Abstract
Children are uniquely susceptible to ozone because airway and lung growth continue for an extensive period after birth. Early-life exposure of the rhesus monkey to repeated ozone cycles results in region-specific disrupted airway/lung growth, but the mediators and mechanisms are poorly understood. Substance P (SP), neurokinin-1 receptor (NK-1R); and nuclear receptor Nur77 (NR4A1) are signaling pathway components involved in ozone-induced cell death. We hypothesize that acute ozone (AO) exposure during postnatal airway development disrupts SP/NK-1R/Nur77 pathway expression and that these changes correlate with increased ozone-induced cell death. Our objectives were to 1) spatially define the normal development of the SP/NK-1R/Nur77 pathway in conducting airways; 2) compare how postnatal age modulates responses to AO exposure; and 3) determine how concomitant, episodic ozone exposure modifies age-specific acute responses. Male infant rhesus monkeys were assigned at age 1 mo to two age groups, 2 or 6 mo, and then to one of three exposure subgroups: filtered air (FA), FA+AO (AO: 8 h/day × 2 days), or episodic biweekly ozone exposure cycles (EAO: 8 h/day × 5 days/14-day cycle+AO). O3 = 0.5 ppm. We found that 1) ozone increases SP/NK-1R/Nur77 pathway expression in conducting airways, 2) an ozone exposure cycle (5 days/cycle) delivered early at age 2 mo resulted in an airway that was hypersensitive to AO exposure at the end of 2 mo, and 3) continued episodic exposure (11 cycles) resulted in an airway that was hyposensitive to AO exposure at 6 mo. These observations collectively associate with greater overall inflammation and epithelial cell death, particularly in early postnatal (2 mo), distal airways.
Keywords: bronchial epithelium, lung, substance P, neurokinin, NK-1R
nearly 34 million children under 18 years of age live in areas that exceed the 1997 8-h ozone National Ambient Air Quality Standard (2). Ozone disrupts lung development and changes lung functional growth in young adults with a history of ozone exposure as children (18, 26, 45). Children are uniquely susceptible to ozone because airway and lung growth continues for an extensive period (8–12 years) after birth (9, 48). We have shown in rhesus monkeys that early-life exposure to repeated cycles of ozone disrupts normal airway (17) and lung growth (3). Despite these observations, the mediators and mechanisms of persistent ozone-induced disruptions in airway and lung growth remain poorly understood.
Inhalation of ozone results in ciliated and type 1 epithelial cell death with autophagic features, inflammation, and repair (10). Acute inhalation of a sufficient cumulative dose of ozone activates bronchopulmonary C-fibers that initiate respiratory reflexes and release neuropeptides, including substance P (SP), at the site of oxidant stress (20). SP, encoded by the gene TAC1, is the predominant neuropeptide in human airways and is the endogenous ligand for neurokinin receptor NK-1 (NK-1R) (7, 21). When activated, the G protein-coupled receptor NK-1 can initiate nonapoptotic epithelial cell death and subsequent cell proliferation (33).
NK-1R-dependent, ozone-induced nonapoptotic epithelial cell death likely involves a MAPK phosphorylated cascade that activates the orphan nuclear receptor, Nur77 (also known as NR4A1, TR3, and NGFI-B) (43, 45). Although it has been shown in a transformed cell line (HEK293) that NK-1R acts through Nur77 to induce nonapoptotic cell death with autophagic features (8), paradoxically NK-1R has also been shown in multiple cell lines to induce cell proliferation through transactivation of epidermal growth factor receptor via a metalloproteinase-dependent pathway (25).
SP/NK-1R/Nur77 pathways are dependent on the abundance and sources of SP and the expression of NK-1R within the airway epithelium. It is not clear how SP and NK-1R change during early postnatal airway development or whether they are affected by acute, as well as repeated, episodes of ozone exposure during this time period. It is known that as the mammalian lung matures, epithelial nerve development follows a proximal-to-distal progression that lags behind the growth of the airways (42, 46). When infant rhesus monkeys were exposed to 11 repeated episodes of ozone exposure, there was evidence of significantly reduced midlevel-to-distal airway epithelial innervation and increased expression of the neural marker, PGP 9.5, within airway epithelial cells (27). These results suggest that early-life episodic exposure to ozone disrupts the normal development of airway epithelial innervation.
In the present study, we hypothesized that the response of the airway epithelium to an acute ozone exposure changes during postnatal development and that this change correlates with changes in SP/NK-1R/Nur77 pathway expression and activity. In addition, we hypothesized that concurrent episodic exposure during this critical development period further disrupts the normal response of the pathway to an acute ozone insult. To test this hypothesis, three objectives were set: 1) spatially define the normal development of the SP/NK-1R pathway in conducting airways; 2) compare how responses to acute ozone exposure are modulated by postnatal age; and 3) determine how any age-specific acute responses are modified by concomitant, episodic ozone exposure. Rhesus monkeys are used because they have similar lung cell morphology, airway architecture, immunology, and development compared with humans (15, 34, 35, 37).
MATERIALS AND METHODS
Animals and exposure protocol.
All protocols were approved by the University of California-Davis Institutional Animal Care and Use Committee and are in compliance with the Animal Welfare Act and Public Health Service Policy on Humane Care and Use of Laboratory Animals. Animals were treated humanely, and care was taken to minimize and/or alleviate any pain or discomfort.
Colony-born rhesus macaques (Macaca mulatta) from the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited California National Primate Research Center began ozone exposure at 1 mo of age, as previously described (32). The exposure paradigm enabled comparison of responses to acute ozone exposure at two postnatal ages and observation of whether responses were modulated by concomitant, repeated ozone exposure. Twenty-four male, infant rhesus monkeys were assigned at 1 mo of age to two age groups, 2 or 6 mo, and then designated to one of three exposure subgroups: filtered air (FA), FA + acute ozone (O3) challenge (AO), or episodic biweekly O3 exposure cycles + AO challenge (EAO). Thus there were eight animals per exposure group, of which four animals were in the 2-mo age group and four animals in the 6-mo age group.
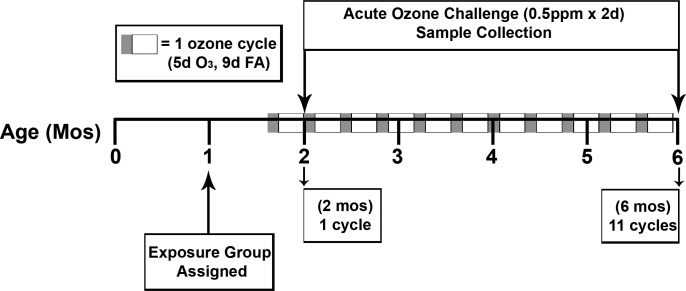
Ozone was monitored with a Dasibi 1003-AH analyzer and generated as previously described (30, 41). The AO exposure protocol consisted of two consecutive, overnight acute O3 exposures (0.5 ppm × 8 h/day × 2 days) just prior to necropsy at 2 or 6 mo of age. The EAO exposure protocol consisted of episodic exposure (0.5 ppm O3 × 8 h/day × 5 days) followed by 9 days of FA, repeated over a 14-day cycle and two consecutive, overnight acute O3 exposures (0.5 ppm × 8 h/day × 2 days) on days 14 and 15 of the last cycle, immediately followed by necropsy at 2 or 6 mo of age. As a result, 2-mo EAO animals underwent one episode of O3 exposure, whereas 6-mo EAO animals underwent 11 episodes of O3 exposure (Fig. 1).
Fig. 1.
Experimental design and exposure regimen. Animals (N = 24, 4/group at each age) were assigned at 1 mo of age to 2 age groups, 2 or 6 mo, and then designated to 1 of 3 exposure subgroups: filtered air (FA), FA + acute ozone (O3) challenge (AO: exposure at 0.5 ppm O3 8 h/day × 2 days), or episodic biweekly O3 exposure cycles + acute O3 challenge (EAO). Each 14-day cycle consisted of 0.5 ppm O3 × 8 h/day × 5 days of exposure, followed by 9 days of filtered air, repeated over a 14-day cycle, and/or 2 consecutive, overnight acute O3 exposures (0.5 ppm × 8 h/day × 2 days) just prior to necropsy at 2 or 6 mo of age. For EAO groups, the 2-day acute overnight challenge took place on days 14 and 15 of the last cycle, immediately followed by necropsy. All exposures took place overnight. EAO 2- and 6-mo animals completed 1 or 11 cycles, respectively.
Airway microdissection and qRT-PCR.
Animals were sedated and deeply anesthetized before being euthanized with an overdose of pentobarbital, as previously reported (31). The right cranial lobe (most proximal to the trachea) was processed, as previously described (32), and inflated with Dulbecco's modified Eagle's medium: Nutrient Mixture F-12 Ham's medium (Sigma) and microdissected on ice. Airway pieces containing intrapulmonary generations ∼5 to 8 (midlevel, ∼2 mm thick) and generations 9 to respiratory bronchioles (distal, ∼1 mm thick) were removed and stored in RNA Later solution (Ambion) at −20°C until being processed for RNA isolation (RNeasy Plus Mini Kit, catalog no. 74134, Qiagen), cDNA generation, and qRT-PCR. TaqMan reagents, probes, and primers were used for both cDNA generation and gene expression via qRT-PCR (Applied BioSystems).
