Abstract
Background
Uterine atony (UA) is recognized as a leading cause of postpartum haemorrhage. However, knowledge of risk factors of haemorrhage-related morbidity among patients diagnosed with UA is uncertain. We investigated risk factors for haemorrhage-related morbidity among patients undergoing Caesarean delivery with UA.
Methods
We conducted a secondary analysis of data sourced from a 4-yr observational study at 19 US academic centres. Patients with UA were identified based on receiving methylergonovine or carboprost. Our primary outcome (haemorrhage-related morbidity) included a composite of intra- or postpartum transfusion; Caesarean hysterectomy; uterine or hypogastric artery ligation; intensive care admission for: pulmonary oedema, coagulopathy, adult respiratory distress syndrome, postoperative ventilation, or invasive line monitoring.
Results
Among 57 182 patients who underwent Caesarean delivery, 2294 (4%) patients developed UA. Haemorrhage-related morbidity occurred in 450 (19.6%) patients with UA. The risk of haemorrhage-related morbidity was increased among African-Americans [adjusted odds ratio (aOR)=2.36; 95% confidence interval (CI)=1.73–3.23], Hispanics (aOR=1.4; 95% CI=1.04–1.9), women with multiple gestations (aOR=1.59; 95% CI=1.06–2.38), placenta praevia (aOR=4.89; 95% CI=3.04–7.87), patients with ASA class III (aOR=1.4; 95 CI=1.03–1.9), or ASA class IV (aOR=5.88; 95% CI=2.48–13.9), exposure to general anaesthesia (GA) (aOR=2.4; 95% CI=1.59–3.62) and combined general and regional anaesthesia (aOR=4.0; 95% CI=2.62–6.09), and ≥2 prior Caesarean deliveries (aOR=1.62; 95% CI=1.1–2.39).
Conclusions
Among patients with UA undergoing Caesarean delivery, the risk of haemorrhage-related morbidity is increased in African-Americans, Hispanics, patients with multiple gestations, placenta praevia, ASA class III or IV, ≥2 prior Caesarean deliveries and those undergoing GA.
Keywords: Caesarean section, morbidity, uterine atony
Editor's key points.
Risk factors for haemorrhage-related morbidity among women undergoing Caesarean delivery who develop refractory uterine atony are uncertain.
This retrospective study investigated these risk factors exploring a large US-based database.
Identified risks were African-American race, Hispanic ethnicity, multiple gestation, placenta praevia, general anaesthesia and ASA class III or IV.
Patients undergoing Caesarean delivery are known to be at increased risk of postpartum haemorrhage (PPH) compared with patients undergoing vaginal delivery.1–3 As rates of Caesarean delivery in the USA have steadily increased (from 20.7% in 1996 to 32.8% in 2010),4 it is speculated that the increasing Caesarean delivery rate has contributed to increase in the rate of PPH.5
Uterine atony (UA) is recognized as the leading cause of PPH.5–8 During Caesarean delivery, pharmacological prophylaxis with uterotonic agents and manual measures (such as uterine massage) is routinely performed to initiate adequate uterine tone and reduce the risk of severe PPH. Despite the incorporation of these prophylactic measures into routine clinical practice, refractory UA may occur during Caesarean delivery requiring the use of second-line uterotonics (such as methylergonovine or carboprost) and other surgical or medical interventions (such as haemostatic brace suturing, interventional radiology, or hysterectomy).9,10 In the setting of refractory UA, women can experience major postpartum bleeding and are at increased risk of severe haemorrhage-related morbidity resulting from profound anaemia, organ hypoperfusion, and complications resulting from invasive medical or surgical intervention for haemorrhage control.11–14
Risk factors for haemorrhage-related morbidity among women who develop refractory UA are uncertain. Identifying specific risk factors for severe haemorrhage-related morbidity may assist obstetricians and anaesthetists in using tailored interventions and care strategies when managing patients with refractory UA. The primary aim of this study was to investigate patient characteristic, obstetric, anaesthetic, and intrapartum risk factors for severe haemorrhage-related morbidity among women who experience UA during Caesarean delivery.
Methods
Study design and data sources
We performed a secondary analysis of data (Caesarean Registry) sourced from a 4-yr observational study conducted by the Eunice Kennedy Shriver National Institute of Child Health and Development Maternal-Fetal Medicine Units (MFMU) Network. Conducted between January 1, 1999 and December 31, 2002 at 19 US academic centres, this study investigated the risk of uterine rupture in women with a prior Caesarean delivery undergoing a trial of labour compared with elective repeat Caesarean delivery; full details of the methodology and study design have been presented previously.15 This study was exempt from Stanford University institutional review board approval as the Caesarean Registry data set contains de-identified data.
