Abstract
Central oxytocin reduces food intake and increases energy expenditure. The ventromedial hypothalamic nucleus (VMN) is associated with energy balance and contains a high density of oxytocin receptors. We hypothesized that oxytocin in the VMN is a negative regulator of energy balance acting to reduce feeding and increase energy expenditure. To test this idea, oxytocin or vehicle was injected directly into the VMN of Sprague-Dawley rats during fasted and nonfasted conditions. Energy expenditure (via indirect calorimetry) and spontaneous physical activity (SPA) were recorded simultaneously. Animals were also exposed to a conditioned taste aversion test, to determine whether oxytocin's effects on food intake were associated with malaise. When food was available during testing, oxytocin-induced elevations in energy expenditure lasted for 1 h, after which overall energy expenditure was reduced. In the absence of food during the testing period, oxytocin similarly increased energy expenditure during the first hour, but differences in 12-h energy expenditure were eliminated, implying that the differences may have been due to the thermic effects of feeding (digestion, absorption, and metabolic processing). Oxytocin acutely elevated SPA and reduced feeding at doses that did not cause a conditioned taste aversion during both the fed and fasted states. Together, these data suggest that oxytocin in the VMN promotes satiety and acutely elevates energy expenditure and SPA and implicates the VMN as a relevant site for the antiobesity effects of oxytocin.
Keywords: oxytocin, ventromedial hypothalamus, energy expenditure, obesity, feeding
oxytocin has antiobesity effects and is currently being tested clinically for use in the treatment of obesity and Type 2 diabetes (34, 65). Although the mechanism for oxytocin's effects have not been fully characterized, an extensive body of work has demonstrated antiobesity effects of oxytocin in rodents (11, 26, 30, 64, 65), as well as humans (65). Behaviorally, central oxytocin has been reported to delay meal onset (2) and reduce intake of sweet foods (22, 27, 33). Additionally, previous studies implicate a physiological role for endogenous oxytocin in reducing meal size (7, 62). In addition to feeding effects, central oxytocin has potent effects on energy metabolism. For example, low doses of intracerebroventricular oxytocin promote weight loss in rats without affecting feeding by elevating fat oxidation in adipose tissue, whereas higher doses of intracerebroventricular oxytocin both reduce feeding and increase lipolysis (11). Conversely, animals deficient in either oxytocin or its receptor show reduced energy expenditure, in some cases with normal feeding (1, 9, 19, 51).
The main sources of oxytocin in the brain are the magnocellular and parvocellular neurons of the hypothalamic paraventricular nucleus (PVN) and the supraoptic nuclei (43, 46). In particular, PVN oxytocin production is essential to maintaining energy balance. This is illustrated by observations that SIM1 haploinsufficiency, which reduces PVN oxytocin expression by 80%, results in an obese hyperphagic phenotype, and is reversed by central oxytocin administration (20). In the PVN, magnocellular neurons release oxytocin both somatodendritically (39) and via axon terminals, most of which project to the posterior pituitary (49), where oxytocin is released into peripheral circulation. Parvocellular oxytocin neurons send projections to the median eminence and additional central locations, including the spinal cord and the brain stem (40, 49). Although still speculative, strong evidence exists implicating the nucleus of the solitary tract (NTS) as a site where oxytocin affects feeding and energy expenditure; however, less well known are the contributions of hypothalamic sites, such as the ventromedial hypothalamic nucleus (VMN) (for review, see Ref. 5).
There is evidence to support that the VMN may be involved in oxytocin effects on energy balance: 1) The VMN, which contains a high percentage of oxytocin receptor (8, 53), has a well-known role in the regulation of energy balance. 2) VMN lesions are associated with reduced sympathetic nervous system activity and delayed satiety leading to obesity (45, 50, 57). Similarly, the prominent characteristic of oxytocin deficiency in mice is reduced energy expenditure due to reduced sympathetic tone (9, 18, 51). 3) Peripheral injections of oxytocin sufficient to induce negative energy balance are associated with elevated c-Fos activation in both the VMN and NTS (64), suggesting that in addition to NTS, VMN may be an important site for oxytocin effects on energy balance. Despite reports of oxytocin signaling in the VMN, immunohistological analyses reveal that very few oxytocin fibers reach this region (21). Two mechanisms have recently been identified for how oxytocin may activate its receptor in the VMN: 1) VMN oxytocin receptor may be activated by oxytocin released dendritically from magnocellular oxytocin neurons (44). On the basis of the proximity of oxytocin neurons to the third ventricle, it has been hypothesized that PVN oxytocin may act in a paracrine manner following dendritic release by entering the ventricular system prior to activating oxytocin receptor in more distal areas of the brain (21, 47). 2) The fiber plexus lateral to the VMN contains axonal-dendritic synapses where oxytocin has been identified in axon terminals (17).
We hypothesized that oxytocin in the VMN is a negative regulator of energy balance acting both to reduce feeding and increase energy expenditure. Here, we show that oxytocin reduces feeding and acutely elevates energy expenditure and conclude that the VMN may be one site where oxytocin acts to regulate energy balance.
METHODS
Animals
Adult male Sprague-Dawley (SD) rats (Charles River, Wilmington, MA) were individually housed in cages and maintained on a 12:12-h light-dark cycle (lights on at 0400). Rooms were maintained at 21–22°C. Animals had ad libitum access to water and standard chow (Harlan Teklad 8604; 14% fat, 32% protein, 54% kcal from carbohydrate), except where indicated. All protocols were approved by the Institutional Animal Care and Use Committee at the Veterans Affairs Medical Center and University of Minnesota prior to experimentation.
