Abstract
The physiological functions of the urinary bladder are to store and periodically expel urine. These tasks are facilitated by the contraction and relaxation of the urinary bladder smooth muscle (UBSM), also known as detrusor smooth muscle, which comprises the bladder wall. The large-conductance voltage- and Ca2+-activated K+ (BK, BKCa, MaxiK, Slo1, or KCa1.1) channel is highly expressed in UBSM and is arguably the most important physiologically relevant K+ channel that regulates UBSM function. Its significance arises from the fact that the BK channel is the only K+ channel that is activated by increases in both voltage and intracellular Ca2+. The BK channels control UBSM excitability and contractility by maintaining the resting membrane potential and shaping the repolarization phase of the spontaneous action potentials that determine UBSM spontaneous rhythmic contractility. In UBSM, these channels have complex regulatory mechanisms involving integrated intracellular Ca2+ signals, protein kinases, phosphodiesterases, and close functional interactions with muscarinic and β-adrenergic receptors. BK channel dysfunction is implicated in some forms of bladder pathologies, such as detrusor overactivity, and related overactive bladder. This review article summarizes the current state of knowledge of the functional role of UBSM BK channels under normal and pathophysiological conditions and provides new insight toward the BK channels as targets for pharmacological or genetic control of UBSM function. Modulation of UBSM BK channels can occur by directly or indirectly targeting their regulatory mechanisms, which has the potential to provide novel therapeutic approaches for bladder dysfunction, such as overactive bladder and detrusor underactivity.
Keywords: KCa1.1 channel, iberiotoxin, paxilline, detrusor, overactive bladder, muscarinic receptors, β-adrenergic receptors
the k+ channels have a key role in maintaining the resting membrane potential and action potentials of urinary bladder smooth muscle (UBSM), also known as detrusor smooth muscle (118). UBSM exhibits spontaneous action potentials (18, 33, 38, 46–48, 103, 120), which determine the phasic (rhythmic) nature of spontaneous contractions in UBSM (21, 27, 46, 51, 53, 54, 58, 60, 119, 120). Ca2+ entry via L-type voltage-dependent Ca2+ channels (VDCCs) is responsible for the depolarization phase of the UBSM action potential and leads to an increase in global intracellular Ca2+, which activates UBSM phasic contractions (44, 48, 65). K+ channels shape the action potentials of UBSM by facilitating repolarization after the initial episode of depolarization (45, 48). Opening of K+ channels causes cell membrane hyperpolarization, limits Ca2+ entry into the UBSM cells via L-type VDCCs, ultimately leading to UBSM relaxation. On the contrary, inhibition of UBSM K+ channels leads to cell membrane depolarization and opening of L-type VDCCs, causing UBSM contraction. Thus, the fundamental purpose of the UBSM K+ channels is to precisely regulate and fine-tune Ca2+ entry into the UBSM cells via L-type VDCCs, hence, the intracellular Ca2+ concentration, which controls UBSM contraction and relaxation. Several families of K+ channels, including Ca2+-activated K+ (KCa) channels, voltage-gated K+ (KV) channels, inward-rectifying ATP-sensitive K+ (Kir, KATP) channels, and two-pore domain K+ (K2P) channels, are expressed and functional in UBSM cells (3, 27, 43, 57, 59, 113, 116, 118, 120, 131). In UBSM, the KCa channel family is represented by three major subtypes of channels, which are classified on the basis of their single-channel conductance: large-conductance voltage- and Ca2+-activated K+ (BK) channels; small-conductance Ca2+-activated K+ channels (SK1-SK3); and intermediate conductance Ca2+-activated K+ (IK) channels (118).
The BK channel is one of the most, if not the most, physiologically important K+ channels that regulates UBSM function in health and disease (21, 47, 48, 51–53, 58, 93, 119, 121, 132, 133, 140). This importance arises from the fact that the BK channel is the only K+ channel that is activated by increases in both intracellular Ca2+ and membrane depolarization, so it is uniquely suited to serve as a Ca2+/voltage signal integrator in the modulation of UBSM cell membrane excitability (31, 117, 118). In UBSM, these channels have intricate regulatory mechanisms involving complex intracellular Ca2+ signals, protein kinases, such as PKA and PKC, phosphodiesterases, and close functional interactions with muscarinic (M) and β-adrenergic receptors (β-ARs). Historically, this channel appears under multiple names—BK, BKCa, MaxiK, Slo1, or KCa1.1—with “BK channel” being the most commonly used, and the “KCa1.1 channel” being the name based on the official channel nomenclature (43). This review article recaps the current state of the knowledge regarding the role of BK channels in urinary bladder physiology and pathophysiology, with special emphasis on recent developments in the field.
UBSM BK Channel Structure and Biophysical Properties
The central role played by the BK channel in urinary bladder function, among all other K+ channels, arises from its dual regulation by both voltage and Ca2+, along with its high unitary single-channel conductance (80). The K+ conducting unit of the BK channel is a tetramer of the pore-forming α-subunit, which is encoded by a single gene (Slo or KCNMA1), consisting of multiple alternative exons (Fig. 1). The BK channel pore-forming α-subunit has the structure of the six-segment (S) transmembrane architecture of the KV channel α-subunit with the addition of an S0 transmembrane segment and a long intracellular domain (S7-S10) at the C-terminus containing the Ca2+ sensor (Fig. 1). Although the BK channel α subunit is encoded by a single gene, a number of splice variants possessing differential regulatory mechanisms have been reported (26). The exact BK channel α subunit splice variants in UBSM are currently unknown. In addition to the pore-forming α-subunits, BK channels may also contain one of the four known tissue-specific regulatory subunits (β1–β4), with β1 being smooth muscle-specific and β4 being predominantly expressed in neurons (Fig. 1). UBSM cells express the pore-forming α-subunits (58, 93), as well as both β1-subunits (58, 119) and β4-subunits (28, 58, 70). Although β1 is the primary ancillary BK channel subunit in UBSM (119), several studies at mRNA and/or protein levels have clearly identified the presence of β4-subunits directly in rat, mouse, and human UBSM cells (28, 58, 70), while confirming the absence of BK channel β2- and β3-subunits (28, 58). BK channels have not been shown to exist in complexes of mixed regulatory β-subunits, such as β1- and β4-subunits present together in the same channel complex. Therefore, one would expect to observe at least three different populations of BK channels in UBSM cells (α/α, α/β1, and α/β4), including one population with slow-gating kinetics due to the presence of the β4-subunit. On the basis of their detailed biophysical characterization, a recent study suggests that the native human UBSM BK channels likely consist of diverse channel complexes (89).
Fig. 1.
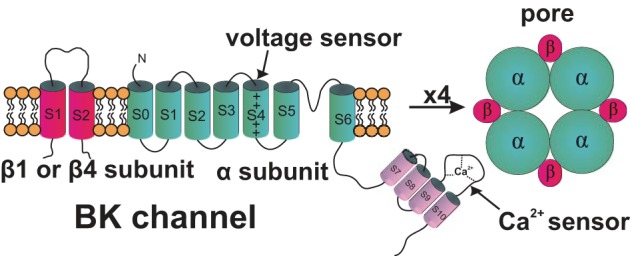
Large-conductance voltage- and Ca2+-activated K+ (BK) channel molecular structure. Four pore-forming α-subunits and the four regulatory β1-subunits or β4-subunits form a functional BK channel in urinary bladder smooth muscle (UBSM) cells. This figure is based on information from Petkov (117, 118).
The UBSM BK channels have unitary single-channel conductance in the range of ∼200 pS, hence, the names “Maxi-K” or “big K+” channel. Depending on the exact experimental conditions, UBSM single BK channel conductance varies from 214 pS in mice (119), 122–244 pS in guinea pigs (48, 63, 81), 170–200 pS in rats (130), 330 pS in rabbits (123, 124), and 136–220 pS in humans (58, 89, 130). A recent study has provided the first systematic characterization of human single BK channel biophysical properties in excised patches of freshly isolated UBSM cells from donor patients (89). The human UBSM single BK channel currents exhibit properties consistent with those reported in other smooth muscle cells: a mean single-channel conductance of about 200 pS, open single-channel probability that is highly dependent on intracellular Ca2+ concentration and voltage, as well as sensitivity to established BK channel inhibitors and activators (58, 89). Furthermore, the human UBSM single BK channel conductance and amplitude is not affected by intracellular Ca2+ concentration or BK channel activators (89).
UBSM BK Channel Physiology and Pharmacology
In its essence, the physiological role of BK channels in controlling UBSM excitability and contractility is to provide a negative-feedback mechanism to limit the amplitude and duration of UBSM action potentials and related phasic contractions (45, 48, 51, 58) (Fig. 2). The initial evidence for a BK channel role in UBSM excitability originally came with the early patch-clamp studies by Klockner and Isenberg in the mid-1980s (71). It is now well established that the BK channel has very high expression levels in UBSM (28, 58, 110, 119). Specifically, it has been shown that in guinea pig UBSM cells, BK channels have an extremely high density of ∼21 BK channels per square micrometer (110). The initial experimental evidence regarding BK channel physiological roles was based exclusively on animal studies, and until recently, our knowledge about the expression and function of the BK channel in human UBSM was very limited to several nonsystematic studies (35, 44, 130, 137). In the past 2 or 3 years, a series of multidisciplinary studies, including combined molecular approaches, patch-clamp electrophysiology, live-cell Ca2+ imaging, and confocal microscopy on freshly isolated human UBSM cells, as well as functional studies on UBSM contractility of tissues, have provided a methodological identification and characterization of the BK channel functional and regulatory roles in native human UBSM (5, 56, 58, 61, 89, 155). It is well known that BK channel properties change quickly upon cell culturing (127) and that BK channel density rapidly decreases in cultured UBSM cells (136). A major advantage of these systematic studies is that they have utilized the perforated patch-clamp technique on native freshly isolated (not cultured) and physiologically active human UBSM cells. The perforated patch-clamp technique enables one to study the BK channel activity with preserved intracellular signal transduction pathways, thus providing novel insights into human UBSM BK channel function. Another innovative feature of these systematic studies is that they have used clinically characterized human UBSM tissues, thus allowing for a correlation of the data from molecular, cellular, and tissue levels with the patients' clinical phenotype, thereby revealing a critical role of the BK channel in human bladder physiology.
Fig. 2.
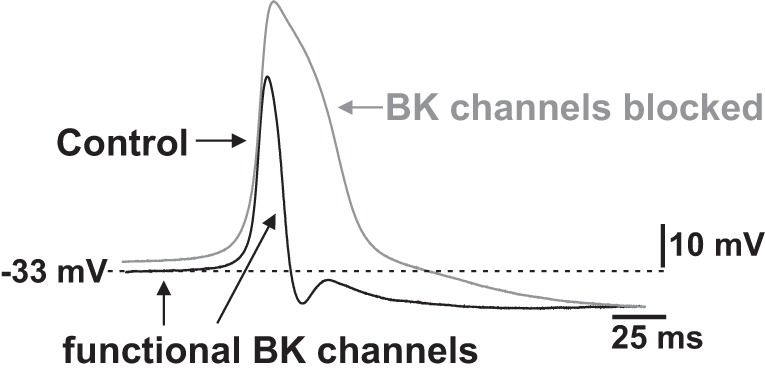
Role of BK channels in determining UBSM cell resting membrane potential and action potential repolarization. BK channels contribute to the initial repolarization phase of the UBSM spontaneous action potential and to the maintenance of the resting membrane potential in UBSM cells. This figure is based on information from Refs. 44, 45, 47, 48, and 58.
