Abstract
Context:
Local drug delivery (LDD) systems have been proposed for the treatment of periodontitis. Curcumin could be a suitable agent as LDD for the treatment of periodontitis.
Aim:
To formulate, evaluate the anti-inflammatory activity and to assess the duration of the action and the efficacy of 2% curcumin gel in the treatment of experimental periodontitis in Wistar albino rat model.
Settings and Design:
Twenty-one Wistar albino rats were randomly assigned to three groups. Periodontitis was induced using ligature model. Group 1: Control; group 2: Plain gel, and group 3: 2% curcumin gel.
Materials and Methods:
About 2% curcumin gel was prepared. The anti-inflammatory activity and duration of action was assessed. Silk ligature 5-0 was used to induce periodontitis. Gingival index (GI) and probing pocket depth (PPD) were measured. Treatment was done. The rats were sacrificed. Morphometric analysis was performed using stereomicroscope and ImageJ software.
Statistical Analysis Used:
Analysis of variance followed by Bonferroni's test, Wilcoxon's test for inter-group comparison, Mann–Whitney test for P value computation was used. The observations are mean ± standard deviation and standard error of the mean. P < 0.01 when compared to control was considered as statistically significant.
Results:
About 2% curcumin gel showed 42.98% inhibition of edema and peak activity was noted at 24 h. There was statistically significant change in the GI and PPD. Morphometric analysis did not show any significant difference between groups. No toxic effects were seen on oral administration of 2000 mg/kg of curcumin.
Conclusions:
About 2% curcumin gel was effective in the treatment of experimental periodontitis.
Keywords: Anti-inflammatory activity, curcumin gel, experimental periodontitis, local drug delivery, morphometric analysis
INTRODUCTION
Periodontitis is one of the most common diseases affecting the teeth. It is seen in individuals who are more susceptible to the disease.[1]
The remnants of plaque and calculus can cause failure in periodontal treatment.[2] Chemotherapeutic agents can be used to enhance the results achieved by mechanical instrumentation or to improve outcomes at sites not responsive to conventional therapy.[3] Several research studies have established their efficacy in the treatment of periodontal diseases.[4]
MATERIALS AND METHODS
Institutional Animal Ethical Committee, approval was obtained. Dosage of the gel was selected based on the results of acute oral toxicity study of Curcuma longa in Wistar albino rats carried out according to Organization for Economic Cooperation and Development Guidelines No. 420. The rats were housed in polypropylene cages under standard conditions with access to food and water ad-libitum.
The aim of this study was to formulate, evaluate the anti-inflammatory activity, to assess the duration of action and the efficacy of 2% curcumin gel in the treatment of experimentally induced periodontitis in Wistar albino rat model.
Preparation of 2% curcumin gel by simple dispersion method
Carbopol-940 was soaked in purified water containing 0.2% w/v sodium benzoate overnight. Using tissue homogenizer hydroxypropyl methylcellulose (HPMC) solution was mixed in propylene glycol. 2 mg of curcumin (Rajesh Chemicals, Mumbai) was transferred into HPMC solution and homogenized. This drug solution was transferred to carbopol solution and homogenized. Triethanolamine was added quantity sufficient (q.s.) to neutralize the pH. Then, distilled water was added to make q.s. to 100 ml. Control gel was prepared in the same manner. The gel was stored at ambient temperature. The formulation of 2% curcumin gel was prepared by Department of Pharmaceutics, NGSM Institute of Pharmaceutical Sciences [Figure 1, Table 1]. Shavetha et al. showed in an in-vitro study that this particular combination showed increased bioavailability and a higher percentage of drug diffusion and good rheological and texture properties.[5]
Figure 1.

Curcumin gel
Table 1.
Formula used to prepare 2% curcumin gel

Evaluation of physicochemical parameters of 2% curcumin gel
The formulations were subjected to tests such as homogeneity, spreadabilty, grittiness, extrudability, pH measurement, drug content, and percentage drug release.
Spreadability
Spreadabilty was measured by modifying an apparatus suggested by Mutimer et al.[6]
Homogeneity
Homogeneity was graded. Grades were allotted as +++ good, ++ fair, and + poor.
