Abstract
Background and Aim:
Use of sedation, analgesia and neuromuscular blocking agents is widely practiced in Intensive Care Units (ICUs). Our aim is to study the current practice patterns related to mobilization, analgesia, relaxants and sedation (MARS) to help in standardizing best practices in these areas in the ICU.
Materials and Methods:
A web-based nationwide survey involving physicians of the Indian Society of Critical Care Medicine (ISCCM) and the Indian Society of Anesthesiologists (ISA) was carried out. A questionnaire included questions on demographics, assessment scales for delirium, sedation and pain, as also the pharmacological agents and the practice methods.
Results:
Most ICUs function in a semi-closed model. Midazolam (94.99%) and Fentanyl (47.04%) were the most common sedative and analgesic agents used, respectively. Vecuronium was the preferred neuromuscular agent. Monitoring of sedation, analgesia and delirium in the ICU. Ramsay's Sedation Scale (56.1%) and Visual Analogue Scale (48.07%) were the preferred sedation and pain scales, respectively. CAM (Confusion Assessment Method)-ICU was the most preferred method of delirium assessment. Haloperidol was the most commonly used agent for delirium. Majority of the respondents were aware of the benefit of early mobilization, but lack of support staff and safety concerns were the main obstacles to its implementation.
Conclusion:
The results of the survey suggest that compliance with existing guidelines is low. Benzodiazepines still remain the predominant ICU sedative. The recommended practice of giving analgesia before sedation is almost non-existent. Delirium remains an underrecognized entity. Monitoring of sedation levels, analgesia and delirium is low and validated and recommended scales for the same are rarely used. Although awareness of the benefits of early mobilization are high, the implementation is low.
Keywords: Analgesia, early mobilization, muscle relaxants, sedation
Introduction
Multiple organ support is an essential aspect of managing critically ill patients in the Intensive Care Unit (ICU). Interventions like endotracheal intubation and mechanical ventilation, invasive catheters, hemodialysis, etc., are commonly performed in the ICUs. With more challenging therapies like prone positioning, high-frequency oscillatory ventilation (HFOV), continuous renal replacement therapy (CRRT) and extracorporeal membrane oxygenation (ECMO), critical care practice has become more physiologically challenging. As most of these modalities of patient care are invasive and may lead to pain, anxiety and physical discomfort, patients need adequate analgesia, sedation and sometimes even muscle relaxation to tolerate these procedures.
Studies have demonstrated a significant effect of inappropriate sedation, analgesia and muscle relaxation on patient's outcome. These practices determine short-term consequences, such as oversedation,[1,2,3,4,5,6,7] hypotension, venous thrombosis, prolonged ventilation,[8,9] increased incidence of bacteremia and ventilator-associated pneumonia,[10,11] increased length of intensive care unit stay (LOS)[12,13] and intermediate- to long-term effects such as critical illness myoneuropathy and post-traumatic stress disorder.
Recent data support early and regular mobilization in the management of critically ill patients. Feasibility of mobilization of a critically ill patient depends on the sedation-analgesia and muscle relaxation practices of the ICU. Sedation, analgesia and muscle relaxation practices are interdependent and overlapping, making it difficult to assess them in isolation. Multiple surveys[14,15,16,17] have been conducted in the past to evaluate the practice of sedation, analgesia, muscle relaxation and early mobilization individually, but only a few have evaluated the combined practice of all the four. We planned to evaluate the current practices of mobilization, analgesia, relaxants and sedation (MARS) in Indian ICUs with a nation-wide web-based survey. No data about such practices in Indian ICUs are available.
Materials and Methods
Designing the survey
The Delphi survey methodology was used. A panel of 10 senior physicians who practice critical care medicine were involved directly in formulating the questionnaire. [SUPPORTING:1] The first step in designing the survey was to prepare a questionnaire with clinical relevance to the aims of the survey. We conducted a literature search through web-based search engines, i.e. PUBMED, MEDLINE, EMBASE and GOOGLE SCHOLAR. The key words included sedation administration/survey, analgesia administration/survey, muscle relaxation administration/survey, mobilization/early mobilization in ICU/critically ill, physical therapy in ICU/critically ill. We shortlisted a set of 75 questions that were discussed among the survey investigators. In the second round, the questionnaire was re-evaluated and only 57 questions that were felt to be more relevant to the aim were included. Re-evaluation of the questions was made for the third time and answer options were evaluated for their relevance to the aim of the survey. Multiple choice options were created with an easy to use “check the box” system to provide responses. As the clinical practices were expected to be different for different group of patients, multiple options were allowed to be chosen.
The set of questions were formatted into two sections, i.e. the first section consisted of 19 questions and on personal details (i.e. age, sex, qualification, specialty, etc.) of the participants, the demographic profile of the concerned ICU (i.e. type of ICU, predominant type of patients, staff and equipment details of the ICU, average length of stay [LOS] and expenditure per day, etc.). The second section consisted of 38 questions on organ dysfunction and severity scores, sedation- analgesia- muscle relaxation practices (i.e. indication, agents, method of administration, monitoring, etc.), evaluation of delirium (incidence, monitoring, drug therapy), weaning and mobilization protocols and practices (i.e. spontaneous breathing trials (SBT), feasibility of mobilization, etc.).
Conduct of survey
The database of the registered members of the Indian Society of Critical Care Medicine (ISCCM) and the Indian Society of Anaesthesiologists (ISA), whose members are distributed across the country, was obtained after due permission. Nine thousand four hundred and fifty-two pooled e-mail addresses were obtained from both databases. Physicians were sent an e-mail link using these e-mail addresses, through which they could access the Internet-based survey (MARS: Mobilization, Analgesia, Relaxation, Sedation). Only those members who did full time or part time intensive care were requested to participate in the survey. The time period allotted to complete the survey was from 1st July 2012 to 1st January 2013 (6 months). Six reminders to complete the survey were sent through e-mail to the non-responders during the study period.
