Abstract
Membrane trafficking depends on transport vesicles and carriers docking and fusing with the target organelle for the delivery of cargo. Membrane tethers and small guanosine triphosphatases (GTPases) mediate the docking of transport vesicles/carriers to enhance the efficiency of the subsequent SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor)-mediated fusion event with the target membrane bilayer. Different classes of membrane tethers and their specific intracellular location throughout the endomembrane system are now well defined. Recent biochemical and structural studies have led to a deeper understanding of the mechanism by which membrane tethers mediate docking of membrane carriers as well as an appreciation of the role of tethers in coordinating the correct SNARE complex and in regulating the organization of membrane compartments. This review will summarize the properties and roles of membrane tethers of both secretory and endocytic systems.
Introduction
Membrane tethering is traditionally considered to define the process associated with the delivery of transport vesicles or carriers laden with protein and lipid cargo to their correct membrane compartment [1-3]. Membrane tethering refers to the initial docking of transport carriers to the target membrane prior to fusion of the two lipid bilayers. Over the past 10 years, it has become clear from both in vivo cell-based studies and in vitro membrane fusion assays that tethering factors are essential for the fidelity of intracellular transport pathways and for the efficient assembly of SNARE (soluble N-ethylmaleimide-sensitive factor [NSF] attachment protein receptor) complexes between the transport vesicle and target membrane and the fusion event. Loss of membrane tethers results in a block in membrane transport and, in many cases, dramatically affects the organization and identity of compartments. Hence, the role of membrane tethers in bridging membranes is important for understanding vesicular trafficking as well as organelle biogenesis.
The key characteristics of membrane tethers that are important for their function are as follows: (a) The majority of membrane tethers are peripheral membrane proteins that are recruited to specific locations by small G proteins [4,5] of the Rab and Arl families [6]. Indeed, membrane tethers represent a major group of Rab and Arl effectors. Recruitment of membrane tethers from the cytoplasm provides a mechanism for the dynamic targeting of these factors to membranes without the requirement for loading in the endoplasmic reticulum (ER), as for membrane proteins, such as SNAREs. This mechanism of recruitment provides the capacity to rapidly generate membrane subdomains and to establish a very restricted distribution of tethers to organelles compared with SNAREs [7,8] which are tailed-anchored proteins inserted initially in the membrane of the ER and then transported to their functional site. (b) There are two major classes of tethering molecules: long coiled-coil proteins and multisubunit complexes [5,9]. Both classes are found throughout the secretory and endocytic pathways, including the ER, Golgi, and endosomes (Figure 1), and both classes are highly conserved throughout the evolution of eukaryotic cells [10], indicating their central roles in regulating the functions of the endomembrane system. (c) Tethering molecules are considered to play a key role in mediating the specificity of interaction of the transport carrier with the correct target membrane and, moreover, in an increasing number of cases have been shown to coordinate the organization of the correct SNAREs at the vesicle docking site [11,12]. (d) In addition to interacting with Rabs and SNAREs, membrane tethers interact with a variety of effectors, and it is apparent that many tethers may have functions in addition to the regulation of membrane fusion [13]. Given their extensive distribution throughout the eukaryotic cell, they may provide a molecular network between neighboring membrane compartments of the secretory and endosomal systems [14,15]. There is growing interest in understanding the potential roles of membrane tethers as molecular communicators between adjacent membrane-bound compartments to coordinate the regulation of membrane flow throughout the membrane transport systems of the cell.
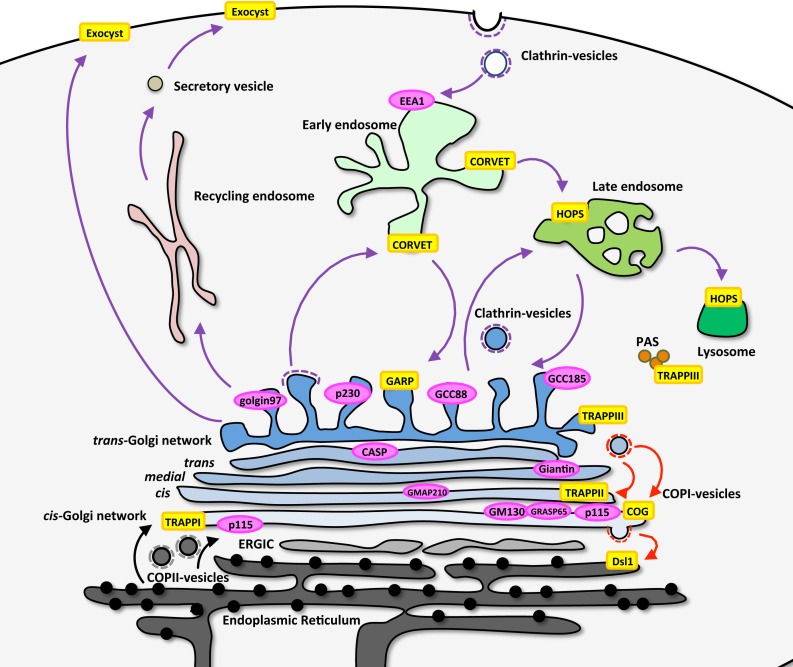
Figure 1. Location of membrane tethers in the trafficking pathways of the cells.
The location and identity of homodimeric coiled-coil tethers (pink ovals) and multiple subunit tethering complexes (yellow rectangles) are shown, as well as some of the transport pathways and transport vesicles that are regulated by the membrane tethers. The majority of the coiled-coil protein tethers are associated with the Golgi apparatus, whereas the multiple subunit tethering complexes are found throughout the secretory and endolysosomal pathways.
Abbreviations: CASP, CCAAT-displacement protein alternatively spliced product; COG, conserved oligomeric Golgi complex; CORVET, class C core vacuole/endosome tethering; EEA1, early endosome antigen-1; ERGIC, endoplasmic reticulum-Golgi intermediate compartment; GARP, Golgi-associated retrograde protein complex; GMAP-210, Golgi microtubule-associated protein of 210 kDa; HOPS, homotypic fusion and vacuole protein sorting; PAS, phagophore assembly site; TRAPP, transport protein particle.
In this review, we describe the classes of membrane tethers and the identity of tethers at the various locations. We highlight recent advances in understanding their role in both vesicle targeting and organelle function. The reader is encouraged to complement this overview with excellent detailed reviews of the individual classes of tethering molecules [5,13,16-20].
Classification of tethering factors
Tethering factors can be broadly divided into two major groups: homodimeric coiled-coil proteins and multisubunit tethering complexes (MTCs). Coiled-coil tethers are large, hydrophilic, homodimeric proteins comprising two globular heads connected by long coiled-coil domains. Owing to their large size, coiled-coil tethers can interact with vesicles over long distances (of more than 200 nm) [17], and the current model postulates that they facilitate the long-range sequestering of vesicles as the initial step in vesicle tethering [21-23]. MTCs, on the other hand, comprise a diverse family of proteins, with each member consisting of 3 to 10 subunits (Figure 2), resulting in an overall molecular weight of approximately 250 to 800 kDa [17]. MTCs interact with vesicles over a shorter distance (up to 30 nm) [20] than the long coiled-coil tethers and are thought to tether a captured vesicle in close apposition to its acceptor compartment and meaning it is poised for fusion.
Figure 2. Models of membrane tethers.
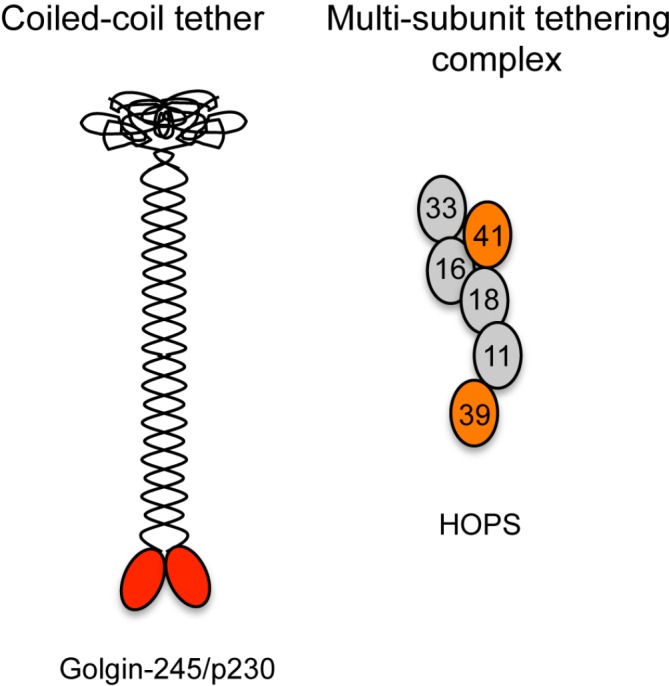
Shown is an example of a long dimeric coiled-coil tether - the TGN golgin, golgin-245/p230, with the Arl1-binding site at the C-terminus (GRIP domain) indicated by the red oval shapes [14] and an example of a multiple subunit tethering complex - HOPS complex, which contains four shared subunits with the CORVET complex (in grey) and two unique subunits (orange), which bind to GTP-Rab7 [16]. The numbers refer to the identity of the Vps subunits.
Abbreviations: CORVET, class C core vacuole/endosome tethering; HOPS, homotypic fusion and vacuole protein sorting; TGN, trans-Golgi-network.
