Abstract
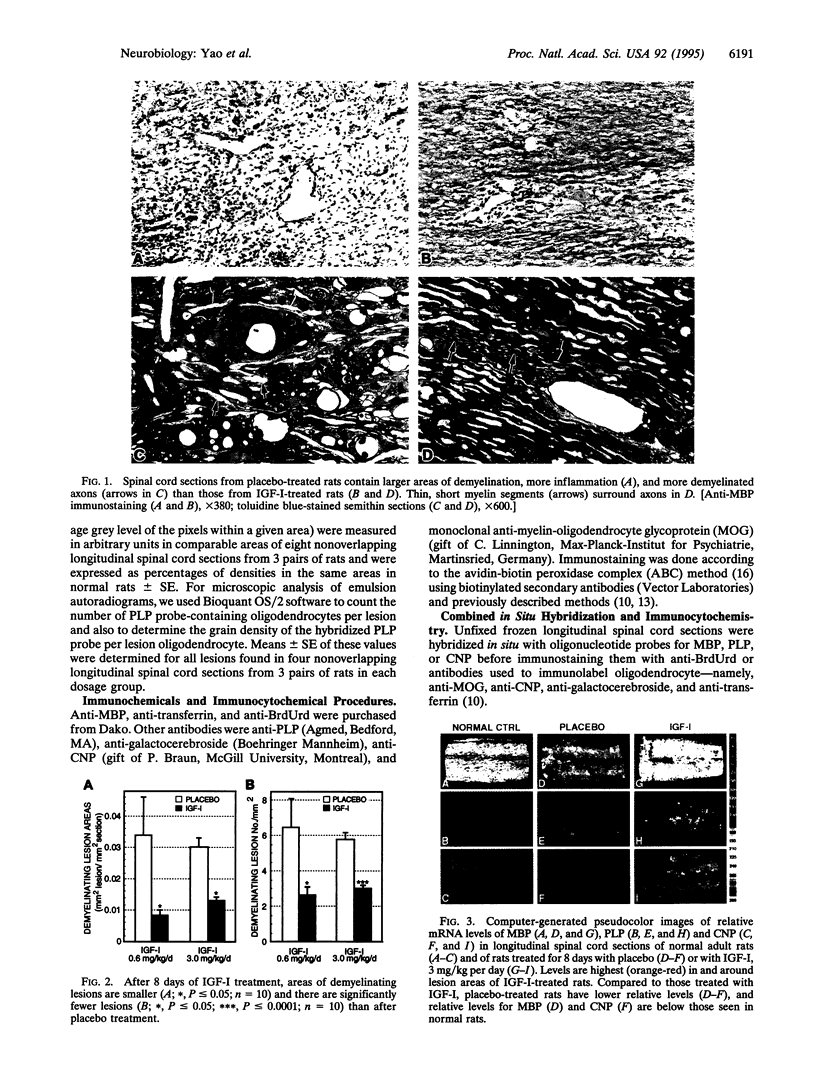
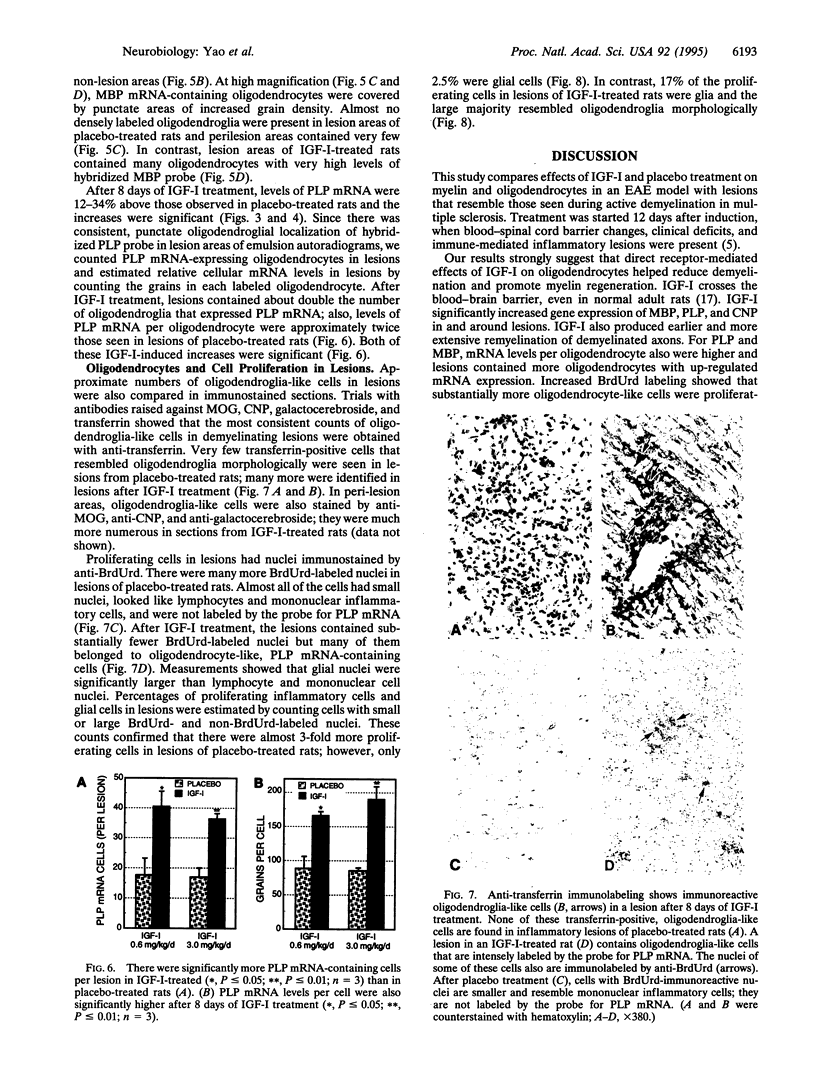
To compare effects of insulin-like growth factor I (IGF-I) and placebo treatment on lesions that resemble those seen during active demyelination in multiple sclerosis, we induced experimental autoimmune encephalomyelitis in Lewis rats with an emulsion containing guinea pig spinal cord and Freund's adjuvant. On day 12-13, pairs of rats with the same degree of weakness were given either IGF-I or placebo intravenously twice daily for 8 days. After 8 days of placebo or IGF-I (200 micrograms/day or 1 mg/day) treatment, the spinal cord lesions were studied by in situ hybridization and with immunocytochemical and morphological methods. IGF-I produced significant reductions in numbers and areas of demyelinating lesions. These lesions contained axons surrounded by regenerating myelin segments instead of demyelinated axons seen in the placebo-treated rats. Relative mRNA levels for myelin basic protein, proteolipid protein (PLP), and 2',3'-cyclic nucleotide 3'-phosphodiesterase in lesions of IGF-I-treated rats were significantly higher than they were in placebo-treated rats. PLP mRNA-containing oligodendroglia also were more numerous and relative PLP mRNA levels per oligodendrocyte were higher in lesions of IGF-I-treated rats. Finally, a significantly higher proportion of proliferating cells were oligodendroglia-like cells in lesions of IGF-I-treated rats. We think that IGF-I effects on oligodendrocytes, myelin protein synthesis, and myelin regeneration reduced lesion severity and promoted clinical recovery in this experimental autoimmune encephalomyelitis model. These IGF-I actions may also benefit patients with multiple sclerosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar R. S., Boes M., Dake B. L., Booth B. A., Henley S. A., Sandra A. Insulin, insulin-like growth factors, and vascular endothelium. Am J Med. 1988 Nov 28;85(5A):59–70. doi: 10.1016/0002-9343(88)90398-1. [DOI] [PubMed] [Google Scholar]
- Bar R. S., Booth B. A., Boes M., Dake B. L. Insulin-like growth factor-binding proteins from vascular endothelial cells: purification, characterization, and intrinsic biological activities. Endocrinology. 1989 Oct;125(4):1910–1920. doi: 10.1210/endo-125-4-1910. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992 Jul 10;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Schmid R., Sendnter M., Raff M. C. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993 May;118(1):283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Beck K. D., Powell-Braxton L., Widmer H. R., Valverde J., Hefti F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995 Apr;14(4):717–730. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- Bondy C. A., Underwood L. E., Clemmons D. R., Guler H. P., Bach M. A., Skarulis M. Clinical uses of insulin-like growth factor I. Ann Intern Med. 1994 Apr 1;120(7):593–601. doi: 10.7326/0003-4819-120-7-199404010-00011. [DOI] [PubMed] [Google Scholar]
- Brück W., Schmied M., Suchanek G., Brück Y., Breitschopf H., Poser S., Piddlesden S., Lassmann H. Oligodendrocytes in the early course of multiple sclerosis. Ann Neurol. 1994 Jan;35(1):65–73. doi: 10.1002/ana.410350111. [DOI] [PubMed] [Google Scholar]
- Carson M. J., Behringer R. R., Brinster R. L., McMorris F. A. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993 Apr;10(4):729–740. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- Clark R., Strasser J., McCabe S., Robbins K., Jardieu P. Insulin-like growth factor-1 stimulation of lymphopoiesis. J Clin Invest. 1993 Aug;92(2):540–548. doi: 10.1172/JCI116621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspan J. B., Stern J., Franceschini B., Yasuda T., Pleasure D. Protein growth factors as potential therapies for central nervous system demyelinative disorders. Ann Neurol. 1994;36 (Suppl):S140–S142. doi: 10.1002/ana.410360734. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Karussis D. M., Lehmann D., Slavin S., Vourka-Karussis U., Mizrachi-Koll R., Ovadia H., Kalland T., Abramsky O. Treatment of chronic-relapsing experimental autoimmune encephalomyelitis with the synthetic immunomodulator linomide (quinoline-3-carboxamide). Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6400–6404. doi: 10.1073/pnas.90.14.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D., Taubenberger J. K., Cannella B., McFarlin D. E., Raine C. S., McFarland H. F. Correlation between magnetic resonance imaging findings and lesion development in chronic, active multiple sclerosis. Ann Neurol. 1993 Nov;34(5):661–669. doi: 10.1002/ana.410340507. [DOI] [PubMed] [Google Scholar]
- Kitz K., Lassmann H., Karcher D., Lowenthal A. Blood-brain barrier in chronic relapsing experimental allergic encephalomyelitis: a correlative study between cerebrospinal fluid protein concentrations and tracer leakage in the central nervous system. Acta Neuropathol. 1984;63(1):41–50. doi: 10.1007/BF00688469. [DOI] [PubMed] [Google Scholar]