Results
Of the 9452 pooled e-mail addresses, 2983 e-mail addresses were common (members common to both societies). Hence, the survey was actually sent to 6469 physicians. Because of various reasons, 526 e-mails were undelivered; hence, e-mails were successfully sent to 5943 physicians. Physicians belonged to different parts of the country. The number of first-phase responders (who responded to a single e-mail) was 378. After a reminder in the next week, 174 more physicians responded and, on further reminders, a total of 659 physicians were enrolled (response rate 11.1%).
Respondent and ICU demographics
Almost 59% (391 responders) of the responders were under the age of 40 years. Majority of the responders (269 responders, 41%) were less than 5 years into their practice in critical care medicine, while only 7% (43 responders) of the responders were into more than 20 years of their practice. Table 1 shows the demographic profile of the respondents and their ICUs, which includes the type of ICU, case mix, per day expenses and use of severity of illness scores. Physicians from ICUs admitting more than 300 patients per year formed 70% (462 responders) of the responders. Majority of the physicians (77%, 509 responders) worked in ICUs that have less than 20 beds. Sepsis with multiorgan dysfunction and respiratory failure were the major reasons for intubation and mechanical ventilation. Majority of the physicians (58%, 382 responders) estimated the average ventilator days to be in the range of 3-5 days and the average length of ICU stay to be in the range of 5-10 days (45%, 300 responders) in their ICU. Only 52% (343 responders) of the physicians reported using a severity scoring system for their patients and APACHE II was the most commonly used score [Table 1]. Seventy-one percent (470 responders) of the respondents had a dedicated physiotherapist in the ICU. Only 19% (122 responders) of the physicians worked in ICUs that had a nurse to patient ratio of 1:1 and 50% (329 responders) reported a nurse to patient ratio of 1:2. When asked about the availability of ventilators in the ICU, 33% (219 responders) of the participants reported to have one ventilator for each bed, 38% (249 responders) of participants had at least one ventilator for two beds and 29% (189 responders) reported to have one ventilator for more than two beds in the ICU.
Table 1.
Respondent and ICU demographics
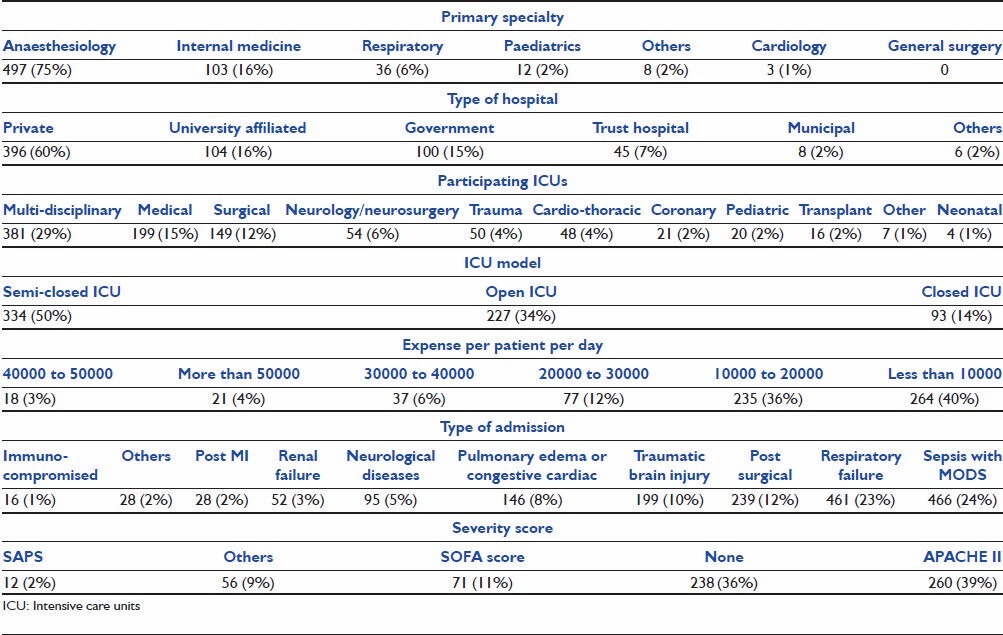
Sedation practices
Midazolam was the most commonly used agent for sedation (95%, 626 responders), followed by Propofol (68.4%, 451 responders). Dexmedetomidine was used by 60.2% (397 responders) of the responders at some time in their practice [Figure 1].
Figure 1.
Sedative agents used
Analgesia practices
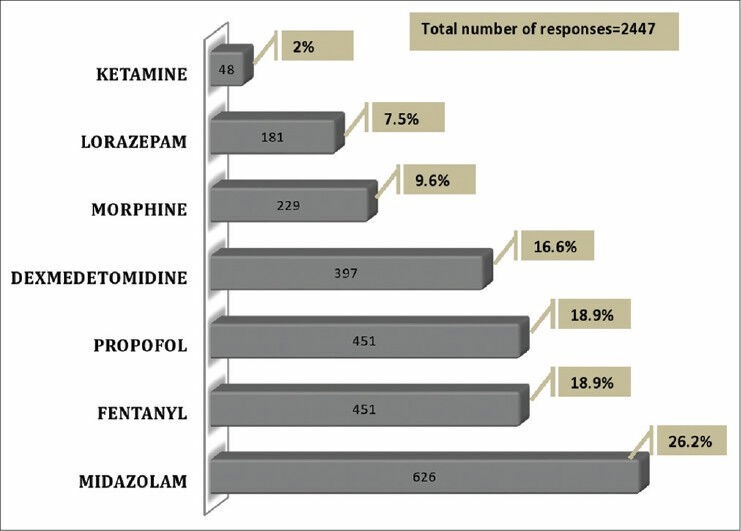
Fentanyl was the most common analgesic agent used (47.04%, 310 responders), followed by Tramadol (16.2%, 103 responders) and Paracetamol (15.2%, 100 responders) [Figure 2]. Among the alternate methods of analgesia, epidural analgesia was most commonly used (62.7%, 407 responders), followed by regional nerve blocks (23%, 151 responders).
Figure 2.
