Abstract
Bile duct ligation (BDL) causes congestive liver failure that initiates hemodynamic changes, resulting in dilutional hyponatremia due to increased water intake and vasopressin release. This project tested the hypothesis that angiotensin signaling at the subfornical organ (SFO) augments drinking behavior in BDL rats. A genetically modified adeno-associated virus containing short hairpin RNA (shRNA) for ANG II receptor subtype 1a (AT1aR) gene was microinjected into the SFO of rats to knock down expression. Two weeks later, BDL or sham surgery was performed. Rats were housed in metabolic chambers for measurement of fluid and food intake and urine output. The rats were euthanized 28 days after BDL surgery for analysis. A group of rats was perfused for immunohistochemistry, and a second group was used for laser-capture microdissection for analysis of SFO AT1aR gene expression. BDL rats showed increased water intake that was attenuated in rats that received SFO microinjection of AT1aR shRNA. Among BDL rats treated with scrambled (control) and AT1aR shRNA, we observed an increased number of vasopressin-positive cells in the supraoptic nucleus that colocalized with ΔFosB staining, suggesting increased vasopressin release in both groups. These results indicate that angiotensin signaling through the SFO contributes to increased water intake, but not dilutional hyponatremia, during congestive liver failure.
Keywords: angiotensin, supraoptic, vasopressin, water intake
hyponatremia is the most frequently occurring clinical electrolyte disorder, and the annual cost of treating patients with hyponatremia in the United States is estimated to be $1.6 to $3.6 billion (8). Hyponatremia is also associated with negative outcomes in many chronic disease states, such as congestive heart failure and cirrhosis. In both liver and heart failure, changes in drinking behavior and the osmoregulation of AVP release from the neurohypophyseal system contribute to water retention and dilutional hyponatremia, thereby increasing morbidity and mortality (12, 51, 62). Treatment with specific V2 receptor antagonists (V2A) does produce solute-free water excretion and improved plasma osmolality in both heart failure and cirrhosis (28, 29, 63); however, there is evidence that increased drinking behavior from disease and possibly as a side effect of V2A treatment also affects mortality and/or rehospitalization (56, 57).
Circulating levels of AVP and oxytocin are primarily determined by the activity of magnocellular neurosecretory cells of the supraoptic (SON) and paraventricular (PVN) nuclei of the hypothalamus that project to the posterior pituitary. The activity of these cells is regulated by extracellular osmolality, and by neuronal projections that respond to changes in plasma osmolality, blood pressure, blood volume, and circulating hormones (3, 9, 22, 30). Increased neurohumoral activation associated with cirrhosis has been attributed to systemic hypotension and hyperdynamic circulatory syndrome (41, 61, 67). Hyperdynamic circulatory syndrome is also reported to activate the renin-angiotensin system during hepatic cirrhosis, which would be consistent with the traditional view that nonosmotic factors induce the dilutional hyponatremia associated with this disease state by stimulating the release of vasopressin despite progressive plasma hyponatremia (20, 73). Increased circulating ANG II is one factor that could contribute to sympatho-excitation, increased AVP release, and elevated fluid intake by acting on the central nervous system (CNS) circumventricular organs (49, 52).
The lamina terminalis contains two circumventricular organs: the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO). Neurons within these forebrain regions lack a functional blood-brain barrier (36, 48), which allows these structures to be sensitive to circulating factors such as plasma osmolality and ANG II (52). The OVLT and SFO both project to the median preoptic nucleus, which is an important integrative center for hydromineral and cardiovascular regulation. ANG II type 1 receptor (AT1R) expression is densely localized to these nuclei (1, 31), and the level of AT1R expression in these regions may be an important excitatory input that also modulates the responsiveness of the brain angiotensin system in regulating body fluid homeostasis.
Chronic bile duct ligation (BDL) in the rat is a commonly used model of hepatic cirrhosis (6, 10). As in the human condition, these rats have elevated circulating vasopressin and increased drinking behavior accompanied by significantly decreased plasma osmolality, increased plasma renin activity, and hypervolemic ascites formation (14). Previously, we observed that the expression of angiotensin type 1a receptors (AT1aRs) was increased in the SFO of BDL rats (70). In that study, losartan was infused chronically into the lateral ventricles of the brains of control and BDL rats to test effects of AT1R blockade on water intake and found that central AT1R blockade resulted in attenuation of the increased water intake-associated BDL (70). This indicates an important role for AT1Rs in regulating fluid intake in BDL. However, the location of AT1Rs involved in thirst and AVP release remain to be determined.
Synaptic activation of vasopressin and oxytocin neurons in the hypothalamus is associated with increased expression of c-Fos and c-Fos-related proteins. The expression of c-Fos has been used to map regions of the CNS that are activated following acute physiological and supraphysiological stimulation (22, 23, 33). Therefore, part of this study examined the expression of FosB in SON to characterize activation of magnocellular neurosecretory cells during BDL and with injection of a neurotrophic adeno-associated virus (AAV) vector containing shRNA against AT1aRs.
The purpose of this study was to evaluate the role of SFO AT1Rs in regulating drinking behavior and activation of SON AVP neurons. Increased expression of AT1aR in the SFO of cirrhotic rats could represent a cellular adaptation that contributes to increased drinking behavior and SON activation. We hypothesize that knockdown of AT1aR in the SFO will attenuate drinking behavior in BDL rats and decrease SON activation. This hypothesis was tested by using an AAV-shRNA vector against AT1aRs to inhibit the observed upregulation of these receptors in the SFO during BDL. We also measured plasma electrolytes and ΔFosB staining to evaluate changes in neurohumoral activation associated with BDL and AAV-shRNA treatment.
METHODS
Animals.
Experiments were conducted on adult male Sprague-Dawley rats (250–350 g, Charles River Laboratories, Wilmington, MA). Rats were individually housed and maintained in a temperature-controlled (23°C) environment under a 12:12-h light cycle with light onset at 0700. Rats had ad libitum access to food and water except during the surgical procedures. All experimental procedures were conducted in accordance with the guidelines of the Public Health Service and were approved by the University of North Texas Health Science Centre Institutional Animal Care and Use Committee.
Bile duct ligation surgery.
Each animal was anesthetized with isoflurane (2% in oxygen). After fully anesthetized, the abdomen was shaved and the area was cleaned with iodine antiseptic. A midline incision was performed on the abdomen, and the common bile duct was isolated by blunt dissection and cut between two ligatures. Each animal was then returned to a cage and monitored daily until used for the experiment. Visual inspection of ascitic fluid in the peritoneal cavity was performed daily after surgery. Any rat showing morbidity (e.g., failure to thrive, significant decrease in food or water intake) was euthanized with thiobutabarbital (Inactin, Sigma, St. Louis, MO; 100 mg/kg ip). Sham-ligated controls were subjected to the same surgical procedure, with the exception that the bile duct was not ligated or cut. All rats from each experimental subgroup were euthanized 4 wk (28 days) after BDL or sham surgery for collection of brain and blood samples. Liver fibrosis was observed in successful bile duct-ligated animals. Liver-to-body weight ratio was used to verify the development of hepatic cirrhosis. Three rats were euthanized after surgery due to illness. The number of remaining animals in each experiment is given in Table 2.
Table 2.
Measurements of serum osmolality and sodium, hematocrit, protein, and liver weight-to-body weight ratio in sham and bile duct ligated rats injected with a viral construct containing either a scrambled control shRNA or a shRNA targeted to AT1aR
| n | Osmolality, mOsm/kg | Serum Sodium, mEq/l | Hematocrit, % | Plasma Protein, g/dl | Liver Wt/Body Wt | Final Body Weight, g | |
|---|---|---|---|---|---|---|---|
| Sham SCM | 12 | 308 ± 1 | 166 ± 5 | 48 ± 1 | 7.6 ± 0.1 | 0.035 ± 0.002 | 484 ± 19 |
| Sham sh-AT1a | 18 | 304 ± 1 | 161 ± 4 | 48 ± 1 | 7.7 ± 0.1 | 0.031 ± 0.001 | 495 ± 9 |
| BDL SCM | 12 | 302 ± 1† | 153 ± 4† | 44 ± 1* | 7.7 ± 0.1 | 0.070 ± 0.002* | 470 ± 8 |
| BDL sh-AT1a | 16 | 300 ± 1* | 146 ± 3* | 44 ± 1* | 7.7 ± 0.1 | 0.075 ± 0.004* | 458 ± 13 |
Data are expressed as means ± SE.
