Abstract
Background:
Plumbago rosea is used in traditional systems of medicine for the preparation of formulations used for treating inflammations, cough, bronchitis, and gastrointestinal disorders, and also in conjunction with cancer chemotherapy. In the present study, the cytotoxic and anti-proliferative effects of plumbagin, and the ethanolic root extract of P. rosea (ETPR) was evaluated on SK-MEL 28 melanoma cell lines and human lymphocytes.
Materials and Methods:
MTT and apoptotic assays were used for the evaluation of cytotoxic and anti-proliferative effects, respectively. In addition, the effect of Plumbagin and ETPR in down regulation of BCL-2 expression is investigated using RT-PCR analysis.
Results:
Both plumbagin and ETPR dose-dependently decreased the cell viability more potently in melanoma cell lines. P. rosea extract demonstrated significant synergy in inhibiting BCL-2 expression than plumbagin. Moreover plumbagin showed more toxicity in human lymphocytes.
Conclusion:
Plumbagin has anti-cancer potential, but the side effects limits its use; yet plumbagin, in combination with other ingredients in Plumbago rosea extract, displays significant synergy leading to a stronger anticancer effect with significantly less toxicity.
Keywords: BCL-2, ethanolic root extract of P. rosea, high performance liquid chromatography, Plumbago rosea, plumbagin, SK-MEL 28
INTRODUCTION
Cancer is one of the leading causes of death in both developed and developing countries and continues to be a major public health problem in many parts of the world.[1] In spite of various efforts to reduce the threat caused by cancer, only modest progress has been made in reducing the morbidity and mortality of this dreadful disease.[2]
Cancer treatment involves various treatment strategies including surgery, radiation therapy, immunotherapy, hormone therapy, or chemotherapy. These treatment regimes are often unsatisfactory and proved unsuccessful with the majority including serious side effects such as cardiac and other toxicities.[3] Tumor cells were found to acquire resistance to apoptosis by various mechanisms that interfere at different levels of apoptosis, such as over-expression of anti-apoptotic genes. Studies have shown a correlation between high levels of BCL-2 expression and the severity of malignancy of human tumors.[4,5,6] Moreover, it has been shown in in vitro and in vivo models that BCL-2 expression confers resistance to many kinds of chemotherapeutic drugs and irradiation.[4,7] In some types of tumors, a high level of BCL-2 expression is associated with a poor response to chemotherapy and seems to be predictive of shorter, disease-free survival.[5,6,8] As a result, research is currently shifting towards traditional herbal drugs. These herbal drugs contain complex mixtures of chemical components[9] that can act individually or synergistically by activating multiple pathways, thus suppressing cancer with minimal toxicity to normal cells.
Plumbago rosea Linn. (syn. Plumbago indica Linn.), commonly known as red chitrak of the family Plumbaginaceae, is a highly reputed Indian medicinal plant mentioned in Ayurvedic literature. It is a perennial shrub widely distributed in the tropics, more specifically in Southern India.[10] The tuberous roots of the plant have been used in traditional medicine for the treatment of rheumatism, edema, piles, common wart, secondary syphilis, and leprosy. It has also been used to treat skin diseases and intestinal worms[10,11,12] these properties are believed to be attributed due to the presence of plumbagin, a natural quinoid constituent. Plumbagin possess various pharmacological activities such as antibacterial antifungal, anticancer,[13,14] and anti-inflammatory activity.[15] In addition to the quinoid constituent plumbagin, several other potential bioactive components such as, sitosterol, stigmasterol, campesterol, napthaquinone, and 5, 6-dihydroxy-2-methyl-1, 4-naphthoquinone (6-hydroxyplumbagin) α-amyrin, β-sitosterol[15,16,17,18] were also reported. However, only a few scientific studies have been conducted to evaluate the efficacy of these constituents. Hence, the present work was intended to compare the effect of purified plumbagin and P. rosea extracts on SK-MEL 28 melanoma cell lines.
MATERIALS AND METHODS
Chemicals and reagents
Plumbagin (CID 10205), MTT (3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyltetrazolium bromide, Fetal bovine serum (FBS), and penicillin were purchased from Sigma Aldrich, USA. Primers for the study were purchased from Synergy Scientific, India. All analytical grade reagents and solvents were purchased from Merck (Mumbai, India).
Collection and extraction of root powder
Whole plants of P. rosea were collected from different parts of Kanyakumari district, Tamil Nadu. The plant was authenticated at Botanical Survey of India, Coimbatore (BSI/SRC/5/23/2010-11/Tech-1282). A voucher specimen was deposited at the Department of Biotechnology, Bharathidasan Institute of Technology, Anna University, Tiruchirappalli for future reference. Roots of the plant were washed thoroughly, air dried, and ground into a powder. Powdered P. rosea roots (75 g) were then subjected to soxhlet extraction with ethanol at 72°C for 24 h. The extracts were then evaporated to dryness in rotary evaporator (Yamato, Japan).
