Abstract
Background
Isoflurane has been reported to induce caspase-3 activation, which may induce neurotoxicity and contribute to the pathogenesis of Alzheimer's disease. However, the underlying mechanism is largely unknown, especially whether or not isoflurane can induce ryanodine receptors (RyRs)-associated endoplasmic reticulum (ER) stress, leading to caspase-3 activation. We therefore assessed the effects of isoflurane on RyRs-associated ER stress.
Methods
We treated primary neurones from wild-type (C57BL/6J) mice with 1% and 2% isoflurane for 1, 3, or 6 h. We then measured levels of C/EBP homologous protein (CHOP) and caspase-12, two ER stress markers, using immunocytochemistry staining and western blotting analysis. Dantrolene (5 μM), the antagonist of RyRs, was used to investigate the role of RyRs in the isoflurane-induced ER stress and caspase-3 activation.
Results
Isoflurane 2% for 6 h treatment increased the levels of CHOP (876% vs 100%, P=0.00009) and caspase-12 (276% vs 100%, P=0.006), and induced caspase-3 activation in the neurones. The administration of 2% isoflurane for 3 h (shorter duration), however, only increased the levels of CHOP (309% vs 100%, P=0.003) and caspase-12 (266% vs 100%, P=0.001), without causing caspase-3 activation. The isoflurane-induced ER stress (CHOP: F=16.64, P=0.0022; caspase-12: F=6.13, P=0.0383) and caspase-3 activation (F=32.06, P=0.0005) were attenuated by the dantrolene treatment.
Conclusions
These data imply that isoflurane might induce caspase-3 activation by causing ER stress through RyRs, and dantrolene could attenuate the isoflurane-induced ER stress and caspase-3 activation. Further investigations of the potential neurotoxicity of isoflurane are needed.
Keywords: endoplasmic reticulum; inhalation anaesthetics, isoflurane; receptors, ryanodine
Editor's key points.
Isoflurane has been suggested to cause neurotoxicity by several mechanisms including by induction of caspase-3.
In this study, isoflurane increased endoplasmic reticulum (ER) stress and activated caspase-3 using mouse neurones.
Effects depended on the concentration and duration of exposure and were attenuated by dantrolene.
These data suggest that caspase 3 activation may be mediated by ryanodine receptors and ER stress.
Further data are required.
Around the world, ∼8.5 million patients with Alzheimer's disease (AD) need surgical care under anaesthesia each year.1 In addition, a much greater number of senior patients who are vulnerable to the development of AD also need surgical care under anaesthesia.2 Anaesthesia, surgery, or both have been suggested to cause cognitive dysfunction, to which AD and senior patients are at risk to develop (Moller and colleagues,3 reviewed in Querfurth and LaFerla4 and Terrando and colleagues).5 Thus, it is significant to study and identify anaesthetics that could potentially advance AD pathology, and to investigate the underlying mechanisms.
The common inhalation anaesthetic isoflurane has been reported to induce caspase-3 activation and other cellular damages in cultured cells and in animals,6–14 which may then cause β-amyloid protein (Aβ) accumulation,14 contributing to AD pathology.15–19 Isoflurane has also been shown to induce caspase-3 activation in the brain tissues of young rodents.20–22 However, the up-stream mechanism by which isoflurane induces caspase-3 activation remains largely unknown. Recent studies have suggested that isoflurane may cause cell death by disrupting intracellular calcium homeostasis.13,23,24 Endoplasmic reticulum (ER) is the main source of cytosolic calcium in neurones and plays an important role in maintaining intracellular calcium homeostasis, protein synthesis, cell survival, and caspase activation.25–28 There are two types of Ca2+-release channels in ER: inositol 1,4,5-triphosphate receptors (IP3R) and ryanodine receptors (RyRs).29 Isoflurane has been shown to induce apoptosis via activation of inositol 1,4,5-trisphosphate receptors.13 However, the effects of isoflurane on the ER still remain largely to be determined; specifically, it is unknown whether isoflurane can induce RyRs-associated ER stress, leading to caspase-3 activation. Such studies would elucidate the underlying up-stream mechanisms of the isoflurane-induced caspase-3 activation and offer the targeted intervention(s). Thus, the outcomes from these studies are novel and important.
ER stress involves the C/EBP homologous protein (CHOP).30,31 CHOP is a proapoptotic transcription factor; its levels are very low under normal conditions but are strongly activated upon ER stress.30 Caspase-12, another ER resident pro-caspase, is proteolysed after ER stress.32 Taken together, we investigated a hypothesis that isoflurane could act on RyRs to increase the levels of CHOP and caspase-12, which then leads to caspase-3 activation in the primary neurones of mice.
Methods
Preparation of primary neurones
The procedure was approved by the Massachusetts General Hospital (Boston, MA, USA) Standing Committee on the Use of Animals in Research and Teaching. The relevant aspects of the ARRIVE guidelines were adhered to as appropriate. We used incremental increases in the concentration of carbon dioxide to kill the wild-type (C57BL/6J) mice at the gestation stage of day 15. The embryos were removed through Caesarean sections and they were decapitated in a 100 mm dish with 20 ml phosphate-buffered saline. We then put the harvested heads in a 100 mm dish, separated out the cortex, and removed meninges. We dissociated the neurones by using trypsinization and trituration. We then re-suspended the dissociated neurones in neurobasal medium with serum for 1 h, and finally, we placed the neurones in serum-free B27/neurobasal medium in six-well plates with a confluent rate of 25%. On the 7–10th day after the harvest, we treated the neurones with isoflurane, dantrolene, or both.
Treatments of primary neurones
We treated the primary neurones with 1% or 2% isoflurane plus 21% O2 and 5% CO2 for 1, 3, and 6 h, as described in our previous studies.10,33 An anaesthesia machine was used to deliver isoflurane to a sealed plastic box in a 37°C incubator. The plastic box contained six-well plates which were seeded with 0.25 million neurones in 1.5 ml neurone culture media. We used the Datex infrared gas analyzer (Puritan-Bennett, Tewksbury, MA, USA) to continuously monitor the delivered concentrations of carbon dioxide, oxygen, and isoflurane. For the interaction studies, we administered dantrolene (5 μM) to the neurones 1 h before the treatment of isoflurane as described in a previous study.34 Dimethyl sulfoxide (DMSO) (1:1500) was used as the solvent of dantrolene.
