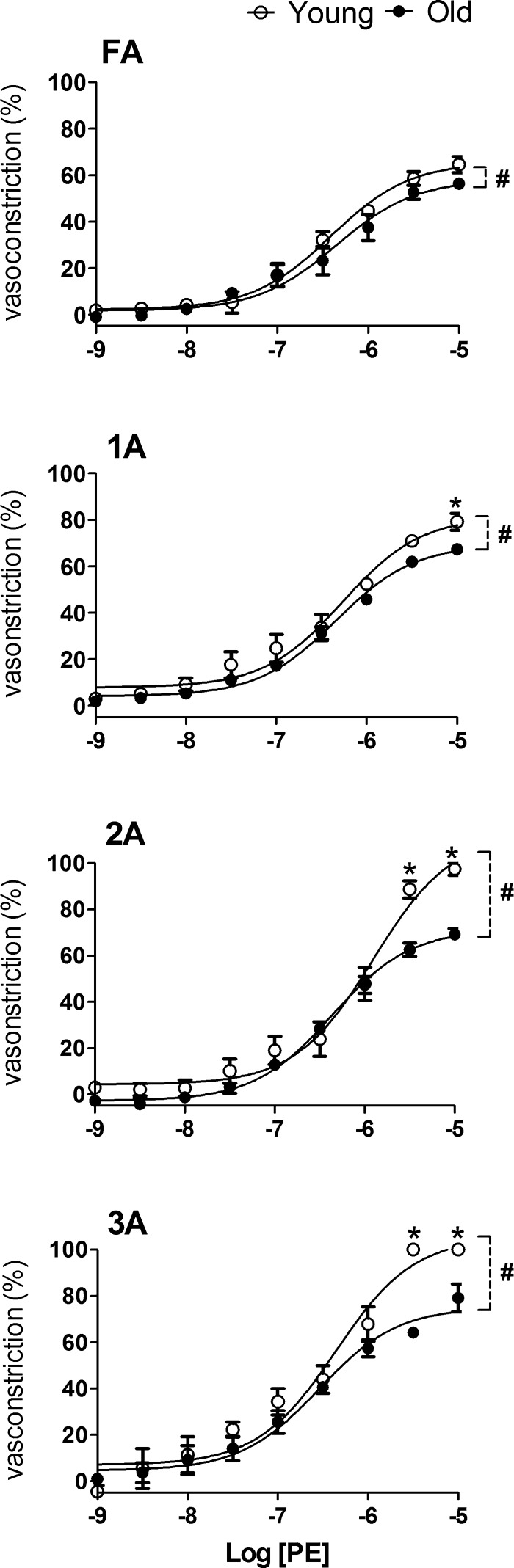
Selective activation of α1ARs with PE induced concentration-dependent vasoconstriction of each branch order (Fig. 3). From 10−9 to 10−6 M, PE induced constrictions within each branch order that were similar in Old and Young (Fig. 3), although an effect of age was apparent at greater [PE]. Peak constrictions in Old were less than in Young, particularly in 2A and 3A where closure occurred in Young (confirmed by absence of red blood cells and disappearance of the lumen) but not in Old (where sluggish flow of red blood cells persisted). The EC50 values for PE were similar between Old and Young across branch orders, with differences between age groups consistently within 0.5 log units (Table 2).
Fig. 3.
Response curves to PE in FA and arterioles of Young and Old. Advanced age attenuated peak vasoconstriction to α1 adrenoreceptor (α1AR) activation, and this effect increased with vessel branch order. Vasoconstriction (in percentage) was calculated for each branch order as [(IDrest − IDssr)/IDrest] × 100%. Summary data are means ± SE; n = 5 per group. #P < 0.05, main effect of age group; *P < 0.05, Old vs. Young.
Table 2.
Log EC50 values for PE, UK 14304, and ACh for FA and arterioles in young and old mice
| PE |
UK 14304 |
ACh |
||||
|---|---|---|---|---|---|---|
| Vessel Branch | Young | Old | Young | Old | Young | Old |
| FA | −6.4 ± 0.1 | −6.4 ± 0.1 | −6.2 ± 0.2 | −6.4 ± 0.3 | −7.5 ± 0.1 | −7.4 ± 0.1 |
| 1A | −6.3 ± 0.1 | −6.3 ± 0.1 | −6.5 ± 0.2 | −6.3 ± 0.2 | −7.0 ± 0.1 | −6.9 ± 0.1 |
| 2A | −5.9 ± 0.1 | −6.4 ± 0.1 | −6.7 ± 0.1 | −6.5 ± 0.2 | −6.4 ± 0.1 | −6.9 ± 0.1 |
| 3A | −6.4 ± 0.1 | −6.5 ± 0.1 | −7.2 ± 0.2 | −6.5 ± 0.2 | −6.2 ± 0.1 | −6.7 ± 0.1 |
Values are means ± SE; n = 5 per group. One vessel from each branch order was studied in each GM preparation. With EC50 values as an index, vascular sensitivity to phenylephrine (PE), UK 14304, and ACh was not significantly different between age groups as determined by using 2-way ANOVA.
Vasoconstriction to UK 14304.