Stereotaxic Surgery and Placement Verification
Rats were anesthetized with intraperitoneal Xylazine (Butler, Dublin, OH; 3.5 mg/kg) and Ketamine (Ketaset, Fort Dodge, IA; 20 mg/kg) and surgically implanted with bilateral 28-gauge stainless-steel guide cannulas (Plastics One, Roanoke, VA) placed 1 mm above the target injection site in the VMN: 0.5 mm lateral, 2.5 posterior to bregma, and 8.6 mm below the skull surface, according to Paxinos and Watson (35). Animals were given 1 wk to recover and at least 4 days of gentle handling and sham injections prior to experimentation. Placement was verified using an neuropeptide Y (NPY) test, as described previously (59). Placement was deemed correct if the animal consumed more than 2 g of chow within 1 h after 100 pmol NPY. Animals who did not respond to NPY were excluded from the study. Because NPY increases feeding in sites other than the VMN, histological staining was performed on a subset of animals, and placement was verified, as described previously (59). Brain tissues were postfixed in 10% formalin solution for 48 h, cryostat sectioned at a thickness of 40 μm, mounted on gelatin-coated slides, stained with 0.1% thionin, and treated with an ethanol gradient (30–100%) and clearing agent (Electron Microscopy Sciences, Hatfield, PA). Placement was deemed correct if the injection site was within 0.25-mm radius from the targeted site. This distance was selected on the basis of diffusion coefficients of the injection volume (28), and our previous data, showing the diffusion radius of 0.5 μl of 0.5% pontamine blue dye (58). Data from animals with misplaced cannulas were excluded from the analyses.
Drug and Injections
Lyophilized oxytocin acetate and NPY were purchased from Bachem Americas (Torrance, CA) and rehydrated in artificial cerebrospinal fluid (aCSF). All doses injected in a volume of 0.5 μl over a period of 30 s, with the injector left in place for an additional 15 s to ensure full delivery. Animals were injected either unilaterally, or bilaterally, as indicated for each experiment.
Spontaneous Physical Activity and Indirect Calorimetry
Acrylic 17 × 17 inch chambers were customized with the capability to simultaneously record energy expenditure and spontaneous physical activity (SPA). Two sets of arrays were affixed to the cage in the x-y plane, and one set was placed 3 inches above for measurement of vertical movement. SPA was defined as the sum of time spent ambulatory and time spent moving in the vertical plane. Stereotypic activity was defined as time spent moving within a defined space around the animal (3.25 × 3.25 inches), as measured by beam breaks (37).
Indirect calorimetry data were collected using Oxymax Lab Animal Monitoring System from Columbus Instruments (Columbus, OH). Prior to testing, chambers were calibrated using a primary gas standard. The chamber was sealed and room air was pumped through at a rate of 3.0–4.7 l/min, depending on the weight of the rat. Gas exchange measurements were automatically recorded every 30 s throughout the 12-h sampling period with the exception of a 5-min 30-s interval every 14.5 min, wherein room air was sampled for reference calibration. Data were recorded as kilocalories per hour for each 30-s interval. To calculate hourly energy expenditure the 30-s interval rates were converted to kcal/30 s interval. The 30-s intervals were then summed to total kilocalories per hour, excluding the sampling period for gas calibration; thus, kilocalories per hour actually represent the kilocalories per hour interval or kcal/43.5 min. SPA and stereotypic activity were simultaneously recorded using customized infrared activity sensors (Med Associates, St. Albans, VT) to detect horizontal and vertical movement, as previously described (52). The first 30 min postinjection was excluded from activity and calorimetry data to account for potential confounds in activity due to handling during injections and to allow for the air in the calorimetry chambers to equilibrate after being sealed.
Calculation of Resting Energy Expenditure and Nonresting Energy Expenditure Components of Total Energy Expenditure
Energy expenditure measurements and SPA were collected at 30-s intervals as described above. Resting energy expenditure (REE) was calculated by averaging the lowest 10 energy expenditure recordings (5 min) over the first 2-h postinjection and verifying them against SPA measurements to be sure that these points reflected times when the animals were not moving. This was necessary because in some cases, we were not able to find enough points of inactivity in the oxytocin-injected animals during the first hour postinjection to make the REE estimation. For validation of our methods, we compared the REE estimates generated from the data in the 2-h immediately postinjection with REE estimates using data from the 12-h testing period using the lowest 10 energy expenditure points. We did not observe unusually low estimations of energy expenditure around the time when room air was sampled, as others have reported previously (16); thus, we did not omit the lowest five points (16). Nonresting energy expenditure (NREE) was calculated as total energy expenditure (TEE) over the first hour of REE. As a final validation, we compared REE and NREE from the same aCSF-treated animals that were used in two different experiments described below (experiment 3 and experiment 4).
Since each hour had three calibration cycles, in which data points were missing for 5 min and 30 s each cycle, it was necessary to extrapolate over that period to get an estimate of 1 h of TEE. We averaged the 30-s energy expenditure measurements (kcal/h) in each subject and multiplied this by the total of the number of 30-s intervals missing due to calibration cycles: Σ measured energy expenditure (kcal) for 43.5 min + average 30-s energy expenditure during 43.5 min × 33 (number of missing 30-s intervals) = TEE. NREE was calculated as estimated TEE − REE.
Conditioned Taste Aversion
We performed a two-bottle conditioned taste aversion test (CTA) (59, 60). The premise for this test is that when rats are exposed to saccharin and water simultaneously, they predominantly prefer saccharin. When animals are exposed to saccharin and water after being given an injection of a drug with aversive properties, their preference for saccharin can be reduced during subsequent exposures, as a sign of drug-induced malaise. In this case, CTA was used to test whether oxytocin in VMN has aversive properties. Sixteen naïve SD rats were deprived of water for 23.5 h and had scheduled water access for 30 min per day for 7 days. Rats were then randomized into three treatment groups and given 15 ml of 0.1% saccharin immediately followed by a VMN injection of aCSF or 0.1 or 1 nmol oxytocin. This conditioned stimulation was repeated once after 48 h. After an additional 48 h, animals were given a two-bottle choice of either water or 0.1% saccharin, and the change in bottle weight was recorded. After a 72-h washout period, the two-bottle test was repeated with the position and presentation order of the bottles reversed. The purpose of repeating the experiment was to account for a potential confound of preference for place and presentation order. Data are presented as the average of the two trials calculated as a percentage of total fluid intake:[(saccharin solution intake)/(saccharin solution intake + water intake)] × 100.