CHOP immunocytochemistry staining
We used the protocol provided by the company (Abcam Inc., Cambridge, MA, USA) to detect intracellular CHOP proteins. Briefly, we placed the neurones on coverslips for the treatments. At the end of the treatments, we fixed the cells in 100% methanol for 20 min on ice. We washed the neurones three times with phosphate-buffered saline, then we incubated the neurones with 0.1% TritonX-100 at 4°C for 10 min. We used 10% normal goat serum for 1 h at room temperature to block the non-specific reaction. Then, we incubated the neurones with anti-CHOP monoclonal antibody (1:200, Abcam Inc.) overnight at 4°C. The next day, we washed the neurones three times with phosphate-buffered saline and incubated the neurones with the secondary antibody (1:1000, goat anti-mouse IgG conjugated to Alexa Fluor® 488, Invitrogen, San Francisco, CA, USA) for 1 h at room temperature. Finally, we incubated the coverslips with Prolong® Gold Antifade Reagent (Invitrogen) and analysed the neurones in mounting medium using a 20× and 60× objective lens fluorescence microscope. We used the Image J (NIH, Bethesda, MD, USA) to determine the immunofluorescence intensity in the cytosol and nucleus. To determine the cytosolic fluorescence, an area surrounding the nucleus was used for counting. For the nuclear fluorescence, the value of fluorescence was acquired from the total nuclear area. Cytosolic CHOP level was expressed as the ratio of cytosolic amount of fluorescence over nuclear amount of fluorescence, which was consistent with the methods described in a previous study.35
Cell lysis and protein amount quantification
The pellets of primary neurones were detergent-extracted on ice with an immunoprecipitation buffer (2 mM EDTA, 150 mM NaCl, 10 mM Tris–HCl, pH 7.4, 0.5% non-idet P-40) plus protease inhibitors (1 μg ml−1 aprotinin, 1 μg ml−1 leupeptin, 1 μg ml−1 pepstatin A). We collected the lysates, centrifuged them at 18 000 g for 15 min, and quantified them for total proteins by using a bicinchoninic acid protein assay kit (Pierce, Iselin, NJ, USA).
Western blotting analysis
The harvested primary neurones were used for western blot analyses as described in our previous study.36 We used CHOP antibody (1:1000 dilution; Abcam Inc.) to recognize CHOP (31 kDa), caspase-12 antibody (1:1000 dilution; Cell Signaling Technology, Inc., Danvers, MA) to recognize caspase-12 (42 kDa), caspase-3 antibody (1:1000 dilution; Cell Signaling Technology, Inc.) to recognize FL-caspase-3 (35–40 kDa) and caspase-3 fragment (17–20 kDa) resulting from cleavage at asparate position 175. Finally, we used anti-β-actin antibody (1:10 000, Sigma, St Louis, MO, USA) to recognize β-actin (42 kDa). Each band in the western blot represented an independent experiment. We averaged results from six to eight independent experiments. The quantification of western blots was performed using the methods described in a previous study.10 Briefly, we used the National Institute of Health image program (National Institute of Health Image 1.62, Bethesda, MD, USA) to analyse the signal intensity. We then quantified the western blots in two steps. First, we used the levels of β-actin to normalize (e.g. determining ratio of FL-caspase-3 amount to β-actin amount) the levels of CHOP, caspase-12, and caspase-3, which may reduce the influence of loading differences in total protein amounts. Secondly, we presented the changes in the levels of CHOP, caspase-12, and caspase-3 in treated neurones as percentages of those in control neurones.
Statistics
There was background of CHOP levels and caspase activation in the neurones; therefore, we did not use absolute values, rather we presented their changes in treated neurones as fold or percentage of those in neurones after the control condition. We expressed the data as mean (sd). The number of samples varied from six to eight, and the samples were normally distributed (data not shown). We used two-way analysis of variance (anova) or t-test to determine the difference between the control and treatments. We considered P-values of <0.05 (*) and 0.01 (**) as statistically significant. The significance testing was two-tailed, and we used Prism 6 software (La Jolla, CA, USA) to analyse the data.
Results
Treatment with 2% isoflurane for 6 h increased CHOP levels and induced caspase-12 activation in primary neurones
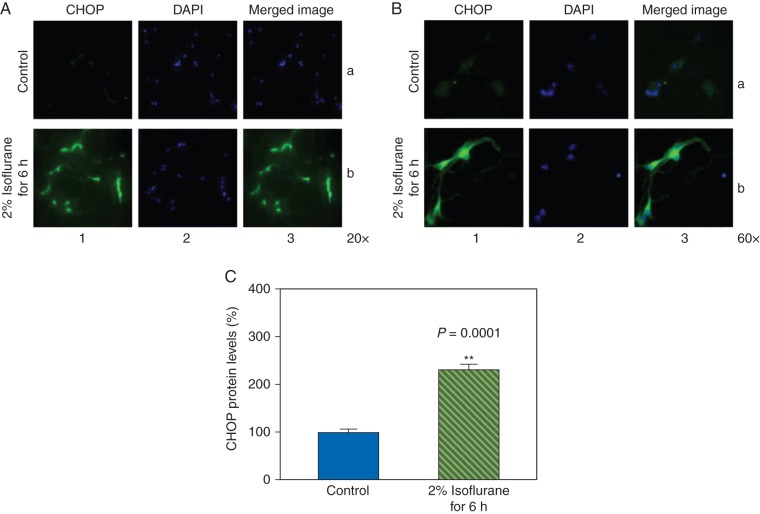
The neurones were harvested at the end of the treatment with 2% isoflurane for 6 h and were subjected to CHOP immunocytochemistry staining (Fig. 1a: 20 × and Fig. 1b: 60 ×). The CHOP immunostaining illustrated that the isoflurane treatment enhanced CHOP levels in cytosol. Specifically, column 1 of Figure 1a and b illustrates the image of CHOP (green), column 2 demonstrates the nuclei of the neurones (blue), and column 3 is the merged image. These images indicated that the levels of CHOP detected by the immunostaining were likely located in the cytosol and the isoflurane treatment (row b of Fig. 1a and b) increased the CHOP levels when compared with the control condition (row a of Fig. 1a and b). Quantification of the immunostaining images demonstrated that the isoflurane treatment enhanced CHOP levels when compared with the control condition: 228% vs 100%, P=0.0001 (Fig. 1c).
Fig 1.
Isoflurane increases CHOP levels in the primary neurones. (a) Immunohistochemistry staining of CHOP (magnification 20 ×). (b) Immunohistochemistry staining of CHOP (magnification 60 ×). Column 1 is the image of CHOP (green), column 2 is the image of nuclei (blue), and column 3 is the merged image. Row a is the cells following the control condition and row b is the cells treated with 2% isoflurane for 6 h. (c) Quantification of the immunohistochemistry staining shows that the isoflurane treatment (green striped bar) increases CHOP levels compared with the control condition (blue bar).