BK channels are blocked with high affinity by the scorpion venoms iberiotoxin and charybdotoxin (39, 94, 134), as well by the Penicillium mycotoxin, paxilline (72). Iberiotoxin is highly selective for the BK channels, whereas charybdotoxin also inhibits the IK channels and some KV channel members of the KV1 family. Intriguingly, UBSM cells do not have functionally active IK channels regulating UBSM excitability under normal physiological conditions (6, 113, 116). However, since iberiotoxin and charybdotoxin do not effectively inhibit the BK channel α/β4 complexes (91), the use of paxilline provides an advantage when equal inhibition of both BK channel α/β1 and α/β4 complexes is desired. It should be noted that all known BK channel splice variants are effectively inhibited by the specific blocker paxilline with similar IC50s (125), thus making paxilline ideal for UBSM BK channel functional studies. Another advantage of paxilline vs. iberiotoxin and charybdotoxin is that paxilline is a small hydrophobic molecule that can easily cross the cell plasma membrane and block the BK channels from inside, while recorded in the cell-attached patch-clamp configuration. The mycotoxin penitrem A is another BK channel inhibitor with inhibitory properties similar to those of paxilline (12, 72, 99). BK channels are also inhibited nonselectively by tetraethylammonium, but with low affinity (21, 53, 117).
Pharmacological blockade of BK channels increases the amplitude and duration of spontaneous action potentials, indicating that the repolarization phase of the UBSM action potential is mediated by BK channel activity (44, 45, 47, 48) (Fig. 2). Although some studies failed to report UBSM resting membrane potential depolarization upon BK channel pharmacological inhibition (44, 45, 47), it is now well documented that blocking BK channels with iberiotoxin or paxilline depolarizes the resting membrane potential in both isolated UBSM cells and intact tissues recorded with patch-clamp or intracellular microelectrodes, respectively (48, 58, 60, 61, 152–155). Further key evidence for a role of the BK channels in controlling the resting membrane potential is that genetic deletion of the BK channel α-subunit causes sustained membrane depolarization in mouse UBSM cells (21, 133). Collectively, these findings clearly indicate that BK channels do control the resting membrane potential in UBSM cells (Fig. 2). In UBSM isolated from various species and humans, pharmacological inhibition of BK channels increases the amplitude, duration, and force of the spontaneous phasic contractions, as well as UBSM tone (21, 35, 37, 51, 56, 58, 60, 61, 65, 109, 119, 134, 152, 153, 155). In contrast, iberiotoxin had no effect on phasic and tonic contractions of UBSM strips isolated from mice that lacked the BK channel pore-forming α-subunit (21), which further confirmed the selectivity of iberiotoxin for UBSM BK channels. Pharmacological inhibition or genetic deletion of BK channels enhances the nerve-evoked contractions in UBSM strips isolated from a number of different species (4, 5, 50, 58, 69, 77, 133, 140, 150). On the other hand, well-established (NS1608, NS8, NS004, and NS1619) and novel highly specific (NS11021 and GoSlo-SR-5–69) BK channel openers increase BK channel open probability and whole cell BK currents, causing membrane hyperpolarization and relaxation of UBSM in a variety of species (24, 61, 63, 65, 81, 88, 101, 109, 123, 124, 129, 130, 132, 143). At the in vivo level, intravenous administration of the BK channel opener, NS8, causes a reduction in micturition frequency and an increase in micturition volume in rats (138).
The ability to genetically manipulate the mouse genome by deleting or overexpressing genes encoding BK channel subunits has allowed us to study their functional effects from cellular to whole organism levels (21, 93, 119, 133, 140, 150). Targeted deletion of the BK channel pore-forming α-subunit or regulatory β1-subunit results in increased UBSM excitability and contractility in mouse models (21, 93, 119) (see also Fig. 3). Combined data from global and smooth muscle-specific BK channel α-subunit knockout (KO) mice indicate that genetic deletion of the BK channel pore-forming α-subunit has profound effects on UBSM function (21, 93, 133, 140, 150). Specifically, UBSM spontaneous and nerve-evoked contractions are dramatically increased upon genetic deletion of the BK channel pore-forming α-subunit, and these mice demonstrate increased urination frequency (21, 93, 133, 140, 150). At the urodynamic level, global BK channel α-subunit KO mice exhibit increased bladder pressures, pressure oscillations, and symptoms of urinary incontinence, such as urine dripping (140). Data from BK channel β1-subunit KO mice indicate that the β1-subunit increases the apparent Ca2+ and voltage sensitivity of UBSM BK channels (119). Whereas the β1-subunit has a key physiological role in bladder physiology (119), the function of the more recently discovered β4-subunit in UBSM is less clear (28, 58, 70), although it might have some role in UBSM pathophysiology (56, 70). It is well known that the β4-subunit makes the BK channel less sensitive to iberiotoxin and charybdotoxin (91). However, all UBSM cells respond to iberiotoxin (53, 58), indicating a predominant expression of the α/α and/or α/β1 BK channel complexes (Fig. 1). Therefore, the α/β4 BK channel complex may have a secondary role in human UBSM function. In general, the β4-subunit decreases the BK channel voltage sensitivity and slows activation kinetics (20). An increased action potential frequency has been reported in dentate granule cells isolated from BK channel β4-subunit KO mice (19). The BK channel β4-subunit may have a similar regulatory role in UBSM, but this needs further investigation.
Fig. 3.
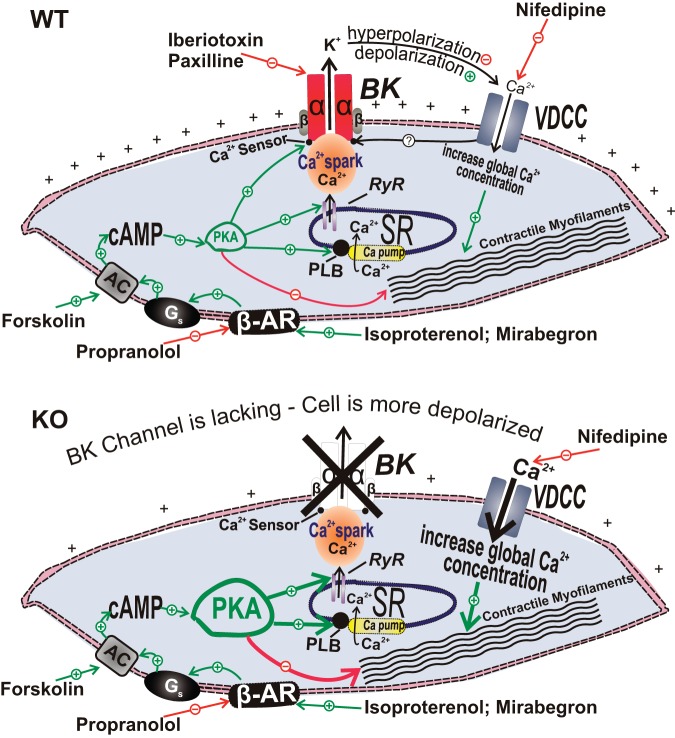
Illustration of the cellular mechanisms by which BK channels mediate β-adrenergic relaxation in UBSM cells with demonstration of the differential outcomes in the wild-type (WT) mouse (top) and the BK-knockout (KO) mouse (bottom), respectively. In UBSM cells from WT mice, functionally active BK channels regulate Ca2+ entry via L-type voltage-dependent Ca2+ channels (VDCC), and, thus, contractility (top). In addition, BK channels are under the local control of the so-called “Ca2+ sparks” caused by Ca2+ release from the ryanodine receptors of the sarcoplasmic reticulum, adjacent to the cell membrane. Following permanent BK channel pore forming α-subunit gene deletion in the BK channel KO mouse, an adaptive compensatory upregulation of the β-AR/cAMP/PKA signaling pathway develops. The enhanced β-AR/PKA activity compensates for the increased Ca2+ entry via L-type VDCC that occurs due to sustained membrane depolarization in the absence of the BK channels. AC, adenylyl cyclase; BK, large-conductance voltage- and Ca2+-activated K+ channels; β-AR, β-adrenergic receptors; VDCC, L-type voltage-dependent Ca2+ channels; Gs, stimulatory G protein; PKA, protein kinase-A; PLB, phospholamban; PLC, phospholipase C; RyR, ryanodine receptor; SR, sarcoplasmic reticulum. This figure is based on information from Brown et al. (21).
UBSM BK Channel Regulation by Ca2+ Signals
Ca2+ is an important regulator not only of UBSM contractility, but also of the BK channels (31, 80). In UBSM, there are two major Ca2+ sources for BK channel activation: 1) Ca2+ entry through L-type VDCCs; and 2) Ca2+ release from the ryanodine receptors (RyRs) of the sarcoplasmic reticulum (SR) (52, 53, 58, 60, 121). BK channels are under the local control of the so-called “Ca2+ sparks”, caused by spontaneous highly localized and transient Ca2+ releases from the RyRs (Figs. 3 and 4). Nelson et al. (108) were the first to postulate that Ca2+ sparks have a role in vascular smooth muscle relaxation through local BK channel activation, causing transient BK currents (TBKCs), originally known as spontaneous transient outward currents (STOCs). Later on Ca2+ sparks were also discovered in UBSM cells, where they occur spontaneously in unstimulated cells and where their primary role is to trigger TBKCs/STOCs without affecting the global intracellular Ca2+ concentration (52, 110, 121, 154). In UBSM cells, both Ca2+ sparks and the associated TBKCs/STOCs are completely inhibited by ryanodine (Figs. 3 and 4) (21, 52, 53, 58, 121). Ryanodine, per se, increases the spontaneous phasic contractions in isolated UBSM strips (51, 101), thus, further underscoring the role of RyR-mediated BK channel regulation at the level of UBSM contractility. Blocking the BK channels with iberiotoxin completely suppresses TBKCs/STOCs in UBSM cells at various recording voltages, which undoubtedly indicates that these transient outward currents are mediated solely by the BK channels (52, 58). Thus, it is now clear that STOCs 1) are not “spontaneous” but rather triggered by Ca2+ sparks and 2) are mediated by the BK channels. In guinea pig UBSM, BK channels and RyRs densely colocalize in spot-like fashion at junctional areas of the cell membrane and SR, where Ca2+ sparks originate to trigger TBKCs (52, 110). Although not all Ca2+ sparks necessarily initiate TBKCs in UBSM cells (52), one Ca2+ spark can elicit no more than one single TBKC by facilitating the coupling of RyRs and BK channels at the junction areas, where 3–100 BK channels can be activated by a single Ca2+ spark (52, 110). Interestingly, UBSM cells isolated from BK channel α-subunit KO mice exhibit Ca2+ sparks, but not TBKCs (93). In guinea pig and human UBSM cells, membrane depolarization causes a significant increase in TBKC amplitude and frequency (52, 56, 58). Herrera et al. (52) have shown that in guinea pig UBSM cells, the coupling strength between Ca2+ sparks and related TBKCs increases with membrane potential depolarization, resulting in much larger TBKCs for a given size Ca2+ spark. Currently, the physiological role of Ca2+ sparks and related TBKCs, which contribute to setting the resting membrane potential, is well documented in UBSM of various animal species (52, 53, 60, 110, 121, 155), including human UBSM (56, 58). At the level of the membrane potential, the TBKCs manifest as spontaneous transient hyperpolarizations (STHs) that collectively contribute to the membrane potential hyperpolarization upon RyR-dependent BK channel activation in UBSM cells (58, 154, 155). In UBSM cells, as in TBKCs, STHs are completely inhibited by BK channel blockers, such as iberiotoxin or paxilline (58, 152–155). There is a tight correlation between the TBKC frequency and STH frequency in guinea pig UBSM cells (155). By switching from voltage-clamp to current-clamp in the same recorded cells, Xin et al. (155) have shown that low-frequency STHs correspond to low-frequency TBKCs, whereas in UBSM cells exhibiting high-frequency TBKCs, the STH frequency is also proportionally high.
