Abstract
Background:
Different therapeutic regimens were used for eradication of Helicobacter pylori, based on the cost, effectiveness and patient's compliance. The aim of this study was the evaluation of licorice compared with bismuth in quadruple regimen on eradication of H. pylori in patients with peptic ulcer disease (PUD).
Materials and Methods:
In a double-blind clinical trial study, 60 patients with PUD and positive rapid urease test were enrolled. The patients were randomly allocated into two equal groups. In first group, licorice, amoxicillin, metronidazole and omeprazole and in the second (control) group, bismuth subsalicylate, amoxicillin, metronidazole and omeprazole were prescribed respectively, and 4 weeks after treatment, in order to evaluate H. pylori eradication, urea breath test was done in all patients. The outcome of the study was the preference usage of licorice as an effective medication for H. pylori eradication.
Results:
Mean age of the patients in the control and case groups were 40.8 ± 15.5 and 42.2 ± 15.8 years, respectively (P = 0.726). Seventeen (56.7%) patients in control group and 16 (53.3%) in the case group were female (P = 0.795). Both groups were similar based on frequency of gastric or duodenal ulcer. Response to treatment were seen in 20 (67%) and 17 (57%) patients of case and control groups, respectively (P > 0.05).
Conclusion:
Our study showed that licorice is as effective as bismuth in H. pylori eradication; therefore, in patients whom bismuth is contraindicated, licorice can be used safely instead.
Keywords: Bismuth, Helicobacter pylori eradication, licorice
INTRODUCTION
Peptic ulcer disease (PUD) is a common and treatable disease worldwide[1] which without eradication of Helicobacter pylori infection, 50-80% recurrence rate will be appeared during the 6-12 months after initial ulcer healing.[2] Esophago-gastro-duodenoscopy (upper endoscopy) is the gold standard method for exact diagnosis of PUD which its accuracy depends on the location of the ulcer and the experience of the endoscopist.[3] PUD can present as epigastric pain or dyspepsia. Dyspepsia is a common and occasionally life-threatening clinical problem. The symptoms of ulcer and nonulcers dyspepsia may be similar. In fact, in endoscopy, the most common diagnosis is functional dyspepsia, but other problems such as ulcer disease or gastric cancer may also be seen.[4] Functional dyspepsia is likely, if symptoms persist after an initial negative investigation. There is not clear relationship between functional dyspepsia and H. pylori infection, although in H. pylori positive patients, improvement of symptoms may be observed in about 15% of the patients after eradication treatment.[5]
Multiple regimens have been evaluated for H. pylori eradication in randomized controlled trials;[6,7] however, despite these studies, the optimal modality of treatment has not been defined yet, due to increasing risk of resistance to treatment.[8] The selected treatment regimen should be effective, as well as considering some issues such as side effects, cost, the number of drugs and duration of treatment.[7]
Bismuth subsalicylate can inhibit growth or eradicate H. pylori in vitro; however, its minimal inhibitory concentration (MIC) is high.[9] Bismuth subsalicylate is effective for suppression of H. pylori as mono-therapy but combination with other antibiotics could increase its efficacy.[10] Licorice (Glycyrrhiza glabra) has been used since many years ago for reliefing of epigastric pain, and healing of gastric ulcers.[11] It stimulates the production of mucus membrane, and may cause symptomatic improvements of peptic ulcer. Moreover, licorice might inhibit leukotriene synthesis and prostaglandin; however, it may also stimulate of adrenocortical axis by inhibition of 11-β hydroxyl steroid dehydrogenase.[12] In vitro antibacterial and antiviral effect of licorice was shown in some studies,[13,14] but by our knowledge there was not any clinical study about anti H. pylori effect of licorice in the patients, so the aim of this study was the evaluation of effect of licorice as one component of quadruple therapy for H. pylori eradication in the patients with dyspepsia.