Analysis of P. rosea extract by HPLC
Analysis of ethanolic extract of P. rosea roots (ETPR) was carried out with high performance liquid chromatography (Gilson, France) using C18-Kromasil column (250 mm × 4.6 mm) attached to Gilson 321 binary gradient pump. Aqueous 0.2 M acetic acid (pH adjusted to 3.5 with trimethylamine) and methanol was used as the mobile phase. The flow rate was 0.8 ml/min. Injection volume was 15 μl and HPLC chromatogram was monitored at 254 nm. Plumbagin in crude root extract was quantified by comparison with the standard curve obtained using the authentic plumbagin (Sigma Aldrich, USA).
Cell culture
SK-MEL 28, human melanoma cell lines were procured from National Centre for Cell Science (NCCS), Pune, India. Cells were cultured in DMEM medium supplemented with 10% heat inactivated FBS, 1% glutamine, and 1% penicillin. The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Human lymphocytes culture was used to evaluate the cytotoxicity of P. rosea. Lymphocytes were isolated using HiSep (HiMedia, India) as per the manufacturer's instruction. Briefly, Hisep media was added to the blood in the ratio 1:3 (media: blood), and centrifuged at 160 g for 20 min. The lymphocytes were then transferred into fresh tube and washed with sterile PBS by centrifuging at 140 g for 15 min. The pellet were cultured in RPMI medium (HiMedia) supplemented with 10% heat inactivated bovine serum, antibiotics (streptomycin, penicillin), NaHCO3. Phytoheamagglutinins were used as a mitogen for the proliferation of lymphocytes. The culture was filtered by using sterilized 0.2-μm pore size cellulose acetate membrane filter (Sartorius). Isolated human peripheral lymphocytes were incubated for 72 h at 37°C.
3-(4, 5dimethythiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay
MTT is a colorimetric assay that measures the reduction of yellow 3-(4, 5dimethythiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) by mitochondrial succinate dehydrogenase. The reduction of MTT by mitochondrial succinate dehydrogenase in to formazan crystals was read spectrophotometrically at 540 nm.
Cell viability was calculated using the formula
% of viability = (Mean absorbance of the sample/Mean absorbance of the control) × 100
Cytotoxicity of ETPR and plumbagin on human lymphocytes
The cytotoxic effects of plumbagin and ETPR were determined in human lymphocytes with MTT assay. Different concentrations of plumbagin (50-100 μg/mL) and ETPR (100-800 μg/mL) were added to the cultured human lymphocytes after 24 h of pre-incubation. The toxicity of cells was determined after 48 h.
Antiproliferative effect of ETPR and plumbagin on SK-MEL 28 melanoma cell lines
SK MEL 28 cells were seeded in tissue culture plates at a concentration of 1 × 105 cells/ml and exposed to increasing concentration of plumbagin (25-200 μg/mL) and ETPR (50-400 μg/mL). Cell viability values were determined after 24 h using MTT cell viability assay as descried before.
Colony formation assay
Cells at the initial density of 1 × 105 in 2 ml medium were seeded in plates containing 2 ml of 0.5% agar with medium. The cultures were incubated with ETPR and plumbagin at 37°C in a humidified 5% CO2 atmosphere for 10 days. The number of colonies were determined by counting them under an inverted phase-contrast microscope at ×40 magnification Colonies consisting of more than 50 cells were fixed with formaldehyde (4%) for 2 h and then stained with trypan blue for 30 min followed by crystal violet and counted.
Acridine orange–ethidium bromide double staining
Acridine orange and ethidium bromide staining confirms the nuclear integrity of treated cells. SK-MEL 28 cells were previously incubated at 37°C in a humidified 5% CO2 atmosphere. ETPR (400 μg/mL) and plumbagin (10 μg/mL) were then added into the wells and further incubated for 24 h. The cells were treated with acridine orange and ethidium bromide (1:1). and viewed under fluorescence microscope (Olympus CKK41) at ×20 magnification.
Percentage viability was determined by using the following formula,
% of viability = (A/B) ×100
Where,
A represents the total number of live cells
B represents the total number of cells.
BCL-2 gene expression in plumbagin- and ETPR-treated cells by RT-PCR method
Expression of anti apoptotic gene, BCL-2 was studied using reverse transcriptase-PCR (RT-PCR). SK-MEL 28 cell lines were previously incubated with ETPR (400 μg/mL) and Plumbagin (10 μg/mL).