Extrudability
The formulation was filled in a clean, lacquered aluminum collapsible one ounce tube with a nasal tip of 5 mm opening. The extrudability was then determined by measuring the amount of gel extruded through the tip when a constant load of 1 kg was placed. The extruded gel was collected and weighed. The percentage of gel extruded was calculated and grades were allotted.[7]
Determination of pH
About 5 ± 0.1 g of gel is taken in a 100 ml in a beaker and the gel was dispersed in 45 ml of water. pH meter was used to determine the pH.
Determination of drug content uniformity
A total of 5 g of the prepared formulation was subjected for analytical assay using UV spectrophotometer at λmax 430 nm.
Drug release studies
Curcumin release from the gel was studied using permeation apparatus.
Evaluation of anti-inflammatory activity of 2% curcumin gel
A total of 18 healthy Wistar albino rats of either sex were randomly allocated to test (2% curcumin gel), standard (Voveron® gel), and control group (plain gel) with six animals (n = 6) in each group. The anti-inflammatory activity was assessed by carrageenan induced paw edema method.
Duration of anti-inflammatory activity of 2% curcumin gel
A total of 36 healthy Wistar albino rats of either sex were divided into six groups (n = 6) based on the time duration at which 2% curcumin gel was applied prior to injection of 0.1% carrageenan. In groups 1-6, 50 mg of 2% curcumin gel was applied at 0, 2, 4, 6, 12, 24, and 48 h prior to administration of carrageenan.
About 50 mg of the 2% gel was divided into two equal parts of 25 mg. The first part of 25 mg gel was applied on the plantar surfaces of their left hind paw surface by gentle rubbing with the index finger approximately 50 times until no gel was seen or felt on the skin. After 5 min, 25 mg gel was applied in a similar manner at 2, 4, 6, 12, 24, and 48 h prior to injection of 0.1 ml of 1% carrageenan solution.[8,9] Three hours after the injection the 3rd h reading was noted. The percentage inflammation and the percent inhibition of edema at each dosing interval were calculated. In the control group (n = 6) the plain gel and standard (Voveron® gel) group (n = 6) gel was applied 3 h prior to the carrageenan injection.
Experimental periodontitis
A total of 21 Wistar albino rats (5-10 weeks old) weighing between 150 and 250 g were used in the study. Five rats weighing 150-250 g were taken in a poly propylene cage each day of the experiment for 9 days. The rats were anesthetized with ketamine anesthesia. Preperiodontal examination was done and the upper second molars were ligated using a sterile braided silk suture (5-0) [Figure 2]. Soft tissue indicators were measured.
Figure 2.
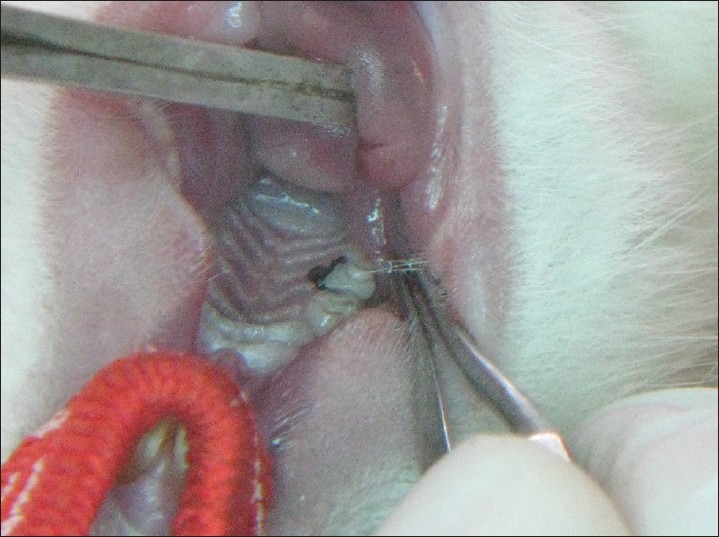
Ligature placed
Treatment of experimental periodontitis