Experiments
Experiment 1: effect of oxytocin in the ventromedial hypothalamus on feeding.
Twelve adult SD rats weighing 550–850 g were maintained on Research Diets control formula (D12450B; 10% fat, 20% protein, 70% kcal carbohydrate) for 2 wk prior to the onset of testing and for the duration of the experiment. This diet was chosen for compatibility with the BioDaq periodic food recording system (Research Diets, New Brunswick, NJ), which was used for measuring food intake. Because of a technical failure of the equipment, we were not able to use the data collected using the BioDaq software, so food intake and spillage were weighed manually in the BioDaq hoppers at each of the indicated time points. Using a repeated-measures design with a 72-h washout period, rats were bilaterally injected with 0.1, 0.5, and 1.0 nmol oxytocin per side or vehicle [artificial cerebrospinal fluid (aCSF)]. Treatments were given in a randomly ordered Latin Square design. Injections were given 30 min prior to the onset of the dark cycle. Food was allowed ad libitum until 1 h prior to injections and immediately postinjection. Body weights were recorded at 0, 24, and 48 h postinjection. One rat was removed from the study due to illness, and his data were excluded from the statistical analyses.
Experiment 2: effect of oxytocin on feeding after fasting.
Twelve naïve adult SD rats weighing 400–800 g were individually housed in wire cages. Bilateral injections of oxytocin were given 3 h into the light cycle after 16 h of food deprivation at doses of 0 (aCSF), 0.1, and 1 nmol per side. Repeated-measures design was used with a 72-h washout period between treatments, as described in experiment 1. Food was made available immediately postinjection, and food intake and spillage were measured at 1, 2, 4, and 24 h. Body weights were measured at baseline and 24 h. Two animals were removed from the statistical analyses, one due to incorrect placement and one due to illness.
Experiment 3: effect of oxytocin in the ventromedial hypothalamus on energy expenditure and spontaneous physical activity.
Eight SD rats weighing 550–950 g were acclimated to customized 17 × 17 in. acrylic chambers until weight stable (5 days). Using a repeated-measures design with 72-h washout period, animals were unilaterally injected with 1 nmol oxytocin or vehicle (aCSF) 30 min before the start of the dark cycle. We switched to a unilateral design for the energy expenditure experiments because the indirect calorimetry chambers take time to seal and calibrate, and we wanted to minimize the amount of time required for making injections to reduce the variability in the timing of the injection for each animal relative to the timing of the light cycle. Rats were placed inside calibrated 17 × 17 inch indirect calorimetry chambers with perforated plastic flooring to allow for spillage collection. Food was removed 1 h prior to injections, and ample (ad libitum) standard chow was placed directly inside the cage immediately postinjection. Water was available ad libitum. Energy expenditure and SPA were recorded for 12 h postinjection during the dark cycle. Food, food spillage, and body weights were measured prior to injections and at 12 h when animals were removed from the chambers.
Experiment 4: effect of oxytocin in the ventromedial hypothalamus on energy expenditure and spontaneous physical activity during fasting.
Experiment 3 was repeated using six rats, with the exception that food was not made available during the testing period. One rat was removed from the study due to equipment failure.
Statistical Analysis
Data analyzed using two-way repeated-measures ANOVA, one-way ANOVA, or two-tailed paired t-tests were analyzed in Prism version 6.0 (GraphPad Software). Since body weights were in some cases minimally different from test to test, analysis of covariance (ANCOVA) were performed for each hour of energy expenditure testing, with body weight as a covariate. For ANCOVA analyses, we used SPSS (version 19.0; IBM, Armonk, NY).
RESULTS
Oxytocin in the Ventromedial Hypothalamus Reduces Feeding Without Causing Taste Aversion
Oxytocin was given 30 min prior to the onset of the dark cycle, when rats would normally begin feeding. At the 0.5- and 1-nmol doses, oxytocin reduced feeding during the first hour by 52 and 66% (food intake was 1.8 ± 0.5 and 1.3 ± 0.4 g for 0.5- and 1-nmol doses, respectively, and 3.7 ± 0.7 for controls; P < 0.05) (Fig. 1A). After 4 h, animals injected with oxytocin had eaten significantly less than controls: 0.1 nmol reduced feeding by 27% (7.0 ± 1.1 g compared with 9.8 ± 1.2 g for controls), 0.5 nmol reduced feeding by 34% (6.4 ± 1.0 g) and 1 nmol reduced feeding by 22% (7.6 ± 0.8 g). Body weight change (−0.6 ± 1.6 g after 0.1 nmol oxytocin injections, −4.0 ± 2.7 g after 0.5-nmol injections and −1.9 ± 1.9 after 1-nmol injections compared with 0.2 ± 2.1 g for control animals; P = 0.5, ns) and cumulative food intake (24.7 ± 1.3 g consumed after 0.1-nmol injections, 25.4 ± 1.6 g after 0.5 nmol and 23.8 ± 1.1 g after 1 nmol compared with 27.6 ± 2.2 g consumed in the control group; P = 0.4, ns) were not different at 24 h postinjection (figure not shown).
Fig. 1.
