Abstract
Background
Estimation suggests that at least 4 million people die, annually, as a result of chronic respiratory disease (CRD). The Global Alliance against Chronic Respiratory Diseases (GARD) was formed following a mandate from the World Health Assembly to address this serious and growing health problem.
Objectives
To investigate the prevalence of CRD in Russian symptomatic patients and to evaluate the frequency of major risk factors for CRD in Russia.
Methods
A cross-sectional, population-based epidemiological study using the GARD questionnaire on adults from 12 regions of the Russian Federation. Common respiratory symptoms and risk factors were recorded. Spirometry was performed in respondents with suspected CRD. Allergic rhinitis (AR) and chronic bronchitis (CB) were defined by the presence of related symptoms according to the Allergic Rhinitis and its Impact on Asthma and the Global Initiative for Obstructive Lung Disease guidelines; asthma was defined based on disease symptoms; chronic obstructive pulmonary disease (COPD) was defined as a post-bronchodilator forced expiratory volume per 1 second/forced vital capacity ratio <0.7 in symptomatic patients, following the Global Initiative for Obstructive Lung Disease guidelines.
Results
The number of questionnaires completed was 7,164 (mean age 43.4 years; 57.2% female). The prevalence of asthma symptoms was 25.7%, AR 18.2%, and CB 8.6%. Based on patient self-reported diagnosis, 6.9% had asthma, 6.5% AR, and 22.2% CB. The prevalence of COPD based on spirometry in patients with respiratory symptoms was estimated as 21.8%.
Conclusion
The prevalence of respiratory diseases and risk factors was high in Russia when compared to available data. For bronchial asthma and AR, the prevalence for related symptoms was higher than self-reported previous diagnosis.
Keywords: chronic respiratory diseases, GARD, Russia, prevalence
Introduction
Chronic respiratory diseases (CRDs) are recognized as being the major cause for premature death in adult populations worldwide. Preventable and treatable CRDs include chronic obstructive pulmonary disease (COPD), asthma, and respiratory allergies.1
In general, the prevalence of CRD is increasing everywhere and in particular amongst children and the elderly.1 The burden of CRD has major adverse effects on the quality of life and disability of affected individuals. It has been predicted that the global burden of CRD will increase considerably in the future, even though many preventable CRDs can be controlled with adequate management in both developed2 and developing countries,3,4 as well as among deprived populations.5,6 However, CRDs remain under-diagnosed and under-treated.7
To address this global health problem, the Global Alliance against Chronic Respiratory Diseases (GARD) was formed following a mandate from the World Health Assembly.8–12 GARD is a voluntary alliance of organizations, institutions, and agencies working towards a common vision to improve global lung health according to local needs. GARD aims to develop a standard way of obtaining relevant data on CRD and risk factors, encourage countries to implement CRD prevention policies and to make recommendations of simple and affordable strategies for CRD management.7
The rationale of this study was to investigate the prevalence of COPD in patients with respiratory symptoms, as well as the prevalence of bronchial asthma (BA), allergic rhinitis (AR), and chronic bronchitis (CB) in the overall Russian population. The frequency of major risk factors for CRD was also evaluated in the same study population.
Methods
This was a cross-sectional population-based epidemiological study conducted in 2010–2011 across 12 regions (Figure S1) of the Russian Federation.
The aim of the study was to recruit 250 adult (≥18 years) respondents in each major Russian city. As a general procedure, the administrative districts of each region participating in the study were selected based on a stratified random cluster sampling procedure. This stratification ensured appropriate weighted representation of each district’s target population in the study sample. The most current census data from the entire region and from each district were collected from official sources in order to proportionally stipulate the number of participants in each district. In a second stage, streets from each previous selected district were also selected by applying a two-step stratified random cluster sampling procedure with a standard random number generator (Microsoft Excel 2010; Microsoft Corporation, Redmond, WA, USA).13 In the last stage, each selected street had also been randomly assigned specific households that would be approached to take part in the study. When blocks of apartments were selected, only several apartments were chosen from the block, and then the interviewers’ team moved to the next block randomly. To ensure inclusion of respondents who could not be available, rounds were conducted during non-working time.
