Abstract
Selective stimulation of inhibitory A1 and facilitatory A2a adenosine receptor subtypes located in the nucleus of the solitary tract (NTS) powerfully inhibits cardiopulmonary chemoreflex (CCR) control of regional sympathetic outputs via different mechanisms: direct inhibition of glutamate release and facilitation of an inhibitory neurotransmitter release, respectively. However, it remains unknown whether adenosine naturally released into the NTS has similar inhibitory effects on the CCR as the exogenous agonists do. Our previous study showed that adenosine is released into the NTS during severe hemorrhage and contributes to reciprocal changes of renal (decreases) and adrenal (increases) sympathetic nerve activity observed in this setting. Both A1 and A2a adenosine receptors are involved. Therefore, we tested the hypothesis that, during severe hemorrhage, CCR control of the two sympathetic outputs is attenuated by adenosine naturally released into the NTS. We compared renal and adrenal sympathoinhibitory responses evoked by right atrial injections of 5HT3 receptor agonist phenylbiguanide (2–8 μg/kg) under control conditions, during hemorrhage, and during hemorrhage preceded by blockade of NTS adenosine receptors with bilateral microinjections of 8-(p-sulfophenyl) theophylline (1 nmol/100 nl) in urethane/chloralose anesthetized rats. CCR-mediated inhibition of renal and adrenal sympathetic activity was significantly attenuated during severe hemorrhage despite reciprocal changes in the baseline activity levels, and this attenuation was removed by bilateral blockade of adenosine receptors in the caudal NTS. This confirmed that adenosine endogenously released into the NTS has a similar modulatory effect on integration of cardiovascular reflexes as stimulation of NTS adenosine receptors with exogenous agonists.
Keywords: nucleus of the solitary tract, purinergic receptors, renal nerve, adrenal nerve, adenosine receptor antagonist
adenosine modulates the integration of central neuronal networks via stimulation of both A1 receptors (which inhibit neuronal activity and synaptic release of neurotransmitters) and A2a receptors (which stimulate neurons and facilitate neurotransmitter release) (16). Usually the inhibitory A1 receptors dominate in most central structures (16, 30); however, in the nucleus of the solitary tract (NTS), facilitatory A2a receptors prevail (1, 3, 13, 14). Adenosine levels in the central nervous system, including the NTS, may also increase several fold as a result of hemodynamic imbalance causing ischemia or hypoxia, which results in catabolism of intracellular ATP to adenosine in the ischemic neurons and glial cells (15, 23, 31, 33, 34). Adenosine levels in the NTS may also increase during the hypothalamic defense response when descending hypothalamic neurons release ATP as a neurotransmitter, which is subsequently degraded to adenosine via ectonucleotidases (5, 7, 24–26, 35). As adenosine rises and envelopes the surrounding neurons, it may then act as a modulator of cardiovascular reflexes integrated within the NTS networks.
The cardiopulmonary chemoreflex (CCR; known also as the Bezold Jarisch reflex) is mediated in rats via vagal C-fibers, which express 5HT3 receptors activated by phenylbiguanide (PBG) (10, 12, 18, 27, 28). This reflex is powerfully inhibited within the NTS by exogenous selective activation of both A1 and A2a adenosine receptor subtypes (8, 11). However, this inhibition occurs via two very different mechanisms: direct inhibition of glutamate release (A1 receptors) and facilitation of an inhibitory neurotransmitter release (A2a receptors), which in turn inhibits neurotransmission in this reflex arc (8, 11). Adenosine is released into the NTS during severe hemorrhage and contributes to the contrasting changes in renal (decreases) and adrenal (increases) sympathetic nerve activity observed in this setting (23). Both A1 and A2a adenosine receptors are involved in these responses (23). To what extent these increases in adenosine levels during severe hemorrhage act to inhibit the cardiopulmonary chemoreflex is unknown. Therefore, we observed the responses in renal and adrenal nerve activity evoked by gradual stimulation of cardiopulmonary chemoreceptors before and during severe hemorrhage performed before and after blockade of both A1 and A2a adenosine receptors in the NTS. Our data confirmed the hypothesis that adenosine release in the NTS during the hypotensive stage of severe hemorrhage attenuates CCR-mediated sympathoinhibition of renal and adrenal sympathetic nerves.
METHODS
All protocols and surgical procedures employed in this study were reviewed and approved by the Institutional Animal Care and Use Committee and were performed in accordance with the Guide for the Care and Use of Laboratory Animals endorsed by the American Physiological Society and published by the National Institutes of Health.
Instrumentation and measurements.