Fig. 4.
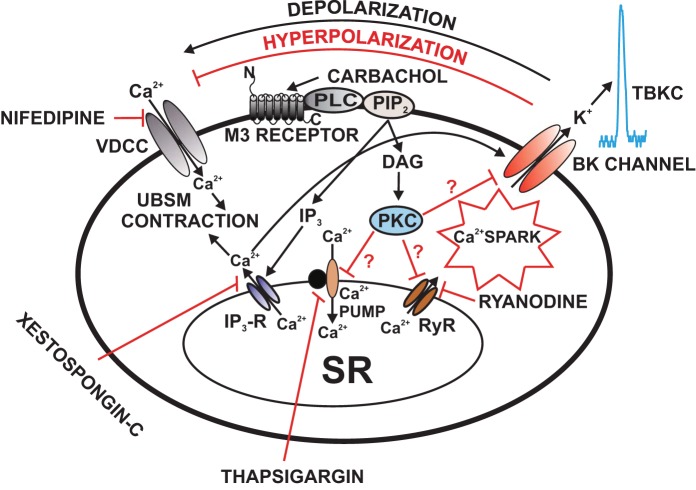
Illustration of the cellular mechanisms by which M3 receptors regulate BK channel function in UBSM cells. Activation of M3 receptors leads to IP3 and DAG production, via a pathway involving phospholipase-C (PLC) and PIP2. IP3 activates IP3 receptors, which releases Ca2+ from the SR. This IP3-induced Ca2+ release transiently activates the BK channels. Over time, depletion of SR Ca2+ upon activation of M3 receptors reduces Ca2+ spark activity, which leads to inhibition of TBKCs and depolarization of UBSM cell resting membrane potential causing activation of VDCC and thus increases UBSM contractility. DAG activates PKC, which may lead to direct inhibition of the SR Ca2+ pump and RyRs resulting in suppression of Ca2+ sparks and TBKCs. Pharmacological tools used in this study to inhibit cellular sources of Ca2+ are indicated. BK, large-conductance voltage- and Ca2+-activated K+ channel; DAG, 1,2-diacylglycerol; IP3, inositol triphosphate; M3 receptors, muscarinic receptors type 3; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase-C; PLC, phospholipase-C; Ryr, ryanodine receptor; TBKCs, transient BK currents; SR, sarcoplasmic reticulum; UBSM, urinary bladder smooth muscle; VDCC, L-type voltage-dependent Ca2+ channel. This figure is based on information from (62, 112, 114).
Unlike the ryanodine-sensitive TBKCs, ryanodine does not affect the depolarization-induced steady-state BK current in UBSM cells (53, 121). In contrast, the latter depends mainly on Ca2+ entry through L-type VDCCs and not Ca2+ release via RyRs as pharmacological inhibition of L-type VDCCs substantially reduces the steady-state BK current (53). Thus, ryanodine has been largely used as a pharmacological tool to separate the TBKCs from the steady-state BK currents in UBSM cells (53, 60, 62, 114, 121, 152).
In UBSM, inositol 1,4,5-trisphosphate (IP3) is produced in response to activation of M receptor type 3, a class of Gq/phospholipase C-coupled receptors (1, 7, 8, 10, 11, 41) (see also Fig. 4). IP3 binds to IP3 receptor (IP3R), which facilitates Ca2+ release from the SR, raising intracellular Ca2+ concentration in the form of Ca2+ transients (105) that are believed to trigger UBSM contractions (7, 95, 145, 159). Recent reports suggest that BK channels may be functionally linked and regulated by the IP3/IP3Rs in some smooth muscle cells, including urethral smooth muscle (76, 157, 160). It is unknown, however, whether IP3 or Ca2+ release from the IP3Rs activates the BK channels in UBSM to regulate cell excitability and contractility. Intriguingly, a recent study has shown that pharmacological activation of M3 receptors with carbachol induces transient BK channel activation, in the form of large outward BK currents with an amplitude much higher than those of the TBKCs and that this phenomenon is eliminated upon inhibiting IP3Rs with xestospongin-C (114). However, the IP3/IP3R-mediated BK channel regulation in UBSM requires further investigation and confirmation.
UBSM BK Channel Regulation by β-AR/cAMP/PKA Signal Transduction Pathway
Unlike cGMP, cAMP has been demonstrated to play a major role in UBSM relaxation (10). Sympathetic nervous system regulation of UBSM membrane excitability and contractility utilizes β-ARs (10). In UBSM, stimulation of β-ARs increases intracellular cAMP concentration, which activates PKA that, in turn, phosphorylates specific proteins resulting in decreased excitability and UBSM relaxation (10, 21, 46, 103). Convincing recent evidence suggests that BK channel activity increases upon pharmacological activation of β-ARs to promote relaxation of UBSM in various species (21, 60, 73, 121). PKA stimulation has been shown to activate Ca2+ sparks in guinea pig UBSM (121, 154). The latter effect appears to be mediated by PKA-induced phosphorylation of phospholamban (PLB), which, when in a phosphorylated state, activates the SR Ca2+-pump, elevates SR Ca2+ load, and, thus, increases RyR and Ca2+ spark activity (Fig. 3).
UBSM expresses all three known β-AR subtypes (β1–β3) with profound species differences in the β-AR subtype expression patterns (10). β3-ARs appear to be the most physiologically relevant in human UBSM since β3-ARs represent ∼97% of all β-AR mRNA expressed in the human bladder (156). In rat UBSM, which also highly expresses β3-ARs β3-ARs and BK channels are functionally coupled to promote UBSM relaxation by a complex Ca2+-dependent mechanism, which involves an increase in TBKC frequency leading to UBSM cell membrane hyperpolarization and inhibition of UBSM spontaneous phasic contractions and tone (60). In rat and human UBSM, β3-AR agonists effectively inhibit both the spontaneous and nerve-evoked UBSM contractions, an inhibitory effect which is significantly reduced by iberiotoxin, suggesting that functional BK channels play a critical role in this process (4, 5, 60, 137). In contrast, although β3-ARs are expressed at the mRNA level in guinea pig UBSM, they serve a negligible role, if any, in UBSM spontaneous and nerve-evoked contractions without affecting cell excitability (2). Unlike rat UBSM (60), the β3-AR agonist BRL37344 does not affect TBKC and steady-state BK channel activity in guinea pig UBSM (2).
A study has suggested an alternative mechanism, according to which, β2-AR subtype can couple directly with the BK channel pore-forming α-subunit and AKAP79/150, a PKA-anchoring protein, necessary for β2-AR regulation of UBSM function (86). The study has further shown that β2-ARs can simultaneously interact with both BK channels and L-type VDCCs, enabling the assembly of a membrane-localized signal transduction complex in rat UBSM cells (86). The recruitment of L-type VDCCs to this complex provides a critical source of Ca2+ for BK channel activation. This complex can potentially mediate both Ca2+-dependent and phosphorylation-dependent modulation of the BK channels in UBSM (86).
The fundamental role of BK channels in β-AR/cAMP/PKA-mediated UBSM relaxation is exemplified by observations that genetic ablation of the BK channel pore-forming α-subunit in transgenic mouse models leads to a compensatory adaptive upregulation of the β-AR/cAMP/PKA signal transduction pathway (21, 133). Inhibition of the BK channel with iberiotoxin in wild-type (WT) mouse UBSM significantly reduces β-AR-mediated and PKA-mediated UBSM relaxations in response to isoproterenol and forskolin, respectively (21). Paradoxically, UBSM from BK channel α-subunit global KO mice showed enhanced sensitivity to isoproterenol and forskolin (21). These paradoxical effects indicate that compensatory mechanisms in the β-AR/cAMP/PKA signal transduction pathway can, at least partially, overcome the permanent loss of BK channels in β-AR-mediated UBSM relaxation (Fig. 3). One limitation of the BK channel genetic ablation approach is the problem of developmental compensation, in which protein products of other genes functionally substitute for a deleted gene, ultimately resulting in an absence of apparent phenotypic abnormalities. Indeed, genetic deletion of the BK channel pore-forming α-subunit in a global KO mouse model causes PKA overexpression to compensate for the channel loss leading to enhanced sensitivity to isoproterenol and forskolin (21) (Fig. 3). These essential findings have also been confirmed independently by a follow-up study in which a smooth muscle-specific BK channel α-subunit KO mouse has been used (133). β-AR agonists can also modulate K+ conductance in human UBSM (137). As β-ARs, β3-ARs, in particular, and BK channels are functionally coupled at the PKA/RyR level to mediate relaxation of UBSM (Fig. 3) (60, 121), alterations in this functional coupling might be involved in bladder pathophysiology. Collectively, these findings support the concept that selective β3-AR agonists can be very effective in controlling UBSM function (4, 5, 9, 60).
UBSM BK Channel Regulation by Phosphodiesterases
A series of recent studies have revealed a critical role for the phosphodiesterases (PDEs) in the regulation of BK channel activity in UBSM (152–155). Specifically, adenylyl cyclases that synthesize cAMP, and PDEs that hydrolyze cAMP, are constitutively active in UBSM cells, and, therefore, pharmacological inhibition of PDEs can lead to a rapid increase in cellular cAMP, which, in turn, utilizes the PKA signaling pathway, described above, in activating the BK channel activity in a RyR-dependent manner (152–155). There are 11 known PDE isoenzymes, of which five (PDE1–PDE5) have been identified in the human urinary bladder (16, 78, 144). In the human bladder, the level of PDE1 mRNA is the second highest among all PDEs following PDE5 (78). PDE1 and PDE4 appear to have particular functional importance in UBSM. A recent study provides evidence that selective pharmacological inhibition of PDE1 suppresses UBSM excitability and contractility via activation of RyRs and an increase in TBKC frequency (155). A more recent study demonstrates that constitutively active PDE4 modulates spontaneous and nerve-evoked UBSM contractions, and that pharmacological inhibition of PDE4 reduces UBSM contractility by increasing the frequency of Ca2+ sparks and the frequency of the functionally coupled TBKCs, while simultaneously decreasing the global intracellular Ca2+ concentration (154). These novel findings on the roles of PDE1 and PDE4 in UBSM function in the context of the BK channel have laid the foundation for further clinical investigations of the therapeutic potential of selective PDE inhibitors in the treatment of bladder dysfunction.