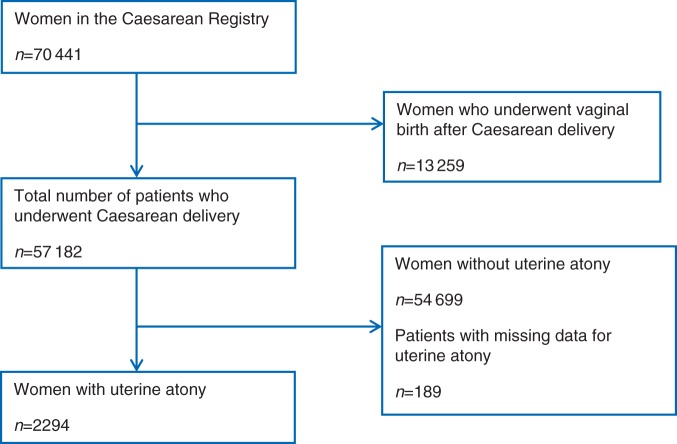
Within the Caesarean Registry, we identified 57 182 patients who underwent Caesarean delivery. We excluded 13 259 patients who had a vaginal birth after prior Caesarean delivery. We defined UA using an approach described in a previous study of UA employing data from the Caesarean Registry.16 UA was determined by: (i) a recorded entry indicating a clinical diagnosis of UA (recorded as a dichotomous variable) and (ii) administration of a second-line uterotonic drug: methylergonovine (methergine), carboprost (hemabate), or both drugs in combination. Figure 1 shows the process of selection for our study population. All participating centres within the MFMU network use oxytocin infusion for atony prophylaxis. Although patient-level data on prophylactic oxytocin dosing regimens were not collected, standard prophylactic oxytocin regimens have been described for a number of designated obstetric centres: nine centres used oxytocin concentration=20 U litre−1 (range=125–250 ml h−1), three centres used 40 U litre−1, and one centre used 10 U litre−1.16
Fig 1.
Study population.
We selected surgical procedures and complications that indicated haemorrhage-related morbidity or that occurred as a consequence of haemorrhage-related morbidity. This conceptual approach has been previously described in studies examining indicators of severe maternal morbidity during delivery hospitalizations.12,17 In order to determine indicators for haemorrhage-related morbidity, we reviewed morbidity studies that included PPH and transfusion as indicators of severe maternal morbidity12,14,17 and population-wide studies of PPH that used blood transfusion and procedures to control bleeding to identify women with severe pregnancy-related morbidity.8,18 Indicators for haemorrhage-related morbidity were then determined based on availability of data within the Caesarean Registry. For our primary outcome, we applied a composite measure for haemorrhage-related morbidity, defined by the presence of any of the following: intraoperative or postpartum red blood cell transfusion; Caesarean hysterectomy; uterine artery ligation; hypogastric artery ligation; or intensive care unit (ICU) admission for at least one of the following criteria: pulmonary oedema, coagulopathy, adult respiratory distress syndrome, postoperative ventilation, presence of an arterial line or central line. The criteria selected for ICU admission were based on studies that have described interventions or complications linked to haemorrhage or transfusion related complications.19 Total estimated blood loss was not reported in the Caesarean Registry.
We selected candidate variables as potential risk factors for haemorrhage-related morbidity. Candidate variables included: maternal age, race/ethnicity, BMI, gestational age at the time of delivery, singleton/multiple gestation, pre-existing diabetes mellitus, hypertensive disorders of pregnancy, chorioamnionitis, placental abruption, placenta praevia, number of prior Caesarean deliveries, presence of labour or attempted induction, ASA class, and mode of anaesthesia for Caesarean delivery. In the Caesarean Registry, obstetric patients were coded as ASA class II, III, or IV only. Using World Health Organization (WHO) classification for BMI class,20 women were grouped into five categories of BMI using height and weight data taken at or within 2 weeks of delivery: normal weight or underweight (<25), overweight (25–29.9), obese class I (30–34.9), obese class II (35–39.9), and obese class III (40 or more). Induction was defined by the presence of any of the following methods: artificial rupture of membranes, cervidil, foley bulb, laminaria, misoprostol, oxytocin, or prostaglandin gel. We classified modes of anaesthesia into five categories: general and regional (spinal or epidural) anaesthesia, general without regional anaesthesia, spinal anaesthesia, epidural anaesthesia, and spinal plus epidural anaesthesia.
Statistical analysis
Univariate analyses were performed using the χ2 test for categorical data to assess the associations between candidate variables and the composite outcome. Candidate variables that were associated with the composite outcome on univariate analysis (P≤0.2) were included as potential covariates in the initial multiple logistic regression model. We used variance inflation factor testing to identify collinearity between independent variables. In order to minimize inequality in numbers within BMI categories and to more clearly elucidate whether an increase in risk occurs with a change in BMI category, we also constructed quintiles for BMI (<27.06, 27.06–29.97, 29.98–33.04, 33.05–37.7, >37.7).
Step-wise backward elimination was performed to construct the parsimonious final model; P<0.05 was required for a variable to be retained in the multivariate model. We constructed separate multivariate models for BMI classes using the WHO criteria and quintiles. Model goodness of fit was evaluated using the Hosmer–Lemoshow statistic. We calculated the area under the receiver-operating characteristic curve (AUCROC) using standard methods to assess the predictive performance of each model.
