Abstract
We tested the hypothesis that infusion of ascorbic acid (AA), a potent antioxidant, would alter vasodilator responses to exercise in human obesity and metabolic syndrome (MetSyn). Forearm blood flow (FBF, Doppler ultrasound) was measured in lean, obese, and MetSyn adults (n = 39, 32 ± 2 yr). A brachial artery catheter was inserted for blood pressure monitoring and local infusion of AA. FBF was measured during dynamic handgrip exercise (15% maximal effort) with and without AA infusion. To account for group differences in blood pressure and forearm size, and to assess vasodilation, forearm vascular conductance (FVC = FBF/mean arterial blood pressure/lean forearm mass) was calculated. We examined the time to achieve steady-state FVC (mean response time, MRT) and the rise in FVC from rest to steady-state exercise (Δ, exercise − rest) before and during acute AA infusion. The MRT (P = 0.26) and steady-state vasodilator responses to exercise (ΔFVC, P = 0.31) were not different between groups. Intra-arterial infusion of AA resulted in a significant increase in plasma total antioxidant capacity (174 ± 37%). AA infusion did not alter MRT or steady-state FVC in any group (P = 0.90 and P = 0.85, respectively). Interestingly, higher levels of C-reactive protein predicted longer MRT (r = 0.52, P < 0.01) and a greater reduction in MRT with AA infusion (r = −0.43, P = 0.02). We concluded that AA infusion during moderate-intensity, rhythmic forearm exercise does not alter the time course or magnitude of exercise-mediated vasodilation in groups of young lean, obese, or MetSyn adults. However, systemic inflammation may limit the MRT to exercise, which can be improved with AA.
Keywords: blood flow, reactive oxygen species, antioxidant
over 25% of younger adults (ages 20–40 yr) are obese, and greater than one-third of the United States population meets the criteria for metabolic syndrome (MetSyn; obesity, hypertension, dyslipidemia, hyperglycemia) (8). Exercise is an effective way to prevent and/or combat obesity and obesity-related disorders, such as MetSyn. Unfortunately, adults with MetSyn exhibit exaggerated blood pressure responses to whole body exercise (30, 38), which may be the result of decreased skeletal muscle vasodilation and/or increased vasoconstriction. In support of this idea, animal models of obesity and MetSyn exhibit blunted vasodilation, increased vasoconstriction, and impaired blood flow distribution within the skeletal muscle microcirculation in response to simulated exercise (2, 10, 13). Such impairments have been linked to increased reactive oxygen species (ROS), such that scavenging of ROS via antioxidant therapies can improve exercise-mediated vasodilation in these animal models (9, 12).
We have shown previously that rapid-onset vasodilation in response to forearm muscle contraction is blunted in human obesity (1). On the other hand, steady-state vasodilator responses to dynamic forearm exercise are preserved, or even increased, in young obese humans and those with MetSyn (25, 26). The potential reasons behind this discrepancy are not completely understood, although mechanisms responsible for vascular responses to exercise onset are unlikely to mirror those important in maintaining blood flow and vascular tone during steady-state exercise (24). Furthermore, data from our laboratory are consistent with the concept that blood flow distribution may be altered in human MetSyn during steady-state exercise (28).
How ROS might influence these vascular responses to exercise in obese and MetSyn humans is largely unknown. As noted above, excessive ROS may impair exercise-mediated vasodilation (11, 21), and recent research suggests that treatment with antioxidants may improve blood flow distribution in animal models (2, 10, 13). Conversely, increased ROS may serve as an important, alternative vasodilator mechanism that compensates for loss of other vascular control mechanisms. Consistent with this concept, ROS signaling is important to the maintenance of blood flow and vascular tone in the coronary circulation of humans with cardiovascular disease (16, 23), such that increasing antioxidant capacity might limit vasodilation in diseased patients.
With these ideas in mind, we aimed to examine the effect of increasing total antioxidant capacity on vasodilator responses to exercise onset and steady-state exercise in human obesity and MetSyn. We hypothesized that acute antioxidant (ascorbic acid, AA) administration would increase vasodilation at exercise onset yet may blunt responses during steady-state, moderate-intensity exercise in obese adults and adults with MetSyn. Furthermore, given that levels of oxidative stress increase with obesity and further rise in MetSyn (14), we hypothesized that acute AA infusion would have a graded effect on exercise-mediated vasodilation, with adults with MetSyn seeing the greatest changes.
MATERIALS AND METHODS
Procedures completed as part of the present study were part of a larger protocol testing resting microvascular endothelial and smooth muscle function (27).
Ethical approval.
Procedures were submitted to and approved by the Institutional Review Board at the University of Wisconsin Madison and conformed to the standards set by the Declaration of Helsinki. Written informed consent was obtained before study participation.
Subjects.
Subjects were between the ages of 18–40 yr to limit the confounding vascular effects of aging (21). Subjects were nonsmokers, generally healthy, and were not taking any cardiovascular medications, as determined by self-report. Two subjects reported consuming supplemental antioxidants [obese n = 1 (acai berry), MetSyn n = 1 (American ginseng, coenzyme Q10)]; however, when excluded from analysis, conclusions were maintained. Therefore, both subjects were included in the present study. Adults were characterized as MetSyn if they met three of the following National Cholesterol Education Program Adult Treatment Panel III criteria as modified by the American Diabetes Association: central obesity (waist circumference >88 cm for women/>102 cm for men), prehypertension (resting blood pressure ≥130 mmHg/≥85 mmHg), hypertriglyceridemia (triglycerides ≥150 mg/dl), hyperglycemia (fasting glucose ≥100 mg/dl), and/or dyslipidemia [high-density lipoprotein (HDL) <50 mg/dl women/<40 mg/dl men] (15). Obese subjects had a body mass index (BMI) ≥30 kg/m2 but were otherwise healthy (not meeting MetSyn criteria). Female subjects were not pregnant and were studied during the early follicular phase (days 1–5) of the menstrual cycle. Hormonal contraception was allowed, and women on contraception (Lean n = 2, Obese n = 1, MetSyn n = 1) were studied during the placebo phase. Subjects were instructed to refrain from exercise, nonsteroidal anti-inflammatory drugs, alcohol, and caffeine for 24 h before the study day.
