Abstract
Background:
Garlic (Allium sativum) has been known to exhibit a wide range of pharmacological activities which are attributed mainly to the organosulfur compounds present in it. Allicin and garlic oil, components obtained from garlic, have been explored and found to be biologically active on various fronts. Allicin is known to have major stability issues due to rapid degradation even at low temperatures, whereas garlic oil, being lipophilic, shows poor bioavailability after oral administration.
Objective:
To develop novel strategies for optimum delivery of allicin and garlic oil so as to achieve effective availability in the physiological system.
Materials and Methods:
Garlic cloves were lyophilized to obtain allicin-releasing garlic powder (ARGP). This powder was analyzed spectrophotometrically and was used to formulate buccal tablets. Garlic oil was obtained by hydrodistillation of garlic cloves and analyzed by gas chromatography. Self-nanoemulsifying systems (SNS) containing garlic oil were prepared using suitable surfactants and cosurfactants. The SNS were adsorbed on Aerosil 200 and filled in hard gelatin capsules. Both the formulations were suitably evaluated.
Results:
Buccal tablets containing ARGP showed satisfactory physical parameters as well as in vitro drug release, mucoadhesive strength, moisture uptake capacity and drug content. Evaluation of capsules containing SNS of garlic oil also gave satisfactory results. The adsorbed SNS when dispersed in water formed nanoemulsions.
Conclusion:
Buccal tablets as well as capsules containing garlic oil SNS provide promising strategies to overcome the difficulties associated with formulation of allicin and garlic oil.
Keywords: Allicin, buccal tablets, garlic oil, nanoemulsions
INTRODUCTION
Garlic (Allium sativum) is a natural plant being used as a food as well as folk medicine for centuries all over the world. In 1996, garlic was described as a plant with various biological properties like antimicrobial, anti-cancer, antioxidant, immunomodulatory, anti-inflammatory, hypoglycemic, and cardiovascular effects.[1] The use of garlic as a remedy for various ailments has been ongoing since ages. According to Ayurvedic and Greek systems of medicine, garlic is one of the established remedies for tuberculosis.[2] As per literature, garlic has been utilized as a preventive and curative remedy during the various epidemic outbreaks such as typhus, dysentery, cholera, influenza.[3]
The widespread biological activities of garlic are attributed to mainly the organosulfur compounds present in it. Alliin, allicin and γ-glutamylcysteines such as γ-glutamyl-S-allylcysteine, γ-glutamyl-S-trans-1-propenylcysteine constitute majority of the sulfur compounds present in whole or crushed garlic.[4] Reports suggest that allicin shows a wide range of pharmacological activities which include antimicrobial, antifungal, antioxidant and antiviral. In addition to these, favorable effects of allicin are also seen in cases of stroke, coronary thrombosis, atherosclerosis, hypertension and malignant diseases.[5,6] Garlic oil has a wide spectrum of actions; not only is it antibacterial, antiviral, antifungal and antiprotozoal but it also has beneficial effects on the cardiovascular and immune systems. Garlic contains unique oil which is very easily digested and is stable to oxidative stress and for these reasons they are useful and healthy for consumption.[7] Garlic oil has shown good pharmacological effects in the treatment of cancer, TB, infectious diseases and diabetes.
The aim of the study was to formulate and evaluate buccal tablets containing allicin and capsules containing self-nanoemulsifying systems of garlic oil.
MATERIALS AND METHODS
Materials
Methocel K4M (Colorcon Ltd.), Carbopol 974P (Lubrizol), Cremophor EL (Gattefosse), Solutol HS15 (Gattefosse), Transcutol P (Gattefosse), Aerosil 200 (Evonik), Hard gelatin capsules (ACG capsules) were obtained as gift samples. Mannitol, Magnesium stearate, Tween 80, PEG 400 and sodium alginate were purchased from SD Fine Chemicals Ltd. All other reagents and chemicals were of AR grade.
Sample procurement and authentication
The bulbs of Allium sativum were purchased from local market in Mumbai, Maharashtra, India. The bulbs were authenticated from Dept. of Botany, St. Xavier's College, Mumbai.