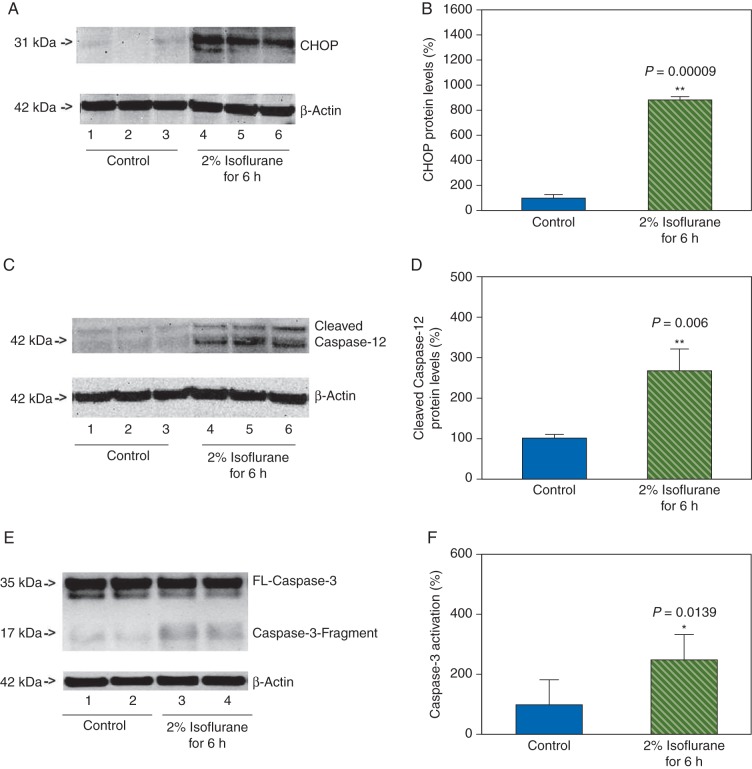
Next, we used western blot analysis to assess the effects of isoflurane on CHOP levels in primary neurones. The CHOP immunoblotting showed that there were observable increases in CHOP levels (31 kDa) after the isoflurane treatment when compared with the control condition in the neurones (Fig. 2a). The quantification of the western blot revealed that the isoflurane treatment increased CHOP levels: 876% vs 100%, P=0.00009 (Fig. 2b). These data suggested that isoflurane might increase CHOP levels, the marker of ER stress.30
Fig 2.
Isoflurane increases the levels of CHOP and caspase-12 in the primary neurones. (a) Treatment with 2% isoflurane for 6 h (lanes 4–6) increases CHOP levels when compared with the control condition (lanes 1–3) in the primary neurones. There is no significant difference in the amounts of β-actin in the control condition- or isoflurane-treated neurones. (b) Quantification of the western blot shows that isoflurane treatment (green striped bar) increases CHOP levels compared with the control condition (blue bar), normalized to β-actin levels. (c) Treatment with 2% isoflurane for 6 h (lanes 4–6) increases cleaved caspase-12 levels when compared with the control condition (lanes 1–3) in the primary neurones. There is no significant difference in the amounts of β-actin in the control condition- or isoflurane-treated neurones. (d) Quantification of the western blot shows that the isoflurane treatment (green striped bar) increases cleaved caspase-12 levels compared with the control condition (blue bar), normalized to β-actin levels. (e) Treatment with 2% isoflurane for 6 h (lanes 3 and 4) increased cleaved caspase-3 levels when compared with the control condition (lanes 1 and 2). There is no significant difference in the amounts of β-actin in the control condition- or isoflurane-treated neurons. (f) The quantification of western blot shows that the isoflurane treatment (green striped bar) induces caspase-3 activation when compared with control condition (blue bar).
The findings that isoflurane might increase CHOP levels in the neurones suggested that isoflurane might induce ER stress. Thus, we assessed whether the isoflurane treatment could also cause activation of caspase-12, another marker of ER stress.32 Caspase-12 immunoblotting demonstrated noticeable increases in cleaved caspase-12 levels (activated) after the isoflurane treatment when compared with the control condition (Fig. 2c) in the neurones. The western blot quantification illustrated that the isoflurane treatment increased cleaved caspase-12 levels: 276% vs 100%, P=0.006 (Fig. 2d). CHOP and caspase-12 are the markers of ER stress;28 thus, these data implied that isoflurane might induce ER stress in the primary neurones. Finally, we found that the treatment with 2% isoflurane for 6 h also induced caspase-3 activation, as evidenced by the enhancement of cleaved caspase-3 (Fig. 2e and f), which was consistent with our previous studies.36
Treatment with 2% isoflurane for 3 h enhanced CHOP levels and induced caspase-12 activation, but not caspase-3 activation
Given that the treatment with 2% isoflurane for 6 h induced ER stress (Figs 1 and 2) and activation of caspase-3 in primary neurones [(Fig. 2e and f) and our previous studies],36 we then assessed whether the isoflurane-induced ER stress could occur before the isoflurane-induced activation of capsase-3. We therefore determined the effects of 2% isoflurane for 3 h (shorter duration) treatment on both ER stress and caspase-3 activation.
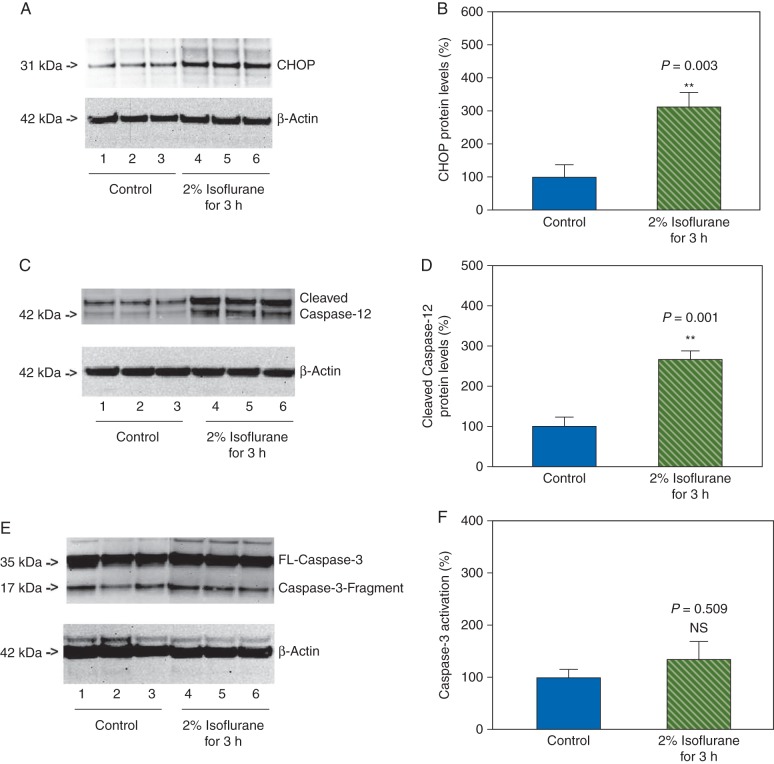
The neurones were harvested at the end of the isoflurane treatment and were exposed to western blot analysis. The CHOP immunoblotting illustrated noticeable enhancement in CHOP levels in the neurones after the treatment with 2% isoflurane for 3 h when compared with the control condition (Fig. 3a). The western blot quantification showed that the isoflurane treatment (2% isoflurane for 3 h) enhanced CHOP levels compared with the control condition: 309% vs 100%, P=0.003 (Fig. 3b). Caspase-12 immunoblotting demonstrated that the 2% isoflurane for 3 h treatment increased the levels of cleaved caspase-12 when compared with control condition (Fig. 3c). The western blot quantification illustrated that the isoflurane treatment (2% isoflurane for 3 h) increased the levels of cleaved caspase-12 when compared with the control condition: 266% vs 100%, P=0.001 (Fig. 3d).