Measurements.
Weight and height were measured, and body composition was determined by waist circumference, BMI (kg/m), and dual-energy X-ray absorptometry (GE Lunar Prodigy; GE Healthcare, Milwaukee, WI). Lean forearm mass was analyzed from whole body DEXA scans using anatomical landmarks (33). Maximal voluntary contraction (MVC, kg) of the nondominant arm was determined as the average of the two highest measurements from five trials using a hand dynamometer. Arterial blood was collected after a 10-h fast, and levels of triglycerides, HDL, low-density lipoprotein (LDL), and glucose were measured immediately (CardioChek; PTS Panels, Indianapolis, IN). Additional plasma samples were collected before study interventions, frozen at −80°C and analyzed at a later date for insulin (Millipore, Billerica, MA), C-reactive protein (CRP; R&D Systems, Minneapolis, MN), thiobarbituric acid-reactive substances (TBARS; Cayman Chemical, Ann Arbor, MI), and total antioxidant capacity (TAC; Ref. 34). A second plasma sample was collected for measures of TAC at the conclusion of study interventions, just before removal of the arterial catheter. A change in TAC is reported relative to baseline [(Intervention − Baseline)/Baseline × 100]. To examine potential insulin resistance, the homeostatic model assessment [HOMA-IR = (Fasting Glucose × Fasting Insulin)/405] and quantitative insulin sensitivity check index [QUICKI = 1/log(Fasting Insulin) + log(Fasting Glucose)] were assessed. To examine physical activity levels, subjects completed the Paffenbarger questionnaire (31), a validated tool for assessing physical activity over the course of a year based on blocks walked, flights of stairs taken, and participation in other sports and recreational activities.
Brachial artery catheterization.
A 20-gauge, 5-cm catheter was placed in the brachial artery of the nondominant forearm under aseptic conditions and after local anesthesia (2% lidocaine). The catheter was used for continuous blood pressure measurement, blood sampling, and local administration of AA (8 mg·dl forearm volume−1·min−1 followed by a 2.5 mg/min maintenance infusion). AA (Bioniche Pharma USA, Lake Forest, IL) was used as a potent antioxidant and was mixed specifically for each study visit. The dose of AA was greater than or equal to that shown previously to increase endothelial-dependent vasodilation (20, 21, 27, 32) and blood flow responses to exercise (7, 21) in humans.
Blood flow.
Forearm blood flow (FBF; artery diameter, blood velocity) was measured using Doppler ultrasound (Vivid 7; General Electric, Milwaukee, WI). The ultrasound probe operator placed the 12-MHz linear array probe medial to the biceps brachii muscle, with position adjustments to maintain a fixed insonation angle of ≤60 degrees, and the sample volume was adjusted to cover the width of the brachial artery (27). A mark was made on the skin over the brachial artery to ensure measurements were taken in the same anatomical position for each trial.
Forearm exercise.
Each subject was supine with the nondominant arm extended to the side, at heart level. Dynamic and rhythmic, nondominant forearm exercise required subjects to squeeze and release two handles together 4–5 cm to raise and lower a weight over a pulley. Exercise was completed at a rate of 20 times per minute (at a duty cycle of 1-s contraction:2-s relaxation) to the rhythm of a metronome. A mild (15% MVC) workload was used to minimize systemic effects of exercise (i.e., increases in sympathetic nervous system activity, baroreflex activation, etc.). This forearm exercise model is similar to that used in several laboratories (3, 21).
Study protocols.
Procedures completed as part of the present study were part of a larger protocol (27). Room temperature was controlled at 21–22°C. Relevant to the present investigation, a total of two study conditions were conducted: 1) 15 min of dynamic forearm exercise, with AA administered during the last 10 min, 2) 5 min of dynamic forearm exercise at 15% MVC during a continuous maintenance dose infusion of AA. In this way, the immediate effect of acute AA administration could be examined during steady-state exercise (Trial 1), in addition to the effect of AA infusion on exercise onset (Trial 2) (21). Two minutes of resting data were acquired at the start of each trial. Trials were separated by a minimum of 30 min of quiet rest, and both exercise trials were preceded by identical intra-arterial infusions of endothelial-dependent and -independent agonists, followed by appropriate wash-out periods, as part of the larger protocol (27). Importantly, we have previously shown vascular responses to steady-state exercise at 15% MVC to be repeatable across exercise trials spanning ∼120 min in both lean subjects and adults with MetSyn (main effect of trial, P = 0.96; unpublished observations in n = 24).
Data acquisition and analysis.
Heart rate (ECG; Datex-Ohmeda, Helsinki, Finland), blood pressure, and brachial artery blood velocity measurements were obtained beat to beat throughout each trial. FBF was determined as the product of mean blood velocity (MBV, cm/s, during the last 30 s of steady-state rest and exercise) and vessel cross sectional area (CSA, radius in cm2) and was reported in ml/min [FBF = (MBV)(CSA)(60 s/min)]. The angle-corrected, intensity-weighted Doppler audio information from the ultrasound system was processed by a commercial interface unit (Multigon Industries, Yonkers, NY) into a blood velocity signal that was sampled in real time using an analog-to-digital data collection system (PowerLab; ADInstruments, Colorado Springs, CO). All hemodynamic data were stored on a computer at 400 Hz. Postprocessing using PowerLab Chart5 application package yielded MBV, blood pressures, and heart rates. To determine vessel CSA, digital video of the brachial artery was recorded, and brachial artery diameters were taken manually as the median of five measurements in late diastole. Arterial diameter was measured on B-mode images in the part of the artery running perpendicular to the ultrasound beam and was identified by strong wall signals in the longitudinal section of the artery in each image by a trained investigator. All measurements were assessed offline.