Most coiled-coil tethers are peripheral membrane proteins, and many have been found to be associated with the Golgi (thus termed “golgins”), although some (such as EEA1) can be found at endosomes. The golgins (approximately 20 members) are regulated by small GTPases of the Rab and Arl families and function in membrane-membrane and membrane-cytoskeleton tethering at the Golgi apparatus [24]. MTCs can be further divided into two general groups: those that function along the secretory pathway—comprising Dsl1p, COG (conserved oligomeric Golgi complex), GARP (Golgi-associated retrograde protein complex), and the exocyst (collectively named complexes associated with tethering containing helical rods, or CATCHR)—and those that function in the endolysosomal pathway, comprising the class C vacuolar protein-sorting (Vps) complexes, HOPS (homotypic fusion and vacuole protein sorting), and CORVET (class C core vacuole/endosome tethering). In addition, eukaryotic cells have TRAPP (transport protein particle) complexes, which are also MTCs but do not readily fit into the two MTC general groups and have tethering roles in both the secretory and endolysosomal pathways as well as a function in autophagy. These tethering factors will hereafter be discussed according to the transport processes they mediate (see Table 1 for summary).
Table 1. Tethering proteins and their proposed function.
| Coiled-coil | Structure/composition | Proposed function |
|---|---|---|
| p115 | 115 kDa protein; homodimer | Tethering of COPI vesicles to Golgi; anterograde trafficking of newly synthesized cargo; Golgi ribbon formation |
| GM130 | 130 kDa protein; homodimer | Tripartite model of p115-dependent tethering |
| Giantin | 400 kDa protein; homodimer | Tripartite model of p115-dependent tethering |
| CASP | 160 kDa protein; homodimer | Retrograde transport within the Golgi |
| GMAP210 | 210 kDa protein; homodimer | Intra-Golgi trafficking and structural maintenance of Golgi |
| GCC185 | 185 kDa protein; homodimer | Endosome-to-TGN trafficking |
| GCC88 | 88 kDa protein; homodimer | Endosome-to-TGN trafficking |
| p230 | 245 kDa protein; homodimer | Anterograde transport from the TGN |
| Golgin97 | 97 kDa protein; homodimer | Anterograde transport from the TGN |
| EEA1 | 162 kDa protein; homodimer | Docking and fusion of vesicles at the early endosome |
| MTCs | Structure/composition | Proposed function |
| COG | 8 subunits in yeast: Cog1-8 | Intra-Golgi trafficking; endosome-to-Golgi retrograde transport |
| Dsl1 | 3 subunits in yeast: Dsl1, Dsl3(Sec39), and Tip20; in mammals: ZW10, RINT-1, and NAG | Golgi-to-ER retrograde transport |
| GARP | 4 subunits: Vps51-54 | Endosome-to-TGN trafficking |
| TRAPPI | 7 subunits in yeast: Bet3A, Bet3B, Bet5, Trs20, Trs23, Trs31, and Trs33 | Tethering of COPII-decorated vesicles from ER at the Golgi |
| TRAPPII | TRAPPI subunits and Trs65, Trs120, and Trs130 | Intra-Golgi transport; binds COPI vesicles; retrograde trafficking from endosomes to Golgi |
| TRAPPIII | TRAPPI subunits and Trs85 | Retrograde endosome-to-TGN transport, autophagy |
| HOPS | 6 subunits in yeast: Vps11, Vps16, Vps18, Vps33, Vps39, and Vps41 | Endolysosomal fusion, homotypic fusion of vacuoles in yeast |
| CORVET | 6 subunits in yeast: Vps3, Vps8, Vps11, Vps16, Vps18, and Vps33 | Acts upstream of HOPS and may tether vesicles or promote homotypic fusion of endosomes |
| Exocyst | 8 subunits in yeast: Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 | Tethering complex for transport carriers from recycling endosomes and the Golgi |
Abbreviations: CASP, CCAAT-displacement protein alternatively spliced product; COG, conserved oligomeric Golgi complex; CORVET, class C core vacuole/endosome tethering; EEA1, early endosome antigen-1; ER, endoplasmic reticulum; GARP, Golgi-associated retrograde protein complex; GCC, Golgi localized coiled-coil protein; GMAP-210, Golgi microtubule-associated protein of 210 kDa; HOPS, homotypic fusion and vacuole protein sorting; MTC, multisubunit tethering complex; TGN, trans-Golgi-network; TRAPP, transport protein particle; Vps, vacuolar protein sorting.
ER-Golgi and intra-Golgi trafficking
Among the first membrane tethers to be identified and analyzed by using in vitro assays were the coiled-coil tether p115 and its yeast homologue Uso1p, shown to have important functions in ER-to-Golgi transport and in the biogenesis of Golgi membranes. However, the mechanism for p115-mediated tethering remains under considerable debate [25]. A tethering model was proposed on the basis of a triad of coiled-coil proteins located at the ER/Golgi: p115, GM130, and giantin [19]. p115 is localized predominantly to the cis-Golgi, ER exit sites, and the ER-Golgi intermediate compartment (ERGIC) [26,27]. GM130 and giantin were subsequently identified as binding partners of p115 via its acidic carboxy domain [28,29]. GM130 was found to associate with Golgi membranes via GRASP65, a 65 kDa protein involved in reassembly of Golgi stacks following mitosis [30,31]. Giantin is a 400 kDa type II integral protein on Golgi membranes and COPI vesicles [29]. The recruitment of p115 and the GM130:GRASP65 complex is thought to be dependent on Rab1 [32,33]. The tripartite tether of p115:GM130:giantin has been proposed to mediate the tethering of recycling COPI vesicles. In the bridging model of tethering, giantin on COPI vesicles binds p115, and p115 binds GM130 on acceptor cis-Golgi membranes, linking COPI vesicles to target Golgi elements [29]. However, subsequent findings have challenged this model, in particular, those that suggest that p115 interaction with GM130 and giantin may not be required directly for membrane tethering [34-37]. Sztul and colleagues [34] have instead proposed a new model for the mechanism of p115-mediated tethering. p115 contains four coiled-coil domains (CC1 to CC4); CC1 and CC4 are both essential for p115 function in Golgi ribbon formation and trafficking of newly synthesized cargo [34,36]. This has led these investigators to suggest that p115-mediated tethering may involve simultaneous interaction of CC1 and CC4 domains of p115 with different subsets of SNAREs, resulting in the “capture” of these SNAREs, effectively concentrating them at fusion sites marked by Rab1 localization. p115 might then catalyze trans-SNARE formation at these sites, leading to the fusion of cargo-laden vesicles with the Golgi [25].
In contrast to these prototypical tethers, other coiled-coil proteins have been implicated in Golgi trafficking. CCAAT-displacement protein alternatively spliced product (CASP) localizes to the Golgi [38] and is important for retrograde traffic within the Golgi, although its exact mechanisms remain undefined [39]. Golgi microtubule-associated protein of 210 kDa (GMAP-210) is a cis-Golgi protein [40] involved in membrane trafficking: overexpression of GMAP-210 blocks anterograde transport of both a soluble form of alkaline phosphatase and hemagglutinin between the ER and cis/medial Golgi stacks [41] and inhibits retrograde transport of Shiga-toxin B subunit between the Golgi and the ER [41]. GMAP-210 is recruited to Golgi membranes by the interaction of a C-terminal domain with Arf1-GTP, whereas the N-terminus can interact directly with curved membranes [42,43], hence the potential to tether membrane vesicles. Overexpression of GMAP-210 induces an enlargement of the Golgi [40], possibly by the accumulation of tethered vesicles unable to undergo fusion. A number of other Golgi-localized coiled-coil proteins, such as golgin-84 and TMF1, have been proposed to play a role in membrane tethering (for review, see [44]).
MTCs also play important roles in Golgi trafficking. The COG complex consists of eight subunits [45,46]. This octameric complex can interact with a gamut of molecules—SNAREs, SNARE-interacting proteins, Rabs, vesicular coats, and molecular motors as well as other tethers of the coiled-coil family—consistent with its role in multiple Golgi trafficking steps [47]. Specifically, COG interacts with Ypt1p (yeast homologue of Rab1), intra-Golgi SNAREs, and the COPI coat, leading to the hypothesis that COG may tether COPI-coated, retrogradely targeted intra-Golgi vesicles to the cis-Golgi [48]. A role in intra-Golgi transport of COG is strongly suggested by findings by Zolov and Lupashin [49], whereby Cog3p deletion in Hela cells led to the accumulation of vesicles carrying the Golgi SNAREs GS15 and GS28 and the cis-Golgi glycoprotein GPP130 and inhibited retrograde transport of Shiga toxin. Another MTC and member of the CATCHR family that has been implicated in Golgi-to-ER retrograde transport is the Dsl1p complex, which in yeast consists of three subunits (Dsl1, Dsl3/Sec39, and Tip20) and forms a stable complex with ER SNAREs Ufe1, Use1, and Sec20 at the ER membrane [50,51]. This interaction is thought to anchor the Dsl1p complex to the ER, where it can tether COPI vesicles [52,53], thereby coupling tethering with SNARE assembly [54]. No Ypt/Rab interaction has been reported for the Dsl1p complex. In addition, Dsl1 can associate with the R-SNAREs Sec22 and Ykt6 [55]; thus, the Dsl1 complex contains many interaction sites for SNAREs that may collectively stabilize Dsl1 at the ER, recognize incoming vesicles, and subsequently promote SNARE-mediated fusion. The mammalian orthologue of the yeast Dsl1 complex comprises ZW10 (Dsl1), RINT-1 (Tip20), and NAG (Sec39/Dsl3) [56]. The ZW10 complex can also associate with ER SNAREs syntaxin 18 (Ufe1), BNIP1 (Sec20), p31 (Use1/Slt1), and Sec22b (Sec22) [56,57]. Interestingly, a recent study by Tagaya and colleagues has shown that RINT-1 can also interact with trans-Golgi-network (TGN) SNAREs and Cog1, a subunit of the COG complex [58], suggesting a degree of promiscuity among CATCHR family complex subunits.