Fig 3.
Treatment with 2% isoflurane for 3 h induces ER stress without caspase-3 activation in the primary neurones. (a) Treatment with 2% isoflurane for 3 h (lanes 4–6) increases CHOP levels when compared with the control condition (lanes 1–3) in the primary neurones. There is no significant difference in the amounts of β-actin in the control condition- or isoflurane-treated neurones. (b). Quantification of the western blot shows that the isoflurane treatment (green striped bar) increases CHOP levels compared with the control condition (blue bar), normalized to β-actin levels. (c) Treatment with 2% isoflurane for 3 h (lanes 4 and 6) increases cleaved caspase-12 levels when compared with the control condition (lanes 1–3) in the primary neurones. There is no significant difference in the amounts of β-actin in the control condition- or isoflurane-treated neurones. (d) Quantification of the western blot shows that the isoflurane treatment (green striped bar) increases the cleaved caspase-12 levels compared with the control condition (blue bar), normalized to β-actin levels. (e) Treatment with 2% isoflurane for 3 h (lanes 4–6) does not induce caspase-3 activation when compared with the control condition (lanes 1–3) in the primary neurones. (f) Quantification of the western blot shows that the isoflurane treatment (green striped bar) does not induce caspase-3 activation compared with the control condition (blue bar), normalized to β-actin levels.
However, the caspase-3 immunoblotting demonstrated that the 2% isoflurane for 3 h treatment did not cause caspase-3 activation when compared with the control condition (Fig. 3e and f). These data, that the treatment with 2% isoflurane for 3 h induced ER stress without caspase-3 activation, suggested that the isoflurane-induced ER stress might precede the isoflurane-induced caspase-3 activation.
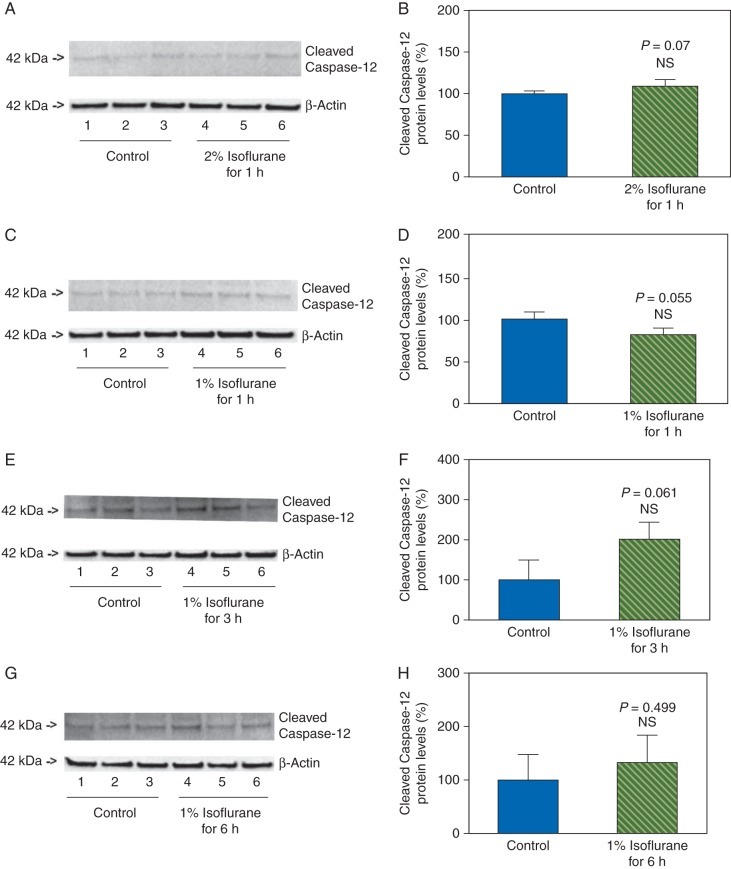
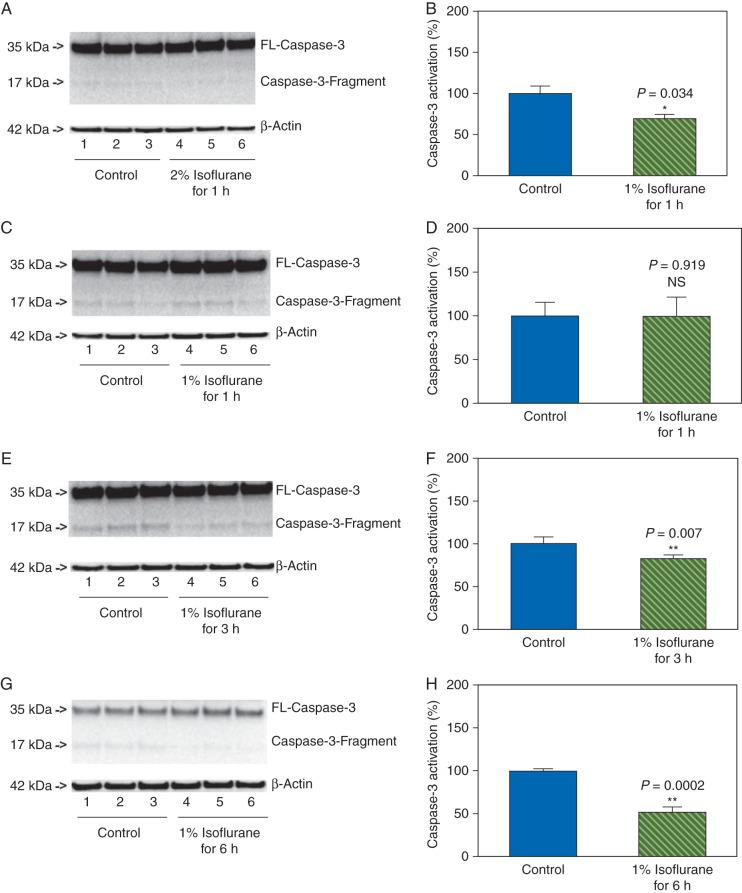
Effects of treatment with 1% or 2% isoflurane for 1, 3, and 6 h on levels of CHOP, caspase-12, and caspase-3 activation in primary neurones of mice
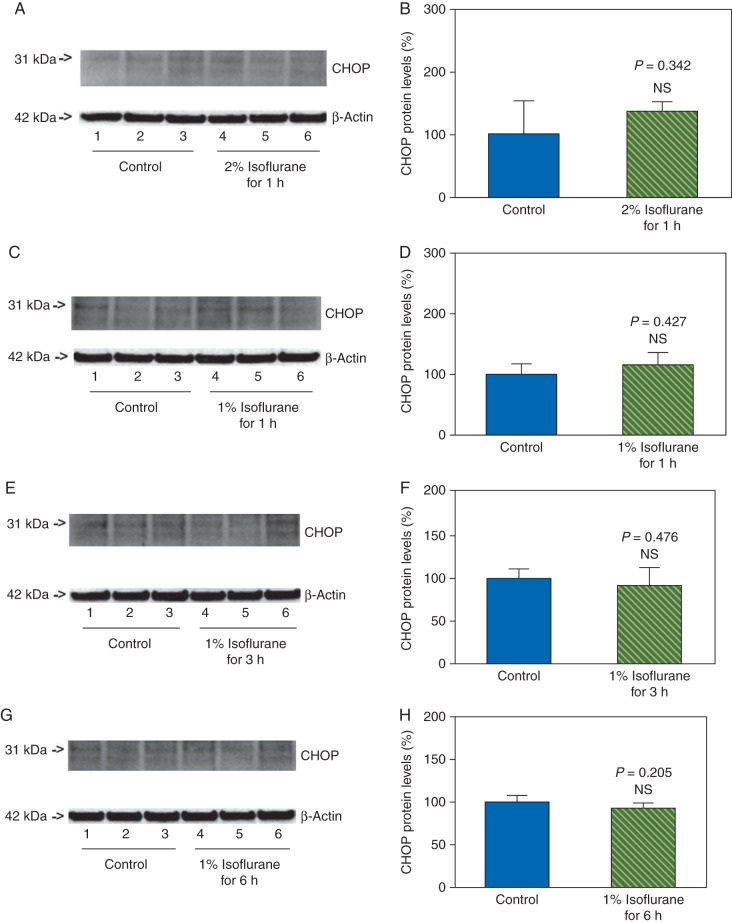
Next, we asked whether the effects of isoflurane on the levels of CHOP, caspase-12, and caspase-3 activation in the primary neurones were concentration- and time-dependent. We therefore assessed the effects of treatment with 2% isoflurane for 1 h, and treatments with 1% isoflurane for 1, 3, and 6 h on the levels of CHOP, caspase-12, and caspase-3 in the primary neurones of mice. We found that these treatments did not increase the levels of CHOP (Fig. 4), and did not induce activation of caspase-12 (Fig. 5) and caspase-3 (Fig. 6) in the neurones. Instead, the treatments with 2% isoflurane for 1 h, 1% isoflurane for 3 h, and 1% isoflurane for 6 h were found to decrease the caspase-3 activation when compared with the control condition. These data suggested that the effects of isoflurane on the levels of CHOP, caspase-12, and caspase-3 activation were concentration- and time-dependent.