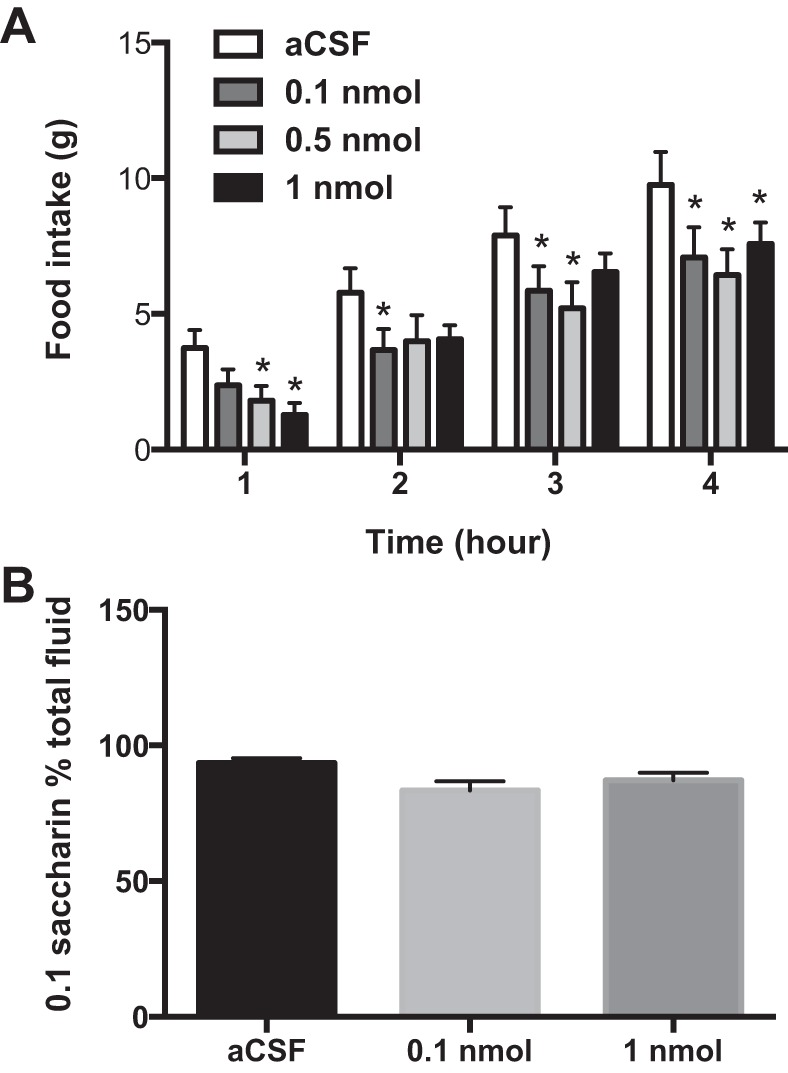
Oxytocin in the ventromedial hypothalamus reduces feeding without causing conditioned taste aversion. Food intake was significantly reduced by doses of 0.5 and 1 nmol oxytocin in the first hour postinjection. A: food intake remained lower until 4 h, at which time feeding was reduced by doses as low as 0.1 nmol. B: oxytocin did not cause conditioned taste aversion, as oxytocin-injected animals did not significantly reduce saccharin intake compared with artificial cerebrospinal fluid (aCSF) controls and more than 75% of total fluid consumed in oxytocin-injected animals was saccharin (84 and 88% of total solution was saccharin in 0.1 nmol and 1 nmol groups, respectively). Two-way repeated-measures ANOVA with Dunnett's test for multiple comparisons (A) or one-way repeated-measures ANOVA (B) was used. *P < 0.05; n = 11 per group (A), n = 6–7 per group (B).
To determine whether oxytocin in the VMN reduces feeding due to malaise, a two-bottle test was performed, as previously described (59, 60). There were no significant differences in saccharin consumption between oxytocin-treated and aCSF-treated animals (Fig. 1B); therefore, it is unlikely that oxytocin caused malaise at the doses given. Together, these data indicate that oxytocin reduced feeding at doses that did not cause conditioned taste aversion.
Oxytocin in the VMN Reduces Feeding After Fasting
After 16 h of fasting, animals injected bilaterally with oxytocin at either 0.1 or 1 nmol ate 38 and 56% less than controls in the first hour (Fig. 2). Control animals ate 5.0 ± 0.9 g during the first hour, compared with 3.6 ± 0.5 and 2.5 ± 0.3 g for animals injected with 0.1 and 1 nmol of oxytocin, respectively (P < 0.05). By 4 h, both groups still had a cumulative reduction in feeding by 18%; control animals ate 9.3 ± 0.8 g and animals injected with 0.1 and 1 nmol oxytocin ate 7.8 ± 0.7 and 7.6 ± 0.5 g, respectively (P < 0.05). After 24 h, food intake (30.0 ± 1.6 g for aCSF, 27.7 ± 1.5 and 27.5 ± 1.6 g after 0.1- and 1-nmol injections, respectively) and body weight change (16.6 ± 2.2 g for aCSF, 10.2 ± 3.3 and 9.2 ± 3.0 g after 0.1- and 1-nmol injections, respectively) were not different (P = 0.2 for food intake, P = 0.08 for body weight change; figure not shown). Together, these data indicate that 1) oxytocin in the VMN acutely reduces feeding during refeeding after fasting without producing compensatory elevations in feeding during the 24 h postinjection and 2) oxytocin effects on feeding are present when the drug is given during the light cycle and in the absence of food.
Fig. 2.

Oxytocin reduces feeding in animals fasted overnight. Bilateral injections of oxytocin were given 3 h into the light cycle after 16 h of food deprivation. Oxytocin reduced 1-h food intake at doses of 0.1 and 1 nmol. After 4 h, feeding was still significantly reduced after doses as low as 0.1 nmol. Two-way repeated-measures ANOVA with Dunnett's test for multiple comparisons. Statistical significance is compared with aCSF *P < 0.05; n = 10.