TAC1, NK-1R, and Nur77 (NR4A1) gene expression were measured by qRT-PCR in microdissected airway, whole lobe, and parenchyma pieces, as previously described by the comparative Ct (2−ΔΔCt) method (4, 31). This approach normalizes the data with a calibrator group to allow relevant comparisons for a gene of interest not only within a group but also across ages, exposure regimens, and compartments within an organ. We selected the 2-mo distal airway filtered air animals as the calibrator group for a few reasons: 1) FA is a control group and it makes logical sense to make comparisons of exposed (AO and EAO) vs. matched controls, 2) the distal airway compartment is the least developed/mature of all compartments analyzed, and 3) 2-mo-old animals are less mature than the 6-mo animals. The FA 2-mo distal FA compartment allows changes to be readily understood in the context of 1) stage of region-specific lung development, 2) age-associated developmental changes, 3) effects of ozone exposure, and 4) associative effects of age and/or compartment on responses to ozone exposure.
All samples were prepared with the same concentration of cDNA and run in triplicate with RPL13A as the internal reference gene (sequence: 5′ primer CACGACGTTGGCTGGAAGT, 3′ primer TCTTTCCTCTTCTCCTCCAAGGT and probe CCAGGCAGTGACAGC) (1). The TAC1, NK-1R, and Nur77 (NR4A1) reactions used TaqMan inventoried probe/primer assays (catalog no. Hs00243225_m1, Rh02787596, and Hs00374226_m1, respectively).
Immunohistochemistry and histology staining.
The left caudal lobe was isolated, cannulated, and lavaged with ethidium homodimer-1 (EthD-1) (4 μM) to assess epithelial cell viability. Cells with compromised membranes fluoresce red, indicating necrotic cell death (50). Owing to ethidium lavage of the left caudal lobe, all sections subjected to fluorescent immunohistochemistry (Figs. 3, 4, and 7) show any EthD-1-positive cells in a given field of view. The lobe was fixed in 1% paraformaldehyde at 25 cm of hydrostatic pressure and partially dissected before paraffin embedment to allow visualization of the axial pathway and schematic mapping of airway generations (29). The lobe was then divided into blocks containing two to three airway generations each and then paraffin embedded. Blocks containing defined axial airway generations were sectioned (5 μm). Paraffin sections from three or four animals/treatment group were immunostained for NK-1R [1:650, purified goat polyclonal antibody NK-1R (N-19): sc-5218, Santa Cruz Biotechnology] or myeloperoxidase (MPO: 1:100, purified rabbit polyclonal antibody MPO Ab-1: RB-373-A, Thermo Scientific) with nickel-enhanced 3,3′-diaminobenzidine tetrahydrochloride (Sigma Chemical) as the chromagen. Sections immunostained for MPO were histostained to identify eosinophils (bright red) and mast cells (bright blue) (CEM stain kit, catalog no. KTCEM, American MasterTech). Additional sections were immunolabeled with Nur77 [1:50, purified goat polyclonal antibody Nur77 (N-19): sc-7014, Santa Cruz Biotechnology] or colabeled with SP (1:150, mouse monoclonal antibody SP-DE4-21: ab14184, Abcam) and PGP 9.5 (1:100, purified rabbit polyclonal antibody protein gene product 9.5: P9102-74A, US Biological) with secondary antibody fluorochromes Alexa 488 (1:150, donkey anti-goat A11055, Invitrogen) or Alexa 488 (1:100, goat anti-mouse A11029, Invitrogen) and Alexa 660 (1:100, goat anti-rabbit A21073, Invitrogen), respectively. Antigen retrieval buffer (AR-10 catalog no. HK057-5K, BioGenex) and a decloaking chamber (BioCare Medical) were used for epitope retrieval. Sections from all groups were run together to minimize run-to-run variability (44). Assays included parallel assessment of treated and negative control slides with negative controls subjected to phosphate-buffered saline in place of primary antibody to demonstrate staining specificity. All negative control slides demonstrated an absence of positive staining (data not shown). Images presented in figures are representative of the mean staining pattern of the group and were selected from approximately six to eight slides per age per exposure group. Each slide contained approximately two to six airways.
Fig. 3.
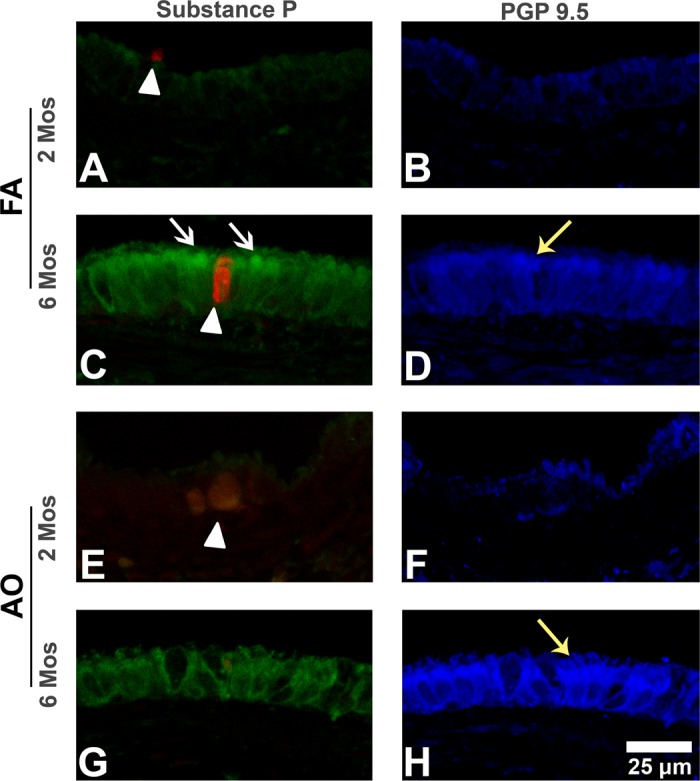
Substance P and protein gene product 9.5 immunohistochemistry (IHC) in midlevel airways. Representative micrographs of sections triple labeled with immunostains substance P (green stain; white arrows) and PGP 9.5 (blue stain; yellow arrows), and cell death marker EthD-1 (red stain; arrowheads) in midlevel airways from FA control 2-mo (A and B) and 6-mo (C and D) and AO-exposed 2 mo (E and F) and 6 mo (G and H) animals. Substance P epithelial peptide expression increases with age (A vs. C). Compared with FA (A, arrowhead), AO at 2 mo increases overall necrotic cell injury (E, arrowhead) but decreases epithelial PGP 9.5-immunopositive cells (B vs. F). At 6 mo, AO decreases substance P epithelial peptide expression (C vs. G, white arrows) and slightly increases epithelial PGP 9.5-immunopositive cells (D vs. H, yellow arrows). EAO images were similar to FA controls (not shown). Representative images selected from ∼4–6 slides/group. Scale bar = 25 μm.
Fig. 4.
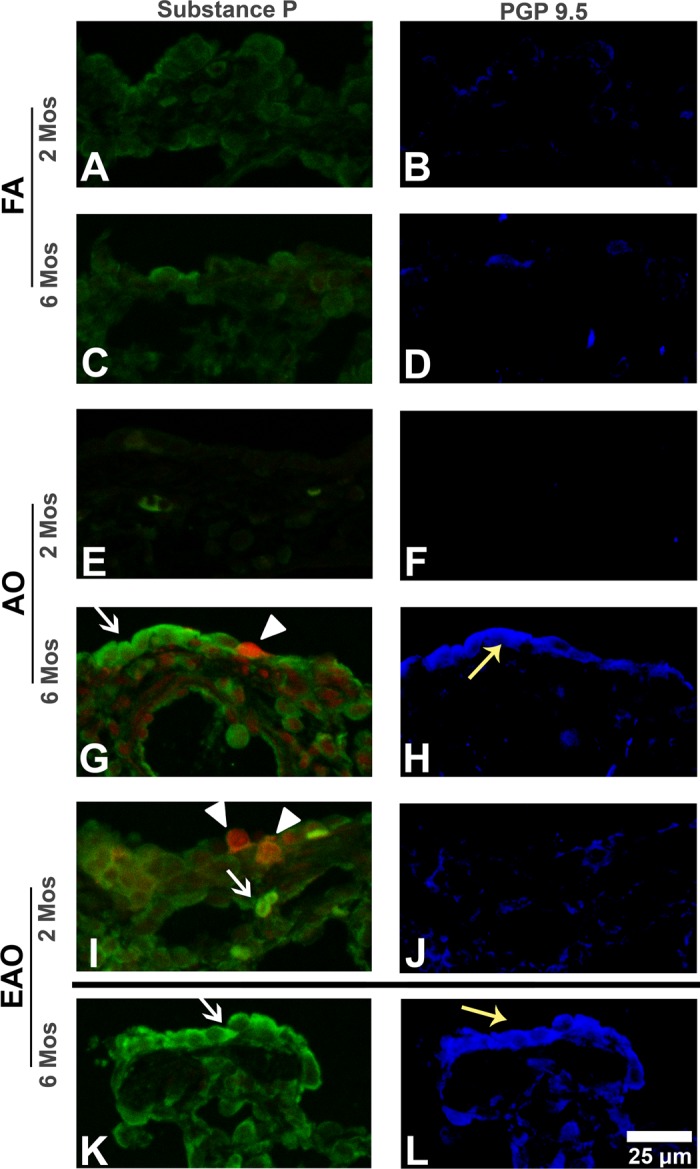
Substance P and protein gene product 9.5 IHC in distal airways. Representative micrographs of sections triple labeled with the immunostains substance P (seen on the apical surface of the epithelium; green stain; white arrows), PGP 9.5 (blue stain; yellow arrows), and cell death marker EthD-1 (red stain; arrowheads) in distal airways from FA control 2-mo (A and B) and 6-mo (C and D), AO-exposed 2-mo (E and F) and 6-mo (G and H), and EAO-exposed 2-mo (I and J) and 6-mo (K and L) animals. Substance P epithelial peptide expression (A vs. C) and PGP 9.5-immunopositive staining increase slightly with age (B vs. D). Compared with FA (A), AO has no effect at 2 mo but markedly increases both substance P (white arrow) and necrotic cell injury (Ethd-1, arrowhead) at 6 mo. Similarly, AO slightly decreases epithelial PGP 9.5-immunopositive staining at 2 mo (F) but markedly increases PGP 9.5 epithelial density at 6 mo (H, yellow arrow). EAO increases epithelial and subepithelial substance P (white arrow) and necrotic cell injury (arrowheads) at 2 mo (A vs. I) and increases substance P at 6 mo (C vs. K). Similarly, EAO slightly increases PGP 9.5-positive subepithelial staining at 2 mo (B vs. J) and markedly at 6 mo (D vs. L, yellow arrow). Representative images selected from ∼4–6 slides/group. Dividing line identifies groups that should not be compared because of differing exposure regimens. Scale bar = 25 μm.