All procedures were described in detail previously (4, 8, 11, 17, 21–23). Briefly, male Sprague-Dawley rats (Charles River) were anesthetized with a mixture of α-chloralose (80 mg/kg) and urethane (500 mg/kg ip), tracheotomized, connected to a small-animal respirator (SAR-830, CWE, Ardmore, PA), and artificially ventilated with 40% oxygen-60% nitrogen mixture. Arterial blood gases were tested occasionally (Radiometer, ABL500, OSM3), and ventilation was adjusted to maintain Po2, Pco2, and pH within normal ranges. Average values measured at the end of each experiment were Po2 = 163.9 ± 7.1 Torr, Pco2 = 34.4 ± 1.4 Torr, and pH = 7.33 ± 0.001. The left femoral artery and vein were catheterized to monitor arterial blood pressure, and to infuse drugs and supplement anesthesia (12–21 mg·kg−1·h−1 of α-chloralose and 76–133 mg·kg−1·h−1 of urethane dissolved in 2.4–4.2 ml·kg−1·h−1 saline), respectively. Additional catheters were inserted into the right atrium via the right jugular vein and the right femoral artery for the intra-atrial PBG injections and for the withdrawal of blood to perform hemorrhage, respectively. The appropriate position of the catheter in the right atrium was confirmed postmortem. Sinoaortic denervation was performed and tested similarly as in the previous studies from our laboratory; special attention was given to preserve the vagus nerve intact (8, 11, 17, 21, 22).
Neural recordings.
In each experiment, simultaneous recordings from renal and adrenal sympathetic nerves (n = 20) were performed as previously described (8, 17, 19–22). Neural signals were initially amplified (×2,000–20,000) with bandwidth set at 100–1,000 Hz, digitized, rectified, averaged in 1-s intervals, and saved on hard disk for further calculations. Signal-to-noise ratio was continuously monitored and kept above 4 during each experiment. If signal-to-noise ratio decreased below 4, the experiment was excluded from further calculations. Background noise was determined after the animal was euthanized yet artificially ventilated to leave unaltered any potential movement artifacts. Resting levels of nerve activity were normalized to 100% before each stimulation of the cardiopulmonary receptors.
Cardiopulmonary chemoreflex stimulus-response curves.
The CCR stimulus-response curves were generated as described in our previous studies (8, 11, 17, 18). Briefly, the cardiopulmonary receptors were stimulated using bolus injections into the right atrium of increasing doses (2, 4, and 8 μg/kg) of serotonin 5HT3 receptor agonist PBG (Sigma, 20 μg/1 ml solution in 0.9% NaCl), with 2.5-min intervals allowed between the injections. Total time to complete the stimulus response curve was ∼6 min. The curves were obtained in two groups of animals: 1) under control conditions, during the last 6 min of a 10-min hypotensive hemorrhage and ∼1 h after the hemorrhage (recovery); and 2) under control conditions and during the last 6 min of the hemorrhage, which was preceded by (∼5 min) bilateral blockade of adenosine receptors in the caudal NTS. The control reflex function curves were obtained at least 30 min before the start of the hemorrhage.
Hemorrhage.
Severe hemorrhage was performed similarly as in our previous study to reversibly induce adenosine release in the NTS (23). Briefly, rapid withdrawal of arterial blood was performed to decrease and maintain MAP at ∼35 mmHg for ∼10 min and was followed by reinfusion. In anesthetized rats, brain adenosine levels increase significantly when MAP decreases below 50 mmHg, i.e., below the cerebral autoregulatory range (31). This rapid, marked hemorrhage (∼12–15 ml/kg) evoked typical de-compensatory decreases in HR and renal sympathetic nerve activity (RSNA), and sustained increases in adrenal sympathetic nerve activity (ASNA), as observed in previous studies performed in anesthetized rats (6, 23, 29, 32). These responses recovered completely ∼60 min after blood reinfusion.
Microinjections into the NTS.