UBSM BK Channel Regulation by M Receptor and PKC
Bladder function is regulated by parasympathetic nerve fibers releasing ACh, the primary excitatory neurotransmitter in the mammalian bladder, which activates M receptors in UBSM cells, initiating physiological phasic contractions that facilitate voiding (10, 107). UBSM expresses all known M receptors (M1–M5), but predominantly M2 and M3, which couple differentially to G proteins (8, 41, 95). M3 receptors are the main determinants of cholinergic response in UBSM tissue, whereas M2 receptors are considered to be of lesser functional importance (1, 7, 95). In general, M3 receptors increase intracellular Ca2+ by mobilizing phosphoinositides that generate IP3 and 1,2-diacylglycerol (DAG), whereas M2 receptors are negatively coupled to adenylyl cyclase, thus reducing cAMP levels (1, 8, 10, 95). Although the expression of M2 predominates over the M3 subtype, the M3 receptors are primarily responsible for UBSM contraction, whereas the M2 receptor role in UBSM contraction is largely indirect by blocking the relaxant responses to cAMP-coupled receptors such as β-ARs (1, 7, 10, 95).
An early study using elevated intracellular Ca2+ concentrations and the conventional whole cell patch-clamp technique on rat UBSM cells reports BK channel inhibition upon M2 receptor stimulation following an initial transient BK channel activation (104). A more recent study also focusing on rat UBSM cells has identified a primary role of the M3 receptor, rather than M2 receptor, in the BK channel inhibitory effects following muscarinic stimulation (114). Specifically, activation of M3 receptors with carbachol leads to initial appearance of large transient outward BK currents followed by sustained inhibition of the spontaneous TBKCs, spontaneous transient hyperpolarizations, and depolarization of UBSM cell resting membrane potential (114). These large carbachol-induced transient outward BK currents are attributed to the IP3-induced SR Ca2+ release, as they are completely blocked by the IP3 R inhibitor xestospongin-C (114). The IP3-induced SR Ca2+ release transiently activates the BK channels and eventually causes a depletion of SR Ca2+. Depletion of SR Ca2+ upon activation of M3 receptors reduces Ca2+ spark activity, which leads to inhibition of the spontaneous TBKCs and depolarization of UBSM cell membrane potential causing enhancement of L-type VDCC activity and UBSM contractility (Fig. 4). Thus, it has now been clarified that under physiological conditions, M3 receptors, but not M2 receptors, are directly involved in BK channel regulation in rat UBSM cells (114). This mechanism of muscarinic inhibition of BK channels has been recently confirmed to operate in human UBSM as well (112). Collectively, these studies have revealed that M3 receptors are functionally coupled to the BK channels in rat and human UBSM (112, 114) (Fig. 4).
The principal downstream effect of the M3 receptor signaling pathway is the activation of phospholipase C via the α subunits of Gq/11 proteins, subsequently leading to the formation of IP3 and DAG, and the latter, in turn, activates PKC (Fig. 4). Thus, the activated PKC may lead to direct inhibition of the SR Ca2+ pumps and RyRs, resulting in suppression of Ca2+ sparks and the associated TBKCs (Fig. 4). Although BK channel regulation by PKC has been known in other cell types for quite some time (128, 141), it was not until very recently when it was shown that PKC can also regulate BK channels in UBSM (62, 64). Specifically, in guinea pig UBSM cells, PKC inhibits BK channel activity indirectly via a Ca2+-dependent mechanism involving the attenuation of the Ca2+ release through RyRs while increasing the global intracellular Ca2+ necessary to activate UBSM contraction (62). Upon pharmacological activation, PKC could phosphorylate proteins in the SR such as RyRs and/or PLB (Fig. 4). This leads to an inhibition of Ca2+ sparks and the associated TBKCs. BK channel inhibition causes UBSM cell membrane potential depolarization, activation of L-type VDCCs, an increase in the global intracellular Ca2+ concentration, and activation of UBSM contractility (62).
UBSM BK Channel Regulation by Other Mechanisms and Substances
In addition to the major BK channel regulatory mechanisms described above, UBSM BK channels are also regulated by a number of other endogenous and exogenous substances, as well as various intrinsic regulatory pathways. For example, new findings provide evidence that prostaglandin E2 stimulatory effects on UBSM excitability and contractility involve inhibition of TBKC activity (115). It has been suggested that the transcription factor known as nuclear factor of activated T cells (NFATc3) regulates UBSM contractility predominantly through an NFATc3-dependent increase in BK channel activity (82). In guinea pig UBSM, BK channels can potentially be activated by 17β-estradiol, leading to UBSM relaxation, but the mechanism of this BK channel modulation by estrogens requires further investigation (158). A recent study revealed that UBSM BK channels are molecular targets for ethanol at physiologically relevant blood alcohol content levels (87). Specifically, ethanol increases BK channel activity by differential mechanisms involving dependence on intracellular Ca2+ and the basal level of TBKC frequency in UBSM cells (87). Thus, BK channels appear to be important mediators of the alcohol-induced UBSM relaxation that allows for increased urine storage capacity accommodating alcohol-induced diuresis.
Role of BK Channels in UBSM Pathophysiology
Many lower urinary tract symptoms (LUTS), including urinary urgency, frequency, nocturia and incontinence, are directly linked to UBSM dysfunction. Overactive bladder (OAB), which is among the top 10 most prevalent chronic health conditions, affects ∼17% of the Western world population, both men and women, and increases with age (32). The current annual cost of OAB treatment is about $66 billion, and by 2015–2020, it may exceed $76.2–82.6 billion annually in the United States alone (40). The mainstay of OAB treatment is antimuscarinic pharmacotherapy, which is limited in efficacy and tolerability (11, 66). Antimuscarinic drugs cause multiple dose-related side effects, including dry mouth, dry eyes, constipation, and tachycardia (66). Some newer therapies, such as intravesical botulinum toxin, are not only invasive and expensive, but also raise a number of safety concerns (9, 23, 75, 79, 97). An attractive alternative is the selective β3-AR agonist, mirabegron, which has been recently approved to treat OAB, and data indicate that it is well tolerated by patients (9, 25, 34). The recent approval of mirabegron as the first β3-AR agonist for the treatment of OAB further solidifies the importance of β3-ARs in the context of the BK channels as pharmacological targets for bladder dysfunction, as outlined earlier (Fig. 3).
LUTS may also derive from detrusor underactivity for which there is no effective therapy (96, 111, 148). OAB and detrusor underactivity are pressing medical issues that have not been well understood (111, 149). Therefore, there is a significant need to identify novel therapeutic treatments for OAB directly targeting UBSM and with fewer side effects. A critical step for the development of a novel, safe, and more effective therapy for OAB is a better understanding of UBSM ion channels, such as BK channels, which control UBSM excitability and contractility under normal and pathophysiological conditions. Thus, in recent years, major research efforts have been directed toward understanding BK channel roles in UBSM pathophysiology, as well as identifying and validating the BK channels and their regulatory mechanisms as novel therapeutic targets for the treatment of LUTS.
The overall physiological function of the BK channel is to reduce membrane excitability and oppose both myogenic and nerve-evoked human UBSM contractions (5, 58, 61, 77, 89). Disrupting this function would lead to increased UBSM contractility and detrusor overactivity (DO). Indeed, the UBSM phasic contractions can be abnormally increased under pathophysiological conditions of DO and related OAB (10, 11, 15, 18, 24, 56, 67, 93, 109, 119, 140) or completely absent as in the case of detrusor underactivity (96, 111). The importance of BK channels in UBSM excitability and contractility suggests that BK channel defects, alterations, or mutations, also known as channelopathies (13, 117), may cause certain forms of LUTS, including DO and detrusor underactivity. Increasing evidence collected during the past decade suggests that alterations in BK channel expression, function, or regulation have very important implications in bladder pathophysiology. In fact, the BK channels seem to be a very likely candidate involved in some forms of DO etiology. BK channel pharmacological inhibition leads to an increase in UBSM excitability (48, 58). Intriguingly, analogous increases in UBSM excitability are observed during experimentally induced partial bladder outlet obstruction (PBOO) in rats (84). In addition, BK channel inhibition with iberiotoxin, paxilline, or charybdotoxin leads to increased UBSM contractility (21, 35, 37, 51, 56, 58, 60, 61, 65, 109, 119, 134, 152, 153, 155), which resembles UBSM contractile behavior upon PBOO. In agreement with these findings, UBSM cells isolated from rats with experimental PBOO also show decreased mRNA expression of the BK channel pore-forming α-subunit and reduced iberiotoxin-sensitive currents, consistent with decreased BK channel activity (14). Decreased BK channel expression has been reported in patients with benign prostatic hyperplasia (BPH) and associated DO due to the BPH-induced PBOO, as well as in rabbits with PBOO-induced DO (24). Genetic ablation of the BK channel pore-forming α-subunit or regulatory β1-subunit cause increased UBSM contractility (21, 93, 119, 133, 140). Overexpression of the BK channel α-subunit using gene transfer techniques can eliminate DO caused by PBOO in rats (30), an observation consistent with increased UBSM contractility in KO mice lacking BK channel subunits (21, 93, 119, 133, 140). Genetic deletion of the BK channel β1-subunit significantly decreases single BK channel open probability, causing DO (119). In UBSM from a rat model of PBOO, BK channel β4-subunit expression decreases gradually with increasing PBOO severity (70). Under the pathological conditions of PBOO, UBSM exhibits increased phasic contractions as BK channel β4-subunit expression decreases (70).
Long-term PBOO leads to alterations in β-AR expression and, thereby, changes in UBSM responses to adrenergic stimuli (100). Expression of RyRs, which control TBKC activity, is decreased in UBSM from rats with experimental PBOO (67). UBSM cells isolated from RyR type-2 KO mice have decreased TBKC frequency, resulting in sustained membrane potential depolarization and ultimately DO (55). Therefore, changes in BK channel functional coupling among β3-ARs, RyR, and/or PKA may be implicated in the pathophysiology of OAB associated with DO (55, 67).
It is well known that OAB prevalence increases with age (11), and our unpublished observations using human UBSM cells indicate that BK channel expression decreases with age (Petkov G, unpublished data). This is consistent with findings that aging reduces BK channel density in the cell membrane of coronary and corporal smooth muscle cells (36, 142).
A recent study utilizing a multidisciplinary approach demonstrated a decrease in the mRNA expression level of the BK channel pore-forming α-subunit in UBSM from patients with neurogenic DO compared with control patients; along with decreased whole cell BK currents and TBKCs in the UBSM cells from neurogenic DO patients using the perforated patch-clamp approach (56). These molecular and cellular findings are consistent with functional studies showing significantly increased UBSM spontaneous phasic contractions and a lack of effect of iberiotoxin on spontaneous contractility in UBSM strips isolated from patients with neurogenic DO (56). These data point to the novel finding that neurogenic DO is associated with decreased UBSM BK channel expression and function, thus leading to increased UBSM excitability and contractility. In another study, UBSM strips isolated from patients with neurogenic DO, consistently did not respond to iberiotoxin or the BK channel opener NS1619, indicating BK channel dysfunction in these patients (109).