- Komoly S., Hudson L. D., Webster H. D., Bondy C. A. Insulin-like growth factor I gene expression is induced in astrocytes during experimental demyelination. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1894–1898. doi: 10.1073/pnas.89.5.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon E. E., Prineas J. W. Blood-brain barrier abnormalities in longstanding multiple sclerosis lesions. An immunohistochemical study. J Neuropathol Exp Neurol. 1994 Nov;53(6):625–636. doi: 10.1097/00005072-199411000-00010. [DOI] [PubMed] [Google Scholar]
- Lassmann H., Rössler K., Zimprich F., Vass K. Expression of adhesion molecules and histocompatibility antigens at the blood-brain barrier. Brain Pathol. 1991 Jan;1(2):115–123. doi: 10.1111/j.1750-3639.1991.tb00648.x. [DOI] [PubMed] [Google Scholar]
- Liu X., Yao D. L., Bondy C. A., Brenner M., Hudson L. D., Zhou J., Webster H. D. Astrocytes express insulin-like growth factor-I (IGF-I) and its binding protein, IGFBP-2, during demyelination induced by experimental autoimmune encephalomyelitis. Mol Cell Neurosci. 1994 Oct;5(5):418–430. doi: 10.1006/mcne.1994.1052. [DOI] [PubMed] [Google Scholar]
- Martin R., McFarland H. F., McFarlin D. E. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- McMorris F. A., Smith T. M., DeSalvo S., Furlanetto R. W. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc Natl Acad Sci U S A. 1986 Feb;83(3):822–826. doi: 10.1073/pnas.83.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozell R. L., McMorris F. A. Insulin-like growth factor I stimulates oligodendrocyte development and myelination in rat brain aggregate cultures. J Neurosci Res. 1991 Oct;30(2):382–390. doi: 10.1002/jnr.490300214. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Immunohistochemical study of allergic encephalomyelitis. Am J Pathol. 1968 Feb;52(2):251–263. [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Suchanek G., Breitschopf H., Brück W., Budka H., Jellinger K., Lassmann H. Patterns of oligodendroglia pathology in multiple sclerosis. Brain. 1994 Dec;117(Pt 6):1311–1322. doi: 10.1093/brain/117.6.1311. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Transport of insulin-related peptides and glucose across the blood-brain barrier. Ann N Y Acad Sci. 1993 Aug 27;692:126–137. doi: 10.1111/j.1749-6632.1993.tb26211.x. [DOI] [PubMed] [Google Scholar]