For internal validation of each model, we used a 10-fold cross-validation procedure that used the full data set for model development. This technique divides the study population into 10 equal groups by sampling randomly without replacement. Each group consisted of a training set (comprising 90% of the data) and a test set (comprising the remaining 10%). A multivariate model with backward elimination was constructed with each training set; a test set was subsequently input into the model, which culminated in the generation of a case-specific prediction for each record for variables of interest. We calculated the AUCROC for the probabilities calculated by 10-fold cross validation. For patients who had a multiple gestation, we accounted for mothers who delivered the first infant in the univariate and multivariate analyses.
It is possible that non-PPH-related maternal and obstetric morbidities may have resulted in the use of invasive monitoring, mechanical ventilation, coagulopathy, adult respiratory distress syndrome, or pulmonary oedema. For a sensitivity analysis, we constructed a second composite outcome measure defined by the presence of any of the following: intraoperative or postpartum red blood cell transfusion; Caesarean hysterectomy; uterine artery ligation; and hypogastric artery ligation. We constructed a multivariate logistic model using this second composite outcome and candidate variables identified in our original analysis. All analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Among all patients who delivered by Caesarean delivery, the overall prevalence of UA was 4% (2294 of 57 182). Patients with missing data for UA (n=189) or who were not diagnosed with UA (n=54 699) were excluded from the analysis (Fig. 1). Among patients who were defined as having UA, 1052 (45.9%) patients received methylergonovine, 843 (36.7%) patients received carboprost, and 399 (17.4%) patients received both methylergonovine and carboprost. Using our composite outcome measure, we identified 450 (19.6%) patients who had UA and haemorrhage-related morbidity (Table 1). The most common complication was postpartum transfusion (13.7%). The rates of pulmonary oedema, coagulopathy, adult respiratory distress syndrome, postoperative ventilation, hypogastric artery ligation, or invasive line placement (arterial line and central line) were low (0.2–1.7%). In total, 79 patients required ICU admission and there were five maternal deaths. Among patients who underwent general anaesthesia (GA), reasons for GA included: failed attempt to perform regional anaesthesia in 27 (7.1%) patients, inadequate surgical anaesthesia after placement of the regional anaesthetic in 68 (17.9%) patients, high spinal in 3 (0.8%) patients, planned (elective) decision in 53 (13.9%) patients, emergency Caesarean delivery in 165 (43.4%) patients, and other indications in 64 (16.9%) patients.
Table 1.
Haemorrhage-related morbidity in women with UA (n=450). Data are n (%). ARDS, adult respiratory distress syndrome; ICU, intensive care unit. As one patient could have >1 condition/criteria for having haemorrhage-related morbidity, the total number given in the table is >450. The percentage values represent the percentage of patients from the entire cohort (n=2294) identified with each morbidity
| Intraoperative transfusion | 184 (8.0%) |
| Postpartum transfusion | 315 (13.7%) |
| Pulmonary oedema—ICU | 15 (0.65%) |
| Coagulopathy—ICU | 21 (0.9%) |
| ARDS—ICU | 5 (0.2%) |
| Postoperative ventilation—ICU | 39 (1.7%) |
| Caesarean hysterectomy | 109 (4.7%) |
| Uterine artery ligation | 117 (5.1%) |
| Hypogastric artery ligation | 7 (0.3%) |
| Radial artery line—ICU | 25 (1.1%) |
| Central line—ICU | 20 (0.9%) |
Maternal, obstetric, anaesthetic, and intrapartum characteristics for patients with morbidity and without morbidity are given in Table 2. Based on the initial univariate analysis, predictors that were considered for multivariate analyses included: maternal age, race/ethnicity, maternal BMI (WHO criteria and quintiles), gestational age, singleton vs multiple gestation, hypertensive disease of pregnancy, placental abruption, placenta praevia, number of prior Caesarean deliveries, presence of labour, or attempted induction, ASA class, and mode of anaesthesia. As there was evidence of collinearity between dystocia and attempted labour or induction of labour, we only included the presence of labour or attempted induction in the final model.
Table 2.