BDL, bile duct ligated; SCM, scrambled control shRNA; sh-AT1a, shRNA targeted to AT1aR.
P < 0.01 compared to sham groups.
P < 0.05 compared to sham SCM group.
Virally mediated AT1aR mRNA interference.
Stereotaxic surgery was used to inject recombinant AAV-containing green fluorescent protein (GFP) and either scrambled shRNA (AAV-SCM) or shRNA against AT1a receptor (AAV-sh-AT1a). The injection volume was 0.3 μl of 1.1 × 1012 genomic particles/ml (GeneDetect, Bradenton, FL). Each rat was anesthetized with isoflurane and placed in a stereotaxic apparatus. To target SFO, 30 gauge injectors oriented vertically were advanced to −1.20 mm (anterior/posterior), 0.00 mm (midline) relative to the bregma, and −4.20 mm (dorsal/ventral) relative to the top of the midsagittal sinus. Each construct was injected in the SFO over a 10-min period. Five minutes thereafter, injectors were then removed, and the scalp incision was closed with sutures. Stereotaxic surgery was performed 2 wk prior to sham ligation or BDL surgery to allow for recovery.
Metabolic cages.
To measure water intake, food intake, and urine output, animals were housed in metabolic cages (Lab Products, Seaford, DE) starting on the 18th day after BDL or sham surgery. The metabolic cage protocol consisted of 10 days and proceeded, as previously described (70). There were four separate randomly allocated groups used for metabolism cage studies: AAV-SCM sham ligated (n = 12), AAV-SCM BDL (n = 12), and AAV-sh-AT1a sham ligated (n = 18), and AAV-sh-AT1a BDL (n = 16). Rats were moved into metabolic cages for measurement of daily food intake, water intake, and urine output on the 18th day after sham ligation or BDL surgery. Food intake, water intake, and urine output were recorded daily at 9:00 AM. Food intake was measured by filling the food containers up to a predetermined weight of ground chow in grams and subtracting the remaining weight 24 h later. Some ground chow was spilled by the rats and captured in the funnel filter. The spilled food was not collected or included in the analysis. Sodium intake was calculated from sodium content of the food (0.32% by weight, cat no. LM485; Teklad Diets, Madison WI). Water intake was determined using graduated cylinders. Urine was collected in 50-ml Falcon centrifuge tubes, and 1 ml from each daily sample was transferred to a 1.5-ml microcentrifuge tube and centrifuged (20 min; 10,000 g). A 10-μl sample was used for measuring urine sodium concentration using a flame photometer (Jenway PFP7, VWR International, Radnor PA). All data were normalized to each rat's body weight at death. At the end of the experiment, each rat was anesthetized with Inactin (100 mg/kg ip) and perfused for immunohistochemistry. A 2- to 3-ml blood sample was taken by cardiac puncture before each perfusion for measuring plasma osmolality, sodium, hematocrit, and plasma protein, as described above. Each rat's liver was removed and weighed, as described above. Sections containing the SFO were analyzed for GFP using an epifluorescence microscopy to determine the location of the injection sites and transfection of SFO neurons. A separate set of rats from each of the four groups (n = 5, each group) was processed for laser capture microdissection, as described above.
Plasma measurements.
Blood collected by cardiac puncture was transferred into a 1.5-ml microcentrifuge tube. Two heparin-containing capillary tubes were filled with blood from this sample for measuring hematocrit. The rest of the sample was centrifuged (5 min; 10,000 g). A 200-μl sample of serum was removed for measuring osmolality using a vapor pressure osmometer (Wescor Logan, UT), and sodium concentration was measured using a flame photometer. Serum protein was measured by refractometery (National Protometer, National Instruments, Baltimore, MD).
ΔFosB immunohistochemistry.
Separate groups of sham-ligated AAV-SCM-injected (n = 9), sham-ligated AAV-sh-AT1a-injected (n = 10), BDL AAV-SCM-injected (n = 7), and BDL AAV-sh-AT1a-injected (n = 8) rats were used in immunohistochemistry studies to determine the effects of SFO AT1a receptor knockdown and BDL on ΔFosB staining in the SON. Four weeks (28 days) after BDL or sham ligation surgery, rats were anesthetized with thiobutabarbital (Inactin, 100 mg/kg ip) and perfused transcardially with 50–100 ml of PBS followed by 300–400 ml of 4% paraformaldehyde in PBS for immunohistochemistry, as previously described (35). The descending aorta and vena cava were clamped below the heart with hemostats, and each liver was removed and weighed.
Alternating sets of 40-μm coronal sections containing the SON were processed for FosB (goat anti-FosB, sc-48-G, 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), vasopressin [polyclonal guinea pig anti-(Arg8)-vasopressin, 1:500; Peninsula Laboratories, San Carlos, CA], and oxytocin (monoclonal mouse anti-oxytocin, MAB5296; 1:5,000; Millipore, Billerica, MA) immunohistochemistry. The primary FosB antibody used in this study does not discriminate between ΔFosB and full-length FosB and will be referred to as ΔFosB staining in the text. After incubation in the anti-FosB antibody for 72 h at 4°C, sections were rinsed and incubated with biotin-conjugated horse anti-goat IgG (1:200; Vector Laboratories, Burlingame, CA), an avidin-peroxidase conjugate (Vectastain ABC kit; Vector Laboratories), and PBS containing 0.04% 3,3′-diaminobenzidine hydrochloride and 0.04% nickel ammonium sulfate, as previously described (35). Next, the sections were incubated with both the vasopressin (guinea pig antivasopressin; Bachem) and oxytocin (mouse anti-oxytocin; Millipore) antibodies for 72 h at 4°C followed by rinsing and incubation with secondary antibodies conjugated to either Cy2 or Cy3 (donkey anti-guinea pig Cy3 and donkey anti-mouse Cy2, 1:250; Jackson ImmunoResearch, West Grove, PA).
A separate set of sections containing the SFO was analyzed to determine the injection sites using GFP fluorescence. Successful injection of the shRNA construct into the SFO was determined by visualizing GFP-labeled neurons in both the dorsolateral outer shell and the ventromedial core reaching ventrally toward the third ventricle. Rats with injection sites that did not contain GFP labeling in the shell and core of the SFO were not included in the analysis. Some rats that were included demonstrated labeling dorsal to the SFO along the cannula track.
Confocal imaging and quantification of immunohistochemistry.
Sections were examined using an Olympus IX-2 DSU confocal microscope equipped for epifluorescence with the appropriate excitation/emission filter sets for imaging ΔFosB, AVP, and oxytocin-positive cells in the SON. Images were captured using a Retiga-SRV camera (Q-imaging, Surrey, British Columbia, Canada). Brain areas were identified using the rat brain atlas by Paxinos and Watson (53). Sections containing the SON located −0.80 mm to −1.80 mm posterior to bregma were analyzed for ΔFosB, AVP, and oxytocin cell numbers and colocalization. Although each section was stained with all three antibodies, ΔFosB colocalization with AVP or oxytocin was determined separately using ImageJ software (v. 1.47; National Institutes of Health, Bethesda, MD). Each grayscale image was adjusted for brightness and contrast and pseudocolored in ImageJ. The ΔFosB images were separately merged with AVP and oxytocin images of the same section. The numbers of ΔFosB, AVP, oxytocin, ΔFosB+AVP, and ΔFosB +oxytocin in each set of images were determined for each section using the cell counting function. Four to six sections were averaged for each rat.
Laser capture microdissection.
A separate set of animals comprising the same four treatment groups was used for the laser capture microdissection studies (n = 5 for all four groups). Each rat was anesthetized with Inactin (100 mg/kg ip) and quickly decapitated 28 days after BDL or sham ligation surgery. Brains were removed and immediately snap frozen in dry-ice cooled isopentane. Serial coronal sections (10 μm) through SFO were cut using a cryostat (CM1950 Leica Microsystems, Buffalo Grove, IL). These sections were mounted onto PEN membrane-coated slides (LCM0522-Arcturus Bioscience, Mountain View, CA) with 2 or 3 forebrain sections on the membrane part of a slide. These slides were then stored in −80°C until further processing.