One of the most common complications of diabetes is diabetic bladder dysfunction, which is multifactorial in nature and involves changes in UBSM that lead to DO (42). About 80% of diabetic patients develop a group of LUTS known as diabetic cystopathy (42, 96). The role of BK channels in diabetic bladder dysfunction is controversial. A study has shown increased contractile responses to iberiotoxin in UBSM strips isolated from streptozotocin-induced diabetic rats but no detectable changes in BK channel α- and β1-subunits expression at mRNA level (102). The authors speculated that the differences in UBSM responses to iberiotoxin may be due to changes in the channel biophysical properties or intracellular Ca2+ concentrations, as no changes in BK channel expression levels have been observed in diabetic UBSM (102). A more recent study, which uses patch-clamp electrophysiology, reported reduction of the depolarization-induced steady-state BK current density, as well as the TBKC frequency and amplitude in UBSM cells isolated from streptozotocin-induced diabetic rats compared with control rats (74). However, unlike the previous study (102), the authors found that the mRNA expression for the BK channel α-subunit increased, whereas the expression of the BK channel β1-subunit decreased in diabetes (74). The authors concluded that these changes in BK channel expression and function enhance UBSM excitability leading to DO/OAB (74). A third study that also uses streptozotocin-induced diabetic rats, reported differential effects of BK channel modulators in isolated UBSM strips from control and diabetic rats, suggesting altered BK channel function and/or expression that may contribute to diabetic cystopathy in this rat model (147). It should be noted that the streptozotocin-induced diabetic rat is an animal model for diabetes Type 1, whereas the role of BK channel in diabetic bladder dysfunction due to diabetes Type 2 has not been studied. Therefore, further studies in this area will be beneficial to clarify the role of this physiologically relevant channel directly in patients with diabetes.
UBSM BK Channel Therapeutic Potential for LUTS
The clinical significance of modulating the BK channels for LUTS treatment is twofold. Targeting the BK channels directly, or their regulatory mechanisms that enhance channel activity, will reduce DO and alleviate OAB symptoms. Alternatively, targeting BK channel regulatory mechanisms that reduce the channel activity or direct application of BK channel-selective inhibitors can stimulate UBSM contractility and, thus, have clinical application for the treatment of LUTS due to detrusor underactivity. To facilitate these novel therapeutic approaches, we first need to better understand BK channel expression, function, and regulation in human UBSM under normal and pathophysiological conditions.
The high level of BK channel expression in UBSM cells and the lack of BK channel expression in the plasma membrane of the cardiac cells, along with their unique properties of dual regulation by voltage and Ca2+, have made the BK channels very attractive pharmacological targets for LUTS. It is also important to consider that BK channels appear to be restricted to UBSM cells with no detectable expression in other components of the UBSM layer. Indeed, BK channels are not functionally expressed in the bladder nerves within the bladder wall (150) or in mouse bladder interstitial cells known as platelet-derived growth factor receptor-α (PDGFRα+) cells (83). Penitrem A-sensitive outward currents, with properties characteristic similar to BK currents have been reported in guinea pig detrusor interstitial cells (90). Although a channel with similar properties to the BK channel has been reported in bladder urothelial cells (85), there is no clear evidence or any confirmation for BK channel expression in bladder urothelium, and this subject needs further investigation. Fry's group has reported that in native urothelial cells, there are spontaneous, oscillating intracellular Ca2+ rises (151) and spontaneous surges in ATP release (135). Whether BK channels contribute to the shaping of these spontaneous oscillatory activities in the urothelium remain to be investigated. Another study from Fry's group suggests the absence of functional BK channels in guinea pig bladder trigone based on the lack of Ca2+ responses to iberiotoxin (applied at lower concentrations of 50 nM) in single trigonal smooth muscle cells (122). The BK channel current density in muscularis mucosae is only about 20% of that seen in UBSM (49), further confirming that BK channels are confined primarily to UBSM cells.
A series of studies clearly suggest that BK channel pharmacological activation with channel-selective openers may represent a novel approach for LUTS treatment (22, 35, 61, 63, 65, 77, 81, 101, 129, 130, 132, 139, 146). A recent study using native freshly isolated human UBSM cells and tissues demonstrates that BK channel pharmacological activation with benzimidazolone NS1619 significantly increases the whole-cell BK current, causes membrane potential hyperpolarization, decreases the level of global intracellular Ca2+, and inhibits myogenic and nerve-evoked contractions in native human UBSM (61). In excised membrane patches from human UBSM cells, NS1619 drastically enhances the single BK channel open probability by about 10-fold with the primary effect occurring on the channel gating (89). A more recent study showed that in vivo administration of the BK channel activator, NS8, results in increased storage capacity of the bladder and reverses acetic acid-induced bladder hyperactivity in rats more efficiently than the antimuscarinic drug oxybutynin (77). One concern of using selective BK channel openers for LUTS treatment is the potential vascular effects, as these channels are also expressed in the vascular smooth muscle. Indeed, through use of several different BK channel openers, Malysz et al. (88) report a lack of tissue selectivity when comparing the effects of the openers between UBSM and vascular smooth muscle tissue strips. However, it should be noted that UBSM is functionally different compared with vascular smooth muscle. Unlike arterial smooth muscle, UBSM exhibits spontaneous action potentials and related phasic contractions (45, 46, 48, 120). Also, the physiological role of the BK channel in UBSM is substantially different from the physiological role of the BK channel in arteries. Unlike arterial smooth muscle, UBSM BK channels play a dual role in the repolarization phase of the action potential, while maintaining the resting membrane potential in parallel (48, 58). It is well known that small changes in the UBSM resting membrane potential, regardless of the cause, can have pronounced inhibitory effects on UBSM contractility (61, 113, 116, 120, 131). It is also known that activation of L-type VDCCs is responsible for the initiation of the action potentials in UBSM (44–46, 48). Action potentials work on the “all or none law”, and the process is activated by reaching a threshold. Because of these reasons, small changes in the resting membrane potential under physiological conditions are sufficient to shift the membrane potential below the threshold for L-type VDCC activation, thereby, decreasing the intracellular Ca2+ levels below that which is necessary to activate UBSM contractions. Recently published data on NS1619 are consistent with this mechanism in native human UBSM (61, 89). Accordingly, NS1619 has a modest effect on the membrane potential; however, this level of hyperpolarization is sufficient to substantially reduce the intracellular Ca2+ levels and related spontaneous phasic contractions in native human UBSM (61). Collectively, this supports the concept that BK channel openers, if applied at lower doses, may affect bladder function, while having no or minimal vascular effects. Finally, the UBSM BK channel is unique at expressing both β1 and β4 regulatory subunits, making it distinct from the BK channels in any other tissues (28, 58, 70).
Therefore, further exploration and clinical investigation of specific BK channel openers are warranted, as they represent a promising therapeutic avenue for treating some types of LUTS in humans. Indeed, there are currently ongoing efforts by both academic institutions and the pharmaceutical industry to develop a new class of more potent and selective BK channel openers (22, 106, 123, 124, 139, 146). On the other hand, targeting BK channels with selective inhibitors to stimulate UBSM contractility as a potential treatment for detrusor underactivity is a completely new area of investigation that still remains largely unexplored at a clinical level.
An alternative approach to targeting the BK channels directly with pharmacological modulators is targeting BK channel regulatory mechanisms. In addition to the selective β3-AR agonist mirabegron, PDE1 and PDE4 are also attractive therapeutic targets for the treatment of LUTS due to their critical role in the regulation of cellular cAMP levels in UBSM (152–155). It has been suggested that pharmacological treatment of OAB with selective PDE1 inhibitors could be a better option with fewer possible adverse effects compared with direct pharmacological activation of the BK channels with selective openers (155). Among 24 different human tissues investigated, the PDE4D mRNA level in human bladder is the highest (78). Notably, PDE4 plays only a minor role in controlling human cardiac cellular cAMP levels (68, 98). In addition, different PDE4 isoforms are localized in distinct cellular compartments by binding different scaffold proteins, such as PKA-anchoring proteins, to form a localized signal transduction complex (17). Therefore, drugs targeting bladder-specific PDE4 isoforms may preferentially stimulate cAMP/PKA pathways in UBSM cells, and thus ultimately regulate UBSM function selectively by targeting the BK channel without significant impact on cardiac function.
UBSM BK channel gene therapy represents another potential clinical application for treatment of LUTS (29). A phase I multicenter study evaluating the safety and potential activity of three increasing doses of the human BK channel α-subunit gene transfer in female participants with OAB and DO, a double blind, imbalance placebo controlled design within three sequential active treatments groups has recently been completed (IND13208). The trial has proven that the recombinant BK channel α-subunit gene can be directly and locally instilled in the human bladder to create a UBSM tissue-specific, long-lasting effects without significant transfer-related side effects (92). This gene transfer approach, uses a “naked” nonviral human cDNA and is different from the conventional viral vector-based gene therapy because it targets a bladder-specific problem (OAB/DO), and, therefore, not every cell of the bladder needs to be altered (29). This is a novel approach to relieve OAB/DO resulting from UBSM increased contractility by enhancing the native molecular and cellular mechanisms that elicit UBSM relaxation (30). The “naked” DNA does not integrate into the chromosome of UBSM cells, but instead exerts its action in the nucleoplasm of UBSM cells, most likely without becoming a permanent component of the UBSM cell's DNA (92). One disadvantage of this novel approach is that the “naked” DNA is not permanent and the transfer needs to be repeated about every 6 mo. On the positive side, it should be noted that with the traditional viral vector-based gene transfer approach, some of the problems associated with viral vectors are due to the allergic reaction caused by the virus itself. This is not the case with the “naked” DNA, which is not allergenic. Thus, the BK channel represents a promising novel opportunity for therapeutic intervention in human UBSM for the treatment of OAB associated with DO.
Perspectives and Significance
BK channels control UBSM cell excitability by maintaining the resting membrane potential and the initial repolarization phase of the spontaneous action potentials that trigger UBSM phasic contractions. Altering UBSM BK channel proteins and function have profound effects on urinary bladder physiology. New data collected in recent years suggest that BK channel dysfunction leads to some forms of OAB associated with DO. Thus, BK channels and their regulatory mechanisms represent a novel opportunity for therapeutic intervention in human UBSM. Increasing BK channel expression levels can reduce DO; alternatively, targeting the BK channels with synthetic channel-opening agents represents a new opportunity for pharmacological manipulation of the bladder to reduce DO. A clinical trial examining the effect of local intravesical bladder instillation of a BK channel α-subunit plasmid showed that there were no serious BK channel gene transfer-related adverse events. This further underscores the potential utility of BK channel genetic manipulation for LUTS treatment. However, there is a need to better understand the BK channel regulatory role in human UBSM under normal and pathophysiological conditions using multilevel approaches, including molecular, cellular, and tissue studies and to correlate the basic science findings with patients' clinical phenotypes. Considering the prevalence of OAB/DO and detrusor underactivity, research in this area remains highly significant, as it will have a positive impact on improving health care and will provide novel therapeutic approaches to help a huge population of patients suffering from LUTS.