Characteristics of women with UA during Caesarean delivery with and without haemorrhage-related morbidity. Data are n (%). *Bivariate analyses did not account for missing data. GA, general anaesthesia; CD, Caesarean delivery; HELLP, Hemolysis Elevated Liver Enzymes and Low Platelets
| UA with morbidity (n=450) | UA without morbidity (n=1844) | P-value* | |
|---|---|---|---|
| Maternal age (yr) | |||
| <20 | 56 (12.5%) | 216 (11.7%) | 0.1 |
| 20–34 | 285 (63.3%) | 1260 (68.3%) | |
| >34 | 109 (24.2%) | 368 (20%) | |
| Race/ethnicity | |||
| African-American | 144 (32%) | 361 (19.6%) | <0.001 |
| Caucasian | 128 (28.4%) | 715 (38.8%) | |
| Hispanic | 156 (34.7%) | 663 (35.9%) | |
| Other | 22 (4.9%) | 105 (5.7%) | |
| BMI at the time of delivery (kg m−2) | |||
| ≤24.9 | 54 (12%) | 142 (7.7%) | 0.007 |
| 25–29.9 | 117 (26%) | 546 (29.6%) | |
| 30–34.9 | 131 (29.1%) | 523 (28.4%) | |
| 35–39.9 | 66 (14.7%) | 287 (15.5%) | |
| ≥40 | 43 (9.5%) | 249 (13.5%) | |
| Missing data | 39 (8.7%) | 97 (5.3%) | |
| BMI at the time of delivery in quintiles (kg m−2) | |||
| <27.06 | 99 (22%) | 331 (17.9%) | 0.08 |
| 27.06–29.97 | 72 (16%) | 357 (19.4%) | |
| 29.98–33.04 | 90 (20%) | 343 (18.6%) | |
| 33.05–37.7 | 75 (16.6%) | 359 (19.5%) | |
| >37.7 | 75 (16.6%) | 357 (19.4%) | |
| Missing data | 39 (8.8%) | 97 (5.2%) | |
| Gestational age (weeks) | |||
| <37 | 144 (32%) | 382 (20.7%) | <0.001 |
| 37–41 | 248 (55.1%) | 1197 (64.9%) | |
| >41 | 56 (12.4%) | 261 (14.2%) | |
| Missing data | 2 (0.5%) | 4 (0.2%) | |
| Type of pregnancy | |||
| Singleton pregnancy | 404 (89.8%) | 1699 (92.1%) | 0.10 |
| Multiple pregnancy | 46 (10.2%) | 145 (7.9%) | |
| Pre-existing diabetes | |||
| Yes | 43 (9.6%) | 208 (11.3%) | 0.30 |
| No | 406 (90.2%) | 1635 (88.6%) | |
| Missing data | 1 (0.2%) | 1 (0.1%) | |
| Hypertensive disease of pregnancy | |||
| None | 352 (78.2%) | 1494 (81%) | 0.08 |
| Gestational Hypertension | 20 (4.5%) | 80 (4.4%) | |
| Pre-eclampsia | 64 (14.2%) | 244 (13.2%) | |
| Eclampsia or HELLP syndrome | 14 (3.1%) | 26 (1.4%) | |
| Chorioamnionitis | |||
| Yes | 71 (15.8%) | 321 (17.4%) | 0.41 |
| No | 379 (84.2%) | 1523 (82.6%) | |
| Placental abruption | |||
| Yes | 32 (7.1%) | 59 (3.2%) | <0.001 |
| No | 418 (92.9%) | 1785 (96.8%) | |
| Placenta praevia | |||
| Yes | 56 (12.4%) | 49 (2.7%) | <0.001 |
| No | 394 (87.6%) | 1795 (97.3%) | |
| Number of prior CDs | |||
| 0 | 258 (57.3%) | 1139 (61.7%) | 0.02 |
| 1 | 117 (26%) | 479 (26%) | |
| ≥2 | 72 (16%) | 210 (11.4%) | |
| Missing data | 3 (0.7%) | 16 (0.9%) | |
| Labour or attempted Induction | |||
| Yes | 264 (58.7%) | 1201 (65.1%) | 0.01 |
| No | 186 (41.3%) | 643 (34.9%) | |
| Dystocia | |||
| Yes | 151 (33.6%) | 809 (43.9%) | <0.001 |
| No | 299 (66.4%) | 1035 (56.1%) | |
| ASA status | |||
| Class II | 325 (72.2%) | 1468 (79.6%) | <0.001 |
| Class III | 98 (21.8%) | 289 (15.7%) | |
| Class IV | 17 (3.8%) | 12 (0.6%) | |
| Missing data | 10 (2.2%) | 75 (4.1%) | |
| Mode of anaesthesia | |||
| GA after regional anaesthesia | 72 (16%) | 100 (5.4%) | <0.001 |
| GA (no regional anaesthesia) | 85 (18.9%) | 123 (6.7%) | |
| Spinal anaesthesia | 115 (25.6%) | 569 (30.9%) | |
| Epidural anaesthesia | 132 (29.3%) | 830 (45%) | |
| Combined spinal-epidural | 45 (10%) | 216 (11.7%) | |
| Missing data | 1 (0.2%) | 6 (0.3%) | |
In the multivariate models, African-American race, Hispanic ethnicity, multiple gestation, placenta praevia, ASA class III, ASA class IV, general with and without prior regional anaesthesia, and two or more prior Caesarean deliveries were significantly associated with haemorrhage-related morbidity (Table 3). In Model 1, BMI 25–29.9, BMI 35–39.9, and BMI≥40 were independently associated with a decreased risk of morbidity compared with patients with BMI<25. In Model 2, patients in the highest BMI quintile were at decreased risk of morbidity vs patients in the lowest BMI quintile (Table 3). Although attempted labour or induction of labour was significantly associated with severe morbidity in the crude analysis, it was not associated with morbidity in the adjusted models. The AUCROCs for multivariate Model 1 and Model 2 were 0.72 (95% CI=0.69–0.75) and 0.71 (95% CI=0.68–0.74), respectively. Using 10-fold cross validation, the AUCROCs for Model 1 and Model 2 were 0.65 and 0.63, respectively, indicating that these models had only modest ability to discriminate between atonic patients with haemorrhage-related morbidity vs those without morbidity.