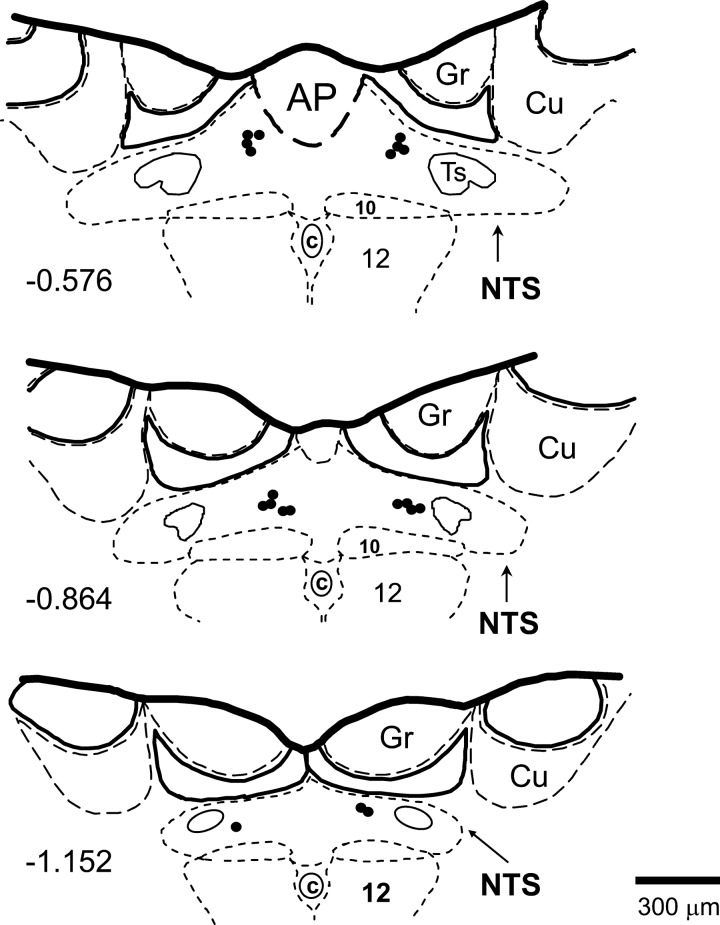
Bilateral microinjections of the nonselective A1 and A2a receptor antagonist 8-(p-sulfophenyl) theophylline (8-SPT) were made with three-barrel glass micropipettes into the medial region of the caudal NTS using the techniques, dose, and volume [1 nmol in 100 nl of artificial cerebrospinal fluid (ACF)] as described previously (4, 8, 9, 11, 19, 21–23). Our previous study showed that this dose of the antagonist reversed the paradoxical renal sympathoinhibition and bradycardia observed during the hypotensive stage of severe hemorrhage (23). All microinjection sites were marked with DiI lypophilic dye (Molecular Probes) and were verified histologically as described previously (4, 8, 9, 11, 19). The microinjection sites are presented in Fig. 1 using diagrams based on the atlas of the rat subpostremal NTS by Barraco et al. (2).
Fig. 1.
The bilateral sites of microinjections of nonselective A1 and A2a receptor antagonist 8-(p-sulfophenyl) theophylline (1 nmol/100 nl) were located within caudal nucleus tractus solitarii (NTS), as shown in schematic diagrams of transverse sections of the medulla oblongata from a rat brain. Scale is shown at bottom; number on left of schematic diagram denotes the rostrocaudal position in millimeters of the section relative to the obex, according to the atlas of the rat subpostremal NTS by Barraco et al. (2). AP, area postrema; c, central canal; 10, dorsal motor nucleus of vagus nerve; 12, nucleus of hypoglossal nerve; Ts, tractus solitarius; Gr, gracile nucleus; Cu, cuneate nucleus.
Data analysis.
The cardiopulmonary reflex stimulus-response curves constructed for MAP, HR, and the regional sympathetic outputs under control conditions were compared with the curves generated during the hypotensive stage of severe hemorrhage and during the hemorrhage preceded with bilateral blockade of adenosine receptors in the caudal NTS. The comparisons were made in two groups of animals: 1) control vs. hemorrhage and control vs. recovery (n = 10) and 2) control vs. NTS adenosine receptor blockade + hemorrhage (n = 10). The reflex decreases of hemodynamic and sympathetic responses were evaluated as integer calculated for the 30-s period following each stimulation of the CCR (see Fig. 2, insets). This method of evaluation of the reflex responses combined both amplitude and time of the response; therefore, it better reflects the reflex reactivity than amplitude alone, especially when baseline levels of all variables were differently altered by hemorrhage.
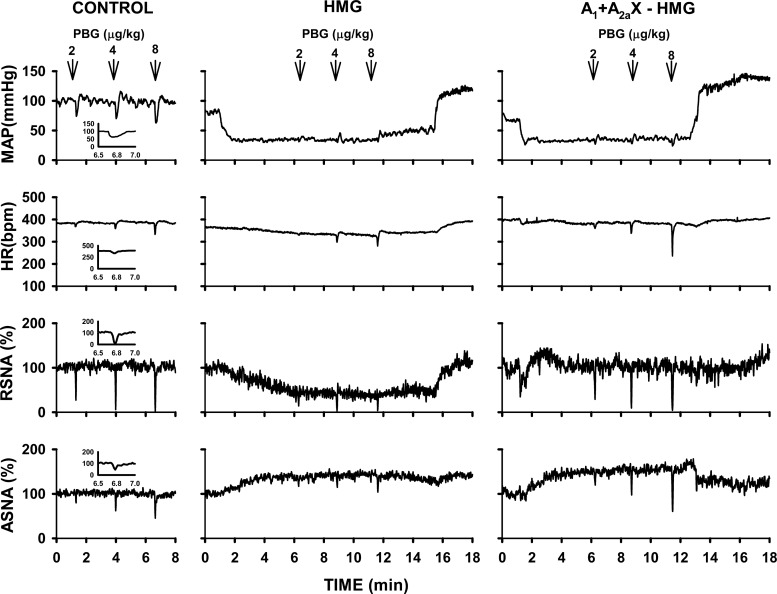
Fig. 2.