Preparation of allicin-releasing garlic powder (ARGP)
It has been known that allicin is not naturally present in garlic until the enzyme, alliinase, has been activated by the crushing of cloves. This allows the transformation of the amino acid, alliin, to allicin and other allylthiosulfinates. When fresh garlic is crushed or blended, allicin formation is complete in approximately 6 seconds, well before consumption.[8] Acidic conditions (pH 3.5 or below) rapidly destroy garlic's alliinase activity and hence prevent the generation of allicin and other thiosulfinates. Allicin is not stable at room temperature it rapidly degrades with time. Allicin is less stable in crushed garlic or aqueous garlic homogenates than it is as a pure compound in water.[9] For these reasons, garlic powder was prepared by a process which prevented the release of allinase and thus allicin formation was avoided during preparation and storage of the powder. Garlic cloves were cut into 4-5 mm thick slices, filled in vials and subjected to lyophilization. When these lyophilized garlic cloves slices were completely desiccated, they were pulverized to obtain ARGP.[10] In the presence of water/salivary fluids, allinase is released that catalyzes conversion of inactive alliin to the physiologically active allicin.
Extraction of garlic oil using hydro-distillation method
Peeled garlic cloves were taken and minced in homogenizer with water. Garlic oil was separated using Clavenger distillation apparatus by heating the homogenized mixture for 2-3 hours at 55-65°C. Traces of water, if any, were removed using sodium sulfate.[11] Garlic oil was stored at 4°C.
Analysis of ARGP for allicin content
A simple and rapid spectrophotometric procedure is available for determination of allicin using the chromogenic thiol 4-mercaptopyridine (4-MP). The assay is based on the reaction of 4-MP (λmax = 324 nm) with the activated disulfide bond of thiosulfinates –S (O)–S–. The 4-MP assay is an easy, sensitive, fast, non-costly, and highly effcient throughput assay of allicin in garlic preparations.[12] Water was added to ARGP and kept for 15 min so as to bring out complete release of allicin. To different concentrations of reconstituted garlic powder, 4-MP solution was added and content of allicin was estimated spectrophotometrically.
The molar concentration of allicin was calculated according the equation:
[allicin] =ΔA324 × dilution/39600
ΔA324 = [A324 -(4MP without allicin)- A324 (4-MP with allicin)]
Analysis of garlic oil by gas chromatography (GC)
Analysis of garlic oil was done by GC (Varian CP 3800) using J and W Scientific, DB624, 30 m × 0.53 mm × 3μ film column. Injector temperature and flame ionization detector temperature were maintained at 150°C and 210°C, respectively. Nitrogen was used as a carrier gas and the flow rate was maintained at 30 ml/min. Five dilutions (0.2, 0.4, 0.6, 0.8 and 2 mg/ml) of garlic oil were prepared to build standard plot using acetone.[11]
Formulation development of buccal tablets containing ARGP
Buccal tablets containing ARGP were formulated. For preparation of buccal tablets, three mucoadhesive polymers were selected: Carbopol 974P, Methocel K4M and sodium alginate. 80 mg ARGP was incorporated in each tablet. Garlic powder to polymer ratio of 1:0.5 was selected for formulation studies of buccal tablets. Combination of Methocel K4M + Carbopol 974P and Methocel K4M + sodium alginate were evaluated for use as mucoadhesive polymers in tablets. Mannitol was used as a diluent. Required amounts of all the ingredients were weighed. Garlic powder was mixed with appropriate amounts of mucoadhesive polymer combination mixture and mannitol. The blend was lubricated with magnesium stearate. The lubricated blend was then compressed into tablets using 8 mm flat punches in a single station tablet punching machine. Each tablet (250 mg) contained 80 mg of ARGP.[13]
Formulation development of hard gelatin capsules containing self-nanoemulsifying systems (SNS) of garlic oil
SNS of garlic oil were developed to increase its bioavailability. When the contents of capsule containing SNS adsorbed on Aerosil 200 were diluted with 10 ml of water and shaken gently, it redispersed back to nanoemulsion form. These conditions simulated the conversion of SNS into nanoemulsion on dilution with GI fluids after oral administration.[14,15] Therefore, it was expected to increase absorption and bioavailability of garlic oil.
Initially, optimization of surfactant: Cosurfactant ratios was done. In this study, Cremophor EL, Solutol HS 15 and Tween 80 were used as surfactants and Polyethylene glycol 400 (PEG 400) and Transcutol P were used as cosurfactants. Content of garlic oil per capsule was fixed at 10 mg. Different ratios of Tween 80 and PEG 400 were mixed with garlic oil and the resultant mixture was further agitated using a cyclomixer with slow addition of distilled water. The nanoemulsions so formed were evaluated for mean globule size by photon correlation spectroscopy (Beckmann N5 coulter). Based on results of these studies, surfactant: Cosurfactant ratios of 2:1 and 3:1 were selected for evaluating suitability of the other surfactant and cosurfactant combinations.