Fig 4.
Treatments with 1% or 2% isoflurane for 1, 3, and 6 h on CHOP levels in primary neurones of mice. Treatment with 2% isoflurane for 1 h does not increase CHOP levels in the neurones (a and b). The treatments with 1% isoflurane for 1 (c and d), 3 (e and f), and 6 (g and h) h do not increase CHOP levels in the neurones.
Fig 5.
Treatments with 1% or 2% isoflurane for 1, 3, and 6 h on caspase-12 activation in primary neurones of mice. Treatment with 2% isoflurane for 1 h does not induce caspase-12 activation in the neurones (a and b). The treatments with 1% isoflurane for 1 (c and d), 3 (e and f), and 6 (g and h) h do not induce caspase-12 activation in the neurones.
Fig 6.
Treatments with 1% or 2% isoflurane for 1, 3, and 6 h on caspase-3 activation in primary neurones of mice. Treatment with 2% isoflurane for 1 h does not induce caspase-3 activation in the neurones (a and b). The treatments with 1% isoflurane for 1 (c and d), 3 (e and f), and 6 (g and h) h do not induce caspase-3 activation in the neurones. The treatments with 2% isoflurane for 1 h (a and b), 1% isoflurane for 3 h (e and f), and 1% isoflurane for 6 h (g and h) decrease the caspase-3 activation.
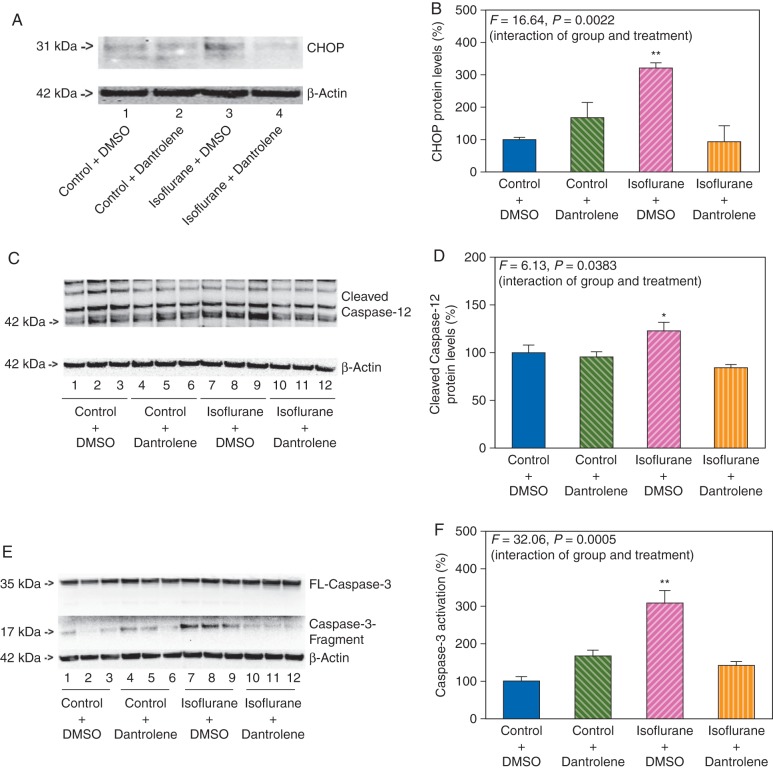
Dantrolene attenuated the isoflurane-induced ER stress and caspase-3 activation in primary neurones
Given the findings that isoflurane induced both ER stress and activation of caspase-3, and the fact that dantrolene is the antagonist of RyRs,37 we then determined whether dantrolene could mitigate the isoflurane-induced ER stress and activation of caspase-3. Dantrolene (5 μM)34 was administered to the primary neurones 1 h before the 2% isoflurane, for 6 h treatment. CHOP immunoblotting illustrated that 2% isoflurane for 6 h treatment enhanced CHOP levels when compared with the control condition in the primary neurones. Dantrolene alone did not significantly alter CHOP levels in the primary neurones when compared with the control condition, but the dantrolene treatment attenuated the isoflurane-induced increases in CHOP levels (Fig. 7a). Two-way anova, based on the quantification of the western blot images, showed the significant interaction of group (control and isoflurane) and treatment (DMSO and dantrolene) (F=16.64, P=0.0022) (Fig. 7b). These data suggested that dantrolene attenuated the isoflurane-induced increases in the CHOP levels.