An example of cardiopulmonary chemoreflex responses in mean arterial pressure (MAP), heart rate (HR), renal (RSNA), and adrenal (ASNA) sympathetic nerve activity evoked by intra-atrial injections of phenylbiguanide (2, 4, and 8 μg/kg) under control conditions (left), during hypotensive stage of severe hemorrhage (HMG; middle), and during hemorrhage evoked after bilateral blockade of adenosine receptors in the NTS (A1 + A2a X − HMG; right). Insets: enlarged responses evoked by injection of 8 μg of phenylbiguanide; the areas below baseline levels represent integers of the responses (measured over 30 s). Severe hemorrhage markedly attenuated hemodynamic and sympathetic responses, and the blockade of NTS adenosine receptors removed this attenuation.
The data were analyzed using the statistical package SYSTAT version 11 (SYSTAT Software, Richmond, CA). Repeated-measures ANOVA for two trial factors (Systat version 11 manual, chapter 3, II-132) was used to compare the reflex responses of each variable across the experimental conditions applied in each group of animals (control vs. hemorrhage and control vs. recovery or control vs. blockade of NTS adenosine receptors + hemorrhage) and across three levels of activation of the CCR (three doses of PBG, 2–8 μg/kg) (Fig. 3). If significant interactions were found (experimental condition × PBG dose), the differences between the means were calculated using post hoc C matrix test for simple effects. In addition, similar comparisons were made across the groups (control vs. control, and hemorrhage vs. blockade of NTS adenosine receptors + hemorrhage) and across three levels of activation of the CCR using two-way ANOVA for independent measures. An α-level of P < 0.05 was used to determine statistical significance.
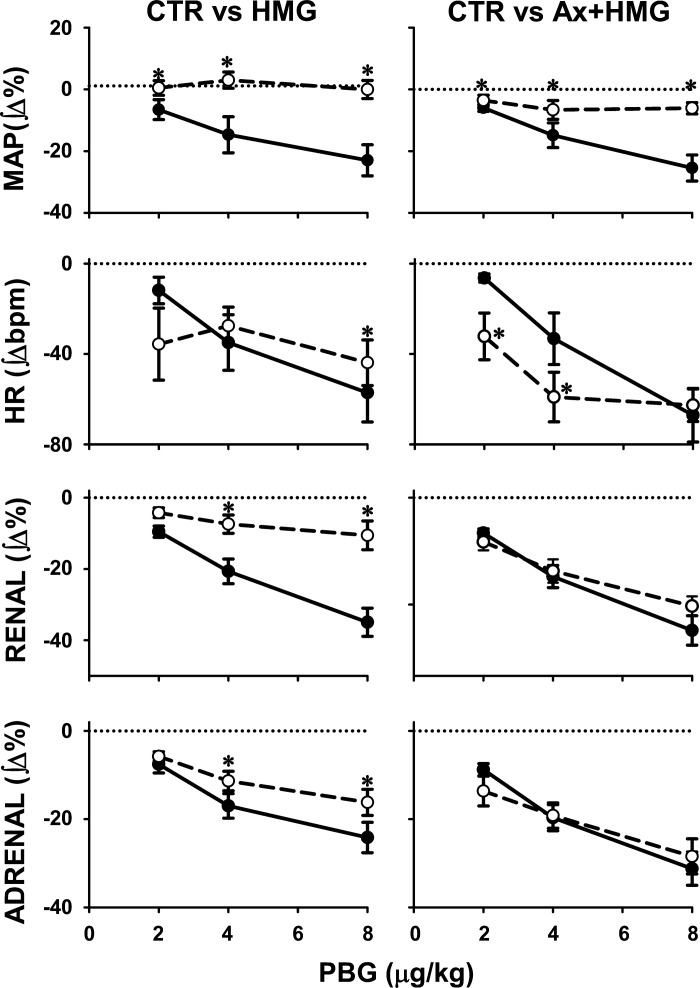
Fig. 3.
Comparisons of cardiopulmonary chemoreflex responses obtained under control conditions (●; solid lines) vs. the responses obtained during hypotensive stage of severe hemorrhage (○; dashed lines) (left) and control vs. hemorrhage evoked after bilateral blockade of adenosine receptors in the NTS (right). Data are mean integers of 30-s reflex responses ± SE. The reflex sympathoinhibitory responses were significantly attenuated during hemorrhage, and this attenuation was removed by bilateral stimulation of NTS adenosine receptors. *Significant difference vs. control (P < 0.05).