Table 3.
Multivariate analysis of haemorrhage-related morbidity among women with UA undergoing Caesarean delivery. OR, odds ratio; CI, confidence interval; CD, Caesarean delivery; GA, general anaesthesia. Model calibration using the Hosmer–Lemoshow test was good for model 1 (P=0.14) and model 2 (P=0.23). Model 1 included pre-delivery BMI categories based on the World Health Organization criteria. Model 2 included pre-delivery BMI categories based on quintiles of pre-delivery BMI
| Variable | Model 1 |
Model 2 |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Race | ||||
| Caucasian | Referent | Referent | ||
| African-American | 2.36 (1.73–3.23) | <0.001 | 2.37 (1.74–3.24) | <0.001 |
| Hispanic | 1.40 (1.04–1.9) | 0.03 | 1.41 (1.04–1.91) | 0.02 |
| Other | 1.23 (0.71–2.15) | 0.46 | 1.23 (0.70–2.15) | 0.46 |
| BMI at the time of delivery (kg m−2) | ||||
| <25 | Referent | |||
| 25–29.9 | 0.63 (0.42–0.95) | 0.03 | ||
| 30–34.9 | 0.72 (0.48–1.08) | 0.11 | ||
| 35–39.9 | 0.62 (0.4–0.98) | 0.04 | ||
| ≥40 | 0.36 (0.22–0.6) | <0.001 | ||
| BMI quintiles (kg m−2) | ||||
| <27.06 | Referent | |||
| 27.06–29.97 | 0.79 (0.55–1.14) | 0.2 | ||
| 29.98–33.04 | 0.94 (0.66–1.34) | 0.74 | ||
| 33.05–37.7 | 0.81 (0.56–1.17) | 0.26 | ||
| >37.7 | 0.56 (0.38–0.82) | 0.005 | ||
| ASA class | ||||
| II | Referent | Referent | ||
| III | 1.4 (1.03–1.9) | 0.03 | 1.40 (1.03–1.90) | 0.03 |
| IV | 5.88 (2.48–13.9) | <0.001 | 5.86 (2.48–13.8) | <0.001 |
| Mode of anaesthesia | ||||
| Spinal | Referent | Referent | ||
| GA+regional | 4.0 (2.62–6.09) | <0.001 | 3.93 (2.58–5.97) | <0.001 |
| GA (no regional) | 2.40 (1.59–3.62) | <0.001 | 2.43 (1.61–3.67) | <0.001 |
| Epidural | 1.02 (0.73–1.42) | 0.93 | 1.01 (0.73–1.42) | 0.92 |
| Epidural+spinal | 1.0 (0.64–1.54) | 0.98 | 1.01 (0.65–1.57) | 0.93 |
| Labour prior to delivery or attempted induction | ||||
| No | Referent | Referent | ||
| Yes | 1.1 (0.81–1.50) | 0.56 | 1.09 (0.80–1.49) | 0.53 |
| Placenta praevia | ||||
| No | Referent | Referent | ||
| Yes | 4.89 (3.04–7.87) | <0.001 | 4.86 (3.02–7.81) | <0.001 |
| Singleton/multiple gestation | ||||
| Singleton | Referent | Referent | ||
| Multiple gestation | 1.59 (1.06–2.38) | 0.03 | 1.57 (1.04–2.36) | 0.04 |
| Number of prior CDs | ||||
| 0 | Referent | Referent | ||
| 1 | 1.22 (0.90–1.65) | 0.20 | 1.21 (0.90–1.64) | 0.21 |
| ≥2 | 1.62 (1.10–2.39) | 0.01 | 1.62 (1.10–2.39) | 0.01 |
Using our second composite outcome measure, which excluded: invasive monitoring, mechanical ventilation, coagulopathy, adult respiratory distress syndrome, or pulmonary oedema, we identified 443 patients (19.3%) with this second composite outcome. In the sensitivity analysis in which we reconstructed Models 1 and 2 using our second composite outcome, we observed minimal changes in the estimates for morbidity using predictors from our original models (results not shown).
Discussion
Although previous population-wide studies have identified UA as a leading cause of PPH,5–8 risk factors for haemorrhage-related morbidity among patients with UA have been poorly investigated. Using clinically rich data abstracted from a US multicentre study of over 57 000 patients, we found that risk factors for haemorrhage-related morbidity among a cohort of patients with UA overlap with a number of established risk factors for severe PPH,3,7,8,21,22 including: African-American race, Hispanic ethnicity, multiple gestation, placenta praevia, and GA. Additionally, patients with poor functional status prior to delivery (ASA class III or IV) were also at increased risk of haemorrhage-related morbidity, whereas morbidly obese patients were at decreased risk of morbidity.