SNS containing optimized ratios of selected surfactant and cosurfactant were adsorbed on different quantities of colloidal silicon dioxide. The SNS adsorbed powders were agitated with distilled water and resultant nanoemulsions were evaluated for mean globule size.
Selected SNS adsorbed powder with desirable mean globule size was filled in size 3 hard gelatin capsule.
Evaluation of buccal tablet containing ARGP
The buccal tablets are evaluated for hardness, thickness, friability and weight variation. In addition to above physical parameters, tablets were also evaluated for drug content, in vitro drug release profile, mucoadhesive strength determination and moisture pick up study.[13,16]
Drug content of buccal tablets was determined spectrophotometrically using the 4-MP method.
The United State Pharmacopoeia (USP) type II dissolution apparatus was used to study the release profile of drug from buccal tablets. The tablets are supposed to release the drug from one side only; therefore, an impermeable backing membrane was placed on the other side of the tablet. Two tablets were further fixed to a 2 × 2 cm glass slide with a solution of cyanoacrylate adhesive. In vitro drug release studies were carried out in 500 ml of phosphate buffer solution pH 6.8 for 8 h using a dissolution paddle apparatus at 50 rpm and 37 ± 0.5°C. At predetermined time intervals, 5 ml aliquots were withdrawn and replaced with fresh 5 ml buffer. The samples were filtered, diluted suitably and then analyzed spectrophotometrically using the 4-MP method.
Ex-vivo mucoadhesive strength of garlic powder buccal tablets was measured using the modified physical balance method. Goat buccal mucosa was used as the model substrate and phosphate buffer pH 6.8 was used as the moistening fluid. Freshly excised goat buccal mucosa was obtained from the local slaughter house and used within 3 hours of slaughter. The tablet was laid onto the model membrane under constant weight of 50 gm for a total constant period of 5 minutes. Bioadhesive strength was measured in terms of weight in grams of water required to detach the tablet from the buccal mucosa. The weight of water required to detach the tablet from buccal mucosa was noted as ex-vivo mucoadhesive strength. Mucoadhesive strength was performed in triplicate and average mucoadhesive strength was determined. All the measurements were carried out at 37 ± 1°C.
The moisture uptake studies were conducted in order to give an indication of the relative moisture absorption capacities of the polymers and an idea whether the formulations maintain their integrity after absorption of moisture. 5% w/v agar was prepared in hot water, transferred into petri plates and allowed to solidify. Six tablets from each formulation series were placed in vacuum oven overnight prior to the study to remove residual moisture if any and laminated on one side with water impermeable backing membrane. The tablets were placed in the petri plates containing agar and then incubated at 37°C for 1 hour, removed and reweighed. The percentage moisture absorption was calculated using the formula:
% moisture absorption = (Final weight – Initial weight) ×100/Initial weight
Evaluation of capsules containing self-nanoemulsifying systems of garlic oil
Capsules were evaluated for weight variation, disintegration time and content of garlic oil.
RESULTS
Analysis of ARGP for allicin content
1 gm of ARGP was found to contain 1.2267 ± 0.0784 mg of allicin.W
Extraction of garlic oil and its analysis by GC
Average yield of garlic oil obtained from various batches was 0.221% w/w. The main aim of this GC analysis was to obtain a relationship between the area under curve values of the components of garlic oil and concentration of garlic oil. Based on area of peaks of separated compounds, standard plot was prepared. GC profile of garlic oil at concentration of 2 mg/ml is shown in Figure 1. The retention time and area of the individual peaks in the chromatogram is summarized in Table 1.
Figure 1.