Oxytocin in the VMN Acutely Increases Energy Expenditure and SPA
To test whether oxytocin increases energy expenditure in the VMN, animals were injected 30 min prior to the onset of the dark cycle, when physical activity normally increases. Total energy expenditure was acutely elevated due to VMN oxytocin injections (Fig. 3A). These effects were abolished after the first hour, and energy expenditure remained lower in the oxytocin animals for the remainder of the 12-h testing period (Fig. 3A, inset). The elevation in energy expenditure during the first hour corresponded to increases in SPA (Fig. 3B) and stereotypic activity (Fig. 3C). The effects of oxytocin on SPA were limited to the first hour postinjection (Fig. 3B). The respiratory exchange ratio (RER) was reduced in animals given oxytocin injections (Fig. 3D). In the 12-h cumulative interval, oxytocin-injected animals ate significantly less than vehicle-treated controls (Fig. 3E) and oxytocin significantly reduced body weight (Fig. 3F).
Fig. 3.
Oxytocin (oxy) in the ventromedial hypothalamus increases energy expenditure (EE) in the first hour (A) and spontaneous physical activity (SPA) (B). Oxytocin increases not only energy expenditure (A), but spontaneous and stereotypic activity as well (C and D) during the first hour postinjection; however, energy expenditure remained lower for most of the remainder of the testing period leading to an overall reduction in 12-h expenditure (A, inset). Respiratory exchange ratio (RER) was reduced by oxytocin injections (D), possibly as a consequence of reduced feeding (E), which shifts nutrient utilization in favor of fat oxidation. Oxytocin injections reduced body weights during the 12-h dark cycle (F). ANCOVA using body weight as a covariate (A), two-way ANOVA (treatment × time) with Sidak's multiple-comparison test (B–D). Two-tailed paired t-test (E and F). n = 8 (repeated measures) *P < 0.05.
Effects of VMN Oxytocin on Energy Expenditure and SPA Without Access to Food
We tested whether differences in feeding could explain the reduced RER and some of the discrepancy between energy expenditure and activity levels observed in the time period after the first hour postinjection. As in the case in which food was available during the testing period, oxytocin significantly increased energy expenditure during the first hour postinjection, but not thereafter (Fig. 4A). There were no differences in 12-h energy expenditure (Fig. 4A, inset), indicating the effects of VMN oxytocin on energy expenditure are acute. Similarly, SPA was elevated during the first hour after oxytocin injection but not vehicle injection (Fig 4B); however, there were no differences in stereotypic activity when food was not available during the testing period (Fig. 4C). This indicates that the presence of food in the cage did not contribute to the elevations in activity level during the first hour. RER was reduced at a similar rate in both groups when neither group had access to food (Fig. 4D).
Fig. 4.
Oxytocin in the ventromedial hypothalamus increases energy expenditure and SPA during fasting. A: oxytocin increased EE during the first hour postinjection. There were no differences in 12-h EE when food was not made available during the testing period (A, inset). SPA was elevated by oxytocin during the first hour (B); however, there were no differences in stereotypic activity (C). D: RER was similarly reduced in both groups. ANCOVA using body weight as a covariate (A), two-way ANOVA (treatment × time) with Sidak's multiple comparisons test (B–D). n = 5 (repeated measures) *P < 0.05.
VMN Oxytocin Effects on Resting and Nonresting Components of Energy Expenditure
To validate the methods for calculating REE and NREE, REE and NREE from aCSF-treated animals over the two experiments were compared, since the same animals were used during both sets of experiments. There were no differences in REE or NREE in aCSF-treated animals during experiments 3 and 4 (Fig. 5). Since elevations in TEE were primarily observed in the first hour, we compared REE and NREE during the first hour postinjection from experiments 3 and 4 (Fig. 5). Oxytocin increased REE, NREE, and TEE when food was available during the testing period (Fig. 5A). When food was not in the cage, oxytocin increased energy expenditure by increasing NREE (Fig. 5B), but not REE.
Fig. 5.

Oxytocin increases both resting and nonresting energy expenditure during the first hour postinjection. A: During nonfasted conditions, oxytocin increases resting energy expenditure (REE) and nonresting energy expenditure (NREE) for 1 h immediately postinjection (n = 8). B: when food was not made available, only NREE was significantly increased by oxytocin in the hour postinjection. In both cases, oxytocin significantly increased total energy expenditure. Two-tailed paired t-test *P < 0.05, oxytocin vs. aCSF.
DISCUSSION
Our results identify the VMN as a novel site where oxytocin affects energy balance. To date, extensive work has discussed the role of the hindbrain in oxytocin-mediated satiety and energy expenditure (3, 4, 6, 26, 30, 38, 56); however, hypothalamic sites for oxytocin effects are less well described. In the NTS, oxytocin modulates the responsiveness to peripheral satiety signals, such as CCK (3, 4, 31) and leptin (6, 61). Though there is evidence for a central PVN-NTS pathway mediating the satiety promoting effects of leptin (36), effects of oxytocin on energy balance persist in leptin receptor-deficient rats (24, 26), suggesting a leptin-independent pathway. Peripheral oxytocin is associated with activation of the NTS, reduced feeding, and weight loss in diet-induced obesity and leptin receptor-deficient rats (11, 23, 26, 64).