Analgesic agents used
Relaxants (neuromuscular blocking [NMB] agents)
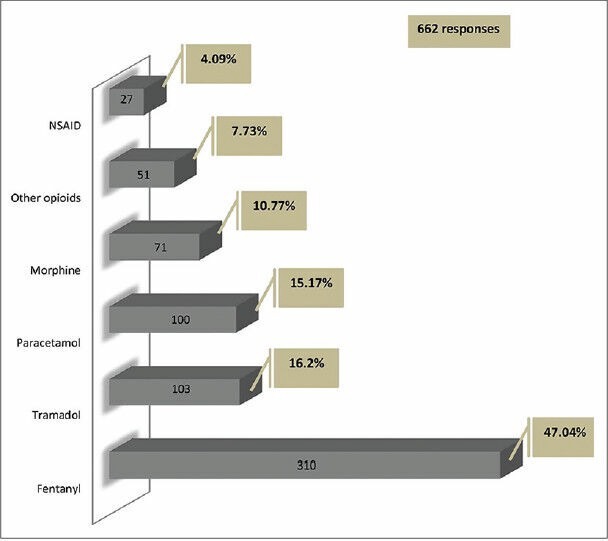
Use of NMB was occasional among 86% (571 responders) of the responders, while only 7% (43 responders) always used NMB during ventilation. The most common indication for its use was severe refractory hypoxia and acute respiratory distress syndrome (ARDS) (71%, 469 responders). Most of the physicians used an NMB agent for less than 48 h (28%, 185 responders) in ARDS. Vecuronium was the most preferred agent (50%, 333 responders), followed by Atracurium (38%, 251 responders) [Figure 3]. “Analgesia first” approach is used by 8% (49 responders) of responders. Analgosedation (simultaneous use of sedation and analgesia) was reported by 66% (437 responders), while 14% (89 responders) reported using sedation, analgesia and NMB agents simultaneously. Only 1% (six responders) of the respondents practiced an “analgesia only” regimen in their ICUs. Continuous drug infusion was used by 54% (357 responders) of the respondents, intermittent boluses by 21% (140 responders) of the respondents and both methods by 25% (162 responders) of the respondents.
Figure 3.
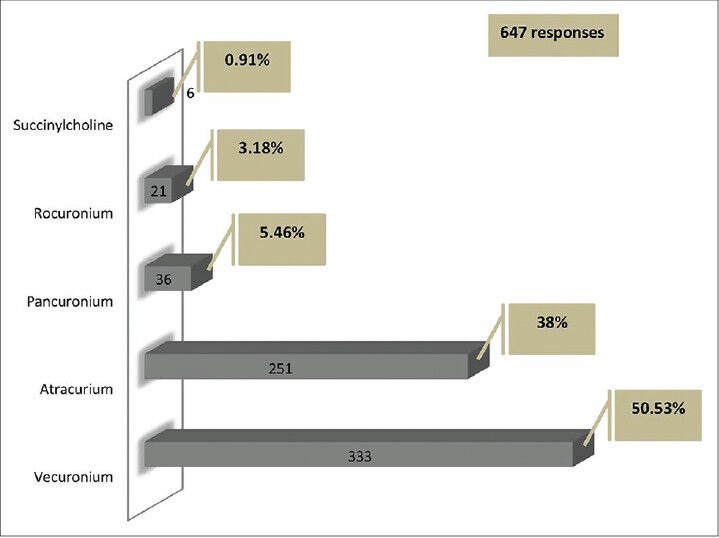
Neuromuscular blocking agents used
Monitoring practices
Routine monitoring of sedation was reported by 58% (381 responders) of the responders. Although there was a wide mix of the type of scale used, Ramsay's Sedation Scale was the most preferred one (56.1%, 247 responders), followed by Richmond agitation sedation scale (RASS) (19.3%, 85 responders) [Figure 4].
Figure 4.
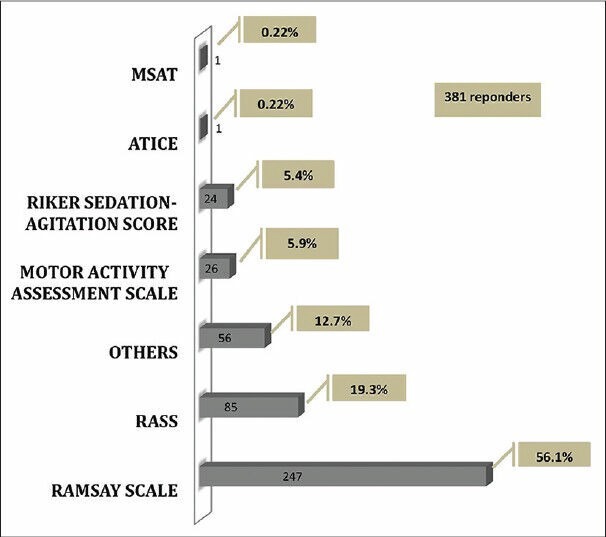
Sedation score used
Sixty-six percent of the responders (442 responders) monitored pain in their ventilated patients. The participants used various pain scales, of which the Visual Analogue Scale (VAS) was the most preferred (48.07%, 299 responders) scale [Figure 5].
Figure 5.
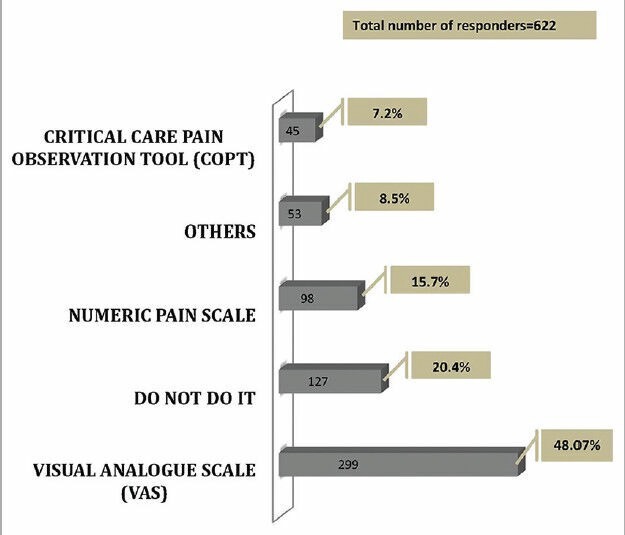
Pain score used
Twenty-seven percent of physicians (178 responders) reported a regular audit to evaluate their compliance in use of sedation, pain and delirium scales. Only 50% of the physicians responded when asked about having a written protocol in their ICU for sedation, pain and delirium management. This 50% consisted of protocols for sedation (18%), analgesia (15%), management of delirium (4%) and none (13%). Only 10% (64 responders) of the participants did a regular post-ICU discharge of all patients who had received sedation, and only 6% of the participants did it occasionally.