Each slide was fixed in ice-cold 100% methanol, as previously described (16). All reagents were prepared with diethylpyrocarbonate-treated water and contained RNase inhibitors. An Arcturus Veritas microdissection instrument, equipped with infrared capture and ultraviolet cutting lasers, was used to laser capture SFO from a single section from each animal. RNA was extracted and purified from each sample as reported earlier (15, 21). Three microliters of total RNA from each sample was amplified as previously published (15, 21) to convert cellular RNA to aminoallyl antisense RNA in the nanogram per microliter range.
The quality of each RNA sample was evaluated using a Nanodrop Spectrophotometer (Nanodrop 2000c spectrophotometer; Thermo Fisher Scientific, Waltham, MA) to measure RNA content and identify contamination. Low 260/280 samples ratios were considered contaminated and not used for reverse transcription.
Quantitative real-time PCR.
Aminoallyl antisense RNA from each sample was reverse-transcribed to cDNA with Sensiscript RT kit reagents (prod. no. 205213; Qiagen, Valencia, CA), as previously described (16). Forward and reverse primers for target genes (Table 1) were obtained from Integrated DNA Technologies (Coralville, IA). For polymerase chain reaction, samples consisted of 2 μl of cDNA, 8.3 μl of RNase/DNase-free water, 2 μl of each primer, and 12.5 μl of iQ SYBR Green Supermix (prod. no. 170–8880; Bio-Rad, Hercules, CA). PCR reactions were performed in a Bio-Rad iQTM5 iCycler system, with the following cyclic parameters: initial denaturation at 95°C for 3 min, followed by 50 cycles of 1.10 min each (10 s at 95°C, followed by 1 minute at 55°C for AT1aR and GAPDH). No-template and -RT controls were performed for each analysis. The housekeeping gene, GAPDH, was used for normalization of mRNA expression. Melt curves generated were analyzed to identify nonspecific products and primer-dimers.
Table 1.
RT-qPCR primer sequences
| Primer | Sequence |
|---|---|
| AT1a forward: | 5′-TTCTCAATCTCGCCTTGGCTGACT-3′ |
| AT1a reverse: | 5′-AAGGAACACACTGGCGTAGAGGTT-3′ |
| GAPDH forward: | 5′-CTCATGACCACAGTCCATGC-3′ |
| GAPDH reverse: | 5′-TACATTGGGGGTAGGAACAC-3′ |
Quantification and analysis of PCR results.
Data were plotted across cycles for detected fluorescence above the background signal. The x coordinate at the threshold of amplification was designated the cycle threshold (Ct) and used as a measure of mRNA abundance. The relative expression of GAPDH mRNA was used for normalization. Data were analyzed by the 2−ΔΔCT method (46, 60).
Statistical analysis.
All results are presented as means ± SE. Daily water and food intake, urine, and sodium excretion values were analyzed using two-way repeated-measures ANOVA. Post hoc analysis of significant main effects was carried out using Student-Newman-Keuls post hoc test (Sigmaplot, San Jose, CA). Other data were analyzed by two-way ANOVA. Significance was set at P < 0.05.
RESULTS
Characterization of BDL experimental model and plasma measurements.
Plasma samples from sham controls and BDL rats were used to measure plasma osmolality, serum sodium, and hematocrit. BDL rats of each treatment group exhibited significantly lower plasma osmolality (P < 0.01) compared with sham control animals of the same group (Table 2). Notably, AAV-sh-AT1a microinjection into the SFO failed to affect plasma osmolality in BDL rats. Similarly, plasma hematocrit was also significantly decreased in BDL compared with sham-ligated rats (P < 0.01, vs. all sham groups). Changes in plasma osmolality and hematocrit are not due to changes in protein, as suggested by the failure to detect differences in plasma protein measurements. Compared with sham, BDL rats that completed the experiment displayed jaundice with elevated serum bilirubin, hepatosplenomegaly, and development of a cirrhotic liver, as evaluated by gross visual examination and significantly increased liver-weight-to-body-weight ratios (P < 0.001). The results demonstrate effective induction of liver failure and dilutional hyponatremia in BDL rats that were not affected by injection of the AAV-sh-AT1a vector.
AT1aR shRNA viral transduction and gene knockdown.
Successful viral delivery of the AAV-shRNA gene expression cassette was verified by confocal microscopy in all animals (Fig. 1). AAV-shRNA vectors contained GFP that functioned as a marker to indicate the site of delivery and cells affected. Six weeks after direct SFO microinjection of AAV-shRNA control and AT1aR vectors, we observed robust GFP expression in the SFO (Fig. 1). GFP-positive cells were also observed in the area surrounding the SFO, notably dorsal to the SFO and occasionally along the injector tracks. All rats included in the study contained GFP labeling in the SFO. Seven animals were excluded from the study due to injection sites that were too dorsal to produce GFP labeling in the SFO. To evaluate the effectiveness of AAV-sh-AT1a transduction to knock down AT1aR expression, 10-μm brain slice sections containing the SFO were used for laser microdissection of the SFO and analysis of AT1aR mRNA abundance by RT-quantitative PCR. AT1aR mRNA abundance was significantly increased in the SFO of BDL rats treated with the control shRNA construct compared with sham-ligated animals injected with either the AAV-SCM or AAV-sh-AT1a viral vectors (Fig. 1; P < 0.01). SFOs from BDL animals injected with AAV-sh-AT1a contained significantly decreased AT1aR mRNA abundance compared with BDL animals receiving the control AAV-SCM shRNA (Fig. 1; P = 0.013, BDL SCM vs. BDL sh-AT1a). Thus, microinjection of the AAV-sh-AT1a vector effectively prevented the increased AT1aR mRNA expression observed in BDL rats.
Fig. 1.
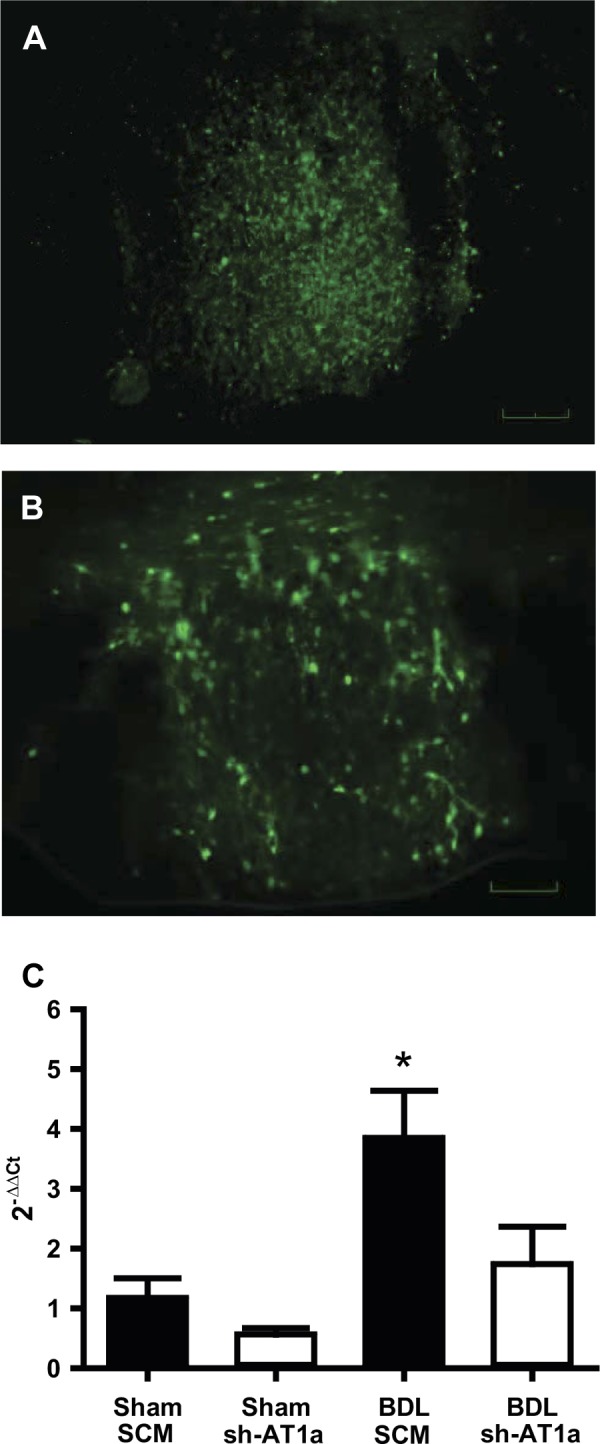
Representative digital images of the subfornical organ (SFO) expressing green fluorescent protein from a rat injected with the viral transduction construct containing either adeno-associated viral-green fluorescent protein-scrambled AAV-short hairpin RNA (AAV-GFP-SCM shRNA) (A) or shRNA against AT1a receptor (AAV-GFP-AT1a shRNA) (B) into the SFO. Scale bar is 50 μm. RT-quantitative PCR analysis (C) of AT1aR mRNA from a laser-microdissected SFO nuclei from sham and BDL rats injected with either a scrambled AAV-shRNA (SCM) or an AAV-shRNA targeted to AT1a gene (sh-AT1a) into the SFO. The data are presented as means ± SE relative mRNA levels as calculated by the 2−ΔΔCT method. *Statistically significant, using ANOVA with Student-Newman-Keuls (SNK) post hoc test. P < 0.02 vs. all other groups; n = 5 for each group.