The TRAPP complex is an atypical tethering factor that cannot be classified under two broad categories of tethers (coiled-coil proteins and MTCs). This complex is found in three forms in yeast (TRAPPI, II, and III), ranging between 300 and 1,000 kDa and reflecting the different subunit compositions. TRAPPI consists of seven subunits (Bet5, Trs20, Bet3A, Bet3B, Trs23, Trs31, and Trs33) [59-62]. An additional three subunits are found in TRAPPII (Trs65, Trs120, and Trs130) [63] and an additional subunit in TRAPPIII, namely Trs85 [64]. The TRAPP complex-associated protein 17 kDa (Tca17) has also been shown to specifically associate with TRAPPII [65], although its role in membrane traffic is unknown. A unique feature of the TRAPP complexes is their ability to act as guanine nucleotide exchange factors (GEFs) for Rab small GTPases [66]. In this regard, the TRAPP complexes differ from other membrane tethers as they have GEF activity and are not Rab/Arl effectors. The core subunits Bet3A, Bet3B, Bet5, and Trs23 interact with Ypt1 to promote nucleotide exchange [67]; thus, the yeast TRAPP complexes function as a multisubunit GEF for the Rab Ypt1. TRAPPI is the most well-studied TRAPP complex thus far, and a tethering function for ER-to-Golgi transport in yeast is proposed. Via an in vitro reconstitution assay, TRAPPI has been shown to be an essential component to tether COPII-decorated ER-derived vesicles with Golgi membranes, and the Bet3 subunit of TRAPPI binds to Sec23, a subunit of the inner layer of the COPII coat [68,69]. In contrast to the role of TRAPPI in ER-Golgi trafficking, TRAPPII has been proposed to mediate intra-Golgi transport in yeast. In line with this proposal, TRAPPII specifically binds COPI, but not COPII, vesicles [69-71]. In addition, mutations in the TRAPPII-specific subunit Trs120 and Trs130 result in trafficking defects within the Golgi and in retrograde trafficking from the endosomes to the Golgi [70].
Yeast TRAPPIII has recently been shown to be required for autophagy. TRAPPIII is localized to the phagophore assembly site (PAS) [64], where it may function to recruit membrane [72]. In addition, Noda and colleagues [73] showed that TRAPPIII functions at the Golgi to receive retrograde cargo from endosomes, including Atg9, a transmembrane protein required for autophagy, and the SNARE protein Snc1. Atg9 is essential for autophagosome formation, and the TRAPPIII-dependent pathway may provide a source of Atg9 for PASs [73]. Hence, TRAPPIII may be localized to more than one site and may play both a direct and an indirect role in phagosome formation.
Less is known about mammalian TRAPP complexes. So far, two mammalian TRAPP complexes have been isolated [61,71,74]. The mammalian TRAPPII complex is associated with COPI-coated vesicular structures and binds to the COP coat adaptor subunit γ1COP [71,75], and as expected from the characteristics of the yeast TRAPP complexes, the mammalian TRAPPII complex has GEF activity for the mammalian Ypt1 homologue, Rab1 [71]. Consistent with a role for the mammalian TRAPPII complex in intra-Golgi trafficking, depletion of TRAPPC10 (mammalian homologue of yeast Trs130) resulted in an accumulation of vesicles adjacent to the early Golgi [71]. More recently, another mammalian TRAPP complex that interacts with COPII has been identified [76].
TGN trafficking
GARP is a heterotetrameric tethering factor that consists of four subunits—Vps51, Vps52, Vps53, and Vps54 [77]—and has been reported to be recruited to the TGN by the Rab6 GTPase in mammalian cells [78,79]. The GARP subunit Vps51 has been shown to interact with the Q-SNARE syntaxin 6 on the TGN and may promote SNARE-complex assembly [80]. There is also evidence that the Vps53 subunit of GARP binds to retrograde transport carriers [80], in line with the ability of this complex to act as a tether linking a transport carrier and acceptor membrane. However, the nature of the interaction between GARP and the transport carriers is currently not known. Importantly, depletion of Vps52 results in a block in endosome-to-TGN retrograde transport of several cargoes, such as CI-MPR, TGN46, and Shiga toxin [81]. In addition, an uncharacterized 164-kDa protein SHIP164 has been found to interact with GARP and syntaxin 6 [82]. Depletion of GARP subunits or overexpression of syntaxin 6 led to the accumulation of SHIP164 to endosomal structures. Intriguingly, several golgins have previously been implicated in the retrograde transport of such cargoes [14]. A model that consolidates these findings is one in which GARP cooperates with golgins at the TGN to tether endosome-derived vesicles.
The homodimeric golgins are found to be predominantly associated with the Golgi, and the golgins recruited specifically to TGN are called TGN golgins. Members of the TGN golgin family all contain the GRIP Golgi-targeting domain and are conserved among mammals to protozoans [83]. There are four mammalian GRIP domain golgins: p230/golgin-245, golgin-97, Golgi localized coiled-coil protein (GCC) 185, and GCC88 [15,83]. In particular, the role of the TGN golgins GCC88 and GCC185 in retrograde trafficking has been well documented. RNA interference (RNAi) silencing revealed that GCC88 and GCC185 were required for the retrograde transport of the TGN38 and Shiga toxin, respectively. In cells lacking GCC185, a specific block in retrograde transport of Shiga toxin and MPR, but not TGN38, was observed [84,85]. GCC185 has also been shown to be required for the transport of MPR by the Rab9-dependent pathway from the late endosomes [86]. In contrast, the silencing of GCC88 dramatically reduced endosome-to-TGN transport of TGN38 and MPR, whereas Shiga toxin was transported at a normal rate. Both TGN38 and MPR accumulated in the early endosome in GCC88-depleted cells, suggesting that GCC88 was required for efficient retrograde transport from the early endosomes [87]. Thus, TGN golgins might exhibit specificity in tethering different retrograde cargo along distinct endosome-to-TGN routes [87,88]. On the other hand, golgin-245/p230 and golgin-97 have instead been implicated in TGN-to-plasma membrane anterograde transport of cargo. Golgin-245/p230 and golgin-97 have been found to be associated with different transport carriers that emerge from the TGN [89,90] and have been shown to selectively regulate the transport of a number of cargoes [89-92]. Hence, the function of golgin-245/p230 and golgin-97 appears to be associated with biogenesis of transport carriers from the TGN or the tethering of TGN-derived transport carriers at their destination (or both). Some studies [93,94] have also implicated golgin-97 and golgin-245/p230 in the regulation of retrograde membrane trafficking between the endosomal system and TGN, although other studies did not confirm these findings [84,87]; it is possible that the perturbation in retrograde transport by these two golgins is an indirect consequence of altered expression levels.
Like the golgins of the Golgi stack, TGN golgins may link the processes of tethering and membrane fusion by interacting with SNARE components or contributing to the retention and assembly of SNAREs at the TGN. GCC185 has been shown to bind syntaxin 16 in vitro [95], and GCC88 is required for the retention of syntaxin 6 to the TGN; in GCC88-depleted cells, syntaxin 6 was located predominantly in dispersed cytoplasmic structures [87]. A number of golgins have been found to interact with components of the cytoskeleton [14]. Notably, GCC185 has been shown to interact with the microtubule-binding proteins CLASPs (cytoplasmic linker associated proteins) that selectively coat non-centrosomal microtubule seeds and are required for the formation of microtubules at the Golgi [96]. TGN golgins have been shown to bind to members of the Rab family: Rab2 (golgin-235 and GCC185), Rab6 (GCC88 and golgin-97), Rab19 (golgin-97), and Rab30 (golgin-97, golgin-245, and GCC88) [15]. Additionally, yeast two-hybrid assays have revealed interactions between GCC185 and various Golgi/TGN-localized Rabs [97]. Given the ability of TGN golgins to interact with such a large array of protein partners, the current view holds that TGN golgins act as membrane scaffold molecules, each generating a distinct membrane domain at the TGN.
Tethers along the endosome/lysosome pathway
Membrane fusion of early endosomes-EEA1
There are multiple sites of membrane fusion along the endocytic pathway. Endocytic vesicles derived from the plasma membrane are tethered to early endosomes by the binding of the coiled-coil protein early endosome antigen-1 (EEA1) [98]; the counterpart in yeast is Vac1[99]. EEA1 is a long coiled-coil dimer of Mr 162 kDa with two cysteine-rich, FYVE finger domains and two Rab5-binding domains at each end [100]. EEA1 associates with early endosomal membranes by the coordinated interaction with GTP-bound Rab5 and with phosphatidylinositol-3-phosphate (PtdIns[3]P) in the membrane of early endosomes via the FYVE finger domains [98,101-103]. Rab5 provides a positive feedback loop as it regulates the production of a local pool of PtdIns(3)P at the early endosomal membrane through its interaction with various phosphate kinases and phosphatases, resulting in the recruitment of more Rab5 and more EEA1 [104,105].