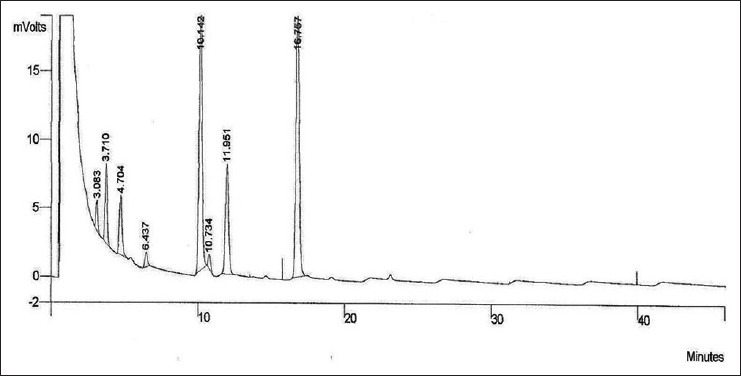
Gas chromatography chromatogram of garlic oil at 2 mg/ml concentration Sample solution of garlic oil was prepared in acetone. As seen, 8 peaks are present in the chromatogram
Table 1.
Gas chromatography data of garlic oil at concentration of 2 mg/ml
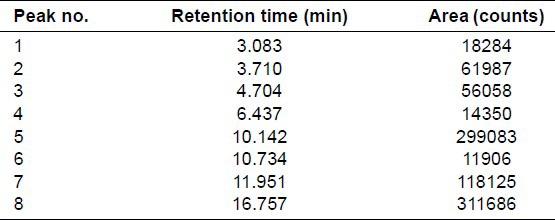
It was observed that total number of peaks reduced from 8 to 5 as concentration of garlic oil was decreased from 2 to 0.2 mg/ml. Peaks 1, 4 and 6 were not detected at lower concentrations. Using gas chromatography under mentioned chromatographic conditions, maximum 8 and minimum 5 compounds were separated from garlic oil. Peak at retention time 10.142 (peak 5) was the sharpest and the most intense one. Also, the peak was present in chromatograms of all garlic oil concentrations. Therefore, this was selected for preparing standard plot. This was designated as substance A. Standard plot of substance A showed best fit linear regression with r2 value of 0.995 and was selected as marker for evaluating content of garlic oil in the formulation.
Formulation development of buccal tablets containing ARGP
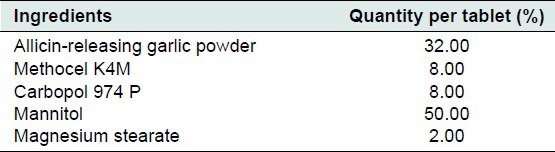
It is difficult to prepare conventional garlic formulations with stable and physiologically available allicin. Allicin is extremely unstable and rapidly degrades with time, even at low temperatures. Oral route of administration is a non-viable option for delivery of allicin because of the degradation of allinase by stomach acid, inactivation of allicin production by intestinal fluids by almost 40% and decomposition of allicin by intestinal cells. Buccal tablets of ARGP were formulated to ensure optimum availability of allicin in the physiological system. Allicin is formed by the action of allinase on alliin when saliva comes in contact with garlic powder. The content of polymer was kept to minimum to prevent the delay in wetting of powder after administration. If high polymer content is used, time required for the saliva to wet the tablet may increase which may retard the rate of formation of allicin. When the combination of sodium alginate and Methocel K4M was used, the tablets did not have enough hardness and disintegrated within 1 hour. Different combinations of Carbopol 974P and Methocel K4M were evaluated. The final quantitative formula for buccal tablets is given in Table 2.
Table 2.
Quantitative formula for buccal tablets containing allicin-releasing garlic powder
Formulation development of hard gelatin capsules containing self-nanoemulsifying systems of garlic oil
SNS can be an excellent drug delivery system for oral administration of poorly permeable and/or highly lipophilic drugs. When converted to nanoemulsions in vivo, the small droplets provide high surface area for drug absorption. Content of garlic oil per capsule was fixed at 10 mg. Tween 80 (surfactant) and PEG 400 (cosurfactant) in proportions, 1:2, 1:1, 2:1 and 3:1 were evaluated for formulation of SNS. Mean globule size obtained after dispersion with water is mentioned in Table 3.
Table 3.
Preliminary optimization of Tween 80 (surfactant) and PEG 400 (cosurfactant) ratio
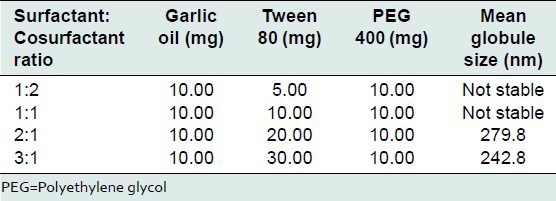
2:1 and 3:1 surfactant: Cosurfactant ratios formed stable nanoemulsion with garlic oil, giving mean globule size in the range of 240-280 nm. These ratios were used for further study.