Four weeks after ligature placement the rats were divided into three groups. The rats in the control group did not receive any treatment, group 2 received plain gel, group 3 received 2% curcumin gel. The gels were applied with a tuberculin syringe with a blunt tip. The application was done every alternate day for 6 days. The soft tissue indicators were measured prior to euthanizing the rats.[10]
Soft tissue indicators of periodontitis
Gingival index
Gingival index (GI) was recorded on maxillary second molar on four surfaces: Mesial, buccal, distal, and palatal surface. GI was recorded 1 week after ligature placement and 1 week after treatment.[11]
Probing pocket depth
Probing pocket depth (PPD) was taken from the gingival margin to the bottom of the pocket using a modified graduated silver cone with the round-ended tip of approximately 0.4 mm in diameter. Six values were recorded and averaged (mesiobuccal, midbuccal, distobuccal, mesiopalatal, midpalatal, and distopalatal). PPD was examined 1 week after ligature placement and 1 week after treatment.[10]
Sacrificial indicator
Morphometric analysis of alveolar bone loss
After the rats were euthanized with ketamine overdose the specimens were dissected carefully to maintain their integrity then, immersed in sodium hypochlorite for 4 h and manual scavenging of the remaining tissue was done. The specimens were stained with methylene blue dye (1 g/100 mL, Sigma-Aldrich, Saint Louis, MO, USA) for 1 min to demarcate the cemento-enamel junction (CEJ) and examined under a stereomicroscope. In order to ensure reproducibility of the alignment of the image, the buccal cusp tip of the first and second molars were placed such that they superimposed on the corresponding lingual/palatal cusp tip. Photographs were obtained with a 6.1-megapixel digital camera (Nikon D100, Ayutthaya, Thailand). Measurements were made on the maxillary second molar in a blinded fashion, 3 times using Java based image processing software, NIH, USA, and the mean values were used in statistical analysis.[12] The distance method applied by Crawford, Taubman, and Smith on digitalized images was used to perform linear measurements from the CEJ to the alveolar bone crest, on half of each root following the axis. Six measurements were obtained for the maxillary second molar [Figures 3 and 4]. The data was subjected to statistical analysis.
Figure 3.
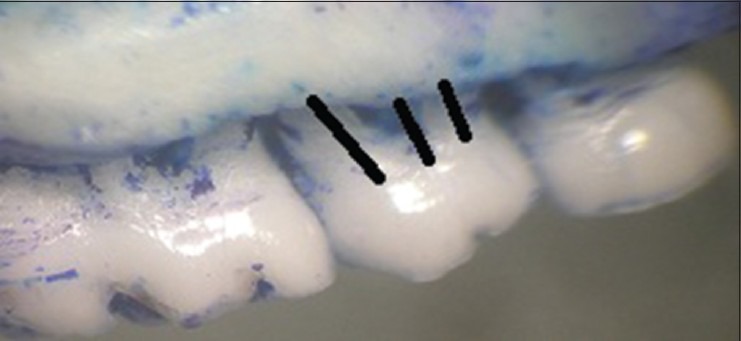
Morphometric analysis: Buccal view
Figure 4.
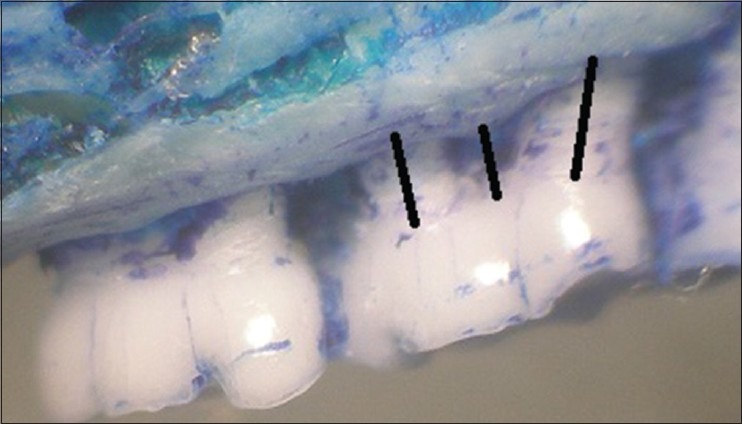
Morphometric analysis: Palatal view
RESULTS
All statistical analysis was performed using InStat-GraphPad software [GraphPad software Inc. CA, USA]. P < 0.001 was considered as highly significant and P < 0.05 was considered as significant.
Evaluation of physicochemical parameters of the 2% curcumin gel
The gels showed optimum spreadability of 15 s, homogeneity of +++ grade, good extrudability. The pH was within the acceptable range of 6.9-7.2 even at the end of 30 days. Curcumin release was 61% [Table 2].
Table 2.