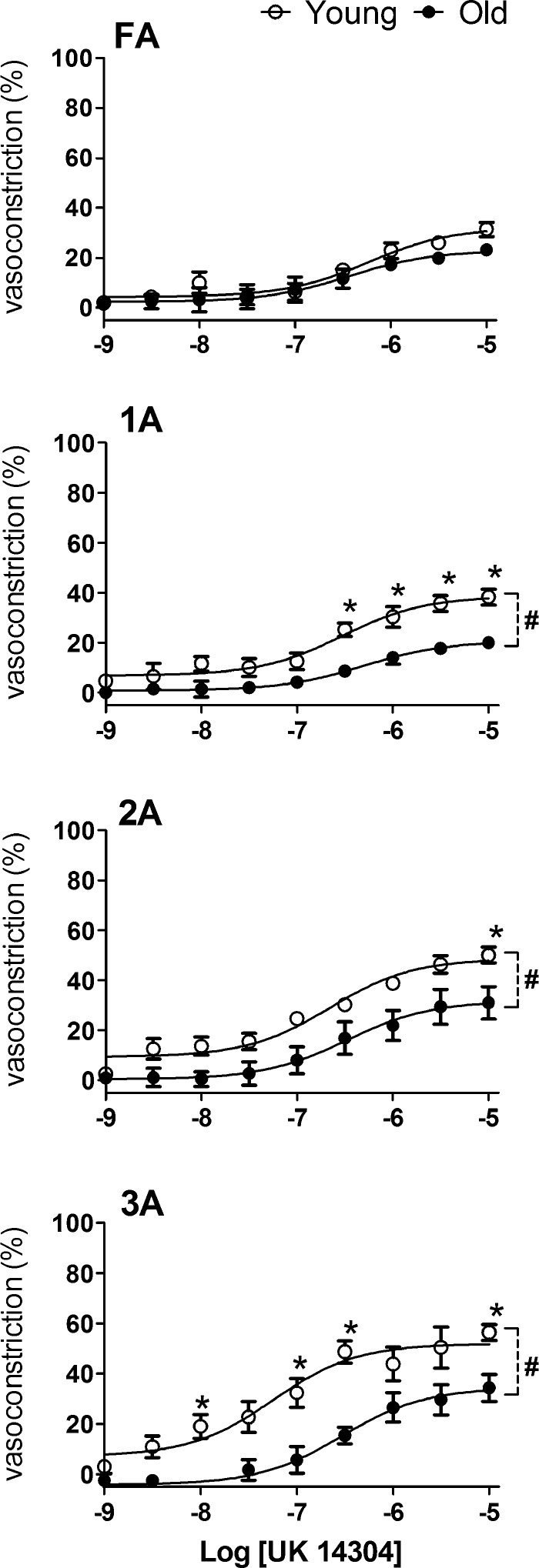
Selective activation of α2ARs with UK 14304 induced a concentration-dependent constriction across vessel branches. The efficacy of α2AR-mediated constrictions increased with branch order from FA to 3A and was greater in Young versus Old in 1A, 2A, and 3A (P < 0.05; Fig. 4). The EC50 values for UK 14304 were similar between Old and Young across branch orders, with differences between age groups typically within 0.5 log units (Table 2).
Fig. 4.
Response curves to UK 14304 in FA and arterioles of Young and Old. Advanced age attenuated vasoconstriction to α2AR activation throughout the arteriolar network despite negligible effect in FA. Vasoconstriction (in percentage) was calculated as in Fig. 3. Summary data are means ± SE; n = 5 per group. #P < 0.05, main effect of age; *P < 0.05, Old vs. Young.
Effects of selective α1AR versus α2AR activation.
Throughout the resistance network of the GM, the magnitude of constriction increased with vessel branch order. Activation of α1ARs with PE consistently produced approximately twofold greater constriction than did activation of α2ARs with UK 14304 (Figs. 3 and 4). Whereas α2AR-mediated constriction was attenuated in all arteriolar branch orders of Old compared with Young (Fig. 4), α1AR responses were maintained in Old although 2A and 3A were resistant to closure (Fig. 3).
Endothelium-dependent dilation.
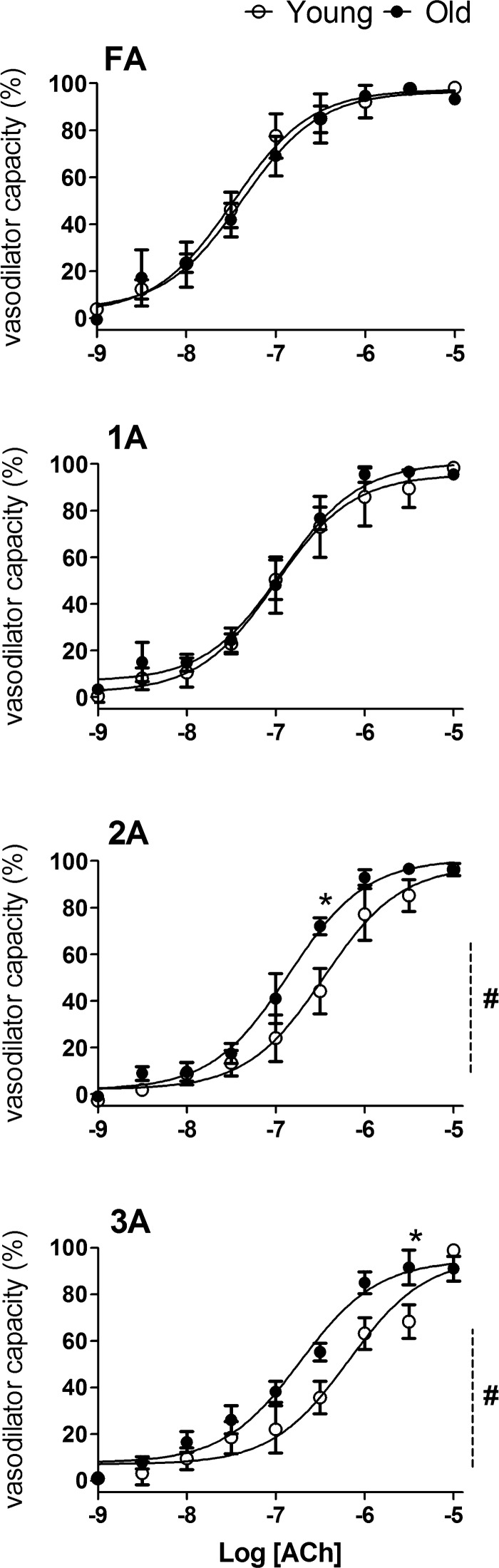
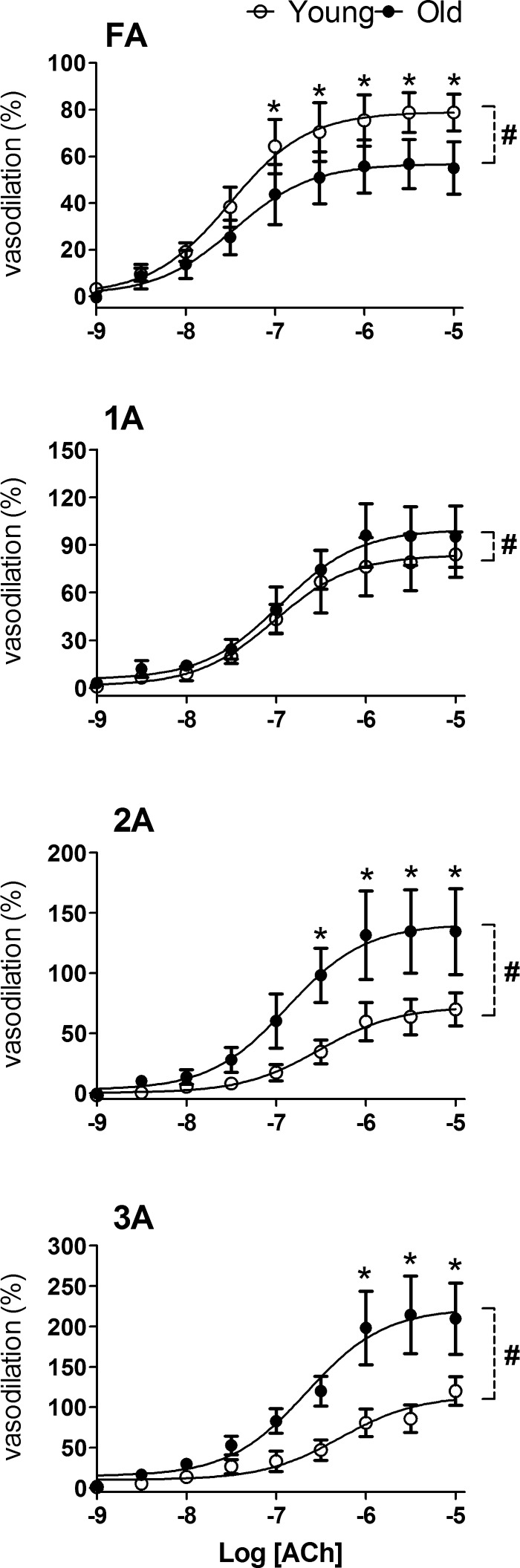