In addition to NTS, there is evidence for hypothalamic involvement in oxytocin effects on energy balance. Oleoylethanolamide (OEA), an ethanolamide produced by the small intestine after feeding, reduces food consumption in both fed (15) and fasted (15, 41) rats via central oxytocin (14). While OEA activates the NTS neurons, recent evidence suggests that the role of NTS in OEA-mediated satiety is via noradrenergic afferent input to PVN oxytocin neurons (42). Gaetani et al. (14) reported that blocking oxytocin receptors in the third ventricle attenuated the anorexigenic effects of OEA without affecting NTS c-Fos activation, suggesting that oxytocin receptor activation in sites other than the NTS can also mediate feeding behavior. Additionally, Zhang and Cai (64) showed previously that the two primary sites activated by oxytocin were VMN and NTS, suggesting a potential role for VMN in oxytocin in feeding behavior. Herein, we report for the first time that oxytocin injections into the VMN reduce feeding in rats in both the fed and fasted state. A conditioned taste aversion test (Fig. 1B) was performed to ensure that oxytocin-injected animals were not avoiding eating due to malaise.
A single unilateral oxytocin injection into the VMN acutely elevated energy expenditure, primarily by increasing activity (Fig. 5). Elevations in stereotypic activity were only evident when food was made available during testing (Figs. 3C and 4C). Stereotypic activity was defined as activity that occurred within a 3-inch box around the animal, and further studies are needed to determine the nature of the stereotypic activity. For example, it is possible that elevations in stereotypic activity were due to activity caused by eating more often (though consuming less during each bout), or by activity due to grooming after eating.
The effects of oxytocin on energy expenditure and activity persisted for 1 h, after which animals tended toward reduced energy expenditure without differences in SPA, leading to an overall significant reduction in energy expenditure for the 12-h period (Fig. 3A, C, D). When food was removed from the cage during the testing period, the differences in 12-h energy expenditure were attenuated, suggesting diet-induced thermogenesis was a contributing factor. For the energy expenditure experiments, to keep the timing of injections similar for each animal relative to the onset of the dark cycle, while accounting for the extra time it takes to seal the indirect calorimetry chambers, we did not perform bilateral injections. It is possible we would have observed a longer duration of elevated energy expenditure or greater increases had we done bilateral injections. For example, Zhang and colleagues (63, 64) observed elevated oxygen consumption that persisted for 4 h following injection of oxytocin into the third ventricle. Future experiments are needed to investigate whether elevated energy expenditure persists longer when both populations of VMN oxytocin receptors are stimulated.
NREE was measured to determine whether physical activity explained oxytocin-induced elevations in energy expenditure during the first hour postinjection. In the absence of food, NREE primarily contributed to the difference in energy expenditure (Fig. 5B). When food was available, both NREE and REE were elevated in oxytocin-treated animals (Fig. 5A), indicating that the presence of food in the cage adds an additional source of oxytocin-induced energy expenditure. It was recently reported that endogenous oxytocin is required for diet-induced energy expenditure (61). In the current study, elevated REE in oxytocin-treated animals during the first hour postinjection could be related to the thermic effect of food, but oxytocin in our study also reduced kilocalorie intake during the first hour postinjection. Alternatively, lack of differences in NREE between oxytocin-treated animals and controls in the absence of food may be due to a floor effect, since NREE was equally low for both animals when food was not made available during testing.
In support of the former (that oxytocin in the VMN increases the thermic effect of food), it was reported that oxytocin in the DMH/VMN is essential for cold-induced thermogenesis (18). Oxytocin receptor-deficient mice have reduced β3-adrenergic receptor expression and elevated expression of α2A-adrenergic receptor in brown adipocytes (18). β3-adrenergic receptor and α2A-adrenergic receptor have opposing actions in brown adipose tissue, β3-adrenergic receptor activation increases thermogenesis, whereas α2A-adrenergic receptor activation inhibits thermogenesis (for review, see Refs. 10 and 18). In addition to cold-induced thermogenesis, the metabolic activity of brown adipocytes is also essential for diet-induced thermogenesis (12). These data support that the finding of elevated REE in oxytocin-injected nonfasted animals during the initial hour postinjection may reflect increases in diet-induced thermogenesis. Peripheral β3-adrenergic receptor activation reduce feeding (54), and the finding that oxytocin alters the expression of adrenergic receptors in brown adipose tissue raises the question as to whether oxytocin in the VMN reduces feeding indirectly via activation of the sympathetic nervous system. Activation of the sympathetic nervous system affects circulating hormones and nutrient metabolites, which influence appetite and satiety. For example, peripheral activation of β3-adrenergic receptors enhances central sensitivity to the anorexigenic hormone leptin (29). If oxytocin acts indirectly to reduce feeding via activation of the sympathetic nervous system, it would explain why animals lacking oxytocin receptor or deficient in oxytocin have normal food intake (1, 9, 19, 51), whereas animals injected with oxytocin have reduced feeding and increased energy expenditure.
PVN oxytocin neurons have previously been shown to exhibit diurnal rhythmicity in oxytocin expression, whereby oxytocin expression is increased during the day and reduced at night (64). Additionally, oxytocin neurons also are affected by energy status. Fasting reduces PVN oxytocin production (5, 13, 20, 55) and refeeding increases oxytocin production (20). The present data show that oxytocin injections reduced feeding when animals have been food-deprived (Fig. 2) 3 h after the onset of the light cycle. Similarly, oxytocin significantly reduced feeding and increased energy expenditure and activity at the onset of the dark cycle. Thus, in the VMN the effects of oxytocin may not be limited by circadian or behavioral influences on signaling at the receptor level, however, since our rats were injected in two different conditions (fed vs. fasted) during different times of the day. Future experiments are necessary to determine circadian effects of oxytocin injections in both the fed and fasted state.