Isolation of RNA
For preparation of total RNA, the phenol–guanidinium thiocyanate-based RNA Sol™ was used. To the cells, 600 μl of RNA sol reagent was added and lysed by repetitive pipetting and allowed to stand for 5 min followed by addition of 200 ml of chloroform: isoamyl alcohol (24:1) for phase separation. The mixture was then vigorously vortexed for 15 sec and allowed to stand for 15 min followed by centrifugation at 12000 g for 15 min at 4°C. The superficial layer containing RNA was transferred to a fresh sterile DEPC-treated microfuge tube. To this, 500 ml of ice cold isopropanol was added, gently mixed, and allowed to stand for 10 min and centrifuged at 12000 g for 15 min at 4°C. The supernatant was discarded and the RNA pellet was washed with 1 ml of 70% ethanol in DEPC-treated water and again centrifuged at 14000 g for 10 min at 4°C to obtain total RNA. This pellet was dissolved in 25 μl of sterile RNase free water by heating at 55°C for 20 min and stored at −20°C until use.
Reverse transcriptase polymerase chain reaction (RT-PCR)
Isolated mRNA was converted to cDNA and amplified using specific primers (BCL-2) as per the manufacturer's instruction (RT PCR kit, Synergy scientific, India). To amplify the cDNA, PCR ready mix was used according to manufacturer's instruction. cDNA synthesis was carried out at 42°C followed by amplification (94°C, 10 min; 94°C, 30 s; 55°C, 30 s; 72°C, 30 s, and final hold at 4°C) for 35 cycles. The final extension was performed at 72°C for 5 min.
PCR products were separated on a 1% agarose gel and stained with fluorescent dye Green view™. Further, the expression of BCL-2 was analyzed semiquantitatively using IMAGE J software.
RESULTS
Analysis of P. rosea extract by HPLC
Ethanolic extraction yielded 0.17g plumbagin/g dry wt. Plumbagin was detected at the retention time of 5.3 min and the total calculated content was 2.53% [Figure 1]. Thus amount of plumbagin extracted from roots of P. rosea corresponds to 4.3 mg/g. dry wt.
Figure 1.
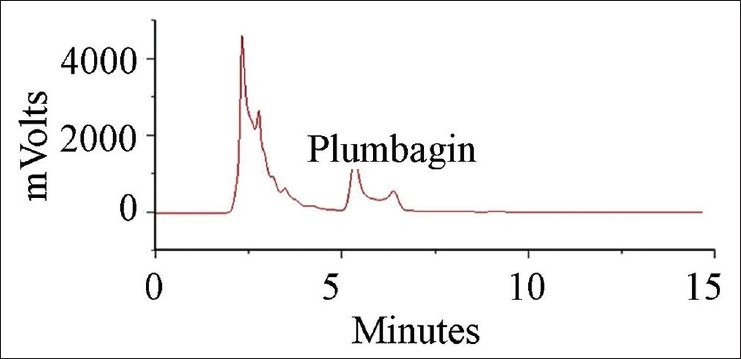
Qunatification of plumbagin in ethanolic extracts of Plumbago rosea roots using HPLC
Cytotoxic effect of ETPR and Plumbagin on human lymphocytes
The cytotoxic effect of plumbagin and ETPR on human lymphocytes was evaluated by MTT assay. Cell lines were sensitive to plumbagin when compared to ETPR, indicating that plumbagin could well induce cytotoxicity to the lymphocyte cell lines. In addition, the LD50 for plumbagin was 46.24 μg/mL when compared to 420.45 μg/mL for ETPR suggesting that very low concentration of plumbagin is sufficient to induce cytotoxic effect on normal cells [Figure 2].
Figure 2.
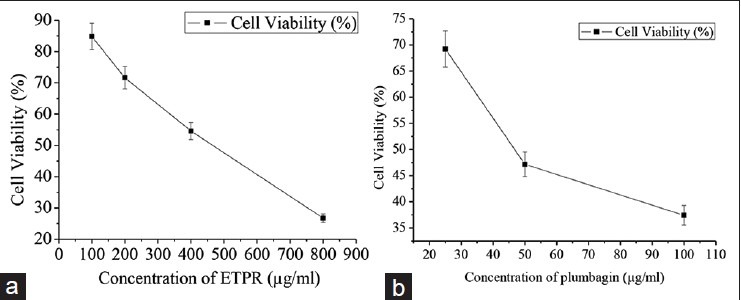
Cytotoxic effect of (a) ETPR, (b) Plumbagin on human lymphocytes cells after 48-h treatment. Each data point represents the mean from three independent experiments (mean ± SE)
Antiproliferative effect of ETPR and plumbagin on SK-MEL cells
To investigate the effect of ETPR and plumbagin on SK-MEL 28 cell proliferation, the cells were treated with various concentrations of ETPR and plumbagin for 24 h. ETPR showed 50% inhibition of cell proliferation only at 84.74 μg/mL whereas with plumbagin, it was observed at a very low concentration of 30.48 μg/mL. In either case, the antiproliferative activity was dose-dependent [Figure 3].