MATERIALS AND METHODS
In a double-blind clinical trial, 60 patients who referred to endoscopy center of Hajar Hospital in Shahrekord (located at Southwest of Iran) were enrolled. Inclusion criteria were: Existence of abdominal pain or dyspepsia and gastric ulcer, duodenal ulcer, gastritis or duodenitis in upper endoscopy and H. pylori positivity in biopsy specimen by rapid urease test. Exclusion criteria were: Noncooperation of the patients during the study, adverse effects of medications during the study, and having liver or renal failure history. The sample size was determined based on availability of patients and our budget limitation. The patients randomly entered into two groups of 30 patients. Two groups were matched based on age, gender, history of cigarette smoking and PUD. While in Group 1 (case group), the patients were treated with metronidazole tablet 500 mg/twice a day bid, amoxicillin 1 g/bid, omeprazole 20 mg/bid and D-Reglis (containing 380 mg licorice/tablet)/bid “manufactured by Irandaruc Pharmacy Company”, in control group, bismuth subsalicylate, 262 mg/tablet (2 tablet/bid) were prescribed instead of licorice. Urea breath test was done for all patients 4 weeks after the end of treatment (by Heliprobe machine, Kibion company, Sweden). Drug regimen was prescribed by Gastroenterologist and evaluation of the patients was done by a resident.
The primary outcome of this study was to evaluate the effect of licorice on H. pylori eradication and secondary outcome was the use of licorice as an effective and safe medication in the combination regimen of PUD treatment. Patients' data were collected by check list and was entered into SPSS 17 (SPSS Inc., Chicago, IL, USA) and were analyzed by using t-test, Chi-square and Fisher's exact test. All data and information were confidential and an informed consent was taken from every patient for obtaining samples and other processes of the study. The study was approved by Ethics Committee of Shahrekord University of Medical sciences.
RESULTS
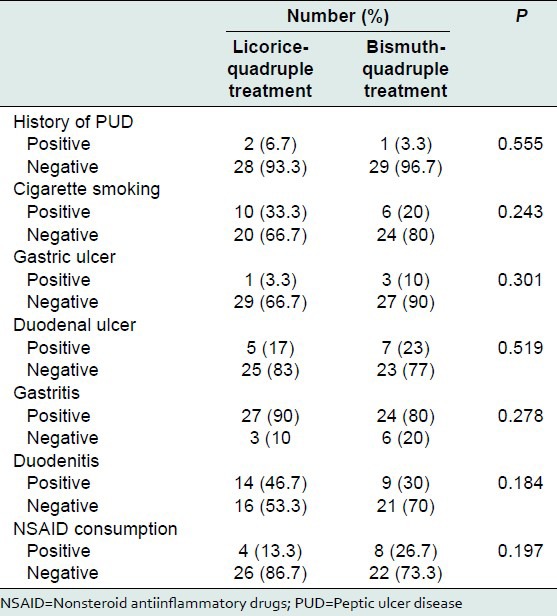
Mean age of the patients in the control and case groups were 40.8 ± 15.5 and 42.2 ± 15.8 years, respectively with no significant difference between two groups (P = 0.726). Seventeen (56.7%) patients in control group and 16 (53.3%) in case group were female (P = 0.795). Table 1 shows the main characteristics of participants. Sixteen patients were smoker (P = 0.243), three patients had history of PUD. There was also history of dyspepsia in 30 patients (P = 0.606). Endoscopy showed gastric ulcer, duodenal ulcer, gastritis and duodenitis in 4, 12, 51 and 23 patients, respectively (P > 0.05). History of nonsteroid antiinflammatory drugs consumption was seen in 12 patients (P = 0.197).
Table 1.
Some criteria of the patients of case and control group
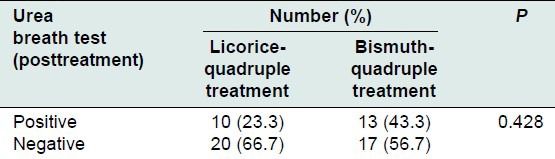
The response to treatment (eradication of H. pylori) was shown in Table 2, with 20 (67%) and 17 (57%) patients among case and control groups, respectively (0.428). There were not any side effects or interactions (such as severe hypertension, muscular weakness or diarrhea), during the study tending to discontinuation of treatment.