In the current study, oxytocin injection during the dark cycle reduced 12-h energy expenditure, but Zhang and Cai (64) reported no change in 12-h energy expenditure under similar conditions. One reason for this discrepancy may be the source of dietary kilocalories: Zhang et al. maintained animals on a high-fat diet, whereas animals were given standard chow in the current study. A higher energy density in the high-fat diet may cause greater elevations in thermic effect of food after oxytocin treatment and, thus, eliminate the energy expenditure deficit caused by reduced caloric intake. Zhang and Cai (64) measured energy expenditure following 1 wk of nightly injections of oxytocin; thus, we cannot exclude the possibility that the discrepancy in our data is due to an effect of acute vs chronic injections. Additionally, in the present study, oxytocin was injected directly into the VMN, whereas Zhang and Cai (64) used intracerebroventricular injections. This raises the possibility that these two data sets are not entirely comparable because regional effects of oxytocin given intracerebroventricularly may counteract each other.
The present study has some limitations. One is the timing of injections during the fed and fasted states. Fasting oxytocin injections were performed during the inactive period, and nonfasting injections were performed at the onset of the dark cycle, when animals would normally eat their first meal. Oxytocin receptors may also have some circadian rhythmicity, and thus, it is not certain whether oxytocin would be similarly anorexigenic in fasted animals that were injected at the onset of the dark cycle. Another limitation of this study is that oxytocin can act as an agonist to the arginine vasopressin V1A receptor (25), which is also present in the hypothalamus; thus, the present data do not distinguish whether oxytocin in the VMN exerts energy regulatory effects via the oxytocin or vasopressin receptor. Future experiments are required to determine which receptor population exogenous oxytocin acts upon as it alters feeding, activity, and energy expenditure.
In summary, the current data show that direct injections of oxytocin in the VMN reduce feeding and elevate energy expenditure during both the fed and fasted state, implicating the VMN as a site where oxytocin has antiobesity effects. It remains to be determined whether the VMN plays a role in endogenous effects of oxytocin on energy balance. On the basis of the current findings, this is worth further investigation.
Perspectives and Significance
Oxytocin is in the beginning stages of the clinical use for the treatment of obesity and diabetes (34, 65), although the mechanism by which oxytocin promotes a negative energy balance is not understood, nor have the sites of oxytocin's effects been characterized. Understanding the site and mechanism of oxytocin effects on feeding and energy expenditure will help in developing targeted therapeutics. In some cases, oxytocin is being used to treat nonmetabolic disorders, such as autism and schizophrenia, for which anorexigenic effects or elevated energy metabolism might be undesirable. In other cases, genetic disorders leading to obesity, such as Prader Willi syndrome—in which oxytocin neurons are reduced (48)—or polyphorphisms of the fat mass and obesity-associated gene—which regulates oxytocin expression (32)—knowing the sites and mechanism of oxytocin effects could help with the development of targeted delivery systems. The current data indicate that in addition to the NTS, the VMN is a central location where oxytocin may exert antiobesity effects.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.E.N. and C.W. conception and design of research; E.E.N. and C.W. performed experiments; E.E.N. and C.W. analyzed data; E.E.N. and C.W. interpreted results of experiments; E.E.N. prepared figures; E.E.N. drafted manuscript; E.E.N., C.J.B., C.M.K., and C.W. edited and revised manuscript; C.J.B., C.M.K., and C.W. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH), and the Department of Veterans Affairs. Additional support was provided by Grant T32DK-083250 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the NIH. We would like to thank Morgan R. Little for her laboratory assistance.
REFERENCES
- 1.Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol 289: R1798–R1806, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav 48: 825–830, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Baskin DG, Kim F, Gelling RW, Russell BJ, Schwartz MW, Morton GJ, Simhan HN, Moralejo DH, Blevins JE. A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology 151: 4207–4213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res 993: 30–41, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Blevins JE, Ho JM. Role of oxytocin signaling in the regulation of body weight. Rev Endocr Metab Disord 14: 311–329, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287: R87–R96, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci 29: 8302–8311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience 253: 155–164, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring) 17: 980–984, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Deblon N, Veyrat-Durebex C, Bourgoin L, Caillon A, Bussier AL, Petrosino S, Piscitelli F, Legros JJ, Geenen V, Foti M, Wahli W, Di Marzo V, Rohner-Jeanrenaud F. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS One 6: e25565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203–209, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Flak JN, Jankord R, Solomon MB, Krause EG, Herman JP. Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol Behav 104: 228–234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaetani S, Fu J, Cassano T, Dipasquale P, Romano A, Righetti L, Cianci S, Laconca L, Giannini E, Scaccianoce S, Mairesse J, Cuomo V, Piomelli D. The fat-induced satiety factor oleoylethanolamide suppresses feeding through central release of oxytocin. J Neurosci 30: 8096–8101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaetani S, Oveisi F, Piomelli D. Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacology 28: 1311–1316, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Gavini CK, Mukherjee S, Shukla C, Britton SL, Koch LG, Shi H, Novak CM. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am J Physiol Endocrinol Metab 306: E635–E647, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin GD, Ferri-Kolwicz SL, Reyes BA, Van Bockstaele EJ, Flanagan-Cato LM. Ovarian hormone-induced reorganization of oxytocin-labeled dendrites and synapses lateral to the hypothalamic ventromedial nucleus in female rats. J Comp Neurol 518: 4531–4545, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasahara Y, Sato K, Takayanagi Y, Mizukami H, Ozawa K, Hidema S, So KH, Kawada T, Inoue N, Ikeda I, Roh SG, Itoi K, Nishimori K. Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology 154: 4305–4315, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Kasahara Y, Takayanagi Y, Kawada T, Itoi K, Nishimori K. Impaired thermoregulatory ability of oxytocin-deficient mice during cold-exposure. Biosci Biotechnol Biochem 71: 3122–3126, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol 22: 1723–1734, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leng G, Onaka T, Caquineau C, Sabatier N, Tobin VA, Takayanagi Y. Oxytocin and appetite. Prog Brain Res 170: 137–151, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Lokrantz CM, Uvnas-Moberg K, Kaplan JM. Effects of central oxytocin administration on intraoral intake of glucose in deprived and nondeprived rats. Physiol Behav 62: 347–352, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 3: 1169–1177, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh IS, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 10: 355–365, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol 24: 609–628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab 302: E134–E144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullis K, Kay K, Williams DL. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res 1513: 85–91, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res 333: 325–329, 1985 [DOI] [PubMed] [Google Scholar]