Fig. 7.
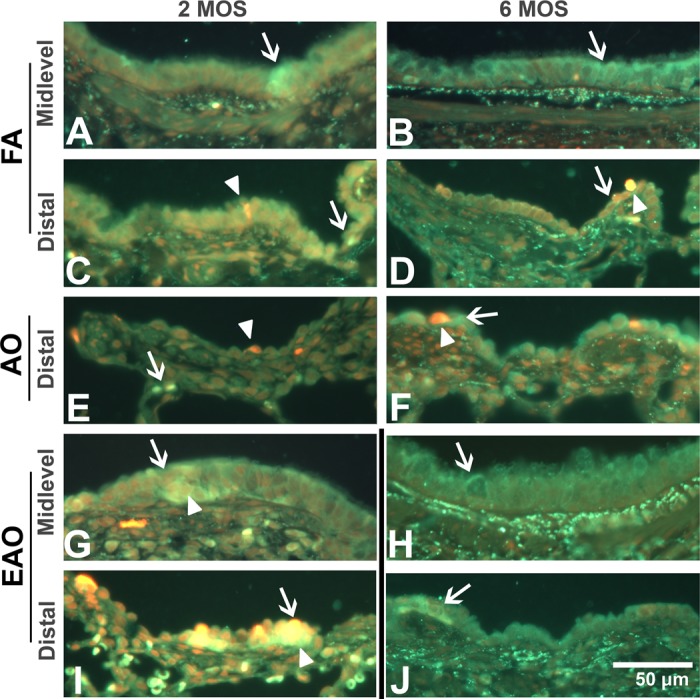
Nur77 receptor protein expression. Representative micrographs of sections double labeled with Nur77 receptor (green stain; arrows) and cell death marker EthD-1 (red stain; arrowheads) from FA control 2-mo midlevel, distal (A and C) and 6-mo midlevel, distal (B and D), AO-exposed 2-mo distal (E) and 6-mo distal (F), and EAO-exposed 2 mo midlevel, distal (G and I) and 6-mo midlevel, distal (H and J) airways. Midlevel (A vs. B) and distal (C vs. D) airway Nur77 receptor immunostaining is comparable in controls at both ages. In distal airways, AO increases subepithelial Nur77 (arrow) at 2 mo (E), epithelial expression at 6 mo (F), and epithelial necrotic cell death (arrowhead) at both ages (E and F). AO has relatively no impact on midlevel airways (not shown). At 2 mo, EAO slightly increases midlevel (G) and distal (I) Nur77 and markedly increases necrotic cell death in both midlevel and distal airways. At 6 mo, EAO slightly increases Nur77 in midlevel (H) and distal (J) epithelia (arrows). Yellow staining indicates colocalization of Nur77 and Ethd-1-positive cells (Fig. 7, G and I). Nur77 in the plasma membrane and cytoplasm and Nur77 in or near the nucleus. Representative images selected from ∼4–6 slides/group. Dividing line identifies groups that should not be compared because of differing exposure regimens. Scale bar = 50 μm.
Microscopy and imaging.
Paraffin sections immunostained with SP and PGP 9.5, and labeled with EthD-1, were imaged by use of a Leica TCS LSI zoom confocal microscope (Leica Microsystems) with excitation lasers at 488, 561, and 635 nm to visualize staining for SP (Alexa 488, Invitrogen), EthD-1, and PGP 9.5 (Alexa 660, Invitrogen), respectively. Histological and other immunostained sections were imaged by light or fluorescent microscopy on an Olympus BH2 microscope (Olympus), equipped with a Leica DFC500 camera (Leica Microsystems) and a ×40 objective.
Statistical analysis.
Within-age comparisons were possible for all exposure groups (e.g., 2-mo FA vs. AO vs. EAO). However, only developmental control (2-mo FA vs. 6-mo FA) and AO-induced (2 mo AO vs. 6 mo AO) trends could be compared across ages owing to identical exposure treatment (i.e., FA or AO just prior to necropsy). Developmental comparison within the EAO-exposure group (2-mo EAO vs. 6-mo EAO) was limited by the cyclic nature of the exposure.
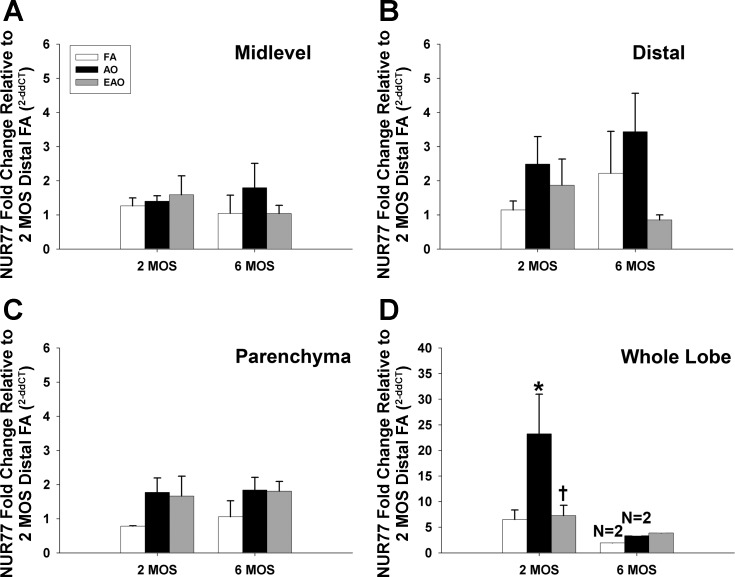
An arbitrary +/− scale was used to illustrate relative qualitative age and treatment differences in gene and protein expression with respect to development or AO/EAO exposure. The following scale designations refer to the number of positive cell clusters per airway cross section: +/− corresponds to 0–2 clusters, + corresponds to 2–3 clusters, and ++ corresponds to 4–5 clusters. Statistically significant changes in gene expression are noted as well. Gene expression in microdissected airways was calculated by the 2−ΔΔCt method, as described previously (28). The N was 3–4 animals per group. Data are expressed as means ± SE, and statistical outliers were eliminated by the extreme studentized deviate method (GraphPad). Multivariate analysis of variance was applied against age, intrapulmonary generation, and exposure factors, when suitable. Fisher's protected least significant difference (PLSD) method was used when multiple comparisons for factors containing more than two levels were performed. Pairwise comparisons were performed individually by using a one-way ANOVA followed by PLSD post hoc analysis with StatView (SAS). P values of ≤ 0.05 were considered statistically significant. Nur77 whole lobe gene expression (Fig. 6D) was excluded from statistical analysis because of lack of sample (N = 2).
Fig. 6.
Nur77 receptor gene expression. Nur77 receptor mRNA expression in midlevel and distal airways (A and B), parenchyma (C), and whole lobe (D) from 2- or 6-mo-old monkeys exposed to FA, AO, or EAO. All mRNA values were analyzed as fold change relative to 2-mo distal FA. Age, AO, and EAO slightly alter distal airway expression at 6 mo, though not significantly (B). AO significantly increases expression at 2 mo in whole lobe (D) compared with age-matched FA (P = 0.0001) and EAO (P = 0.0001) and treatment-matched 6 mo AO (P = 0.0001). Whole lobe 2-mo values are significantly greater than other age-matched compartments for FA [P = 0.05 vs. midlevel (A); P = 0.05 vs. distal (B); P = 0.05 vs. parenchyma (C)], AO [P = 0.0001 vs. midlevel (A); P = 0.0001 vs. distal (B); P = 0.0001 vs. parenchyma (C)], and EAO [P = 0.03 vs. midlevel (A); P = 0.04 vs. distal (B); P = 0.04 vs. parenchyma (C)]. Data are expressed as means ± SE. N = 3–4/group at each age (except whole lobe 6-mo FA and AO where N = 2 due to depleted sample), significance is claimed when P ≤ 0.05. *Greater than age-matched FA group; †less than age-matched AO group.
RESULTS
Gene and protein expression of SP-neurokinin-1 receptor pathway.