RESULTS
Average resting MAP and HR values measured before the control CCR stimulus-response curves (n = 20) were 83.9 ± 2.3 mmHg and 391.0 ± 8.3 beats/min, respectively, and returned to basal values (81.4 ± 2.0 mmHg and 397.2 ± 8.4 beats/min; P > 0.05) before the next perturbations. Individual and group mean responses are shown in Figs. 2 and 3. Hemorrhage caused the typical, opposite changes in baseline renal and adrenal sympathetic nerve activity (decreases and increases, respectively). The depressor, cardiac slowing, and sympathoinhibitory responses evoked by gradual activation of the CCR under control conditions were markedly attenuated during hemorrhage. Two-way ANOVA showed significant experimental effects for both renal and adrenal nerve responses (P = 0.001 and P = 0.002, respectively). Note that during hemorrhage the CCR sympathoinhibitory responses were attenuated despite opposite changes in baseline renal and adrenal sympathetic nerve activity. This attenuation was abolished after bilateral inhibition of adenosine receptors in the caudal NTS; no significant differences between control and hemorrhage reflex function curves were observed after the blockade for renal and adrenal nerve responses (experimental effects, P = 0.375 and P = 0.798, respectively). The effects of adenosine receptor antagonist (8-spt) on baseline MAP, HR, RSNA, and ASNA were −11.5 ± 3.1 mmHg, −6.4 ± 4.8 beats/min, −12.9 ± 6.7 Δ%, and −13.3 ± 5.7 Δ%, respectively. Only the decreases in MAP and renal nerve activity were significantly different from zero (P < 0.05). These effects were similar to those we reported previously (8, 9, 23) and were not different from spontaneous fluctuations in these parameters observed during experiments. No difference in total blood volume withdrawn needed to decrease MAP to ∼35 mmHg was observed between control and blockade conditions (14.0 ± 2.6 vs. 16.9 ± 1.9 ml/kg, respectively; P = 0.389). The reflex HR responses were attenuated to a lesser extent compared with either of the individual sympathetic nerve activity responses. Blockade of NTS adenosine receptors facilitated the HR responses compared with those observed under control conditions. The attenuation of the reflex reactivity evoked by hemorrhage was reversible; the reflex responses evoked ∼1 h after the hemorrhage were not significantly different from control responses evoked before the hemorrhage (Table 1).
Table 1.
Averaged cardiopulmonary chemoreflex responses to PBG (2–8 μg/kg) evoked under control conditions and during recovery
| Control |
Recovery |
|||||||
|---|---|---|---|---|---|---|---|---|
| PBG, μg/kg | MAP, Δ% | HR, Δbeats/min | RSNA, ∫Δ% | ASNA, ∫Δ% | MAP, ∫Δ% | HR, Δbeats/min | RSNA, ∫Δ% | ASNA, ∫Δ% |
| 2 | −6.6 ± 3.2 | −11.8 ± 5.9 | −9.5 ± 1.6 | −7.6 ± 2.0 | −5.6 ± 1.5 | −12.7 ± 5.4 | −10.5 ± 1.3 | −6.8 ± 1.2 |
| 4 | −14.7 ± 5.8 | −34.9 ± 12.3 | −20.7 ± 3.5 | −17.0 ± 2.8 | −8.8 ± 1.5 | −29.5 ± 10.2 | −20.1 ± 2.2 | −11.6 ± 1.5 |
| 8 | −23.0 ± 5.0 | −57.1 ± 12.9 | −35.0 ± 4.0 | −24.2 ± 3.4 | −14.0 ± 2.5* | −50.3 ± 11.3 | −29.3 ± 2.9 | −18.6 ± 2.5 |
Values are means ± SE. Control, control conditions; Recovery, ∼ 1 h after hemorrhage; PBG, phenylbiguanide; HR, heart rate; MAP, mean arterial pressure; RSNA, renal sympathetic nerve activity; ASNA, adrenal sympathetic nerve activity.
Significant difference vs. control (P < 0.05).
Comparisons of reflex sympathoinhibitory responses across the groups showed significant accentuation of reactivity after blockade of NTS adenosine receptors (downward shift of reflex response curves) compared with the curves obtained during hemorrhage alone with intact NTS adenosine receptors (experimental condition effect, P < 0.0001) for both renal and adrenal nerves. There were no differences between control stimulus response curves for both nerves across groups (P = 0.121 and P = 0.590 for adrenal and renal nerve, respectively). HR responses tended to be accentuated during hemorrhage after blockade of NTS adenosine receptors compared with hemorrhage alone (experimental condition effect, P = 0.088) without differences between control responses across the groups (P = 0.914).