The primary analysis was to test whether exercise-mediated vasodilation was altered after AA infusion and whether vascular responses to AA infusion were different between groups. To account for group differences in resting blood pressure and forearm size and to assess vasodilation, the main dependent variable was a change in relative forearm vascular conductance (FVC) [FBF measurements (ml/min) normalized for blood pressure and lean forearm mass (ml·min−1·100 mmHg−1·100 g−1)]. In addition to steady-state vasodilation, we examined time to steady-state responses (Fig. 1A). For this analysis, beat-by-beat data were averaged into 3-s time bins (length of the exercise duty cycle) and fit using a single component model [ΔFVC (t) = G0 + G1(1 − e−(t − TD)/τ)], with G1 representing the amplitude, TD representing time delay, and τ representing the time constant of the response. Thus, ΔFVC (t) is indicative of the time-dependent change in FVC from baseline (G0) relative to lean forearm mass (29). The mean response time (MRT, time taken to reach ∼63% of the increase to steady state) was then calculated as MRT = TD + τ (29).
Fig. 1.
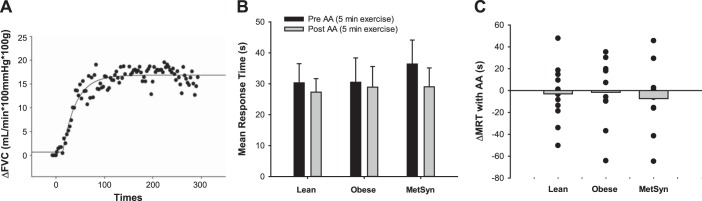
Effect of acute ascorbic acid (AA) infusion on mean response time (MRT). A: change in forearm vascular conductance (FVC) averaged in 3-s bins during 5 min of dynamic forearm exercise. Data from a representative healthy control before AA infusion (Trial 1). B: Lean (n = 13), Obese (n = 10), metabolic syndrome (MetSyn) (n = 9). MRT was not different between groups (main effect of group: P = 0.26). MRT was not altered when AA was infused (Trial 2), compared with exercise alone (Trial 1) (main effect of AA: P = 0.50). C: change in MRT with AA infusion (Δ) was not different between groups (interaction between group and AA: P = 0.90) although individual responses were variable (a negative value indicates MRT decreased with AA infusion).
Statistical analysis was done using SigmaPlot Version 12.0 (Systat Software, Evanston, IL). Subject characteristics were analyzed using a one-way ANOVA. Hemodynamic variables during 15 min of exercise (Trial 1) were analyzed using a two-way ANOVA approach to determine the significance of the fixed effect of group (Lean, Obese, MetSyn) and/or exercise (Rest, Exercise, Exercise + AA) on parameters of interest. Hemodynamic variables during the first 5 min of exercise (Trial 1 vs. Trial 2) were analyzed using a three-way ANOVA to determine the significance of the fixed effect of group (Lean, Obese, MetSyn), exercise (Rest, Exercise), and/or AA (Pre-AA, Post-AA) on parameters of interest. MRT and changes (Δ, Exercise − Rest) in main outcome variables were assessed using a two-way ANOVA to determine the significance of the fixed effect of group (Lean, Obese, MetSyn) and/or AA (Pre-AA, Post-AA). Distributional assumptions were assessed, and log transformations were used as appropriate. Bonferroni or Dunn's post hoc comparisons were performed when significant effects were observed. Pearson's product-moment correlations were conducted post hoc to determine the association between main outcome variables (Δ FVC and MRT) and measures of cardiovascular disease risk (age, weight, waist circumference, body fat percentage, plasma glucose, HDL cholesterol, triglycerides, TBARS, CRP, TAC, physical activity, insulin, HOMA-IR, and QUICKI). The number of subjects was determined a priori based on previous research from older adults (21), which showed that n = 15 per group would provide 0.92, and n = 10 per group would provide 0.75 power, to detect a 32 ± 7% increase in steady-state FVC with infusion of AA. All data are presented as means ± SE, and significance was determined a priori at P < 0.05.
RESULTS
Subject characteristics.
Subject characteristics are summarized in Table 1. Fourteen adults with MetSyn, 10 obese, and 15 lean healthy adults completed the present study (74% White non-Hispanic, 5% White Hispanic, 13% Asian American, 3% African American, 5% not disclosed). Five additional subjects (4 obese, 1 MetSyn) participated in the present investigation but were excluded because of inability to obtain measures of lean forearm mass as a result of limitations of the DXA scanner. There were no significant differences between groups in regard to age, height, lean forearm mass, MVC, total cholesterol, LDL cholesterol, HDL cholesterol, TBARS, TAC, or physical activity (P > 0.05). Subjects in the obese and MetSyn groups were clinically obese, displaying significantly higher weight, BMI, waist circumference, and percentage of body fat (P < 0.05) compared with lean adults. Glucose, HOMA-IR, QUICKI, triglycerides, resting mean blood pressure, and CRP were not significantly different between lean and obese adults (P > 0.05). Adults with MetSyn exhibited higher fasting glucose, insulin, triglycerides, HOMA-IR, QUICKI, resting mean blood pressure, and CRP compared with lean and/or obese adults (P < 0.05). Intra-arterial infusion of AA resulted in a significant increase in plasma TAC (174 ± 37%, n = 28 attributable to sample loss), and the increase was not different between groups (P > 0.05).
Table 1.