Gene expression patterns of key NK-1R pathway components were defined in midlevel (generations 5–8) and distal (generation 9–respiratory bronchioles) conducting airways. For each gene, mRNA values were analyzed as fold change relative to that measured in the distal airways of the 2-mo FA group. Overall, SP/NK-1R/Nur77 pathway gene and protein expression increased with postnatal age (summarized in Table 1). The effects of ozone exposure at 2 or 6 mo of age are summarized in Tables 2 and 3, respectively.
Table 1.
Pattern of expression during development compared with 2 mo
| TAC1/SP | NK-1R | Nur77 | EthD-1 | ||
|---|---|---|---|---|---|
| Midlevel | 2 mo | +/− | + | + | +/− |
| 6 mo | + | ++ | + | +/− | |
| Distal | 2 mo | +/− | +/− | + | + |
| 6 mo | ++ | + | ++ | + | |
| P = 0.04 vs. 2 mo Distal P = 0.03 vs. 6 mo Midlevel |
SP, substance P; NK-1R, neurokinin-1 receptor; EthD-1, ethidium homodimer-1. Note: +/−0–2, +2–3, ++ 4–5 positive clusters per airway cross section. qRT-PCR P values listed when significant.
Table 2.
Results for AO and EAO compared with FA control at 2 mo (see Table 1)
| TAC1/SP | NK-1R | Nur77 | EthD-1 | Inflammation | ||
|---|---|---|---|---|---|---|
| 2 mo | AO | No change | ↑Midlevel | ↑Distal | ↑Distal | ↑Distal†,‡ |
| EAO | ↑Distal | ↑Midlevel | ↑Midlevel* | ↑Midlevel | ↑Midlevel†,‡,§ | |
| ↑Distal | ↑Distal | ↑Distal†,‡ |
AO, acute O3; EAO, episodic + acute O3; FA, filtered air.
Change reflected in protein expression only. †, ‡, §: Presence of polymorphonuclear cells (PMN, †), eosinophils (‡), or mast cells (§).
Table 3.
Results for AO and EAO compared with FA control at 6 mo (see Table 1)
| TAC1/SP | NK-1R | Nur77 | EthD-1 | Inflammation | ||
|---|---|---|---|---|---|---|
| 6 mo | AO | ↑Distal | ↑Midlevel | ↑Distal | ↑Distal | ↑Distal†,‡ |
| P = 0.0001 vs. 2 mo Distal | P = 0.05 vs. FA | |||||
| P = 0.0004 vs. 6 mo Midlevel | P = 0.03 vs. EAO | |||||
| ↑Distal | ||||||
| EAO | ↓ Distal | ↑Midlevel* | ↑Distal* | No Change | ↑Midlevel†,‡,§ | |
| P = 0.02 vs. FA | ||||||
| P = 0.0001 vs. AO | ↑Distal†,‡ | |||||
| ↑Distal* |
Change reflected in protein expression only. Underline: differs from matched 2 mo. qRT-PCR P values listed when significant. †, ‡, §: Presence of polymorphonuclear cells (PMN, †), eosinophils (‡), or mast cells (§).
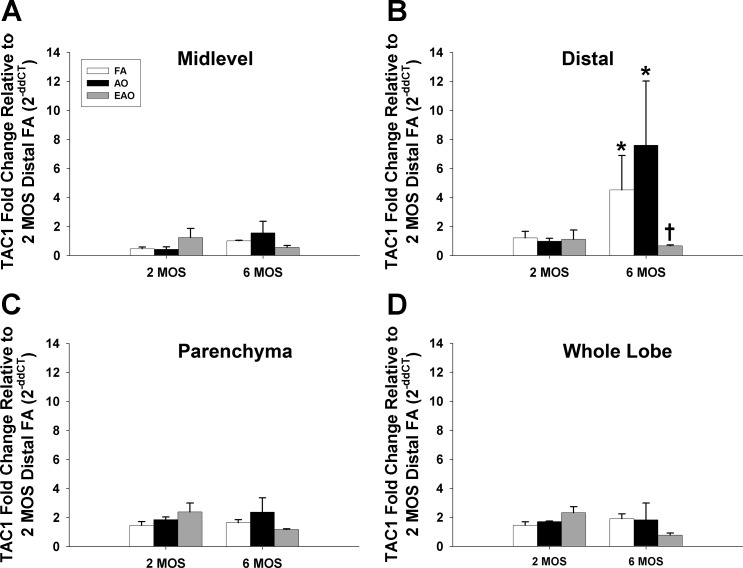
SP precursor peptide TAC1.
The gene encoding protachykinin-1 (SP precursor peptide) TAC1 was used as a surrogate to identify transcriptional changes in nonneural airway SP. Age and ozone exposure drove significant differences in TAC1 mRNA expression found in the distal conducting airways. As FA control animals aged, TAC1 gene expression significantly increased (Table 1, Fig. 2B; 2 mo vs. 6 mo). Animals that underwent AO exposure also had significantly increased TAC1 gene expression in the distal airways at 6 mo compared with 2 mo (Table 3, Fig. 2B). EAO exposure significantly stunted distal mRNA levels at 6 mo compared with FA and AO exposures (Table 2, Fig. 2B).
Fig. 2.
TAC1 gene expression. Substance P precursor gene TAC1 mRNA expression in midlevel (A) and distal (B) airway, parenchyma (C), and whole lobe (D) compartments from 2- or 6-mo-old monkeys exposed to FA, AO, or EAO. All mRNA values were analyzed as fold change relative to 2-mo distal FA. Age and compartment significantly increase distal airway expression compared with age-matched midlevel (FA P = 0.03) and compartment-matched 2-mo (FA P = 0.04), and AO enhances this trend at 6 mo (P = 0.0004 vs. AO midlevel; P = 0.0001 vs. 2 mo AO). EAO significantly decreases 6-mo distal expression compared with age-matched FA (P = 0.02) and AO (P = 0.0001) groups. Data are expressed as means ± SE. N = 3–4/group at each age; significance is claimed when P < 0.05 (*greater than treatment matched 2 mo group; †less than age-matched FA and AO groups).
SP and PGP 9.5.
In control animals exposed to FA, the density of immunopositive cells for SP peptide (Fig. 3, A and C) and protein gene product 9.5 (PGP 9.5) (Fig. 3, B and D) increased with age in midlevel airways (Table 1). AO exposure resulted in a similar distribution and relative abundance of SP- and PGP 9.5-positive cells in midlevel airways (Tables 2 and 3, Fig. 3, E–H). In contrast, in the distal airways, AO exposure appeared to increase both SP and PGP 9.5 immunostaining at 6 mo of age compared with 2 mo of age (Tables 2 and 3, Fig. 4, E–H). EAO did not impact SP peptide expression in midlevel airway epithelia (data not shown). In distal airways, EAO exposure enhanced the density of both SP- and PGP 9.5-immunopositive cells at 6 mo compared with 2 mo (Tables 2 and 3, Fig. 4, I–L).
The relative abundance of Ethd-1-positive necrotic cells per airway observed was increased (qualitatively assessed similarly to immunohistochemistry data) in the distal airways at 6 mo with AO exposure (Table 3, Fig. 4G) and at 2 mo with EAO exposure (Table 2, Fig. 4I) compared with age-matched FA control and the earlier (for AO) or later (for EAO) exposure time point (see Nur77 and EthD-1).
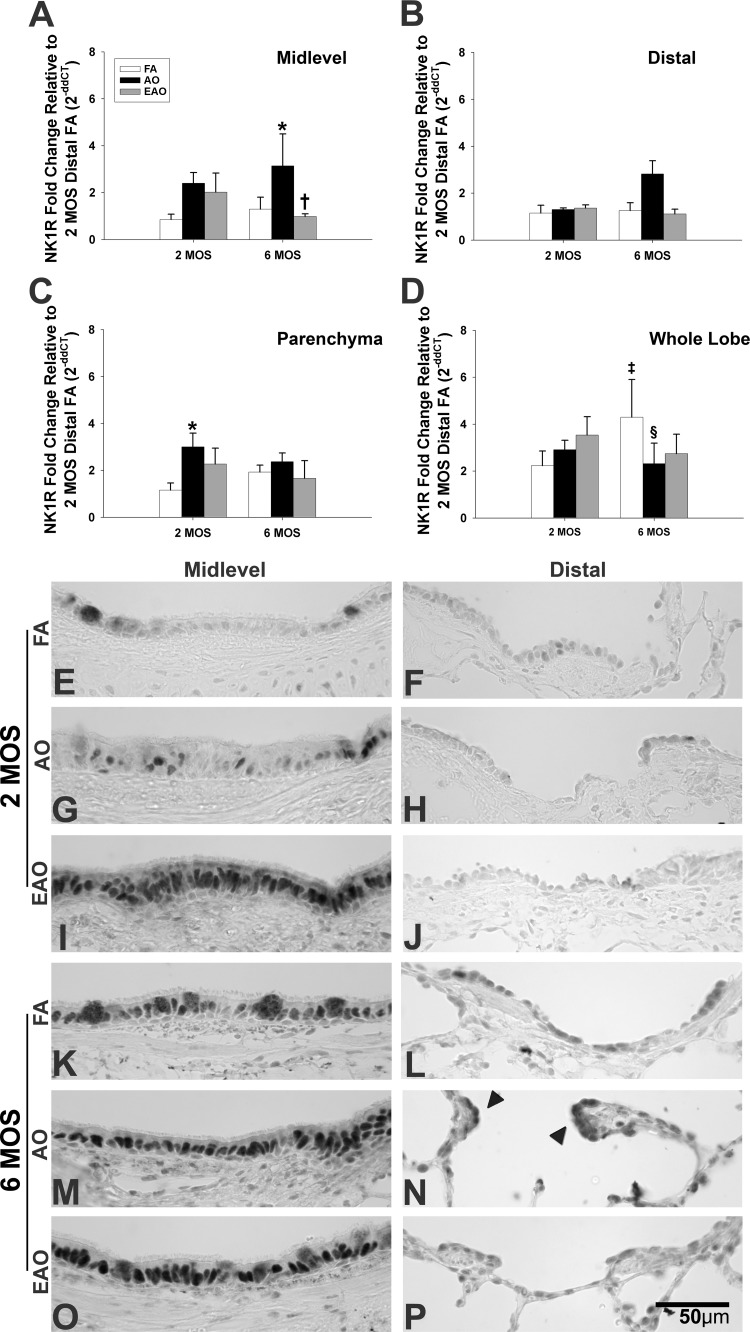
NK-1R.