DISCUSSION
The present study showed for the first time that adenosine naturally released into the NTS modulates cardiopulmonary chemoreflex responses. Regional sympathetic responses evoked by stimulation of cardiopulmonary chemoreceptors were inhibited during severe posthemorrhagic hypotension, and this inhibition was abolished by blockade of adenosine receptor subtypes within the primary site of initial central integration of the reflex, the caudal NTS. This adenosinergic inhibition of CCR mechanisms that occurs during posthemorrhagic hypotension was similar to that our laboratory observed previously following selective stimulation of NTS adenosine receptor subtypes using exogenous agonists (8, 11). The posthemorrhagic attenuation of CCR reflex control of sympathetic outputs was independent of the normal reciprocal changes in the baseline levels of regional sympathetic activity observed during hemorrhage (increases in adrenal and decreases in renal nerve activity) (6, 23, 29, 32). This indicates that adenosine released during severe hemorrhage exerts active inhibition of the CCR networks within the NTS.
Selective stimulation of NTS A1 and A2a adenosine receptors with exogenous agonists exerts powerful inhibition of CCR sympathetic responses via two different mechanisms: direct inhibition of neurotransmission in the reflex arc and facilitation of an inhibitory neurotransmitter release, which in turn inhibits the reflex arc, respectively (8, 11). Adenosine operating via NTS A1 (but not A2a) adenosine receptors also inhibits arterial baroreflex control of regional sympathetic outputs (9, 19). Taken together, these data indicate that adenosine released into the NTS during life-threatening hypotension plays a physiological role attenuating sympathoinhibitory and depressor reflexes.
The inhibition of HR responses evoked by the CCR during hemorrhage was much weaker than that observed in the regional sympathetic reflex responses. Interestingly, after blockade of adenosine receptors in the NTS, the hemorrhage significantly facilitated the CCR HR responses. CCR-evoked bradycardia is mediated mostly via the parasympathetic component of the reflex. Therefore, it is likely that hemorrhage accentuated the parasympathetic mechanism of the reflex via some unknown mechanism(s) (for example, serotoninergic), whereas adenosine released into the NTS during hemorrhage attenuated this facilitation. Since the inhibition of the sympathetic component of the CCR responses has a much smaller contribution to the reflex bradycardia, adenosine released during hemorrhage in animals with intact NTS adenosine receptors did not markedly inhibit HR responses vs. peripheral sympathetic responses.
Under normal conditions, blockade of NTS adenosine receptors does not affect arterial baroreflex or CCR control of regional sympathetic outputs (8, 9, 11). This indicates that, under physiological conditions, adenosine levels in the NTS are too low to exert visible effects on sympathoinhibitory reflexes integrated in the NTS. However, during severe hypotension, adenosine levels in the NTS increase markedly (23, 31), revealing adenosinergic inhibition on these reflexes. It should be stressed that selective exogenous activation of NTS A1 and A2a adenosine receptors evokes powerful, contrasting changes in baseline activity of regional sympathetic outputs (renal, adrenal, and lumbar). Stimulation of NTS A1 receptors differentially increases regional sympathetic activity (adrenal > renal ≥ lumbar) (22), whereas stimulation of NTS A2a receptors increases adrenal, decreases renal, and does not alter lumbar sympathetic nerve activity (20, 21). This indicates that NTS adenosine receptor subtypes may powerfully modulate sympathetic activity in a regionally specific manner during hemodynamic imbalance. However, this potential action of adenosine is not visible at normal conditions when adenosine levels within the NTS are low.
Limitations of the method.
During hemorrhage, MAP was experimentally controlled and maintained at relatively low levels (∼35 mmHg), which are well beyond the reflex reactivity. Although the residual MAP reflex responses were visible during hemorrhage, especially after blockade of NTS adenosine receptors (Fig. 2), it was impossible to distinguish to what extent the attenuation of MAP responses was the result of mechanical restriction vs. the result of adenosinergic inhibition during hemorrhage. Therefore, it was difficult to estimate the relative contribution of HR vs. regional sympathetic activity to adenosinergic modulation of MAP control during hemorrhage. However, the adenosinergic attenuation of the sympathetic CCR responses was much greater than the attenuation of the HR responses. This suggests that adenosinergic attenuation of sympathoinhibitory responses may prevent further decreases in MAP during hemorrhage.
Baseline renal sympathetic nerve activity was decreased during hemorrhage, which might have limited the reflex reactivity independently of adenosine. However, adrenal baseline activity increased during hemorrhage, and despite this increase the adrenal CCR responses were significantly attenuated. This indicates that adenosine did contribute to attenuation of sympathetic reflex responses. Note that the blockade of adenosine receptors within the NTS completely removed inhibition of CCR renal and adrenal responses during hemorrhage. In the present study, adenosine levels within the NTS were not measured. Therefore, we cannot estimate at what specific concentration adenosine starts inhibiting the CCR network. However, it is known that, during hemorrhage, central levels of adenosine increase proportionally to decreases in MAP and time of the hemorrhage (31). Our previous study suggested that short-lasting hemorrhage (5 min) tends to facilitate cardiopulmonary reflex bradycardia and sympathoinhibition (23), whereas in the present study a longer hemorrhage (10 min) inhibited the CCR responses. Taken together, these data suggest that adenosine effects on integration of sympathoinhibitory reflexes within the NTS are concentration dependent.
