Abstract
Introduction:
Chagas disease is a zoonotic disease caused by Trypanosoma cruzi and dogs are one of the main domestic reservoirs.
Materials and Methods:
One molecular (OligoC-TesT, Coris Bioconcept) and one serological (T. cruzi-Detect, Inbios) rapid tests were evaluated as infection markers for T. cruzi in 102 dogs living in eight villages endemic for Chagas in Costa Rica.
Results:
T. cruzi-Detect performed well as screening tool with 23.3% positive samples. The large number of invalid results (66.7%) observed in samples tested with OligoC-TesT precluded assessing the use of this new method as epidemiological tool to detect T. cruzi infection in dogs.
KEY WORDS: Asymptomatic infections, Chagas, Costa Rica, diagnostic tools, T. cruzi-Detect, OligoC-TesT, rapid test, Trypanosoma cruzi
INTRODUCTION
Trypanosoma cruzi is the etiological agent of Chagas disease; one of the most important vector-borne diseases in Latin America. In endemic areas, even if other transmission routes exist, T. cruzi is mainly spread by triatomine bugs. In Costa Rica Triatoma dimidiata is the main vector of T. cruzi at domiciliary and peridomiciliary levels.[1] In humans, Chagas disease is characterized by a brief acute phase usually asymptomatic followed by a lifelong chronic phase often associated with cardiac and digestive lesions. In Costa Rica T. cruzi infection tends to cluster in poor rural settings where Chagas prevalence is higher.[2] Dogs have been considered the main domestic reservoirs of T. cruzi in most Latin American countries and in some areas of USA.[3,4,5,6] However dogs are also common victims of the disease developing chronic cardiac manifestations similar to those detected in humans.[3] Actually dogs are considered to be at higher risk of T. cruzi infection than humans.[7] In a previous study, 27.7% (15/54) and 5.5% (3/54) of asymptomatic dogs from Costa Rica were found seropositive and positive to xenodiagnosis, respectively.[6] The seroprevalences observed in dogs from other endemic areas are highly variable: 4.3% in West Indies,[8] 6.4% in Tennessee, USA,[9] 7.6% in Mexico,[7] 16.2% in Panama,[10] from 11% to 89% in North-Eastern Brazil[11,12] and between 6.9% and 67.6% in Venezuela.[5,13] However it is difficult to compare those results as the sampling and analytical methods varied. Finally, the number of studies reporting molecular diagnostic methods to detect T. cruzi in asymptomatic dogs is limited, e.g.,: 5.1% polymerase chain reaction (PCR) positive dogs in Panama.[10]
A number of rapid diagnostic tests (RDT) for Chagas disease are now available. Immunochromatographic tests (ICTs) are RDT that can be performed in the field and provide a result on the spot. ICTs have been used for T. cruzi infection in dogs in USA[8] and Argentina.[14] More recently, an oligochromatographic (OligoC-TesT) test was developed to diagnose Chagas disease in humans.[15] T. cruzi OligoC-TesT, a simplified and standardized PCR format, has been validated for use in humans.[16] However the T. cruzi OligoC-TesT has not yet been evaluated in dogs. The aim of this study was to evaluate one serological (T. cruzi-Detect) and one molecular (OligoC-TesT) RDT for T. cruzi infection in dogs in Costa Rica.
MATERIALS AND METHODS
Study population and samples
Dogs from 8 villages in Costa Rica were included in the study [Figure 1]. These villages were selected based on previous evidence of T. cruzi infection in dogs and vectors and/or previous presence of human cases of Chagas from published papers or reports from the Ministry of Health or from the Universidad de Costa Rica. To maximize the number of dogs included in the study, blood samples (6 ml) from all dogs (1) attending the villages’ veterinary clinic on the day of the visit and (2) identified during house to house surveys in the study villages were collected by cephalic venepuncture in June 2011. Three ml were collected in a potassium- EDTA vacutainer tube mixed immediately and stored at 4°C before being processed for genomic DNA extraction. The other 3 ml were transferred to a Z-serum clot activator vacutainer tube, incubated for 20 min at room-temperature and centrifugated for 10 min at 3000 rpm. The serum was collected and stored at −20°C for serological analyses.
Figure 1.