Table 2.
Frequency of response to treatment in the case and control group
DISCUSSION
This study showed that low dose of licorice (380 mg/bid) against H. pylori in quadruple therapy could be as effective as bismuth. After endoscopy, there was no difference between two groups based on duodenal ulcer, gastric ulcer, duodenitis or gastritis. In addition, response to treatment in patients of two groups was similar.
Almost one-third of patients with dyspepsia may have PUD. In addition, due to susceptibility of these patients to the development of gastric adenocarcinoma, eradication treatment might be recommended in these patients. Hence, treatment of H. pylori infection should be considered in patients with gastric or duodenal ulcer, although eradication of H. pylori in nonulcer dyspepsia remains debatable.[15,16,17] Furthermore, various regimens have been used for H. pylori eradication with success rate of 75-80%.[18] In our study, the patients were under treatment of traditional quadruple therapy against H. pylori because of high efficacy, low price, and minimal side-effects of this regimen.
Anti H. pylori and gastric mucosal protective effect of licorice and bismuth is somewhat similar,[19] therefore we used licorice (D-reglis) instead of bismuth in traditional quadruple therapy, and showed that licorice is at least as efficient as bismuth in quadruple regimen for H. pylori eradication. Antimicrobial effect of licorice has been evaluated in some studies such as the study of Tsukiyama, which found that licorice was effective against Gram-positive bacteria and especially against all Bacillus species.[13] In addition, Fukai showed the effectiveness of licorice against methicillin-resistant Staphylococcus aureus.[20] In a review article, Bouras described many therapeutic effects of licorice, such as anti-PUD and gastric mucosal protection, antimicrobial and antiviral property, antitumor and antiinflammatory effects, decreasing of low density lipoprotein cholesterol and improvement of sore throat.[21]
In a few studies, anti H. pylori effect of licorice were evaluated in vitro, for example Nariman showed that licorice could be effective against H. pylori.[22] Moreover, Fukai proved its anti H. pylori effect and concluded that licorice may be useful for prevention of peptic ulcer or gastric cancer in H. pylori-infected patients.[14] Jafarian et al. evaluated anti H. pylori effect of licorice on isolated strains from gastric biopsy of the patients, and concluded that none of strains had resistance to the licorice extracts.
In fact, H. pylori may show susceptibility to licorice extract in MIC (The MIC was defined as the lowest concentration of a drug that completely inhibited the growth on the agar plates) that is achievable in the stomach under therapeutic concentration. As licorice had not adverse effects on the normal bacterial flora of gastrointestinal tract, therefore the authors suggested that licorice extract may be an effective agent for the treatment of all forms of H. pylori spices in vivo, due to susceptibility of all H. pylori strains to licorice extracts.[23] Similar results were obtained by Krausse et al. which showed bactericidal activity of licorice to kill all the five strains of H. pylori rapidly; they suggested its using as an alternative agent in clarithromycin-resistant strains of H. pylori.[24] In another study, Aly et al. evaluated antiinflammatory effect of licorice and concluded that licorice can increase antiinflammatory effect of Na diclofenac as well as adding antiulcer property.[25] Beil et al. also approved antiinflammatory and anti H. pylori effect of two flavonoids of licorice that stimulated production of prostaglandin E2 in isolated gastric mucosal cells, and inhibited growth of H. pylori in culture media; therefore they suggested consumption of licorice in the patients with gastrointestinal diseases associated with H. pylori infection.[26] Usual recommended dose by Producer Company for D-reglis in PUD is two tablets, 2 or 3 times/day, however; in this study lower dose of drug was effective too. This dosage of licorice is associated with lower side effect as in this study, and none of patients had any complication of treatment. Krahenbuhl also showed that the use of less than 500 mg of glicirinic acid daily had minimal and transient effect on blood pressure elevation, but larger amounts could lead to permanent side effects.[27] Furthermore, all previous studies on anti H. pylori effect of licorice were done in vitro and in laboratory environment. Antimicrobial effect of antibiotics in vivo in the acidic condition of stomach may be different from their efficacy in medium in laboratory, for example amoxicillin is a very potent anti H. pylori in vitro but its effect significantly decreased in acidic PH of stomach.[28]
Our study has several limitations such as small sample size, short duration of follow-up and lack of control group with placebo consumption. Therefore, we recommend conducting studies with larger sample size, longer duration of follow-up for detection of recurrence and use of third group with placebo and finally use of licorice in other therapeutic modality such as triple regimen.