In our study, approximately one in five patients with severe UA were identified with haemorrhage-related morbidity. This is in keeping with current knowledge that links PPH to morbidity during the delivery and the postpartum periods,23 and the increasing incidence of UA as a major contributor to rising rates of PPH.5 Specific to Caesarean delivery, the risk factors for morbidity in our study are similar to those reported in prior studies investigating risk factors for PPH, notably: placenta praevia, GA, and multiple gestation.1,22,24–26 However, risk factors for severe atonic PPH were not reported in these studies. Our findings that African-American race and Hispanic ethnicity were independently associated with an increased risk of haemorrhage-related morbidity are supported by prior work investigating ethnic/racial disparities for atonic PPH. Using US administrative data, Bryant and colleagues21 observed that African-Americans and Hispanics were at increased risk of atonic PPH and transfusion or hysterectomy compared with a Caucasian referent group. These findings suggest that racial/ethnic disparities for haemorrhage-related morbidity may exist, yet it is unclear whether these associations may be related to biological differences or to differences in the quality of hospital care between racial/ethnic groups.
We observed an increased risk of morbidity in women with a history of two or more Caesarean deliveries compared with no prior Caesarean deliveries. Our data contrast with those of other PPH studies of women undergoing Caesarean delivery.22,24 In these studies, a history of prior Caesarean delivery was associated either with no increased risk24 or a decreased risk22 of PPH. However, these studies did not specifically focus on women with UA and selected predictors for PPH were not consistent between studies. Other population-wide studies suggest that a history of prior Caesarean delivery may be associated with an increased risk of PPH.6,27 Furthermore, the risk of severe obstetric morbidity, including red blood cell transfusion ≥4 units, is known to increase progressively with an increase in the number of prior Caesarean deliveries.28
In our study, GA was a risk factor for haemorrhage-related morbidity. This finding is in accordance with prior work that has linked GA to an increased risk of PPH vs regional anaesthesia.22,24,26,29 The most likely mechanism is that inhalation anaesthetic agents induce a concentration-dependent inhibitory effect on spontaneous myometrial contractility,30 thereby increasing the risk of UA. We observed that the odds for morbidity were increased two- and four-fold in women who received only GA and general with regional anaesthesia, respectively. One possible explanation is that GA combined with regional anaesthesia may culminate in a greater degree of maternal hypotension and organ hypoperfusion resulting in increased obstetric morbidity.
Patients with an ASA class III or IV were independently associated with an increased risk of haemorrhage-related morbidity compared with ASA class II patients, which suggests that patients with poor functional status may have reduced physiological reserve for tolerating major postpartum blood loss, anaemia and hypoperfusion compared with healthier patients. No ASA I patients were described in the Caesarean Registry. In the USA, practitioners may modify ASA classification class I–II in the setting of pregnancy because there is no pregnancy-specific modifier.31
Women in the highest BMI class (using both WHO criteria and quintiles) were at significantly reduced risk of haemorrhage-related morbidity compared with women in the lowest BMI class or quintile. There is controversy regarding the associations between obesity with UA and atonic PPH. The risk of atonic PPH has previously been found to be increased by 14, 47, and 114% in obesity classes I, II, and III, respectively compared with a non-obese group.32 However, in keeping with our results, Paglia and colleagues33 reported that women with a BMI of >30 were associated with a decreased risk of PPH, and Rouse and colleagues16 observed no differences in BMI at delivery among patients diagnosed with UA compared with patients without atony. As obese patients tend to have a more hypercoagulable state (because of higher fibrinogen, factor VII, factor VIII, von Willebrand factor, and plasminogen activator inhibitor levels),34 enhanced maternal hypercoagulability may provide a protective effect against major blood loss. As previous studies investigating the association between maternal BMI and PPH have used maternal weight and height measurements before pregnancy32 and during pregnancy,33 more prospective research is needed to more clearly elucidate the direction of the associations between maternal pre-pregnancy BMI and pre-delivery BMI and PPH and haemorrhage-related morbidity.
