Abstract
Recent findings demonstrate that inhaled cigarette smoke, the predominant lung carcinogen, elicits a T helper 17 (Th17) inflammatory phenotype. Interleukin-17A (IL-17), the hallmark cytokine of Th17 inflammation, displays pro- and antitumorigenic properties in a manner that varies according to tumor type and assay system. To investigate the role of IL-17 in lung tumor growth, we used an autochthonous tumor model (K-RasLA1 mice) with lung delivery of a recombinant adenovirus that expresses IL-17A. Virus-mediated expression of IL-17A in K-RasLA1 mice at 8–10 wk of age doubled lung tumor growth in 3 wk relative to littermates that received a green fluorescent protein-expressing control adenovirus. IL-17 induced matrix metalloproteinase-9 (MMP-9) expression in vivo and in vitro. In accord with this finding, selective and specific inhibitors of MMP-9 repressed the increased motility and invasiveness of IL-17-treated lung tumor cells in culture. Knockdown or mutation of p53 promoted the motility of murine lung tumor cells and abrogated the promigratory role of IL-17. Coexpression of siRNA-resistant wild-type, but not mutant, human p53 rescued both IL-17-mediated migration and MMP-9 mRNA induction in p53 knockdown lung tumor cells. IL-17 increased MMP-9 mRNA stability by reducing interaction with the mRNA destabilizing serine/arginine-rich splicing factor 1 (SRSF1). Taken together, our results indicate that IL-17 stimulates lung tumor growth and regulates MMP-9 mRNA levels in a p53- and SRSF1-dependent manner.
Keywords: IL-17, MMP-9, p53, SRSF1, lung tumor growth
the interleukin-17 family consists of six members, IL-17A to IL-17F. Although the family members are structurally related, they originate from different cell types and have diverse biological functions (28). IL-17 family members bind to a family of IL-17 receptors (IL-17 receptor A to E) that form hetero- and homodimers with bound ligand to activate downstream signaling (14). Because these IL-17 receptor family members have different ligand-binding specificities and variable tissue distributions, the IL-17 cytokine family associates with multiple inflammatory responses. IL-17A, commonly called IL-17, is the first identified and by far the most well-studied IL-17 family member. T helper 17 (Th17) cells are the primary source of IL-17A and homologous family member IL-17F (56). In both humans and mice, IL-17A and IL-17F bind and activate a heterodimeric receptor formed by IL-17RA and IL-17RC (24, 30). IL-17RA is expressed ubiquitously, whereas IL-17RC is mainly expressed in epithelial cells and fibroblasts (47).
Clinical findings with cancers of the stomach (78), prostate (59), colon (34), and lung (38) demonstrate that elevated levels of IL-17 correlate with a worse prognosis. However, in experimental models, the role of IL-17 in tumor growth depends on context. In many models, particularly in immunodeficient mice, IL-17 promotes tumorigenesis, and enhanced angiogenesis appears to account, in large part, for this protumorigenic effect (52). However, in immunocompetent mice, IL-17 impairs growth of tumor allografts by stimulating antitumor immunity (29, 42). A possible explanation for these disparate findings is that tumor graft models are inadequate in testing the effect of IL-17 in tumorigenesis. In autochthonous models of prostate and lung cancer in immunocompetent mice, IL-17 deficiency impairs tumor growth (3, 79). In an autochthonous model of pancreatic cancer, IL-17 overexpression accelerates tumorigenesis (44).
In lung cancer, dissection of the inflammatory response to lung carcinogens could be used to identify specific inflammatory mediators that promote lung tumor progression. In support of this view, lung inflammation induced by cigarette smoke accelerates progression of lung adenocarcinoma in mice (69). Since cigarette smoke elicits Th17 inflammation (4, 61), tumors arising in the lung must adapt to this inflammatory phenotype. This observation prompted us to determine the consequence of overexpression of IL-17A, the prototypical Th17 cytokine, on progression of mutant K-Ras-driven lung adenocarcinoma.
MATERIALS AND METHODS
Animal model.
K-RasLA1 mice in the C57BL/6 background were provided by Dr. Tyler Jacks through the National Cancer Institute Mouse Repository. Mice were maintained under pathogen-free conditions and experimental protocols were approved by the Tulane University Institutional Animal Care and Use Committee following guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care.
Plasmids.
Plasmids pCMV-p53-wt (60) and pCMV-p53-R175H express the wild-type human p53 and dominant negative R175H mutant human p53, respectively, from the CMV promoter. The pCMV-p53-R175H plasmid was constructed by digesting the SPC-p53-R175H plasmid (49) with BamHI and adding EcoRI linkers after filling in the restricted DNA. After digestion with HindIII, the R175H mutant p53 cDNA was subcloned into the pCMV12S.FS plasmid (50) at the EcoRI-HindIII sites after removal of the EIA cDNA insert.
Adenovirus administration to mice and assessment of tumor progression.
Lung tumor-bearing K-RasLA1 mice 8–10 wk of age were anesthetized with isoflurane before being administered 1×108 pfu IL-17-expressing recombinant adenovirus [AdV-IL-17 (58)] by oropharyngeal aspiration (32). Control K-RasLA1 mice received an identical amount of GFP-expressing adenovirus [AdV-GFP (13)]. Three weeks after treatment the mice were euthanized and the lungs were inflated by perfusion with 10% formalin at 30 cm pressure for 20 min before removal. After overnight fixation, the number of lung tumors on the pleural surface was quantified without knowledge of the sample identity. Tissue sections prepared from paraffin-embedded lung tissue were stained with hematoxylin and eosin (H&E) before evaluation of tumor burden. The tumor burden [defined as the ratio of hyperplastic lesion area to total lung section area on H&E-stained sections (27)] was quantified with an Aperio ScanScope slide scanner.
Cell culture.
mK-Ras-LE cells, a murine lung cancer epithelial cell line, were established from a lung tumor-bearing K-RasLA1 mouse (35). The mK-Ras-LE cells form tumors in syngeneic mice and express the lung epithelial cell markers surfactant protein C and E-cadherin but fail to express Clara cell secretory protein or N-cadherin (data not shown). mK-Ras-R172H-LE cells were established from a lung tumor-bearing K-RasLA1 mouse that was also heterozygous for a R172H knockin mutation of p53 (33). The line had been backcrossed to the C57BL/6 inbred strain for more than 10 generations before the cells were prepared. The mK-Ras-R172H-LE cells are positive for SPC and cytokeratin but negative for E-cadherin and slightly positive for vimentin (our unpublished observation). Both cell lines were cultured in RPMI medium with 10% FBS and 1% penicillin-streptomycin (complete medium) at 37°C with 5% CO2 (35).
Immunohistochemistry.
Immunohistochemistry was performed as described (17) with some modifications. Lung tissue sections were blocked in PBS with 3% BSA overnight at 4°C before incubation overnight at 4°C with the primary antibody against matrix metalloproteinase-9 (MMP-9; 1:200 dilution) (NBP1–57940; Novus Biologicals, Littleton, CO) diluted in PBS with 3% BSA. The negative control tissue sections were incubated with normal rabbit serum replacing the primary antibody diluted to the same concentration. After one washing with 3% BSA in PBS, the sections were incubated with biotin-conjugated donkey anti-rabbit secondary antibody (1:2,500 dilution) (711-065-152; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at room temperature. After three washes with 3% BSA in PBS, the sections were incubated with streptavidin-conjugated horseradish peroxidase (1:2,000 dilution) (016-030-084; Jackson ImmunoResearch Laboratories) in the same solution for 1 h at room temperature. Visualization with diaminobenzidine and counterstaining were as previously described (17).
Wound healing assay.
Cells were seeded on 12-well plates with RPMI complete medium. When the cells reached about 80% confluence, the medium was replaced with serum-free RPMI followed by overnight incubation. Then a single artificial wound was made by scratching the center of the monolayer of cells with a 200-μl pipet tip at time 0. After wounding, the cells were washed with PBS to remove detached cells and fresh serum-free RPMI was added containing increasing concentrations of mouse IL-17. During the postwounding period, images within the same area of the scratches were taken with a phase-contrast microscope. Ten measurements of wound width were taken for each scratch and were averaged. Percent of wound closure was calculated as the distance (μm) the cells migrated relative to the initial scratch width. In some experiments, a selective inhibitor of MMP-9 or an antibody to MMP-9 was added simultaneously with IL-17. The MMP-9 selective inhibitor (MMP-9 Inhibitor I, CAS 1177749-58-4, Millipore, Billerica, MA) was added at the dose of 10 nM. The MMP-9 antibody (AB19016; Millipore) was added at the dose of 12 μg/ml to inhibit MMP-9 and the same amount of rabbit IgG was used as the negative control. In the migration assay using cells infected with recombinant adenovirus expressing reversion-inducing-cysteine-rich protein with kazal motifs [AdV-RECK (63), a cellular repressor of metalloproteinases, including MMP-9 (2)], or AdV-GFP, cells were infected (MOI = 10) for 24 h before wounding and addition of IL-17. For the migration assays with p53 knockdown experiments, cells growing in 1 ml RPMI containing 10% FBS in 24-well plate were transfected with 5 pm siRNA using Lipofectamine diluted in 50 μl Opti-MEM according to the supplier's (Invitrogen) specifications. For the p53 knockdown-restoration experiments, cells growing in 1 ml RPMI containing 10% FBS in 24-well plate were transfected with 5 pm siRNA plus 150 ng pCMV-p53-wt plasmid or pCMV-p53-R175H plasmid using Lipofectamine diluted in 50 μl Opti-MEM according to the supplier's (Invitrogen) specifications. The protocol for the wound healing assay in p53 knockdown or knockdown-restoration experiments was the same as in untransfected cells, except the treatment incubation time was 30 h. Silencer Select siRNAs specifically targeting mouse p53 (gene ID: s75472) and Silencer Select Negative Control no. 1 siRNA were purchased from Invitrogen.
Transwell migration assays.