Physicochemical characters of 2% curcumin gel

Evaluation of anti-inflammatory action of 2% curcumin gel
Percentage inflammation was calculated using the formula:
Percentage inflammation = {V − Vi/Vi} × 100
where Vi = 0 h reading and V = 3 h reading. The average paw thickness in the drug treated group was compared within the control group.
Percent inhibition of the edema was calculated using the formula:
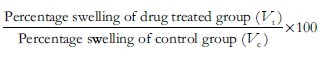
The mean weight of the control group was 238.33 ± 9.83 g, the test group was 237.50 ± 22.30 g and the standard was 227.33 ± 62.19 g. Analysis of variance was used for multiple comparisons. F value was 0.151. P value was 0.861. There was no statistically significant difference between the groups.
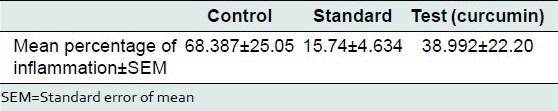
The mean percentage of inflammation as measured using change in paw thickness in the control, standard, and test group was 68.38 ± 25.05 mm, 15.74 ± 4.63 mm, and 38.99 ± 22.20 mm, respectively. Percent inhibition of edema in the standard group was 76.97% and in the test group was 42.98%. The test group showed moderate anti-inflammatory activity [Tables 3-5].
Table 3.
Weight of the animals selected for the study
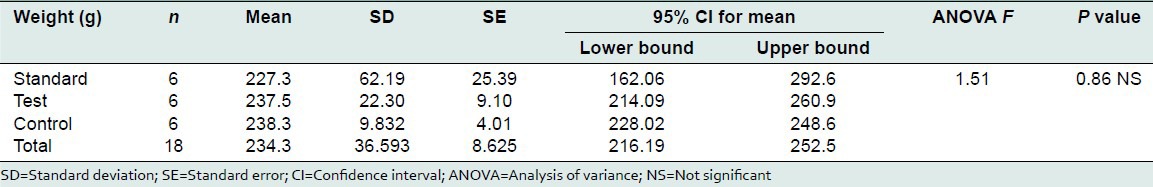
Table 5.
Percent inhibition of edema
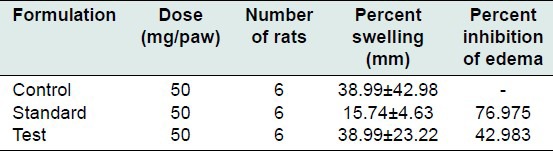
Table 4.
Percentage inflammation in control, standard, and test (curcumin) group
Duration of the anti-inflammatory activity of 2% curcumin gel
The gel base was used as control, 1% Voveron® gel was used as standard and the test formulation was 2% curcumin gel. The test groups 1–6, the mean percentage of inflammation was 36.65 ± 11.98 mm, 23.05 ± 4.79, 22.46 ± 6.63, 21.78 ± 7.91, 19.88 ± 14.69, and 48.79 ± 15.72 mm. Percent inhibition of edema was 46.39%, 66.28%, 67.14%, 68.15%, 70.91%, and 28.65%, respectively [Table 6].
Table 6.
Duration of anti-inflammatory action of 2% curcumin gel

Soft tissue indicators of periodontitis
Gingival index
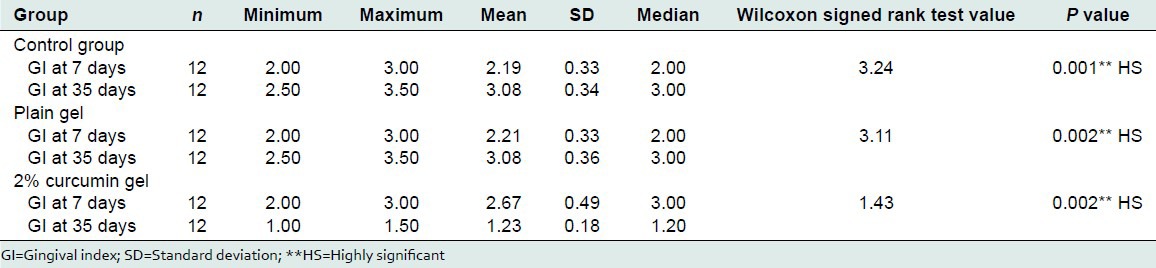
The mean GI at the end of 7 days was in the control, plain gel, and curcumin gel group was 2.19 ± 0.33, 2.21 ± 0.33, and 2.67 ± 0.49. The mean GI at the end of 35 days in the control, plain gel, and curcumin gel group was 3.08 ± 0.34, 3.08 ± 0.36, and 1.23 ± 0.18. The mean percentage change was −40.35%, −39.62%, and 53.75%. There was a statistically significant change in the GI at the end of 35 days, P value was 0.001 [Table 7].