ACh evoked concentration-dependent vasodilation in all vessel branches of Old and Young GM (Figs. 5 and 6). When expressed relative to vasodilator capacity for respective branches to evaluate sensitivity, FA and 1A responses were not different between Young and Old, whereas EDD of 2A and 3A in Old were shifted slightly (<0.5 log unit) but significantly to the left when compared with Young (Table 2 and Fig. 5). When expressed relative to respective resting control diameters, the magnitude of EDD of FA was less (P < 0.05) in Old compared with Young (Fig. 6). In contrast, EDD of 2A and 3A was approximately twofold greater (P < 0.05) in Old compared with Young (Fig. 6).
Fig. 5.
Response curves to ACh in FA and arterioles of Young and Old relative to maximal changes in diameter. Vasodilator capacity (in percentage) was calculated as [(IDssr − IDrest)/(IDmax − IDrest)] × 100% and was preserved with advanced age across vessel branches with greater potency for Old versus Young in 2A and 3A. Summary data are means ± SE; n = 5 per group. #P < 0.05, main effect of age; *P < 0.05, Old vs. Young.
Fig. 6.
Response curves to ACh in FA and arterioles of Young and Old relative to resting diameters. Functional vasodilation (in percentage) was calculated as [(IDssr − IDrest)/IDrest] × 100% and increased with branch order from FA to 3A (note difference in ordinate scales). Whereas endothelium-dependent dilation of FA was attenuated in Old versus Young, endothelium-dependent dilation in arterioles was enhanced with advanced age, particularly in 2A and 3A. Summary data are means ± SE; n = 5 per group. #P < 0.05, main effect of age; *P < 0.05, Old vs. Young.
Branch order differences.