Fig 7.
Dantrolene attenuates the isoflurane-induced ER stress and caspase-3 activation in the primary neurones. (a) Treatment with 2% isoflurane plus DMSO for 6 h (lane 3) increases CHOP levels when compared with the control plus DMSO condition (lane 1) in the primary neurones. Treatment with isoflurane plus dantrolene (lane 4) leads to a reduction in the CHOP levels when compared with the treatment with isoflurane plus DMSO (lane 3). There is no significant difference in amounts of β-actin among the different groups. (b) Quantification of the western blot shows that isoflurane plus DMSO treatment (pink striped bar) increases CHOP levels when compared with the control plus DMSO condition (blue bar), normalized to β-actin levels, whereas dantrolene (orange striped bar) attenuates the isoflurane-induced increases in the CHOP levels, normalized to β-actin levels. (c) Treatment with 2% isoflurane plus DMSO for 6 h (lanes 7–9) increases the cleaved caspase-12 levels when compared with the control plus DMSO condition (lanes 1–3) in the primary neurones. Treatment with isoflurane plus dantrolene (lanes 10–12) leads to a lesser degree of cleaved caspase-12 levels compared with the treatment with isoflurane plus DMSO (lanes 7–9). There is no significant difference in amounts of β-actin among different groups. (d) Quantification of the western blot shows that isoflurane plus DMSO treatment (pink striped bar) increases the cleaved caspase-12 levels compared with the control plus DMSO condition (blue bar), normalized to β-actin levels, whereas dantrolene (orange striped bar) attenuates the activation of caspase-12 induced by isoflurane plus DMSO (pink striped bar), normalized to β-actin levels. (e) Treatment with 2% isoflurane plus DMSO for 6 h (lanes 7–9) induces caspase-3 activation when compared with the control plus DMSO condition (lanes 1–3) in the primary neurones. Treatment with isoflurane plus dantrolene (lanes 10–12) induces a lesser degree of caspase-3 activation when compared with the treatment with isoflurane plus DMSO (lanes 7–9). There is no significant difference in the amounts of β-actin among different groups. (f) Quantification of the western blot shows that isoflurane plus DMSO treatment (pink striped bar) induces caspase-3 activation compared with the control plus DMSO condition (blue bar), normalized to β-actin levels, whereas dantrolene (orange striped bar) attenuates the isoflurane-induced caspase-3 activation, normalized to β-actin levels.
We then asked whether dantrolene could also attenuate the isoflurane-induced activation of caspase-12. Quantitative western blot analysis demonstrated that the dantrolene treatment attenuated the isoflurane-induced activation of caspase-12 (F=6.13, P=0.0383, two-way anova) (Fig. 7c and d).
Given that dantrolene rescued the ER stress induced by isoflurane, we asked whether dantrolene could also attenuate the isoflurane-induced caspase-3 activation in the primary neurones. As shown in Figure 7e, 2% isoflurane for 6 h treatment (lanes 7–9) caused activation of caspase-3 when compared with the control condition (lanes 1–3) in the primary neurones. Treatment with isoflurane plus dantrolene (lanes 10–12) led to a lesser degree of caspase-3 activation compared with the treatment with isoflurane plus DMSO (lanes 7–9). The western blot quantification showed that the dantrolene treatment attenuated the isoflurane-induced activation of caspase-3: F=32.06, P=0.0005 (two-way anova) (Fig. 7f).
Discussion
Given that CHOP and caspase-12 are the markers of ER stress, we assessed the effects of isoflurane on the levels of CHOP, caspase-12, and caspase-3 in the primary neurones from wild-type mice. We found that 2% isoflurane for 6 h of treatment increased the levels of CHOP (Figs 1a–c and 2a and b), and cleaved caspase-12 (Fig. 2c and d) in the primary neurones. These results suggested that isoflurane might induce ER stress.
We then found that isoflurane could induce activation of caspase-3 in the neurones (Fig. 2), and more importantly, treatment with isoflurane for a shorter time only induced ER stress and not activation of caspase-3 in the current experiments (Fig. 3). The data suggested that the isoflurane-induced ER stress preceded the isoflurane-caused activation of caspase-3, and furthermore, isoflurane might produce activation of caspase-3 via ER stress.
Moreover, we found that the effects of isoflurane on the levels of CHOP, and activation of caspase-12, and caspase-3 in the neurones were concentration- and time-dependent, and treatment with 2% isoflurane for 1 h, and treatments with 1% isoflurane for 1, 3, and 6 h did not increase the levels of CHOP (Fig. 4), and did not induce activation of caspase-12 (Fig. 5) and caspase-3 (Fig. 6) in the neurones.
Finally, we found that dantrolene, the antagonist of RyRs,37 attenuated the isoflurane-induced ER stress and the isoflurane-caused activation of caspase-3 (Fig. 7). These findings further implied that isoflurane might cause activation of capase-3 via RyRs-associated ER stress.