Delirium in ICU
About two-thirds of the responders (65%, 430 responders) felt that the incidence of delirium in mechanically ventilated patients is less than 10%. The incidence was reported to be in the range of 10-50% by 31% (201 responders) of the responders, and only 2% of the responders felt that more than half of their mechanically ventilated patients experience delirium. Only 480 physicians (73%) responded when asked about use of delirium score. Majority (65.6%, 315 responders) reported not assessing delirium in the ICU. Only 22% (107 responders) of them used formal scales to measure delirium, The CAM-ICU score being the most preferred one (20%) [Figure 6]. Haloperidol was the most commonly used drug (77%, 507 responders) for the treatment of delirium.
Figure 6.
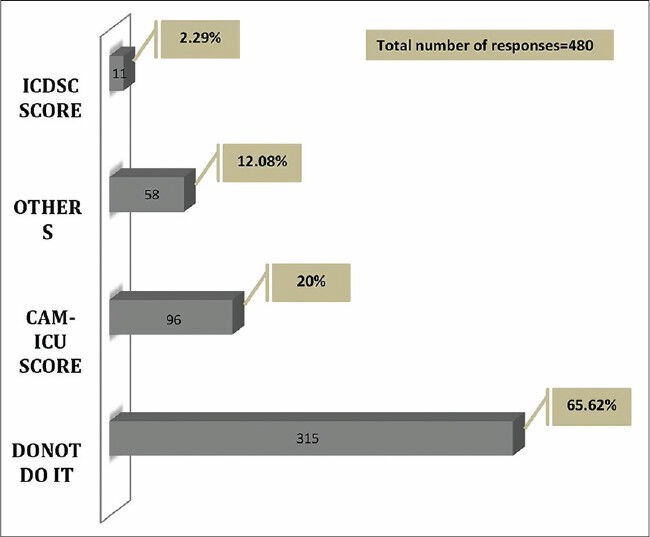
Delirium score used
Weaning methodology
Eighty-three percent of physicians (551 responders) said that they practiced a protocolized weaning in their ICUs. Eighty percent (533 responders) of the weaning practices were physician driven as opposed to only 5% driven by critical care nurses.
Seventy-nine percent (524 responders) reported that in their ICU, a spontaneous awakening trial (SAT) was performed prior to weaning efforts and 91% (605 responders) of the physicians weaned using a regular spontaneous breathing trial (SBT). Sixty-two percent (406 responders) of physicians tried SAT sometime during the first 48 h on mechanical ventilation and 16% (105 responders) did so in the first 24 h. SBT was performed within the first 24 h by 13% (86 responders) of the physicians and 53% (350 responders) of the physicians subjected their patients to SBT within 48 h.
Mobilization practices
Ninety-two percent (614 responders) opined that early mobilization did have a role in patient management and outcome in ICU. While 85% (558 responders) of the physicians order some form of mobilization regularly, only 20% (134 responders) believed that patients in the ICU can be mobilized safely while on ventilators and with invasive monitoring. Apprehensions related to dislodgement of tubes and lines remained the major limiting factor in non-mobilization of ICU patients (22%, 142 responders). The level of mobilization reported was as follows: 86% (572 responders) had bed side, 70%(462 responders) to a wheel chair and 67% (441 responders) to some walking. When asked about the possible reason for non-mobilization, only 569 physicians responded. 33.7% (192 responders), i.e 192 responders, reported that mobilization in ICU is not applicable and is not feasible due to staffing issues (non-availability of trained staff). 13.7% (90 responders) of the responders were of the opinion that they could not mobilize critically ill patients with equipment attached (ventilators, lines, etc.) and 7.8% (52 responders) were afraid of complications that might occur as a result of it.
Discussion
Our survey had 659 responders. The data from the survey suggest that compliance with existing guidelines is low.[18] Benzodiazepines are the predominant sedative used in the ICU. The practice of giving analgesia before sedation is almost non-existent. Delirium remains an under-recognized entity. Monitoring of sedation levels, analgesia and delirium is low and validated and recommended scales for the same are infrequently used.
Only 659 (11.12%) of the 5943 physicians responded to the survey. However, this cannot be considered as the actual response rate of the survey, as we had requested only those who do full time or part time intensive care among them to respond. Hence, many physicians for whom this survey was not applicable did not respond and this number is unknown. We adopted this method of sending e-mails to all members of both societies as we did not know how many physicians among them practice some form of intensive care. Although our method avoided a selection bias, the actual number of those practicing some form of intensive care for whom the survey was designed is yet unknown. This number would be the true denominator to determine the actual response rate. This true denominator, however, may be small considering several members of both societies practice their primary specialty (being anesthesiology or general medicine) and some members of the ISCCM include surgeons, microbiologists and physiologists (memberships require an interest in critical care and not necessarily practice in the specialty).
Surveys on sedation and analgesia in critically ill patients have a varied response rate from 20% to 65%. Most of the previous surveys identified the number of units rather than the number of physicians in the response rate calculations. These surveys yielded a high response rate (65%,[14] 63.5%[15] ). However, surveys conducted by sending the questionnaire directly to physicians (such as ours) reported a wide variation in response rates ranging from 20% to 60%.[16,17]
A high percentage (59%) of the responders was under 40 years of age. In a Canadian survey, majority of the responders (47%) were in the age group of 40-49 years. Majority of the responders were less than 5 years into their critical care practice as compared with the west, where almost equal number of responders were less than 5 years, 5-10 years and 10-15 years into their practice.[17] Majority of the responding doctors were practicing critical care medicine in private sector hospitals and almost two-thirds of the physicians were trained in anesthesiology. Only a few of the relevant surveys in the past have collected the demographic profile of the participants. In a survey in Canada, the majority of the participating physicians were from internal medicine (56%), and anesthesiologists constituted 26% of the respondents.[17]
Although the true response rate is unknown, the actual numbers of respondents was good. The 659 respondents with varying levels of experience in critical care were from private, public, trust hospitals and both, with and without university affiliation and formal critical care courses, which covers the diverse background of the critical care practice in India. Patients with sepsis and respiratory failure represent the majority of patients among the ICUs of respondents with an average of 3-5 ventilated days. This shows that a majority of the respondents were practicing in ICUs dealing with sick patients, needing higher level of support, where analgesia and sedation practices would be applicable. A severity scoring system was used by 64% of the responding physicians, mostly APACHE II. None of the previous surveys have evaluated the use of severity scoring in the corresponding ICUs.