EEA1 was one of the first membrane tethers to be analyzed by using in vitro fusion assays. Homotypic endosome fusion assays directly demonstrated a role for EEA1 in endosome docking and fusion [98,106,107]. More recently, a synthetic endosome system has been reconstituted by using artificial proteoliposomes, and, in this fully reconstituted system, EEA1 was shown to be an essential component of the core machinery required for membrane fusion [11]. Together with GTP-Rab5, membrane-bound EEA1 forms a large complex that includes Rabaptin5-Rabex5, NSF, and the Q-SNARE syntaxin 13 [108]. The multiple interactions with EEA1 suggest that this membrane tether may function as a scaffold to bring together the various factors necessary to ensure productive and specific fusion of membranes with early endosomes.
Coordinating trafficking of the endosomes and lysosomes: CORVET and HOPS
The MTCs of the endosomes, HOPS and CORVET, have attracted considerable interest recently. Originally identified in yeast as a class of the Vps mutants, variants have now been identified in a range of higher eukaryotes [16], including humans [109]. CORVET and HOPS act sequentially and coordinate fusion events associated with the early/late endosomes and the lysosome. Both complexes are heterohexamers and share a common set of four Vps proteins (VPS11, VPS16, VPS18, and VPS33 [110,111]), and both interact with GTP-Rabs and SNAREs. Two isoforms of VPS16 and VPS33 have been described in metazoans [109,112-114]. Mutation in any of the core subunits results in loss of both CORVET and HOPS complexes and severe defects in endosomal biogenesis and vacuolar morphology in yeast [18,115]. Loss-of-function mutants in higher eukaryotes for HOPS or CORVET either are embryonic lethal or cause severe developmental defects [116-119]. Furthermore, mutations in VPS33 and 16 have been linked to diseases, including cancer [120,121]. In addition to the four common core subunits, both complexes contain two additional subunits (CORVET Vps8 and VPs3 and HOPS Vps41 and Vps39) that are unique to each complex and account for their specific Rab-binding properties [110,122,123]. CORVET is a Rab5 effector, whereas HOPS is a Rab7 effector. The interactions with Rabs and SNAREs account for their capacity to tether membranes and for their defined location on early or late endosomes/lysosomes, respectively.
Of the two tethers, HOPS is the better defined structurally and functionally. HOPS tethers membranes by binding to Rab7 (or the yeast homologue Ypt7) on late endosomes and vacuoles/lysosomes [124,125]. Recent structural data based on electron microscopic (EM) tomography of single particles [126] and analysis of protein interactions between HOPS subunits and Rab proteins [123,127,128] have provided a low resolution of the HOPS complex and the identification of the Rab-binding sites, offering insight into the tethering function of this complex. An elegant in vitro system has been established, and HOPS was shown to stimulate SNARE-mediated fusion of proteoliposomes in vitro [129,130]. Moreover, HOPS has been shown to bind SNAREs [130,131]. The fusion of proteoliposomes by using the in vitro system requires four yeast SNAREs: Ypt7p/Rab7, HOPS, the SNARE chaperones Sec17p/Sec18p, and regulatory phosphoinositide lipids [130]. The primary function of Ypt7 in this in vitro system is the recruitment of HOPS to membranes, and membrane fusion can occur independently of Ypt7 [129]. Interestingly, three of the SNAREs (Q-SNARE) are required for tethering and these findings are compatible with the concept of proofreading by the HOPS complex [12]. One of the subunits of HOPS, namely Vps33, is an SM (Sec1/Munc-18-like SNARE master) protein, a member of the family of proteins known to bind trans-SNARE complexes and direct their fusogenic activity [111,132]. Recent crystal structural analyses confirmed Vps33 to be an SM and identified the basis of the interaction of Vps33 with Vps16 and the HOPS complex [133,134]. Hence, HOPS is considered important to ensure the correct arrangement of the set of SNAREs and to promote fusogenic activity rather than lysis, which can occur in SNARE-mediated events in the absence of SM proteins [129,135]. The emerging view is that HOPS tethers by binding Ytp7p/Rab7 on one membrane and SNAREs on the other [124,129]. In addition to late endosome/lysosome fusion, HOPS interacts with components of the AP3-coated vesicles derived from the TGN and may promote the tethering of these AP3 vesicles with late endosomes/lysosomes [136].
Additional functions for HOPS have been suggested on the basis of identified effectors, including a role in autophagy and antigen presentation. HOPS interacts with components of the autophagosome machinery [137] and may regulate the fusion of autophagosomes with lysosomes, as for late endosome-lysosome fusion. Another HOPS effector is Arl8b that promotes the membrane transport of the major histocompatibility complex molecule CD1 to the lysosomes from the Golgi to mediate antigen binding and subsequent antigen presentation at the cell surface [138].
In comparison with the function of HOPS, that of CORVET is less well understood. CORVET functions upstream of HOPS, probably in early endosomes as it binds to GTP-Rab5, and may tether vesicles or act as a tether to promote homotypic endosome fusion, a process necessary for the generation of multivesicular bodies [16,139]. CORVET appears to act independently of the coiled-coil early endosome tether EEA1 (Vac1) [140]. Interestingly, there is evidence that CORVET and HOPS may be intimately aligned with the switch from Rab5 to Rab7. The pathway of this conversion involves the interaction of HOPS with the effector SAND1/Mon1, which displaces Rab5 GEF from membranes and moreover is a component of the Mon1-Ccz1 complex [141], which has been identified as the GEF for yeast Rab7 (Ypt7) [142]. Thus, HOPS may be tightly integrated with the switch from GTP-Rab5 to GTP-Rab7 during late endosome biogenesis.
Post-Golgi secretion
Exocyst is an MTC with eight subunits, and as for HOPS and CORVET, subunits were originally identified in screens for yeast mutants [143], in this case for a block in secretion, and subsequently defined as components of a membrane-associated complex required for exocytosis [9,144]. Exocyst is a member of the CATCHR complexes, which share conserved structural features and which include the previously discussed GARP COG and Dsl1. The octameric exocyst complex consists of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 and has been proposed to function as a tethering complex for transport carriers derived from the recycling endosomes [145,146] and the Golgi [147,148] to dock and fuse with the plasma membrane [9,149]. Binding partners have been identified for each of the exocyst subunits [144]. The Exo70 and Sec3 subunits interact with the lipid PtdIns(4,5)P2 and Rho GTP at the cell surface [150,151]. On the other hand, Sec15 has been shown to interact with Rab11 on transport vesicles [152], consistent with the known role of Rab11 in mediating the delivery of transport carriers to the cell surface. There is also emerging evidence indicating a direct interaction between exocyst subunits and Q-SNAREs and the SM protein, Sec1 [153]. Therefore, the exocyst may also be involved in regulating SNARE assembly, as for a number of the other membrane tethers.
The exocyst has been associated with a range of functions associated with the development in various organisms [144]. These functions include synapse formation, cilia development, and axon outgrowth, and a recent report describes the importance of the exocyst in branching morphogenesis in drosophila tracheal cells [154]. A key role of the exocyst in polarized delivery of membrane to the cell surface was identified originally in the earlier studies in yeast [155], and the range of functions now associated with the exocyst is likely to reflect the importance in the delivery of membrane and cargo to specific domains at the cell surface. However, it should be noted that not all secretory processes are dependent on exocyst; for example, synaptic vesicle fusion is an exocyst-independent process [156].
Conclusions
Membrane tethers are central for both the regulation of membrane traffic and biogenesis of organelles. It is now clear that both homodimeric coiled-coil tethers and MTC tethers interact not only with small G proteins (Rabs and Arls) but also with SNAREs and function to mediate the docking of transport carriers as well as, in some cases, the assembly of SNARE complexes. Reconstituted proteoliposome systems have been particularly instructive for providing direct evidence of a tethering function and for understanding the nature of interactions and the mechanism by which the tethers EEA1 and HOPS, in particular, regulate efficient and specific fusion events. More definitive evidence of a tethering function is required for many of the other membrane tethers using such reconstitution systems. In addition, many of the tethers have been studied to date in isolation of other membrane tethers, and it is important to better appreciate the network of interactions mediated by tethers and the potential coordination of their collective functions in vivo. The adoption of a more systems-based in vivo approach should help to connect the individual roles of membrane tethers to yield advances in our understanding of how the membrane tethers act as a network to coordinate membrane trafficking, membrane flux, and organelle biogenesis throughout the endomembrane system of the cell.
Acknowledgments
This work was supported by funding from the National Health and Medical Research Council of Australia and the Australian Research Council.