Our previous studies have suggested that isoflurane may cause mitochondrial dysfunction as evidenced by opening of mitochondria permeability transition pores and facilitating release of cytochrome c from the mitochondria to the cytosol.1,36 The current findings suggested that isoflurane might also induce ER stress. The RyRs family has three isoforms, which are expressed in the brain. The RyRs have multiple allosteric Ca2+ binding sites that are responsible for prompting Ca2+-induced Ca2+ release to the cytosol.38 The findings that dantrolene, the antagonist of RyRs, attenuated the isoflurane-induced ER stress and activation of caspase-3 suggested that isoflurane might act on RyRs in the ER of the primary neurones, leading to ER stress and activation of caspase-3. Previous studies showed that reduction in IP3 receptor could attenuate the isoflurane-induced caspase-3 activation.13,24 The current findings suggested that antagonism of either IP3 receptor or RyRs alone was sufficient in attenuating the isoflurane-induced ER stress-associated caspase-3 activation. However, it remains to be investigated whether the isoflurane-induced mitochondrial dysfunction and the isoflurane-induced IP3 receptor or RyRs-associated ER stress can interact with each other (potentiation or attenuation), leading to various degrees of caspase-3 activation and cellular toxicity.
ER stress and activation of RyRs contribute to malignant hyperthermia, a life-threatening disease with a dramatic increase in body temperature and skeletal muscle rigidity. Malignant hyperthermia can be triggered by inhalation anaesthetics including isoflurane. Dantrolene is the only medicine for the treatment of malignant hyperthermia and a recent study has suggested that dantrolene can ameliorate the cognitive decline and neuropathology in AD transgenic mice.39,40 In the current study, dantrolene was shown to inhibit the isoflurane-induced ER stress and caspase-3 activation. Isoflurane-induced caspase-3 activation has been suggested to contribute to cognitive impairment in animals,41 and isoflurane has also been suggested to be associated with postoperative cognitive dysfunction in humans.41 Collectively, these findings imply the potential association between malignant hyperthermia and cognitive impairment or postoperative cognitive dysfunction. We therefore have postulated that the patients who have a history of malignant hyperthermia may have a higher risk in developing postoperative cognitive dysfunction, pending further studies. Future experiments to test this hypothesis are needed.
Even though isoflurane has been reported to induce caspase activation and cause apoptosis, other reports suggest that isoflurane may protect against apoptosis.42–51 This discrepancy could be due to differences in the duration and concentration of isoflurane exposure as demonstrated in other studies.52–54 Specifically, our previous studies showed that low concentration and short treatment time of isoflurane attenuated while high concentration and long isoflurane treatment time potentiated the hypoxia- and Aβ-induced caspase-3 activation.52–54 Consistently, a recent study by Shu and colleagues20 showed that prolonged exposure to isoflurane plus nitrous oxide also caused caspase-3 activation in brain tissues of 7-day-old rats. Taken together, we hypothesize that isoflurane may have concentration- and time-dependent dual effects (attenuation vs potentiation) on neurotoxicity, which has been supported by a recent study.55 Future research to test this hypothesis is warranted.
One caveat of the current study is that we cannot extrapolate the in vitro findings to the brain. However, the majority of previous in vitro studies of isoflurane neurotoxicity used cultured tumour cells. Therefore, the outcomes from the current studies in primary neurones would be considered more clinically relevant. Nevertheless, future experiments are required to investigate the in vivo relevance of these in vitro findings, which may include the studies to assess whether dantrolene can mitigate the isoflurane-induced cognitive impairment in rodents. Secondly, the CHOP levels in the experiments varied even in the control condition. The variations most likely resulted from different exposure times with super strength reagents of western blot analysis. Nevertheless, the data were still able to illustrate the dose- and time-dependent effects of isoflurane on the level of CHOP in the primary neurones of mice.
In conclusion, we found that isoflurane could cause ER stress (enhancing the levels of CHOP and inducing caspase-12 activation) by acting on RyRs in primary neurones. The isoflurane-induced ER stress might precede the isoflurane-induced activation of caspase-3. RyRs antagonist dantrolene attenuated the isoflurane-induced ER stress and activation of caspase-3. These data suggested that ER stress could be one of the up-stream mechanisms by which isoflurane caused activation of caspase-3. Finally, mitigation of RyRs-associated ER stress could be a potential target for the treatment of anaesthesia neurotoxicity. More studies are needed to determine anaesthesia neurotoxicity, especially the underlying mechanisms, and targeted interventions.
Authors’ contributions
H.W., Y.D., J.Z., G.W., Y.Z., and Z. Xie: conceived and designed the experiments. H.W., Y.D., J.Z., and Z. Xu: performed the experiments. J.Z. and Y.D.: analysed the data. Z. Xie, C.S., and Y.Z.: wrote the paper.
Declaration of interest
None declared.
Funding
This research was supported by R21AG029856, R21AG038994, R01 GM088801, and R01 AG041274 from National Institutes of Health, Bethesda, MD, Investigator-initiated Research grant from Alzheimer's Association, Chicago, IL, and Cure Alzheimer's Fund, Wellesley, MA to Z. Xie.
Acknowledgements
Anaesthetic isoflurane was generously provided by the Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA. These studies are attributed to the Department of Anaesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School.