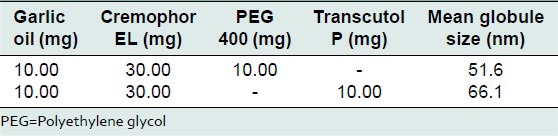
As compared to systems with Tween 80 and Solutol HS 15, systems with Cremophor EL showed lower particle size in the range of 50-80 nm. Therefore, further optimization was done using Cremophor EL. Systems with Cremophor EL and either PEG 400 or Transcutol P in a ratio of 2:1 gave stable nanoemuslsions with mean globule size in range of 55-77 nm. The data is summarized in Table 4. Systems with Cremophor EL and either PEG 400 or Transcutol P in ratio of 3:1 gave stable nanoemuslsions with mean globule size in range of 50-68 nm. The data is shown in Table 5. Based on globule size data and stability, it was concluded that Cremophor EL is a superior surfactant for SNS containing garlic oil when used in combination with PEG 400 or Transcutol P as cosurfactant in 2:1 and 3:1 ratios. Such systems gave stable nanoemulsions when dispersed in water.
Table 4.
Optimization of self-nanoemulsifying systems using Cremophor EL, Solutol HS 15 as surfactant and PEG 400, Transcutol P as cosurfactant at 2:1 surfactant: Cosurfactant ratio
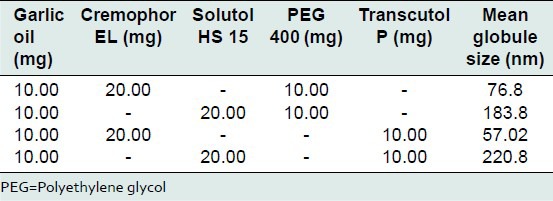
Table 5.
Optimization of self-nanoemulsifying systems using Cremophor EL as surfactant and PEG 400, Transcutol P as cosurfactant at 3:1 surfactant: Cosurfactant ratio
Formulations containing optimized quantities of surfactant and cosurfactant were adsorbed on different quantities of Aerosil 200. The adsorbed blend was dispersed in water and evaluated for mean globule size. As evident from Table 6, formulations having surfactant: Cosurfactant ratio of 3:1 showed best mean globule size in 425-500 nm range after being adsorbed on Aerosil 200. Formulation containing Transcutol P as cosurfactant (formulation 5) was selected as final prototype due to the narrow particle size range as compared to others. Also formulation 5 contained 30 mg Aerosil 200 which provided improved ease of processing of adsorbed blend. This prototype formulation was filled in size 3 capsules. Quantitative formula for the prototype formulation is given in Table 7.
Table 6.
Optimization of quantity of Aerosil 200 for adsorbing self-nanoemulsifying systems
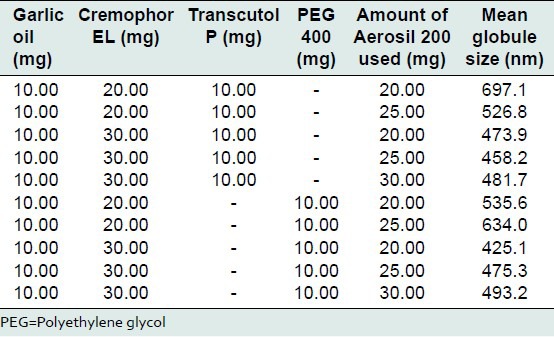
Table 7.
Quantitative formula of capsules containing garlic oil

Evaluation of buccal tablets containing ARGP
Buccal tablets showed hardness in the range of 4.5-5 kg/cm2 and thickness of 3.475 ± 0.0288 mm. Friability and weight variation were well within the USP limits. The results of the evaluation are summarized in Table 8.
Table 8.
Evaluation of buccal tablets containing allicin-releasing garlic powder

Thus, in addition to good physical and chemical parameters, the tablets showed satisfactory drug content and good mucoadhesive strength. Tablets also showed sufficient moisture absorption which implies that the formation of allicin would be rapid as soon the tablets come in contact with saliva.
In vitro allicin release profile in pH 6.8 phosphate buffer from buccal tablets containing ARGP is shown in Figure 2. It was observed that maximum allicin release of 93% occurred at time point of 4 hours after which decrease in allicin concentration is observed, possibly due to degradation of allicin.