Figure 3.

Antiproliferative effect of (a) ETPR, (b) Plumbagin on SK-MEL 28 cells after 24-h treatment. Data are expressed as a percentage of control (statistics data). Each data point represents the mean of four wells from three independent experiments (mean ± SE)
Colony formation assay
The assay for colony formation assay was carried out to compare the long term effect of ETPR and plumbagin on SK MEL 28 cell growth. The cells were incubated individually with ETPR and plumbagin for 10 days, the colonies were counted and photographed. After 10 days incubation of the indicated concentration of plumbagin and ETPR, the colony numbers were counted and the colony photos were taken. The results showed that ETPR-treated cells resulted in significantly lesser cell growth and colony formation when compared with plumbagin-treated cells.
Although reduced cell growth (35.23%) was observed with ETPR, the concentration measured was 400 μg/mL, whereas with plumbagin 10 μg/mL was sufficient to reduce the cell growth to 61.17% [Figure 4].
Figure 4.
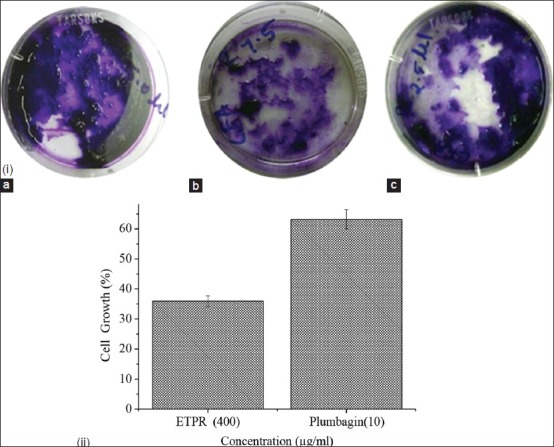
(i) Colony formation and cell growth in SK-MEL28 melanoma cell lines treated with plumbagin & ETPR, (a) control, (b) 400 μg/mL of ETPR, (c) 10 μg/mL of plumbagin in DMEM containing 10% FBS, (ii) Cell numbers determined by hemocytometer. Values are expressed as mean ± SE from three independent experiments
Double staining of SK-MEL 28 cell lines with acridine orange and ethidium orange
In order to determine whether the decrease in cell viability was due to apoptosis, a morphological change at cellular and nuclear levels was assessed with ETPR and plumbagin-treated cells. Cellular and nuclear morphological changes of treated cells were assessed to determine whether observed decrease in cell viability were consequence of an apoptosis process. Acridine orange selectively stains the living cells as green whereas ethidium bromide stains dead cell DNA as red [Figure 5]. The untreated control SK-MEL 28 cell lines were characterized by bright green nucleus with uniform intensity and the absence of ethidium bromide uptake. From the results it can be observed that ETPR-treated cells showed higher percentage of apoptotic cells compared to plumbagin, which confirms the synergistic anticancer potential of crude extract [Figure 5].
Figure 5.
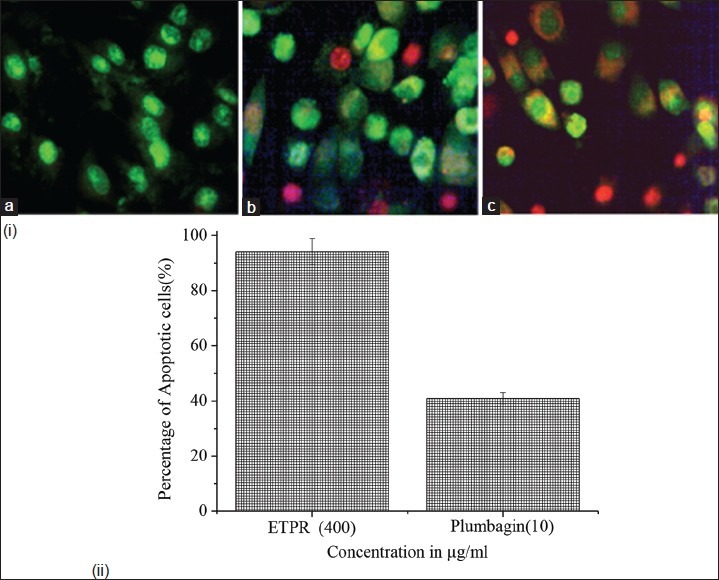
(i) Fluorescence photomicrographs of SK-MEL 28 melanoma cell lines stained with acridine orange and ethidium bromide, after being treated with ETPR and plumbagin. (a) Control cells with bright red nucleus, (b) cells treated with 10 μg/ml plumbagin, (c) cells treated with 400 μg/ml ETPR. ii) The bar graph shows the percentage of apoptotic cells. The baseline apoptosis in the untreated group was normalized with data from the treated group. The data shown are representative of the combined means from three independent experiments
Analysis of BCL-2 mRNA expression
BCL-2 mRNA expression was analyzed in both plumbagin- and ETPR-treated SK-MEL 28 cells. Plumbagin- and ETPR-treated SK MEL 28 cells showed increased amounts of RNA transcripts of the BCL-2 gene compared to the untreated cells. Levels of RNA transcript of BCL-2 was decreased markedly in ETPR-treated cells (400 μg/ml) when compared to plumbagin-treated cells (10 μg/ml) [Figure 6]. GAPDH was used as internal control.