Cells at about 80% confluence were incubated overnight with serum-free RPMI. The next day, the cells were trypsinized and resuspended in serum-free RPMI before seeding 2.5 × 105 cells in 200 μl in 24-well Transwell migration inserts (8-μm pore, BD Biosciences, San Jose, CA). Serum-free RPMI with or without 10 ng/ml mouse IL-17 was added to the lower chamber. After 24 h, the cells on the upper surface of the insert were removed by scraping with cotton swabs and the cells that migrated to the lower surface were fixed and stained with the HEMA-3 staining kit (Thermo Fisher Scientific, Waltham, MA). After air drying, the inserts were mounted with Permount on glass slides. At least five random images were taken at ×200 magnification under a light microscope. The number of migrated cells were quantified per image and averaged per well.
Transwell invasion assays.
The invasion assays were performed with 24-well BD BioCoat Matrigel Invasion Chambers as described by the supplier (BD Biosciences). Briefly, cells were seeded in the inserts coated with growth factor reduced Matrigel at a density of 2.5 × 105 cells/well in 200 μl serum-free RPMI. RPMI containing 0.5% FBS with or without 10 ng/ml mouse IL-17 was added to the lower chamber. After 48 h, the inserts were stained and photographed as described above and the number of cells that invaded through the Matrigel was quantified as described above.
RNA quantification.
Total RNA was extracted from cultured cells or mouse lung tissue with TriPure Isolation Reagent (Roche Applied Science, Mannheim, Germany) and purified by use of the RNeasy Mini Kit (Qiagen, Valencia, CA), followed by TURBO DNase treatment (Invitrogen, Carlsbad, CA) as described by the supplier. RNA purity and concentration were measured using a NanoDrop Spectrophotometer (Thermo Scientific). First-strand cDNA was generated by reverse transcription using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative PCR of the MMP-9 and β-actin cDNAs was performed with primer sets (MMP-9 forward 5′-CAATCCTTGCAATGTGGATG-3′ and MMP-9 reverse 5′-TAAGGAAGGGGCC CTGTAAT-3′, β-actin forward 5′-TCTACGAGGGCTATGCTCTCC-3′, β-actin reverse 5′-GGATGCCACAGGATTCCATAC-3′) by use of iQ SYBR Green Supermix (Bio-Rad). PCR conditions were 95°C for 3 min, followed by 45 cycles at 95°C for 15 s, 60°C for 30 s, and 72°C for 15 s. After PCR, a melting curve validated the specificity of the amplification. Relative expression of the MMP-9 mRNA was normalized against the internal control mouse β-actin mRNA by the 2−ΔΔCt method (39).
Cell viability assay.
mK-Ras-LE cells were seeded into 96-well plates in RPMI complete medium for 24 h. Then the complete medium was removed and serum-free RPMI was added followed by incubation overnight. The next day the medium was replaced with serum-free RPMI with increasing concentrations of mouse IL-17. After 48 h, the cell viability was determined by using the MTT Cell Proliferation Assay kit (ATCC, Manassas, VA) according to the manufacturer's protocol.
Bronchoalveolar lavage.
Bronchoalveolar lavage (BAL) was performed after intubation of mice with a 20-gauge 1.25-in. catheter secured in place with a suture (17). Mice were lavaged with 5 × 0.8 ml of ice-cold lavage buffer (0.137 M sodium chloride, 2.7 mM potassium chloride, 12 mM phosphate buffer, 0.4 mM EDTA, pH 7.4). After removal of the cells from the first lavage by centrifugation at 1,500 g for 5 min, the samples were aliquoted and stored at −70°C. The cells from the first lavage were combined with lavages 2–5 and the collected cell pellet was resuspended in 500 μl of ice-cold lavage buffer. The total cell count was recorded by mixing 10 μl of the resuspended cells 1:1 with Trypan blue (MP Biomedicals, Solon, OH) and counted on a Bright-Line Hemacytometer. Then 5 × 104 cells were cytospun onto slides by using a Shandon Cytospin 3 at 600 RPM for 3 min. The slides were allowed to dry before staining with Hema 3 (Fisher Scientific, Pittsburgh, PA) followed by dehydration with xylene and mounting with Permount (Fisher Scientific). Differential cell counts were performed on 200 cells on randomly selected fields per sample by an investigator who was unaware sample identity. The first aliquot of BAL fluid was analyzed for IL-17 protein expression with a Mouse IL-17A ELISA kit (BioLegend, San Diego, CA) according to the manufacturer's instruction.
Gelatin zymography.
MMP-9 levels in cell culture media or BAL fluid from mice was determined by gelatin zymography (55). The samples were loaded on a Novex 10% Zymogram (Gelatin) Gel (Invitrogen). The gel was run at constant voltage (∼100 V) at 4°C until the bromophenol blue tracking marker reached the bottom. Then the gel was incubated in 1× Zymogram Renaturing Buffer (2.5% Triton X-100 in water) for 30 min at room temperature with gentle agitation and subsequently incubated in 1× Zymogram Developing Buffer (50 mM Tris, 5 mM CaCl2, 0.2 M NaCl) for another 30 min. The gel was incubated in fresh 1× developing buffer overnight at 37°C for maximum sensitivity. Then the gel was stained with 0.5% Coomassie blue in methanol-acetic acid-water, 50:10:40, for 45 min and destained in the same solution without dye to detect the clear area of protease activity.
mRNA stability assay.
mK-Ras-LE cells were pretreated in serum-free RPMI with or without 10 ng/ml IL-17 for 2 h. At time 0 total RNA was prepared from the cells with Tripure (Roche) and RNeasy Mini Kit (Qiagen) as described above then the medium was replaced with fresh serum-free RPMI medium containing 10 μg/ml actinomycin D (Sigma-Aldrich, St. Louis, MO) or the same concentration actinomycin D plus 10 ng/ml IL-17 for 8 h before preparation of total RNA. The abundance of mRNA for MMP-9, β-actin, CXCL-1, and CXCL-2 at time 0 and 8 h was determined by quantitative RT-PCR (CXCL-1 forward 5′-GGGCGCCTATCGCCAAT-3′, CXCL-1 reverse 5′-ACCTTCAAGCTCTGGA TGTTCTTG-3′; CXCL-2 forward 5′-TGTCAATGCCTGAAGACCCTGCC-3′, CXCL-2 reverse 5′-AACTTTTTGACCGCCC TTGAGA-3′). PCR conditions for CXCL-1 and CXCL-2 were 95°C for 3 min followed by 40 cycles at 95°C for 15 s, 60°C for 1 min.
siRNA transfection.
Cells growing in 2 ml RPMI containing 10% FBS in a six-well plate were transfected with 30 pm siRNA by using Lipofectamine 2000 transfection reagent (Invitrogen) diluted in 500 μl of Opti-MEM I reduced-serum medium (Invitrogen) according to the manufacturer's recommendations. Silencer Select siRNAs specifically targeting serine/arginine-rich splicing factor 1 (SRSF1) (gene ID: s200965) and Silencer Select Negative Control no. 1 siRNA were purchased from Invitrogen. After 48 h, total cellular RNA was prepared and subjected to quantitative RT-PCR as described above.
RNA immunoprecipitation.
RNA coimmunoprecipitation assays were performed as described previously with some modifications (19). mK-Ras-LE cells were incubated in serum-free RPMI medium overnight and treated with or without 10 ng/ml IL-17 for another 48 h. After treatment, 4 × 106 cells were harvested by trypsinization and fixed in PBS solution with 0.1% formalin for 15 min. The fixation procedure was quenched by incubating in 10 ml PBS with 0.25 M glycine (pH 7) for 5 min. Then the cells were washed with PBS and resuspended in 1 ml RIPA buffer (50 mM Tris-Cl pH 8, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) containing 1× protease inhibitors (Roche), and sonicated (30% power, 12 s; 40% power, 12 s; 50% power, 5 s twice) with a Branson Sonifier. After removal of insoluble material by centrifugation at 16,000 g for 15 min, 1 ml of each cell extract was incubated with 5 μg antibody to SRSF1 (Santa Cruz Biotechnology, Santa Cruz, CA) or the same volume of PBS for 1 h. Then 20 μl BSA preblocked protein A/G Plus-Agarose beads (Santa Cruz) were added and incubated on a rotating incubator overnight at 4°C. The next day, the agarose beads were collected by centrifugation at 3,000 g, 4°C for 5 min. After being washed three times with 1 ml RIPA buffer, the beads were resuspended in 200 μl elution buffer (50 mM Tris-Cl pH 7, 5 mM EDTA, 10 mM DTT, 1% SDS) and incubated at 70°C for 1 h to reverse formalin cross-linking. RNA was extracted from the eluate by using TriPure (Roche) reagent according to the manufacturer's protocol. cDNA was prepared as described above. The same volume of each cDNA product (1.5 out of 40 μl) was subjected to quantitative RT-PCR for measuring CXCL-2, β-actin, and MMP-9 mRNA levels as described above.
Statistical analysis.
Data are presented as means ± SE. Data were analyzed by using Student's t-test or Mann-Whitney test when appropriate with GraphPad Prism 5 software. P values lower than 0.05 were considered as statistically significant.
RESULTS
IL-17 overexpression increases lung tumor growth in K-RasLA1 mice.