Figure 2.
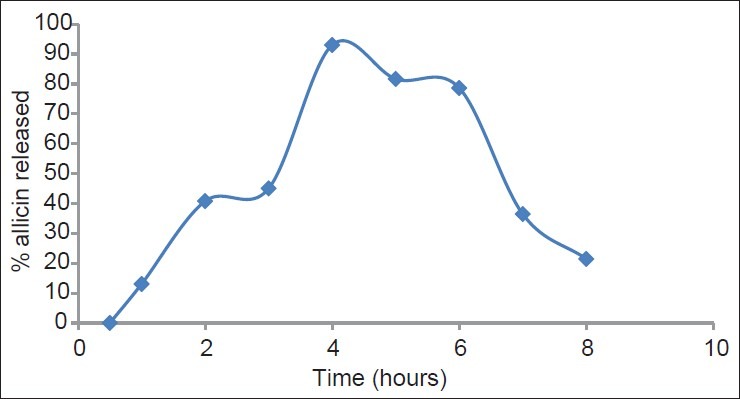
In vitro release profile of allicin in pH 6.8 phosphate buffer from buccal tablets Around 93% of allicin was released in 4 hours after which there was decrease in allicin concentration, possibly due to degradation of allicin
Evaluation of capsules containing garlic oil
Weight variation and disintegration time of the capsules were well within USP limits. The content of garlic oil in the formulation was calculated by using the linear regression equation for standard curve for substance A. GC profile of formulation is given in Figure 3. From GC analysis, the average content of garlic oil per capsule was found to be 9.30 mg.
Figure 3.
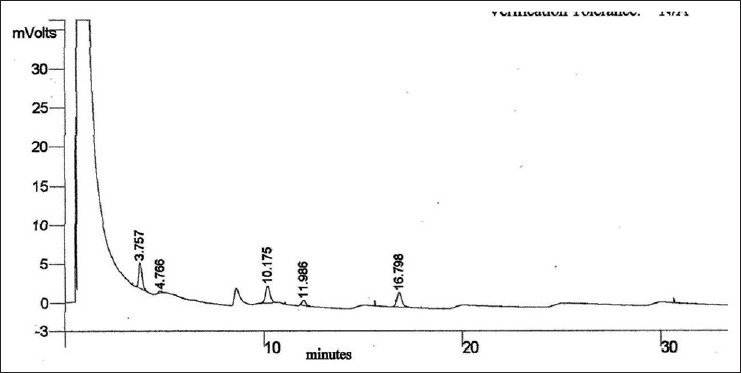
Gas chromatography chromatogram of capsule containing garlic oil
CONCLUSIONS
Garlic bulbs were obtained from local market and authenticated. Allicin as well as garlic oil have been reported to exhibit widespread pharmacological actions. Allicin is known to be extremely unstable. It is produced by the action of the enzyme allinase on the precursor alliin, a naturally occurring compound in whole garlic cloves. Formulations containing allicin are difficult to prepare due to extreme instability of allicin, even at low temperatures. Conventional formulations aimed at formation of allicin in vivo also pose a major challenge as alliinase is destroyed by stomach acid. In addition, intestinal fluid inhibits production of allicin from powder by 40% and intestinal epithelial cells also decompose allicin. Thus, delivery of allicin to the physiological system to achieve a therapeutically significant concentration is difficult. To overcome the problems, buccal tablets containing lyophilized garlic powder were prepared. In our formulation, lyophilization inhibited the action of allinase in the powder due to absence of water; whereas formulation of buccal tablet gave a potential advantage of by-passing the above-mentioned issues associated with oral delivery of allicin. The buccal tablets showed satisfactory physico-chemical profiles. Garlic oil, being lipophilic would pose a bioavailability problem when delivered via oral route. Self-nanoemulsifying systems containing garlic oil were formulated. The nanoemulsions were adsorbed onto colloidal silicon dioxide and filled into hard gelatin capsules to enable easy administration by oral route. The capsules would release the contents into the gastric fluid which would redisperse to form the nanoemulsion. The nanoemulsions formed in vivo would provide a high surface area for absorption of the oil thus increasing the bioavailability.
In a nutshell, the study provides potential strategies to deliver therapeutically promising components (Allicin and garlic oil) obtained from Allium sativum in a viable way such that they reach the systemic circulation at the desired rate and extent.