Fluid intake and renal sodium excretion.
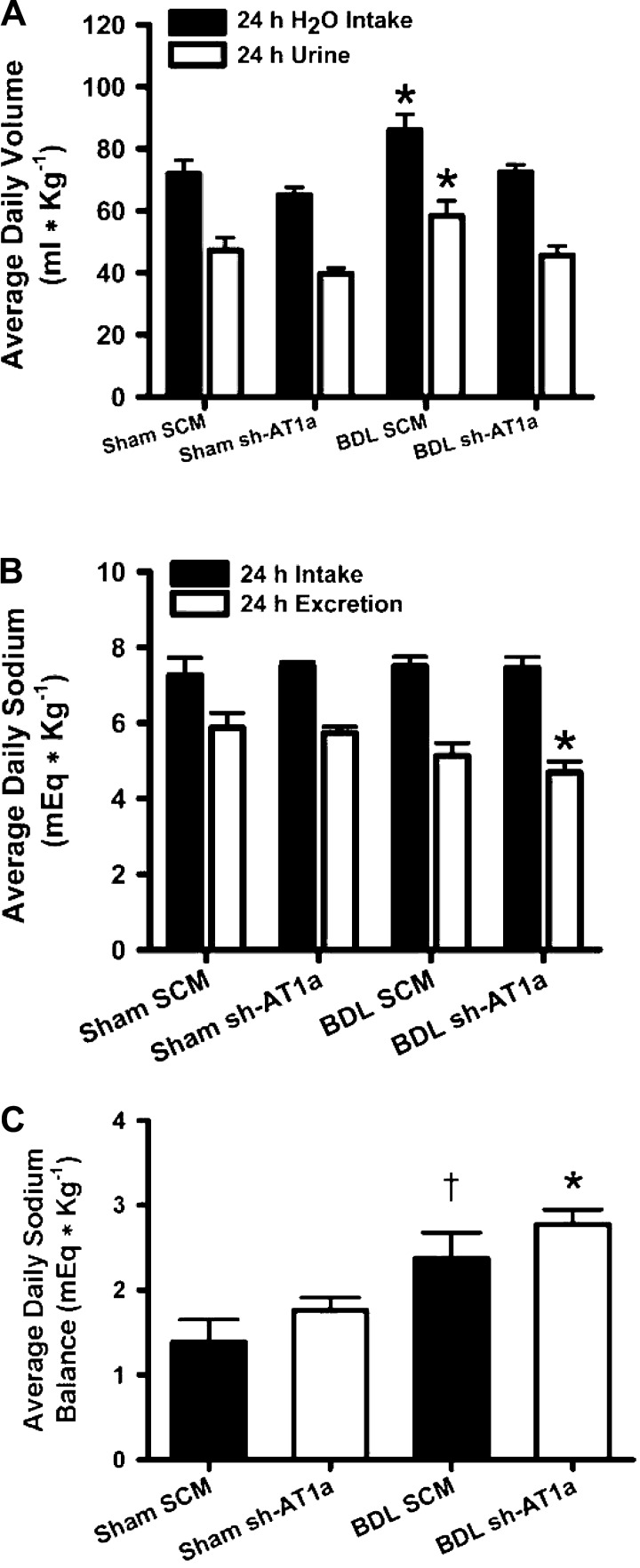
There was no significant change in water intake or renal sodium excretion between sham animals treated with either the control SCM shRNA or the AT1aR shRNA. We observed a significant increase in average daily water consumption of BDL rats injected with the control shRNA compared with sham-ligated animals treated with both control and AT1aR shRNA vectors (Fig. 2; P < 0.05, two-way repeated-measures ANOVA). This increased daily water intake was attenuated to sham levels by injection of AT1aR shRNA into SFO (Fig. 2; BDL SCM vs. BDL AAV-sh-AT1a, P < 0.05). Therefore, knockdown of SFO AT1aR mRNA expression is associated with a normalization of drinking behavior in BDL rats. Interestingly, BDL animals injected with AAV-SCM shRNA showed greater urine volume output compared with sham animals (Fig. 2), possibly due to vasopressin and/or aldosterone escape along with the increased water intake. However, water balance between the two BDL animal groups was not different (BDL SCM: 27.6 ± 1.3 ml kg−1 day−1 vs. BDL sh-AT1a: 27.5 ± 1.4 ml kg−1 day−1).
Fig. 2.
A: average daily water intake and urine output from Sham and BDL rats injected with either a scrambled control AAV-shRNA (SCM) or an AAV-shRNA targeted to AT1a gene (sh-AT1a) into the SFO. Values are expressed as means ± SE. *Statistically significant, two-way repeated-measures ANOVA with post hoc interaction test (SNK). P < 0.05 vs. all other groups. Animal numbers range from 12 to 18 for each group. B: average daily sodium intake and renal sodium excretion from sham and BDL rats injected with either a scrambled AAV-shRNA (SCM) or an AAV-shRNA targeted to AT1a gene (sh-AT1a) into the SFO. Values are expressed as means ± SE. *Statistically significant, two-way repeated-measures ANOVA with post hoc interaction test (SNK) P < 0.05 vs. sham groups. Animal numbers are 12–18 for each group. C: average daily sodium balance from sham and BDL rats injected with either a scrambled AAV-shRNA (SCM) or an AAV-shRNA targeted to AT1a gene (sh-AT1a) into the SFO. Values are expressed as means ± SE. *Statistically significant, P < 0.01 vs. sham groups. †Statistically significant, P < 0.05 vs. sham SCM group. Statistical analysis was by two-way repeated-measures ANOVA with post hoc interaction test (SNK). Animal numbers are 12–18 for each group.
We observed a decreased renal sodium excretion in BDL animals injected with the AAV-sh-AT1a construct compared with both sham-ligated treatment groups (Fig. 2; P < 0.05). We did not observe any difference in renal sodium excretion between BDL animals injected with the control AAV-SCM transduction vector and sham-ligated animals of both viral vector treatment groups (Fig. 2). There was no significant difference in food intake between all of the treatment groups (data not shown) or, consequently, in sodium intake (Fig. 2). Both BDL animal groups also showed an increased sodium balance (Fig. 2; BDL shAT1a: P < 0.05 compared with both sham groups; BDL SCM: P < 0.05 compared with sham SCM).
ΔFosB in the SON of BDL rats.