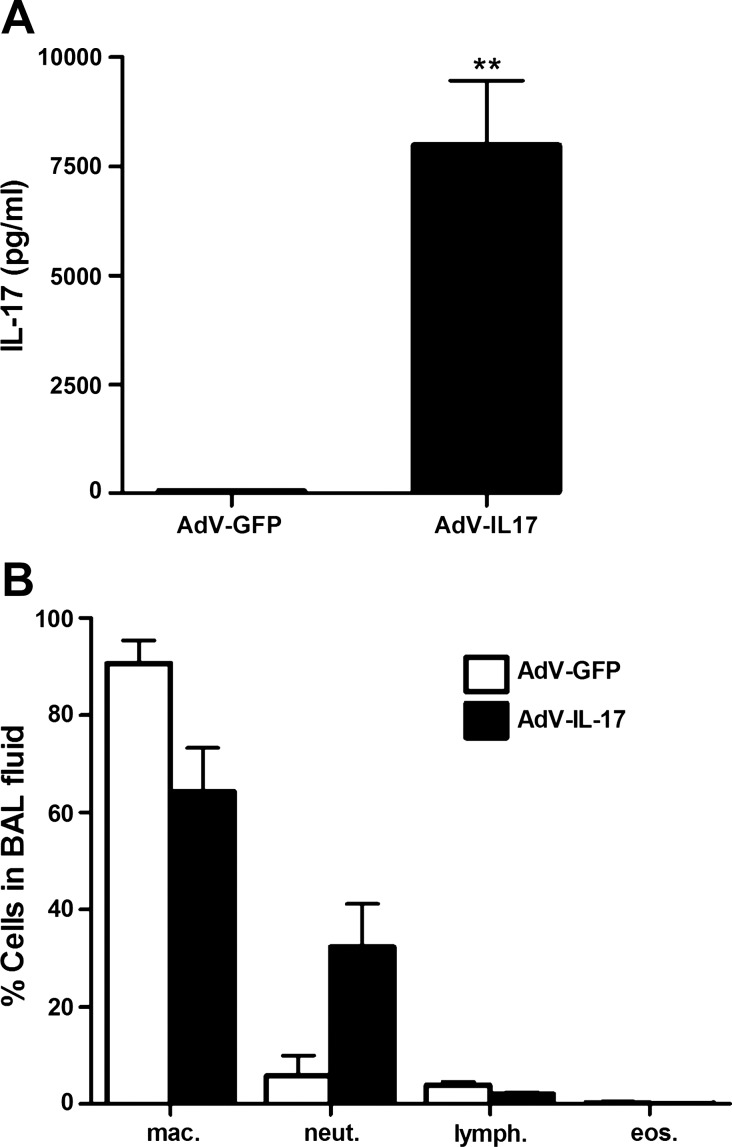
To overexpress IL-17 in the lungs of mice we administered an IL-17A-expressing recombinant adenovirus (AdV-IL-17) to 8- to 10-wk-old wild-type mice by oropharyngeal aspiration. This route of delivery produces expression of the transduced gene in epithelial cells throughout the lung (17). For control purposes, an equivalent amount of AdV-GFP was delivered to littermates. One week after virus delivery, a 150-fold increase in IL-17 levels (Fig. 1A) and a corresponding fivefold increase in lung neutrophilia (Fig. 1B) were detected in the BAL fluid from mice treated with AdV-IL-17 compared with that of the AdV-GFP-treated control group.
Fig. 1.
Expression of IL-17 and lung neutrophilia in IL-17-expressing recombinant adenovirus (AdV-IL-17)-treated mice. A: AdV-IL-17 or an equivalent amount (1×108 pfu) of green fluorescent protein-expressing recombinant adenovirus (AdV-GFP) was delivered to C57BL/6 mice by oropharyngeal aspiration. One week posttreatment, bronchoalveolar lavage (BAL) was performed. The levels of IL-17 in the BAL fluid were determined by ELISA. Graph shows mean levels ± SE IL-17 in the first 0.8 ml aliquot of BAL fluid (**P < 0.01 vs. AdV-GFP group, n = 5 per group). B: differential cell counts of BAL cells. Cytospin samples of the cells recovered by BAL were stained with Hema 3 (Fisher). Cells were visualized by microscopy, and 200 cells were counted from each sample. Graph shows percentage of the indicated cell type (means ± SE) in the cells recovered from the BAL fluid from mice treated with AdV-IL-17 (solid bars) and AdV-GFP (open bars). P = 0.07, percentage neutrophils AdV-GFP vs. AdV-IL-17, n = 5 per group.
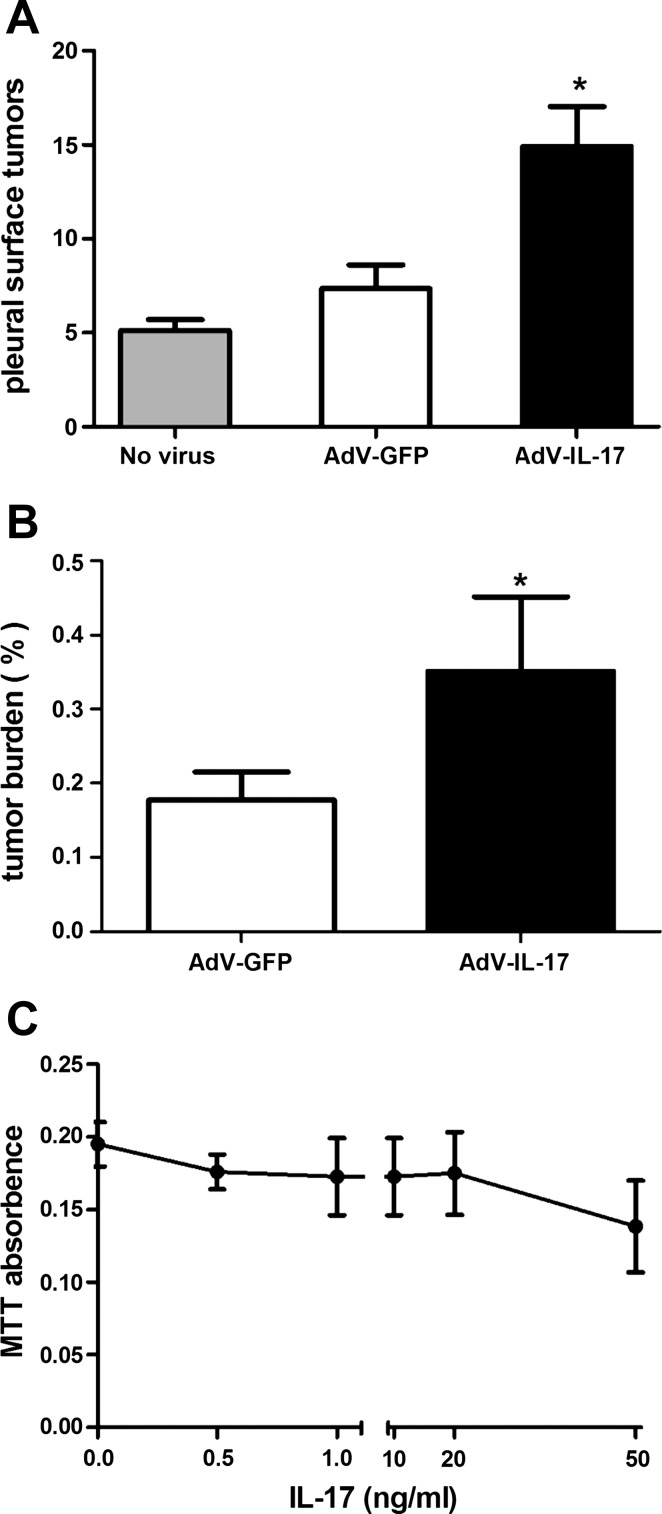
To evaluate the effect of IL-17A on lung tumor growth, AdV-IL-17 or an equivalent amount of AdV-GFP was delivered to the lungs of tumor-bearing K-RasLA1 mice at 8 to 10 wk of age. Three weeks after adenovirus treatment, the number of visible tumors on the pleural surface of AdV-IL-17-treated mice doubled relative to that in AdV-GFP-treated littermates (Fig. 2A). K-RasLA1 littermates that did not receive adenovirus had a comparable number of tumors on the pleural surface relative to that of the AdV-GFP-treated control group. To confirm IL-17-mediated acceleration of lung tumor growth, we used a slide scanner to quantify tumor burden on H&E-stained lung tissue sections from the adenovirus-treated animals. Consistent with quantification of tumors on the pleural surface, tumor burden expressed as the ratio of tumor lesion area to total lung area in H&E-stained tissue sections (27) nearly doubled in K-RasLA1 mice overexpressing IL-17 (AdV-IL-17) relative to the AdV-GFP control group (Fig. 2B). These data confirmed that IL-17 overexpression stimulated a rapid increase in lung tumor growth in vivo over a relatively brief 3-wk period. However, IL-17 failed to stimulate proliferation of serum-starved mutant K-Ras-expressing lung tumor cells [prepared from a K-RasLA1 mouse (35)] in cell culture (Fig. 2C).
Fig. 2.
Overexpression of IL-17 promotes lung tumor growth in K-RasLA1 mice. A: quantification of tumor nodules on the lung pleural surfaces of K-RasLA1 mice after AdV-IL-17 treatment. K-RasLA1 mice at 8–10 wk received 1×108 pfu IL-17-expressing recombinant adenovirus (AdV-IL-17) (n = 9), green fluorescent protein expressing adenovirus (AdV-GFP) (n = 6), or no virus treatment (n = 3) by oropharyngeal aspiration. Three weeks after adenovirus treatment, the mice were evaluated for lung tumor nodules on the pleural surface. Graph shows the mean number (± SE) of tumor modules on the pleural surface of fixed lung tissue from K-RasLA1 mice untreated (shaded bar, n = 3), or treated with control virus (AdV-GFP, open bar, n = 6), or treated with IL-17-expressing adenovirus (AdV-IL-17, solid bar, n = 9). *P < 0.05 AdV-IL-17 vs. AdV-GFP. B: evaluation of tumor burden. The area of hyperplastic lesions and total area of lung tissue examined was quantified on hematoxylin and eosin (H&E)-stained tissue sections from each mouse. Graph shows the mean lung tumor burden (± SE) as measured by the ratio (percent) of the tumor area vs. total area evaluated. Open bar represents the tumor burden of AdV-GFP treated K-RasLA1 mice (n = 6) and solid bar represents the tumor burden of AdV-IL-17-treated littermates (n = 9). *P < 0.05 AdV-IL-17 vs. AdV-GFP. C: serum-starved mK-Ras-LE cells were treated with increasing concentrations of mouse IL-17 for 48 h. Relative cell number was assessed by MTT assay. The experiment was repeated twice in triplicate. Data shown are means ± SE.
IL-17 enhances MMP-9 expression and lung tumor cell motility.