Footnotes
Source of Support: The funding for the project was provided by All India Council for Technical Education (AICTE), Delhi
Conflict of Interest: None declared
REFERENCES
- 1.Reuter HD, Koch HP, Lawson LD. Therapeutic effects and applications of garlic and its preparations. In: Koch HP, Lawson LD, editors. Garlic: The Science and Therapeutic Application of Allium sativum L. and Related Species. Baltimore: Williams and Wilkins; 1996. pp. 135–512. [Google Scholar]
- 2.Hannan A, Ullah MI, Usman M, Hussain S, Absar M, Javed K. Anti-mycobacterial activity of Garlic (Allium sativum) against multi-drug resistant and non-multi-drug resistant Mycobacterium tuberculosis. Pak J Pharm Sci. 2011;24:81–5. [PubMed] [Google Scholar]
- 3.Petrovska BB, Cekovska S. Extracts from the history and medical properties of garlic. Pharmacogn Rev. 2010;4:106–10. doi: 10.4103/0973-7847.65321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iciek M, Kwiecień I, Włodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen. 2009;50:247–65. doi: 10.1002/em.20474. [DOI] [PubMed] [Google Scholar]
- 5.Ilić DP, Nikolić VD, Nikolić LB, Stanković MZ, Stanojević LP, Cakić MD. Allicin and Related Compounds: Biosynthesis, Synthesis and Pharmacological Activity. Facta Universitatis. 2011;9:9–20. [Google Scholar]
- 6.Oommen S, Anto RJ, Srinivas G, Karunagaran D. Allicin (from garlic) induces caspase-mediated apoptosis in cancer cells. Eur J Pharmacol. 2004;485:97–103. doi: 10.1016/j.ejphar.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 7.Ross ZM, O’Gara EA, Hill DJ, Sleightolme HV, Maslin DJ. Antimicrobial Properties of Garlic Oil against Human Enteric Bacteria: Evaluation of Methodologies and Comparisons with Garlic Oil Sulfides and Garlic Powder. Appl Environ Microbiol. 2009;67:475–80. doi: 10.1128/AEM.67.1.475-480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson LD, Gardner CD. Composition, Stability, and Bioavailability of Garlic Products Being Used in a Clinical Trial. J Agric Food Chem. 2005;53:6254–61. doi: 10.1021/jf050536+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawson LD, Hughes BG. Characterization of the formation of allicin and other thiosulfinates from garlic. Planta Med. 1992;58:345–50. doi: 10.1055/s-2006-961482. [DOI] [PubMed] [Google Scholar]
- 10.Lawson LD, Wang ZJ. Low Allicin Release from Garlic Supplements: A Major Problem Due to the Sensitivities of Alliinase Activity. J Agric Food Chem. 2001;49:2592–9. doi: 10.1021/jf001287m. [DOI] [PubMed] [Google Scholar]
- 11.Sowbhagya HB, Kaul TP, Florence SP, Appurao AG, Srinivas P. Evaluation of enzyme-assisted extraction on quality of garlic volatile oil. Food Chem. 2009;113:1234–38. [Google Scholar]
- 12.Miron T, Shin I, Feigenblat G, Weiner L, Mirelman D, Wilchek M, et al. A spectrophotometric assay for allicin, alliin, and alliinase (alliin lyase) with a chromogenic thiol: Reaction of 4-mercaptopyridine with thiosulfinates. Anal Biochem. 2002;307:76–83. doi: 10.1016/s0003-2697(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 13.Nagaraju K, Velmurugan S, Deepika B, Vinushitha S. Formulation and In-vitro evaluation of Buccal Tablets of Metoprolol Tartrate. Int J Pharm Pharm Sci. 2011;3:239–46. [Google Scholar]
- 14.Date AA, Nagarsenker MS. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int J Pharm. 2007;329:166–72. doi: 10.1016/j.ijpharm.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Dixit RP, Nagarsenker MS. Self-nanoemulsifying granules of ezetimibe: Design, optimization and evaluation. Eur J Pharm Sci. 2008;35:183–92. doi: 10.1016/j.ejps.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Bhanja S, Ellaiah P, Martha SK, Sahu PK, Tiwari SP, Panigrahi BB. Formulation and In vitro evaluation of mucoadhesive buccal tablets of Timolol maleate. Int J Pharm Biomed Res. 2010;1:129–34. [Google Scholar]