- 29.Okamatsu-Ogura Y, Nio-Kobayashi J, Iwanaga T, Terao A, Kimura K, Saito M. Possible involvement of uncoupling protein 1 in appetite control by leptin. Exp Biol Med (Maywood) 236: 1274–1281, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12: 113–118, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology 129: 785–791, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Olszewski PK, Fredriksson R, Eriksson JD, Mitra A, Radomska KJ, Gosnell BA, Solvang MN, Levine AS, Schioth HB. Fto colocalizes with a satiety mediator oxytocin in the brain and upregulates oxytocin gene expression. Biochem Biophys Res Commun 408: 422–426, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Olszewski PK, Klockars A, Schioth HB, Levine AS. Oxytocin as feeding inhibitor: maintaining homeostasis in consummatory behavior. Pharmacol Biochem Behav 97: 47–54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, Hallschmid M. Oxytocin reduces reward-driven food intake in humans. Diabetes 62: 3418–3425, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Boston, MA: Elsevier Academic, 2007 [Google Scholar]
- 36.Perello M, Raingo J. Leptin activates oxytocin neurons of the hypothalamic paraventricular nucleus in both control and diet-induced obese rodents. PLoS One 8: e59625, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez-Leighton CE, Boland K, Billington CJ, Kotz CM. High and low activity rats: elevated intrinsic physical activity drives resistance to diet-induced obesity in non-bred rats. Obesity (Silver Spring) 21: 353–360, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci 28: 11731–11740, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pow DV, Morris JF. Differential distribution of acetylcholinesterase activity among vasopressin- and oxytocin-containing supraoptic magnocellular neurons. Neuroscience 28: 109–119, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol 399: 101–109, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, Murillo-Rodriguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D. An anorexic lipid mediator regulated by feeding. Nature 414: 209–212, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Romano A, Cassano T, Tempesta B, Cianci S, Dipasquale P, Coccurello R, Cuomo V, Gaetani S. The satiety signal oleoylethanolamide stimulates oxytocin neurosecretion from rat hypothalamic neurons. Peptides 49: 21–26, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Rosen GJ, de Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience 155: 809–817, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabatier N, Leng G, Menzies J. Oxytocin feeding, and satiety. Front Endocrinol (Lausanne) 4: 35, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakaguchi T, Arase K, Bray GA. Sympathetic activity and food intake of rats with ventromedial hypothalamic lesions. Int J Obes 12: 285–291, 1988 [PubMed] [Google Scholar]
- 46.Sokol HW, Zimmerman EA, Sawyer WH, Robinson AG. The hypothalamic-neurohypophysial system of the rat: localization and quantitation of neurophysin by light microscopic immunocytochemistry in normal rats and in Brattleboro rats deficient in vasopressin and a neurophysin. Endocrinology 98: 1176–1188, 1976 [DOI] [PubMed] [Google Scholar]
- 47.Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol 32: 426–450, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metab 80: 573–579, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980 [DOI] [PubMed] [Google Scholar]
- 50.Takahashi A, Ishimaru H, Ikarashi Y, Kishi E, Maruyama Y. Effects of ventromedial hypothalamus stimulation on glycogenolysis in rat liver using in vivo microdialysis. Metab Clin Exp 46: 897–901, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 19: 951–955, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol 291: R889–R899, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Barberis C, Jard S. Oxytocin receptors in the central nervous system. Distribution, development, and species differences. Ann NY Acad Sci 652: 29–38, 1992 [DOI] [PubMed] [Google Scholar]
- 54.Tsujii S, Bray GA. A β-3 adrenergic agonist (BRL-37,344) decreases food intake. Physiol Behav 63: 723–728, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Tung YC, Ma M, Piper S, Coll A, O'Rahilly S, Yeo GS. Novel leptin-regulated genes revealed by transcriptional profiling of the hypothalamic paraventricular nucleus. J Neurosci 28: 12419–12426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uchoa ET, Zahm DS, de Carvalho Borges B, Rorato R, Antunes-Rodrigues J, Elias LL. Oxytocin projections to the nucleus of the solitary tract contribute to the increased meal-related satiety responses in primary adrenal insufficiency. Exp Physiol 98: 1495–1504, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vander Tuig JG, Knehans AW, Romsos DR. Reduced sympathetic nervous system activity in rats with ventromedial hypothalamic lesions. Life Sci 30: 913–920, 1982 [DOI] [PubMed] [Google Scholar]
- 58.Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am J Physiol Regul Integr Comp Physiol 293: R992–R1002, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol 293: R1037–R1045, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Kotz CM. Urocortin in the lateral septal area modulates feeding induced by orexin A in the lateral hypothalamus. Am J Physiol Regul Integr Comp Physiol 283: R358–R367, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Wu Z, Xu Y, Zhu Y, Sutton AK, Zhao R, Lowell BB, Olson DP, Tong Q. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS One 7: e45167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamashita M, Takayanagi Y, Yoshida M, Nishimori K, Kusama M, Onaka T. Involvement of prolactin-releasing peptide in the activation of oxytocin neurones in response to food intake. J Neuroendocrinol 25: 455–465, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang G, Bai H, Zhang H, Dean C, Wu Q, Li J, Guariglia S, Meng Q, Cai D. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron 69: 523–535, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang G, Cai D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am J Physiol Endocrinol Metab 301: E1004–E1012, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J, Cai D. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One 8: e61477, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]