Subject demographics
| Lean | Obese | MetSyn | |
|---|---|---|---|
| Sex, M/F | 12/3 | 8/2 | 10/4 |
| Age, yr | 30 ± 3 | 33 ± 3 | 33 ± 3 |
| Height, cm | 174 ± 2 | 174 ± 3 | 176 ± 3 |
| Weight, kg | 70 ± 3 | 110 ± 7* | 122 ± 8* |
| BMI, kg/m2 | 23 ± 1 | 36 ± 2* | 39 ± 2* |
| Waist, cm | 79 ± 3 | 114 ± 4* | 119 ± 5* |
| Body fat, % | 22 ± 2 | 40 ± 2* | 41 ± 2* |
| Total forearm mass, g | 1028 ± 63 | 1436 ± 93* | 1457 ± 175 |
| Lean forearm mass, g | 970 ± 59 | 1063 ± 68 | 1040 ± 114 |
| MVC, kg | 39 ± 2 | 46 ± 3 | 41 ± 3 |
| MABP, mmHg | 90 ± 2 | 98 ± 4 | 105 ± 2* |
| Glucose, mg/dl | 81 ± 3 | 75 ± 3 | 92 ± 5† |
| Insulin, μU/ml | 12 ± 1 | 31 ± 9* | 43 ± 9* |
| HOMA-IR, AU | 3 ± 1 | 6 ± 2 | 10 ± 2* |
| QUICKI, AU | 0.34 ± 0.01 | 0.31 ± 0.01 | 0.28 ± 0.01* |
| Total cholesterol, mg/dl | 151 ± 8 | 162 ± 14 | 173 ± 12 |
| Triglyceride, mg/dl | 76 ± 7 | 98 ± 12 | 189 ± 28*† |
| HDL, mg/dl | 46 ± 4 | 32 ± 4 | 37 ± 4 |
| LDL, mg/dl | 91 ± 6 | 115 ± 8 | 99 ± 10 |
| CRP, mg/l | 0.4 ± 0.1 | 1.4 ± 0.4 | 2.1 ± 0.5* |
| TBARS, MDA units | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.9 ± 0.3 |
| TAC, Trolox, μM | 3.9 ± 1.5 | 2.3 ± 0.1 | 2.2 ± 0.2 |
| TAC increase with AA, % | 224 ± 97 | 137 ± 34 | 166 ± 57 |
| Physical activity, kcal/wk | 2354 ± 523 | 1528 ± 233 | 1889 ± 461 |
Values are means ± SE. Lean (n = 15), Obese (n = 10), metabolic syndrome (MetSyn) (n = 14), unless otherwise noted: Waist (Lean n = 14), Body Fat (Obese n = 9), Insulin/homeostatic model assessment of insulin resistance (HOMA-IR)/quantitative insulin sensitivity check index (QUICKI) (Lean n = 11, Obese n = 8, MetSyn n = 11), low-density lipoprotein (LDL) (Lean n = 13, Obese n = 6), high-density lipoprotein (HDL) (Obese n = 9), C-reactive protein (CRP) (Lean n = 14), thiobarbituric acid reactive substances (TBARS) (Lean n = 14), total antioxidant capacity (TAC) (Lean n = 8, Obese n = 8, MetSyn n = 12), physical activity (MetSyn n = 12). Effect of group:
P < 0.05 vs. Lean,
P < 0.05 vs. Obese. BMI, body mass index; MVC, maximal voluntary contraction; MABP, mean arterial blood pressure; MDA, malondialdehyde; AA, ascorbic acid.
Systemic hemodynamic responses to steady-state exercise.
See Tables 2 and 3. Brachial artery diameter was larger in obese and MetSyn adults when compared with controls (main effect of group, P < 0.05) and did not change with exercise or AA infusion (main effect of exercise/AA, P < 0.05). Mean arterial blood pressure was greatest in adults with MetSyn (main effect of group, P < 0.05) and did not change with exercise (main effect of exercise, P > 0.05). Mean arterial blood pressure increased from Trial 1 to Trial 2 (∼5 mmHg; main effect of AA, P < 0.01; Table 2) although responses were not different between groups (interaction between group and AA, P > 0.05).
Table 2.
Steady-state responses to 15-min forearm exercise with intra-arterial infusion of AA (Trial 1)
| Lean | Obese | MetSyn | |
|---|---|---|---|
| Mean arterial blood pressure, mmHg | |||
| Rest | 97 ± 3 | 101 ± 3 | 105 ± 2* |
| Exercise | 100 ± 3 | 101 ± 3 | 106 ± 3* |
| Exercise + AA | 105 ± 4 | 104 ± 4 | 109 ± 3* |
| Brachial artery diameter, cm | |||
| Rest | 0.40 ± 0.02 | 0.44 ± 0.02 | 0.43 ± 0.02 |
| Exercise | 0.42 ± 0.01 | 0.46 ± 0.02 | 0.45 ± 0.02 |
| Exercise + AA | 0.43 ± 0.01 | 0.47 ± 0.02 | 0.46 ± 0.02 |
| Forearm blood flow, ml/min × 100 g | |||
| Rest | 7 ± 1 | 9 ± 2 | 14 ± 2* |
| Exercise† | 26 ± 2 | 33 ± 5 | 43 ± 7* |
| Exercise + AA† | 31 ± 2 | 37 ± 5 | 46 ± 7* |
| Forearm vascular conductance, ml/min × 100 mmHg × 100 g | |||
| Rest | 7 ± 1 | 9 ± 2 | 13 ± 3* |
| Exercise† | 27 ± 2 | 33 ± 5 | 41 ± 6* |
| Exercise + AA† | 30 ± 3 | 37 ± 5 | 43 ± 6* |
Values are means ± SE. Lean (n = 14), Obese (n = 10), MetSyn (n = 13) (2 subjects did not complete all 15 min of exercise). Main effect of group:
P < 0.05 vs. Lean. Main effect of exercise:
P < 0.05 vs. Rest.
Table 3.