Abbreviations
- CATCHR
complexes associated with tethering containing helical rods
- COG
conserved oligomeric Golgi complex
- CORVET
class C core vacuole/endosome tethering
- EEA1
early endosome antigen-1
- ER
endoplasmic reticulum
- GARP
Golgi-associated retrograde protein complex
- GCC
Golgi localized coiled-coil protein
- GEF
guanine nucleotide exchange factor
- GMAP-210
Golgi microtubule-associated protein of 210 kDa
- HOPS
homotypic fusion and vacuole protein sorting
- MTC
multisubunit tethering complex
- NSF
N-ethylmaleimide-sensitive factor
- PAS
phagophore assembly site
- PtdIns(3)P
phosphatidylinositol-3-phosphate
- SM
Sec1/Munc-18-like soluble N-ethylmaleimide-sensitive factor attachment protein receptor master
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- TGN
trans-Golgi-network
- TRAPP
transport protein particle
- Vps
vacuolar protein sorting
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/6/74
References
- 1.Barlowe C. Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J Cell Biol. 1997;139:1097–108. doi: 10.1083/jcb.139.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao X, Ballew N, Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 1998;17:2156–65. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waters MG, Pfeffer SR. Membrane tethering in intracellular transport. Curr Opin Cell Biol. 1999;11:453–9. doi: 10.1016/S0955-0674(99)80065-9. [DOI] [PubMed] [Google Scholar]
- 4.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–82. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Gillingham AK, Munro S. Long coiled-coil proteins and membrane traffic. Biochim Biophys Acta. 2003;1641:71–85. doi: 10.1016/S0167-4889(03)00088-0. [DOI] [PubMed] [Google Scholar]
- 6.Derby MC, Gleeson PA. New insights into membrane trafficking and protein sorting. Int Rev Cytol. 2007;261:47–116. doi: 10.1016/S0074-7696(07)61002-X. [DOI] [PubMed] [Google Scholar]
- 7.Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- 8.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 9.Whyte James R C, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115:2627–37. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- 10.Wideman JG, Leung KF, Field MC, Dacks JB. The cell biology of the endocytic system from an evolutionary perspective. Cold Spring Harb Perspect Biol. 2014;6:a016998. doi: 10.1101/cshperspect.a016998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohya T, Miaczynska M, Coskun U, Lommer B, Runge A, Drechsel D, Kalaidzidis Y, Zerial M. Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes. Nature. 2009;459:1091–7. doi: 10.1038/nature08107. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1160876
- 12.Starai VJ, Hickey CM, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell. 2008;19:2500–8. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chia Pei Zhi Cheryl, Gleeson PA. The regulation of endosome-to-Golgi retrograde transport by tethers and scaffolds. Traffic. 2011;12:939–47. doi: 10.1111/j.1600-0854.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 14.Goud B, Gleeson PA. TGN golgins, Rabs and cytoskeleton: regulating the Golgi trafficking highways. Trends Cell Biol. 2010;20:329–36. doi: 10.1016/j.tcb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Sinka R, Gillingham AK, Kondylis V, Munro S. Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J Cell Biol. 2008;183:607–15. doi: 10.1083/jcb.200808018. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1127086
- 16.Balderhaar Henning J kleine, Ungermann C. CORVET and HOPS tethering complexes - coordinators of endosome and lysosome fusion. J Cell Sci. 2013;126:1307–16. doi: 10.1242/jcs.107805. [DOI] [PubMed] [Google Scholar]
- 17.Bröcker C, Engelbrecht-Vandré S, Ungermann C. Multisubunit tethering complexes and their role in membrane fusion. Curr Biol. 2010;20:R943–52. doi: 10.1016/j.cub.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Solinger JA, Spang A. Tethering complexes in the endocytic pathway: CORVET and HOPS. FEBS J. 2013;280:2743–57. doi: 10.1111/febs.12151. [DOI] [PubMed] [Google Scholar]
- 19.Sztul E, Lupashin V. Role of tethering factors in secretory membrane traffic. Am J Physiol Cell Physiol. 2006;290:C11–26. doi: 10.1152/ajpcell.00293.2005. [DOI] [PubMed] [Google Scholar]
- 20.Yu I, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–56. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- 21.Cottam NP, Ungar D. Retrograde vesicle transport in the Golgi. Protoplasma. 2012;249:943–55. doi: 10.1007/s00709-011-0361-7. [DOI] [PubMed] [Google Scholar]
- 22.Munro S. The golgin coiled-coil proteins of the Golgi apparatus. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez IB, Lowe M. Golgins and GRASPs: holding the Golgi together. Semin Cell Dev Biol. 2009;20:770–9. doi: 10.1016/j.semcdb.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Barr FA, Short B. Golgins in the structure and dynamics of the Golgi apparatus. Curr Opin Cell Biol. 2003;15:405–13. doi: 10.1016/S0955-0674(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 25.Grabski R, Hay J, Sztul E. Tethering factor P115: a new model for tether-SNARE interactions. Bioarchitecture. 2012;2:175–80. doi: 10.4161/bioa.21702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson DS, Alvarez C, Gao YS, García-Mata R, Fialkowski E, Sztul E. The membrane transport factor TAP/p115 cycles between the Golgi and earlier secretory compartments and contains distinct domains required for its localization and function. J Cell Biol. 1998;143:319–31. doi: 10.1083/jcb.143.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waters MG, Clary DO, Rothman JE. A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J Cell Biol. 1992;118:1015–26. doi: 10.1083/jcb.118.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura N, Lowe M, Levine TP, Rabouille C, Warren G. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell. 1997;89:445–55. doi: 10.1016/S0092-8674(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 29.Sönnichsen B, Lowe M, Levine T, Jämsä E, Dirac-Svejstrup B, Warren G. A role for giantin in docking COPI vesicles to Golgi membranes. J Cell Biol. 1998;140:1013–21. doi: 10.1083/jcb.140.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barr FA, Nakamura N, Warren G. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J. 1998;17:3258–68. doi: 10.1093/emboj/17.12.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91:253–62. doi: 10.1016/S0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- 32.Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 2000;289:444–8. doi: 10.1126/science.289.5478.444. [DOI] [PubMed] [Google Scholar]
- 33.Moyer BD, Allan BB, Balch WE. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis--Golgi tethering. Traffic. 2001;2:268–76. doi: 10.1034/j.1600-0854.2001.1o007.x. [DOI] [PubMed] [Google Scholar]
- 34.Grabski R, Balklava Z, Wyrozumska P, Szul T, Brandon E, Alvarez C, Holloway ZG, Sztul E. Identification of a functional domain within the p115 tethering factor that is required for Golgi ribbon assembly and membrane trafficking. J Cell Sci. 2012;125:1896–909. doi: 10.1242/jcs.090571. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13884956
- 35.Kondylis V, Pizette S, Rabouille C. The early secretory pathway in development: a tale of proteins and mRNAs. Semin Cell Dev Biol. 2009;20:817–27. doi: 10.1016/j.semcdb.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Puthenveedu MA, Linstedt AD. Gene replacement reveals that p115/SNARE interactions are essential for Golgi biogenesis. Proc Natl Acad Sci USA. 2004;101:1253–6. doi: 10.1073/pnas.0306373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasile E, Perez T, Nakamura N, Krieger M. Structural integrity of the Golgi is temperature sensitive in conditional-lethal mutants with no detectable GM130. Traffic. 2003;4:254–72. doi: 10.1034/j.1600-0854.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 38.Gillingham AK, Pfeifer AC, Munro S. CASP, the alternatively spliced product of the gene encoding the CCAAT-displacement protein transcription factor, is a Golgi membrane protein related to giantin. Mol Biol Cell. 2002;13:3761–74. doi: 10.1091/mbc.E02-06-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malsam J, Satoh A, Pelletier L, Warren G. Golgin tethers define subpopulations of COPI vesicles. Science. 2005;307:1095–8. doi: 10.1126/science.1108061. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1024178
- 40.Infante C, Ramos-Morales F, Fedriani C, Bornens M, Rios RM. GMAP-210, A cis-Golgi network-associated protein, is a minus end microtubule-binding protein. J Cell Biol. 1999;145:83–98. doi: 10.1083/jcb.145.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pernet-Gallay K, Antony C, Johannes L, Bornens M, Goud B, Rios RM. The overexpression of GMAP-210 blocks anterograde and retrograde transport between the ER and the Golgi apparatus. Traffic. 2002;3:822–32. doi: 10.1034/j.1600-0854.2002.31107.x. [DOI] [PubMed] [Google Scholar]
- 42.Drin G, Morello V, Casella J, Gounon P, Antonny B. Asymmetric tethering of flat and curved lipid membranes by a golgin. Science. 2008;320:670–3. doi: 10.1126/science.1155821. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1109106