Figure 6.

(i) Levels of RNA transcript of BCL-2 was decreased markedly in 400 μg/ml ETPR-treated cells when compared to 10 μg/ml plumbagintreated cells. GAPDH was used as internal control, (ii) Densitometric analysis of RNA transcript using IMAGE VIEW™ software. Represented data values were obtained from triplicate analysis and expressed as the mean ± SD
To validate the synergistic effect of phytochemicals present in ETPR by inhibiting the expression of BCl-2, mRNA isolated from SK-MEL cell lines exposed to ETPR was transcribed to cDNA using primers supplied by sigma RT-PCR amplification mix (Synergy scientific, India). The cDNA presence was confirmed by agarose gel electrophoresis. The band intensities were visualized using UV transilluminator. From the results it can be observed that P. rosea extract has shown significant inhibition of BCL-2 when compared with plumbagin alone. The intensity of expression was analyzed using IMAGE J VIEW software.
DISCUSSION
Skin cancer is the third most common human malignancy. Increase of the incidence of malignant skin cancer is rising at an alarming rate.[19,20] However, the main problem to treat melanoma is its resistance to radiation and conventional chemotherapeutic agents.[20,21] Hence, novel therapies are needed to reduce the effects of the increasing incidence in human melanoma.
Plumbago rosea Linn a shrubby perennial herb, commonly known as rakta chitrak, grows in wild and in abundance in India.[22] It is heavily used in traditional systems of medicine for the preparation of formulations used to treat a variety of disease conditions. The juice of the leaves and roots mixed with oil is employed as an application for rheumatism paralysis and leprosy.[22] The roots are used in dyspepsia, colic, inflammations, cough, bronchitis, hemorrhoids, elephantiasis chronic intermittent fever, ring worm, skin diseases diarrhea, and piles.[23] The anticancer potential of the plant P. rosea has been previously explored in animal models.[24]
Plumbagin, is a natural napthaquinone, possessing various pharmacological activities namely antimalarial, antimicrobial, anticancer, cardiotonic, antibiotic, and antineoplastic activities.[11,13,25] Potential role of plumbagin, as an anti-cancer agent has been recognize[24,26,27,28] and its anti-cancer effects have been reported in diverse cancer models such as prostate[29], lung[30,31], cervical[32,33], ovarian[34] as well as melanoma.[34]
SK MEL 28 cells and lymphocytes showed growth inhibition in a dose-dependent manner when treated with plumbagin. Plumbagin was found to be slightly more toxic to normal lymphocytes. Recently, much focus has been attributed to multi-component phyto or natural compounds as anticancer agents because of their prominent pharmacological activity at low concentrations and minimal toxicity on normal cells.[35] In addition these phyto extracts would increase the efficacy of drugs by modulating several molecular targets. Extracts of the medicinal plants are believed to contain a wide array of polyphenolic compounds, which might possess cancer preventive and/or therapeutic properties.[36]
Crude extracts exert the antitumor activities through regulation of different cell signaling pathways. SK MEL cells treated with ETPR for 24 h showed growth inhibition in a dose-dependent manner. The LD50 value of ETPR on SK MEL cells was found to be 124 μg/ml. It is well-established that herbal extracts have lesser degree of toxicity compared to purified constituents. In the light of this fact, we assessed the effect of ETPR on lymphocytes (as normal). Our results are consistent with previous studies, wherein Angelica sinensis extracts showed better responses compared with that of the single purified phthalides.[37]
The nuclear morphology analyzed quantitatively by staining suggested that an apoptotic cell death mechanism was more potentially involved in the antiproliferative effect of ETPR compared to plumbagin. As apoptosis is controlled by complex interplay between regulatory proteins of bcl-2 family. Therefore, Bcl-2 proteins have emerged as attractive targets for the development of novel anticancer agents as down regulation of Bcl-2 by plumbagin has been previously reported. An attempt has been taken to explore the synergistic potential of crude extracts by comparing the extent of down regulation of Bcl-2 by plumbagin and crude extracts. The extent of inhibition of Bcl-2 was found to be higher in crude extracts when compared to plumbagin alone. This might be due to the potentiating of activity of plumbagin by the other bioactive constituents present in crude extracts.