The sedation practices have varied widely among the published surveys of the past. A survey in the UK found that the use of a particular sedative is guided by the required duration of sedation. For short-term sedation (less than 24 h), Propofol was the preferred agent, and Midazolam when the anticipated sedation duration was more than 24 h. When weaning was considered, the sedation used predominantly was Propofol.[15] Similar studies from Australia and Canada found Midazolam to be the most commonly used agent, followed by Propofol.[14,17]
The current recommendation is in favor of no non benzodiazepine (NBZD) sedative agents over benzodiazepines (BZD).[18] Multiple studies from the United States have shown that there is a progressive increase in the usage of Dexmedetomidine.[19,20,21,22,23,24] Riker et al. showed that Dexmedetomidine was associated with more ventilator-free days (3.7 days vs. 5.6 days) as compared with midazolam.[25] The meta-analyses by the American College of Critical Care Medicine (ACCM) and the Society of Critical Care Medicine (SCCM),[18] and a study by P and Haripande et al.[26] showed that BZD is associated with increased ICU LOS. A head-to-head trial concluded that Propofol is better than Lorazepam,[27] and a similar study advocated Dexmedetomedine over Midazolam for minimizing the duration of mechanical ventilation. Moreover, the same study noted a higher rate of delirium with BZD (Midazolam) than Dexmedetomidine. The cost-effectiveness of Dexmeditomidine has been demonstrated in the past.[28]
Our survey found that Midazolam is still the most commonly used sedative agent in India. With the current evidence about increased delirium with the use of BZD, this may be of some concern. It is closely followed by use of Propofol and Dexmedetomidine. Although BZD use is still predominant, as opposed to the current recommendations, it was encouraging to see up to 60.2% of the responders reporting the use of Dexmedetomidine at least on one occasion in their practice.
The present guidelines advocate the routine use of sedation scales as it has been found to improve patient outcome.[18,29,30,31] Multiple studies have demonstrated that a combined use of protocolized sedation, sedation scale and NBZD agents lead to improved patient outcome in ICUs, decreased length of mechanical ventilation, ICU and hospital LOS.[32,33,34,35,36,37,38,39,40,41,42] Only 58% of the 381 responders routinely monitored sedation levels in the ICU. One of the reasons for this may be related to staffing issues. RASS and Riker sedation agitation scale (SAS) are the most validated and recommended scales for sedation assessment in the ICU.[18] In our survey, however, the Ramsay Sedation Scale (RAS) was preferred by most. This may be explained due to the lack of awareness of more validated scales or RAS, as it is easier to use and interpret. Similar results have been found in previous surveys.[15,17]
Intravenous opioids are the recommended agents for non-neuropathic pain in critically ill patients. The co-administration of non-opioid analgesia and non-pharmacological means of analgesia reduces the amount of opioid use and its adverse effects. Thoracic epidural anesthesia/analgesia is recommended as an alternate mode for post-operative pain management in patients undergoing abdominal aortic surgery.[18] In our survey, opioids (mostly Fentanyl) were the predominant analgesic used, with non-opioids like non-steroidal anti-inflammatory drugs and paracetamol used as alternatives or adjunct analgesics. Epidural and regional block were the predominant alternate methods of analgesia. The gold standard for pain assessment is the severity reported by the patient himself.[18] The use of Behavioural Pain scale (BPS) in ICUs has been demonstrated to improve patient management and outcome, which included duration of mechanical ventilation and LOS in ICU.[43,44,45] As most of the patients in the ICU fail to self-report, BPS and Critical-Care Pain Observation Tool (CPOT) are the most validated pain scales to be used in critical care, which include medical, post-operative and trauma patients.[18] VAS is the most commonly used pain scale probably due to its simplicity. Our survey shows that the more reliable pain scales like CPOT and BPS are hardly used (4%) in Indian ICUs. This may be due to a lack of awareness or the greater complexity of these scales in comparison with VAS.
Few surveys previously evaluated the practice of using NMB agents in ICUs. In the Canadian survey,[17] Pancuronium was the most commonly used NMB agent followed by Rocuronium and Vecuronium. Among physicians who used cis-atracurium, intermittent and continuous dosing was equally distributed. Paralytic infusion was interrupted for assessment of patients by 64% of the users. The most common indications for their use were: The use of unconventional modes of ventilation, decreased lung compliance and refractory hypoxemia. Twenty-two percent of the participants who used NMB agents follow an institutional protocol and 84% employ the Train-of-four (TOF) monitoring. Interestingly, up to 41% use an agent for reversal of paralysis sometime during the treatment.[17] On the other hand, a survey in England found that the predominant use of NMB agents was restricted to neurological and neurosurgical ICUs. The use of NMB agents was comparatively infrequent (7% of participants use NMB agents for > 10% of cases).[15]
In our survey, the routine use of NMB was low (7%) and occasional use was among 86% respondents, with the predominant indication for use being hypoxia and ARDS. Only 9% of participants reported that the most common indication was head injury. The most common agent used was Vecuronium, followed by Atracurium.
The currently recommended[18] “Analgesia first” approach is practiced only by a very small proportion of respondents (8%, 49 responders), with a majority still using sedation and analgesia together. Recent data suggest that an analgesia-only regimen may be beneficial in certain cases.[46] Often, analgesic medications are added as a supplement to sedation. It reduced doses of both agents and, in some cases, was a more effective therapy.[47] In our current survey, analogosedation was the most common practice used by the responders and analgesia only was practiced only by 1% (six responders) of the physicians. Only 21% responders used intermittent drug boluses, while most (79%) used continuous drug infusions or a combination of both strategies. Lack of adequate nursing staff in most of the ICUs may be the reason for this.