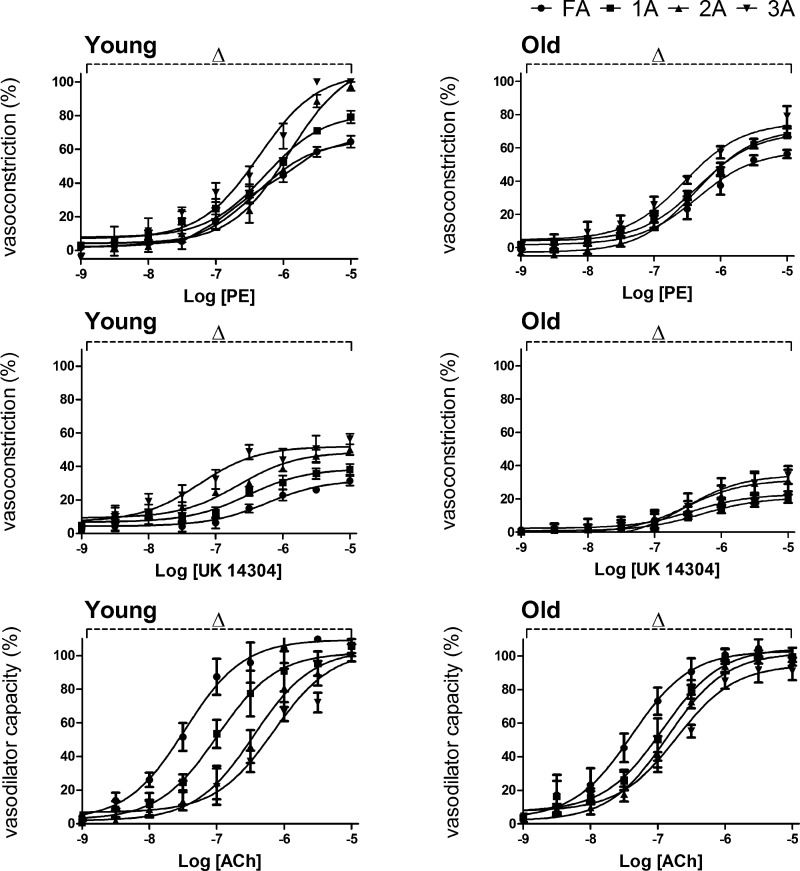
In addition to differences between Old and Young within individual branch orders, vasomotor responses to respective agonists exhibited differences between branch orders within age groups. In Young, [PE] > 10−7 M induced greater α1AR-mediated constriction in distal versus proximal branches such that 3A > 2A > 1A > FA (Fig. 7). In Old, this effect of branch order was maintained though less robust. For [UK 14304] > 10−8 M, α2AR-mediated constriction also increased with vessel branch order with 3A > 2A > 1A > FA, and this relationship was also maintained (although less robust) in Old (Fig. 7). For EDD, the trend was reversed from that seen during vasoconstriction such that the sensitivity to ACh was greatest in proximal vessels with FA > 1A > 2A > 3A. As seen with vasoconstriction, differences between branch orders were more robust in Young than in Old (Fig. 7). For each agonist, there was a significant effect (P < 0.05) of vessel branch order as well as concentration.
Fig. 7.
Branch order differences in reactivity to agonists in Young and Old. In Young (left), α1AR-mediated constriction increased with PE >10−7 M and was greater in distal versus proximal branches (3A > 2A > 1A > FA). With lower efficacy, a similar trend resulted from α2AR-mediated constriction in response to UK 14304. In contrast, proximal vessels exhibited greater sensitivity during EDD with ACh such that FA > 1A > 2A > 3A. In Old (right), respective patterns of branch order differences persisted but encompassed a smaller range. Summary data are means ± SE; n = 5 per group. ΔP < 0.05, main effect of branch order. Data from Figs. 3–5 are replotted here to facilitate comparisons across branch orders for respective age groups and agonists. Respective EC50 values are given in Table 2.
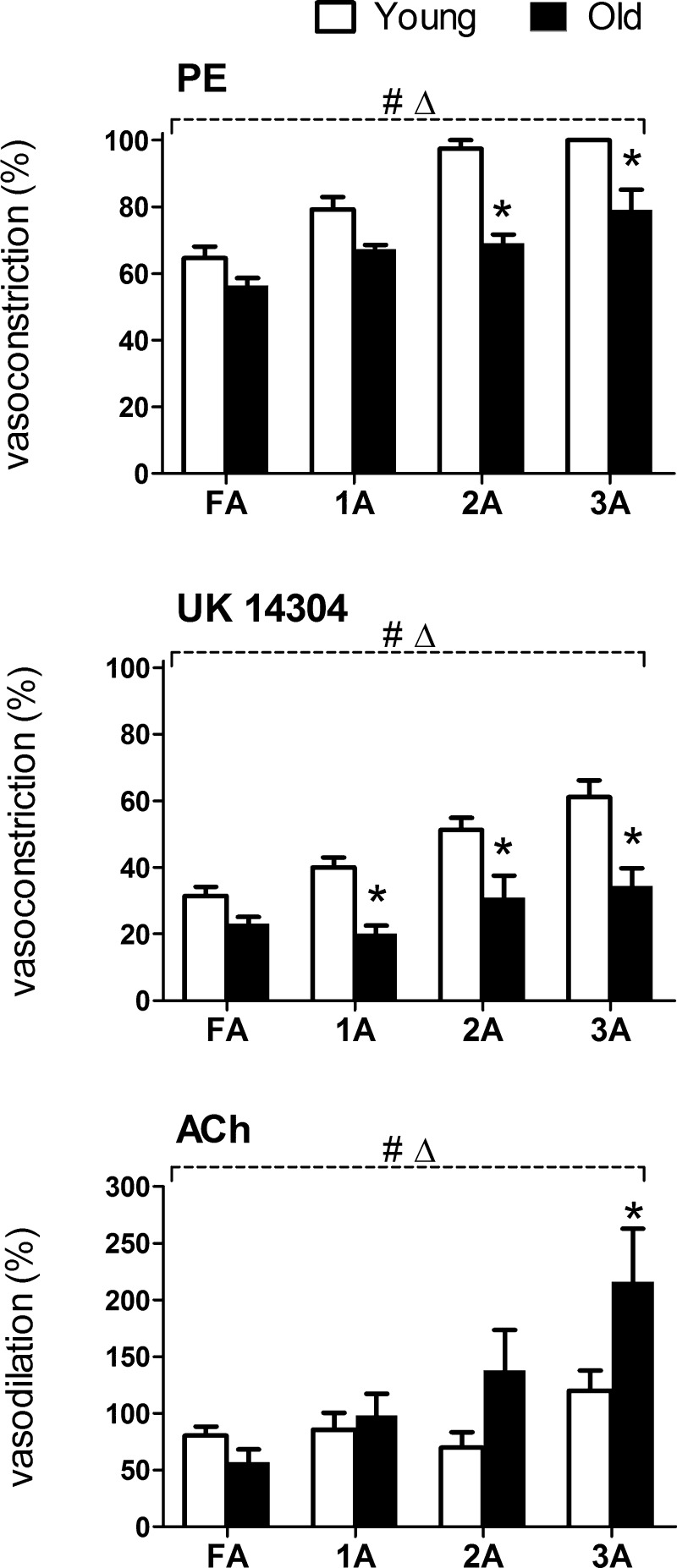
Maximal responses to agonists.