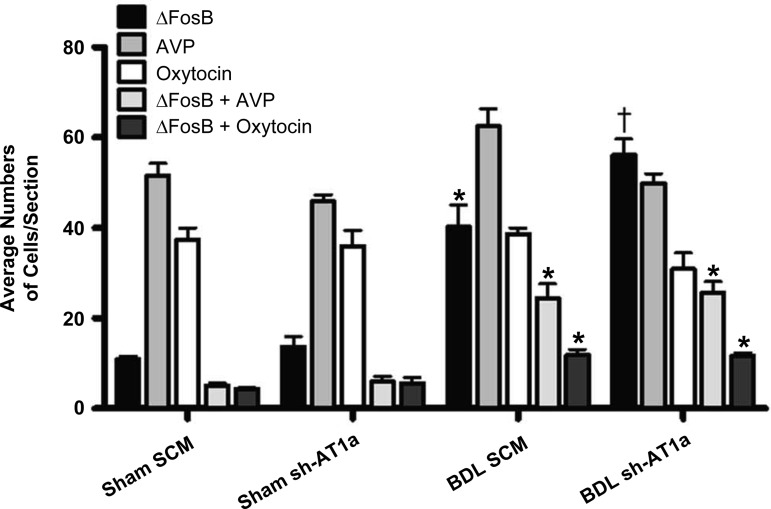
BDL increased ΔFosB staining and colocalization of ΔFosB with vasopressin in the SON (Figs. 3 and 4). The numbers of ΔFosB-positive cells in the SON was significantly increased 4 wk after BDL in both viral treatment groups compared with both sham-ligated controls (Figs. 3 and 4). Furthermore, we observed increased numbers of ΔFosB-positive cells in the SON of BDL AAV-sh-AT1a animals compared with BDL AAV-SCM animals (Figs. 3 and 4). We also observed a significant independent main effect of both BDL and virus treatments on the numbers of vasopressin-positive cells in the SON, indicating that BDL and virus treatment are independently associated with an increase of this cell type (sham: 48.7 ± 1.7 vs. BDL: 56.2 ± 1.9; F = 8.36, P < 0.01; SCM: 57.0 ± 1.9 vs. shAT1a: 47.9 ± 1.8; F = 12.45, P < 0.01). Although we failed to find a significant interaction between BDL and the vector treatment using two-way ANOVA, the numbers of vasopressin-positive cells increased with BDL surgery and decreased with AAV-sh-AT1a viral knockdown (Figs. 3 and 4). In both BDL virus treatment groups, we observed increased numbers of ΔFosB and vasopressin double-labeled cells compared with both sham treatment groups (Figs. 3 and 4). In BDL rats treated with the control AAV-SCM or the AAV-AT1a shRNA transduction vector, 61% and 55%, respectively, of the ΔFosB-positive cells also were vasopressin-positive compared with 47% and 39% in sham-ligated controls injected with the AAV-SCM or AAV-AT1a shRNA, respectively.
Fig. 3.
Effects of bile duct ligation and AAV-AT1a shRNA injection into the SFO on the numbers of cells stained for ΔFosB, vasopressin (AVP), oxytocin, and colocalized ΔFosB and vasopressin or ΔFosB and oxytocin in the supraoptic nucleus of rats in the several treatment groups. *Significantly different from sham groups, P < 0.001. †Significantly different from all other treatment groups, P < 0.01. Animal numbers range from 7 to 9 for each group.
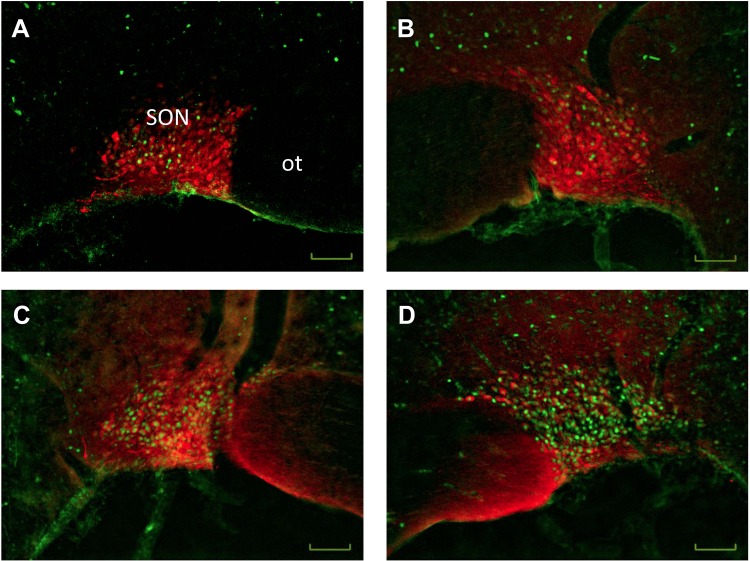
Fig. 4.
Representative digital images of vasopressin immunofluorescence (red) merged with pseudocolored ΔFosB/FosB staining (green) of the supraoptic nucleus from a sham-ligated control (A and B) and a bile duct-ligated rat (C and D) injected with either a viral construct containing scrambled control shRNA (left) or a viral construct containing the AT1aR shRNA (right). SON, supraoptic nucleus; ot, optic tract. Scale bar is 50 μm for each image.
The results obtained from oxytocin and ΔFosB immunohistochemistry are shown in Figs. 3 and 5. We observed an increased number of cells with colocalization of ΔFosB and oxytocin in the SON. The number of oxytocin-positive cells was similar in all treatment groups. In BDL rats treated with control AAV-SCM or the AAV-AT1a shRNA, 29% and 39%, respectively, of the ΔFosB-positive cells also were oxytocin-positive compared with 40% and 45% in sham-ligated controls injected with the AAV-SCM or AAV-AT1a shRNA, respectively. An increased percentage of ΔFosB-positive cells that colocalized with oxytocin in sham-ligated rats compared with BDL is due to the significantly decreased numbers of ΔFosB in sham-ligated rats.
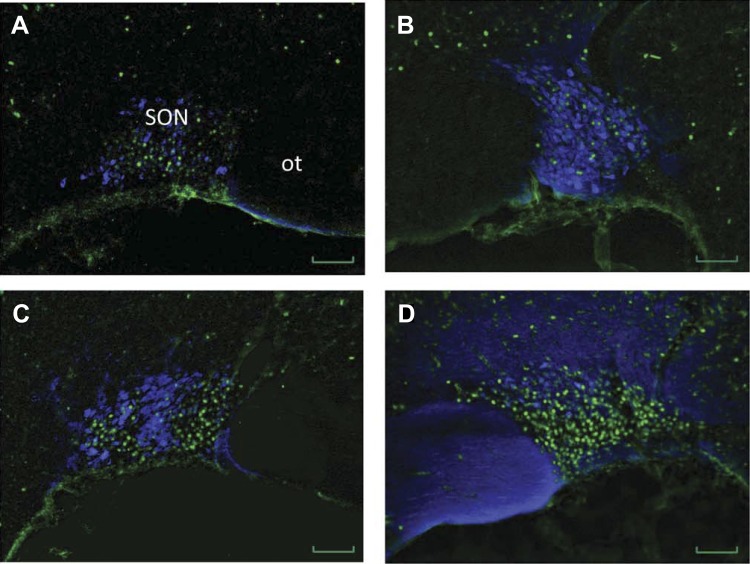
Fig. 5.
Representative digital images of oxytocin immunofluorescence (blue) merged with pseudocolored ΔFosB/FosB staining (green) of the supraoptic nucleus (SON) from a sham-ligated control (A and B) and a bile duct-ligated rat (C and D) injected with either a viral construct containing scrambled control shRNA (left) or a viral construct containing the AT1aR shRNA (right). ot, optic tract. Scale bar is 50 μm for each image.
DISCUSSION
We employed a neurotrophic AAV-sh-AT1a shRNA vector to determine whether increased expression of AT1aR in the SFO contributes to the changes in drinking behavior associated with BDL. Previous studies using this vector have shown that its effects are primarily neurotrophic, and it reliably knocks down AT1R expression within 12 days and persists for more than 50 days (24, 49, 96). Even though it was only recently developed, the use of shRNA to induce stable and localized knockdown AT1aR expression in neurons of the CNS has emerged as a powerful tool to decipher the molecular mechanisms involved in the neural control of blood pressure and body fluid homeostasis. Therefore, we used this approach to test the hypothesis that AT1aRs in the SFO may be involved in the central modulation of drinking behavior.
The main findings of this study are that 1) the knockdown of SFO AT1aRs by injection of AAV-sh-AT1a prevented the increased water intake associated with BDL, 2) injection of AAV-sh-AT1a resulted in a more pronounced increase in ΔFosB cell counts in the SON of BDL animals, and 3) while AT1aR knockdown in the SFO significantly reduced water intake, this treatment had no effect on plasma osmolality or sodium. This failure to influence plasma sodium and osmolality occurred despite a decrease in urinary sodium excretion, an increase in sodium balance, and reduced water intake observed in BDL rats with AT1aR knockdown in the SFO. Persistent dilutional hyponatremia in the presence of increased sodium balance of BDL rats treated with AAV-AT1a shRNA may be due to increased fecal sodium loss or the maintenance of ascites. Interestingly, despite decreased water intake, water balance was not different between the BDL treatment groups. These observations indicate that SFO AT1aR signaling may not be necessary for the activation of SON vasopressin neurons or the concomitant dilutional hyponatremia that is observed in BDL rats but does mediate the increase in water intake.