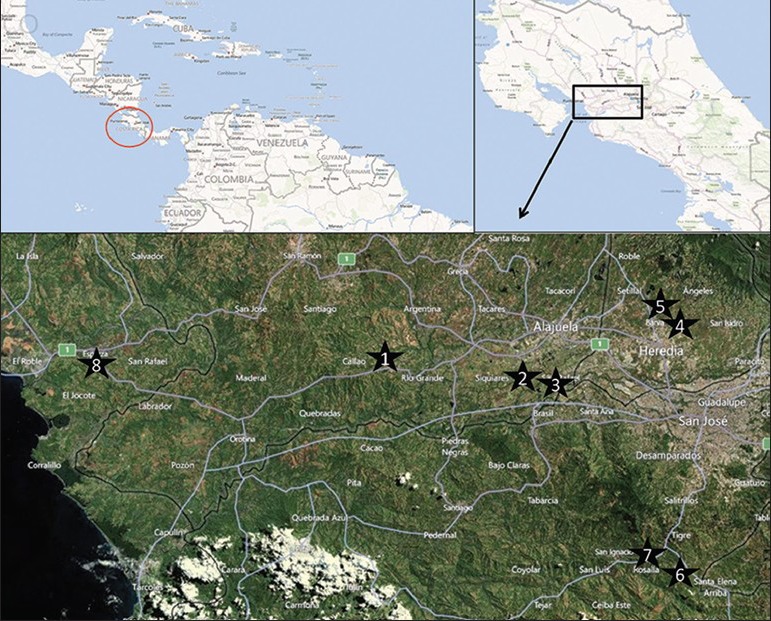
Location of the study villages in Costa Rica: (1) Atenas (Province of Alajuela), (2) la Guácima (Province of Alajuela), (3) San Rafael (Province of Alajuela), (4) San Rafael (Province of Heredia), (5) Getsemani (Province of Heredia), (6) San Gabriel de Aserri (Province of San Jose) and (7) Vuelta de Jorco (Province of San Jose) in the Central Valley and (8) Esparza (Province of Puntarenas) on the western part of the country, 80 km from the capital, San José
Trypanosoma cruzi OligoC-TesT
Total DNA was extracted from whole blood samples (300 μl of whole blood per animal) using the Wizard Genomic DNA Purification kit (Promega, Southampton, UK) following the manufacturer's recommendations with two modifications: Incubation in isopropanol was prolonged (overnight), and the final DNA pellet was resuspended in a smaller volume (50 μl) of DNA rehydratation solution. Extracted DNA was stored at −20°C.
OligoC-TesT kits were provided by the manufacturer (Coris Bioconcept, Gembloux, Belgium) and were used according to product recommendations for detection of T. cruzi DNA.[15] Each PCR reaction consisted of 47.3 μl of the T. cruzi ampli-mix, 0.2 μl (1 unit) of Hot Start Taq DNA (Qiagen, Manchester, UK) and 2.5 μl of extracted sample DNA. 2.5 μl of T. cruzi ampli-control were used as positive control and 2.5 μl of PCR-grade water were used as negative control. Thermal cycling conditions were as follows: 94°C for 15 min, 40 cycles of 94°C for 30 s, 65°C for 20 s, 72°C for 20 s, and one cycle of 72°C for 1 min and 94°C for 30 s. All PCR reactions were performed in a Thermal cycler (Applied Biosystems, Madrid, Spain). Amplified products were kept at −20°C until oligochromatographic detection was performed. Prior to detection, the multi-plate was preheated in the thermal cycler (94°C for 30 s). Briefly, 40 μl of PCR products were mixed with 40 μl of migration buffer in individual assay tubes. A T. cruzi Oligo-Strip was immediately dipped in this mixture. A positive result was recorded after 5 min of incubation at 55°C when both the T. cruzi test line and the internal control line were visible, together with migration control lines, which indicates correct running of the test strip buffer. A positive result was also recorded when only the T. cruzi test line and migration control lines were visible. Negative results were recorded when only the internal control line and migration control lines were visible. PCR inhibition (“invalid test”) was recorded when migration control lines were visible but neither T. cruzi test nor internal control lines were visible.
Trypanosoma cruzi-Detect-Canine dipstick test
The dipstick test (T. cruzi-Detect-Canine, Inbios, Seattle, WA) was carried out according to the manufacturer's instructions. Serum (20 μl) and the provided chase buffer solution (150-200 μl) were added onto the dipsticks. After 10 min, a red control line and if the result was positive, a second line appeared on the test field. Only 90 T. cruzi-Detect-Canine tests were available.
Ethical considerations
Ethical clearance for this study was obtained from the Ethics Committee of the School of Veterinary Medicine of Heredia, Costa Rica. Informed consent was obtained from the dogs’ owners before including them in the study.
RESULTS AND DISCUSSION
A blood sample was obtained from 102 dogs living in eight Chagas endemic villages [Figure 1]. 73 (71%) of the samples were collected during the house to house surveys and the rest (n = 29) were obtained from dogs attending veterinary clinics. The majority of dogs included in the study were males (59.8%) and had the following age distribution: 22.5% puppies (0–1 years), 41.2% young adults (2–4 years), 14.7% adults (5–6 years) and 21.6% old adults (≥7 years).