To evaluate how advanced age influenced the dynamic range of vasoconstriction and vasodilation in respective vessel branch orders, maximal responses to the activation of α1ARs (PE), α2ARs (UK 14304), and muscarinic receptors (ACh) are presented for respective agonists in Fig. 8. Across vessel branch orders, PE consistently evoked approximately twofold greater vasoconstriction than did UK 14304. Maximal vasoconstriction to the activation of either α1ARs or α2ARs was reduced in Old versus Young (P < 0.05), particularly in arterioles. EDD increased with branch order in arterioles of Old but not in arterioles of Young. Irrespective of age group, maximal diameters with ACh were similar to those with SNP (Table 1).
Fig. 8.
Maximal responses to agonists in FA and arterioles supplying the GM of Young and Old. Maximal vasoconstrictions to PE (from Fig. 3) and to UK 14304 (from Fig. 4) were attenuated throughout the resistance network of GM in Old versus Young; this effect increased with arteriolar branch order. Maximal dilation to ACh (from Fig. 6) was depressed in FA of Old versus Young; in arterioles, maximal vasodilation increased with branch order with Old progressively greater than Young. Summary data are means ± SE; n = 5 per group. ΔP < 0.05, main effect of branch order; *P < 0.05, Old vs. Young.
DISCUSSION
The present study has defined microvascular reactivity along resistance networks of skeletal muscle in Young and Old. Using intravital microscopy to study the mouse GM, we evaluated internal vessel diameters, vasomotor tone, and responses to defined vasoactive stimuli. Under resting conditions, Young and Old exhibited similar diameters for FA and respective arteriolar branches. During maximal dilation, diameters of distal arterioles (i.e., 2A and 3A) tended to be larger in Old compared with Young, reflecting greater spontaneous vasomotor tone. Nevertheless, vasoconstriction in response to elevated O2 was attenuated throughout the networks of Old compared with Young. Furthermore, although α1ARs were twice as effective as α2ARs in evoking constrictions, advanced age attenuated responses to both AR subtypes, particularly in distal arterioles. Remarkably, with similar resting diameters, vasodilation to ACh (i.e., EDD) was greater in distal arterioles of Old compared with Young. Thus the effect of advanced age on the reactivity of microvessels supplying skeletal muscle varies with branch order and nature of the vasoactive stimulus.
Vessel diameters.
In accord with Poiseuilles′ law, the IDs of resistance arteries and arterioles are principal determinants of both tissue perfusion and peripheral resistance. Consistent with previous studies of microvascular resistance networks (4, 31, 33), vessel diameter decreased as branch order increased with FA > 1A > 2A > 3A in Young and Old (Fig. 2). At rest under control conditions, the IDs of respective branch orders were not different between age groups (Fig. 2A). Although these hierarchical relationships were maintained during maximal dilation, arteriolar diameters tended to be larger in Old compared with Young as branch order increased (Fig. 2B). As a consequence, and despite no difference in resting diameters, vasomotor tone increased with vessel branch order, particularly in 2A and 3A of Old when compared with those in Young (Fig. 2C). This scenario contrasts with hypertension and diabetes, where thickening, narrowing, and rarefaction of arterioles (with tissue ischemia) are found (18, 19, 41, 42). Recent findings have shown no difference in systolic blood pressure in male C57BL/6 mice at 3 vs. 24 mo (49); thus the present data appear most applicable to “healthy” aging in contrast with conditions associated with vascular disease.
Oxygen response.
As O2 delivery increases relative to demand, resistance microvessels of skeletal muscle constrict as a mechanism of negative feedback. Raising the O2 content of the superfusion solution provides a source of O2 in addition to that carried in the bloodstream (11, 29, 51). In turn, constriction of arterioles in response to elevating superfusion Po2 is an exquisitely sensitive index of the functional integrity of exposed microvessels (11, 23, 33). Typically, O2 responses are evaluated in a single arteriole within a preparation before beginning experiments to assess viability of the preparation (1, 21, 33). Consistent with such criteria, constriction of each branch of the GM resistance network during equilibration with 21% O2 confirmed the integrity of our preparations irrespective of age group. Because the O2 sensor may be located within the tissue (22), our finding that constriction during elevation of superfusion Po2 encompassed FAs external to the muscle is consistent with the ability of O2 to depolarize arteriolar SMCs (54) and evoke conducted vasoconstriction (22). Through evaluating entire resistance networks, we demonstrate that the relative magnitude of constriction in response to elevated PO2 increased with vessel branch order, from FA to 3A (Fig. 2D). Consistent with earlier findings focused on 2A of the GM (1, 21), the O2 response was depressed in all branch orders in Old versus Young (Fig. 2D). Thus the ability of O2 to evoke constriction is attenuated throughout the resistance vasculature with aging. Given the similarities in vessel IDs at rest between age groups (Fig. 2A), greater spontaneous vasomotor tone in 2A and 3A of Old versus Young (Fig. 2C) suggests that the role of PO2 in governing SMC activation is reduced in advanced age. In light of tissue oxygenation being integral to capillary perfusion and blood flow regulation (11, 29, 51), attenuated O2 responses in Old (Fig. 2D) imply alterations in the regulation of muscle blood flow during advanced age, e.g., as manifested through a greater role for reactive oxygen species (3, 34, 44).