GARD study received favorable opinion from the National Ethics Committee, Russian Ministry of Health. Prior to initiating a face-to-face interview by a team that consisted of either a nurse or a physician, subjects gave written consent for the use of the anonymized data reported in the questionnaire (Figure S1) and for pulmonary function testing – applicable to respondents with suspected CRD based on self-reported symptoms. Upon availability of hospital records, patient-reported diagnoses were checked.
The presence of BA symptoms was considered if patients experienced an attack of wheezing, or wheezing/whistling that resulted in breathlessness. AR symptoms were adapted from the Allergic Rhinitis and its Impact on Asthma criteria, according to which the presence of running nose with sneezing or nasal obstruction indicates rhinitis and the presence of running nose alone might also indicate rhinitis.14 CB was defined as the presence of cough and sputum production for at least 3 months in 2 years.15
COPD was defined following the Global Initiative for Obstructive Lung Disease definition of post-bronchodilator FEV1/FVC <0.7. The identification of symptomatic patients included clinical diagnosis of dyspnea, chronic cough or sputum production,15 as well as those who were active smokers for more than 1 year, or those exposed to biomass or occupational hazards.
These selection criteria were checked by a doctor/pulmonologist using the GARD questionnaire which was developed in the respondent’s native language. As the questionnaire was self-completed by respondents and did not cover all of the information necessary to check the above criteria, the doctor/pulmonologist briefly interviewed the respondents regarding their medical history to establish the pulmonary origin of dyspnea and collect details on allergies and current health condition. In addition, all subjects who had an acute respiratory viral infection at the time of the interview were excluded to ensure reliable pulmonary function tests. Spirometry was performed at the investigational center and in accordance with international standards, including bronchodilator challenge.16 For post-bronchodilator measurements, investigators were recommended to perform spirometry 15 minutes after two–four puffs of salbutamol (200–400 μg) via metered-dose inhaler with spacer.
Statistical analysis
The primary study endpoint was to establish prevalence of COPD, BA, and AR in accordance with the current diagnostics standards in the representative population. Using a two-tailed binomial test with a significance level of 5%, the study was designed to have 80% power to establish prevalence for each disease under study with the significance level not less than 1% on each side. The calculation of the sample size was made using Stata 12 package (sampsi) (StataCorp LP, College Station, TX, USA). The target sample size of 7,164 respondents was determined using the following assumptions:
Binomial distribution of the prevalence
The maximum prevalence for each study indication according to literature was 20%
Maximum acceptable one-sided error for prevalence determination was 5%, which was a 1% one-sided error for a maximum prevalence of 20%
Two-sided type 1 error of 5%, which was the risk to incorrectly accept the false null hypothesis of non-equivalence of sample prevalence and estimated population prevalence
An 80% probability to detect non-equivalence of the sample prevalence and the estimated population prevalence in case of true non-equivalence
A 10% probability that patients did not show up for the functional testing at the investigational site after the completion of the questionnaire.
The comparison of the qualitative parameters in different groups (by age, sex, etc) was carried out using chi-square test, in the case of two groups, where possible, Fisher’s exact test was used. Taking into consideration the cross-sectional nature of the study, odds ratio was calculated and 95% confidence intervals (CI) for the odds ratio to estimate the statistical significance of associations between risk factors and diseases. As spirometry was performed only in a sub-set population with symptoms and/or risk factors, this association was not calculated. All results were considered statistically significant at the level of P<0.05.
Results
A total of 7,164 questionnaires were completed. The mean age of respondents was 43.4 years, ranging from 18 to 88 years, and 57.2% were female. Of the respondents, 64.2% were employed at the time of the survey, and 3.4% were migrants from other countries.
Respondents’ characteristics are shown in Table 1.
Table 1.