There are a number of limitations to our study. No data were collected for blood loss or haematological indices immediately prior to transfusion, which limited our ability to assess PPH severity and infer decision-making practices for blood transfusions. Although haematological indices influence transfusion decision-making, time delays often occur because of transport of blood tubes and laboratory processing of blood samples during the course of a major PPH. As a result, physicians may rely upon clinical indications for transfusion such as maternal vital signs, rate and magnitude of blood loss, and evidence of organ hypoperfusion. Additionally, no data were available for specific interventions or therapies for atonic PPH including: haemostatic (B-lynch) suture placement, intrauterine balloon tamponade, interventional radiological techniques (such as uterine artery balloon catheterization and uterine artery embolization), or pro-thrombotic drugs (such as recombinant factor VIIa). Although we identified clinical factors associated with haemorrhage-related morbidity, there may be other factors linked to haemorrhage-related morbidity that were not available in the Caesarean Registry (such as the presence of uterine leimyomata). Missing data were present for a number of variables that we could not consider in our regression models, such as: type of uterine incision: 381 (16.6%) patients; and oxytocin use in labour: 1180 (51.4%) patients. The association of GA with haemorrhage-related morbidity may have been overestimated, as it is possible that some patients underwent pre- or intraoperative conversion from regional to GA in order to optimize maternal oxygen delivery and cardiac output after major peripartum bleeding occurred. It is also possible that some patients who underwent a spinal anaesthetic may have required conversion to GA if the duration of surgery became prolonged and outlasted the duration of the spinal anaesthetic. The Caesarean Registry does not contain specific drug data for GA or regional anaesthesia, and therefore, we were not able to determine the influence of different drugs, doses, or concentrations of anaesthetic agents on the risk of haemorrhage-related morbidity. Finally, the values of AURROCs were between 0.63 and 0.72, which demonstrate limited discrimination.
In conclusion, within a cohort of 2294 patients who had UA at Caesarean delivery, the risk of atonic haemorrhage-related morbidity is increased in African-Americans, Hispanics, patients with multiple gestations, placenta praevia, impaired pre-delivery ASA functional status, a history of two or more Caesarean deliveries, and patients undergoing GA. Prospective clinical studies are needed to further validate these associations and elucidate other factors that may be linked with haemorrhage-related morbidity among patients with UA. Vigilance and early therapeutic intervention are advocated for patients who undergo Caesarean delivery with patient characteristic, medical, or clinical risk factors for PPH-related morbidity related to UA. The use of regional anaesthesia (not in combination with GA) is recommended for patients who may have risk factors for UA as an approach to reduce haemorrhage-related morbidity.
Authors' contributions
A.J.B. conceived the study hypothesis. A.J.B. and Y.Y.E.-S. designed the study. Database management and statistical analysis was performed by A.J.B. The manuscript was drafted by A.J.B., and revised critically for intellectual content by B.C. and Y.Y.E.-S. All the authors read and approved the final version. A.J.B. is a recipient of an award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1K23HD070972).
Declaration of interest
We acknowledge the assistance of NICHD, the MFMU Network, and the Protocol Subcommittee in making the database available on behalf of the project. The contents of this report represent the views of the authors and do not represent the views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network or the National Institutes of Health.
Funding
This study was internally funded by the Department of Obstetrics and Gynaecology and the Department of Anaesthesia at Stanford University School of Medicine.
References
- 1.Rossen J, Okland I, Nilsen OB, Eggebo TM. Is there an increase of postpartum hemorrhage, and is severe hemorrhage associated with more frequent use of obstetric interventions? Acta Obstet Gynecol Scand. 2010;89:1248–55. doi: 10.3109/00016349.2010.514324. [DOI] [PubMed] [Google Scholar]
- 2.Stones RW, Paterson CM, Saunders NJ. Risk factors for major obstetric haemorrhage. Eur J Obstet Gynecol Reprod Biol. 1993;48:15–8. doi: 10.1016/0028-2243(93)90047-g. [DOI] [PubMed] [Google Scholar]
- 3.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265–72. doi: 10.1111/j.1471-0528.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 4.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Mathews TJ. Births: Final Data for 2010. Natl Vital Stat Rep. 2012;61:1. [PubMed] [Google Scholar]
- 5.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol. 2010;202:353e1–6. doi: 10.1016/j.ajog.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Effects of onset of labor and mode of delivery on severe postpartum hemorrhage. Am J Obstet Gynecol. 2009;201:273e1–9. doi: 10.1016/j.ajog.2009.06.007. –. [DOI] [PubMed] [Google Scholar]
- 7.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110:1368–73. doi: 10.1213/ANE.0b013e3181d74898. [DOI] [PubMed] [Google Scholar]