Adrenergic reactivity.
In human subjects, femoral arterial blood flow and vascular conductance were 30–40% lower in older (∼63 years) compared with young (∼28 years) males in association with tonic elevation of muscle SNA and the activation of αARs (5, 8). Although comparable recordings of SNA with aging are lacking in mice, the activities of key enzymes governing catecholamine synthesis (e.g., tyrosine hydroxylase) were approximately twofold higher in the adrenal glands of Old (28 mo) compared with Young (4 mo) male mice and rats (40). Independent studies found norepinephrine release from sympathetic nerves to be greater at rest and during stress in Old (24 mo) versus Young (3 mo) male Fischer-344 rats (32). In light of enhanced constitutive αAR activation in GM of Old (21), findings collectively suggest a greater level of SNA in Old versus Young. Adrenergic vasoconstriction is mediated postsynaptically by two AR subtypes on vascular SMCs: α1ARs and α2ARs (13, 33). The selectivity of PE and UK 14304 on respective αAR subtypes has been confirmed in the mouse GM (33). In both Young and Old studied here, the activation of α1ARs with PE constricted all vessel branches to a greater extent than did activation of α2ARs with UK 14304 (Figs. 3 and 4). Thus although both receptor subtypes mediate vasoconstriction in the GM microcirculation, α1ARs consistently displayed approximately twofold greater efficacy when compared with α2ARs. Furthermore, although the ability of α1ARs and of α2ARs to evoke constriction increased with vessel branch order (Fig. 8), there were no consistent differences between age groups in their sensitivity (i.e., EC50 values) to respective agonists (Table 2).
In humans, reductions in forearm blood flow during α1AR activation (via intra-arterial infusion of PE) were blunted in older (∼65 years) compared with younger (∼26 years) males, whereas blood flow reductions to α2AR activation with clonidine infusion were similar between age groups (7). Thus attenuated reductions in forearm blood flow in older versus younger men during tyramine infusion (to evoke release of endogenous norepinephrine) were attributed to reduced responsiveness of α1ARs (7). During exposure to PE, we observed that 2A and 3A of Old were unable to constrict to the same extent observed in Young (Fig. 3). This new finding illustrates an important functional limitation that may help to explain the reduced response to activation of α1ARs in the human forearm with aging. In turn, the inability to fully constrict 2A and 3A of Old during maximal activation of α1ARs (Fig. 3) may reflect tissue remodeling with aging (57). For example, an increase in the amount, orientation, and/or stiffness of the extracellular matrix (24, 27) may resist lumen closure of smaller arterioles in Old that are otherwise able to do so in younger animals. Further studies at the ultrastructural level are required to provide greater insight into the nature of this adaptation to advanced age.
During tyramine infusion into the femoral artery, reductions in leg blood flow were also attenuated in older (∼62 years) versus younger (∼24 years) men and attributed to the attenuation of both α1AR- and α2AR-mediated responses with aging (47). The effect of aging on reducing maximal constriction was greater for α2AR versus α1AR activation in the mouse GM (Fig. 8). The activation of α1ARs was more efficacious than that of α2ARs in evoking vasoconstriction and was relatively less affected by advanced age, particularly in FA and 1A (Fig. 8). Thus AR activation was still able to restrict muscle blood flow in Old. Indeed, relative to attenuated α2AR-mediated constriction of arterioles with aging, α1AR-mediated constriction was maintained at all but the highest PE concentrations (Fig. 3), which may help to ensure the maintenance of peripheral resistance and arterial blood pressure during exercise (48).
Endothelium-dependent dilation.
As demonstrated in humans (9, 17, 44) and in resistance vessels isolated from rats (2, 35, 55, 56), advanced aging is associated with attenuated EDD, typically characterized by activating muscarinic receptors on the endothelium. Nevertheless, intravital studies of the mouse GM had indicated no difference in the ability of ACh to dilate 2A of Young versus Old in vivo (1). To investigate the effect of advanced age on EDD along resistance networks, we quantified responses to ACh in two ways. First, to address the sensitivity of EDD, changes in ID at each ACh concentration were normalized to the corresponding maximal change in ID. These data indicate that the vasodilator capacity of FA and of arterioles in Old was not different from that of Young (Fig. 5). Furthermore, there was a trend for greater sensitivity to ACh in 2A and 3A of Old compared with Young (Fig. 5). Finding that the maximal ID of each branch order to ACh was similar to that evoked by the nitric oxide donor SNP (Table 1) indicates that the endothelium lining resistance networks of Old were fully able to drive vascular smooth muscle relaxation. In contrast, as an index for the dynamic range of blood flow control, normalizing ID changes to respective resting diameters (i.e., functional vasodilation) revealed that EDD was depressed significantly in FA of Old while enhanced in 2A and 3A of the same networks (Fig. 6). Whereas reduced EDD in FA of Old versus Young is consistent with impaired EDD of conduit and resistance arteries with advanced age (12, 44, 53), enhanced EDD of the smaller downstream arterioles in Old suggests that the effect of advanced age on EDD varies with microvessel branch order in vivo. Such regional differences within the microcirculation have not been documented previously. In part this is attributable to the focus on FA and 1A in previous studies (2, 35, 50, 55). Furthermore, in vivo studies evaluating ACh-induced EDD in human skeletal muscle with aging were based on changes in limb blood flow (9, 10, 17). In such cases, specific branch orders within the microcirculation as emphasized here could not be accounted for.