GRANTS
This study was supported by a grant from the National Institutes of Health R01 DK-084284 to G. V. Petkov.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
Author contributions: G.V.P. conception and design of research; G.V.P. performed experiments; G.V.P. analyzed data; G.V.P. interpreted results of experiments; G.V.P. prepared figures; G.V.P. drafted manuscript; G.V.P. edited and revised manuscript; G.V.P. approved final version of manuscript.
ACKNOWLEDGMENTS
I would like to thank the members of my research group Drs. J. Malysz, K. Hristov, S. Parajuli, W. Xin, Mr. A. Provence, and Mr. V. Fernandes for the critical evaluation of the manuscript.
REFERENCES
- 1.Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, Kay G, Laties A, Nathanson NM, Pasricha PJ, Wein AJ. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol 148: 565–578, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afeli SA, Hristov KL, Petkov GV. Do β3-adrenergic receptors play a role in guinea pig detrusor smooth muscle excitability and contractility? Am J Physiol Renal Physiol 302: F251–F263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afeli SA, Malysz J, Petkov GV. Molecular expression and pharmacological evidence for a functional role of Kv7 channel subtypes in Guinea pig urinary bladder smooth muscle. PLoS One 8: e75875, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afeli SA, Petkov GV. Functional BK channels facilitate the beta3-adrenoceptor agonist-mediated relaxation of nerve-evoked contractions in rat urinary bladder smooth muscle isolated strips. Eur J Pharmacol 711: 50–56, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afeli SA, Rovner ES, Petkov GV. BRL37344, a β3-adrenergic receptor agonist, decreases nerve-evoked contractions in human detrusor smooth muscle isolated strips: role of BK channels. Urology 82: 744 e741–747, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afeli SA, Rovner ES, Petkov GV. SK but not IK channels regulate human detrusor smooth muscle spontaneous and nerve-evoked contractions. Am J Physiol Renal Physiol 303: F559–F568, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An JY, Yun HS, Lee YP, Yang SJ, Shim JO, Jeong JH, Shin CY, Kim JH, Kim DS, Sohn UD. The intracellular pathway of the acetylcholine-induced contraction in cat detrusor muscle cells. Br J Pharmacol 137: 1001–1010, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson KE. Muscarinic acetylcholine receptors in the urinary tract. Hand Exp Pharmacol 202: 319–344, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Andersson KE. New developments in the management of overactive bladder: focus on mirabegron and onabotulinumtoxinA. Ther Clin Risk Manag 9: 161–170, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56: 581–631, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Asano S, Bratz IN, Berwick ZC, Fancher IS, Tune JD, Dick GM. Penitrem A as a tool for understanding the role of large conductance Ca2+/voltage-sensitive K+ channels in vascular function. J Pharmacol Exp Ther 342: 453–460, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashcroft FM. From molecule to malady. Nature 440: 440–447, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Aydin M, Wang HZ, Zhang X, Chua R, Downing K, Melman A, DiSanto ME. Large-conductance calcium-activated potassium channel activity, as determined by whole-cell patch clamp recording, is decreased in urinary bladder smooth muscle cells from male rats with partial urethral obstruction. BJU Int 110: E402–E408, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Baker SA, Hatton WJ, Han J, Hennig GW, Britton FC, Koh SD. Role of TREK-1 potassium channel in bladder overactivity after partial bladder outlet obstruction in mouse. J Urol 183: 793–800, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Bingham J, Sudarsanam S, Srinivasan S. Profiling human phosphodiesterase genes and splice isoforms. Biochem Biophys Res Commun 350: 25–32, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Blackman BE, Horner K, Heidmann J, Wang D, Richter W, Rich TC, Conti M. PDE4D and PDE4B function in distinct subcellular compartments in mouse embryonic fibroblasts. J Biol Chem 286: 12590–12601, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570: 13–22, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci 8: 1752–1759, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. β-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butera JA, Jenkins DJ, Lennox JR, Sheldon JH, Norton NW, Warga D, Argentieri TM. Synthesis and bladder smooth muscle relaxing properties of substituted 3-amino-4-aryl-(and aralkyl-)cyclobut-3-ene-1,2-diones. Bioorg Med Chem Lett 15: 2495–2501, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Chancellor MB, Patel V, Leng WW, Shenot PJ, Lam W, Globe DR, Loeb AL, Chapple CR. OnabotulinumtoxinA improves quality of life in patients with neurogenic detrusor overactivity. Neurology 81: 841–848, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang S, Gomes CM, Hypolite JA, Marx J, Alanzi J, Zderic SA, Malkowicz B, Wein AJ, Chacko S. Detrusor overactivity is associated with downregulation of large-conductance calcium- and voltage-activated potassium channel protein. Am J Physiol Renal Physiol 298: F1416–F1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn 33: 17–30, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Tian LJ, MacDonald SHF, McClafferty H, Hammond MSL, Huibant JM, Ruth P, Knaus HG, Shipston MJ. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) α-subunits generated from a single site of splicing. J Biol Chem 280: 33599–33609, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Kellett WF, Petkov GV. Voltage-gated K+ channels sensitive to stromatoxin-1 regulate myogenic and neurogenic contractions of rat urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 299: R177–R184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory β4 subunit in rat and mouse bladder smooth muscle. J Urol 182: 374–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christ GJ. Potential applications of gene therapy/transfer to the treatment of lower urinary tract diseases/disorders. Hand Exp Pharmacol 202: 255–265, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Christ GJ, Day NS, Day M, Santizo C, Zhao W, Sclafani T, Zinman J, Hsieh K, Venkateswarlu K, Valcic M, Melman A. Bladder injection of “naked” hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am J Physiol Regul Integr Comp Physiol 281: R1699–R1709, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Contreras GF, Castillo K, Enrique N, Carrasquel-Ursulaez W, Castillo JP, Milesi V, Neely A, Alvarez O, Ferreira G, Gonzalez C, Latorre R. A BK (Slo1) channel journey from molecule to physiology. Channels (Austin) 7: 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coyne KS, Sexton CC, Bell JA, Thompson CL, Dmochowski R, Bavendam T, Chen CI, Clemens JQ. The prevalence of lower urinary tract symptoms (LUTS) and overactive bladder (OAB) by racial/ethnic group and age: Results from OAB-POLL. Neurourol Urodyn 32: 230–237, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Creed KE, Ishikawa S, Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol 338: 149–164, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui YS, Zong HT, Yang CC, Yan HL, Zhang Y. The efficacy and safety of mirabegron in treating OAB: a systematic review and meta-analysis of phase III trials. Int Urol Nephrol 46: 275–284, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Darblade B, Behr-Roussel D, Oger S, Hieble JP, Lebret T, Gorny D, Benoit G, Alexandre L, Giuliano F. Effects of potassium channel modulators on human detrusor smooth muscle myogenic phasic contractile activity: potential therapeutic targets for overactive bladder. Urology 68: 442–448, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Davies KP, Stanevsky Y, Tar MT, Chang JS, Chance MR, Melman A. Ageing causes cytoplasmic retention of MaxiK channels in rat corporal smooth muscle cells. Int J Impot Res 19: 371–377, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeFarias FP, Carvalho MF, Lee SH, Kaczorowski GJ, SuarezKurtz G. Effects of the K+ channel blockers paspalitrem-C and paxilline on mammalian smooth muscle. Eur J Pharmacol 314: 123–128, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Fujii K, Foster CD, Brading AF, Parekh AB. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol 99: 779–785, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem 265: 11083–11090, 1990 [PubMed] [Google Scholar]
- 40.Ganz ML, Smalarz AM, Krupski TL, Anger JT, Hu JC, Wittrup-Jensen KU, Pashos CL. Economic costs of overactive bladder in the United States. Urology 75: 526–532, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Giglio D, Tobin G. Muscarinic receptor subtypes in the lower urinary tract. Pharmacology 83: 259–269, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Gomez CS, Kanagarajah P, Gousse AE. Bladder dysfunction in patients with diabetes. Curr Urol Rep 12: 419–426, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, Covarriubias M, Desir GV, Furuichi K, Ganetzky B, Garcia ML, Grissmer S, Jan LY, Karschin A, Kim D, Kuperschmidt S, Kurachi Y, Lazdunski M, Lesage F, Lester HA, McKinnon D, Nichols CG, O'Kelly I, Robbins J, Robertson GA, Rudy B, Sanguinetti M, Seino S, Stuehmer W, Tamkun MM, Vandenberg CA, Wei A, Wulff H, Wymore RS. International Union of Pharmacology. XLI Compendium of Voltage-Gated Ion Channels: Potassium Channels. Pharmacol Rev 55: 583–586, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 140: 159–169, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayase M, Hashitani H, Kohri K, Suzuki H. Role of K+ channels in regulating spontaneous activity in detrusor smooth muscle in situ in the mouse bladder. J Urol 181: 2355–2365, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Heppner TJ, Layne JJ, Pearson JM, Sarkissian H, Nelson MT. Unique properties of muscularis mucosae smooth muscle in guinea pig urinary bladder. Am J Physiol Regul Integr Comp Physiol 301: R351–R362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 289: R402–R409, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C481–C490, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol 541: 483–492, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hotta S, Morimura K, Ohya S, Muraki K, Takeshima H, Imaizumi Y. Ryanodine receptor type 2 deficiency changes excitation-contraction coupling and membrane potential in urinary bladder smooth muscle. J Physiol 582: 489–506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hristov KL, Afeli SA, Parajuli SP, Cheng Q, Rovner ES, Petkov GV. Neurogenic detrusor overactivity is associated with decreased expression and function of the large-conductance voltage- and Ca2+-activated K+ channels. PLoS One 8: e68052, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hristov KL, Chen M, Afeli SA, Cheng Q, Rovner ES, Petkov GV. Expression and function of KV2-containing channels in human urinary bladder smooth muscle. Am J Physiol Cell Physiol 302: C1599–C1608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hristov KL, Chen M, Soder RP, Parajuli SP, Cheng Q, Kellett WF, Petkov GV. KV2.1 and electrically silent KV channel subunits control excitability and contractility of guinea pig detrusor smooth muscle. Am J Physiol Cell Physiol 302: C360–C372, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hristov KL, Parajuli SP, Soder RP, Cheng Q, Rovner ES, Petkov GV. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 302: C1632–C1641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hristov KL, Smith AC, Parajuli SP, Malysz J, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channel regulation by protein kinase C in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 306: C460–C470, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu S, Kim HS. On the mechanism of the differential effects of NS004 and NS1608 in smooth muscle cells from guinea pig bladder. Eur J Pharmacol 318: 461–468, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Hypolite JA, Lei Q, Chang S, Zderic SA, Butler S, Wein AJ, Malykhina AP, Chacko S. Spontaneous and evoked contractions are regulated by PKC-mediated signaling in detrusor smooth muscle: involvement of BK channels. Am J Physiol Renal Physiol 304: F451–F462, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Imai T, Okamoto T, Yamamoto Y, Tanaka H, Koike K, Shigenobu K, Tanaka Y. Effects of different types of K+ channel modulators on the spontaneous myogenic contraction of guinea-pig urinary bladder smooth muscle. Acta Physiol Scand 173: 323–333, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Jayarajan J, Radomski SB. Pharmacotherapy of overactive bladder in adults: a review of efficacy, tolerability, and quality of life. Res Rep Urol 6: 1–16, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang HH, Song B, Lu GS, Wen QJ, Jin XY. Loss of ryanodine receptor calcium-release channel expression associated with overactive urinary bladder smooth muscle contractions in a detrusor instability model. BJU Int 96: 428–433, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Johnson WB, Katugampola S, Able S, Napier C, Harding SE. Profiling of cAMP and cGMP phosphodiesterases in isolated ventricular cardiomyocytes from human hearts: comparison with rat and guinea pig. Life Sci 90: 328–336, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Kellett WF, Petkov GV. Role of Ca2+-activated K+ channel in the neurogenic contractions induced by electrical field stimulation in detrusor smooth muscle isolated from rats and guinea pigs. Biophys J 98: 125a–126a 2010 [Google Scholar]
- 70.Kita M, Yunoki T, Takimoto K, Miyazato M, Kita K, de Groat WC, Kakizaki H, Yoshimura N. Effects of bladder outlet obstruction on properties of Ca2+-activated K+ channels in rat bladder. Am J Physiol Regul Integr Comp Physiol 298: R1310–R1319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klockner U, Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflügers Arch 405: 329–339, 1985 [DOI] [PubMed] [Google Scholar]
- 72.Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB, 3rd, Kaczorowski GJ, Smith M, Garcia ML. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 33: 5819–5828, 1994 [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi H, Adachi-Akahane S, Nagao T. Involvement of BKCa channels in the relaxation of detrusor muscle via β-adrenoceptors. Eur J Pharmacol 404: 231–238, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Kryshtal DA, Paduraru OM, Boldyriev OI, Kit O, Rekalov VV, Shuba Ia M. [Changes in calcium-dependent potassium channels of isolated smooth muscle cells of the bladder in rats with experimental diabetes] Fiziol Zh 57: 25–32, 2011 [PubMed] [Google Scholar]
- 75.Kuo HC, Liu HT, Chuang YC, Birder LA, Chancellor MB. Pilot study of liposome-encapsulated onabotulinumtoxin A for patients with overactive bladder: A single-center study. Eur Urol 65: 1117–1124, 2014 [DOI] [PubMed] [Google Scholar]
- 76.Kyle BD, Bradley E, Large R, Sergeant GP, McHale NG, Thornbury KD, Hollywood MA. Mechanisms underlying activation of transient BK current in rabbit urethral smooth muscle cells and its modulation by IP3-generating agonists. Am J Physiol Cell Physiol 305: C609–C622, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.La Fuente JM, Fernandez A, Cuevas P, Gonzalez-Corrochano R, Chen MX, Angulo J. Stimulation of large-conductance calcium-activated potassium channels inhibits neurogenic contraction of human bladder from patients with urinary symptoms and reverses acetic acid-induced bladder hyperactivity in rats. Eur J Pharmacol 735C: 68–76, 2014 [DOI] [PubMed] [Google Scholar]
- 78.Lakics V, Karran EH, Boess FG. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology 59: 367–374, 2010 [DOI] [PubMed] [Google Scholar]
- 79.Lamin E, Smith AL. Urologic agents for treatment of bladder dysfunction in neurologic disease. Curr Treat Options Neurol 16: 280, 2014 [DOI] [PubMed] [Google Scholar]
- 80.Latorre R, Brauchi S. Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ voltage. Biol Res 39: 385–401, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Layne JJ, Nausch B, Olesen SP, Nelson MT. BK channel activation by NS11021 decreases excitability and contractility of urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 298: R378–R384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Layne JJ, Werner ME, Hill-Eubanks DC, Nelson MT. NFATc3 regulates BK channel function in murine urinary bladder smooth muscle. Am J Physiol Cell Physiol 295: C611–C623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee H, Koh BH, Peri LE, Sanders KM, Koh SD. Functional expression of SK channels in murine detrusor PDGFR+ cells. J Physiol 591: 503–513, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Jiang C, Hao P, Li W, Fan L, Zhou Z, Song B. Changes in T-type calcium channel and its subtypes in overactive detrusor of the rats with partial bladder outflow obstruction. Neurourol Urodyn 26: 870–878, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Li M, Sun Y, Simard JM, Wang JY, Chai TC. Augmented bladder urothelial polyamine signaling and block of BK channel in the pathophysiology of overactive bladder syndrome. Am J Physiol Cell Physiol 297: C1445–C1451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu G, Shi J, Yang L, Cao L, Park SM, Cui J, Marx SO. Assembly of a Ca2+-dependent BK channel signaling complex by binding to β2 adrenergic receptor. EMBO J 23: 2196–2205, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malysz J, Afeli SA, Provence A, Petkov GV. Ethanol-mediated relaxation of guinea pig urinary bladder smooth muscle: involvement of BK and L-type Ca2+ channels. Am J Physiol Cell Physiol 306: C45–C58, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malysz J, Buckner SA, Daza AV, Milicic I, Perez-Medrano A, Gopalakrishnan M. Functional characterization of large conductance calcium-activated K+ channel openers in bladder and vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 369: 481–489, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Malysz J, Rovner ES, Petkov GV. Single-channel biophysical and pharmacological characterizations of native human large-conductance calcium-activated potassium channels in freshly isolated detrusor smooth muscle cells. Pflügers Arch 465: 965–975, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCloskey KD. Characterization of outward currents in interstitial cells from the guinea pig bladder. J Urol 173: 296–301, 2005 [DOI] [PubMed] [Google Scholar]
- 91.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 97: 5562–5567, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. Plasmid-based gene transfer for treatment of erectile dysfunction and overactive bladder: results of a phase I trial. Isr Med Assoc J 9: 143–146, 2007 [PubMed] [Google Scholar]
- 93.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Miller C, Moczydlowski E, Latorre R, Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature 313: 316–318, 1985 [DOI] [PubMed] [Google Scholar]
- 95.Mimata H, Nomura Y, Emoto A, Latifpour J, Wheeler M, Weiss RM. Muscarinic receptor subtypes and receptor-coupled phosphatidylinositol hydrolysis in rat bladder smooth muscle. Int J Urol 4: 591–596, 1997 [DOI] [PubMed] [Google Scholar]
- 96.Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol 15: 11–22, 2013 [PMC free article] [PubMed] [Google Scholar]
- 97.Mohee A, Khan A, Harris N, Eardley I. Long-term outcome of the use of intravesical botulinum toxin for the treatment of overactive bladder (OAB). BJU Int 111: 106–113, 2013 [DOI] [PubMed] [Google Scholar]
- 98.Molenaar P, Christ T, Hussain RI, Engel A, Berk E, Gillette KT, Chen L, Galindo-Tovar A, Krobert KA, Ravens U, Levy FO, Kaumann AJ. PDE3, but not PDE4, reduces β1 - and β2-adrenoceptor-mediated inotropic and lusitropic effects in failing ventricle from metoprolol-treated patients. Br J Pharmacol 169: 528–538, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Molinari EJ, Sullivan JP, Wan Y, Brioni JD, Gopalakrishnan M. Characterization and modulation of [125I]iberiotoxin-D19Y/Y36F binding in the guinea-pig urinary bladder. Eur J Pharmacol 388: 155–161, 2000 [DOI] [PubMed] [Google Scholar]
- 100.Moore CK, Levendusky M, Longhurst PA. Relationship of mass of obstructed rat bladders and responsiveness to adrenergic stimulation. J Urol 168: 1621–1625, 2002 [DOI] [PubMed] [Google Scholar]
- 101.Mora TC, Suarez-Kurtz G. Effects of NS1608, a BKCa channel agonist, on the contractility of guinea-pig urinary bladder in vitro. Br J Pharmacol 144: 636–641, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakahara T, Mitani A, Kubota Y, Maruko T, Sakamoto K, Tanaka Y, Koike K, Shigenobu K, Ishii K. MaxiK channel-triggered negative feedback system is preserved in the urinary bladder smooth muscle from streptozotocin-induced diabetic rats. J Smooth Muscle Res 40: 97–109, 2004 [DOI] [PubMed] [Google Scholar]
- 103.Nakahira Y, Hashitani H, Fukuta H, Sasaki S, Kohri K, Suzuki H. Effects of isoproterenol on spontaneous excitations in detrusor smooth muscle cells of the guinea pig. J Urol 166: 335–340, 2001 [PubMed] [Google Scholar]
- 104.Nakamura T, Kimura J, Yamaguchi O. Muscarinic M2 receptors inhibit Ca2+-activated K+ channels in rat bladder smooth muscle. Int J Urol 9: 689–696, 2002 [DOI] [PubMed] [Google Scholar]
- 105.Narayanan D, Adebiyi A, Jaggar JH. Inositol trisphosphate receptors in smooth muscle cells. Am J Physiol Heart Circ Physiol 302: H2190–H2210, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nardi A, Olesen SP. BK channel modulators: a comprehensive overview. Curr Med Chem 15: 1126–1146, 2008 [DOI] [PubMed] [Google Scholar]
- 107.Nausch B, Heppner TJ, Nelson MT. Nerve-released acetylcholine contracts urinary bladder smooth muscle by inducing action potentials independently of IP3-mediated calcium release. Am J Physiol Regul Integr Comp Physiol 299: R878–R888, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995 [DOI] [PubMed] [Google Scholar]
- 109.Oger S, Behr-Roussel D, Gorny D, Bernabe J, Comperat E, Chartier-Kastler E, Denys P, Giuliano F. Effects of potassium channel modulators on myogenic spontaneous phasic contractile activity in human detrusor from neurogenic patients. BJU Int 108: 604–611, 2011 [DOI] [PubMed] [Google Scholar]