Demographic characteristics of sample respondents
| Subjects (n) | Male
|
Female
|
Total
|
|---|---|---|---|
| 3,067 (42.8%) (95% CI: 41.7–44.0) | 4,093 (57.2%) (95% CI: 56.0–58.3) | 7,164a (n, %) | |
| Age (years)b | |||
| Below 20 years | 105 (3.4%) | 118 (2.9%) | 223 (3.0%) |
| 20–29 years | 769 (25.1%) | 842 (20.6%) | 1,611 (22.5%) |
| 30–39 years | 576 (18.8%) | 713 (17.4%) | 1,289 (18.0%) |
| 40–49 years | 536 (17.5%) | 764 (18.7%) | 1,300 (18.2%) |
| 50–59 years | 591 (19.3%) | 928 (22.7%) | 1,519 (21.2%) |
| 60–69 years | 325 (10.6%) | 484 (11.8%) | 809 (11.3%) |
| 70–79 years | 139 (4.5%) | 212 (5.2%) | 351 (4.9%) |
| 80 years and older | 25 (0.8%) | 31 (0.8%) | 56 (0.8%) |
| Smoking status | |||
| Have ever smoked | 2,132 (69.5%) (95% CI: 67.9–71.1) | 1,152 (28.1%) (95% CI: 26.8–29.6) | 3,284 (45.9%) (95% CI: 44.7–47.0) |
| Current smokers | 1,608 (52.4%) (95% CI: 50.6–54.2) | 792 (19.4%) (95% CI: 18.1–20.6) | 2,400 (33.5%) (95% CI: 32.4–34.6) |
| Exposure to workplace dust | 956 (31.2%) (95% CI: 29.5–32.8) | 630 (15.4%) (95% CI: 14.3–16.5) | 1,586 (22.2%) (95% CI: 21.2–23.1) |
| Exposure to biomass | 960 (31.3%) (95% CI: 29.7–33.0) | 1,473 (36.0%) (95% CI: 34.5–37.5) | 2,433 (34.0%) (95% CI: 32.9–35.1) |
| Migration from another country | 121 (3.9%) (95% CI: 3.3–4.7) | 125 (3.1%) (95% CI: 2.5–3.6) | 246 (3.4%) (95% CI: 3.0–3.9) |
| Employment status | |||
| Employed | 2,039 (66.5%) (95% CI: 64.8–68.2) | 2,562 (62.6%) (95% CI: 61.1–64.1) | 4,601 (64.3%) (95% CI: 63.1–65.3) |
| Unemployed | 1,027 (33.5%) (95% CI: 31.8–35.2) | 1,530 (37.4%) (95% CI: 35.9–38.9) | 2,557 (35.7%) (95% CI: 34.6–36.8) |
Notes:
Four respondents did not indicate their sex
six respondents did not indicate their age.
Abbreviation: CI, confidence interval.
Prevalence of BA, AR, and CB related symptoms
Prevalence of disease related symptoms is shown in Table 2. When respondents were asked if they had ever experienced attacks of wheezing or whistling accompanied with the feeling of breathlessness, 25.7% (95% CI: 24.7–26.7) responded affirmatively. Out of those, 78.7% confirmed they had two or more attacks.
Table 2.
Prevalence of respiratory symptoms in the total sample
| Frequency of positive answer (n=7,164) | |
|---|---|
| Asthma | |
| Attack of wheezing or whistling with breathlessness | 25.7% (95% CI: 24.7–26.7) |
| Allergic rhinitis | |
| Running nose alone | 19.2% (95% CI: 18.3–20.1) |
| Running nose with sneezing or nasal obstruction | 18.2% (95% CI: 17.3–19.2) |
| Ocular symptoms in respondents with running nose with sneezing or nasal obstruction | 52.9% (95% CI: 50.4–55.3) |
| Chronic bronchitis | |
| Cough and sputum production most of the days of the week ≥3 consecutive months ≥2 years | 8.6% (95% CI: 7.9–9.3) |
Abbreviation: CI, confidence interval.