Increased renal sodium retention is well described in both humans and animal models of cirrhosis (6, 42, 94). However, renal sodium retention is not sufficient to prevent the progression of dilutional hyponatremia. The mechanism by which sodium is retained in cirrhosis is likely due to both actions of angiotensin at the proximal tubule and aldosterone on the distal tubule (42, 83, 94). Increased renal sympathetic nerve activity and the intrarenal RAS also contribute to sodium retention in BDL rats (66). Increased renal sodium retention contributes to ascites formation and signals a worsening prognosis for patients with cirrhosis (41). We confirmed this association between cirrhosis and decreased renal sodium excretion. BDL animals exhibited significantly increased sodium balance compared with sham-ligated animals of the same virus treatment group. Injection of the AAV-sh-AT1a had no net effect on renal sodium excretion for either sham or BDL animals. BDL animals treated with the AAV-sh-AT1a drank significantly less water without a concomitant increase in plasma osmolality or serum sodium, despite a significant increase in renal sodium retention. This is a result of the failure of the kidneys to excrete dilute urine, as demonstrated by the observation that there was no difference in the water balance between the BDL viral treatment groups. Taken together, this confirms previous observations that, despite the observed increase in renal sodium retention, insidious solute-free water retention—presumably due to the actions of increased circulating AVP—is the main determinant of dilutional hyponatremia in the context of BDL cirrhosis.
As previously stated, injections of AAV-sh-AT1a failed to affect dilutional hyponatremia despite altering the BDL animal's water intake. We previously reported similar results in BDL rats given chronic intracerebroventricular infusions of the AT1R antagonist losartan (91). In that experiment, however, it could not be determined whether losartan crossed the cerebrospinal fluid-circumventricular organ barrier to block AT1Rs in the SFO. Therefore, neural activation of SON AVP secretion through a mechanism that included angiotensin signaling at the level of the SFO could not be ruled out. In a previous experiment, our laboratory provided a group of BDL rats with 2% saline to drink to decrease plasma renin activity (PRA) (21) by increasing sodium delivery to the distal tubule of the kidney. This manipulation was associated with a significant decrease in PRA and circulating vasopressin compared with BDL rats provided only with water (21). This finding provided correlational evidence that the renin-angiotensin system may contribute to increased plasma vasopressin concentrations in BDL rats. However, the current study demonstrated that injection of the AAV-AT1a shRNA in SFO did not prevent the reduction of plasma osmolality associated with BDL. While we specifically targeted the SFO in this study, other circumventricular organs, such as the OVLT and the area postrema, which contain AT1Rs (36, 48), could contribute to dilutional hyponatremia in this model. In addition, Davisson (24) showed that brain AT1b receptors are important mediators of drinking behavior in response to central administration of ANG II. However, it was also shown that central AT1aR overexpression increased both baseline and ANG II-induced drinking behavior (42). Both of these studies were performed in mice, and it is has been shown that systemic ANG II is not dipsogenic in some strains of mice (37). Studies in AT1aR knockout mice have produced conflicting results regarding the role of this receptor and AVP release. Initial studies in AT1aR knockouts reported no change in basal circulating levels of AVP and enhanced AVP release to hyperosmotic stimulation, leading the authors to propose a role for the AT1bR (17). In contrast, recent publications have reported deficits in the regulation of AVP in AT1aR knockout mice (44, 45). Therefore, studies that include knockdown of AT1bR in SFO may be helpful to further identify the role that these receptors play in AVP release in BDL rats.
Furthermore, in response to decreased blood pressure or perfusion, angiotensin signaling can be potentiated through the actions of both ANG II and aldosterone in SFO. A previous study from our laboratory reported the association between BDL, increased drinking behavior, and increased expression of AT1R protein and mRNA in the SFO (70). The current study confirms the relationship between increased SFO AT1aR expression and water intake in BDL rats. These data are similar to other studies that showed increased abundance of AT1R in the SFO of animal models that had elevated peripheral renin-angiotensin system activation (71, 72). Studies using transgenic mice have shown that the increased production of SFO ANG II increased water intake (58) and that chronic brain-restricted overexpression of AT1R results in higher baseline water intake and an increased water intake response to intracerebroventricular ANG II (42). Studies of spontaneously hypertensive rats (32, 54), rats deprived of water (5, 59), and rats with congestive heart failure (71, 72) have also shown increased expression of AT1R in the SFO, which were associated with increased water intake where measured. Both ANG II and aldosterone have been shown to increase the expression of AT1Rs in cardiovascular regulatory nuclei by acting through mitogen-activated protein kinases (71, 76, 77). Aldosterone also upregulates AT1Rs through the mineralocorticoid receptor (75), possibly by acting on the hormone response element in the promoter region of the AT1R gene (27, 34). The mechanism responsible for the increase in SFO AT1aR expression associated with BDL remains to be determined.
Our results indicate that SFO AT1Rs are important mediators of the increased water intake, despite decreased plasma osmolality during BDL-induced liver cirrhosis in rats. While animals normally regulate plasma osmolality during acute osmotic challenges through differential vasopressin release to alter renal sodium-free water clearance, fluid intake is ultimately needed for continued regulation of plasma osmolality. As a result, body fluid and electrolyte balance is determined by the interaction of physiological and behavioral effector systems. In the physiological context of progressively decreasing plasma osmolality associated with BDL cirrhosis, normal or increased water intake, along with inappropriate vasopressin release, could contribute to dilutional hyponatremia.
Our results demonstrate that ΔFosB-positive profiles are increased in BDL rats treated with AAV-sh-AT1a. This result was not anticipated, given that previous studies have demonstrated that circulating ANG II acting through the SFO facilitates the release of neurohypophysial hormones (25). Although a majority of the ΔFosB-positive SON neurons in BDL rats appear to be vasopressinergic, activation of oxytocin neurons was also evident. Vasopressin and oxytocin are released from different populations of magnocellular neurosecretory cells, which demonstrate different electrophysiological characteristics (3, 4, 30, 55). ANG II can promote AVP secretion by both a multisynaptic mechanism through AT1Rs in the SFO, and as a direct neural efferent from the SFO acting via AT1Rs in the SON (2, 25, 49). Electrophysiological studies of the SFO to SON pathway have shown that stimulation of the SFO has complex effects on AVP and oxytocin neurons (25, 26, 64). It could be that AT1aR knockdown in SFO removed both excitatory and inhibitory influences on SON resulting in greater ΔFosB staining through alternative neural mechanisms. Synaptic or osmotic activation of AVP and oxytocin neurons in the hypothalamus is associated with increased expression of ΔFosB (38, 39), an AP-1 transcription factor that has been shown to play a critical role in the adaptation of neural networks that regulate sympathetic outflow during dehydration (19). Previously, we showed that inhibition of ΔFosB with a dominant negative adeno-associated viral construct resulted in diuresis and attenuation of dilutional hyponatremia (21). Therefore, increased expression of ΔFosB in vasopressin-positive SON neurons of cirrhotic rats could be more than a marker of activation, but could mediate cellular adaptations that contribute to increased vasopressin release. The role of c-fos and c-Fos-related proteins in the regulation of AVP and oxytocin gene expression remains controversial (11). Recent studies have suggested that the rat AVP gene promoter contains a functional AP-1 regulatory site (74) and that c-fos gene expression and AVP heterogenous nuclear RNA (hnRNA) expression in both SON and PVN are significantly correlated with changes in plasma osmolality (39). However, direct SON injections of putative neurotransmitter agonists produced differential effects on c-fos mRNA and vasopressin hnRNA expression (38). The failure to observe concurrent increases in c-fos and vasopressin hnRNA expression led the authors to suggest the induction of these genes following acute osmotic stimulation likely involves different mechanisms. It is possible that other downstream targets of ΔFosB, such as transient receptor potential vanilloid channels (14, 50) or changes in synaptic function (47, 69) are involved in the sustained activation and/or hormone release that results in dilutional hyponatremia.