- 43.Mesmin B, Drin G, Levi S, Rawet M, Cassel D, Bigay J, Antonny B. Two lipid-packing sensor motifs contribute to the sensitivity of ArfGAP1 to membrane curvature. Biochemistry. 2007;46:1779–90. doi: 10.1021/bi062288w. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1067718
- 44.Lupashin V, Sztul E. Golgi tethering factors. Biochim Biophys Acta. 2005;1744:325–39. doi: 10.1016/j.bbamcr.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Ungar D, Oka T, Brittle EE, Vasile E, Lupashin VV, Chatterton JE, Heuser JE, Krieger M, Waters MG. Characterization of a mammalian Golgi-localized protein complex, COG, that is required for normal Golgi morphology and function. J Cell Biol. 2002;157:405–15. doi: 10.1083/jcb.200202016. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1006561
- 46.Whyte JR, Munro S. The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev Cell. 2001;1:527–37. doi: 10.1016/S1534-5807(01)00063-6. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1002397
- 47.Willett R, Ungar D, Lupashin V. The Golgi puppet master: COG complex at center stage of membrane trafficking interactions. Histochem Cell Biol. 2013;140:271–83. doi: 10.1007/s00418-013-1117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suvorova ES, Duden R, Lupashin VV. The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins. J Cell Biol. 2002;157:631–43. doi: 10.1083/jcb.200111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zolov SN, Lupashin VV. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J Cell Biol. 2005;168:747–59. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1024218
- 50.Kraynack BA, Chan A, Rosenthal E, Essid M, Umansky B, Waters MG, Schmitt HD. Dsl1p, Tip20p, and the novel Dsl3(Sec39) protein are required for the stability of the Q/t-SNARE complex at the endoplasmic reticulum in yeast. Mol Biol Cell. 2005;16:3963–77. doi: 10.1091/mbc.E05-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripathi A, Ren Y, Jeffrey PD, Hughson FM. Structural characterization of Tip20p and Dsl1p, subunits of the Dsl1p vesicle tethering complex. Nat Struct Mol Biol. 2009;16:114–23. doi: 10.1038/nsmb.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1168258
- 52.Andag U, Schmitt HD. Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J Biol Chem. 2003;278:51722–34. doi: 10.1074/jbc.M308740200. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1016687
- 53.Zink S, Wenzel D, Wurm CA, Schmitt HD. A link between ER tethering and COP-I vesicle uncoating. Dev Cell. 2009;17:403–16. doi: 10.1016/j.devcel.2009.07.012. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1165363
- 54.Ren Y, Yip CK, Tripathi A, Huie D, Jeffrey PD, Walz T, Hughson FM. A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1. Cell. 2009;139:1119–29. doi: 10.1016/j.cell.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1346957
- 55.Meiringer Christoph T A, Rethmeier R, Auffarth K, Wilson J, Perz A, Barlowe C, Schmitt HD, Ungermann C. The Dsl1 protein tethering complex is a resident endoplasmic reticulum complex, which interacts with five soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptors (SNAREs): implications for fusion and fusion regulation. J Biol Chem. 2011;286:25039–46. doi: 10.1074/jbc.M110.215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aoki T, Ichimura S, Itoh A, Kuramoto M, Shinkawa T, Isobe T, Tagaya M. Identification of the neuroblastoma-amplified gene product as a component of the syntaxin 18 complex implicated in Golgi-to-endoplasmic reticulum retrograde transport. Mol Biol Cell. 2009;20:2639–49. doi: 10.1091/mbc.E08-11-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirose H, Arasaki K, Dohmae N, Takio K, Hatsuzawa K, Nagahama M, Tani K, Yamamoto A, Tohyama M, Tagaya M. Implication of ZW10 in membrane trafficking between the endoplasmic reticulum and Golgi. EMBO J. 2004;23:1267–78. doi: 10.1038/sj.emboj.7600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arasaki K, Takagi D, Furuno A, Sohda M, Misumi Y, Wakana Y, Inoue H, Tagaya M. A new role for RINT-1 in SNARE complex assembly at the trans-Golgi network in coordination with the COG complex. Mol Biol Cell. 2013;24:2907–17. doi: 10.1091/mbc.E13-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718047167
- 59.Jiang Y, Scarpa A, Zhang L, Stone S, Feliciano E, Ferro-Novick S. A high copy suppressor screen reveals genetic interactions between BET3 and a new gene. Evidence for a novel complex in ER-to-Golgi transport. Genetics. 1998;149:833–41. doi: 10.1093/genetics/149.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim Y, Raunser S, Munger C, Wagner J, Song Y, Cygler M, Walz T, Oh B, Sacher M. The architecture of the multisubunit TRAPP I complex suggests a model for vesicle tethering. Cell. 2006;127:817–30. doi: 10.1016/j.cell.2006.09.029. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529680
- 61.Sacher M, Barrowman J, Schieltz D, Yates JR, Ferro-Novick S. Identification and characterization of five new subunits of TRAPP. Eur J Cell Biol. 2000;79:71–80. doi: 10.1078/S0171-9335(04)70009-6. [DOI] [PubMed] [Google Scholar]
- 62.Sacher M, Jiang Y, Barrowman J, Scarpa A, Burston J, Zhang L, Schieltz D, Yates JR, Abeliovich H, Ferro-Novick S. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 1998;17:2494–503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sacher M, Barrowman J, Wang W, Horecka J, Zhang Y, Pypaert M, Ferro-Novick S. TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol Cell. 2001;7:433–42. doi: 10.1016/S1097-2765(01)00190-3. [DOI] [PubMed] [Google Scholar]
- 64.Lynch-Day MA, Bhandari D, Menon S, Huang J, Cai H, Bartholomew CR, Brumell JH, Ferro-Novick S, Klionsky DJ. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci USA. 2010;107:7811–6. doi: 10.1073/pnas.1000063107. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/3197958
- 65.Montpetit B, Conibear E. Identification of the novel TRAPP associated protein Tca17. Traffic. 2009;10:713–23. doi: 10.1111/j.1600-0854.2009.00895.x. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529682
- 66.Jones S, Newman C, Liu F, Segev N. The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Mol Biol Cell. 2000;11:4403–11. doi: 10.1091/mbc.11.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529683
- 67.Cai Y, Chin HF, Lazarova D, Menon S, Fu C, Cai H, Sclafani A, Rodgers DW, De La Cruz, Enrique M, Ferro-Novick S, Reinisch KM. The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes. Cell. 2008;133:1202–13. doi: 10.1016/j.cell.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529684
- 68.Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay JC, Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–4. doi: 10.1038/nature05527. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1066779
- 69.Sacher M, Ferro-Novick S. Purification of TRAPP from Saccharomyces cerevisiae and identification of its mammalian counterpart. Meth Enzymol. 2001;329:234–41. doi: 10.1016/S0076-6879(01)29083-1. [DOI] [PubMed] [Google Scholar]
- 70.Cai H, Zhang Y, Pypaert M, Walker L, Ferro-Novick S. Mutants in trs120 disrupt traffic from the early endosome to the late Golgi. J Cell Biol. 2005;171:823–33. doi: 10.1083/jcb.200505145. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1030690
- 71.Yamasaki A, Menon S, Yu S, Barrowman J, Meerloo T, Oorschot V, Klumperman J, Satoh A, Ferro-Novick S. mTrs130 is a component of a mammalian TRAPPII complex, a Rab1 GEF that binds to COPI-coated vesicles. Mol Biol Cell. 2009;20:4205–15. doi: 10.1091/mbc.E09-05-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1163370
- 72.Tan D, Cai Y, Wang J, Zhang J, Menon S, Chou H, Ferro-Novick S, Reinisch KM, Walz T. The EM structure of the TRAPPIII complex leads to the identification of a requirement for COPII vesicles on the macroautophagy pathway. Proc Natl Acad Sci USA. 2013;110:19432–7. doi: 10.1073/pnas.1316356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shirahama-Noda K, Kira S, Yoshimori T, Noda T. TRAPPIII is responsible for vesicular transport from early endosomes to Golgi, facilitating Atg9 cycling in autophagy. J Cell Sci. 2013;126:4963–73. doi: 10.1242/jcs.131318. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718089076
- 74.Loh E, Peter F, Subramaniam VN, Hong W. Mammalian Bet3 functions as a cytosolic factor participating in transport from the ER to the Golgi apparatus. J Cell Sci. 2005;118:1209–22. doi: 10.1242/jcs.01723. [DOI] [PubMed] [Google Scholar]
- 75.Gwynn B, Smith RS, Rowe LB, Taylor BA, Peters LL. A mouse TRAPP-related protein is involved in pigmentation. Genomics. 2006;88:196–203. doi: 10.1016/j.ygeno.2006.04.002. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529685
- 76.Bassik MC, Kampmann M, Lebbink RJ, Wang S, Hein MY, Poser I, Weibezahn J, Horlbeck MA, Chen S, Mann M, Hyman AA, Leproust EM, McManus MT, Weissman JS. A systematic mammalian genetic interaction map reveals pathways underlying ricin susceptibility. Cell. 2013;152:909–22. doi: 10.1016/j.cell.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717985933