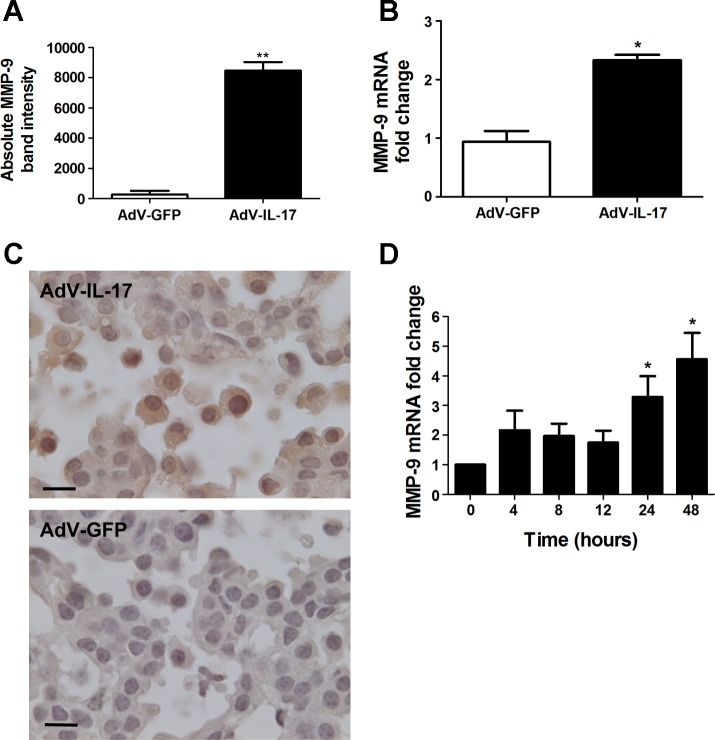
IL-17 can stimulate expression of MMP-9 (1, 18, 36), and this may partially account for the selective stimulation of lung tumor growth in vivo. Consequently, we evaluated gelatinase activity in the BAL fluid from adenovirus-treated mice by zymography. One week after virus delivery, BAL fluid and lung tissue were prepared from wild-type mice treated with AdV-IL-17 or AdV-GFP. Gelatin-zymography revealed a less than twofold increase in MMP-2 and approximately a 30-fold increase in MMP-9 in the BAL fluid from AdV-IL-17-treated mice relative to that from AdV-GFP-treated littermates (Fig. 3A). The increased amount of MMP-9 in the BAL fluid correlated with more than a twofold increase in MMP-9 mRNA in total lung RNA (Fig. 3B). Immunohistochemistry with tissue sections prepared from K-RasLA1 mice 1 wk after AdV-IL-17 administration revealed expression of MMP-9 in a variety of lung cells including both tumor cells and immune cells infiltrating the tumor (Fig. 3C). These data are consistent with the possibility that induction of MMP-9 could at least partially account for lung tumor growth mediated by IL-17 overexpression. Since MMP-9 expression correlates with progression of lung adenocarcinoma in a number of studies (6, 7, 26, 43, 70, 73, 74), we addressed the mechanism of MMP-9 activation by IL-17 in cell culture. Gelatin zymography of the cell culture medium from serum-starved mK-Ras-LE cells treated with IL-17 displayed a time- and concentration-dependent increase in MMP-9 (data not shown). In accord with these findings, IL-17 treatment increased MMP-9 mRNA levels more than fourfold relative to β-actin mRNA in mK-Ras-LE cells (Fig. 3E). Thus IL-17 enhanced expression of MMP-9 in murine lung tumor cells.
Fig. 3.
Induction of MMP-9 by IL-17. A: wild-type mice were treated with 1×108 pfu AdV-IL-17 or AdV-GFP by oropharyngeal aspiration. One week after treatment, the mice were euthanized and evaluated for MMP-9 expression. Equal volumes of BAL fluid from AdV-IL-17- or AdV-GFP-treated mice were assessed by gelatin zymography for MMP activity (n = 4 per group). Bands corresponding to MMP-9 on the zymogram were quantified by densitometry. Graph shows the MMP-9 band intensity from the BAL fluid of AdV-GFP- (open bar) or AdV-IL-17-treated (solid bar) mice. Data shown are means ± SE. **P < 0.01 AdV-IL-17 vs. AdV-GFP. B: total lung RNA prepared from the mice in A was assessed by qRT-PCR for MMP-9 mRNA levels with β-actin mRNA as the internal control. Mice treated with AdV-IL-17 (solid bar) showed a 2.3-fold increase (2−ΔΔCT method) in MMP-9 mRNA levels compared with AdV-GFP treated littermates (open bar). Data shown are means ± SE; n = 3 per group. *P < 0.05 AdV-IL-17 vs. AdV-GFP. C: K-RasLA1 mice were treated with AdV-IL-17 as described above. One week postexposure the lungs of the treated mice were fixed and paraffin embedded. Lung tissue sections were immunostained with an antibody to MMP-9 by the diaminobenzidine method. MMP-9-positive (brown) staining tumor and immune cells did not appear in the tumor area on an adjacent section stained with the negative control antibody. D: total RNA was prepared from mK-Ras-LE cells at increasing times after treatment with 10 ng/ml IL-17. Graph shows mean levels of MMP-9 mRNA relative to β-actin (2−ΔΔCT method) at the indicated time after addition of IL-17 to the serum-starved cells. The bars represent means ± SE (n ≥ 4). *P < 0.05 relative to the 0 time point.
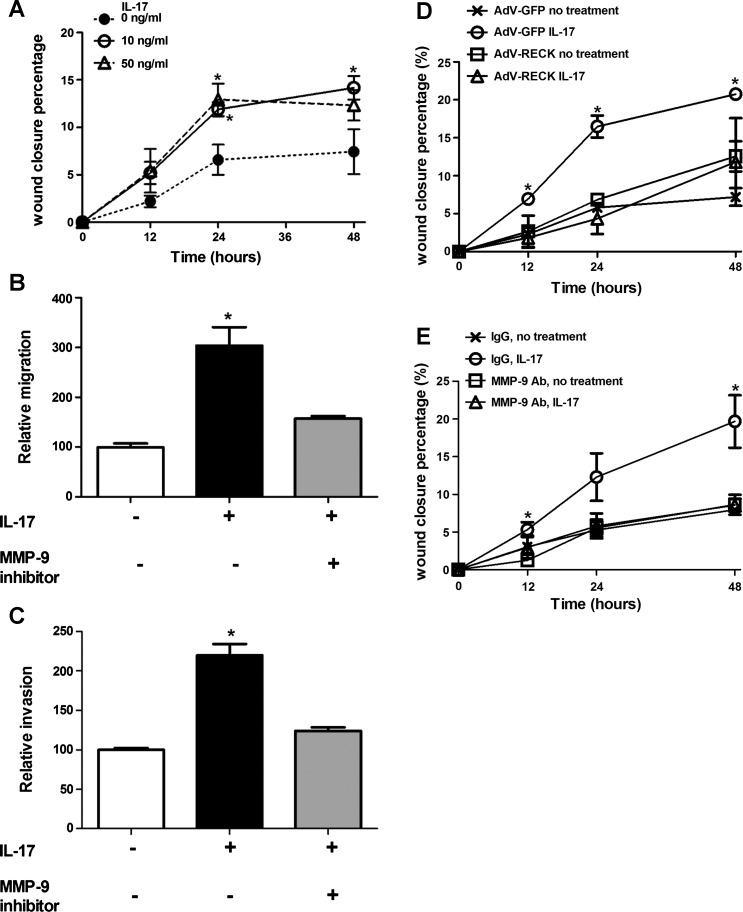
MMP-9 can increase cell motility and invasion (64). Therefore, we determined whether IL-17 could increase the motility and invasiveness of mK-Ras-LE cells. IL-17 promoted migration of mK-Ras-LE cells in a scratch-wound closure assay (Fig. 4A). In agreement with the concept that the enhanced motility and invasion of IL-17-treated mK-Ras-LE cells required MMP-9, a selective MMP-9 inhibitor prevented augmented migration mediated by IL-17 (Fig. 4B). In addition, enhanced invasion of IL-17-treated mK-Ras-LE cells through a Matrigel matrix in Transwell invasion assays was also repressed by the MMP-9 inhibitor (Fig. 4C). Although these results implicate MMP-9 in the enhanced motility and invasiveness of IL-17-treated mK-Ras-LE cells, the lack of inhibitor specificity precludes the conclusion that the enhanced motility is MMP-9 dependent. Two additional strategies were employed to further test role of MMP-9 in IL-17-mediated motility. In the first approach, mK-Ras-LE cells were infected with a recombinant adenovirus that expresses reversion-inducing-cysteine-rich protein with kazal motifs [RECK, a cellular repressor of MMP-2, MMP-9, and MMP-14 (2, 54)] and the effect of IL-17 on the motility of the RECK-expressing cells was compared with that of IL-17-treated control cells infected with AdV-GFP. As expected, IL-17 increased wound closure of AdV-GFP-infected mK-Ras-LE cells. AdV-RECK infection increased RECK protein expression ∼130-fold compared with that in AdV-GFP infected mK-Ras-LE cells and significantly repressed the induction of motility mediated by IL-17 (Fig. 4D). Similarly, addition of an antibody that inhibits MMP-9 activity (15) also repressed the increased motility of IL-17-treated mK-Ras-LE cells (Fig. 4E). These data demonstrate that induction of lung tumor cell motility by IL-17 is MMP-9 dependent.
Fig. 4.