Various studies on ICU delirium have found the incidence to be as high as 80% in mechanically ventilated patients.[48,49,50,51] Delirium has been proven to be a major predictor of mortality, hospital LOS, cost of ICU care and long-term impairment of cognition.[50,52,53,54,55,56] The recommended measures for prevention of delirium include early mobilization, pharmacologic and combined strategies. The present survey suggests that delirium is still an under-recognized entity in India. Sixty-five percent of the participants state that the incidence of delirium in mechanically ventilated patients was up to 10%. Routine monitoring of delirium is recommended.[18] Even among the 34.38% (165 responders) of the 480 respondents who claimed to assess delirium in their patients, only 22.29% (107) used formal scales to measure delirium. CAM-ICU and ICDSC are the most valid and reliable scales for delirium screening in critically ill patients,[18] and the former appears to be the preferred option among the few survey participants who assess delirium.
Although there is no clear evidence for recommended treatment options for delirium in critically ill patients, antipsychotic agents are routinely used as per the recommendations from some guidelines.[57,58,59] Use of Haloperidol for the treatment of delirium has a level C recommendation and was most commonly used in our survey.
Early mobilization is being increasingly used as a non-pharmacological method[60] and is associated with decreased depth of sedation, improved outcomes such as increased number of ventilator-free days and reduced hospital and ICU LOS. Multiple studies have demonstrated the safety of early mobilization of critically ill patients.[60,61,62,63] In our survey, the majority of respondents were aware of the benefit of mobilization but were limited in implementing it due to lack of support staff and concerns of safety, particularly in patients with multiple lines and those receiving ventilatory support.
Limitations of the study
A major limitation of this study is that the actual response rate cannot be determined, as the actual number of physicians who do at least part time intensive care (the denominator) is unknown. However, the number of respondents being large (659) and with varying levels of experience in critical care, cover the diverse spectrum of intensive care practices in India. Another inherent limitation of such surveys is that the reliability of individual responses cannot be ensured.
Strengths of the study
This survey includes the largest number of physicians that has participated among similar surveys conducted elsewhere.[16,17] This nation-wide survey is the first of its kind in the field of critical care medicine in India. Although we cannot claim with certainty that it is truly representative of practices in India, this study give us some idea of the MARS practices in Indian ICUs. Future prospective studies targeted at practicing intensivists will give us more accurate information on these practices. It is also clear from the results of this study that some aspects of care can be improved by increasing knowledge and awareness (analgesia first, use of non-BZD sedatives, presence of delirium, etc.), while other aspects such as early mobilization, the benefits of which though well known, need better infrastructural support for implementation.
Conclusion
Despite the inherent limitation of our survey, the data represent the diverse nature of sedation practices in Indian ICUs. The results of this survey suggest that compliance with existing guidelines is low. BZDs still remain the predominant sedative used in the ICU. Giving analgesia before sedation is almost non-existent. Delirium remains an under-recognized entity. Monitoring of sedation levels, analgesia and delirium is low and validated and recommended scales for the same are rarely used. Although awareness of the benefits of early mobilization is high, the implementation is low.
Footnotes
Source of Support: Indian Society of Critical Care Medicine(ISCCM)
Conflict of Interest: None declared.
References
- 1.Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27:2609–15. doi: 10.1097/00003246-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial. Lancet. 2008;371:126–34. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 3.Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of seda-tive infusions in critically ill patients undergoing mechanical ventila-tion. N Engl J Med. 2000;342:1471–7. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 4.Treggiari MM, Romand JA, Yanez ND, Deem SA, Goldberg J, Hudson L, et al. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med. 2009;37:2527–34. doi: 10.1097/CCM.0b013e3181a5689f. [DOI] [PubMed] [Google Scholar]
- 5.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous i.v. seda tion is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–8. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 6.Fraser GL, Prato BS, Riker RR, Berthiaume D, Wilkins ML. Frequency, severity, and treat-ment of agitation in young versus elderly patients in the ICU. Phar-macotherapy. 2000;20:75–82. doi: 10.1592/phco.20.1.75.34663. [DOI] [PubMed] [Google Scholar]
- 7.Conti J, Smith D. Haemodynamic responses to extubation after cardiac surgery with and without continued sedation. Br J Anaesth. 1998;80:834–6. doi: 10.1093/bja/80.6.834. [DOI] [PubMed] [Google Scholar]
- 8.Barr J, Zomorodi K, Bertaccini EJ, Shafer SL, Geller E. A double-blind, random ized comparison of i.v.lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model. Anesthesiology. 2001;95:286–98. doi: 10.1097/00000542-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Swart EL, Zuideveld KP, de Jongh J, Danhof M, Thijs LG, Strack van Schijndel RM. Comparative population pharmacokinetics of lorazepam and midazolam during long-term continuous infusion in critically ill patients. Br J Clin Pharmacol. 2004;57:135–45. doi: 10.1046/j.1365-2125.2003.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rello J, Lode H, Cornaglia G, Masterton R. VAP Care Bundle Contributors: A European care bundle for prevention of ventilator-associated pneu-monia. Intensive Care Med. 2010;36:773–80. doi: 10.1007/s00134-010-1841-5. [DOI] [PubMed] [Google Scholar]