CONCLUSION
Licorice is a safe, inexpensive and effective agent for H. pylori eradication in quadruple regimen, so we could use it in some conditions such as ones with intolerance or contraindication of Bismuth use. Meanwhile, it may also be effective in resistant strains or recurrent PUD.
ACKNOWLEDGMENT
The authors acknowledge all staff of Hajar Endoscopy Center for their cooperation in this study.
None of authors have any conflict of interest with regard to this article. This study funded and supported by Research Deputy of Shahrekord University of medical Sciences.
Footnotes
Source of Support: This study funded and supported by Research Deputy of Shahrekord University of medical Sciences
Conflict of Interest: None declared
REFERENCES
- 1.Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9(Suppl 2):33–9. [PubMed] [Google Scholar]
- 2.Current status of maintenance therapy in peptic ulcer disease. The ACG Committee on FDA-Related Matters. Am J Gastroenterol. 1988;83:607–17. [PubMed] [Google Scholar]
- 3.Cotton PB, Shorvon PJ. Analysis of endoscopy and radiography in the diagnosis, follow-up and treatment of peptic ulcer disease. Clin Gastroenterol. 1984;13:383–403. [PubMed] [Google Scholar]
- 4.Mason JM, Delaney B, Moayyedi P, Thomas M, Walt R North of England Dyspepsia Guideline Development Group. Managing dyspepsia without alarm signs in primary care: New national guidance for England and Wales. Aliment Pharmacol Ther. 2005;21:1135–43. doi: 10.1111/j.1365-2036.2005.02445.x. [DOI] [PubMed] [Google Scholar]
- 5.Chey WD, Wong BC Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 6.Gisbert JP, Gonzalez L, Calvet X. Systematic review and meta-analysis: Proton pump inhibitor vs. ranitidine bismuth citrate plus two antibiotics in Helicobacter pylori eradication. Helicobacter. 2005;10:157–71. doi: 10.1111/j.1523-5378.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 7.Fischbach LA, van Zanten S, Dickason J. Meta-analysis: The efficacy, adverse events, and adherence related to first-line anti Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther. 2004;20:1071–82. doi: 10.1111/j.1365-2036.2004.02248.x. [DOI] [PubMed] [Google Scholar]
- 8.Hojo M, Miwa H, Nagahara A, Sato N. Pooled analysis on the efficacy of the second-line treatment regimens for Helicobacter pylori infection. Scand J Gastroenterol. 2001;36:690–700. doi: 10.1080/003655201300191941. [DOI] [PubMed] [Google Scholar]
- 9.Vogt K, Warrelmann M, Hahn H. The minimum inhibitory concentrations of various bismuth salts against Campylobacter pylori. Zentralbl Bakteriol. 1989;271:304–10. doi: 10.1016/s0934-8840(89)80028-3. [DOI] [PubMed] [Google Scholar]
- 10.Marshall BJ, Valenzuela JE, McCallum RW, Dooley CP, Guerrant RL, Cohen H, et al. Bismuth subsalicylate suppression of Helicobacter pylori in nonulcer dyspepsia: A double-blind placebo-controlled trial. Dig Dis Sci. 1993;38:1674–80. doi: 10.1007/BF01303177. [DOI] [PubMed] [Google Scholar]
- 11.Shibata S. A drug over the millennia: Pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000;120:849–62. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- 12.Balakrishnan V, Pillai MV, Raveendran PM, Nair CS. Deglycyrrhizinated liquorice in the treatment of chronic duodenal ulcer. J Assoc Physicians India. 1978;26:811–4. [PubMed] [Google Scholar]