IL-17 promotes MMP-9-dependent migration and invasion of mK-ras-LE cells. A: confluent mK-Ras-LE cells in 24-well plates were serum-starved overnight, then a scratch wound was made at time 0 (0 h) and fresh serum-free medium or serum-free medium supplemented with 10 or 50 ng/ml IL-17 was added before returning the cells to the incubator. Cell motility was measured as the ratio of wound closure relative to initial wound width. Graph shows percentage of wound closure vs. time for cells incubated in 0 (●, dotted line), 10 (○, solid line) and 50 (△, dashed line) ng/ml IL-17. Data shown are means ± SE (n = 4). *P < 0.05 vs. negative control group at the same time point. B: Transwell migration assays were performed with (solid and shaded bars) or without (open bar) 10 ng/ml IL-17 added to the bottom of the Transwells. The extent of migration is expressed as the number of cells on the underside of the Transwell in the treated group relative to the number of cells on the underside of the Transwell in the negative control group, which was normalized to 100. Enhanced migration mediated by IL-17 (solid bar) was reduced by the inhibitor of MMP-9 (shaded bar). Data shown are means ± SE (n = 4). *P < 0.05 vs. negative control or IL-17 and MMP-9 inhibitor cotreatment. C: mK-Ras-LE cells were plated onto a Matrigel-coated porous membrane without serum. Transwell invasion assays were performed with (solid and shaded bars) or without (open bar) addition of 10 ng/ml IL-17 added to the bottom of the Transwells. The extent of invasion is expressed as the number of cells on the underside of the Transwell in the treated group relative to the number of cells on the underside of the Transwell in the negative control group, which was normalized to 100. Enhanced invasion mediated by IL-17 (solid bar) was reduced by the inhibitor of MMP-9 (shaded bar). Data shown are means ± SE (n = 4). *P < 0.05 vs. negative control or IL-17 and MMP-9 inhibitor cotreatment. D: a migration assay was performed as described in A except the cells were infected at a MOI of 10 with recombinant adenovirus expressing reversion-inducing-cysteine-rich protein with kazal motifs (AdV-RECK) or AdV-GFP 24 h before wounding and addition of IL-17. Graph shows percentage wound closure at the indicated times in cells infected with AdV-GFP (×) or AdV-RECK (□) in the absence of IL-17 and in cells transfected with AdV-GFP (○) or AdV-RECK (△) in the presence of 10 ng/ml IL-17. Data shown are means ± SE (n = 3). *P < 0.05 vs. negative control group at the same time point. E: a migration assay was performed as described in A except an MMP-9 antibody (12 μg/ml, Millipore AB19016) was used to inhibit MMP-9. In place of the MMP-9 antibody, the same amount of rabbit IgG was used as the negative control. Graph shows percentage wound closure at the indicated times in cells treated with rabbit IgG (×) or MMP-9 antibody (□) in the absence of 10 ng/ml IL-17, and in cells treated with rabbit IgG (○) or MMP-9 antibody (△) in the presence of 10 ng/ml IL-17. Data shown are means ± SE (n = 3). *P < 0.05 vs. negative control group at the same time point.
Knockdown or mutation of p53 abrogates promotion of lung tumor cell motility by IL-17.
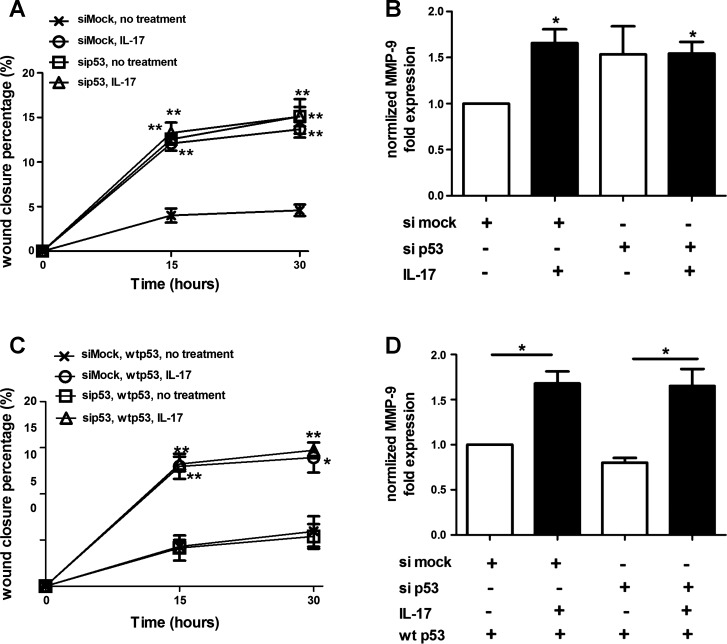
Lung tumors harboring mutations in both K-Ras and the p53 tumor suppressor protein grow more rapidly and metastasize more readily than lung tumors with mutations only in K-Ras (25, 33). To test the effect of p53 mutation on the response of lung tumor cells to IL-17, we determined the consequences of p53 knockdown in mK-Ras-LE cells upon promotion of migration by IL-17. Knockdown of p53 (∼90% knockdown efficiency confirmed by immunoblotting) enhanced migration of mK-Ras-LE cells and produced no additional effect on enhanced migration mediated by IL-17 (Fig. 5A). In contrast, mK-Ras-LE cells transfected with a mock siRNA migrated more slowly than the p53 siRNA-transfected counterparts and retained the response to IL-17. In accord with these findings, IL-17 increased MMP-9 mRNA levels in the mock siRNA-transfected cells, whereas p53 knockdown increased MMP-9 mRNA levels and IL-17 had no additional effect (Fig. 5B). Thus the effect of the p53 siRNA on migration and response to IL-17 correlated with a similar effect on MMP-9 expression. Restoration of p53 by cotransfection of the mouse p53 siRNA with a plasmid that expresses a siRNA resistant wild-type human p53 into mK-Ras-LE cells repressed migration and restored the enhanced migratory response to IL-17 (Fig. 5C). Furthermore, restoration of wild-type p53 rescued IL-17-mediated induction of MMP-9 mRNA (Fig. 5D). These experiments demonstrated that IL-17-mediated induction of MMP-9 and migration is dependent on p53.
Fig. 5.
IL-17 fails to enhance migration and MMP-9 expression in p53-knockdown lung tumor cells. A: knockdown of p53 prevents enhanced migration of IL-17-treated cells. mK-Ras-LE cells were transfected with a p53-targeting siRNA (sip53) or a non-targeting control siRNA (siMock) before growth to confluence. A scratch wound was made in the confluent cultures at time 0 (0 h) and fresh serum-free medium or serum-free medium supplemented with 10 ng/ml IL-17 was added before returning the cells to the incubator. At 15 and 30 h after the addition of IL-17, the percentage of wound closure was assessed for mock- (×) and p53-targeting (□) siRNA-transfected cells incubated without IL-17 or mock- (○) and p53-targeting (△) siRNA-transfected cells incubated with 10 ng/ml IL-17. Data shown are means ± SE (n = 4). **P < 0.01 vs. negative control at the same time point. B: RNA was prepared from the cells at the 30-h time point in A and levels of MMP-9 mRNA were determined by qRT-PCR. Graph shows the mean fold change (± SE) of MMP-9 mRNA levels relative to β-actin (2−ΔΔCT method) with (solid bars) or without (open bars) IL-17. For normalization purposes, the MMP-9/β-actin mRNA ratio in siMock-transfected, untreated cells was made equal to 1. *P < 0.05 vs. negative control. C: restoration of wild-type p53 rescues enhanced motility mediated by IL-17. mK-Ras-LE cells were cotransfected with a p53-targeting siRNA (sip53) or a nontargeting control siRNA (siMock) with a plasmid (pCMV-p53-wt) that expresses wild-type human p53, which is siRNA resistant. Graph shows percentage wound closure at the indicated times after wild-type human p53 expression in cells transfected with mock siRNA (×) or p53 siRNA (□) in the absence of 10 ng/ml IL-17 and in cells transfected with mock siRNA (○) or p53 siRNA (△) in the presence of 10 ng/ml IL-17. Data shown are means ± SE (n = 4). *P < 0.05, **P < 0.01 vs. negative control at the same time point. D: restoration of wild-type p53 rescues induction of MMP-9 mRNA by IL-17. Same as B, except the levels of MMP-9 mRNA at the 30-h time point from the cells in C were determined. *P < 0.05.
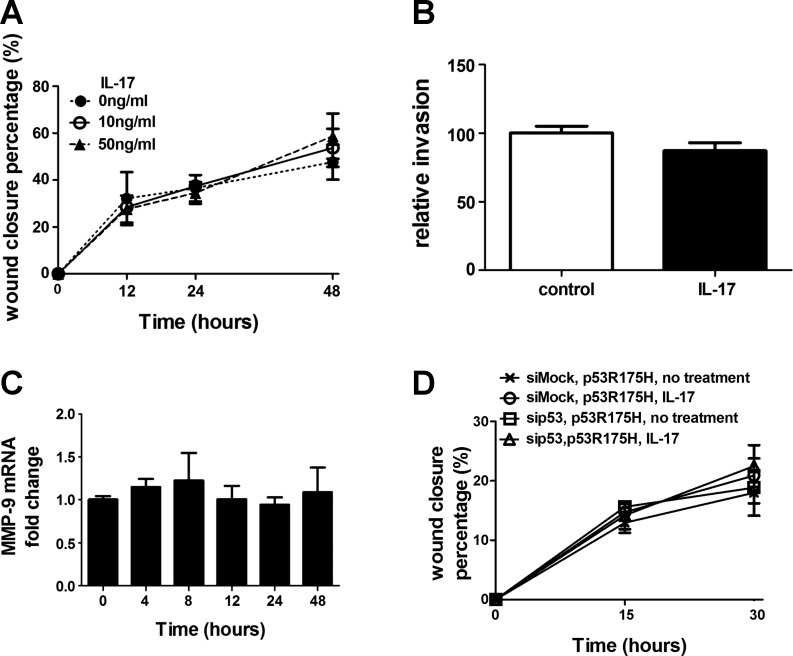
Although p53 deletion promotes tumor progression, most tumor-promoting mutations of p53 are missense mutations that lead to expression of a mutant protein (10). To address the effects of mutant p53 upon lung tumor promotion by IL-17, we prepared a lung tumor cell line, mK-Ras-R172H-LE cells, from K-RasLA1 mice that were also heterozygous knockin for the tumor promoting R172H mutation of p53 (33). Treatment of mK-Ras-R172H-LE cells with IL-17 had no effect on migration (Fig. 6A) or invasion (Fig. 6B) and IL-17 failed to increase MMP-9 mRNA levels in mK-Ras-R172H-LE cells (Fig. 6C). To confirm that IL-17 does not promote migration of mutant p53-expressing cells, we attempted a similar p53 rescue experiment like that shown in Fig. 5 with a mutant p53-expressing plasmid. mK-Ras-LE cells cotransfected with the mouse p53 siRNA and a plasmid that expresses a siRNA-resistant mutant human p53 R175H (equivalent to the mouse R172H mutant) failed to display enhanced migration upon IL-17 treatment (Fig. 6D). Taken together, these data suggest that IL-17 enhances migration of lung tumor cells through a MMP-9-dependent mechanism and that wild-type, but not mutant, p53 mediates the response to IL-17.