The results show a high variability between the two tests [Table 1]. The prevalence reported using T. cruzi-Detect was 23.3% (21/90) was similar to seroprevalences reported in other studies in Central America using serological tests.[6,10] However, this figure should be interpreted with caution as the sampling was not random. T. cruzi-Detect is rapid and easy to use and it has been suggested as a good screening tool for T. cruzi infection in dogs in the field.[8,14,17] However serological tests should be coupled to confirmatory tests.[17,18] The observed prevalence using OligoC-TesT was lower (8.8%; 9/102). It is however remarkable that 66.7% of the samples tested (68/102) gave “invalid” results. The OligoC-TesT positive prevalence would be 26.5% (9/34), if the invalid samples were discarded. This high number of invalid OligoC-TesT results is surprising and is probably caused by inhibitory components in the DNA extracts. The OligoC-TesT has never been evaluated in dogs and this study indicates that the test is not compatible with blood samples from dogs and needs further optimization. Molecular tests like OligoC-TesT are potential confirmatory tests as they are more specific than serological tests,[15] which are subjected to cross reaction with other pathogens.[5,19,20] The poor performance of the OligoC-TesT made difficult comparing both tests. Nevertheless, the agreement between serological tests and molecular tests was very poor. Out of nine OligoC-TesT positive samples, eight were T. cruzi-Detect negative and one did not have a serological result. Discordant molecular and serological results has already been reported in other studies using PCR and serological tests for T. cruzi infection in humans[21] and other kinetoplastid diseases like leishmaniasis.[22]
Table 1.
Results of the T. cruzi-detect and T. cruzi OligoC-TesT in dogs living in Chagas endemic villages in Costa Rica

Despite the risk of cross-reactivity, T. cruzi-Detect seems to be an appropriate tool to screen for T. cruzi infection in dogs in epidemiological studies. Unfortunately the large number of invalid results in this study precluded assessing the use of OligoC-TesT as marker of T. cruzi infection in dogs. Nevertheless, the OligoC-TesT technology represents and interesting alternative/complement to serological tests.
ACKNOWLEDGMENTS
We are grateful to InBios International, Inc. that provided Trypanosoma cruzi-Detect-Canine dipsticks for free and to Coris Bioconcept that provided T. cruzi OligoC-Tests at a reduced cost for the study. We would like to thank the personnel from UNA (Sergio Alfaro) and UCR (Cristian Fonseca y Heriberto Gutierrez) who assisted us. We would also thank the veterinarians and pet owners participating in the study.
Footnotes
Source of Support: Royal Society (International Travel Grants - 2010/R3). S. Deborggraeve is a postdoctoral fellow of the Research Foundation Flanders (FWO)
Conflict of Interest: None declared.
REFERENCES
- 1.Hashimoto K, Schofield CJ. Elimination of Rhodnius prolixus in Central America. Parasit Vectors. 2012;5:45. doi: 10.1186/1756-3305-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeledón R, Solano G, Burstin L, Swartzwelder JC. Epidemiological pattern of Chagas’ disease in an endemic area of Costa Rica. Am J Trop Med Hyg. 1975;24:214–25. doi: 10.4269/ajtmh.1975.24.214. [DOI] [PubMed] [Google Scholar]
- 3.Barr SC. Canine Chagas’ disease (American trypanosomiasis) in North America. Vet Clin North Am Small Anim Pract. 2009;39:1055–64. doi: 10.1016/j.cvsm.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Gürtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134:69–82. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crisante G, Rojas A, Teixeira MM, Añez N. Infected dogs as a risk factor in the transmission of human Trypanosoma cruzi infection in western Venezuela. Acta Trop. 2006;98:247–54. doi: 10.1016/j.actatropica.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Montenegro VM, Jimenez M, Dias JC, Zeledon R. Chagas disease in dogs from endemic areas of Costa Rica. Mem Inst Oswaldo Cruz. 2002;97:491–4. doi: 10.1590/s0074-02762002000400006. [DOI] [PubMed] [Google Scholar]
- 7.Balan LU, Yerbes IM, Piña MA, Balmes J, Pascual A, Hernández O, et al. Higher seroprevalence of Trypanosoma cruzi infection in dogs than in humans in an urban area of Campeche, Mexico. Vector Borne Zoonotic Dis. 2011;11:843–4. doi: 10.1089/vbz.2010.0039. [DOI] [PubMed] [Google Scholar]