- 11.Rello J, Lorente C, Bodí M, Diaz E, Ricart M, Kollef MH. Why do physicians not follow evi-dence-based guidelines for preventing ventilator-associated pneu-monia. A survey based on the opinions of an international panel of intensivists? Chest. 2002;122:656–61. doi: 10.1378/chest.122.2.656. [DOI] [PubMed] [Google Scholar]
- 12.Akça O, Melischek M, Scheck T, Hellwagner K, Arkiliç CF, Kurz A, et al. Postoperative pain and subcu-taneous oxygen tension. Lancet. 1999;354:41–2. doi: 10.1016/S0140-6736(99)00874-0. [DOI] [PubMed] [Google Scholar]
- 13.Hedderich R, Ness TJ. Analgesia for trauma and burns. Crit Care Clin. 1999;15:167–84. doi: 10.1016/s0749-0704(05)70046-4. [DOI] [PubMed] [Google Scholar]
- 14.Botha J, Le Blanc V. The State of Sedation in the Nation: Results of an Australian Survey. Crit Care Resusc. 2005;7:92–6. [PubMed] [Google Scholar]
- 15.Reschreiter H, Maiden M, Kapila A. Sedation practice in the intensive care unit: A UK national survey. Crit Care. 2000;12:R152. doi: 10.1186/cc7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soliman HM, Mélot C, Vincent JL. Sedative and analgesic practice in the intensive care unit: The results of a European survey. Br J Anaesth. 2001;87:186–92. doi: 10.1093/bja/87.2.186. [DOI] [PubMed] [Google Scholar]
- 17.Mehta S, Burry L, Fischer S, Martinez-Motta JC, Hallett D, Bowman D, et al. Canadian Critical Care Trials Group. Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Crit Care Med. 2006;34:374–80. doi: 10.1097/01.ccm.0000196830.61965.f1. [DOI] [PubMed] [Google Scholar]
- 18.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 19.Wunsch H, Kahn JM, Kramer AA, Rubenfeld GD. Use of intravenous infu-sion sedation among mechanically ventilated patients in the United States. Crit Care Med. 2009;37:3031–9. doi: 10.1097/CCM.0b013e3181b02eff. [DOI] [PubMed] [Google Scholar]
- 20.Szumita PM, Baroletti SA, Anger KE, Wechsler ME. Sedation and analgesia in the intensive care unit: Evaluating the role of dexmedetomidine. Am J Health Syst Pharm. 2007;64:37–44. doi: 10.2146/ajhp050508. [DOI] [PubMed] [Google Scholar]
- 21.Tan JA, Ho KM. Use of dexmedetomidine as a sedative and anal-gesic agent in critically ill adult patients: A meta-analysis. Intensive Care Med. 2010;36:926–39. doi: 10.1007/s00134-010-1877-6. [DOI] [PubMed] [Google Scholar]
- 22.Oldenhof H, de Jong M, Steenhoek A, Janknegt R. Clinical pharmacokinet-ics of midazolam in intensive care patients, a wide interpatient vari-ability? Clin Pharmacol Ther. 1988;43:263–9. doi: 10.1038/clpt.1988.31. [DOI] [PubMed] [Google Scholar]
- 23.Triltsch AE, Welte M, von Homeyer P, Grosse J, Genähr A, Moshirzadeh M, et al. Bispectral index-guided sedation with dexmedetomidine in intensive care: A prospective, randomized, double blind, placebo-controlled phase II study. Crit Care Med. 2002;30:1007–14. doi: 10.1097/00003246-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dex medetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–33. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. SEDCOM (Safety and Efficacy of Dexmedetomidine Compared with Midazolam) Study Group: Dexmedetomidinevs midazolam for sedation of critically ill patients: A randomized trial. JAMA. 2009;301:489–99. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 26.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidinevslorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized con-trolled trial. JAMA. 2007;298:2644–53. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 27.Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, et al. A randomized trial of inter-mittent lorazepam versus propofol with daily interruption in mechani-cally ventilated patients. Crit Care Med. 2006;34:1326–32. doi: 10.1097/01.CCM.0000215513.63207.7F. [DOI] [PubMed] [Google Scholar]
- 28.Dasta JF, Kane-Gill SL, Pencina M, Shehabi Y, Bokesch PM, Wisemandle W, et al. A cost-minimization analysis of dexmedetomidine compared with midazolam for long-term seda-tion in the intensive care unit. Crit Care Med. 2010;38:497–503. doi: 10.1097/CCM.0b013e3181bc81c9. [DOI] [PubMed] [Google Scholar]
- 29.Detriche O, Berré J, Massaut J, Vincent JL. The Brussels sedation scale: Use of a simple clinical sedation scale can avoid excessive sedation in patients undergoing mechanical ventilation in the intensive care unit. Br J Anaesth. 1999;83:698–701. doi: 10.1093/bja/83.5.698. [DOI] [PubMed] [Google Scholar]
- 30.Brattebø G, Hofoss D, Flaatten H, Muri AK, Gjerde S, Plsek PE. Effect of a scoring system and protocol for sedation on duration of patients' needs for ventilator support in a surgical intensive care unit. BMJ. 2002;324:1386–9. doi: 10.1136/bmj.324.7350.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacLaren R, Plamondon JM, Ramsay KB, Rocker GM, Patrick WD, Hall RI. A prospective evaluation of empiric versus protocol-base sedation and analgesia. Pharmacotherapy. 2000;20:662–72. doi: 10.1592/phco.20.7.662.35172. [DOI] [PubMed] [Google Scholar]
- 32.Arabi Y, Haddad S, Hawes R, Moore T, Pillay M, Naidu B. Changing sedation practices in the intensive care unit-Protocol implementation, multifaceted mul-tidisciplinary approach and teamwork. Middle East J Anesthesiol. 2007;19:429–47. [PubMed] [Google Scholar]
- 33.Arias-Rivera S, Sánchez-Sánchez Mdel M, Santos-Díaz R, Gallardo-Murillo J, Sánchez-Izquierdo R, Frutos-Vivar F, et al. Effect of a nursing-implemented sedation protocol on weaning outcome. Crit Care Med. 2008;36:2054–60. doi: 10.1097/CCM.0b013e31817bfd60. [DOI] [PubMed] [Google Scholar]
- 34.Brattebø G, Hofoss D, Flaatten H, Muri AK, Gjerde S, Plsek PE. Effect of a scoring system and protocol for sedation on duration of patients' need for ven-tilator support in a surgical intensive care unit. BMJ. 2002;324:1386–9. doi: 10.1136/bmj.324.7350.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quenot JP, Ladoire S, Devoucoux F, Doise JM, Cailliod R, Cunin N. Effect of a nurse-imple-mented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med. 2007;35:2031–6. doi: 10.1097/01.ccm.0000282733.83089.4d. [DOI] [PubMed] [Google Scholar]
- 36.Robinson BR, Mueller EW, Henson K, Branson RD, Barsoum S, Tsuei BJ. An analgesia-delirium-sedation protocol for critically ill trauma patients reduces ventilator days and hospital length of stay. J Trauma. 2008;65:517–26. doi: 10.1097/TA.0b013e318181b8f6. [DOI] [PubMed] [Google Scholar]