Fig. 6.
Mutant p53 alters IL-17-mediated promotion of migration and induction of MMP-9. A: effect of IL-17 on migration of mutant p53-expressing cells. Same as Fig. 4A except confluent mK-Ras-R172H-LE cells were assessed for migration in serum-free medium (●, dotted line) or serum-free medium supplemented with 10 (○, solid line) or 50 (△, dashed line) ng/ml IL-17. Graph shows percentage of wound closure vs. time. Data shown are means ± SE (n = 4). B: IL-17 does not promote invasion of mutant p53-expressing cells. Transwell migration assays (see Fig. 4B) were performed with mK-Ras-R172H-LE cells in serum-free media with (solid bar) or without (open bar) 10 ng/ml IL-17. Data shown are means ± SE (n = 4). C: total RNA was prepared from mK-Ras-R172H-LE cells at increasing times after treatment with 10 ng/ml IL-17. Graph shows the mean level of MMP-9 mRNA relative to β-actin (2−ΔΔCT method) at the indicated time after addition of IL-17 to the serum-starved cells. Data shown are means ± SE (n ≥ 5). D: same as Fig. 5C, except the cotransfected plasmid (pCMV-p53R175H) expressed the R175H mutant of human p53 in the K-Ras-LE cells with p53 knocked down. Graph shows percentage wound closure at the indicated times with mutant human p53 expression in cells transfected with mock siRNA (×) or p53 siRNA (□) in the absence IL-17 and in cells transfected with mock siRNA (○) or p53 siRNA (△) in the presence of 10 ng/ml IL-17. Data shown are means ± SE (n = 4).
IL-17 upregulates MMP-9 expression via mRNA stabilization.
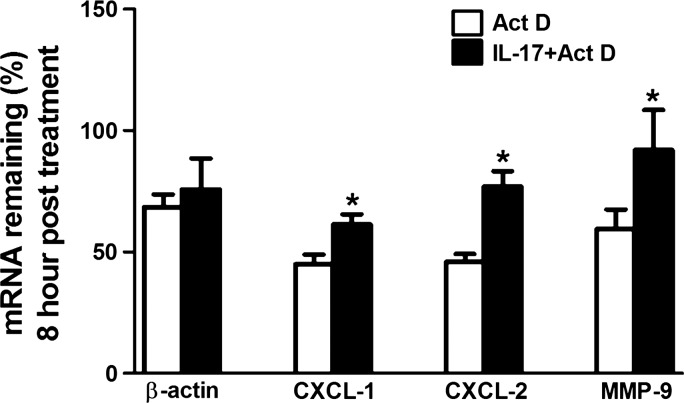
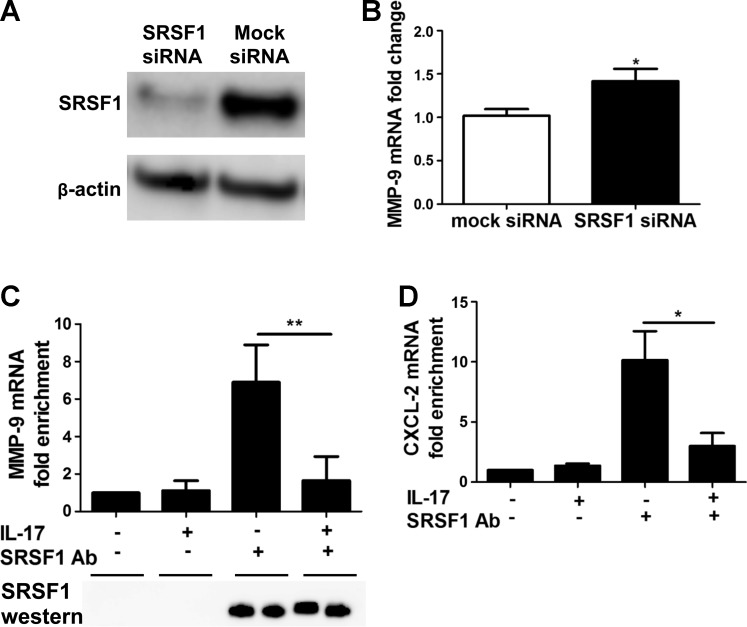
A previous report demonstrated that IL-17 enhanced the stability of chemokine mRNAs (67). To test whether induction of MMP-9 by IL-17 also involved stabilization of MMP-9 mRNA, we treated mK-Ras-LE cells with IL-17 for 2 h before inhibiting transcription with 10 μg/ml actinomycin D. The amount of β-actin mRNA remaining appeared similar in mK-Ras-LE cells in the presence and absence of IL-17 after 8 h of actinomycin D treatment (Fig. 7). Consistent with previous findings, IL-17 treatment increased CXCL-1 and CXCL-2 chemokine mRNA levels 8 h after inhibition of mRNA synthesis (Fig. 7). Similarly, IL-17 treatment also stabilized MMP-9 mRNA (Fig. 7). Chemokine mRNA stabilization by IL-17 requires the serine/arginine-rich splicing factor 1, SRSF1 (67). To determine whether SRSF1 altered the stability of MMP-9 mRNA, we transfected mK-Ras-LE cells with a siRNA that targeted SRSF1. After 48 h, Western blots showed ∼80% knockdown efficiency of the SRSF1 protein in mK-Ras-LE cells (Fig. 8A). The SRSF1-targeting siRNA increased MMP-9 mRNA ∼1.4-fold in transfected mK-Ras-LE cells relative to control cells transfected with scrambled siRNA (Fig. 8B). To demonstrate IL-17-regulated interaction between SRSF1 and MMP-9 mRNA, we performed RNA coimmunoprecipitation assays followed by mRNA quantification by qRT-PCR. An antibody to SRSF1 coimmunoprecipitated approximately sevenfold more MMP-9 mRNA than the negative control from whole cell extracts of untreated serum-starved mK-Ras-LE cells (Fig. 8C). Treatment of the cells with IL-17 reduced the amount of MMP-9 mRNA that coimmunoprecipitated with the antibody to SRSF1 to levels approximating the negative control. Immunoblots confirmed that equal amounts of SRSF1 immunoprecipitated specifically with or without IL-17 treatment (Fig. 8C). Additional assays did not show an association between β-actin mRNA with SRSF1 in extracts from untreated or IL-17-treated cells (data not shown). Positive control experiments replicated previous findings (19), demonstrating an IL-17-dependent association between SRSF1 and CXCL-2 mRNA (Fig. 8D). These observations support the concept that IL-17 increased MMP-9 mRNA stability by reducing interaction with SRSF1. These data agree with our conclusion that IL-17 increases expression of MMP-9 in lung tumor cells via posttranscriptional stabilization of the MMP-9 mRNA by reducing interaction with SRSF1.
Fig. 7.
IL-17 enhanced MMP-9 mRNA stability in mK-Ras-LE cells. Serum-starved mK-Ras-LE cells were untreated (open bars) or treated with 10 ng/ml IL-17 (solid bars) for 2 h. Then 10 μg/ml actinomycin D (Act D) was added to both groups at time 0 and total RNA was prepared. Equal amounts of total RNA prepared at time 0 and 8 h from duplicate or triplicate samples were assessed for the target mRNAs (β-actin, CXCL-1, CXCL-2, and MMP-9) by qRT-PCR. Results are presented as percent of the indicated mRNA remaining relative to the amount at time 0. The mRNA levels before actinomycin D treatment were set to 1. Data shown are means ± SE (n ≥ 4). *P < 0.05 for IL-17 treated vs. untreated control.
Fig. 8.
IL-17 regulates MMP-9 mRNA levels via serine/arginine-rich splicing factor 1 (SRSF1). A: mK-Ras-LE cells were transfected with a scrambled siRNA or a siRNA targeted to SRSF1. After 48 h, cell lysates were prepared and subjected to Western blot. Western blots confirmed siRNA knockdown of SRSF1 compared with the level in cells transfected with a Mock siRNA. B: same as A except total RNA was analyzed for MMP-9 mRNA levels in mK-Ras-LE cells transfected with a siRNA targeting SRSF1. Reduction of SRSF1 with a siRNA increased the MMP-9 mRNA level 1.4-fold (solid bar) relative to that in cells transfected with the control siRNA (open bar). The levels of MMP-9 mRNA were determined by qRT-PCR (2−ΔΔCT method) relative to β-actin mRNA. Results are means ± SE of 3 independent experiments. *P < 0.05 vs. mock siRNA-transfected group. C: serum-starved mK-Ras-LE cells were treated with or without 10 ng/ml IL-17 for 48 h. The cells were briefly fixed with formalin prior to preparation of cell lysates as described in materials and methods. Coimmunoprecipitated RNA was extracted from the protein A/G beads and cross-linking was reversed prior to conversion to cDNA. The amount of MMP-9 cDNA in each sample was quantified by real-time PCR. The results obtained with protein A/G beads alone with extracts from untreated cells were normalized to one. Data are presented as fold enrichment of MMP-9 mRNA compared with the negative control samples. Data shown are means ± SE (n = 4). **P < 0.01. Treatment of mK-Ras-LE cells with 10 ng/ml IL-17 reduced the amount of MMP-9 mRNA that could be selectively immunoprecipitated with an antibody to SRSF1 to near negative control levels. Bottom: immunoblotting showed that equal amounts of SRSF1 were immunoprecipitated from the extracts of IL-17-treated and untreated cells. D: same as C, except coimmunoprecipitation of CXCL-2 mRNA with an antibody to SRSF1 was assessed. Data are presented as fold enrichment of CXCL-2 mRNA compared with the negative control samples. Data are means ± SE (n = 4). *P < 0.05.