- 8.Mehrabadi A, Hutcheon JA, Lee L, Kramer MS, Liston RM, Joseph KS. Epidemiological investigation of a temporal increase in atonic postpartum haemorrhage: a population-based retrospective cohort study. BJOG. 2013;120:853–62. doi: 10.1111/1471-0528.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039–47. doi: 10.1097/00006250-200610000-00046. [DOI] [PubMed] [Google Scholar]
- 10.Royal College of Obstetricians and Gynaecologists. Green-top Guideline No. 52: Prevention and Management of Postpartum Haemorrhage. London: RCOG Guidelines and Audit Committee; 2011. [Google Scholar]
- 11.Berg CJ, Harper MA, Atkinson SM, et al. Preventability of pregnancy-related deaths: results of a state-wide review. Obstet Gynecol. 2005;106:1228–34. doi: 10.1097/01.AOG.0000187894.71913.e8. [DOI] [PubMed] [Google Scholar]
- 12.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–36. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 13.Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: case-control study. Br Med J. 2001;322:1089–93. doi: 10.1136/bmj.322.7294.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuklina EV, Meikle SF, Jamieson DJ, et al. Severe obstetric morbidity in the United States: 1998–2005. Obstet Gynecol. 2009;113:293–9. doi: 10.1097/AOG.0b013e3181954e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landon MB, Hauth JC, Leveno KJ, et al. Maternal and perinatal outcomes associated with a trial of labor after prior Cesarean delivery. N Engl J Med. 2004;351:2581–9. doi: 10.1056/NEJMoa040405. [DOI] [PubMed] [Google Scholar]
- 16.Rouse DJ, Leindecker S, Landon M, et al. The MFMU Cesarean Registry: uterine atony after primary Cesarean delivery. Am J Obstet Gynecol. 2005;193:1056–60. doi: 10.1016/j.ajog.2005.07.077. [DOI] [PubMed] [Google Scholar]
- 17.Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. Am J Obstet Gynecol. 2008;199:133e1–8. doi: 10.1016/j.ajog.2007.12.020. –. [DOI] [PubMed] [Google Scholar]
- 18.Roberts CL, Cameron CA, Bell JC, Algert CS, Morris JM. Measuring maternal morbidity in routinely collected health data: development and validation of a maternal morbidity outcome indicator. Med Care. 2008;46:786–94. doi: 10.1097/MLR.0b013e318178eae4. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet MP, Deneux-Tharaux C, Bouvier-Colle MH. Critical care and transfusion management in maternal deaths from postpartum haemorrhage. Eur J Obstet Gynecol Reprod Biol. 2011;158:183–8. doi: 10.1016/j.ejogrb.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Obesity: preventing and managing the global epidemic. 2000. Geneva: WHO technical report series 894. [PubMed]
- 21.Bryant A, Mhyre JM, Leffert LR, Hoban RA, Yakoob MY, Bateman BT. The association of maternal race and ethnicity and the risk of postpartum hemorrhage. Anesth Analg. 2012;115:1127–36. doi: 10.1213/ANE.0b013e3182691e62. [DOI] [PubMed] [Google Scholar]
- 22.Skjeldestad FE, Oian P. Blood loss after Cesarean delivery: a registry-based study in Norway, 1999–2008. Am J Obstet Gynecol. 2012;206:76e1–7. doi: 10.1016/j.ajog.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 23.Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstet Gynecol. 2009;113:1075–81. doi: 10.1097/AOG.0b013e3181a09fc0. [DOI] [PubMed] [Google Scholar]
- 24.Combs CA, Murphy EL, Laros RK., Jr Factors associated with hemorrhage in Cesarean deliveries. Obstet Gynecol. 1991;77:77–82. [PubMed] [Google Scholar]
- 25.Kolas T, Oian P, Skjeldestad FE. Risks for peroperative excessive blood loss in Cesarean delivery. Acta Obstet Gynecol Scand. 2010;89:658–63. doi: 10.3109/00016341003605727. [DOI] [PubMed] [Google Scholar]
- 26.Magann EF, Evans S, Hutchinson M, Collins R, Lanneau G, Morrison JC. Postpartum hemorrhage after Cesarean delivery: an analysis of risk factors. South Med J. 2005;98:681–5. doi: 10.1097/01.SMJ.0000163309.53317.B8. [DOI] [PubMed] [Google Scholar]
- 27.Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209:449e1–7. doi: 10.1016/j.ajog.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat Cesarean deliveries. Obstet Gynecol. 2006;107:1226–32. doi: 10.1097/01.AOG.0000219750.79480.84. [DOI] [PubMed] [Google Scholar]
- 29.Chang CC, Wang IT, Chen YH, Lin HC. Anesthetic management as a risk factor for postpartum hemorrhage after Cesarean deliveries. Am J Obstet Gynecol. 2011;205:462e1–7. doi: 10.1016/j.ajog.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 30.Yoo KY, Lee JC, Yoon MH, et al. The effects of volatile anesthetics on spontaneous contractility of isolated human pregnant uterine muscle: a comparison among sevoflurane, desflurane, isoflurane, and halothane. Anesth Analg. 2006;103:443–7. doi: 10.1213/01.ane.0000236785.17606.58. [DOI] [PubMed] [Google Scholar]
- 31.Barbeito A, Muir HA, Gan TJ, et al. Use of a modifier reduces inconsistency in the American Society of Anesthesiologists Physical Status Classification in parturients. Anesth Analg. 2006;102:1231–3. doi: 10.1213/01.ane.0000198564.59290.ee. [DOI] [PubMed] [Google Scholar]
- 32.Blomberg M. Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol. 2011;118:561–8. doi: 10.1097/AOG.0b013e31822a6c59. [DOI] [PubMed] [Google Scholar]
- 33.Paglia MJ, Grotegut CA, Johnson LN, Thames B, James AH. Body mass index and severe postpartum hemorrhage. Gynecol Obstet Invest. 2012;73:70–4. doi: 10.1159/000329335. [DOI] [PubMed] [Google Scholar]
- 34.Mertens I, Van Gaal LF. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3:85–101. doi: 10.1046/j.1467-789x.2002.00056.x. [DOI] [PubMed] [Google Scholar]