Steady-state responses to 5-min forearm exercise before and after intra-arterial infusion of AA, Trial 1 (Pre-AA) vs. Trial 2 (Post-AA)
| Lean |
Obese |
MetSyn |
||||
|---|---|---|---|---|---|---|
| Pre-AA | Post-AA | Pre-AA | Post-AA | Pre-AA | Post-AA | |
| Mean arterial blood pressure, mmHg | ||||||
| Rest | 96 ± 3 | 100 ± 3‡ | 101 ± 3 | 104 ± 3‡ | 105 ± 2*† | 113 ± 3*†‡ |
| Exercise | 99 ± 3 | 104 ± 3‡ | 101 ± 3 | 105 ± 3‡ | 106 ± 2*† | 112 ± 3*†‡ |
| Brachial artery diameter, cm | ||||||
| Rest | 0.40 ± 0.02 | 0.40 ± 0.01 | 0.44 ± 0.02* | 0.44 ± 0.02* | 0.44 ± 0.02* | 0.44 ± 0.02* |
| Exercise | 0.42 ± 0.01 | 0.42 ± 0.01 | 0.46 ± 0.02* | 0.47 ± 0.02* | 0.46 ± 0.02* | 0.46 ± 0.02* |
| Forearm blood flow, ml/min × 100 g | ||||||
| Rest | 7 ± 1 | 8 ± 1 | 9 ± 2 | 12 ± 2 | 16 ± 3*† | 15 ± 2*† |
| Exercise | 27 ± 2 | 30 ± 2 | 33 ± 5 | 37 ± 4 | 45 ± 6*† | 43 ± 5*† |
| Forearm vascular conductance, ml/min × 100 mmHg × 100 g | ||||||
| Rest | 7 ± 1 | 8 ± 1 | 9 ± 2 | 11 ± 2 | 15 ± 3* | 14 ± 2* |
| Exercise | 27 ± 2 | 30 ± 3 | 33 ± 5 | 36 ± 4 | 42 ± 6* | 39 ± 5* |
Values are means ± SE. Lean (n = 15), Obese (n = 10), MetSyn (n = 14). Main effect of group:
P < 0.05 vs. Lean,
P < 0.05 vs. Obese. Main effect of AA:
P < 0.05 versus Pre AA.
AA infusion and time to achieve steady-state exercise-mediated vasodilation.
Data are summarized in Fig. 1. Time course data were analyzed from a subset of participants (Lean n = 13, Obese n = 10, MetSyn n = 9) because of incomplete blood velocity data during the transition from rest to steady state (defined as <90% of data points available for analysis, as a result of repositioning the ultrasound probe). MRT was not different between groups (main effect of group, P = 0.26). AA infusion did not alter the time to achieve steady state in any group (MRT: main effect of AA, P = 0.50; interaction between group and AA, P = 0.90).
AA infusion and steady-state exercise-mediated vasodilation.
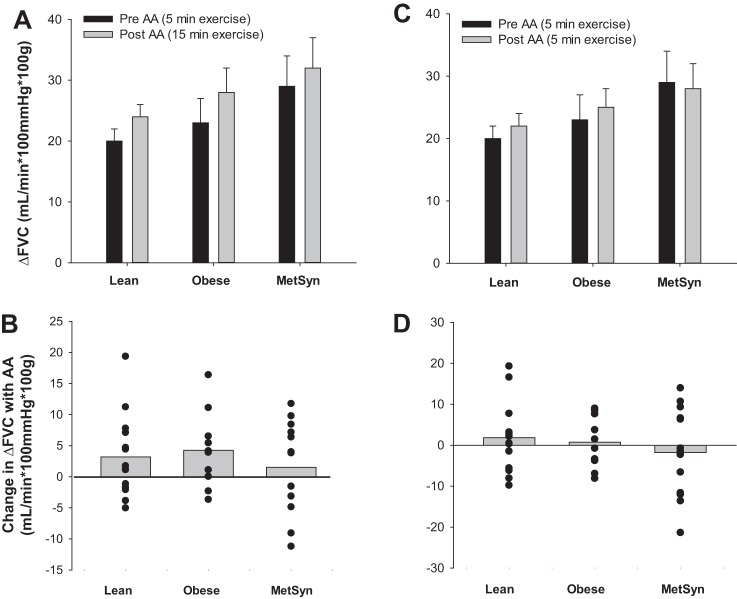
Data are summarized in Table 3. FBF and FVC were greatest in adults with MetSyn (main effect of group, P < 0.05). FBF and FVC increased with exercise (main effect of exercise, P < 0.01), and the rise in blood flow and conductance with exercise (Δ) was not different between groups (Fig. 2, A and C). There appeared to be no effect of AA infusion on steady-state FBF or FVC (main effect of AA, P > 0.05) regardless of whether AA was infused during steady-state exercise (Table 2, Fig. 2, A and B) or before exercise onset (Table 3, Fig. 2, C and D).
Fig. 2.
Effect of acute ascorbic acid infusion on the exercise-mediated increase in FVC. A: Lean (n = 14), Obese (n = 10), MetSyn (n = 13). The exercise-mediated increase FVC from rest (Δ, Exercise − Rest) was not altered when AA was infused (Trial 1, Min 15), compared with exercise alone (Trial 1, Min 5) (main effect of AA: P = 0.34), and responses were not different between groups (main effect of group: P = 0.16). B: change in the exercise-mediated increase in FVC with AA infusion was not different between groups (interaction between group and AA: P = 0.94) although individual responses were variable (a positive value indicates FVC increased after AA infusion). C: Lean (n = 15), Obese (n = 10), MetSyn (n = 14). The exercise-mediated increase in FVC from rest (Δ) was not different between groups (main effect of group, P = 0.31), and responses did not increase when AA was infused (Trial 2, Min 5), compared with exercise alone (Trial 1, Min 5) (main effect of AA: P = 0.93). D: change in the exercise-mediated increase in FVC with AA infusion was not different between groups (interaction between group and AA: P = 0.85) although individual responses were variable (a positive value indicates FVC increased after AA infusion).
Relationship between cardiovascular disease risk factors and exercise hyperemia.