AO exposure caused NK-1R gene expression to significantly increase in the midlevel airways (Table 3, Fig. 5A) at 6 mo and parenchyma at 2 mo (Table 2, Fig. 5C) compared with the age-matched FA control. EAO exposure was associated with a significant reduction in NK-1R gene expression in the midlevel airways compared with AO exposure (Table 3, Fig. 5A) at 6 mo. Neither age nor exposure regimen significantly altered distal NK-1R mRNA levels (Fig. 5B). NK-1R gene expression was significantly increased in the whole lung lobe with normal lung maturation (Fig. 5D, FA 2 mo vs. FA 6 mo) but AO caused NK-1R mRNA levels to significantly decrease at 6 mo compared with the FA control (Fig. 5D).
Fig. 5.
Neurokinin-1 receptor (NK-1R) expression. NK-1R mRNA expression in midlevel and distal airways (A and B), parenchyma (C), and whole lobe (D) from 2- or 6-mo-old monkeys exposed to FA, AO, or EAO. All mRNA values were analyzed as fold change relative to 2-mo distal FA. AO significantly increases midlevel (A) 6-mo expression compared with age-matched FA (P = 0.05) and EAO (P = 0.03) animals and increases parenchyma (C) 2-mo expression compared with age-matched FA (P = 0.05). Age and compartment significantly increase 6-mo whole lobe (D) expression [P = 0.03 vs. 2 mo; P = 0.002 vs. midlevel (A); P = 0.002 vs. distal (B); P = 0.02 vs. parenchyma (C)]. AO significantly decreases this trend at 6 mo in whole lobe (P = 0.04). Representative micrographs of NK-1R immunostained (seen in plasma membrane and cytoplasm) midlevel and distal airways from FA control 2-mo (E and F) and 6-mo (K and L), AO-exposed 2-mo (G and H) and 6-mo (M and N), and EAO-exposed 2-mo (I and J) and 6-mo (O and P) animals. Compared with 2 mo, airway NK-1R immunostaining is greater at 6 mo. AO and EAO increase NK-1R expression in midlevel airways at both ages relative to age-matched FA and selectively in AO-exposed, 6-mo distal airways (N, arrowheads). Data are expressed as means ± SE. N = 3–4/group at each age; significance is claimed when P ≤ 0.05. ‡Greater than treatment matched 2 mo group; *greater than age-matched FA group; §less than age-matched FA group; †less than age-matched AO group. Representative images selected from ∼4–6 slides/group. Scale bar = 50 μm.
NK-1R protein expression increased with age during normal development (FA controls), with a greater number of immunopositive epithelial cells in both midlevel (Table 1, Fig. 5, E and K) and distal airways (Table 1, Fig. 5, F and L). Midlevel airways consistently had more pronounced staining than distal airways. Both AO and EAO exposure increased NK-1R protein expression in 2-mo midlevel epithelium with EAO driving the more robust increase (Table 2, Fig. 5, G and I). Similarly, AO and EAO enhanced the developmental increase of NK-1R protein in 6-mo midlevel epithelium (Table 3, Fig. 5, M and O) compared with the age-matched control (Fig. 5K). Distal NK-1R protein appeared unaffected by O3 exposure at 2 mo (Table 2, Fig. 5, H and J) but notably increased with AO at the 6-mo time (Table 3, Fig. 5, N and P).
Nur77 and EthD-1.
Similar to NK-1R gene expression, Nur77 did not change with normal aging in the lower airways of the control animals (Table 1, Fig. 6). Age and exposure regimen had no significant effects on mRNA levels in midlevel and distal airway or parenchyma compartments (Fig. 6, A–C). AO exposure resulted in whole lobe Nur77 gene expression being significantly increased at 2 mo compared with FA exposure (Table 2, Fig. 6D). In contrast, EAO exposure resulted in Nur77 mRNA whole lobe levels being significantly reduced compared with the AO exposure at 2 mo (Fig. 6D). Changes in whole lobe mRNA levels that were not reflected in the other compartments we specifically evaluated may be attributed to robust responses in proximal airways. Whole lobe samples were collected from a cross section of tissue containing conducting airway (proximal, midlevel, and distal) and parenchyma regions with the larger, proximal airways comprising the greatest area of airways sampled. Any notable changes in proximal airways would bias whole lobe mRNA levels toward those observed in proximal regions, potentially diluting changes in gene expression observed in more distal regions.
Nur77 protein expression appeared to slightly increase in the epithelium of midlevel airways with age, as seen in the FA control animals (Table 1, Fig. 7, A and B); however, age did not alter the expression or distribution of Nur77 in the distal airways (Fig. 7, C and D). AO exposure had relatively no impact on Nur77 protein expression in midlevel airways (data not shown). In distal airways, AO exposure increased Nur77 protein expression in the subepithelium at 2 mo (Table 2, Fig. 7E, arrows) and epithelium at 6 mo (Table 3, Fig. 7F) compared with age-matched FA controls. EAO exposure increased Nur77 protein expression at 2 mo in midlevel (Table 2, Fig. 7G) and distal (Table 2, Fig. 7I) airways. EAO exposure only slightly increased Nur77 in 6-mo distal (Table 3, Fig. 7J) airways, where it had shifted from the typical epithelial apex to a basolateral arrangement following repeat ozone exposure.
Overall necrotic cell death, as indicated by Ethd-1 incorporation into compromised cell membranes, was characterized in FA control animals by sparsely distributed single positive cells throughout the midlevel airway epithelium and slightly more individual cells in distal airway epithelium, with age having no influence in distribution or abundance (Fig. 7, A–D). AO and EAO induced similar changes to those observed in similar sections presented in Fig. 4. Colocalization of Nur77 and Ethd-1 is indicated by yellow staining (Fig. 7, G and I).
Inflammation.
Both midlevel and distal airway sections were stained immunohistochemically for MPO to indicate neutrophils, and histologically to denote the presence of mast cells or eosinophils (Fig. 8). Resident mast cells (blue stain, arrowheads) and a few eosinophils (red stain, blue arrows) were present in the subepithelial airway zone of FA midlevel (Fig. 8, A and B) and distal (Fig. 8, C and D) airways at both 2 and 6 mo. AO exposure had no significant impact on midlevel airways (not shown). However, AO exposure markedly increased the presence of MPO-positive cells (black arrows), indicating neutrophil influx, in the epithelium and subepithelium of the distal airways at 2 mo (Table 2, Fig. 8E) and the epithelium of distal 6 mo (Table 3, Fig. 8F) animals compared with age-matched FA controls. A slight increase in epithelial and subepithelial eosinophils (blue arrows) was found with AO exposure in distal airways (Fig. 8, E and F). EAO exposure increased the overall presence of innate inflammatory cells compared with age-matched FA controls, with neutrophils, mast cells and eosinophils in the subepithelium of 2 mo midlevel airways (Table 2, Fig. 8G). EAO exposure also increased the three inflammatory infiltrates, especially neutrophils, in midlevel airway epithelium at 6 mo (Table 3, Fig. 8H) compared with age-matched controls. The inflammatory response in the distal airways following EAO exposure was less pronounced compared with AO exposure. Subepithelial eosinophils were slightly increased at 2 mo (Fig. 8I) and 6 mo (Fig. 8J). EAO exposure also slightly increased epithelial neutrophils in the distal airways at 6 mo (Fig. 8J).
Fig. 8.