DISCUSSION
Our data show that IL-17A overexpression promotes rapid growth of mutant K-Ras-driven lung cancers. Coincident with stimulation of tumorigenesis in vivo, IL-17 stimulates the expression of MMP-9 in the lung and in lung epithelial cells in culture. Consistently, IL-17-treated mK-Ras-LE cells display enhanced migration and invasiveness that is MMP-9 dependent, as demonstrated by selective (pharmacological inhibitor and RECK overexpression) and specific (antibody) inhibition of MMP-9. A knockdown-restoration strategy demonstrated that IL-17-mediated migration and induction of MMP-9 depend on wild-type p53. In contrast, IL-17 does not enhance MMP-9 expression or consequent motility and invasion of mutant p53-expressing lung tumor cells. IL-17 increases MMP-9 mRNA stability. In accord with posttranscriptional regulation, siRNA-mediated knockdown of SRSF1 increases levels of MMP-9 mRNA. Moreover, MMP-9 mRNA binds to SRSF1 in a manner that is regulated by IL-17. We conclude that IL-17 enhances migration of wild-type p53-expressing lung tumor cells in a MMP-9-dependent manner that includes dissociation of the SRSF1-destabilizing factor from the MMP-9 mRNA.
IL-17A displays dichotomous roles in tumor progression. For example, it has been proposed that IL-17A enhances antitumor immunity in immunocompetent mice but increases tumor growth in the absence of an adaptive immune response (41), but this distinction is not so clear cut (40). Tumor type appears to be an important determinant of the prognostic significance of Th17 inflammation on clinical outcome (12). In addition, our data suggest that p53 status affects IL-17-mediated promotion of lung tumorigenesis. SRSF1 overexpression can induce p53 (11). Consequently, the ability of the activated IL-17 receptor to sequester SRSF1 (67) may be related to the opposing effects of IL-17 and p53 in MMP-9 regulation shown here. Consistent with our findings, prior studies have identified a protumorigenic role for IL-17 in models of lung adenocarcinoma (3, 37, 38, 57). However, with one exception (3), these previous investigations did not examine the effects of IL-17A in an autochthonous lung tumor model and studies with tumor grafts have produced results that were often contradictory (40, 72). Our results agree with, and extend, previous findings demonstrating that Th17 inflammation accelerates lung tumorigenesis in an autochthonous model of mutant K-Ras-expressing lung cancer (3). However, the approach here differs by overexpressing IL-17 to identify the cytokine-tumor relationship rather than using IL-17-deficient mice, which lack the homeostatic functions of IL-17. Although IL-17 overexpression more accurately reflects the clinical scenario of lung inflammation than the use of genetically deficient mice, the epithelial source of IL-17 overexpression consequent to adenovirus infection does not model the Th17 cell source of IL-17 that occurs in the lung exposed to carcinogens. IL-17-mediated lung neutrophilia likely increases levels of neutrophil elastase, an established inducer of lung tumor growth (23). Indeed, IL-17 recruits Gr-1+-CD11b+ myeloid cells to the lung and their depletion suppresses growth of K-Ras-driven lung tumors (3).
Typically, lung tumors develop in the context of chronic inflammation associated with inhaled carcinogens, primarily cigarette smoke. Consistent with the approach here, cigarette smoke is a Th17 adjuvant (4, 61) and inflammation associated with cigarette smoke promotes progression of mutant K-Ras-driven lung tumors (69). Moreover, a bacterial pathogen promotes chronic Th17 lung inflammation and accelerates lung tumorigenesis in mutant K-Ras-expressing mice (3, 48). These observations are consistent with the dependence of mutant K-Ras-expressing lung tumors on signaling by the proinflammatory transcription factor nuclear factor-κB [NF-κB (45, 75)]. Enhanced tumor progression associated with inflammation occurs in other tumor types driven by mutant K-Ras (6, 16). In a model of pancreatic cancer, inflammation initiates an NF-κB-dependent positive feedback loop that amplifies K-Ras activity (6). Moreover, tumors harboring mutant K-Ras develop in an inflammatory microenvironment fostered by the oncoprotein (44, 65, 68), and this selective pressure likely contributes to adaptation and escape from antitumor immunity.
High serum levels of MMP-9 (76) or immunohistochemical detection of MMP-9 in tumor specimens (80) are poor prognostic indicators in lung cancer. Moreover, increasing serum MMP-9 concentrations correlate with disease severity in chronic obstructive pulmonary disease, COPD (51), a disease that predisposes lung cancer (21). Chemokine-mediated release of MMP-9 stored in tertiary granules of neutrophils infiltrating the lung can exacerbate COPD (71) and lung cancer (22). Indeed, tumor-associated leukocytes appear to be a major source of MMP-9 during stimulation of tumor growth by AdV-IL-17 (Fig. 3C). Active MMP-9 can release growth factors, promote angiogenesis, accelerate tumor cell migration into surrounding normal tissue, and prepare the premetastatic niche (8, 20, 66, 77), which likely contributes to the association of MMP-9 expression with increasing lung tumor grade (46). Our data demonstrating MMP-9-dependent migration of lung epithelial cells agree with previous findings that addition of active MMP-9 accelerates migration of A549 cells (62) and that MMP-9 expression by alveolar type II cells is essential for wound healing (53). Knockdown or mutation of p53 increases lung tumor cell motility and abrogates IL-17-mediated induction of MMP-9. In addition, IL-17-mediated stabilization of MMP-9 mRNA by disruption of SRSF1 interaction coincides with previous findings demonstrating this mechanism of mRNA regulation for chemokine mRNAs (67).
Improving the survival rates in non-small cell lung cancer (NSCLC) will require the identification of novel therapeutic targets. Our data are consistent with the view that cigarette smoke promotes progression of NSCLC by inducing expression of IL-17. One mechanism that could account for enhanced proliferation in response to IL-17 is induction of MMP-9. Our data also suggest that, once p53 is inactivated by mutation, lung tumor promotion would be independent of IL-17. In NSCLC, serum MMP-9 levels potentially serve as a prognostic marker (31) and an indicator of response to chemotherapy (9). Although inhibitors of matrix metalloproteinases have not improved survival in patients with NSCLC, clinical trial end points and the late stage of disease in study participants have complicated the interpretation of these findings (5). Toward a goal of personalized therapy, our results suggest that MMP-9 inhibition in lung tumors with high levels of IL-17 and wild-type p53 warrants reinvestigation.
GRANTS
This work was supported by National Institutes of Health Grant CA132603 (B. Shan, D. E. Sullivan, G. F. Morris) and by the Wetmore Foundation. B. Xu received fellowship support from the Foundation in Lung Pathobiology and Matching Funds from the Tulane Cancer Center. Z. You received grants from the National Institutes of Health (P20GM103518 and R01CA174714) and Department of Defense (PC121647, PC13448, and PC130118).
DISCLOSURES
J. Kolls is employed by Vitae and got grants from Amgen and Constellation Pharmaceuticals.
AUTHOR CONTRIBUTIONS
B.X., D.A.P., J.K.K., B.C., B.S., D.E.S., and G.F.M. conception and design of research; B.X., J.F.G., Y.W., B.S., D.E.S., and G.F.M. performed experiments; B.X., B.S., D.E.S., and G.F.M. analyzed data; B.X., Z.Y., B.C., B.S., D.E.S., and G.F.M. interpreted results of experiments; B.X. and G.F.M. prepared figures; B.X. and G.F.M. drafted manuscript; B.X., D.A.P., B.S., D.E.S., and G.F.M. edited and revised manuscript; B.X., J.F.G., D.A.P., Y.W., J.K.K., Z.Y., B.C., B.S., D.E.S., and G.F.M. approved final version of manuscript.