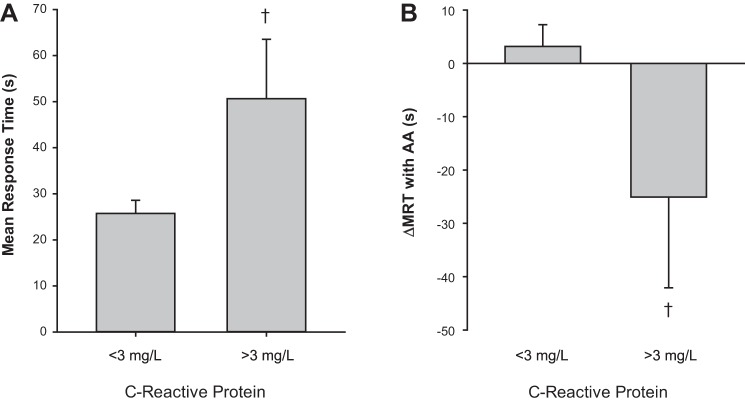
Relationships between main outcome variables and measures of cardiovascular disease risk were examined using data pooled from all groups. A significant positive relationship was observed between MRT and CRP (r = 0.52, P < 0.01; n = 31), such that subjects with the highest CRP levels exhibited the longest MRT before infusion of AA. Additionally, CRP was negatively correlated with Δ MRT after AA infusion (r = −0.43, P = 0.02, n = 31), indicating that subjects with the highest CRP experienced the greatest reduction in MRT during acute AA infusion. Consistent with clinical guidelines, those subjects with CRP levels >3 mg/l (n = 6) tended to exhibit longer baseline MRT (P = 0.09) and a greater reduction in MRT with AA infusion (P = 0.07) (Fig. 3). A trend was also observed between the change in steady-state FVC with AA infusion (Δ FVC) and physical activity (r = −0.31, P = 0.07; n = 36). In this way, those subjects with the lowest level of physical activity exhibited the greatest increase in Δ FVC with infusion of AA.
Fig. 3.
Effect of inflammation on MRT and change with acute ascorbic acid infusion. A: C-reactive protein (CRP) <3 mg/l (n = 25), CRP >3 mg/l (n = 6). Consistent with clinical guidelines, those subjects with CRP levels >3 mg/l tended to exhibit longer baseline MRT (P = 0.09). B: those subjects with CRP levels >3 mg/l tended to exhibit a greater reduction in MRT (Δ) with ascorbic acid infusion (P = 0.07) compared with adults with CRP levels <3 mg/l. †P < 0.10 vs. CRP <3 mg/l.
DISCUSSION
This study directly examined exercise-mediated vasodilation before and during acute AA infusion in young (∼32 yr old) obese adults and adults with MetSyn. Results from the present study confirm previous findings that steady-state vascular responses to dynamic forearm exercise are not impaired in obese adults and adults with MetSyn and further suggest that the time to achieve steady-state is also preserved. Contrary to our hypothesis, increasing total antioxidant capacity did not alter the time course or steady-state vascular responses during dynamic, moderate-intensity exercise in groups of lean or obese humans or those with MetSyn. Despite the lack of group effect, individual analyses suggest that the impact of increasing total antioxidant capacity on exercise-mediated vasodilator responses differs considerably across subjects in all groups studied, with greatest effect in adults with high levels of systemic inflammation.
Time course of vasodilator responses to exercise and impact of acute AA infusion.
Rodent models of MetSyn exhibit impaired vasodilation within 30 s of the onset of contraction during simulated exercise (9). Thus we proposed that vasodilator responses to exercise onset and the time to achieve steady-state vasodilation would be impaired in obese adults and adults with MetSyn. In contrast, the MRT to dynamic forearm exercise was not altered in obese adults and adults with MetSyn (Fig. 1B); rather, MRT across all groups was similar to those published previously in young, healthy men using a similar exercise model (18). Such findings were surprising, given that contraction-induced rapid (<6 s) vasodilation is blunted in human obesity (1). Furthermore, research from healthy humans suggests that rapid vasodilation is primarily potassium channel and nitric oxide mediated (3, 6), and both mechanisms are altered in animal models of obesity and MetSyn (12, 17). In this context, differences in key vascular control mechanisms between rapid-onset vasodilation and the transition to and maintenance of steady-state exercise hyperemia may explain the discrepancy between findings. However, mechanisms mediating the transition from rest to steady state were previously unexamined in human obesity and MetSyn.
On the basis of rationale from animal studies in which acute antioxidant administration altered contraction-induced vasodilation (9), we examined the effect of acute AA infusion on MRT in obese adults and adults with MetSyn. Interestingly, there appeared to be no significant effect of an increase in total antioxidant capacity on hemodynamic responses in any group (Fig. 1B). Despite these initial findings, with closer inspection, our data suggest that chronic inflammation may play a modest role in determining the time course of the exercise vasodilator response. Specifically, when data from all groups were combined, adults with the highest CRP levels took the longest time to achieve steady-state exercise-mediated vasodilation, suggesting that inflammation may account for ∼27% (r = 0.52, r2 = 0.27, P < 0.01) of the variance in MRT. Along these lines, adults with MetSyn exhibited significantly higher CRP and an ∼20% greater MRT to exercise before AA infusion compared with lean and obese individuals (Fig. 1; Pre-AA, Lean = 30 ± 6; Obese = 31 ± 8; MetSyn = 36 ± 8 s). Interestingly, acute AA infusion was able to improve MRT in those individuals with high CRP, exposing a potential relationship between inflammation, ROS, and the time course of the exercise vasodilator response independent of obesity and/or MetSyn (Fig. 3). Given that a slower vasodilator response to exercise may result in earlier onset of fatigue and poor exercise tolerance (18), such findings have important clinical implications.
Steady-state exercise-mediated vasodilation and impact of acute AA infusion.
Confirming our previous observations, steady-state vasodilator responses to moderate-intensity, dynamic forearm exercise are not impaired in human obesity (25) and MetSyn (26) and, if anything, are increased in obese and MetSyn adults (Tables 2–3, Fig. 2, A and C). Although not directly examined, previous research from our laboratory is consistent with impaired blood flow distribution in human MetSyn (28), and such ideas are supported by recent research from animal models (2, 10, 13). In this way, impaired flow distribution may result in ventilation-perfusion mismatch, thus requiring a greater whole limb exercise blood flow response to meet the metabolic demand of working tissues. Increased ROS has been shown to contribute to the observed increase in flow heterogeneity in response to simulated exercise in animal models of MetSyn (2, 10, 13). Thus we hypothesized that infusion of AA may contribute to a reduction in exercise blood flow in MetSyn, possibly as a result of improved flow distribution.
Alternatively, increased levels of ROS commonly observed in obesity and MetSyn may act as compensatory mechanisms important in preserving exercise hyperemia in response to a loss of primary vascular control mechanisms. In support of this idea, ROS signaling is known to play a role in the maintenance of blood flow in the coronary circulation of humans with cardiovascular disease (16, 23). Along these lines, oral antioxidant administration may actually reduce exercise-mediated vasodilation even in young healthy adults (35), supporting a potential contribution of ROS to the normal vasodilator response to exercise in humans.