Circulating oxytocin could contribute to hyponatremia due to its structural similarity to vasopressin and its ability to stimulate renal vasopressin receptors (18, 43). Although oxytocin has been shown to increase sodium excretion and inhibit sodium appetite (7, 65, 68), these effects would not be consistent with the increase in sodium balance observed in BDL rats in the present study. As mentioned above, the effects of increased sympathetic tone to the kidney and the direct renal action of ANG II and aldosterone could be responsible for the sodium retention in this model. In a previous study, experimental cirrhosis in the rat was not associated with increased oxytocin gene expression in the hypothalamus, although the study used a different model of cirrhosis (40). We did not observe a significant increase in the numbers of oxytocin-positive neurons following BDL in the current study or in previous publications (21, 50).
Perspectives and Significance
We tested the contribution of AT1aRs in the SFO to the changes in body fluid regulation produced by BDL. The results demonstrate that increased water intake associated with BDL was prevented by knockdown of AT1aRs in the SFO. In contrast, knockdown of AT1aRs did not increase urine output or raise plasma osmolality and hematocrit as would be expected if AVP release were significantly decreased. This suggests that preventing the upregulation of SFO AT1aRs is not sufficient to reverse or delay the development of dilutional hyponatremia produced by BDL. ΔFosB studies of the putative neural activity in the SON further confirm the observation that SFO AT1aRs do not significantly contribute to dilutional hyponatremia during this disease model. Nonetheless, these findings do help identify a potential role for SFO AT1aRs in excess drinking during liver failure. Although angiotensin signaling at the SFO does not appear to be involved in the nonosmotic release of vasopressin, there is considerable evidence that increased drinking behavior from disease and as a side effect of V2A treatment affects mortality and/or rehospitalization (56, 57). Therefore, it is possible that this research might contribute to the palliation of suffering of patients from excess thirst during cirrhosis or heart failure, even though it might not lead to a direct treatment of dilutional hyponatremia. Better understanding of the central mechanisms that regulate water intake and vasopressin release through osmotic and nonosmotic modalities will assist in the development of treatment protocols for dilutional hyponatremia. Future experiments will consider the possible role of hindbrain neural afferents to vasopressin-secreting cells in the development of this disorder.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.D.W., T.P.N., and J.T.C. conception and design of research; J.D.W. and T.P.N. performed experiments; J.D.W. and T.P.N. analyzed data; J.D.W., T.P.N., and J.T.C. interpreted results of experiments; J.D.W. prepared figures; J.D.W. drafted manuscript; T.P.N. and J.T.C. edited and revised manuscript; J.T.C. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by R56 HL-62569 (to J. T. Cunningham), and F32 DK-083884 (to J. D. Walch). The authors would like to thank J. T. Little for his technical expertise with the animal experiments and histology.
REFERENCES
- 1.Allen AM, Moeller I, Jenkins TA, Zhuo J, Aldred GP, Chai SY, Mendelsohn FAO. Angiotensin receptors in the nervous system. Brain Res Bull 47: 17–28, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Anderson JW, Smith PM, Ferguson AV. Subfornical organ neurons projecting to paraventricular nucleus: Whole-cell properties. Brain Res 921: 78–85, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog Neurobiol 47: 291–339, 1995 [PubMed] [Google Scholar]
- 4.Armstrong WE. The neurophysiology of neurosecretory cells. J Physiol 585: 645–647, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth SW, Gerstberger R. Differential regulation of angiotensinogen and AT1A receptor mRNA within the rat subfornical organ during dehydration. Brain Res Mol Brain Res 64: 151–164, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Better OS, Aisenbrey GA, Berl T, Anderson RJ, Handelman WA, Linas SL, Guggenheim SJ, Schrier RW. Role of antidiuretic hormone in impaired urinary dilution associated with chronic bile-duct ligation. Clin Sci (Lond) 58: 493–500, 1980 [DOI] [PubMed] [Google Scholar]
- 7.Blackburn RE, Samson WK, Fulton RJ, Stricker EM, Verbalis JG. Central oxytocin inhibition of salt appetite in rats: evidence for differential sensing of plasma sodium and osmolality. Proc Natl Acad Sci USA 90: 10380–10384., 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boscoe A, Paramore C, Verbalis JG. Cost of illness of hyponatremia in the United States. Cost Eff Resour Alloc 4: 10, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourque CW, Voisin DL, Chakfe Y. Stretch-inactivated cation channels: cellular targets for modulation of osmosensitivity in supraoptic neurons. Prog Brain Res 139: 85–94, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Brond L, Hadrup N, Salling N, Torp M, Graebe M, Christensen S, Nielsen S, Jonassen TE. Uncoupling of vasopressin signaling in collecting ducts from rats with CBL-induced liver cirrhosis. Am J Physiol Renal Physiol 287: F806–F815, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev 81: 1197–1267, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Cárdenas A, Arroyo V. Mechanisms of water and sodium retention in cirrhosis and the pathogenesis of ascites. Best Pract Res Clin Endocrinol Metab 17: 607–622, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Carreño FR, Ji LL, Cunningham JT. Altered central TRPV4 expression and lipid raft association related to inappropriate vasopressin secretion in cirrhotic rats. Am J Physiol Regul Integr Comp Physiol 296: R454–R466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carreño FR, Walch J, Cunningham JT. BDNF-TrkB pathway mediates NMDA receptor NR2B subunit phosphorylation in the supraoptic nuclei following progressive dehydration. J Neuroendocrinol 23: 894–905, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carreño FR, Walch JD, Dutta M, Nedungadi TP, Cunningham JT. Brain-derived neurotrophic factor-tyrosine kinase B pathway mediates NMDA receptor NR2B subunit phosphorylation in the supraoptic nuclei following progressive dehydration. J Neuroendocrinol 23: 894–905, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Chen H, Morris M. Enhanced osmotic responsiveness in angiotensin AT1a receptor-deficient mice: evidence for a role for AT1b receptors. Exp Physiol 90: 739–746, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Chou CL, DiGiovanni SR, Mejia R, Nielsen S, Knepper MA. Oxytocin as an antidiuretic hormone. I. Concentration dependence of action. Am J Physiol Renal Fluid Electrolyte Physiol 269: F70–F77, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Colombari DS, Colombari E, Freiria-Oliveira AH, Antunes V, Yao ST, Hindmarch C, Ferguson AV, Fry M, Murphy D, Paton JF. Switching control of sympathetic activity from forebrain to hindbrain in chronic dehydration. J Physiol 589: 4457–4471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croquet V, Moal F, Veal N, Wang J, Oberti F, Roux J, Vuillemin E, Gallois Y, Douay O, Chappard D, Cales P. Hemodynamic and antifibrotic effects of losartan in rats with liver fibrosis and/or portal hypertension. J Hepatol 37: 773–780, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Cunningham JT, Nedungadi TP, Walch JD, Nestler EJ, Gottlieb HB. ΔFosB in the supraoptic nucleus contributes to hyponatremia in rats with cirrhosis. Am J Physiol Regul Integr Comp Physiol 303: R177–R185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham JT, Penny ML, Murphy D. Cardiovascular regulation of supraoptic neurons in the rat: synaptic inputs and cellular signals. Prog Biophys Mol Biol 84: 183–196, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Dampney RA, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol 71: 359–384, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Davisson RL. Divergent functions of angiotensin II receptor isoforms in the brain. J Clin Invest 106: 103–106, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson AV. Systemic angiotensin acts at subfornical organ to facilitate activity of neurohypophysial neurons. Am J Physiol Regul Integr Comp Physiol 251: R712–R717, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Ferguson AV, Latchford KJ. Local circuitry regulates the excitability of rat neurohypophysial neurones. Exp Physiol 85 Spec No: 153S–161S, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Funder JW. Minireview: Aldosterone and mineralocorticoid receptors: Past, present, and future. Endocrinology 151: 5098–5102, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Gheorghiade M, Gottlieb SS, Udelson JE, Konstam MA, Czerwiec F, Ouyang J, Orlandi C. Vasopressin V2 receptor blockade with tolvaptan versus fluid restriction in the treatment of hyponatremia. Am J Cardiol 97: 1064–1067, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Gheorghiade M, Niazi I, Ouyang J, Czerwiec F, Kambayashi J, Zampino M, Orlandi C. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation 107: 2690–2696, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Grindstaff RR, Cunningham JT. Cardiovascular regulation of vasopressin neurons in the supraoptic nucleus. Exp Neurol 171: 219–226, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Grob M, Trottier JF, Mouginot D. Heterogeneous co-localization of AT1A receptor and Fos protein in forebrain neuronal populations responding to acute hydromineral deficit. Brain Res 996: 81–88, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Gutkind JS, Kurihara M, Castren E, Saavedra JM. Increased concentration of angiotensin II binding sites in selected brain areas of spontaneously hypertensive rats. J Hypertens 6: 79–84, 1988 [DOI] [PubMed] [Google Scholar]