Double staining revealed the percentage of apoptotic cells being increased in crude extract-treated cell lines when compared with the purified constituent-treated cells. The presence of phenolic compounds in plant extracts might be responsible for chemopreventive properties such as antioxidant, anticarcinogenic, or antimutagenic and anti-inflammatory effects. Phenolic compounds also contribute to their apoptosis property by arresting cell cycle, regulating carcinogen metabolism and ontogenesis expression, inhibiting DNA binding and cell adhesion, migration, proliferation or differentiation, and blocking signaling pathways.[38,39,40,41]
The in vitro and in vivo colorectal anticancer study of Tian-Xian liquid (crude herbal medicine) showed significant multi-targeted to down regulation of metastatic markers than its bioactive fractions.[42] Tomoxifen showed drug resistance in breast cancer patients; therefore, MCF-7 cells were treated with tomoxifen combined with coptis extracts. The result showed that tomoxifen possesses significant promising potential anticancer effect and it triggers many anticancer pathways due to the synergistic effect.[43] Similarly, P. rosea extract also demonstrated a synergistic effect with a significantly stronger antiproliferative activity on skin cancer cells than plumbagin. The results suggested that P. rosea might have a wider pharmacological application because in addition to the active compound plumbagin, other components in P. rosea might also contribute to the herbal anti-tumor effect and/or enhance the action in a synergistic manner. These results are also in line with the long-established principle of traditional Ayurvedic practice, which recommends, instead of using purified ingredient, a single or mixture of herbs containing various active compounds in specific proportions can be used to treat and prevent diseases by operating synergistically.[44,45] Every country has its own traditional medicinal system and it should be studied by the recent scientific techniques[46] because sometimes herbal preparation may meet with problems such as identifying plants, variable growing conditions, and batch-to-batch variations.[47]
CONCLUSION
The present study concluded that the P. rosea extracts exhibited selective cytotoxic and anti-proliferative effects in human skin cancer SK MEL-28 cells. Furthermore, for the first time, a significant synergy of cytotoxicity towards cancer cells was demonstrated in the use of P. rosea extract due to interactions among plumbagin and other components present in the herb. Further mechanistic studies and in vivo anti-cancer models need to be done to fully assess the anticancer potential of plumbagin and herbal extracts. Therefore, P. rosea is worthy to be further investigated and developed as herbal medicine for potential use in cancer therapy.
ACKNOWLEDGMENT
The authors would like to thank “Biogenix Research Centre, Trivandrum, Kerala” for providing the facility to carry out this work. Dr.K. Satheesh Kumar, Scientist-E2, Biotechnology & Bioinformatics Division, Tropical Botanical Garden and Research Institute, Kerala for his support to carry out this work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Jemal A, Bray F, Center MM, Frelay J, Ward E, Forman D. Global Cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hail N., Jr Mitochondria: A novel target for the chemoprevention of cancer. Apoptosis. 2005;10:687–705. doi: 10.1007/s10495-005-0792-8. [DOI] [PubMed] [Google Scholar]
- 3.Kim GP, Alberts SR, Tschetter LK. Annual Meeting of the-American Society of Clinical Oncology. 14. Vol. 22. New Orleans: LA; 2004. Jun 5-8, Gemcitabine and aocetaxel in patients with measurable unresectable or metastatic hepatocellular carcinoma, a North Central Cancer Treatment Group (NCCTG) phase II trial (abstract 4270) [Google Scholar]
- 4.Weller M, Malipiero U, Aguzzi A, Reed JC, Fontana A. Protooncogene bcl-2 gene transfer abrogates Fas/APO-1 antibody-mediated apoptosis of human malignant glioma cells and confers resistance to chemotherapeutic drugs and therapeutic irradiation. J Clin Invest. 1995;95:2633–43. doi: 10.1172/JCI117965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81:3091–6. [PubMed] [Google Scholar]
- 6.Hermine O, Haioun C, Lepage E, Agay MF, Briere J, Lavignac C, et al. Prognostic significance of bcl-2 protein expression in aggressive non- Hodgkin's lymphoma. Groupe d’Etude des Lymphomes de l’ Adulte (GELA) Blood. 1996;87:265–72. [PubMed] [Google Scholar]