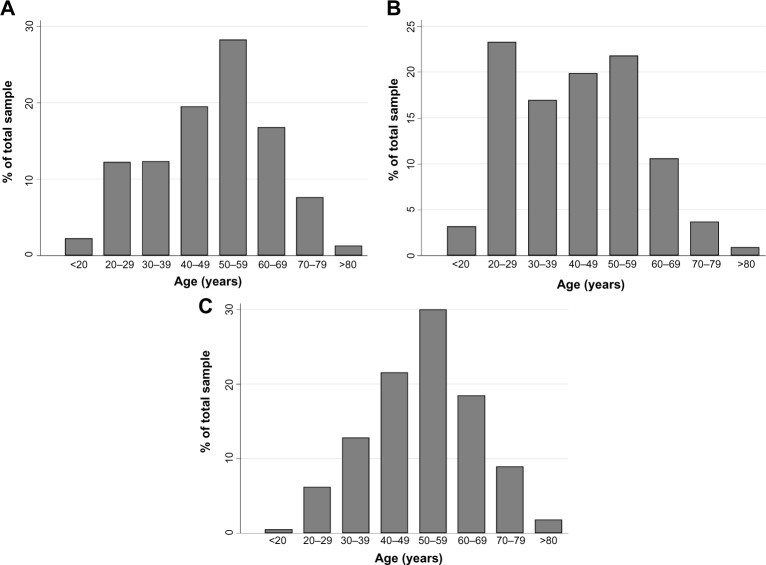
The presence of a running nose along with the presence of at least one of the symptoms of sneezing or nasal congestion was reported among 18.2% (95% CI: 17.3–19.2) of the study population. Of these, 52.9% also had ocular symptoms. The distribution of respondents with respiratory symptoms, by age group, is shown in Figure 1.
Figure 1.
The distribution of respondents with respiratory symptoms for bronchial asthma, allergic rhinitis, and chronic bronchitis.
Notes: (A) Asthma. (B) Allergic rhinitis. (C) Chronic bronchitis.
Cough and expectoration, occurring in the majority of the week for more than three consecutive months in a year, lasting more than 2 years, compatible with CB, was experienced by 8.6% of all respondents (95% CI: 7.9–9.3). Frequencies of respiratory symptoms are shown in Table 2.
Prevalence of previous diagnosis
Out of all respondents participating in the survey, 6.9% (95% CI: 6.3–7.5) reported a previous diagnosis of BA at some point in their life. Previous AR diagnosis was also reported by 6.5% of respondents (95% CI: 5.9–7.1). The highest percentage was found among those respondents who had a previous diagnosis of CB (22.2%; 95% CI: 21.2%–23.2%).
Of patients with proven COPD based on spirometry results, 51.4% (95% CI: 45.0–57.7) and 6.8% (95% CI: 4.0–10.6) had also self-reported previous CB and emphysema diagnosis, respectively. When analyzing the pool of respondents with symptoms compatible with CB, only 23.5% (95% CI: 21.5–25.7) reported to have a previous diagnosis. Out of those, 25.9% (95% CI: 20.6–31.8) had positive COPD diagnosis after spirometry. Distribution of respondents with COPD within spirometry population, by age group, is shown in Figure 2.
Figure 2.
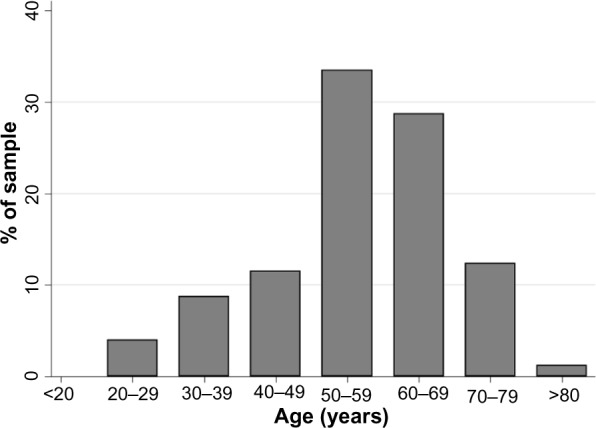
Distribution of respondents with COPD based on GOLD guidelines, by age group.
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Obstructive Lung Disease.
Prevalence of COPD based on spirometry
Spirometry data was recorded in 16% (251) of the total study sample in patients with suspected CRD. This information was based on both historical and newly performed spirometry data collected as part of the study.