Table 7.
Comparison of GI before and after treatment
Probing pocket depth
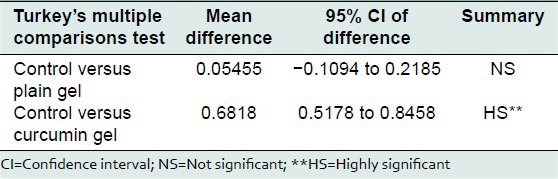
Mean PPD in control group, plain gel group, curcumin group, and Tulsi group, 1 week after placement of ligature, was 2.75 ± 0.121, 2.73 ± 0.120 and 2.681 ± 0.098 mm, respectively. Mean PPD in control group, plain gel group 1 week after placement of ligature, in the control group was, plain gel group and curcumin group was 2.79 ± 0.137, 2.73 ± 0.120 and 1.79 ± 0.094, respectively. On comparison between control and plain gel and curcumin gel using Tukey's multiple comparison 1 week after ligature placement, mean difference was 0.018, 0.072, and 0.027, respectively. There was no statistical significant difference between groups [Tables 8 and 9].
Table 8.
Probing pocket depth
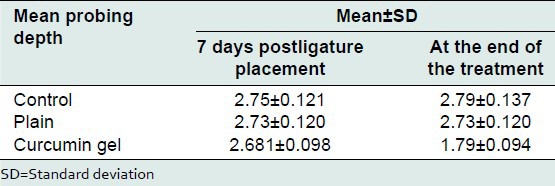
Table 9.
Comparison between control and treatment group at the end of treatment
Sacrificial indicators
Morphometric analysis
The mean bone loss at mesiobuccal, midbuccal, distobuccal, mesiopalatal, midpalatal, and distopalatal site treated with plain gel was 0.30 ± 0.05 mm, 0.28 ± 0.05, 0.22 ± 0.05, 0.37 ± 0.06, 0.29 ± 0.03, and 0.29 ± 0.05 mm respectively.
The mean bone loss at mesiobuccal, midbuccal, distobuccal, mesiopalatal, midpalatal, and distopalatal site treated with 2% curcumin gel was 0.25 ± 0.04 mm, 0.20 ± 0.05, 0.14 ± 0.03, 0.35 ± 0.17, 0.25 ± 0.12, and 0.25 ± 0.12 mm respectively.
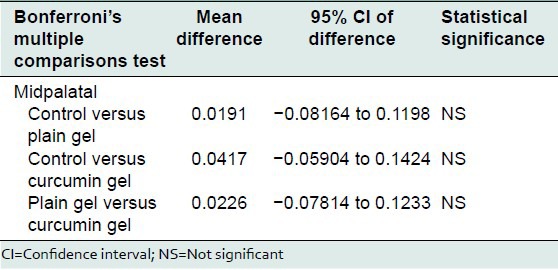
On comparison there was no statistically significant bone loss between the various groups at the mesiobuccal, midbuccal, distobuccal, mesiopalatal, midpalatal, and distopalatal site.
Bonferroni's multiple comparisons test was used to compare the effect of control, plain gel and 2% curcumin gel at mesiobuccal, midbuccal, mesiopalatal, midpalatal, and distopalatal sites. There was no statistically significant difference between the mean bone loss at mesiobuccal, midbuccal, mesiopalatal, midpalatal, and distopalatal sites treated with control, plain gel, and 2% curcumin gel [Tables 10-16].
Table 10.
Mean bone level at sites treated with control, plain gel, and 2% curcumin gel
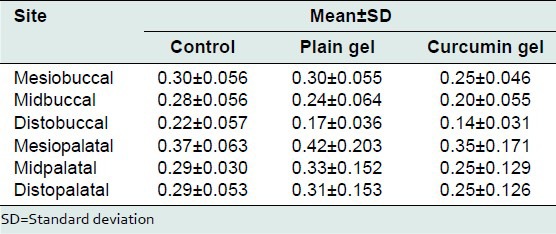
Table 16.