Despite the above rationale, increasing total antioxidant capacity via intra-arterial infusion of AA neither increases nor significantly limits steady-state exercise hyperemia in younger lean, obese, or MetSyn adults during steady-state exercise (Trial 1) or before exercise onset (Trial 2). Consistent with our findings, antioxidant supplementation does not affect total limb blood flow or flow distribution in older rats during treadmill exercise (5), nor does it impact the oxygen delivery/utilization balance in skeletal muscle of young healthy rats (4). It is important to note that, when mechanisms important to vascular control (e.g., ROS) are blunted, redundant signals may compensate to produce a “normal” hyperemic response (36). Thus, by examining acute responses to AA infusion during exercise, in addition to the effect of AA infusion before exercise onset, we can rule out the possibility of the presence of a compensatory mechanism masking the normal contribution of ROS to exercise-mediated vasodilation in human obesity and/or MetSyn in the present study.
Interestingly, post hoc analysis revealed a possible relationship between physical activity and FVC, such that adults with lower levels of physical activity tend to exhibit increased steady-state exercise-mediated vasodilation with infusion of AA (r = −0.31, P = 0.07). Although speculative, these results may suggest that, rather than obesity-related disease per se, ROS-mediated impairments in vascular responses to exercise might be the result of relatively sedentary behaviors observed in the current research cohort. Along these lines, physical activity has been shown to increase production of endogenous antioxidant enzymes, which may be important to counteract the presence of chronic and/or exercise-mediated ROS production (19). Consistent with this idea, low levels of physical activity are associated with increased oxidative stress, inflammation, impaired endothelial function, and increased cardiovascular disease risk (37). It is important to note that all subjects were relatively inactive (<3 h of organized activity each week), typical physical activity consisted of low-intensity walking, and subjects refrained from exercise for a minimum of 24 h before participation (a standard protocol for these types of studies). It may be possible, although unlikely, that physical activity completed more than 24 h before study participation modified the observed relationship between low physical activity and increased steady-state vasodilation with AA. Future studies are necessary to explore this idea, in addition to examining the effect of initiation of an exercise training program in sedentary individuals.
Experimental considerations.
As summarized above, group data indicate that increasing total antioxidant capacity with AA infusion does not alter time course (MRT) or magnitude (Δ FVC) of vasodilator responses to moderate-intensity, dynamic forearm exercise. This observation may be the result of the relatively young age and likely short disease duration in the present study population, resulting in only small group differences in baseline measures of systemic oxidative stress, antioxidant capacity, and inflammation (Table 1). However, the range of individual responses to exercise and AA infusion within the present data, independent of group, is consistent with the concept of unique physiological phenotypes. Specifically, when the change in vascular responses to acute AA infusion was examined, MRT and FVC to exercise increased, decreased, or did not change (Figs. 1C, and 2, B and D). Furthermore, our data suggest that systemic measures of oxidative stress may not be representative of what is occurring locally within the skeletal muscle vasculature, and thus large differences in systemic oxidative stress may not be necessary to observe impairments in vascular control and/or improvements with infusion of AA. Thus physiological changes likely occur in some subjects very early in the disease process, before large impairments in standard clinical measures. Considering that ROS scavenging improves exercise-mediated vasodilation in a group of healthy older adults (21) and endothelial-dependent vasodilation in older obese adults (32), our data indicate a complex transition between early disease processes and advancing age, with the potential for age-by-disease and/or lifestyle interactions. These speculations require more rigorous testing both acutely and longitudinally.
It is important to note that CRP is a marker of systemic inflammation and CRP elevations likely occur in response to disturbances in IL-1, TNF-α, and IL-6 signaling. Thus CRP may not be mediating the observed impairments in MRT to dynamic handgrip exercise and may not be an appropriate direct target for future therapies (22); however, our results are suggestive of the contribution of an inflammatory pathway in observed responses. Furthermore, although we observed significant increases in TAC, we did not directly measure changes in ROS during study interventions. Taken together, future studies will be necessary to better understand the specific mechanisms behind inflammation-mediated impairments in MRT and the direct effect of ROS scavenging (both acute and chronic therapies) on measures of exercise-mediated vasodilation.
Conclusion.
In summary, our findings add to the growing body of knowledge indicating that the ability to achieve steady-state vasodilation in response to moderate-intensity, dynamic forearm exercise is not impaired by obesity or MetSyn in younger (∼32 yr) humans. Despite preserved exercise responses, factors linked to cardiovascular disease risk (e.g., inflammation) might alter control mechanisms important to exercise-mediated vasodilation. Furthermore, the variety of both time-course and steady-state vasodilator responses to exercise, as well as the range of individual responses to acute AA infusion, suggest that a “normal” exercise response can be achieved by numerous physiological mechanisms, some of which may be ROS mediated. Interestingly, averaged responses across groups suggest that the collective effects of obesity and/or MetSyn per se are not sufficient to impart negative ROS-mediated vasodilator responses similar to those reported in other human conditions, such as aging. In this way, future work will be necessary to systematically test alternative mechanisms, in addition to disease duration and/or disease-by-age interactions and their influence on exercise-mediated vasodilation.
GRANTS
This study was supported by the American Heart Association predoctoral awards 10PRE3870000 (J. Limberg) and 11PRE7390038 (J. Harrell), and the National Institutes of Health (NIH) HL091397 (W. Schrage) and HL105820 (W. Schrage). In addition, we acknowledge the involvement of WNPRC Assay Services and the partial support of NIH grant RR000167. This study was also supported by the Clinical and Translational Science Award program through the NIH National Center for Advancing Translational Sciences Grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.K.L., J.W.H., R.J., M.W.E., L.T.P., J.J.S., and W.G.S. performed experiments; J.K.L., J.M.K., J.W.H., and R.J. analyzed data; J.K.L., J.M.K., J.W.H., R.J., M.W.E., L.T.P., J.J.S., and W.G.S. interpreted results of experiments; J.K.L. and J.M.K. prepared figures; J.K.L. and W.G.S. drafted manuscript; J.K.L., J.M.K., J.W.H., R.J., and W.G.S. edited and revised manuscript; J.K.L., J.M.K., J.W.H., R.J., M.W.E., L.T.P., J.J.S., and W.G.S. approved final version of manuscript; M.W.E., L.T.P., J.J.S., and W.G.S. conception and design of research.