A post-bronchodilator test was performed in 94.4% of these subjects. Subjects with a post-bronchodilator test had a significantly reduced vital capacity and FEV1 when compared to the total population who had spirometry, and also had a significantly higher age (P<0.001).
The prevalence of COPD in patients with respiratory symptoms, or risk factors, was 21.8% (95% CI: 19.5%–24.5%). By extrapolation, the prevalence of symptomatic COPD in the total study population was 15.3%.
Risk factors
The prevalence of smoking was quite high, with 45.7% of the total population responding that they had a smoking history, of which 73.1% were current smokers. Smoking history was measured if patients have consumed at least 200 packs in their life-time. Regarding workplace dust, 22.2% of the total population responded that they had been exposed to workplace dust for more than a year. For indoor use of an open fire for heating or cooking, 34.0% of the total population responded that they used one. The association between these selected risk factors and respiratory symptoms is shown in Table 3.
Table 3.
Association between risk factors and chronic respiratory diseases
| Symptom odds ratio (95% CI); P-value
|
|||
|---|---|---|---|
| Occupational hazard | Smoking | Biomass exposure | |
| Bronchial asthma | 1.979 (1.737–2.254) <0.0001 |
1.116 (0.992–1.255) 0.0633 |
1.431 (1.268–1.614) <0.0001 |
| Chronic bronchitis | 2.584 (2.168–3.080) <0.0001 |
2.617 (2.189–3.129) <0.0001 |
1.677 (1.415–1.988) <0.0001 |
| Allergic rhinitis | 1.327 (1.167–1.509) <0.0001 |
0.760 (0.671–0.860) <0.0001 |
0.979 (0.871–1.10) 0.7161 |
Abbreviation: CI, confidence interval.
Discussion
The GARD study was the first cross-sectional population-based epidemiological study among a representative sample, using a standardized methodology and validated questionnaire, to evaluate the prevalence of respiratory diseases in several regions of the Russian Federation.
Partial use of data collected from previously completed GARD questionnaires from 2009–2010 was approved by the study steering committee. The GARD questionnaire has been used in several studies and has been shown to be an accurate and reliable diagnostic tool.17,18
The prevalence of respiratory symptoms in the population sampled was found to be high. The percentage of patients with asthma related symptoms was 25.7%, AR 18.2% and CB 8.6%.
Based on spirometry-confirmed diagnosis, 21.8% of respondents with respiratory symptoms had COPD, and, by extrapolations, 15.3% of the overall population suffered from the disease.
CRDs are recognized as a major public health problem with an increasing morbidity and mortality. With such a high burden on the health care system, emphasis on better diagnosis and management of these diseases must be achieved, and reliable epidemiological data on the prevalence and severity of diseases, such as COPD and its exacerbations, are crucial to guide health care policy.19
In the Russian Federation, it has been estimated from earlier epidemiological studies that the prevalence of CRD ranges from 17% to 21%. This includes the prevalence of asthma which ranges from 6%–8% for adults and up to 12% for children, and for COPD between 6%–7%, and other miscellaneous disease of 2%.20
COPD is the fourth cause of death worldwide.21 An estimation from the World Health Organization suggests that COPD will be the third cause of death by 2030.22 The association between COPD and CB may lead to a more severe COPD prognostic, which encompasses a poorer lung function, exacerbation, a worse quality of life and, consequently, a higher economic burden.23 We used the Global Initiative for Obstructive Lung Disease strategy definition of COPD in symptomatic subjects, which represents a simplified case definition for epidemiological purposes, rather than a definitive clinical diagnosis; this may have resulted in patients with COPD not being diagnosed. The limitation of our study is that a large proportion of patients with COPD are asymptomatic; the study may have underestimated the prevalence of COPD as the spirometry was conducted only in symptomatic patients.24
The problem of COPD under-diagnosis is well known. Only about one-third of all cases with COPD are recognized by the health care professional,25–27 and the proportion of undiagnosed cases decreases with increasing disease severity.28 The prevalence of COPD has often been reported in the range of 6%–10% of the total adult population.29 However, for the PLATINO study,18 the crude prevalence of COPD was estimated to be up to 19.7% in population ≥40 years in Montevideo, especially in elderly men. Other studies have also reported prevalence of up to 20%, dependent on the definition used.24,30,31 In the BOLD (Burden of Obstructive Lung Disease) Study, prevalence of non-flow obstruction was observed in up to 80% with variation of COPD prevalence from 0.9% to 15.5% among cities, depending on the disease stage.32
As the main objective of the GARD study was to assess COPD prevalence in symptomatic patients, the estimation of crude prevalence of 15.3% should be analyzed carefully, even though our results are compatible with what has been seen in different populations.