References
- 1.Zhang Y, Xu Z, Wang H, et al. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–98. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlisides P, Xie Z. Neurotoxicity of general anesthetics: an update. Curr Pharm Des. 2012;18:6232–40. doi: 10.2174/138161212803832344. [DOI] [PubMed] [Google Scholar]
- 3.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 4.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 5.Terrando N, Brzezinski M, Degos V, et al. Perioperative cognitive decline in the aging population. Mayo Clin Proc. 2011;86:885–93. doi: 10.4065/mcp.2011.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–9. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–41. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie Z, Culley DJ, Dong Y, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–27. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Z, Dong Y, Maeda U, et al. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–94. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Xie Z, Dong Y, Maeda U, et al. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61:1300–6. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 11.Wei H, Kang B, Wei W, et al. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–47. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Loop T, Dovi-Akue D, Frick M, et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102:1147–57. doi: 10.1097/00000542-200506000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Wei H, Liang G, Yang H, et al. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–60. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- 14.Xie Z, Dong Y, Maeda U, et al. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–54. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gervais FG, Xu D, Robertson GS, et al. Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- 16.Stadelmann C, Deckwerth TL, Srinivasan A, et al. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer's disease. Evidence for apoptotic cell death. Am J Pathol. 1999;155:1459–66. doi: 10.1016/S0002-9440(10)65460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louneva N, Cohen JW, Han LY, et al. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer's disease. Am J Pathol. 2008;173:1488–95. doi: 10.2353/ajpath.2008.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Amelio M, Cavallucci V, Middei S, et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nat Neurosci. 2011;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 19.Burguillos MA, Deierborg T, Kavanagh E, et al. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–24. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- 20.Shu Y, Zhou Z, Wan Y, et al. Nociceptive stimuli enhance anesthetic-induced neuroapoptosis in the rat developing brain. Neurobiol Dis. 2012;45:743–50. doi: 10.1016/j.nbd.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Sanders RD, Hassell J, Davidson AJ, Robertson NJ, Ma D. Impact of anaesthetics and surgery on neurodevelopment: an update. Br J Anaesth. 2013;110(Suppl. 1)):i53–72. doi: 10.1093/bja/aet054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jevtovic-Todorovic V, Absalom AR, Blomgren K,, et al. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111:143–51. doi: 10.1093/bja/aet177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–50. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang G, Dong Y, Zhang B,, et al. Isoflurane-induced caspase-3 activation is dependent on cytosolic calcium and can be attenuated by memantine. J Neurosci. 2008;28:4551–60. doi: 10.1523/JNEUROSCI.5694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–25. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 26.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–64. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–92. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 28.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 29.Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends Pharmacol Sci. 1994;15:145–9. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 30.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 31.Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–94. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B, Dong Y, Zhang G, et al. The inhalation anesthetic desflurane induces caspase activation and increases amyloid beta-protein levels under hypoxic conditions. J Biol Chem. 2008;283:11866–75. doi: 10.1074/jbc.M800199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Liang G, Chen Q, et al. Anesthetic-induced neurodegeneration mediated via inositol 1,4,5-trisphosphate receptors. J Pharmacol Exp Ther. 2010;333:14–22. doi: 10.1124/jpet.109.161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dzeja PP, Bortolon R, Perez-Terzic C, Holmuhamedov EL, Terzic A. Energetic communication between mitochondria and nucleus directed by catalyzed phosphotransfer. Proc Natl Acad Sci USA. 2002;99:10156–61. doi: 10.1073/pnas.152259999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Dong Y, Wu X, et al. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–37. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inui M, Saito A, Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987;262:15637–42. [PubMed] [Google Scholar]
- 38.Ruiz A, Matute C, Alberdi E. Intracellular Ca2+ release through ryanodine receptors contributes to AMPA receptor-mediated mitochondrial dysfunction and ER stress in oligodendrocytes. Cell Death Dis. 2010;1:e54. doi: 10.1038/cddis.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellinger AM, Mongillo M, Marks AR. Stressed out: the skeletal muscle ryanodine receptor as a target of stress. J Clin Invest. 2008;118:445–53. doi: 10.1172/JCI34006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng J, Liang G, Inan S, et al. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett. 2012;516:274–9. doi: 10.1016/j.neulet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B, Tian M, Zhen Y, et al. The effects of isoflurane and desflurane on cognitive function in humans. Anesth Analg. 2012;114:410–5. doi: 10.1213/ANE.0b013e31823b2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu X, Feng J, Zuo Z. Isoflurane preconditioning reduces the rat NR8383 macrophage injury induced by lipopolysaccharide and interferon gamma. Anesthesiology. 2008;108:643–50. doi: 10.1097/ALN.0b013e318167aeb4. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Peng L, Zuo Z. Isoflurane preconditioning increases B-cell lymphoma-2 expression and reduces cytochrome c release from the mitochondria in the ischemic penumbra of rat brain. Eur J Pharmacol. 2008;586:106–13. doi: 10.1016/j.ejphar.2008.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raphael J, Zuo Z, Abedat S, Beeri R, Gozal Y. Isoflurane preconditioning decreases myocardial infarction in rabbits via up-regulation of hypoxia inducible factor 1 that is mediated by mammalian target of rapamycin. Anesthesiology. 2008;108:415–25. doi: 10.1097/ALN.0b013e318164cab1. [DOI] [PubMed] [Google Scholar]
- 45.Zaugg M, Jamali NZ, Lucchinetti E, Shafiq SA, Siddiqui MA. Norepinephrine-induced apoptosis is inhibited in adult rat ventricular myocytes exposed to volatile anesthetics. Anesthesiology. 2000;93:209–18. doi: 10.1097/00000542-200007000-00032. [DOI] [PubMed] [Google Scholar]
- 46.Tyther R, Fanning N, Halligan M, Wang J, Redmond HP, Shorten G. The effect of the anaesthetic agent isoflurane on the rate of neutrophil apoptosis in vitro. Ir J Med Sci. 2001;170:41–4. doi: 10.1007/BF03167720. [DOI] [PubMed] [Google Scholar]
- 47.Wise-Faberowski L, Raizada MK, Sumners C. Oxygen and glucose deprivation-induced neuronal apoptosis is attenuated by halothane and isoflurane. Anesth Analg. 2001;93:1281–7. doi: 10.1097/00000539-200111000-00051. [DOI] [PubMed] [Google Scholar]
- 48.Wise-Faberowski L, Aono M, Pearlstein RD, Warner DS. Apoptosis is not enhanced in primary mixed neuronal/glial cultures protected by isoflurane against N-methyl-d-aspartate excitotoxicity. Anesth Analg. 2004;99:1708–14. doi: 10.1213/01.ANE.0000136474.35627.FF. table of contents. [DOI] [PubMed] [Google Scholar]
- 49.de Klaver MJ, Manning L, Palmer LA, Rich GF. Isoflurane pretreatment inhibits cytokine-induced cell death in cultured rat smooth muscle cells and human endothelial cells. Anesthesiology. 2002;97:24–32. doi: 10.1097/00000542-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Kawaguchi M, Drummond JC, Cole DJ, Kelly PJ, Spurlock MP, Patel PM. Effect of isoflurane on neuronal apoptosis in rats subjected to focal cerebral ischemia. Anesth Analg. 2004;98:798–805. doi: 10.1213/01.ane.0000105872.76747.f6. table of contents. [DOI] [PubMed] [Google Scholar]
- 51.Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA. Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca2+ and mitogen-activated protein kinases. Anesthesiology. 2005;102:606–15. doi: 10.1097/00000542-200503000-00020. [DOI] [PubMed] [Google Scholar]
- 52.Xu Z, Dong Y, Wu X, et al. The potential dual effects of anesthetic isoflurane on Abeta-induced apoptosis. Curr Alzheimer Res. 2011;8:741–52. doi: 10.2174/156720511797633223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan C, Xu Z, Dong Y, et al. The potential dual effects of anesthetic isoflurane on hypoxia-induced caspase-3 activation and increases in beta-site amyloid precursor protein-cleaving enzyme levels. Anesth Analg. 2011;113:145–52. doi: 10.1213/ANE.0b013e3182185fee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–62. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao X, Yang Z, Liang G, et al. Dual effects of isoflurane on proliferation, differentiation, and survival in human neuroprogenitor cells. Anesthesiology. 2013;118:537–49. doi: 10.1097/ALN.0b013e3182833fae. [DOI] [PMC free article] [PubMed] [Google Scholar]