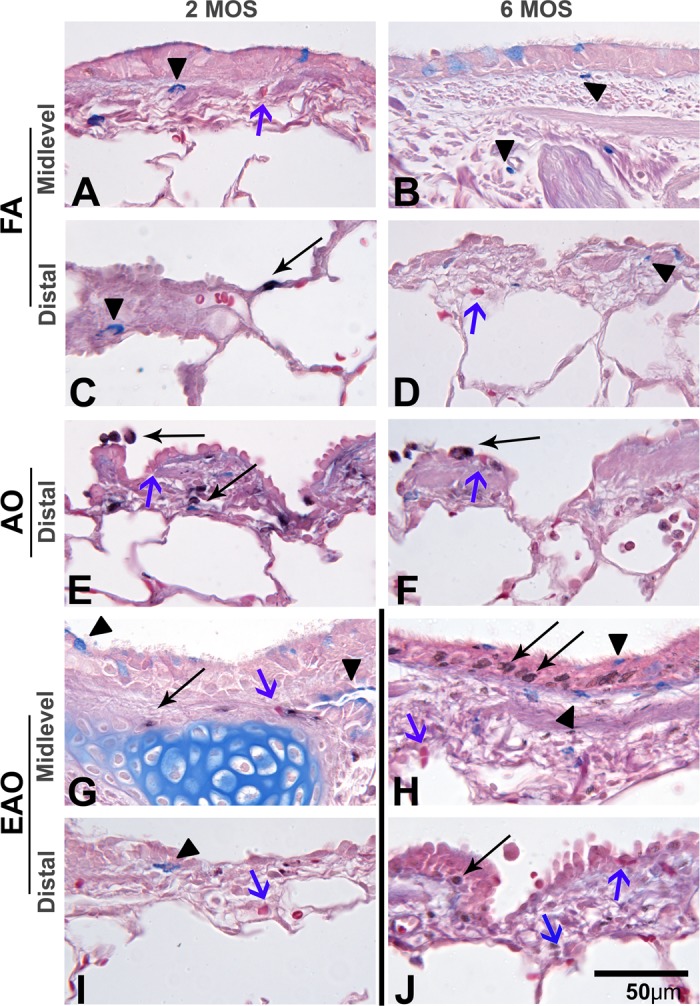
Leukocyte infiltration. Representative micrographs of midlevel and distal airway sections evaluated for relative presence of mast cells (bright blue; arrowheads), eosinophils (bright red; blue arrows), and myeloperoxidase-positive- neutrophils (black; thin arrows) from FA control 2-mo midlevel, distal (A and C) and 6-mo midlevel, distal (B and D), AO-exposed 2-mo distal (E) and 6-mo distal (F) and EAO-exposed 2-mo midlevel, distal (G and I) and 6-mo midlevel, distal (H and J) airways. AO midlevel images were similar to FA controls (not shown). AO increases the presence of periepithelial eosinophils (blue arrows) and epithelial neutrophils (thin arrows) in distal airways (E and F). EAO enhances the presence of periepithelial neutrophils, eosinophils, and mast cells in both midlevel (G and H) and distal airways (I and J) with a more pronounced neutrophilic influx in 6-mo midlevel airway epithelia (H). Representative images selected from ∼4–6 slides/group. Dividing line identifies groups that should not be compared because of differing exposure regimens. Scale bar = 50 μm.
DISCUSSION
Ozone inhalation during postnatal lung development is known to cause long-term changes to the lung. The pattern of how early life exposure affects exposure outcomes during critical periods of airway growth is not well understood. In this study, we examined the spatial distribution of the SP/NK-1R/Nur77 pathway in conducting airway epithelium at two early postnatal ages in rhesus monkeys and how 1) acute ozone exposure impacts this spatial distribution and 2) episodic exposure, in tandem with the development process, modifies this spatial distribution and subsequent ozone responses. We observed that the SP/NK-1R/Nur77 pathway distribution changes with the developing airway epithelium. Individual constituent gene and protein expression is modulated by age, lung compartment, and type of ozone exposure regimen. These findings collectively suggest 1) that the SP/NK-1R/Nur77 pathway closely associates with changes in airway response to an acute ozone exposure and 2) that repeat, episodic ozone exposure occurring in tandem with acute ozone challenge disrupts the airway's set response to the principal, acute insult.
Ozone exposure during a susceptible and dynamic lung growth period (e.g., 6 mo of age) disrupts neurokinin-mediated cellular processes, particularly in the conducting airway epithelium (31). Because the lung grows and matures in a proximal-to-distal lung direction, we took care to define cellular responses in specific lung regions. We found that ozone increases SP/NK-1R/Nur77 pathway expression in conducting airways and overall inflammation and epithelial cell death in distal airways. In addition, we found that an episode of ozone exposure (5 days/cycle) delivered early in the second month of life resulted in an airway that was hypersensitive to acute ozone exposure at the end of the second month of life and that continued episodic exposure (11 cycles) resulted in an airway that was hyposensitive to acute ozone exposure at 6 mo.
Prior work conducted by our laboratory and others in the same monkey model used here has determined that the early postnatal period (0–6 mo) represents a distinct window of increased susceptibility to ozone-induced effects, including airway remodeling (24, 27, 32) and altered neuromediated ligand receptor interactions (5, 17, 30). We selected the 0.5 ppm ozone dose based on our previous research because it elicits consistent responses across a heterogeneous primate population. Although this dose exceeds the 1-h peak U.S. ozone standard (0.12 ppm) by fourfold (14), it represents a real-world exposure that may be encountered on a high-ozone day in heavily polluted metropolitan areas, including Mexico City (40). As previously described, our regimen of 5 days of O3 followed by 9 days of FA mimics the scenario in which unhealthy levels of ozone occur for several consecutive days followed by several days within attainment standards (31, 32).
Oslund and colleagues (33) have previously demonstrated in rats that NK-1 receptors are activated following an acute ozone exposure (1 ppm × 8 h) and that activated NK-1 receptors participate in the epithelial injury and repair cycle, including cell proliferation. When using airway explants from episodic-ozone exposed postnatal monkeys subjected to in vitro oxidant challenge with lipid ozonide, we found upregulation in both NK-1R gene and protein expression (31). Thus we surmised that the SP/NK-1R/Nur77 pathway contributes to the intrinsic ability of the epithelial mesenchymal trophic unit (EMTU) to respond to an oxidant challenge. The EMTU is defined as being composed of highly interactive structural components, with inherent signaling, neuromodulatory, muscular, and cellular capabilities (16). Here we built on this previous research by examining the expression pattern of both upstream and downstream components of the SP/NK-1R/Nur77 pathway in a structurally and functionally relevant model of childhood development under severe, but real-world, poor air quality conditions.
SP/NK-1R/Nur77 pathway expression is increased during the postnatal period.
Overall, SP/NK-1R/Nur77 pathway gene and protein expression increased with development. We observed an increase in SP-immunopositive epithelial cells in both midlevel and distal conducting airways with increasing postnatal age. This suggests that the epithelium acts as an alternate depot for SP in the rhesus monkey airway during the postnatal period studied and may play a direct role in NK-1R activation and normal airway proliferation and repair processes. NK-1R, a plasma membrane G protein-coupled receptor, is activated upon SP binding. The SP/NK-1R complex is important during development (24, 26, 40, 52, 58) and acute airway injury because it can initiate nonapoptotic epithelial cell death (via a Nur77-dependent pathway) and subsequent cell proliferation, ultimately helping to stabilize the airway. The importance of the SP/NK-1R/Nur77 pathway in stabilizing the airway during acute injury is illustrated by numerous studies that show exacerbated airway injury and inflammatory responses when this pathway is disrupted (13, 39, 43, 49).
Postnatal age and conducting airway region drive acute ozone response.
Acute ozone inhalation induces site-specific necrotic cell death, particularly in ciliated airway epithelial cells (5, 36, 38). Injured and necrotic cells release various inflammatory mediators, including chemokines and cytokines, that induce neutrophil and eosinophil responses (10). We observed that necrotic cell death associates with neutrophil and eosinophil influx in the distal airways of 2- and 6-mo-old rhesus monkeys following acute ozone exposure (Figs. 7 and 8). The distal columnar epithelium of AO-exposed airways was disrupted, with individual epithelial cells appearing less defined and more squamated or flattened, particularly at 2 mo, suggesting site-specific ozone-induced disruption (Fig. 6, E and F). Plopper and colleagues (36) observed similar irregularities in 3-yr-old monkeys acutely exposed to ozone (0.4 ppm × 2 h) with epithelial cell loss and increased inflammatory cell infiltration.
In parallel with necrosis, acute ozone inhalation increased Nur77 gene and protein expression, a modulator of programmed cell death (11), in distal airways at both ages and 2-mo whole lobe mRNA levels (more than 20-fold), compared with age-matched distal expression (Fig. 6). Acute ozone inhalation shifted Nur77 protein nuclear distribution to the subepithelial compartment of 2-mo distal airways, whereas at 6 mo the pattern was more focal and exclusively within the epithelium. The role of Nur77 in cell fate is associated with its location in the cell. Nuclear retention of Nur77 is often associated with cell survival, whereas translocation to intracellular organelles, including mitochondria, leads to cell death (51).
Age coupled with airway level strongly influenced the degree and direction of acute ozone exposure response, with the greatest impact observed in distal airways of 6-mo-old animals (TAC1/SP, NK-1R) compared with acute ozone exposure in 2-mo-old (NK-1R) and age-matched controls. Shifts in SP epithelial or subepithelial density that mirror changes in acute inflammatory influx, such as those observed in distal airways of 2-mo-old animals following acute ozone exposure (Figs. 4 and 8), indicate that nonneural SP may participate in the oxidant stress-induced inflammatory response.
Acute ozone exposure significantly increased both gene and protein NK-1R expression in 6-mo airways, implying an acute, robust upregulation of the NK-1R pathway. NK-1R protein expression was a mixture of both cytoplasmic and perinuclear presentations, illustrating both unoccupied NK-1 receptors and a spectrum of possibly liganded SP-NK-1R complexes in a variety of endocytotic stages (19).
Factors driving postepisodic responses to acute challenge are multifactorial.