- 77.Conibear E, Stevens TH. Vps52p, Vps53p, and Vps54p form a novel multisubunit complex required for protein sorting at the yeast late Golgi. Mol Biol Cell. 2000;11:305–23. doi: 10.1091/mbc.11.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liewen H, Meinhold-Heerlein I, Oliveira V, Schwarzenbacher R, Luo G, Wadle A, Jung M, Pfreundschuh M, Stenner-Liewen F. Characterization of the human GARP (Golgi associated retrograde protein) complex. Exp Cell Res. 2005;306:24–34. doi: 10.1016/j.yexcr.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 79.Siniossoglou S, Pelham HR. An effector of Ypt6p binds the SNARE Tlg1p and mediates selective fusion of vesicles with late Golgi membranes. EMBO J. 2001;20:5991–8. doi: 10.1093/emboj/20.21.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pérez-Victoria FJ, Bonifacino JS. Dual roles of the mammalian GARP complex in tethering and SNARE complex assembly at the trans-golgi network. Mol Cell Biol. 2009;29:5251–63. doi: 10.1128/MCB.00495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529686
- 81.Pérez-Victoria FJ, Mardones GA, Bonifacino JS. Requirement of the human GARP complex for mannose 6-phosphate-receptor-dependent sorting of cathepsin D to lysosomes. Mol Biol Cell. 2008;19:2350–62. doi: 10.1091/mbc.E07-11-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529687
- 82.Otto GP, Razi M, Morvan J, Stenner F, Tooze SA. A novel syntaxin 6-interacting protein, SHIP164, regulates syntaxin 6-dependent sorting from early endosomes. Traffic. 2010;11:688–705. doi: 10.1111/j.1600-0854.2010.01049.x. [DOI] [PubMed] [Google Scholar]
- 83.Gleeson PA, Lock JG, Luke MR, Stow JL. Domains of the TGN: coats, tethers and G proteins. Traffic. 2004;5:315–26. doi: 10.1111/j.1398-9219.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 84.Derby MC, Lieu ZZ, Brown D, Stow JL, Goud B, Gleeson PA. The trans-Golgi network golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure. Traffic. 2007;8:758–73. doi: 10.1111/j.1600-0854.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 85.Reddy JV, Burguete AS, Sridevi K, Ganley IG, Nottingham RM, Pfeffer SR. A functional role for the GCC185 golgin in mannose 6-phosphate receptor recycling. Mol Biol Cell. 2006;17:4353–63. doi: 10.1091/mbc.E06-02-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 1993;12:677–82. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lieu ZZ, Derby MC, Teasdale RD, Hart C, Gunn P, Gleeson PA. The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network. Mol Biol Cell. 2007;18:4979–91. doi: 10.1091/mbc.E07-06-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lieu ZZ, Gleeson PA. Identification of different itineraries and retromer components for endosome-to-Golgi transport of TGN38 and Shiga toxin. Eur J Cell Biol. 2010;89:379–93. doi: 10.1016/j.ejcb.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 89.Lieu ZZ, Lock JG, Hammond LA, La Gruta Nicole L, Stow JL, Gleeson PA. A trans-Golgi network golgin is required for the regulated secretion of TNF in activated macrophages in vivo. Proc Natl Acad Sci USA. 2008;105:3351–6. doi: 10.1073/pnas.0800137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lock JG, Hammond LA, Houghton F, Gleeson PA, Stow JL. E-cadherin transport from the trans-Golgi network in tubulovesicular carriers is selectively regulated by golgin-97. Traffic. 2005;6:1142–56. doi: 10.1111/j.1600-0854.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 91.Brémond A, Meynet O, Mahiddine K, Coito S, Tichet M, Scotlandi K, Breittmayer J, Gounon P, Gleeson PA, Bernard A, Bernard G. Regulation of HLA class I surface expression requires CD99 and p230/golgin-245 interaction. Blood. 2009;113:347–57. doi: 10.1182/blood-2008-02-137745. [DOI] [PubMed] [Google Scholar]
- 92.Kakinuma T, Ichikawa H, Tsukada Y, Nakamura T, Toh B. Interaction between p230 and MACF1 is associated with transport of a glycosyl phosphatidyl inositol-anchored protein from the Golgi to the cell periphery. Exp Cell Res. 2004;298:388–98. doi: 10.1016/j.yexcr.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 93.Lu L, Tai G, Hong W. Autoantigen Golgin-97, an effector of Arl1 GTPase, participates in traffic from the endosome to the trans-golgi network. Mol Biol Cell. 2004;15:4426–43. doi: 10.1091/mbc.E03-12-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoshino A, Setty Subba Rao Gangi, Poynton C, Whiteman EL, Saint-Pol A, Burd CG, Johannes L, Holzbaur EL, Koval M, McCaffery JM, Marks MS. tGolgin-1 (p230, golgin-245) modulates Shiga-toxin transport to the Golgi and Golgi motility towards the microtubule-organizing centre. J Cell Sci. 2005;118:2279–93. doi: 10.1242/jcs.02358. [DOI] [PubMed] [Google Scholar]
- 95.Ganley IG, Espinosa E, Pfeffer SR. A syntaxin 10-SNARE complex distinguishes two distinct transport routes from endosomes to the trans-Golgi in human cells. J Cell Biol. 2008;180:159–72. doi: 10.1083/jcb.200707136. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1104233
- 96.Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia Ana R R, McLeod IX, Yates JR, Maiato H, Khodjakov A, Akhmanova A, Kaverina I. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell. 2007;12:917–30. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1087850
- 97.Hayes GL, Brown FC, Haas AK, Nottingham RM, Barr FA, Pfeffer SR. Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans-Golgi. Mol Biol Cell. 2009;20:209–17. doi: 10.1091/mbc.E08-07-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529688
- 98.Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–5. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- 99.Peterson MR, Burd CG, Emr SD. Vac1p coordinates Rab and phosphatidylinositol 3-kinase signaling in Vps45p-dependent vesicle docking/fusion at the endosome. Curr Biol. 1999;9:159–62. doi: 10.1016/S0960-9822(99)80071-2. [DOI] [PubMed] [Google Scholar]
- 100.Callaghan J, Simonsen A, Gaullier JM, Toh BH, Stenmark H. The endosome fusion regulator early-endosomal autoantigen 1 (EEA1) is a dimer. Biochem J. 1999;338(Pt 2):539–43. doi: 10.1042/0264-6021:3380539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stenmark H, Aasland R, Toh BH, D'Arrigo A. Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J Biol Chem. 1996;271:24048–54. doi: 10.1074/jbc.271.39.24048. [DOI] [PubMed] [Google Scholar]
- 102.Burd CG, Emr SD. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol Cell. 1998;2:157–62. doi: 10.1016/S1097-2765(00)80125-2. [DOI] [PubMed] [Google Scholar]
- 103.Kutateladze TG, Ogburn KD, Watson WT, Beer T de, Emr SD, Burd CG, Overduin M. Phosphatidylinositol 3-phosphate recognition by the FYVE domain. Mol Cell. 1999;3:805–11. doi: 10.1016/S1097-2765(01)80013-7. [DOI] [PubMed] [Google Scholar]
- 104.Murray JT, Panaretou C, Stenmark H, Miaczynska M, Backer JM. Role of Rab5 in the recruitment of hVps34/p150 to the early endosome. Traffic. 2002;3:416–27. doi: 10.1034/j.1600-0854.2002.30605.x. [DOI] [PubMed] [Google Scholar]
- 105.Shin H, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR, Modregger J, Uttenweiler-Joseph S, Wilm M, Nystuen A, Frankel WN, Solimena M, Camilli P de, Zerial M. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol. 2005;170:607–18. doi: 10.1083/jcb.200505128. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1027488
- 106.Mills IG, Jones AT, Clague MJ. Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr Biol. 1998;8:881–4. doi: 10.1016/S0960-9822(07)00351-X. [DOI] [PubMed] [Google Scholar]
- 107.Simonsen A, Lippé R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–8. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 108.McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98:377–86. doi: 10.1016/S0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- 109.Huizing M, Didier A, Walenta J, Anikster Y, Gahl WA, Krämer H. Molecular cloning and characterization of human VPS18, VPS 11, VPS16, and VPS33. Gene. 2001;264:241–7. doi: 10.1016/S0378-1119(01)00333-X. [DOI] [PubMed] [Google Scholar]
- 110.Peplowska K, Markgraf DF, Ostrowicz CW, Bange G, Ungermann C. The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endo-lysosomal biogenesis. Dev Cell. 2007;12:739–50. doi: 10.1016/j.devcel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 111.Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97:9402–7. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pulipparacharuvil S, Akbar MA, Ray S, Sevrioukov EA, Haberman AS, Rohrer J, Krämer H. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J Cell Sci. 2005;118:3663–73. doi: 10.1242/jcs.02502. [DOI] [PubMed] [Google Scholar]
- 113.Gissen P, Johnson CA, Gentle D, Hurst LD, Doherty AJ, O'Kane CJ, Kelly DA, Maher ER. Comparative evolutionary analysis of VPS33 homologues: genetic and functional insights. Hum Mol Genet. 2005;14:1261–70. doi: 10.1093/hmg/ddi137. [DOI] [PubMed] [Google Scholar]
- 114.Zhu G, Salazar G, Zlatic SA, Fiza B, Doucette MM, Heilman CJ, Levey AI, Faundez V, L'hernault SW. SPE-39 family proteins interact with the HOPS complex and function in lysosomal delivery. Mol Biol Cell. 2009;20:1223–40. doi: 10.1091/mbc.E08-07-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aoyama M, Sun-Wada G, Yamamoto A, Yamamoto M, Hamada H, Wada Y. Spatial restriction of bone morphogenetic protein signaling in mouse gastrula through the mVam2-dependent endocytic pathway. Dev Cell. 2012;22:1163–75. doi: 10.1016/j.devcel.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 117.Kawamura N, Sun-Wada G, Aoyama M, Harada A, Takasuga S, Sasaki T, Wada Y. Delivery of endosomes to lysosomes via microautophagy in the visceral endoderm of mouse embryos. Nat Commun. 2012;3:1071. doi: 10.1038/ncomms2069. [DOI] [PubMed] [Google Scholar]