- 33.Herdegen T, Zimmermann M. Immediate early genes (IEGs) encoding for inducible transcription factors (ITFs) and neuropeptides in the nervous system: functional network for long-term plasticity and pain. Prog Brain Res 104: 299–321, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Huang BS, Leenen FHH. Mineralocorticoid actions in the brain and hypertension. Curr Hypertens Rep 13: 214–220, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Ji LL, Fleming T, Penny ML, Toney GM, Cunningham JT. Effects of water deprivation and rehydration on c-Fos and FosB staining in the rat supraoptic nucleus and lamina terminalis region. Am J Physiol Regul Integr Comp Physiol 288: R311–R321, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Johnson AK. The sensory psychobiology of thirst and salt appetite. Med Sci Sports Exer 39: 1388–1400, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Johnson RF, Beltz TG, Thunhorst RL, Johnson AK. Investigations on the physiological controls of water and saline intake in C57BL/6 mice. Am J Physiol Regul Integr Comp Physiol 285: R394–R403, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Kawasaki M, Ponzio TA, Yue C, Fields RL, Gainer H. Neurotransmitter regulation of c-fos and vasopressin gene expression in the rat supraoptic nucleus. Exp Neurol 219: 212–222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawasaki M, Yamaguchi K, Saito J, Ozaki Y, Mera T, Hashimoto H, Fujihara H, Okimoto N, Ohnishi H, Nakamura T, Ueta Y. Expression of immediate early genes and vasopressin heteronuclear RNA in the paraventricular and supraoptic nuclei of rats after acute osmotic stimulus. J Neuroendocrinol 17: 227–237, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Kim JK, Summer SN, Howard RL, Schrier RW. Vasopressin gene expression in rats with experimental cirrhosis. Hepatology 17: 143–147, 1993 [PubMed] [Google Scholar]
- 41.Kim MY, Baik SK, Lee SS. Hemodynamic alterations in cirrhosis and portal hypertension. Korean J Hepatol 16: 347–352, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazartigues E, Sinnayah P, Augoyard G, Gharib C, Johnson AK, Davisson RL. Enhanced water and salt intake in transgenic mice with brain-restricted overexpression of angiotensin (AT1) receptors. Am J Physiol Regul Integr Comp Physiol 295: R1539–R1545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C, Wang W, Summer SN, Westfall TD, Brooks DP, Falk S, Schrier RW. Molecular mechanisms of antidiuretic effect of oxytocin. J Am Soc Nephrol 19: 225–232, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li XC, Shao Y, Zhuo JL. AT1a receptor knockout in mice impairs urine concentration by reducing basal vasopressin levels and its receptor signaling proteins in the inner medulla. Kidney Int 76: 169–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li XC, Shao Y, Zhuo JL. AT1a receptor signaling is required for basal and water deprivation-induced urine concentration in AT1a receptor-deficient mice. Am J Physiol Renal Physiol 303: F746–F756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 47.McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. [Delta]FosB: a molecular switch for long-term adaptation in the brain. Mol Brain Res 132: 146, 2004 [DOI] [PubMed] [Google Scholar]
- 48.McKinley MJ, Johnson AK. The physiological regulation of thirst and fluid intake. News Physiol Sci 19: 1–6, 2004 [DOI] [PubMed] [Google Scholar]
- 49.McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, Vivas L, Wade JD, Oldfield BJ. Vasopressin secretion: Osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol 16: 340–347, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Nedungadi TP, Carreno FR, Walch JD, Bathina CS, Cunningham JT. Region-specific changes in TRPV channel expression in the vasopressin magnocellular system in hepatic cirrhosis induced hyponatremia. J Neuroendocrinol 24: 642–652, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oren RM. Hyponatremia in congestive heart failure. Am J Cardiol 95: 2B–7B, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: Converging signals for neurogenic hypertension. Curr Hypertens Rep 9: 228–235, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1997 [Google Scholar]
- 54.Pérez-Delgado MDM, Carmona-Calero E, Marrero-Gordillo N, Pérez-González H, Castañeyra-Perdomo A. Effect of hypertension on the angiotensin II fibres arriving at the posterior lobe of the hypophysis of the rat An immunohistochemical study. Histol Histopathol 15: 73–77, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Renaud LP. CNS pathways mediating cardiovascular regulation of vasopressin. Clin Exp Pharmacol Physiol 23: 157–160, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Rossi J, Bayram M, Udelson JE, Lloyd-Jones D, Adams KF, Oconnor CM, Stough WG, Ouyang J, Shin DD, Orlandi C, Gheorghiade M. Improvement in hyponatremia during hospitalization for worsening heart failure is associated with improved outcomes: insights from the acute and chronic therapeutic impact of a vasopressin antagonist in chronic heart failure (ACTIV in CHF) trial. Acute Card Care 9: 82–86, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Rozen-Zvi B, Yahav D, Gheorghiade M, Korzets A, Leibovici L, Gafter U. Vasopressin receptor antagonists for the treatment of hyponatremia: Systematic review and meta-analysis. Am J Kidney Dis 56: 325–337, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest 117: 1088–1095, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanvitto GL, Jöhren O, Häuser W, Saavedra JM. Water deprivation upregulates ANG II AT1 binding and mRNA in rat subfornical organ and anterior pituitary. Am J Physiol Endocrinol Metab 273: E156–E163, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protocols 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Schrier RW. Primary systemic arterial vasodilation in cirrhotic patients. Kidney Int 78: 619, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Schrier RW. Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med 119: S47–S53, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355: 2099–2112, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Sgro S, Ferguson AV, Renaud LP. Subfornical organ—supraoptic nucleus connections: an electrophysiologic study in the rat. Brain Res 303: 7–13, 1984 [DOI] [PubMed] [Google Scholar]
- 65.Shirley DG, Walter MF, Keeler BD, Waters NJ, Walter SJ. Selective blockade of oxytocin and vasopressin V1a receptors in anaesthetised rats: evidence that activation of oxytocin receptors rather than V(1a) receptors increases sodium excretion. Nephron Physiol 117: 21–26, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Veelken R, Hilgers KF, Porst M, Krause H, Hartner A, Schmieder RE. Effects of sympathetic nerves and angiotensin II on renal sodium and water handling in rats with common bile duct ligature. Am J Physiol Renal Physiol 288: F1267–F1275, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab 17: 471–503, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Verbalis JG, Mangione MP, Stricker EM. Oxytocin produces natriuresis in rats at physiological plasma concentrations. Endocrinology 128: 1317–1322, 1991 [DOI] [PubMed] [Google Scholar]
- 69.Vialou V, Robison AJ, LaPlant QC, Covington HE, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. ΔFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 13: 745–752, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walch JD, Carreno FR, Cunningham JT. Intracerebroventricular losartan infusion modulates angiotensin type 1 receptor (AT1R) expression in the subfornical organ (SFO) and drinking behaviour in bile duct ligated rats. Exp Physiol 98: 922–933, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei SG, Yu Y, Zhang ZH, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol 296: H1425–H1433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Mitogen-activated protein kinases mediate upregulation of hypothalamic angiotensin II type 1 receptors in heart failure rats. Hypertension 52: 679–686, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang YY, Lin HC, Huang YT, Lee TY, Hou MC, Lee FY, Liu RS, Chang FY, Lee SD. Effect of 1-week losartan administration on bile duct-ligated cirrhotic rats with portal hypertension. J Hepatol 36: 600–606, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Yoshida M, Iwasaki Y, Asai M, Takayasu S, Taguchi T, Itoi K, Hashimoto K, Oiso Y. Identification of a functional AP1 element in the rat vasopressin gene promoter. Endocrinology 147: 2850–2863, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension 51: 727–733, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294: H1067–H1074, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Zhang ZH, Yu Y, Wei SG, Felder RB. Aldosterone-induced brain MAPK signaling and sympathetic excitation are angiotensin II type-1 receptor dependent. Am J Physiol Heart Circ Physiol 302: H742–H751, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]