Acute ozone challenge, coupled with a history of episodic exposure, significantly deviates the normal developmental SP/NK-1R/Nur77 pathway pattern during postnatal months 1–6. Both midlevel and distal conducting airways were impacted, with the latter being the most disrupted. When SP binds to NK-1R at the cell surface, the SP-NK-1R complex formed becomes enclosed in a vesicle and is then transferred to a perinuclear, acidified endosome where SP is degraded and the NK-1R is recycled and returned to the cell surface (19). Variables, including age and relative abundance of ligand to receptor, may dictate whether the SP-NK-1R complex is internalized and degraded. These factors may explain why SP-positive cells were observed in the subepithelial compartment of EAO-exposed 2-mo (Fig. 4I) and the epithelial compartment of EAO-exposed 6-mo distal airways (Fig. 4, G and K). The lack of robust EAO NK-1R gene expression is likely attributed to the episodic nature of repeat insult where an initial surge leads to enhanced protein expression but then returns to basal levels. NK-1R can be recycled and returned to the plasma membrane for subsequent activation, thus diminishing the need to sustain high mRNA levels to elicit a response. Airways repeatedly exposed to ozone show perinuclear NK-1R protein, suggesting that ozone-induced increases in SP may associate with increased SP-NK-1R complex formation and subsequent SP degradation via endocytotic pathways.
The mechanisms underlying increased cell death and inflammatory cell infiltration are multifactorial and beyond the scope of this study; however, site-specific ozone dose and resident antioxidant capability could also be contributors to region- and age-selective epithelial disruption, since areas of greater ozone concentration correlate with glutathione depletion following inhaled exposure (36). The less mature 2-mo animals demonstrated significant disruption in airway SP/NK-1R/Nur77 pathway expression, epithelial cell death, and inflammatory processes in response to acute challenge concurrent with repeat exposure. Necrotic cell death and Nur77 colocalization was greatest in the distal airways of 2-mo EAO animals (Fig. 7) and, similar to the age-matched AO group, showed pervasive leukocyte influx with neutrophils, eosinophils, and mast cells (Fig. 8). Jorres and colleagues (23) observed similar findings in adult humans noting that airway inflammation persists after repeat ozone exposure. In contrast, 6-mo EAO animals acutely exposed to ozone following 11 repeat 5-day exposure cycles had the least amount of necrotic cell death and greatest neutrophilic influx of any other exposure group. The more mature animal's ability to launch a superior neutrophilic response following acute ozone exposure likely minimized the ozone-induced cellular injury response we observed in 2-mo animals, since neutrophils play a key role in ozone-related acute epithelial injury and repair (22). Interestingly, these same animals presented with enhanced nuclear Nur77 expression and neutrophils within the epithelium, suggesting possible disruptions of both the Nur77-associated necrotic cell death pathway and injury/inflammation relationship within the airway.
Regardless of age, mast cells were present in combination with Nur77 in midlevel airways of EAO animals. Mast cells reside and mature in the lung mucosa and are aptly placed to respond to ozone-induced injury. Upon stimulation by immunomodulators, such as SP, mast cells degranulate and release a variety of lipid mediators, biogenic amines, and cytokines that facilitate both acute and persistent inflammatory processes (6, 12). This multi-inflammatory cell influx was observed in human subjects following repeat ozone exposure with mast cells, neutrophils, and eosinophils increasingly present following cessation of exposure (36 h) (47).
The hyper- vs. hyposensitivity of airways to acute ozone challenge following episodic exposure in 2- vs. 6-mo animals is novel. However, it is important to acknowledge some limitations when comparing ages. Our exposure regimen defines how the acute response at a given age is modified by the absence or history of a previous episodic or “priming” exposure. However, we cannot determine the degree to which this response observed at 6-mo EAO is the result of age-dependent immunocellular maturation or the consequence of completing 11 (vs. 1 cycle for 2-mo EAO) repeated exposure cycles. Future studies can partially examine this question by investigating how animals subjected to a single repeat exposure cycle at 2 mo respond to an AO challenge at 6 mo, just prior to airway sampling. Conversely, subjecting 6-mo animals to a single repeat exposure cycle just prior to AO challenge and subsequent necropsy would determine how varying exposure histories modulate the more mature postnatal lung's response to an acute ozone challenge. Additionally, samples collected for gene expression represent a “snapshot” in time whereas tissue sections used for protein expression assessment may reflect changes that occurred over a period of time; hence, changes in gene expression may have preceded changes in protein expression and returned to basal levels prior to the time of collection.
We conclude that 1) postnatal months 2–6 represent a particularly vulnerable window where ozone induces increases in TAC1/SP, NK-1R, and Nur77; 2) susceptibility to adverse acute ozone effects is modulated by age (2 vs. 6 mo) and lung region, including airway level; 3) whether the airway demonstrates a heightened or depressed response to an acute oxidant challenge is driven by age as well as ozone exposure history; and 4) ozone-induced changes in the SP/NK-1R/Nur77 pathway correlate with an altered inflammatory profile in the airway wall and increased airway epithelial cell death, particularly in early postnatal (2 mo), distal airways. Characterization of the ability of the monkey airway to respond to acute ozone challenge when there is a history of previous ozone exposure during early development provides a model for understanding how children living in polluted metropolitan areas during key stages of development may respond to subsequent ozone exposures later in life.
GRANTS
This work was supported by the NIH Program Project Grant “Pulmonary Effects of Environmental Oxidant Pollutants” NIEHS ES000628 and NIH National Center for Research Resources Grant NCRR RR000169, a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. This work was developed under Science To Achieve Results (STAR) Fellowship FP917122 awarded by the United States Environmental Protection Agency (EPA) to S. R. Murphy. The research described in this article has been funded in part by the US EPA but has not been subject to the Agency's required peer and policy review and therefore does not necessarily reflect the values of the Agency and no official endorsement should be inferred.
DISCLOSURES
The authors, with the exception of Dr. Van Winkle, declare no competing financial interests. Dr. Van Winkle receives grant funding from the American Petroleum Institute (API).
AUTHOR CONTRIBUTIONS
S.R.M. and E.S.S. performed experiments; S.R.M., K.L.O., and L.S.V.W. analyzed data; S.R.M., K.L.O., D.M.H., L.S.V.W., and E.S.S. interpreted results of experiments; S.R.M. prepared figures; S.R.M. drafted manuscript; S.R.M., K.L.O., D.M.H., L.A.M., L.S.V.W., and E.S.S. edited and revised manuscript; S.R.M., K.L.O., D.M.H., L.A.M., L.S.V.W., and E.S.S. approved final version of manuscript; D.M.H., L.A.M., and E.S.S. conception and design of research.
ACKNOWLEDGMENTS
Much of this project was conducted at the Cellular and Molecular Imaging Core at the Center for Health and the Environment, UC Davis. We thank Patricia Edwards for skilled technical assistance. The rhesus monkey model of childhood asthma is the product of all the efforts of faculty and staff in the California National Primate Research Center Respiratory Diseases Unit. We also thank Suzette Smiley-Jewell for critical editorial review of this manuscript.