- 7.Findley HW, Gu L, Yeager AM, Zhou M. Expression and regulation of BCL-2, BCL-XL, and BAX correlate with p53 status and sensitivity to apoptosis in childhood acute lymphoblastic leukemia. Blood. 1997;89:2986–93. [PubMed] [Google Scholar]
- 8.Coustan-Smith E, Kitanaka A, Pui C, McNinch L, Evans WE, Raimondi SC, et al. Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia. Blood. 1996;87:1140–6. [PubMed] [Google Scholar]
- 9.Nostro A, Germanò MP, D’angelo V, Marino A, Cannatelli MA. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol. 2000;30:379–84. doi: 10.1046/j.1472-765x.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- 10.A dictionary of Indian raw materials and industrial products. II. New Delhi: CSIR; 1989. Anonymons, The Wealth of India; pp. 163–4. [Google Scholar]
- 11.Pillai NG, Menon TV, Pillai G, Rajasekharan S, Nair CR. Effect of plumbagin in charmakeela (common wart) a case report. J Res Ayurveda Siddha. 1981;2:120–6. [Google Scholar]
- 12.Agarwal VS, Barin G. India: Kalyani Publications; 1985. Drug plants of India: Root drugs; p. 204. [Google Scholar]
- 13.Krishnaswamy M, Purushothaman KK. Plumbagin: A study of its anticancer, antibacterial and antifungal properties. Indian J Exp Biol. 1980;18:876–7. [PubMed] [Google Scholar]
- 14.Purushothaman KK, Mohana KT, Susan Biological profile of plumbagin. Bull Medico Ethno Botan Res. 1985;6:177–88. [Google Scholar]
- 15.Rahul C, Deepak S, Santosh KS, Subrahmanyam G, Sunil K, Poduval TB, et al. Plumbagin inhibits proliferative and inflammatory responses of T cells independent of ROS generation but by modulating intracellular thiols. J Cell Biochem. 2010;110:1082–93. doi: 10.1002/jcb.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinda B, Das SK, Hajra AK, Bhattacharya A, CheL GK, Achari B. Chemical constituents of P. indica. roots and reaction of plumbagin. Indian J Chem. 1999;38:577–82. [Google Scholar]
- 17.Dinda B, Chel G. 6-Hydroxyplumbagin, a naphthoquinone from Plumbago indica. Phytochemistry. 1992;31:3652–3. [Google Scholar]
- 18.Hafeez BB, Zhong W, Mustafa A, Fischer JW, Witkowsky O, Verma AK. Plumbagin inhibits prostate cancer development in TRAMP mice via targeting PKCε, Stat3 and neuroendocrine markers. Carcinogenesis. 2012;33:2586–92. doi: 10.1093/carcin/bgs291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 20.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 21.Cummins DL, Cummins JM, Pantle H, Silverman MA, Leonard AL, Chanmugam A. Cutaneous malignant melanoma. Mayo Clini Proc. 2006;81:500–7. doi: 10.4065/81.4.500. [DOI] [PubMed] [Google Scholar]
- 22.Jayaweera DM. Part IV. Colombo: National Science Council of Srilanka; 1982. Medicinal plants used in Ceylon. [Google Scholar]
- 23.Sathyavati GV, Gupta AK, Tandon N. Indian Council of Medicinal research. II. New Delhi: 1987. Medicinal Plants of India. [Google Scholar]
- 24.Parimala R, Sachdanandam P. Effect of Plumbagin on some glucose metabolizing enzymes studied in rats in experimental hepatoma. Mol Cell Biochem. 1993;12:59–63. doi: 10.1007/BF00926835. [DOI] [PubMed] [Google Scholar]
- 25.Didry N, Dubrevil L, Pinkas M. Activity of anthraquinonic and naphthoquinonic compounds on oral bacteria. Pharmazie. 1994;49:681–3. [PubMed] [Google Scholar]
- 26.Hazra B, Sarkar R, Bhattacharyya S, Ghosh PK, Chel G, Dinda B. Synthesis of plumbagin derivatives and their inhibitory activities against Ehrlich ascites carcinoma in vivo and Leishmania donovani Promastigotes In Vitro. Phytother Res. 2002;16:133–7. doi: 10.1002/ptr.867. [DOI] [PubMed] [Google Scholar]
- 27.Naresh RA, Udupa N, Devi PU. Niosomal plumbagin with reduced toxicity and improved anticancer activity in BALB/C mice. J Pharm Pharmacol. 1996;48:1128–32. doi: 10.1111/j.2042-7158.1996.tb03907.x. [DOI] [PubMed] [Google Scholar]
- 28.Sugie S, Okamoto K, Rahman KM, Tanaka T, Kawai K, Yamahara J, et al. Inhibitory effects of plumbagin and juglone on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1998;127:177–83. doi: 10.1016/s0304-3835(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 29.Powolny AA, Singh SV. Plumbagin-induced apoptosis in human prostate cancer cells is associated with modulation of cellular redox status and generation of reactive oxygen species. Pharm Res. 2008;25:2171–80. doi: 10.1007/s11095-008-9533-3. [DOI] [PubMed] [Google Scholar]