Summary and Perspective
Studies in humans have illustrated that skeletal muscle blood flow is reduced with advanced age in association with augmented SNA (8, 14) along with impaired EDD (9, 44). However, such studies are based primarily upon evaluating diameter and velocity in conduit (e.g., brachial and femoral) arteries, where changes in blood flow reflect regulatory events occurring further downstream, i.e., in the microcirculation. Such measurements in humans are thereby limited by the inability to directly observe those vessels actually responsible for controlling tissue perfusion and peripheral resistance. Thus using rodents as a model system provides an opportunity to extend direct observations of blood flow control into the mammalian microcirculation. The effect of aging on resistance microvessels of rats has focused on FAs (50, 55, 56) and the large arterioles (e.g., 1A) (2, 35). These proximal microvessels are most readily isolated and studied in vitro because they are of sufficient diameter and length to enable microdissection and cannulation for evaluation using pressure myography. Where the effect of aging on arterioles has been studied in vivo, observations have focused on 1A and 2A in muscles of rats and mice (1, 6). In contrast, this study is the first to examine the effects of aging on vascular reactivity throughout microvascular resistance networks of skeletal muscle in vivo, from proximal FA through 3A further downstream.
Using the GM preparation in male C57BL/6 mice, we report that advanced age (i.e., 24 vs. 4 mo) blunted the sensitivity to O2 throughout networks with the greatest effect in distal (2A, 3A) arterioles. Advanced age also prevented closure 2A and 3A arterioles during maximal activation of α1ARs, which may be explained by tissue remodeling and associated changes in the extracellular matrix. Nearly all constrictions to α2AR activation were attenuated throughout arteriolar networks but not in FAs, highlighting regional differences in microvascular adaptations to aging. We speculate that the loss of arteriolar α2AR reactivity with aging may result from a tonic increase in receptor activation (21, 40), consistent with findings in humans (5, 8). Remarkably, whereas constriction of the smaller (2A, 3A) arterioles was impaired consistently in Old versus Young, these same arteriolar branches in Old exhibited enhanced EDD and greater spontaneous vasomotor tone. Thus distal arterioles exhibiting the greatest attenuation of constrictor responses simultaneously exhibited the greatest dilator responses. Nevertheless, maintained constriction (or impaired EDD) of proximal FA can restrict muscle blood flow even when distal arterioles are dilated maximally (21, 52). Unlike studies in humans, which have relied on indirect measurements of limb blood flow, intravital studies in mice enable direct observations of respective microvessels that control flow magnitude (FA, 1A) as well as its distribution within the tissue (2A, 3A).
The consistency of our present findings in the mouse GM with those from earlier studies of human subjects suggests that the GM is a viable model for investigating how aging affects the microcirculation of skeletal muscle. As typical of human skeletal muscles (28, 43), the mouse GM is of mixed fiber type (26, 30). Thus the insight gained from understanding where and how advanced age affects adrenergic vasoconstriction, EDD, and blood flow control in respective microvascular branch orders of the mouse GM in vivo may well be applied toward developing selective therapeutic strategies for promoting muscle blood flow in aging humans.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant R01-HL086483. S. S. Segal is supported by NHLBI MERIT Award R37-HL041026.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.Y.S. and S.S.S. conception and design of research; S.Y.S. performed experiments; S.Y.S. and S.S.S. analyzed data; S.Y.S. and S.S.S. interpreted results of experiments; S.Y.S. prepared figures; S.Y.S. drafted manuscript; S.Y.S. and S.S.S. edited and revised manuscript; S.Y.S. and S.S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Drs. Erika Boerman and Matthew Socha provided a critical review of this work.