The assumption of this prevalence was based on the fact that symptomatic patients who have not undergone spirometry (due to the exclusion criteria being matched or refusals/further contact failure after the first assessment by questionnaire) would also have the same frequency of cases observed in those patients with similar clinical characteristics who underwent spirometry. For the remaining asymptomatic population who did not meet clinical diagnosis criteria, our assumption was that the result of spirometry would be ≥0.7.
COPD itself is a predictor of mortality as it has been shown that this is significantly higher amongst subjects with COPD compared to subjects without COPD (P<0.001).33 This study reinforces the need for the provision of adequate standard care management in order to improve the quality of life of patients and to decrease exacerbations and hospitalizations.34–36 Our results suggest that prevalence of COPD in Russia is higher than previously suggested, which also results in a greater health issue.
The prevalence of CB in COPD patients varies substantially.23,37 In the GARD study, more than half of the patients with COPD also had CB. In a recent European study,38 CB prevalence varies from 0.7% to 9.7%, going up to 20.1%–56.9% among current smoker respondents, as smoking is one of the major risk factors for developing CB, which can also be seem from our study results. Our findings suggested that prevalence based on CB symptoms is 8.6%, which is consistent with the findings in European populations.
The prevalence of BA may vary considerably in different countries, from 4% to 18% of populations.39 Even though BA is much more present in developed countries, its prevalence in less industrialized regions is growing.40 Our findings suggest that in Russia, the prevalence of BA related-symptoms is higher than originally estimated.20 Asthma is one of the most common chronic diseases in the world and it is estimated to be accountable for about one in every 250 deaths worldwide.41 A study performed by Brogger et al suggested a 3-fold increase in the prevalence of self-reported diagnosis in 26 years, which could be due to a better standard of care and an easier access to physicians.42
As for other CRDs, AR is also experiencing an increase of epidemic proportion,43 which leads to an intensification in the socioeconomic burden of the disease across the world. Prevalence of AR has been reported as high as 21% in Europe.44 Asthma and rhinitis have been reported to have similarities related to their epidemiological and patho-physiological background.45 If untreated, rhinitis may have considerable economic and quality of life implications.46,47
A high proportion of the Russian population is exposed to risk factors which could drive specific public health initiatives. In this study, the odds ratios between BA, CB, and AR and occupational hazard, smoking, and biomass exposure were estimated as an attempt to collect information on the major risk factors for those CRDs. The present study did not intend to assess risk factor related to COPD, nor the difference pattern between genders and cities. The prevalence of major risk factors including smoking, occupational hazards, and biomass exposure was high.
The major effects of smoking on CRDs have been extensively recorded over more than 40 years.48 The associations found in this study were generally consistent with findings from other epidemiological studies where the GARD questionnaire has been used.17,18 It should be noted, however, that this study design (cross-sectional) was not performed to evaluate causality between risk factors and respiratory disease already established in other studies. Among all three risk factors, the occurrence of an occupational hazard demonstrated a statistically significant association with all respiratory symptoms (P<0.0001). Biomass exposure occurrence had a positive association with CB and BA, which was also statistically significant (P<0.0001). The negative association between smoking and AR should be analyzed carefully. Some risk factors may be obscured due to changes in smoke habits due to the occurrence of respiratory symptoms. Also, for those respondents with a history of respiratory symptoms, there is a possibility of reluctance to start smoking. All of these factors may create a bias in the risk factors association analysis due to the cross-sectional design study type.