Comparison of the effect of control, plain gel, and 2% curcumin gel on bone loss (distopalatal site)
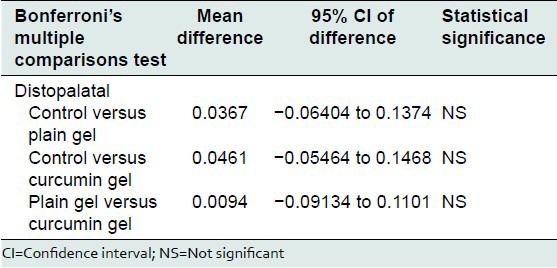
Table 11.
Comparison of the effect of control, plain gel, and 2% curcumin gel on bone loss (mesiobuccal site)
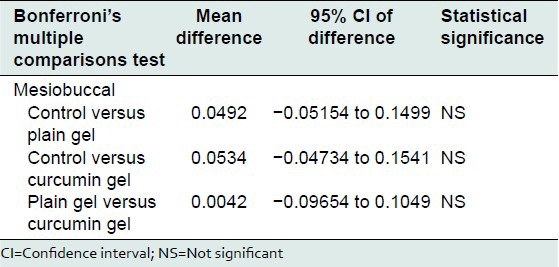
Table 12.
Comparison of the effect of control, plain gel, and 2% curcumin gel on bone loss (midbuccal site)
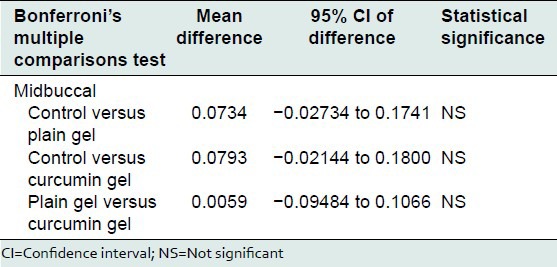
Table 13.
Comparison of the effect of control, plain gel and 2% curcumin gel on bone loss (distobuccal site)
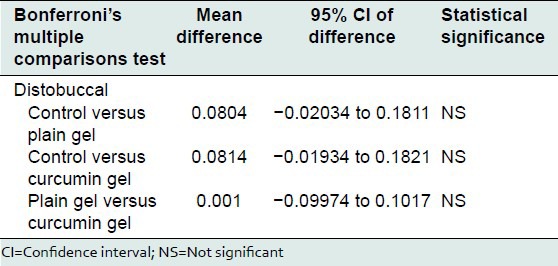
Table 14.
Comparison of the effect of control, plain gel, and 2% curcumin gel on bone loss (mesiopalatal site)
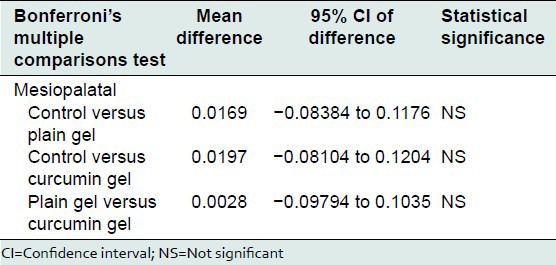
Table 15.
Comparison of the effect of control, plain gel, and 2% curcumin gel on bone loss (mid palatal site)
Acute oral toxicity
All the animals survived for the period of 14 days. They appeared healthy throughout the study. All the animals appeared to gain weight during the observation period of 14 days. There were no signs of gross toxicity, adverse pharmacological events or changes in behavior. Gross necropsy findings did not show any abnormalities.