REFERENCES
- 1.Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol 561: 535–545, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behnke BJ, Delp MD. Aging blunts the dynamics of vasodilation in isolated skeletal muscle resistance vessels. J Appl Physiol 108: 14–20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behringer EJ, Shaw RL, Westcott EB, Socha MJ, Segal SS. Aging impairs electrical conduction along endothelium of resistance arteries through enhanced Ca2+-activated K+ channel activation. Arterioscler Thromb Vasc Biol 33: 1892–1901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boegehold MA, Johnson PC. Response of arteriolar network of skeletal muscle to sympathetic nerve stimulation. Am J Physiol Heart Circ Physiol 254: H919–H928, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Casey DP, Joyner MJ. Influence of alpha-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol 113: 1201–1212, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook JJ, Wailgum TD, Vasthare US, Mayrovitz HN, Tuma RF. Age-related alterations in the arterial microvasculature of skeletal muscle. J Gerontol 47: B83–B88, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res 27: 669–678, 1970 [DOI] [PubMed] [Google Scholar]
- 12.Egashira K, Inou T, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Kuga T, Urabe Y, Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation 88: 77–81, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Faber JE. In situ analysis of α-adrenoceptors on arteriolar and venular smooth muscle in rat skeletal muscle microcirculation. Circ Res 62: 37–50, 1988 [DOI] [PubMed] [Google Scholar]
- 14.Fisher JP, Paton JF. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens 26: 463–475, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Flurkey K, Currer J, Harrison D. The mouse in aging research. In: The Mouse in Biomedical Research, edited by Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL. Burlington: American College Laboratory Animal Medicine (Elsevier), 2007, p. 637–672 [Google Scholar]
- 16.Haug SJ, Segal SS. Sympathetic neural inhibition of conducted vasodilatation along hamster feed arteries: complementary effects of α1- and α2-adrenoreceptor activation. J Physiol 563: 541–555, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis 186: 390–395, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hutchins PM. Arteriolar rarefaction in hypertension. Bibl Anat 166–168, 1979 [PubMed] [Google Scholar]
- 19.Hutchins PM, Lynch CD, Cooney PT, Curseen KA. The microcirculation in experimental hypertension and aging. Cardiovasc Res 32: 772–780, 1996 [PubMed] [Google Scholar]
- 20.Irion GL, Vasthare US, Tuma RF. Age-related change in skeletal muscle blood flow in the rat. J Gerontol 42: 660–665, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol 588: 2269–2282, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson WF. Arteriolar oxygen reactivity: where is the sensor? Am J Physiol Heart Circ Physiol 253: H1120–H1126, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Jackson WF, Duling BR. The oxygen sensitivity of hamster cheek pouch arterioles. In vitro and in situ studies. Circ Res 53: 515–525, 1983 [DOI] [PubMed] [Google Scholar]
- 24.Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res 95: 194–204, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Lampa SJ, Potluri S, Norton AS, Laskowski MB. A morphological technique for exploring neuromuscular topography expressed in the mouse gluteus maximus muscle. J Neurosci Methods 138: 51–56, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension 45: 1050–1055, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50 Spec No: 11–16, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Lindbom L, Tuma RF, Arfors KE. Influence of oxygen on perfused capillary density and capillary red cell velocity in rabbit skeletal muscle. Microvasc Res 19: 197–208, 1980 [DOI] [PubMed] [Google Scholar]
- 30.Manttari S, Jarvilehto M. Comparative analysis of mouse skeletal muscle fibre type composition and contractile responses to calcium channel blocker. BMC physiology 5: 4, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall JM. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol 332: 169–186, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarty R, Pacak K, Goldstein DS, Eisenhofer G. Regulation of peripheral catecholamine responses to acute stress in young adult and aged F-344 rats. Stress 2: 113–122, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Moore AW, Jackson WF, Segal SS. Regional heterogeneity of α-adrenoreceptor subtypes in arteriolar networks of mouse skeletal muscle. J Physiol 588: 4261–4274, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller-Delp JM, Gurovich AN, Christou DD, Leeuwenburgh C. Redox balance in the aging microcirculation: new friends, new foes, and new clinical directions. Microcirculation 19: 19–28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46: 454–462, 2005 [DOI] [PubMed] [Google Scholar]
- 37.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res 110: 1097–1108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohyanagi M, Faber JE, Nishigaki K. Differential activation of α1- and α2-adrenoceptors on microvascular smooth muscle during sympathetic nerve stimulation. Circ Res 68: 232–244, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Proctor DN, Le KU, Ridout SJ. Age and regional specificity of peak limb vascular conductance in men. J Appl Physiol 98: 193–202, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Reis DJ, Ross RA, Joh TH. Changes in the activity and amounts of enzymes synthesizing catecholamines and acetylcholine in brain, adrenal medulla, and sympathetic ganglia of aged rat and mouse. Brain Res 136: 465–474, 1977 [DOI] [PubMed] [Google Scholar]
- 41.Rizzoni D, Agabiti-Rosei E. Structural abnormalities of small resistance arteries in essential hypertension. Intern Emerg Med 7: 205–212, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Rizzoni D, Rosei EA. Small artery remodeling in diabetes mellitus. Nutr Metab Cardiovasc Dis 19: 587–592, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Saltin B, Henriksson J, Nygaard E, Andersen P, Jansson E. Fiber types and metabolic potentials of skeletal muscles in sedentary man and endurance runners. Ann NY Acad Sci 301: 3–29, 1977 [DOI] [PubMed] [Google Scholar]
- 44.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci 120: 357–375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation 12: 33–45, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol 536: 937–946, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol 582: 63–71, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol 97: 731–738, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trott DW, Luttrell MJ, Seawright JW, Woodman CR. Aging impairs PI3K/Akt signaling and NO-mediated dilation in soleus muscle feed arteries. Eur J Appl Physiol 113: 2039–2046, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Tuma RF, Lindbom L, Arfors KE. Dependence of reactive hyperemia in skeletal muscle on oxygen tension. Am J Physiol Heart Circ Physiol 233: H289–H294, 1977 [DOI] [PubMed] [Google Scholar]
- 52.VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol 550: 563–574, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vargas F, Osuna A, Fernandez-Rivas A. Abnormal renal vascular reactivity to acetylcholine and nitroprusside in aging rats. Gen Pharmacol 28: 133–137, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Welsh DG, Jackson WF, Segal SS. Oxygen induces electromechanical coupling in arteriolar smooth muscle cells: a role for L-type Ca2+ channels. Am J Physiol Heart Circ Physiol 274: H2018–H2024, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 93: 1685–1690, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Woodman CR, Price EM, Laughlin MH. Selected contribution: aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol 95: 2164–2170, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Yildiz O. Vascular smooth muscle and endothelial functions in aging. Ann NY Acad Sci 1100: 353–360, 2007 [DOI] [PubMed] [Google Scholar]