- 118.Messler S, Kropp S, Episkopou V, Felici A, Würthner J, Lemke R, Jerabek-Willemsen M, Willecke R, Scheu S, Pfeffer K, Wurthner JU. The TGF-β signaling modulators TRAP1/TGFBRAP1 and VPS39/Vam6/TLP are essential for early embryonic development. Immunobiology. 2011;216:343–50. doi: 10.1016/j.imbio.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 119.Schonthaler HB, Fleisch VC, Biehlmaier O, Makhankov Y, Rinner O, Bahadori R, Geisler R, Schwarz H, Neuhauss Stephan C F, Dahm R. The zebrafish mutant lbk/vam6 resembles human multisystemic disorders caused by aberrant trafficking of endosomal vesicles. Development. 2008;135:387–99. doi: 10.1242/dev.006098. [DOI] [PubMed] [Google Scholar]
- 120.Gissen P, Johnson CA, Morgan NV, Stapelbroek JM, Forshew T, Cooper WN, McKiernan PJ, Klomp Leo W J, Morris Andrew A M, Wraith JE, McClean P, Lynch SA, Thompson RJ, Lo B, Quarrell OW, Di Rocco M, Trembath RC, Mandel H, Wali S, Karet FE, Knisely AS, Houwen Roderick H J, Kelly DA, Maher ER. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat Genet. 2004;36:400–4. doi: 10.1038/ng1325. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718529689
- 121.Roy D, Sin S, Damania B, Dittmer DP. Tumor suppressor genes FHIT and WWOX are deleted in primary effusion lymphoma (PEL) cell lines. Blood. 2011;118:e32–9. doi: 10.1182/blood-2010-12-323659. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718529690
- 122.Price A, Wickner W, Ungermann C. Proteins needed for vesicle budding from the Golgi complex are also required for the docking step of homotypic vacuole fusion. J Cell Biol. 2000;148:1223–9. doi: 10.1083/jcb.148.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol. 2000;151:551–62. doi: 10.1083/jcb.151.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hickey CM, Stroupe C, Wickner W. The major role of the Rab Ypt7p in vacuole fusion is supporting HOPS membrane association. J Biol Chem. 2009;284:16118–25. doi: 10.1074/jbc.M109.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1159475
- 125.Zick M, Wickner W. Phosphorylation of the effector complex HOPS by the vacuolar kinase Yck3p confers Rab nucleotide specificity for vacuole docking and fusion. Mol Biol Cell. 2012;23:3429–37. doi: 10.1091/mbc.E12-04-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bröcker C, Kuhlee A, Gatsogiannis C, Balderhaar Henning J kleine, Hönscher C, Engelbrecht-Vandré S, Ungermann C, Raunser S. Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting (HOPS) tethering complex. Proc Natl Acad Sci USA. 2012;109:1991–6. doi: 10.1073/pnas.1117797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ostrowicz CW, Bröcker C, Ahnert F, Nordmann M, Lachmann J, Peplowska K, Perz A, Auffarth K, Engelbrecht-Vandré S, Ungermann C. Defined subunit arrangement and rab interactions are required for functionality of the HOPS tethering complex. Traffic. 2010;11:1334–46. doi: 10.1111/j.1600-0854.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- 128.Plemel RL, Lobingier BT, Brett CL, Angers CG, Nickerson DP, Paulsel A, Sprague D, Merz AJ. Subunit organization and Rab interactions of Vps-C protein complexes that control endolysosomal membrane traffic. Mol Biol Cell. 2011;22:1353–63. doi: 10.1091/mbc.E10-03-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stroupe C, Hickey CM, Mima J, Burfeind AS, Wickner W. Minimal membrane docking requirements revealed by reconstitution of Rab GTPase-dependent membrane fusion from purified components. Proc Natl Acad Sci USA. 2009;106:17626–33. doi: 10.1073/pnas.0903801106. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1260962
- 130.Mima J, Hickey CM, Xu H, Jun Y, Wickner W. Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. EMBO J. 2008;27:2031–42. doi: 10.1038/emboj.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1119878
- 131.Collins KM, Thorngren NL, Fratti RA, Wickner WT. Sec17p and HOPS, in distinct SNARE complexes, mediate SNARE complex disruption or assembly for fusion. EMBO J. 2005;24:1775–86. doi: 10.1038/sj.emboj.7600658. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1026025
- 132.Pieren M, Schmidt A, Mayer A. The SM protein Vps33 and the t-SNARE H(abc) domain promote fusion pore opening. Nat Struct Mol Biol. 2010;17:710–7. doi: 10.1038/nsmb.1809. [DOI] [PubMed] [Google Scholar]
- 133.Baker RW, Jeffrey PD, Hughson FM. Crystal Structures of the Sec1/Munc18 (SM) Protein Vps33, Alone and Bound to the Homotypic Fusion and Vacuolar Protein Sorting (HOPS) Subunit Vps16*. PLoS ONE. 2013;8:e67409. doi: 10.1371/journal.pone.0067409. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718030146
- 134.Graham SC, Wartosch L, Gray SR, Scourfield EJ, Deane JE, Luzio JP, Owen DJ. Structural basis of Vps33A recruitment to the human HOPS complex by Vps16. Proc Natl Acad Sci USA. 2013;110:13345–50. doi: 10.1073/pnas.1307074110. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718052352
- 135.Dennison SM, Bowen ME, Brunger AT, Lentz BR. Neuronal SNAREs do not trigger fusion between synthetic membranes but do promote PEG-mediated membrane fusion. Biophys J. 2006;90:1661–75. doi: 10.1529/biophysj.105.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1031093
- 136.Angers CG, Merz AJ. HOPS interacts with Apl5 at the vacuole membrane and is required for consumption of AP-3 transport vesicles. Mol Biol Cell. 2009;20:4563–74. doi: 10.1091/mbc.E09-04-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718529691
- 137.Liang C, Lee J, Inn K, Gack MU, Li Q, Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C, Jung JU. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol. 2008;10:776–87. doi: 10.1038/ncb1740. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1116294
- 138.Garg S, Sharma M, Ung C, Tuli A, Barral DC, Hava DL, Veerapen N, Besra GS, Hacohen N, Brenner MB. Lysosomal trafficking, antigen presentation, and microbial killing are controlled by the Arf-like GTPase Arl8b. Immunity. 2011;35:182–93. doi: 10.1016/j.immuni.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13294020
- 139.Balderhaar Henning J kleine, Lachmann J, Yavavli E, Bröcker C, Lürick A, Ungermann C. The CORVET complex promotes tethering and fusion of Rab5/Vps21-positive membranes. Proc Natl Acad Sci USA. 2013;110:3823–8. doi: 10.1073/pnas.1221785110. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717979705
- 140.Cabrera M, Arlt H, Epp N, Lachmann J, Griffith J, Perz A, Reggiori F, Ungermann C. Functional separation of endosomal fusion factors and the class C core vacuole/endosome tethering (CORVET) complex in endosome biogenesis. J Biol Chem. 2013;288:5166–75. doi: 10.1074/jbc.M112.431536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/4015972
- 142.Nordmann M, Cabrera M, Perz A, Bröcker C, Ostrowicz C, Engelbrecht-Vandré S, Ungermann C. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol. 2010;20:1654–9. doi: 10.1016/j.cub.2010.08.002. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/5326957
- 143.Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–15. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 144.Heider MR, Munson M. Exorcising the exocyst complex. Traffic. 2012;13:898–907. doi: 10.1111/j.1600-0854.2012.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Prigent M, Dubois T, Raposo G, Derrien V, Tenza D, Rossé C, Camonis J, Chavrier P. ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J Cell Biol. 2003;163:1111–21. doi: 10.1083/jcb.200305029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oztan A, Silvis M, Weisz OA, Bradbury NA, Hsu S, Goldenring JR, Yeaman C, Apodaca G. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol Biol Cell. 2007;18:3978–92. doi: 10.1091/mbc.E07-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yeaman C, Grindstaff KK, Wright JR, Nelson WJ. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J Cell Biol. 2001;155:593–604. doi: 10.1083/jcb.200107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ponnambalam S. Protein secretion and the Golgi apparatus. Mol Membr Biol. 2003;20:97–8. doi: 10.1080/096878031000104935. [DOI] [PubMed] [Google Scholar]
- 149.Grote E, Carr CM, Novick PJ. Ordering the final events in yeast exocytosis. J Cell Biol. 2000;151:439–52. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol. 2008;180:145–58. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 2007;26:4053–65. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang X, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004;279:43027–34. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
- 153.Sivaram Mylavarapu V S, Furgason Melonnie L M, Brewer DN, Munson M. The structure of the exocyst subunit Sec6p defines a conserved architecture with diverse roles. Nat Struct Mol Biol. 2006;13:555–6. doi: 10.1038/nsmb1096. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1033940
- 154.Jones TA, Nikolova LS, Schjelderup A, Metzstein MM. Exocyst-mediated membrane trafficking is required for branch outgrowth in Drosophila tracheal terminal cells. Dev Biol. 2014;390:41–50. doi: 10.1016/j.ydbio.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718305051
- 155.TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–94. [PMC free article] [PubMed] [Google Scholar]
- 156.Murthy M, Garza D, Scheller RH, Schwarz TL. Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron. 2003;37:433–47. doi: 10.1016/S0896-6273(03)00031-X. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1011944