- 37.Bucknall TK, Manias E, Presneill JJ. A randomized trial of protocol-directed sedation management for mechanical ventilation in an Aus-tralian intensive care unit. Crit Care Med. 2008;36:1444–50. doi: 10.1097/CCM.0b013e318168f82d. [DOI] [PubMed] [Google Scholar]
- 38.Elliott R, McKinley S, Aitken LM, Hendrikz J. The effect of an algorithm-based sedation guideline on the duration of mechanical ventilation in an Aus-tralian intensive care unit. Intensive Care Med. 2006;32:1506–14. doi: 10.1007/s00134-006-0309-0. [DOI] [PubMed] [Google Scholar]
- 39.Mascia MF, Koch M, Medicis JJ. Pharmacoeconomic impact of rational use guidelines on the provision of analgesia, sedation, and neuromuscular blockade in critical care. Crit Care Med. 2000;28:2300–6. doi: 10.1097/00003246-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Marshall J, Finn CA, Theodore AC. Impact of a clinical pharma-cist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med. 2008;36:427–33. doi: 10.1097/01.CCM.0000300275.63811.B3. [DOI] [PubMed] [Google Scholar]
- 41.DuBose JJ, Inaba K, Shiflett A, Trankiem C, Teixeira PG, Salim A, et al. Measurable outcomes of quality improvement in the trauma intensive care unit: The impact of a daily quality rounding checklist. J Trauma. 2008;64:22–7. doi: 10.1097/TA.0b013e318161b0c8. [DOI] [PubMed] [Google Scholar]
- 42.Devlin JW, Holbrook AM, Fuller HD. The effect of ICU sedation guidelines and pharmacist interventions on clinic. Ann Pharmacother. 1997;31:689–95. doi: 10.1177/106002809703100604. [DOI] [PubMed] [Google Scholar]
- 43.Chanques G, Jaber S, Barbotte E, Violet S, Sebbane M, Perrigault PF. Impact of systematic evalu-ation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34:1691–9. doi: 10.1097/01.CCM.0000218416.62457.56. [DOI] [PubMed] [Google Scholar]
- 44.Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J. DOLOREA Investigators: Pain assessment is associated with decreased duration of mechani cal ventilation in the intensive care unit: A post Hoc analysis of the DOLOREA study. Anesthesiology. 2009;111:1308–16. doi: 10.1097/ALN.0b013e3181c0d4f0. [DOI] [PubMed] [Google Scholar]
- 45.Arbour C, Gélinas C, Michaud C. Impact of the implementation of the Critical-Care Pain Observation Tool (CPOT) on pain management and clinical outcomes in mechanically ventilated trauma intensive care unit patients: A pilot study. J Trauma Nurs. 2011;18:52–60. [Google Scholar]
- 46.Strøm T, Martinussen T, Toft P. A protocol of no sedation for criti-cally ill patients receiving mechanical ventilation: A randomised trial. Lancet. 2010;375:475–80. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 47.Breen D, Karabinis A, Malbrain M, Morais R, Albrecht S, Jarnvig IL. Decreased duration of mechanical ventilation when comparing analgesia-based seda-tion using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: A randomised trial [ISRCTN47583497] Crit Care. 2005;9:R200–10. doi: 10.1186/cc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.WSJ.com. Changing intensive care to improve life afterward. [Last accessed on 2014 Aug 07]. Available from: http://online.wsj.com/article/SB10001424052748704081604576144321242020948.html .
- 49.Hospitals fight a form of delirium that often strikes ICU patients-The Washington Post. [Last accessed on 2014 Aug 07]. Available from: http://www.washingtonpost.com ; http://www.washingtonpost.com/national/health/hospitalsfight-a-form-of-delirium-that-often-strikes-icu-patients/2011/03/23/AF518nMD_story.html .
- 50.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–62. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 51.McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK, et al. Delirium in the intensive care unit: Occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51:591–8. doi: 10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 52.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. SEDCOM (Safety and Efficacy of Dexmedetomidine Compared with Midazolam) Study Group: Delirium duration and mortality in lightly sedated, mechani-cally ventilated intensive care patients. Crit Care Med. 2010;38:2311–8. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 53.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–7. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, et al. Delirium as a predictor of mor-tality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–62. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 55.Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a pre-dictor of long-term cognitive impairment in survivors of critical ill-ness. Crit Care Med. 2010;38:1513–20. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin J, Heymann A, Bäsell K, Baron R, Biniek R, Bürkle H, et al. Evidence and consensus-based German guidelines for the management of analgesia, sedation and delirium in intensive care-Short version. Ger Med Sci. 2010;8:1–31. doi: 10.3205/000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michaud L, Büla C, Berney A, Camus V, Voellinger R, Stiefel F, et al. Delirium: Guidelines for general hospitals. J Psychosom Res. 2007;62:371–83. doi: 10.1016/j.jpsychores.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Tropea J, Slee JA, Brand CA, Gray L, Snell T. Clinical practice guidelines for the management of delirium in older people in Australia. Australas J Ageing. 2008;27:150–6. doi: 10.1111/j.1741-6612.2008.00301.x. [DOI] [PubMed] [Google Scholar]
- 60.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 2009;373:1874–82. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bailey P, Thomsen GE, Spuhler VJ, Blair R, Jewkes J, Bezdjian L, et al. Early activityis feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–45. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 62.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, et al. Early intensivecare unit mobility therapy in the treatment of acuterespiratory failure. Crit Care Med. 2008;36:2238–43. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 63.Stiller K, Phillips AC, Lambert P. The safety of mobilisationand its effects on haemodynamic and respiratorystatus of intensive care patients. Physio Theory Pract. 2004;20:175–85. [Google Scholar]