ACKNOWLEDGMENTS
We thank all our participants. In addition, we thank Trent Evans, Molly Dixon, Caitlin Zillner, Jessica Danielson, and Meghan Crain for technical assistance.
REFERENCES
- 1.Blain GM, Limberg JK, Mortensen GF, Schrage WG. Rapid onset vasodilatation is blunted in obese humans. Acta Physiol (Oxf) 205: 103–112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butcher JT, Goodwill AG, Stanley SC, Frisbee JC. Blunted temporal activity of microvascular perfusion heterogeneity in metabolic syndrome: a new attractor for peripheral vascular disease? Am J Physiol Heart Circ Physiol 304: H547–H558, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casey DP, Mohamed EA, Joyner MJ. Role of nitric oxide and adenosine in the onset of vasodilation during dynamic forearm exercise. Eur J Appl Physiol 113: 295–303, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Copp SW, Ferreira LF, Herspring KF, Hirai DM, Snyder BS, Poole DC, Musch TI. The effects of antioxidants on microvascular oxygenation and blood flow in skeletal muscle of young rats. Exp Physiol 94: 961–971, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Copp SW, Schwagerl PJ, Hirai DM, Poole DC, Musch TI. Acute ascorbic acid and hindlimb skeletal muscle blood flow distribution in old rats: rest and exercise. Can J Physiol Pharmacol 90: 1498–1505, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305: H29–H40, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes 2: 180–193, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Frisbee JC. Impaired skeletal muscle perfusion in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 285: R1124–R1134, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Frisbee JC, Hollander JM, Brock RW, Yu HG, Boegehold MA. Integration of skeletal muscle resistance arteriolar reactivity for perfusion responses in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 296: R1771–R1782, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisbee JC, Maier KG, Stepp DW. Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol 283: H2160–H2168, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Frisbee JC, Stepp DW. Impaired NO-dependent dilation of skeletal muscle arterioles in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol 281: H1304–H1311, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Frisbee JC, Wu F, Goodwill AG, Butcher JT, Beard DA. Spatial heterogeneity in skeletal muscle microvascular blood flow distribution is increased in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 301: R975–R986, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita K, Nishizawa H, Funahashi T, Shimomura I, Shimabukuro M. Systemic oxidative stress is associated with visceral fat accumulation and the metabolic syndrome. Circ J 70: 1437–1442, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109: 433–438, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Gutterman DD, Miura H, Liu Y. Redox modulation of vascular tone: focus of potassium channel mechanisms of dilation. Arterioscler Thromb Vasc Biol 25: 671–678, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Haddock RE, Grayson TH, Morris MJ, Howitt L, Chadha PS, Sandow SL. Diet-induced obesity impairs endothelium-derived hyperpolarization via altered potassium channel signaling mechanisms. PLoS One 6: e16423, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughson RL, Shoemaker JK, Tschakovsky ME, Kowalchuk JM. Dependence of muscle VO2 on blood flow dynamics at onset of forearm exercise. J Appl Physiol 81: 1619–1626, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Ji LL. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic Biol Med 44: 142–152, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Vasodilatory responsiveness to adenosine triphosphate in ageing humans. J Physiol 588: 4017–4027, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane T, Wassef N, Poole S, Mistry Y, Lachmann HJ, Gillmore JD, Hawkins PN, Pepys MB. Infusion of pharmaceutical-grade natural human C-reactive protein is not proinflammatory in healthy adult human volunteers. Circ Res 114: 672–676, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Larsen BT, Bubolz AH, Mendoza SA, Pritchard KA, Jr, Gutterman DD. Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol 29: 739–745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2: 321–447, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Limberg JK, De Vita MD, Blain GM, Schrage WG. Muscle blood flow responses to dynamic exercise in young obese humans. J Appl Physiol 108: 349–355, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Limberg JK, Evans TD, Blain GM, Pegelow DF, Danielson JR, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Effect of obesity and metabolic syndrome on hypoxic vasodilation. Eur J Appl Physiol 112: 699–709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limberg JK, Harrell JW, Johansson RE, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Microvascular function in younger adults with obesity and metabolic syndrome: role of oxidative stress. Am J Physiol Heart Circ Physiol 305: H1230–H1237, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limberg JK, Morgan BJ, Sebranek JJ, Proctor LT, Eldridge MW, Schrage WG. Neural control of blood flow during exercise in human metabolic syndrome. Exp Physiol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macdonald M, Pedersen PK, Hughson RL. Acceleration of VO2 kinetics in heavy submaximal exercise by hyperoxia and prior high-intensity exercise. J Appl Physiol 83: 1318–1325, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Miyai N, Shiozaki M, Yabu M, Utsumi M, Morioka I, Miyashita K, Arita M. Increased mean arterial pressure response to dynamic exercise in normotensive subjects with multiple metabolic risk factors. Hypertens Res 36: 534–539, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc 25: 60–70, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes 50: 159–165, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol 83: 1383–1388, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Rice-Evans C, Miller NJ. Total antioxidant status in plasma and body fluids. Methods Enzymol 234: 279–293, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S, Bailey DM. Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol 292: H1516–H1522, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol 587: 5541–5549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsioufis C, Kasiakogias A, Tsiachris D, Kordalis A, Thomopoulos C, Giakoumis M, Bounas P, Pittaras A, Michaelides A, Stefanadis C. Metabolic syndrome and exaggerated blood pressure response to exercise in newly diagnosed hypertensive patients. Eur J Prev Cardiol 19: 467–473, 2012 [DOI] [PubMed] [Google Scholar]