- 30.Hsu YL, Cho CY, Kuo PL, Huang YT, Lin CC. Plumbagin (5-hydroxy- 2-methyl-1,4-naphthoquinone) induces apoptosis and cell cycle arrest in A549 cells through p53 accumulation via c-Jun NH2-terminal kinase-mediated phosphorylation at serine 15 In Vitro and in vivo. J Pharmacol Exp Ther. 2006;318:484–94. doi: 10.1124/jpet.105.098863. [DOI] [PubMed] [Google Scholar]
- 31.Gomathinayagam R, Sowmyalakshmi S, Mardhatillah F, Kumar R, Akbarsha MA, Damodaran C. Anticancer mechanism of plumbagin, a natural compound, on non-small cell lung cancer cells. Anticancer Res. 2008;28:785–92. [PubMed] [Google Scholar]
- 32.Srinivas P, Gopinath G, Banerji A, Dinakar A, Srinivas G. Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol Carcinog. 2004;40:201–11. doi: 10.1002/mc.20031. [DOI] [PubMed] [Google Scholar]
- 33.Nair S, Nair RR, Srinivas P, Srinivas G, Pillai MR. Radiosensitizing effects of plumbagin in cervical cancer cells is through modulation of apoptotic pathway. Mol Carcinog. 2008;47:22–33. doi: 10.1002/mc.20359. [DOI] [PubMed] [Google Scholar]
- 34.Wang CC, Chiang YM, Sung SC, Hsu YL, Chang JK, Kuo PL. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun Nterminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008;259:82–98. doi: 10.1016/j.canlet.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Gostner JM, Wrulich OA, Marcel J, Dietmar F, Ueberall F. An update on the strategies in multicomponent activity monitoring within the phytopharmaceutical field. BMC Complement Altern Med. 2012;12:1–11. doi: 10.1186/1472-6882-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai J, Mumper RJ. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–52. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winnie L, Ting K, Chi HC, John AR, Ge L. Study of the antiproliferative effects and synergy of phthalides from Angelica sinensis on colon cancer cells. J Ethnopharmacol. 2008;120:36–43. doi: 10.1016/j.jep.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Baravalia Y, Vaghasiya Y, Chanda S. Hepatoprotective effect of Woodfordia fruticosa Kurz flowers on diclofenac sodium induced liver toxicity in rats. Asian Pac J Trop Med. 2011;4:342–6. doi: 10.1016/S1995-7645(11)60100-4. [DOI] [PubMed] [Google Scholar]
- 39.Karthikeyan R, Somasundaram ST, Manivasagam T, Balasubramanian T, Anantharaman P. Hepatoprotective activity of brown alga Padina boergesenii against CCl 4 induced oxidative damage in Wistar rats. Asian Pac J Trop Med. 2010;3:696–701. [Google Scholar]
- 40.Li ZY, Wang Y, Shen WT, Zhou P. Content determination of benzyl glucosinolate and anti-cancer activity of its hydrolysis product in Carica papaya L. Asian Pac J Trop Med. 2012;5:231–33. doi: 10.1016/S1995-7645(12)60030-3. [DOI] [PubMed] [Google Scholar]
- 41.Saeed S, Abasalt B. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacogn Res. 2014;6:99–107. doi: 10.4103/0974-8490.128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu ES, Sze SC, Cheung HP, Wong KL, Liu Q, Ng TB, et al. Differential effects of anti-metastatic mechanism of Tian-Xian liquid (TXL) and its bioactive fractions on human colorectal cancer models. J Ethnopharmacol. 2011;137:403–13. doi: 10.1016/j.jep.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 43.Jing L, Chengwei H, Keyuan Z, Jingdong W, Jing XK. Coptis extracts enhance the anticancer effect of estrogen receptor antagonist on human breast cancer cells. Biochem Biophys Res Commun. 2012;378:174–8. doi: 10.1016/j.bbrc.2008.10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samudralwar DL, Garg AN. Minor and trace elemental determination in the Indian herbal and other medicinal preparations. Biol Trace Elem Res. 1996;54:113–21. doi: 10.1007/BF02786258. [DOI] [PubMed] [Google Scholar]
- 45.Sharma MK, Kumar M, Kumar A. Ocimum sanctum aqueous leaf extract provides protection against mercury induced toxicity in Swiss albino mice. Indian J Exp Biol. 2002;40:1079–82. [PubMed] [Google Scholar]
- 46.Jayaram KK. Fuzzy logic and modern analytical tool coupling in comprehensive evaluation of traditional and complementary medicine. Pharmacogn Mag. 2006;2:202–3. [Google Scholar]
- 47.Paula C, Liliana TG, Marcelino AG, Carlos CL, Francisco GL, Gabriela AG, et al. Hepatoprotective effect of commercial herbal extracts on carbon tetrachloride-induced liver damage in wister rats. Pharmacogn Mag. 2013;5:150–6. doi: 10.4103/0974-8490.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]