REFERENCES
- 1.Benevides L, Cardoso CR, Tiezzi DG, Marana HR, Andrade JM, Silva JS. Enrichment of regulatory T cells in invasive breast tumor correlates with the upregulation of IL-17A expression and invasiveness of the tumor. Eur J Immunol 43: 1518–1528, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Chang CK, Hung WC, Chang HC. The Kazal motifs of RECK protein inhibit MMP-9 secretion and activity and reduce metastasis of lung cancer cells in vitro and in vivo. J Cell Mol Med 12: 2781–2789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, Moghaddam SJ, Dong C. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci USA 111: 5664–5669, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls JK, Zheng M. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One 6: e20333, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295: 2387–2392, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S, Cruz-Monserrate Z, Wang H, Ji B, Logsdon CD. An NF-kappaB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest 122: 1519–1528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diament MJ, Peluffo GD, Stillitani I, Cerchietti LC, Navigante A, Ranuncolo SM, Klein SM. Inhibition of tumor progression and paraneoplastic syndrome development in a murine lung adenocarcinoma by medroxyprogesterone acetate and indomethacin. Cancer Invest 24: 126–131, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2: 161–174, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Ertan E, Soydinc H, Yazar A, Ustuner Z, Tas F, Yasasever V. Matrix metalloproteinase-9 decreased after chemotherapy in patients with non-small cell lung cancer. Tumori 97: 286–289, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev 26: 1268–1286, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fregoso OI, Das S, Akerman M, Krainer AR. Splicing-factor oncoprotein SRSF1 stabilizes p53 via RPL5 and induces cellular senescence. Mol Cell 50: 56–66, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12: 298–306, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Friedlander PL, Delaune CL, Abadie JM, Toups M, LaCour J, Marrero L, Zhong Q, Kolls JK. Efficacy of CD40 ligand gene therapy in malignant mesothelioma. Am J Respir Cell Mol Biol 29: 321–330, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9: 556–567, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest 118: 3012–3024, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491: 254–258, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guenther JF, Cameron JE, Nguyen HT, Wang Y, Sullivan DE, Shan B, Lasky JA, Flemington EK, Morris GF. Modulation of lung inflammation by the Epstein-Barr virus protein Zta. Am J Physiol Lung Cell Mol Physiol 299: L771–L784, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemdan NY. Anti-cancer vs. cancer-promoting effects of the interleukin-17-producing T helper cells. Immunol Lett 149: 123–133, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Herjan T, Yao P, Qian W, Li X, Liu C, Bulek K, Sun D, Yang WP, Zhu J, He A, Carman JA, Erzurum SC, Lipshitz HD, Fox PL, Hamilton TA. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J Immunol 191: 640–649, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2: 289–300, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer 13: 233–245, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Houghton AM. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle 9: 1732–1737, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, Jenkins KM, Beaulieu KA, Mouded M, Frank SJ, Wong KK, Shapiro SD. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med 16: 219–223, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Y, Ota N, Peng I, Refino CJ, Danilenko DM, Caplazi P, Ouyang W. IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol 184: 4307–4316, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, Jacks T. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res 65: 10280–10288, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kao SJ, Su JL, Chen CK, Yu MC, Bai KJ, Chang JH, Bien MY, Yang SF, Chien MH. Osthole inhibits the invasive ability of human lung adenocarcinoma cells via suppression of NF-kappaB-mediated matrix metalloproteinase-9 expression. Toxicol Appl Pharmacol 261: 105–115, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Kasinski AL, Slack FJ. miRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53-induced mouse model of lung adenocarcinoma. Cancer Res 72: 5576–5587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity 21: 467–476, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 114: 357–359, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol 179: 5462–5473, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laack E, Kohler A, Kugler C, Dierlamm T, Knuffmann C, Vohwinkel G, Niestroy A, Dahlmann N, Peters A, Berger J, Fiedler W, Hossfeld DK. Pretreatment serum levels of matrix metalloproteinase-9 and vascular endothelial growth factor in non-small-cell lung cancer. Ann Oncol 13: 1550–1557, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse compared with intratracheal instillation. Exp Lung Res 32: 181–199, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, El-Naggar AK, Lozano G. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119: 861–872, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Le Gouvello S, Bastuji-Garin S, Aloulou N, Mansour H, Chaumette MT, Berrehar F, Seikour A, Charachon A, Karoui M, Leroy K, Farcet JP, Sobhani I. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut 57: 772–779, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Li C, Nguyen HT, Zhuang Y, Lin Y, Flemington EK, Guo W, Guenther J, Burow ME, Morris GF, Sullivan D, Shan B. Post-transcriptional up-regulation of miR-21 by type I collagen. Mol Carcinog 50: 563–570, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Li J, Lau GK, Chen L, Dong SS, Lan HY, Huang XR, Li Y, Luk JM, Yuan YF, Guan XY. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One 6: e21816, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Han Y, Fei G, Guo Z, Ren T, Liu Z. IL-17 promoted metastasis of non-small-cell lung cancer cells. Immunol Lett 148: 144–150, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Cao ZY, Sun B, Wang GY, Fu Z, Liu YM, Kong QF, Wang JH, Zhang Y, Xu XY, Li HL. Effects of IL-17A on the occurrence of lung adenocarcinoma. Cancer Biol Ther 12: 610–616, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Maniati E, Soper R, Hagemann T. Up for Mischief? IL-17/Th17 in the tumour microenvironment. Oncogene 29: 5653–5662, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin-Orozco N, Dong C. The IL-17/IL-23 axis of inflammation in cancer: friend or foe? Curr Opin Investig Drugs 10: 543–549, 2009 [PubMed] [Google Scholar]
- 42.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31: 787–798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martins SJ, Takagaki TY, Silva AG, Gallo CP, Silva FB, Capelozzi VL. Prognostic relevance of TTF-1 and MMP-9 expression in advanced lung adenocarcinoma. Lung Cancer 64: 105–109, 2009 [DOI] [PubMed] [Google Scholar]
- 44.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, Jaffee EM, Drake CG, Housseau F, Maitra A, Kolls JK, Sears CL, Pardoll DM, Leach SD. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 25: 621–637, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, Jacks T. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature 462: 104–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minamoto H, Antonangelo L, da Silva AG, Gallo CP, de Andrade e Silva FB, Fenezelian S, Rodrigues OR, Jatene F, Saldiva P, Capelozzi VL. Tumour cell and stromal features in metastatic and non-metastatic non-small cell lung carcinomas. Histopathology 43: 427–443, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 11: 763–776, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Moghaddam SJ, Li H, Cho SN, Dishop MK, Wistuba II, Ji L, Kurie JM, Dickey BF, Demayo FJ. Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am J Respir Cell Mol Biol 40: 443–453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris GF, Hoyle GW, Athas GB, Lei WH, Xu J, Morris CB, Friedman M. Lung-specific expression in mice of a dominant negative mutant form of the p53 tumor suppressor protein. J La State Med Soc 150: 179–185, 1998 [PubMed] [Google Scholar]
- 50.Morris GF, Labrie C, Mathews MB. Modulation of transcriptional activation of the proliferating cell nuclear antigen promoter by the adenovirus E1A 243-residue oncoprotein depends on proximal activators. Mol Cell Biol 14: 543–553, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navratilova Z, Zatloukal J, Kriegova E, Kolek V, Petrek M. Simultaneous up-regulation of matrix metalloproteinases 1, 2, 3, 7, 8, 9 and tissue inhibitors of metalloproteinases 1, 4 in serum of patients with chronic obstructive pulmonary disease. Respirology 17: 1006–1012, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol 175: 6177–6189, 2005 [DOI] [PubMed] [Google Scholar]
- 53.O'Kane CM, McKeown SW, Perkins GD, Bassford CR, Gao F, Thickett DR, McAuley DF. Salbutamol up-regulates matrix metalloproteinase-9 in the alveolar space in the acute respiratory distress syndrome. Crit Care Med 37: 2242–2249, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 107: 789–800, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Okamoto T, Valacchi G, Gohil K, Akaike T, van der Vliet A. S-nitrosothiols inhibit cytokine-mediated induction of matrix metalloproteinase-9 in airway epithelial cells. Am J Respir Cell Mol Biol 27: 463–473, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6: 1133–1141, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reppert S, Boross I, Koslowski M, Tureci O, Koch S, Lehr HA, Finotto S. A role for T-bet-mediated tumour immune surveillance in anti-IL-17A treatment of lung cancer. Nat Commun 2: 600, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol 161: 6383–6389, 1998 [PubMed] [Google Scholar]
- 59.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res 14: 3254–3261, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shan B, Morris GF. Binding sequence-dependent regulation of the human proliferating cell nuclear antigen promoter by p53. Exp Cell Res 305: 10–22, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Shan M, Yuan X, Song LZ, Roberts L, Zarinkamar N, Seryshev A, Zhang Y, Hilsenbeck S, Chang SH, Dong C, Corry DB, Kheradmand F. Cigarette smoke induction of osteopontin (SPP1) mediates TH17 inflammation in human and experimental emphysema. Sci Transl Med 4: 117ra119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shyamsundar M, McAuley DF, Ingram RJ, Gibson DS, O'Kane D, McKeown ST, Edwards A, Taggart C, Elborn JS, Calfee CS, Matthay MA, O'Kane CM. Keratinocyte growth-factor promotes epithelial survival and resolution in a human model of lung injury. Am J Respir Crit Care Med 189: 1520–1529, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siddesha JM, Valente AJ, Sakamuri SS, Yoshida T, Gardner JD, Somanna N, Takahashi C, Noda M, Chandrasekar B. Angiotensin II stimulates cardiac fibroblast migration via the differential regulation of matrixins and RECK. J Mol Cell Cardiol 65: 9–18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sossey-Alaoui K, Ranalli TA, Li X, Bakin AV, Cowell JK. WAVE3 promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res 308: 135–145, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 6: 447–458, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 200: 448–464, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF). Nat Immunol 12: 853–860, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sunaga N, Imai H, Shimizu K, Shames DS, Kakegawa S, Girard L, Sato M, Kaira K, Ishizuka T, Gazdar AF, Minna JD, Mori M. Oncogenic KRAS-induced interleukin-8 overexpression promotes cell growth and migration and contributes to aggressive phenotypes of non-small cell lung cancer. Int J Cancer 130: 1733–1744, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell 17: 89–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.To Y, Dohi M, Matsumoto K, Tanaka R, Sato A, Nakagome K, Nakamura T, Yamamoto K. A two-way interaction between hepatocyte growth factor and interleukin-6 in tissue invasion of lung cancer cell line. Am J Respir Cell Mol Biol 27: 220–226, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Vlahos R, Wark PA, Anderson GP, Bozinovski S. Glucocorticosteroids differentially regulate MMP-9 and neutrophil elastase in COPD. PLoS One 7: e33277, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W. Th17 cells in cancer: help or hindrance? Carcinogenesis 32: 643–649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu KC, Yang ST, Hsia TC, Yang JS, Chiou SM, Lu CC, Wu RS, Chung JG. Suppression of cell invasion and migration by propofol are involved in down-regulating matrix metalloproteinase-2 and p38 MAPK signaling in A549 human lung adenocarcinoma epithelial cells. Anticancer Res 32: 4833–4842, 2012 [PubMed] [Google Scholar]
- 74.Wu MH, Hong TM, Cheng HW, Pan SH, Liang YR, Hong HC, Chiang WF, Wong TY, Shieh DB, Shiau AL, Jin YT, Chen YL. Galectin-1-mediated tumor invasion and metastasis, up-regulated matrix metalloproteinase expression, and reorganized actin cytoskeletons. Mol Cancer Res 7: 311–318, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Xue W, Meylan E, Oliver TG, Feldser DM, Winslow MM, Bronson R, Jacks T. Response and resistance to NF-kappaB inhibitors in mouse models of lung adenocarcinoma. Cancer Discov 1: 236–247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ylisirnio S, Hoyhtya M, Turpeenniemi-Hujanen T. Serum matrix metalloproteinases -2, -9 and tissue inhibitors of metalloproteinases -1, -2 in lung cancer–TIMP-1 as a prognostic marker. Anticancer Res 20: 1311–1316, 2000 [PubMed] [Google Scholar]
- 77.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev 13: 35–48, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun 374: 533–537, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Zhang Q, Liu S, Ge D, Xue Y, Xiong Z, Abdel-Mageed AB, Myers L, Hill SM, Rowan BG, Sartor O, Melamed J, Chen Z, You Z. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res 72: 2589–2599, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng S, Chang Y, Hodges KB, Sun Y, Ma X, Xue Y, Williamson SR, Lopez-Beltran A, Montironi R, Cheng L. Expression of KISS1 and MMP-9 in non-small cell lung cancer and their relations to metastasis and survival. Anticancer Res 30: 713–718, 2010 [PubMed] [Google Scholar]