There were no significant differences between total blood withdrawal under control vs. blockade conditions in the present and previous studies (23). This suggests that the effective concentration of PBG at the chemoreceptor afferents was similar in the two conditions. Furthermore, the similarity in the hemorrhage volumes indicates that attenuation of renal sympathoinhibition after the blockade did not significantly alter the rate of MAP decrease during hemorrhage, which therefore may depend more on nonrenal sympathetic outputs. Although a similar volume of blood was shed under control and following blockade of NTS adenosine receptors, large differences in the reactivity between these two experimental conditions were observed. This demonstrates that the attenuation of sympathetic reflex responses observed during hemorrhage was caused by adenosine endogenously released into the NTS.
Perspectives
Stimulation of adenosine receptor subtypes in the NTS evokes differential shifts in baseline levels of MAP and regional sympathetic activity (20–22). Studies utilizing microinjections into the NTS of exogenous agonists of adenosine A1 and A2a receptors indicated that these receptors differentially inhibit the sympathoinhibitory reflexes integrated in the NTS. The CCR was inhibited to a much greater extent (via both A1 and A2a receptors) compared with the inhibition of arterial baroreflex, which was inhibited only via A1 receptors, which are less potent in the NTS than A2a receptors (8, 9, 11, 19). The present study confirmed that the effects observed with stimulation of adenosine receptor subtypes using exogenous agonists also occur with adenosine naturally released into the NTS during severe hemorrhage, which exerted similar effects on reflex reactivity to those using the agonists. These findings stress a physiological role of adenosine in modulation of cardiovascular reflexes at the level of brain stem cardiovascular centers during severe hemodynamic imbalance. Further studies in this field may have important clinical applications in emergency medicine.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-67814.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.M., C.L., and T.J.S. performed experiments; Z.M. and C.L. analyzed data; Z.M., D.S.O., and T.J.S. interpreted results of experiments; Z.M. and C.L. prepared figures; Z.M. drafted manuscript; Z.M., D.S.O., and T.J.S. edited and revised manuscript; D.S.O. and T.J.S. approved final version of manuscript; T.J.S. conception and design of research.
REFERENCES
- 1.Abdel-Rahman AA, Tao S. Differential alteration of neuronal and cardiovascular responses to adenosine microinjected into the nucleus tractus solitarius of spontaneously hypertensive rats. Hypertension 27: 939–948, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Barraco R, el Ridi M, Ergene E, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull 29: 703–765, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Barraco RA, el Ridi MR, Ergene E, Phillis JW. Adenosine receptor subtypes in the brainstem mediate distinct cardiovascular response patterns. Brain Res Bull 26: 59–84, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Barraco RA, O'Leary DS, Ergene E, Scislo TJ. Activation of purinergic receptor subtypes in the nucleus tractus solitarius elicits specific regional vascular response patterns. J Auton Nerv Syst 59: 113–124, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Carlsson S, Skarphedinsson JO, Delle M, Hoffman P, Thoren P. Reflex changes in post- and preganglionic sympathetic adrenal nerve activity and postganglionic sympathetic renal nerve activity upon arterial baroreceptor activation and during severe haemorrhage in the rat. Acta Physiol Scand 144: 317–323, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Dale N, Gourine AV, Llaudet E, Bulmer D, Thomas T, Spyer KM. Rapid adenosine release in the nucleus tractus solitarii during defence response in rats: real-time measurement in vivo. J Physiol 544: 149–160, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichinose TK, Minic Z, Li C, O'Leary DS, Scislo TJ. Activation of NTS A1 adenosine receptors inhibits regional sympathetic responses evoked by activation of cardiopulmonary chemoreflex. Am J Physiol Regul Integr Comp Physiol 303: R539–R550, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichinose TK, O'Leary DS, Scislo TJ. Activation of NTS A2a adenosine receptors differentially resets baroreflex control of renal vs. adrenal sympathetic nerve activity. Am J Physiol Heart Circ Physiol 296: H1058–H1068, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeggo RD, Kellett DO, Wang Y, Ramage AG, Jordan D. The role of central 5-HT3 receptors in vagal reflex inputs to neurones in the nucleus tractus solitarius of anaesthetized rats. J Physiol 566: 939–953, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minic Z, O'Leary DS, Scislo TJ. Nucleus tractus solitarii A2a adenosine receptors inhibit cardiopulmonary chemoreflex control of sympathetic outputs. Auton Neurosci 180: 32–42, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira TS, Takakura AC, Colombari E, Guyenet PG. Activation of 5-hydroxytryptamine type 3 receptor-expressing C-fiber vagal afferents inhibits retrotrapezoid nucleus chemoreceptors in rats. J Neurophysiol 98: 3627–3637, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Mosqueda-Garcia R, Tseng CJ, Appalsamy M, Beck C, Robertson D. Cardiovascular excitatory effects of adenosine in the nucleus of the solitary tract. Hypertension 18: 494–502, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Mosqueda-Garcia R, Tseng CJ, Appalsamy M, Robertson D. Modulatory effects of adenosine on baroreflex activation in the brainstem of normotensive rats. Eur J Pharmacol 174: 119–122, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Phillis JW, Walter GA, O'Regan MH, Stair RE. Increases in cerebral cortical perfusate adenosine and inosine concentrations during hypoxia and ischemia. J Cereb Blood Flow Metab 7: 679–686, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 17.Scislo TJ, DiCarlo SE. Gender difference in cardiopulmonary reflex inhibition of sympathetic nerve activity. Am J Physiol Heart Circ Physiol 267: H1537–H1543, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Scislo TJ, DiCarlo SE, Collins HL. Daily spontaneous running did not alter vagal afferent reactivity. Am J Physiol Heart Circ Physiol 265: H1564–H1570, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Scislo TJ, Ichinose TK, O'Leary DS. Stimulation of NTS A1 adenosine receptors differentially resets baroreflex control of regional sympathetic outputs. Am J Physiol Heart Circ Physiol 294: H172–H182, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Scislo TJ, O'Leary DS. Activation of A2a adenosine receptors in the nucleus tractus solitarius inhibits renal but not lumbar sympathetic nerve activity. J Auton Nerv Syst 68: 145–152, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Scislo TJ, O'Leary DS. Differential control of renal vs. adrenal sympathetic nerve activity by NTS A2a and P2x purinoceptors. Am J Physiol Heart Circ Physiol 275: H2130–H2139, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Scislo TJ, O'Leary DS. Mechanisms mediating regional sympathoactivatory responses to stimulation of NTS A1 adenosine receptors. Am J Physiol Heart Circ Physiol 283: H1588–H1599, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Scislo TJ, O'Leary DS. Adenosine receptors located in the NTS contribute to renal sympathoinhibition during hypotensive phase of severe hemorrhage in anesthetized rats. Am J Physiol Heart Circ Physiol 291: H2453–H2461, 2006 [DOI] [PubMed] [Google Scholar]
- 24.St Lambert JH, Dashwood MR, Spyer KM. Role of brainstem adenosine A1 receptors in the cardiovascular response to hypothalamic defence area stimulation in the anaesthetized rat. Br J Pharmacol 117: 277–282, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Lambert JH, Dawid-Milner MS, Silva-Carvalho L, Spyer KM. Action of adenosine receptor antagonists on the cardiovascular response to defence area stimulation in the rat. Br J Pharmacol 113: 159–164, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St Lambert JH, Thomas T, Burnstock G, Spyer KM. A source of adenosine involved in cardiovascular responses to defense area stimulation. Am J Physiol Regul Integr Comp Physiol 272: R195–R200, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Thoren P, Noresson E, Ricksten SE. Cardiac receptors with non-medullated vagal afferents in the rat. Acta Physiol Scand 105: 295–303, 1979 [DOI] [PubMed] [Google Scholar]
- 28.Thoren P, Noresson E, Ricksten SE. Resetting of cardiac C-fiber endings in the spontaneously hypertensive rat. Acta Physiol Scand 107: 13–18, 1979 [DOI] [PubMed] [Google Scholar]
- 29.Togashi H, Yoshioka M, Tochihara M, Matsumoto M, Saito H. Differential effects of hemorrhage on adrenal and renal nerve activity in anesthetized rats. Am J Physiol Heart Circ Physiol 259: H1134–H1141, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Tupone D, Madden CJ, Morrison SF. Highlights in basic autonomic neurosciences: central adenosine A1 receptor: the key to a hypometabolic state and therapeutic hypothermia? Auton Neurosci 176: 1–2, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Wylen DG, Park TS, Rubio R, Berne RM. Cerebral blood flow and interstitial fluid adenosine during hemorrhagic hypotension. Am J Physiol Heart Circ Physiol 255: H1211–H1218, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Victor RG, Thoren P, Morgan DA, Mark AL. Differential control of adrenal and renal sympathetic nerve activity during hemorrhagic hypotension in rats. Circ Res 64: 686–694, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Winn HR, Rubio R, Berne RM. Brain adenosine production in the rat during 60 seconds of ischemia. Circ Res 45: 486–492, 1979 [DOI] [PubMed] [Google Scholar]
- 34.Yan S, Laferriere A, Zhang C, Moss IR. Microdialyzed adenosine in nucleus tractus solitarii and ventilatory response to hypoxia in piglets. J Appl Physiol 79: 405–410, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol 49: 589–618, 1996 [DOI] [PubMed] [Google Scholar]