DISCUSSION
Periodontitis is a chronic inflammatory disease caused by bacterial infection of the supporting tissues around the teeth.[13,14] The concept of locally delivering chemotherapeutic agents to the periodontal pocket as a method to treat periodontal disease has been studied for over few decades. Several herbal drugs have been an area of interest in the treatment of periodontal diseases.[4]
Periodontal disease can be induced in the rats by tying a ligature of 2-0–5-0 braided silk around the cervix of the maxillary or mandibular molars or by injecting lipopolysaccharides into the papilla or combination of both.[15] Souza et al. used 4 weeks for periodontitis induction in the maxilla. It was similar to the study period used in our study.[16]
Studies have supported the positive role of anti-inflammatory agents in the treatment of periodontal disease.[17] The anti-inflammatory properties of curcumin are mediated by the modulating the activity of signaling pathways and transcription factors, especially nuclear factor kappa-β, activating protein-1 and mitogen-activated protein kinases. Curcumin suppresses the expression of interleukin (IL)-6, IL-1β, tumor necrosis factor-α, prostaglandins, matrix metalloproteinase 2 (MMP-2) and MMP-9.[18,19,20,21,22,23] Curcumin was also shown to improve wound healing by increasing collagen deposition, angiogenesis and the density of fibroblasts, reducing the radiation-induced delay in wound repair.[24] These pharmacological properties make it an effective drug to treat periodontal disease and hence, we fabricated a 2% curcumin gel by simple dispersion method.
The gel showed a standard physicochemical profile with a drug release of 61% inhibition of edema was 42.98%. Maximum inhibition was seen at 24 h. The anti-inflammatory activity lasted for 48 h. Hence, the gel was applied on alternate days to treat experimental periodontitis.
The GI scores in the control group and plain gel group increased indicating that the inflammation progressed. In the 2% curcumin gel group, a highly significant mean percentage decrease was noted. This showed that the curcumin gel had good anti-inflammatory effect.
Morphometric analysis did not show any significant difference between the groups. Whether or not the local drug delivery (LDD) system results in bone regeneration is controversial. In our study, we did not notice any significant difference in the residual periodontal bone level among various groups.
Limitations of the study
Pressure sensitive probe and stent needs to be used
No scaling and root planning was done
Microbial profiling, biochemical, and immuno-histochemical parameters have to be evaluated
It is worthwhile to do chronic toxicity study. But, the animals were monitored closely during the entire study period for any signs of toxicity. No signs of chronic toxicity were noted.
CONCLUSION
About 2% curcumin gel has shown good anti-inflammatory effect for 24-48 h despite the limitations in our study. The 2% curcumin gel showed significant reduction in gingival inflammation and pocket depth. The periodontal bone loss was not statistically significant. This is consistent with the use of LDD systems as they help in disease limitation and not significant bone regeneration. Hence, 2% curcumin can be used as useful adjunct to enhance the results of standard periodontal therapy.
ACKNOWLEDGMENTS
I would like to acknowledge the technical and intellectual support provided by Dr. B. H. Sripathi Rao, Principal and Dean, Dr. A. Shashikanth Hegde, Prof. and Head, Dr. K. S. Rajesh, Sr. Professor, Department of Periodontics, Dr. Maji Jose, Prof. and Head, Department of Oral and Maxillofacial Pathology, Yenepoya Dental College, Mangalore, Mr. E. P. Rejeesh, Department of Pharmacology, Yenepoya Medical College, Dr. Sucharita Suresh, statistician, Father Mullers College, Mangalore.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Ryan ME. Nonsurgical approaches for the treatment of periodontal diseases. Dent Clin North Am. 2005;49:611–36. doi: 10.1016/j.cden.2005.03.010. vii. [DOI] [PubMed] [Google Scholar]
- 2.Rabbani GM, Ash MM, Jr, Caffesse RG. The effectiveness of subgingival scaling and root planing in calculus removal. J Periodontol. 1981;52:119–23. doi: 10.1902/jop.1981.52.3.119. [DOI] [PubMed] [Google Scholar]