- 110.Ohi Y, Yamamura H, Nagano N, Ohya S, Muraki K, Watanabe M, Imaizumi Y. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J Physiol 534: 313–326, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Osman NI, Chapple CR, Abrams P, Dmochowski R, Haab F, Nitti V, Koelbl H, van Kerrebroeck P, Wein AJ. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol 65: 389–398, 2014 [DOI] [PubMed] [Google Scholar]
- 112.Parajuli SP, Hristov KL, Cheng Q, Malysz J, Rovner ES, Petkov GV. Functional link between muscarinic receptors and large-conductance Ca2+-activated K+ channels in freshly-isolated human detrusor smooth muscle cells. Pflügers Arch In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Parajuli SP, Hristov KL, Soder RP, Kellett WF, Petkov GV. NS309 decreases rat detrusor smooth muscle membrane potential and phasic contractions by activating SK3 channels. Br J Pharmacol 168: 1611–1625, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Parajuli SP, Petkov GV. Activation of muscarinic M3 receptors inhibits large-conductance voltage- and Ca2+-activated K+ channels in rat urinary bladder smooth muscle cells. Am J Physiol Cell Physiol 305: C207–C214, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parajuli SP, Provence A, Petkov GV. Prostaglandin E2 excitatory effects on guinea pig urinary bladder smooth muscle: A novel regulatory mechanism mediated by large-conductance voltage- and Ca2+-activated K+ channels. Eur J Pharmacol 738: 179–185, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parajuli SP, Soder RP, Hristov KL, Petkov GV. Pharmacological activation of small conductance calcium-activated potassium channels with naphtho[1,2-d]thiazol-2-ylamine decreases guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther 340: 114–123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petkov GV. Ion Channels. In: Pharmacology: Principles and Practice, edited by Hacker M, Messer W, Bachmann K. chap. 16, p. 387–427, 2009 [Google Scholar]
- 118.Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. β1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of KATP channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427–R1433, 2001 [DOI] [PubMed] [Google Scholar]
- 121.Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005 [DOI] [PubMed] [Google Scholar]
- 122.Roosen A, Wu C, Sui G, Chowdhury RA, Patel PM, Fry CH. Characteristics of spontaneous activity in the bladder trigone. Eur Urol 56: 346–353, 2009 [DOI] [PubMed] [Google Scholar]
- 123.Roy S, Large RJ, Akande AM, Kshatri A, Webb TI, Domene C, Sergeant GP, McHale NG, Thornbury KD, Hollywood MA. Development of GoSlo-SR-5–69, a potent activator of large-conductance Ca2+-activated K+ (BK) channels. Eur J Med Chem 75: 426–437, 2014 [DOI] [PubMed] [Google Scholar]
- 124.Roy S, Morayo Akande A, Large RJ, Webb TI, Camarasu C, Sergeant GP, McHale NG, Thornbury KD, Hollywood MA. Structure-activity relationships of a novel group of large-conductance Ca2+-activated K+ (BK) channel modulators: the GoSlo-SR family. ChemMedChem 7: 1763–1769, 2012 [DOI] [PubMed] [Google Scholar]
- 125.Saleem F, Rowe ICM, Shipston MJ. Characterization of BK channel splice variants using membrane potential dyes. Br J Pharmacol 156: 143–152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sand C, Michel MC. Bradykinin contracts rat urinary bladder largely independently of phospholipase C. J Pharmacol Exp Ther 348: 25–31, 2014 [DOI] [PubMed] [Google Scholar]
- 127.Sandow SL, Grayson TH. Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol 297: H1–H7, 2009 [DOI] [PubMed] [Google Scholar]
- 128.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci 22: 505–512, 2001 [DOI] [PubMed] [Google Scholar]
- 129.Sheldon JH, Norton NW, Argentieri TM. Inhibition of guinea pig detrusor contraction by NS-1619 is associated with activation of BKCa and inhibition of calcium currents. J Pharmacol Exp Ther 283: 1193–1200, 1997 [PubMed] [Google Scholar]
- 130.Siemer C, Bushfield M, Newgreen D, Grissmer S. Effects of NS1608 on MaxiK channels in smooth muscle cells from urinary bladder. J Membr Biol 173: 57–66, 2000 [DOI] [PubMed] [Google Scholar]
- 131.Soder RP, Parajuli SP, Hristov KL, Rovner ES, Petkov GV. SK channel-selective opening by SKA-31 induces hyperpolarization and decreases contractility in human urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 304: R155–R163, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Soder RP, Petkov GV. Large-conductance Ca2+-activated K+ channel activation with NS1619 decreases myogenic and neurogenic contractions of rat detrusor smooth muscle. Eur J Pharmacol 670: 252–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sprossmann F, Pankert P, Sausbier U, Wirth A, Zhou XB, Madlung J, Zhao H, Bucurenciu I, Jakob A, Lamkemeyer T, Neuhuber W, Offermanns S, Shipston MJ, Korth M, Nordheim A, Ruth P, Sausbier M. Inducible knockout mutagenesis reveals compensatory mechanisms elicited by constitutive BK channel deficiency in overactive murine bladder. FEBS J 276: 1680–1697, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suarez-Kurtz G, Garcia ML, Kaczorowski GJ. Effects of charybdotoxin and iberiotoxin on the spontaneous motility and tonus of different guinea pig smooth muscle tissues. J Pharmacol Exp Ther 259: 439–443, 1991 [PubMed] [Google Scholar]
- 135.Sui G, Fry CH, Montgomery B, Roberts M, Wu R, Wu C. Purinergic and muscarinic modulation of ATP release from the urothelium and its paracrine actions. Am J Physiol Renal Physiol 306: F286–F298, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sui GP, Wu C, Fry CH. The electrophysiological properties of cultured and freshly isolated detrusor smooth muscle cells. J Urol 165: 627–632, 2001 [DOI] [PubMed] [Google Scholar]
- 137.Takemoto J, Masumiya H, Nunoki K, Sato T, Nakagawa H, Ikeda Y, Arai Y, Yanagisawa T. Potentiation of potassium currents by β-adrenoceptor agonists in human urinary bladder smooth muscle cells: a possible electrical mechanism of relaxation. Pharmacology 81: 251–258, 2008 [DOI] [PubMed] [Google Scholar]
- 138.Tanaka M, Sasaki Y, Kimura Y, Fukui T, Hamada K, Ukai Y. A novel pyrrole derivative, NS-8, suppresses the rat micturition reflex by inhibiting afferent pelvic nerve activity. BJU Int 92: 1031–1036, 2003 [DOI] [PubMed] [Google Scholar]
- 139.Tashima T, Toriumi Y, Mochizuki Y, Nonomura T, Nagaoka S, Furukawa K, Tsuru H, Adachi-Akahane S, Ohwada T. Design, synthesis, and BK channel-opening activity of hexahydrodibenzazepinone derivatives. Bioorg Med Chem 14: 8014–8031, 2006 [DOI] [PubMed] [Google Scholar]
- 140.Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 289: F604–F610, 2005 [DOI] [PubMed] [Google Scholar]
- 141.Toro L, Li M, Zhang Z, Singh H, Wu Y, Stefani E. MaxiK channel and cell signalling. Pflügers Arch 466: 875–886, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Toro L, Marijic J, Nishimaru K, Tanaka Y, Song M, Stefani E. Aging, ion channel expression, and vascular function. Vascul Pharmacol 38: 73–80, 2002 [DOI] [PubMed] [Google Scholar]
- 143.Trivedi S, Potterlee L, Li JH, Yasay GD, Russell K, Empfield JR, Trainor DA, Kau ST. Calcium-dependent K-channels in guinea pig and human urinary bladder. Biochem Biophys Res Commun 213: 404–409, 1995 [DOI] [PubMed] [Google Scholar]
- 144.Truss MC, Uckert S, Stief CG, Kuczyk M, Jonas U. Cyclic nucleotide phosphodiesterase (PDE) isoenzymes in the human detrusor smooth muscle. I. Identification and characterization. Urol Res 24: 123–128, 1996 [DOI] [PubMed] [Google Scholar]
- 145.Tugba Durlu-Kandilci N, Brading AF. Intracellular calcium stores in beta-escin skinned rat and guinea-pig bladders. Eur J Pharmacol 566: 172–180, 2007 [DOI] [PubMed] [Google Scholar]
- 146.Turner SC, Carroll WA, White TK, Gopalakrishnan M, Coghlan MJ, Shieh CC, Zhang XF, Parihar AS, Buckner SA, Milicic I, Sullivan JP. The discovery of a new class of large-conductance Ca2+-activated K+ channel opener targeted for overactive bladder: synthesis and structure-activity relationships of 2-amino-4-azaindoles. Bioorg Med Chem Lett 13: 2003–2007, 2003 [DOI] [PubMed] [Google Scholar]
- 147.Vahabi B, Lawson K, McKay NG, Sellers DJ. Phasic activity of urinary bladder smooth muscle in the streptozotocin-induced diabetic rat: Effect of potassium channel modulators. Eur J Pharmacol 660: 431–437, 2011 [DOI] [PubMed] [Google Scholar]
- 148.van Koeveringe GA, Vahabi B, Andersson KE, Kirschner-Herrmans R, Oelke M. Detrusor underactivity: a plea for new approaches to a common bladder dysfunction. Neurourol Urodyn 30: 723–728, 2011 [DOI] [PubMed] [Google Scholar]
- 149.Wein A. What are the barriers and how do we manage them? A review of progress over the last 10 years. Can Urol Assoc J 7: S172–S173, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol 292: R616–R624, 2007 [DOI] [PubMed] [Google Scholar]
- 151.Wu C, Gui GP, Fry CH. Intracellular Ca2+ regulation and electrophysiolgical properties of bladder urothelium subjected to stretch and exogenous agonists. Cell Calcium 49: 395–399, 2011 [DOI] [PubMed] [Google Scholar]
- 152.Xin W, Cheng Q, Soder RP, Petkov GV. Inhibition of phosphodiesterases relaxes detrusor smooth muscle via activation of the large-conductance voltage- and Ca2+-activated K+ channel. Am J Physiol Cell Physiol 302: C1361–C1370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xin W, Cheng Q, Soder RP, Rovner ES, Petkov GV. Constitutively active phosphodiesterase activity regulates urinary bladder smooth muscle function: critical role of KCa1.1 channel. Am J Physiol Renal Physiol 303: F1300–F1306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xin W, Li N, Cheng Q, Petkov GV. BK channel-mediated relaxation of urinary bladder smooth muscle: a novel paradigm for phosphodiesterase type 4 regulation of bladder function. J Pharmacol Exp Ther 349: 56–65, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xin W, Soder RP, Cheng Q, Rovner ES, Petkov GV. Selective inhibition of phosphodiesterase 1 relaxes urinary bladder smooth muscle: role for ryanodine receptor-mediated BK channel activation. Am J Physiol Cell Physiol 303: C1079–C1089, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamaguchi O, Chapple CR. β3-adrenoceptors in urinary bladder. Neurourol Urodyn 26: 752–756, 2007 [DOI] [PubMed] [Google Scholar]
- 157.Yang Y, Li PY, Cheng J, Cai F, Lei M, Tan XQ, Li ML, Liu ZF, Zeng XR. IP3 decreases coronary artery tone via activating the BKCa channel of coronary artery smooth muscle cells in pigs. Biochem Biophys Res Commun 439: 363–368, 2013 [DOI] [PubMed] [Google Scholar]
- 158.Yasay GD, Kau ST, Li JH. Mechanoinhibitory effect of estradiol in guinea pig urinary bladder smooth muscles. Pharmacology 51: 273–280, 1995 [DOI] [PubMed] [Google Scholar]
- 159.Yu W, Sun X, Robson SC, Hill WG. Extracellular UDP enhances P2X-mediated bladder smooth muscle contractility via P2Y6 activation of the phospholipase C/inositol trisphosphate pathway. FASEB J 27: 1895–1903, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao G, Neeb ZP, Leo MD, Pachuau J, Adebiyi A, Ouyang K, Chen J, Jaggar JH. Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells. J Gen Physiol 136: 283–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]