- Prineas J. W., Barnard R. O., Kwon E. E., Sharer L. R., Cho E. S. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993 Feb;33(2):137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- Racke M. K., Sriram S., Carlino J., Cannella B., Raine C. S., McFarlin D. E. Long-term treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 2. J Neuroimmunol. 1993 Jul;46(1-2):175–183. doi: 10.1016/0165-5728(93)90247-v. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Cannella B., Duijvestijn A. M., Cross A. H. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990 Oct;63(4):476–489. [PubMed] [Google Scholar]
- Raine C. S. The Dale E. McFarlin Memorial Lecture: the immunology of the multiple sclerosis lesion. Ann Neurol. 1994;36 (Suppl):S61–S72. doi: 10.1002/ana.410360716. [DOI] [PubMed] [Google Scholar]
- Reinhardt R. R., Bondy C. A. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994 Nov;135(5):1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- Robbins K., McCabe S., Scheiner T., Strasser J., Clark R., Jardieu P. Immunological effects of insulin-like growth factor-I--enhancement of immunoglobulin synthesis. Clin Exp Immunol. 1994 Feb;95(2):337–342. doi: 10.1111/j.1365-2249.1994.tb06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld R. G., Pham H., Keller B. T., Borchardt R. T., Pardridge W. M. Demonstration and structural comparison of receptors for insulin-like growth factor-I and -II (IGF-I and -II) in brain and blood-brain barrier. Biochem Biophys Res Commun. 1987 Nov 30;149(1):159–166. doi: 10.1016/0006-291x(87)91618-4. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cross A. H. Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol. 1991 Nov;30(5):694–700. doi: 10.1002/ana.410300510. [DOI] [PubMed] [Google Scholar]
- Tapson V. F., Boni-Schnetzler M., Pilch P. F., Center D. M., Berman J. S. Structural and functional characterization of the human T lymphocyte receptor for insulin-like growth factor I in vitro. J Clin Invest. 1988 Sep;82(3):950–957. doi: 10.1172/JCI113703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D. L., Webster H. D., Hudson L. D., Brenner M., Liu D. S., Escobar A. I., Komoly S. Concentric sclerosis (Baló): morphometric and in situ hybridization study of lesions in six patients. Ann Neurol. 1994 Jan;35(1):18–30. doi: 10.1002/ana.410350105. [DOI] [PubMed] [Google Scholar]
- Yao D. L., West N. R., Bondy C. A., Brenner M., Hudson L. D., Zhou J., Collins G. H., Webster H. D. Cryogenic spinal cord injury induces astrocytic gene expression of insulin-like growth factor I and insulin-like growth factor binding protein 2 during myelin regeneration. J Neurosci Res. 1995 Apr 1;40(5):647–659. doi: 10.1002/jnr.490400510. [DOI] [PubMed] [Google Scholar]