- 8.Rosypal AC, Tripp S, Kinlaw C, Sharma RN, Stone D, Dubey JP. Seroprevalence of canine leishmaniasis and American trypanosomiasis in dogs from Grenada, West Indies. J Parasitol. 2010;96:228–9. doi: 10.1645/GE-2238.1. [DOI] [PubMed] [Google Scholar]
- 9.Rowland ME, Maloney J, Cohen S, Yabsley MJ, Huang J, Kranz M, et al. Factors associated with Trypanosoma cruzi exposure among domestic canines in Tennessee. J Parasitol. 2010;96:547–51. doi: 10.1645/GE-2299.1. [DOI] [PubMed] [Google Scholar]
- 10.Pineda V, Saldaña A, Monfante I, Santamaría A, Gottdenker NL, Yabsley MJ, et al. Prevalence of trypanosome infections in dogs from Chagas disease endemic regions in Panama, Central America. Vet Parasitol. 2011;178:360–3. doi: 10.1016/j.vetpar.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 11.Xavier SC, Roque AL, Lima Vdos S, Monteiro KJ, Otaviano JC, Ferreira da Silva LF, et al. Lower richness of small wild mammal species and chagas disease risk. PLoS Negl Trop Dis. 2012;6:e1647. doi: 10.1371/journal.pntd.0001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lima MM, Sarquis O, de Oliveira TG, Gomes TF, Coutinho C, Daflon-Teixeira NF, et al. Investigation of Chagas disease in four periurban areas in northeastern Brazil: Epidemiologic survey in man, vectors, non-human hosts and reservoirs. Trans R Soc Trop Med Hyg. 2012;106:143–9. doi: 10.1016/j.trstmh.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Bonfante-Cabarcas R, Rodríguez-Bonfante C, Vielma BO, García D, Saldivia AM, Aldana E, et al. Seroprevalence for Trypanosoma cruzi infection and associated factors in an endemic area of Venezuela. Cad Saude Publica. 2011;27:1917–29. doi: 10.1590/s0102-311x2011001000005. [DOI] [PubMed] [Google Scholar]
- 14.Cardinal MV, Reithinger R, Gürtler RE. Use of an immunochromatographic dipstick test for rapid detection of Trypanosoma cruzi in sera from animal reservoir hosts. J Clin Microbiol. 2006;44:3005–7. doi: 10.1128/JCM.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deborggraeve S, Coronado X, Solari A, Zulantay I, Apt W, Mertens P, et al. T. cruzi OligoC-TesT: A simplified and standardized polymerase chain reaction format for diagnosis of Chagas disease. PLoS Negl Trop Dis. 2009;3:e450. doi: 10.1371/journal.pntd.0000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Winne K, Büscher P, Luquetti AO, Tavares SB, Oliveira RA, Solari A, et al. The Trypanosoma cruzi satellite DNA OligoC-TesT and Trypanosoma cruzi kinetoplast DNA OligoC-TesT for diagnosis of Chagas disease: A multi-cohort comparative evaluation study. PLoS Negl Trop Dis. 2014;8:e2633. doi: 10.1371/journal.pntd.0002633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieto PD, Boughton R, Dorn PL, Steurer F, Raychaudhuri S, Esfandiari J, et al. Comparison of two immunochromatographic assays and the indirect immunofluorescence antibody test for diagnosis of Trypanosoma cruzi infection in dogs in south central Louisiana. Vet Parasitol. 2009;165:241–7. doi: 10.1016/j.vetpar.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Picka MC, Meira DA, de Carvalho TB, Peresi E, Marcondes-Machado J. Definition of a diagnostic routine in individuals with inconclusive serology for Chagas disease. Braz J Infect Dis. 2007;11:226–33. doi: 10.1590/s1413-86702007000200012. [DOI] [PubMed] [Google Scholar]
- 19.Alves AS, Mouta-Confort E, Figueiredo FB, Oliveira RV, Schubach AO, Madeira MF. Evaluation of serological cross-reactivity between canine visceral leishmaniasis and natural infection by Trypanosoma caninum. Res Vet Sci. 2012;93:1329–33. doi: 10.1016/j.rvsc.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Umezawa ES, Souza AI, Pinedo-Cancino V, Marcondes M, Marcili A, Camargo LM, et al. TESA-blot for the diagnosis of Chagas disease in dogs from co-endemic regions for Trypanosoma cruzi, Trypanosoma evansi and Leishmania chagasi. Acta Trop. 2009;111:15–20. doi: 10.1016/j.actatropica.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Salomone OA, Basquiera AL, Sembaj A, Aguerri AM, Reyes ME, Omelianuk M, et al. Trypanosoma cruzi in persons without serologic evidence of disease, Argentina. Emerg Infect Dis. 2003;9:1558–62. doi: 10.3201/eid0912.030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattarai NR, Van der Auwera G, Khanal B, De Doncker S, Rijal S, Das ML, et al. PCR and direct agglutination as Leishmania infection markers among healthy Nepalese subjects living in areas endemic for Kala-Azar. Trop Med Int Health. 2009;14:404–11. doi: 10.1111/j.1365-3156.2009.02242.x. [DOI] [PubMed] [Google Scholar]