- 13.Tsukiyama R, Katsura H, Tokuriki N, Kobayashi M. Antibacterial activity of licochalcone A against spore-forming bacteria. Antimicrob Agents Chemother. 2002;46:1226–30. doi: 10.1128/AAC.46.5.1226-1230.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T. Anti Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002;71:1449–63. doi: 10.1016/s0024-3205(02)01864-7. [DOI] [PubMed] [Google Scholar]
- 15.Moayyedi P, Soo S, Deeks J, Delaney B, Harris A, Innes M, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2001;16:D002096. doi: 10.1002/14651858.CD002096. [DOI] [PubMed] [Google Scholar]
- 16.McColl K, Murray L, El-Omar E, Dickson A, El-Nujumi A, Wirz A, et al. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med. 1998;339:1869–74. doi: 10.1056/NEJM199812243392601. [DOI] [PubMed] [Google Scholar]
- 17.Malfertheiner P, MOssner J, Fischbach W, Layer P, Leodolter A, Stolte M, et al. Helicobacter pylori eradication is beneficial in the treatment of functional dyspepsia. Aliment Pharmacol Ther. 2003;18:615–25. doi: 10.1046/j.1365-2036.2003.01695.x. [DOI] [PubMed] [Google Scholar]
- 18.Houben MH, van de Beek D, Hensen EF, de Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy: The impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047–55. doi: 10.1046/j.1365-2036.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 19.Romano M, Cuomo A. Eradication of Helicobacter pylori: A clinical update. MedGenMed. 2004;6:19. [PMC free article] [PubMed] [Google Scholar]
- 20.Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T. Antimicrobial activity of licorice flavonoids against methicillin-resistant Staphylococcus aureus. Fitoterapia. 2002;73:536–9. doi: 10.1016/s0367-326x(02)00168-5. [DOI] [PubMed] [Google Scholar]
- 21.Bouras P, Tsonas S. Licorice: A traditional herb and its modern effects on humans. Sci J. 2001;7:135–40. [Google Scholar]
- 22.Nariman F, Eftekhar F, Habibi Z, Falsafi T. Anti Helicobacter pylori activities of six Iranian plants. Helicobacter. 2004;9:146–51. doi: 10.1111/j.1083-4389.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 23.Malek Jafarian M, Ghazvini K. In vitro susceptibility of Helicobacter pylori to licorice extract. Iran J Pharm Res. 2007;6:69–72. [Google Scholar]
- 24.Krausse R, Bielenberg J, Blaschek W, Ullmann U. In vitro anti Helicobacter pylori activity of Extractum liquiritiae, glycyrrhizin and its metabolites. J Antimicrob Chemother. 2004;54:243–6. doi: 10.1093/jac/dkh287. [DOI] [PubMed] [Google Scholar]
- 25.Aly AM, Al-Alousi L, Salem HA. Licorice: A possible antiinflammatory and antiulcer drug. AAPS PharmSciTech. 2005;6:E74–82. doi: 10.1208/pt060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beil W, Birkholz C, Sewing KF. Effects of flavonoids on parietal cell acid secretion, gastric mucosal prostaglandin production and Helicobacter pylori growth. Arzneimittelforschung. 1995;45:697–700. [PubMed] [Google Scholar]
- 27.Krähenbühl S, Hasler F, Frey BM, Frey FJ, Brenneisen R, Krapf R. Kinetics and dynamics of orally administered 18 beta-glycyrrhetinic acid in humans. J Clin Endocrinol Metab. 1994;78:581–5. doi: 10.1210/jcem.78.3.8126129. [DOI] [PubMed] [Google Scholar]
- 28.Gerrits MM, Schuijffel D, van Zwet AA, Kuipers EJ, Vandenbroucke-Grauls CM, Kusters JG. Alterations in penicillin-binding protein 1A confer resistance to beta-lactam antibiotics in Helicobacter pylori. Antimicrob Agents Chemother. 2002;46:2229–33. doi: 10.1128/AAC.46.7.2229-2233.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