- 3.Greenstein G, Polson A. The role of local drug delivery in the management of periodontal diseases: A comprehensive review. J Periodontol. 1998;69:507–20. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 4.Hirasawa M, Takada K, Makimura M, Otake S. Improvement of periodontal status by green tea catechin using a local delivery system: A clinical pilot study. J Periodontal Res. 2002;37:433–8. doi: 10.1034/j.1600-0765.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Shwetha P, Upendra KJ. Development and evaluation of topical Curcumin from different combination of polymers formulation and evaluation of herbal gel. Int J Pharm Pharm Sci. 2012;4:4452–6. [Google Scholar]
- 6.Mutimer MN, Riffkin C, Hill JA, Glickman ME, Cyr GN. Modern ointment base technology. II. Comparative evaluation of bases. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1956;45:218–8. doi: 10.1002/jps.3030450406. [DOI] [PubMed] [Google Scholar]
- 7.Vijaybhaskar D, Kumar NS, Rao KP, Pratima S. Histopathological effect of mucoadhesive herbal gel on tobacco carcinogenicity. Int J Pharm Biol Sci Arch. 2012;3:394–9. [Google Scholar]
- 8.Brinker F. Sandy. OR: Eclectic Institute Publishers; 1998. Herb Contraindications and Drug Interactions; p. 33. [Google Scholar]
- 9.Pereira SL, de Oliveira JW, Angelo KK, da Costa AM, Costa F. Clinical effect of a mouth rinse containing Ocimum gratissimum on plaque and gingivitis control. J Contemp Dent Pract. 2011;12:350–5. [PubMed] [Google Scholar]
- 10.Liu R, Li N, Liu N, Zhou X, Dong ZM, Wen XJ, et al. Effects of systemic ornidazole, systemic and local compound ornidazole and pefloxacin mesylate on experimental periodontitis in rats. Med Sci Monit. 2012;18:BR95–102. doi: 10.12659/MSM.882514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;8(6 Suppl):610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 12.Amer M, Elverdin JC, Fernández-Solari J, Medina VA, Chiarenza AP, Vacas MI. Reduced methacholine-induced submandibular salivary secretion in rats with experimental periodontitis. Arch Oral Biol. 2011;56:421–7. doi: 10.1016/j.archoralbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Evans AS. Epidemiological concepts. In: Evans AS, Brachmen PS, editors. Bacterial Infections of Humans Epidemiology and Control. New York: Plenum; 1991. pp. 1–58. [Google Scholar]
- 14.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–48. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 15.Struillou X, Boutigny H, Soueidan A, Layrolle P. Experimental animal models in periodontology: A review. Open Dent J. 2010;4:37–47. doi: 10.2174/1874210601004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souza DM, Prado Fde A, Prado Mde A, Rocha RF, Carvalho YR. Evaluation of two morphometric methods of bone loss percentages caused by periodontitis in rats in different locations. J Appl Oral Sci. 2010;18:493–7. doi: 10.1590/S1678-77572010000500011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drisko CH. Non-surgical pocket therapy: Pharmacotherapeutics. Ann Periodontol. 1996;1:491–566. doi: 10.1902/annals.1996.1.1.491. [DOI] [PubMed] [Google Scholar]
- 18.Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, Moon DO, et al. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005;174:8116–24. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 19.Gulcubuk A, Altunatmaz K, Sonmez K, Haktanir-Yatkin D, Uzun H, Gurel A, et al. Effects of curcumin on tumour necrosis factor-alpha and interleukin-6 in the late phase of experimental acute pancreatitis. J Vet Med A Physiol Pathol Clin Med. 2006;53:49–54. doi: 10.1111/j.1439-0442.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 20.Mun SH, Kim HS, Kim JW, Ko NY, Kim do K, Lee BY, et al. Oral administration of curcumin suppresses production of matrix metalloproteinase (MMP)-1 and MMP-3 to ameliorate collagen-induced arthritis: Inhibition of the PKCdelta/JNK/c-Jun pathway. J Pharmacol Sci. 2009;111:13–21. doi: 10.1254/jphs.09134fp. [DOI] [PubMed] [Google Scholar]
- 21.Swarnakar S, Ganguly K, Kundu P, Banerjee A, Maity P, Sharma AV. Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. J Biol Chem. 2005;280:9409–15. doi: 10.1074/jbc.M413398200. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekaran CV, Sundarajan K, Edwin JR, Gururaja GM, Mundkinajeddu D, Agarwal A. Immune-stimulatory and anti-inflammatory activities of Curcuma longa extract and its polysaccharide fraction. Pharmacognosy Res. 2013;5:71–9. doi: 10.4103/0974-8490.110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghodasara J, Pawar A, Deshmukh C, Kuchekar B. Inhibitory effect of rutin and curcumin on experimentally-induced calcium oxalate urolithiasis in rats. Pharmacognosy Res. 2010;2:388–92. doi: 10.4103/0974-8490.75462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanai H, Sugimoto K. Curcumin has bright prospects for the treatment of inflammatory bowel disease. Curr Pharm Des. 2009;15:2087–94. doi: 10.2174/138161209788489177. [DOI] [PubMed] [Google Scholar]