REFERENCES
- 1.Ahn K, Huh JW, Park SJ, Kim DS, Ha HS, Kim YJ, Lee JR, Chang KT, Kim HS. Selection of internal reference genes for SYBR green qRT-PCR studies of rhesus monkey (Macaca mulatta) tissues. BMC Mol Biol 9: 78, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Lung Association. State of the Air Report 2014. Chicago, IL: American Lung Association, 2014 [Google Scholar]
- 3.Avdalovic MV, Tyler NK, Putney L, Nishio SJ, Quesenberry S, Singh PJ, Miller LA, Schelegle ES, Plopper CG, Vu T, Hyde DM. Ozone exposure during the early postnatal period alters the timing and pattern of alveolar growth and development in nonhuman primates. Anat Rec 295: 1707–1716, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker GL, Shultz MA, Fanucchi MV, Morin DM, Buckpitt AR, Plopper CG. Assessing gene expression in lung subcompartments utilizing in situ RNA preservation. Toxicol Sci 77: 135–141, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Barr BC, Hyde DM, Plopper CG, Dungworth DL. A comparison of terminal airway remodeling in chronic daily vs. episodic ozone exposure. Toxicol Appl Pharmacol 106: 384–407, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Berger P, Girodet PO, Begueret H, Ousova O, Perng DW, Marthan R, Walls AF, Tunon de Lara JM. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. FASEB J 17: 2139–2141, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bleecker ER. Cholinergic and neurogenic mechanisms in obstructive airways disease. Am J Med 81: 93–102, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Bouzas-Rodriguez J, Zarraga-Granados G, Sanchez-Carbente M, del R, Rodriguez-Valentin R, Gracida X, Anell-Rendon D, Covarrubias L, Castro-Obregon S. The nuclear receptor NR4A1 induces a form of cell death dependent on autophagy in mammalian cells. PLoS One 7: e46422, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burri PH. Structural aspects of postnatal lung development — alveolar formation and growth. Biol Neonate 89: 313–322, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Castleman WL, Tyler WS, Dungworth DL. Lesions in respiratory bronchioles and conducting airways of monkeys exposed to ambient levels of ozone. Exp Mol Pathol 26: 384–400, 1977 [DOI] [PubMed] [Google Scholar]
- 11.Castro-Obregon S, Rao RV, del Rio G, Chen SF, Poksay KS, Rabizadeh S, Vesce S, Zhang XK, Swanson RA, Bredesen DE. Alternative, nonapoptotic programmed cell death. J Biol Chem 279: 17543–17553, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Cyphert JM, Kovarova M, Allen IC, Hartney JM, Murphy DL, Wess J, Koller BH. Cooperation between mast cells and neurons is essential for antigen-mediated bronchoconstriction. J Immunol 182: 7430–7439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dib M, Zsengeller Z, Mitsialis A, Lu B, Craig S, Gerard C, Gerard NP. A paradoxical protective role for the proinflammatory peptide substance P receptor (NK1R) in acute hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 297: L687–L697, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Environmental Protection Agency. Air Quality Criteria for Ozone and Related Photochemical Oxidants (2006 Final). Washington, DC: U. S. Environmental Protection Agency, 2006. EPA/600/R-05/004aF-cF [Google Scholar]
- 15.Evans MJ, Fanucchi MV, Plopper CG, Hyde DM. Postnatal development of the lamina reticularis in primate airways. Anat Rec 293: 947–954, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. The attenuated fibroblast sheath of the respiratory tract epithelial-mesenchymal trophic unit. Am J Respir Cell Mol Biol 21: 655–657, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, Schelegle ES. Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am J Physiol Lung Cell Mol Physiol 291: L644–L650, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Frischer T, Studnicka M, Gartner C, Tauber E, Horak F, Veiter A, Spengler J, Kuhr J, Urbanek R. Lung function growth and ambient ozone: a three-year population study in school children. Am J Respir Crit Care Med 160: 390–396, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Grady EF, Garland AM, Gamp PD, Lovett M, Payan DG, Bunnett NW. Delineation of the endocytic pathway of substance P and its seven-transmembrane domain NK1 receptor. Mol Biol Cell 6: 509–524, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groneberg DA, Quarcoo D, Frossard N, Fischer A. Neurogenic mechanisms in bronchial inflammatory diseases. Allergy 59: 1139–1152, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Helyes Z, Elekes K, Sándor K, Szitter I, Kereskai L, Pintér E, Kemény Á, Szolcsányi J, McLaughlin L, Vasiliou S, Kipar A, Zimmer A, Hunt SP, Stewart JP, Quinn JP. Involvement of preprotachykinin A gene-encoded peptides and the neurokinin 1 receptor in endotoxin-induced murine airway inflammation. Neuropeptides 44: 399–406, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Hyde DM, Miller LA, McDonald RJ, Stovall MY, Wong V, Pinkerton KE, Wegner CD, Rothlein R, Plopper CG. Neutrophils enhance clearance of necrotic epithelial cells in ozone-induced lung injury in rhesus monkeys. Am J Physiol Lung Cell Mol Physiol 277: L1190–L1198, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Jorres RA, Holz O, Zachgo W, Timm P, Koschyk S, Muller B, Grimminger F, Seeger W, Kelly FJ, Dunster C, Frischer T, Lubec G, Waschewski M, Niendorf A, Magnussen H. The effect of repeated ozone exposures on inflammatory markers in bronchoalveolar lavage fluid and mucosal biopsies. Am J Respir Crit Care Med 161: 1855–1861, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Kajekar R, Pieczarka EM, Smiley-Jewell SM, Schelegle ES, Fanucchi MV, Plopper CG. Early postnatal exposure to allergen and ozone leads to hyperinnervation of the pulmonary epithelium. Respir Physiol Neurobiol 155: 55–63, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Koon HW, Zhao D, Na X, Moyer MP, Pothoulakis C. Metalloproteinases and transforming growth factor-α mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem 279: 45519–45527, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kunzli N, Kelly T, Balmes J, Tager IB. Reproducibility of retrospective assessment of outdoor time-activity patterns as an individual determinant of long-term ambient ozone exposure. Int J Epidemiol 26: 1258–1271, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Larson SD, Schelegle ES, Walby WF, Gershwin LJ, Fanuccihi MV, Evans MJ, Joad JP, Tarkington BK, Hyde DM, Plopper CG. Postnatal remodeling of the neural components of the epithelial-mesenchymal trophic unit in the proximal airways of infant rhesus monkeys exposed to ozone and allergen. Toxicol Appl Pharmacol 194: 211–220, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Miller LA, Hurst SD, Coffman RL, Tyler NK, Stovall MY, Chou DL, Putney LF, Gershwin LJ, Schelegle ES, Plopper CG, Hyde DM. Airway generation-specific differences in the spatial distribution of immune cells and cytokines in allergen-challenged rhesus monkeys. Clin Exp Allergy 35: 894–906, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore BD, Hyde D, Miller L, Wong E, Frelinger J, Schelegle ES. Allergen and ozone exacerbate serotonin-induced increases in airway smooth muscle contraction in a model of childhood asthma. Respiration 83: 529–542, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy SR, Schelegle ES, Edwards PC, Miller LA, Hyde DM, Van Winkle LS. Postnatal exposure history and airways: oxidant stress responses in airway explants. Am J Respir Cell Mol Biol 47: 815–823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy SR, Schelegle ES, Miller LA, Hyde DM, Winkle LSV. Ozone exposure alters serotonin and serotonin receptor expression in the developing lung. Toxicol Sci 134: 168–179, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oslund KL, Hyde DM, Putney LF, Alfaro MF, Walby WF, Tyler NK, Schelegle ES. Activation of neurokinin-1 receptors during ozone inhalation contributes to epithelial injury and repair. Am J Respir Cell Mol Biol 39: 279–288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plopper C, St George J, Cardoso W, Wu R, Pinkerton K, Buckpitt A. Development of airway epithelium. Patterns of expression for markers of differentiation. Chest 101: 2S–5S, 1992 [PubMed] [Google Scholar]
- 35.Plopper CG, Alley JL, Weir AJ. Differentiation of tracheal epithelium during fetal lung maturation in the rhesus monkey Macaca mulatta. Am J Anat 175: 59–71, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Plopper CG, Hatch GE, Wong V, Duan X, Weir AJ, Tarkington BK, Devlin RB, Becker S, Buckpitt AR. Relationship of inhaled ozone concentration to acute tracheobronchial epithelial injury, site-specific ozone dose, and glutathione depletion in rhesus monkeys. Am J Respir Cell Mol Biol 19: 387–399, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Plopper CG, Heidsiek JG, Weir AJ, George JA, Hyde DM. Tracheobronchial epithelium in the adult rhesus monkey: a quantitative histochemical and ultrastructural study. Am J Anat 184: 31–40, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Postlethwait EM, Joad JP, Hyde DM, Schelegle ES, Bric JM, Weir AJ, Putney LF, Wong VJ, Velsor LW, Plopper CG. Three-dimensional mapping of ozone-induced acute cytotoxicity in tracheobronchial airways of isolated perfused rat lung. Am J Respir Cell Mol Biol 22: 191–199, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Robledo RF, Witten ML. NK1-receptor activation prevents hydrocarbon-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 276: L229–L238, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Sánchez JA, Garfias Ayala FJ. Recent trend in ozone levels in the metropolitan zone of Mexico City. J Mex Chem Soc 52: 256–262, 2008 [Google Scholar]
- 41.Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, Walby WF, Mitchell V, Tarkington BK, Wong VJ, Baker GL, Pantle LM, Joad JP, Pinkerton KE, Wu R, Evans MJ, Hyde DM, Plopper CG. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol Appl Pharmacol 191: 74–85, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Sorokin SP, Ebina M, Hoyt RF., Jr Development of PGP 9.5- and calcitonin gene-related peptide-like immunoreactivity in organ cultured fetal rat lungs. Anat Rec 236: 213–225, 1993 [DOI] [PubMed] [Google Scholar]
- 43.Sterner-Kock A, Vesely KR, Stovall MY, Schelegle ES, Green JF, Hyde DM. Neonatal capsaicin treatment increases the severity of ozone-induced lung injury. Am J Respir Crit Care Med 153: 436–443, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Sutherland KM, Combs TJ, Edwards PC, Van Winkle LS. Site-specific differences in gene expression of secreted proteins in the mouse lung: comparison of methods to show differences by location. J Histochem Cytochem 58: 1107–1119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tager IB, Balmes J, Lurmann F, Ngo L, Alcorn S, Kunzli N. Chronic exposure to ambient ozone and lung function in young adults. Epidemiology 16: 751–759, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Tollet J, Everett AW, Sparrow MP. Development of neural tissue and airway smooth muscle in fetal mouse lung explants: a role for glial-derived neurotrophic factor in lung innervation. Am J Respir Cell Mol Biol 26: 420–429, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Torres A, Utell MJ, Morow PE, Voter KZ, Whitin JC, Cox C, Looney RJ, Speers DM, Tsai Y, Frampton MW. Airway inflammation in smokers and nonsmokers with varying responsiveness to ozone. Am J Respir Crit Care Med 156: 728–736, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Tschanz SA, Burri PH. Postnatal lung development and its impairment by glucocorticoids. Pediatr Pulm 16: 247–249, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Vesely KR, Schelegle ES, Stovall MY, Harkema JR, Green JF, Hyde DM. Breathing pattern response and epithelial labeling in ozone-induced airway injury in neutrophil-depleted rats. Am J Respir Cell Mol Biol 20: 699–709, 1999 [DOI] [PubMed] [Google Scholar]
- 50.West JA, Van Winkle LS, Morin D, Fleschner CA, Forman HJ, Plopper CG. Repeated inhalation exposures to the bioactivated cytotoxicant naphthalene (NA) produce airway-specific Clara cell tolerance in mice. Toxicol Sci 75: 161–168, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Wingate AD, Arthur JS. Post-translational control of Nur77. Biochem Soc Trans 34: 1107–1109, 2006 [DOI] [PubMed] [Google Scholar]