This study has substantial strengths. To the best of our knowledge, it is the first comprehensive prevalence study conducted in Russia for CRD. Using probabilistic sampling strategy, the study was able to capture the prevalence of symptomatic CRD and the major risk factors in the Russian Federation. There are also potential limitations of the study, which included only symptomatic patients and did not represent the total number of COPD patients. Moreover not all self-reported symptomatic patients underwent spirometry due to the reasons previously explained.
Furthermore, as for any questionnaire based study, the study outcomes are based on the willingness of the respondents to report their diseases. Furthermore, by also having the prevalence of self-reported symptoms we are minimizing the subjectivity of self-report diagnosis due to the different diagnosis criteria that may be used by physicians. There is also a potential bias of non/incomplete-responders, as we did not adjust for subjects who did not complete all of the questions during the visit. In addition, no adjustments for age or sex have been made. Our sample includes 57% females which could have an impact on prevalence as there may be important sex differences on the perception of dyspnea, health status, and physical activity limitation.49
Conclusion
The prevalence of respiratory symptoms in the Russian Federation was found to be high. For asthma, the overall asthmatic symptoms were present in 25.7% of respondents. AR symptoms were presented in 18.2% and CB in 8.6%.
The estimated prevalence of 21.8% for COPD in symptomatic patients and 15.3% in the overall population may still be an underestimate as this was only estimated by spirometry from symptomatic patients. COPD can be present in asymptomatic patients which also may reflect a misrepresentation of prevalence present in this study. There was a considerable discrepancy between self-reported diagnosis and disease based-symptoms for AR and BA, which may suggest that respondents were under-diagnosed. The discrepancy between self-reported symptoms and previous diagnosis highlights the fact that CDR may not be recognized, or may have a late diagnosis which might lead to economic and health impacts. This data will be used to monitor the course and health care utilization of these diseases and to evaluate the impact of future educational programs on assessing and treating patients with CRDs.
Supplementary material
Burden of major respiratory diseases: chronic respiratory diseases core questionnaire.
Acknowledgments
The authors wish to acknowledge Diana Jones of Cambrian Clinical Associates Ltd for the development of the first draft of the manuscript, editorial suggestions to draft versions of this paper, assembling tables and figures, collating author comments, and referencing. This assistance was funded by GlaxoSmithKline.
The study was supported from the grant provided by GlaxoSmithKline Russia to the contract-research organization “Worldwide Clinical Trials”. Worldwide Clinical Trials has conducted the study and assisted in developing the study protocol to investigators. This study was supported by ZAO GlaxoSmithKline Trading Ltd.
Author contributions
All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. Conception and design: AGC, IVD, DVG, NSA, NK and LGM. Analysis and interpretation: AGS. All authors were responsible for drafting and editing the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization . Global Surveillance, Prevention and Control of Chronic Respiratory Diseases. A Comprehensive Approach. Geneva: World Health Organization; 2007. [Accessed July 17, 2014]. Available from: http://www.who.int/gard/publications/GARD%20Book%202007.pdf. [Google Scholar]
- 2.Haahtela T, Tuomisto LE, Pietinalho A, et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61(8):663–670. doi: 10.1136/thx.2005.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fairall LR, Zwarenstein M, Bateman ED, et al. Effect of educational outreach to nurses on tuberculosis case detection and primary care of respiratory illness: pragmatic cluster randomised controlled trial. BMJ. 2005;331(7519):750–754. doi: 10.1136/bmj.331.7519.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer GB, Camargos PA, Mocelin HT. The burden of asthma in children: a Latin American perspective. Paediatr Respir Rev. 2005;6(1):8–13. doi: 10.1016/j.prrv.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Evans R, III, Gergen PJ, Mitchell H, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135(3):332–338. doi: 10.1016/s0022-3476(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 6.Cloutier MM, Hall CB, Wakefield DB, Bailit H. Use of asthma guidelines by primary care providers to reduce hospitalizations and emergency department visits in poor, minority, urban children. J Pediatr. 2005;146(5):591–597. doi: 10.1016/j.jpeds.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J, Dahl R, Khaltaev N. Global alliance against chronic respiratory diseases. Allergy. 2007;62(3):216–223. doi: 10.1111/j.1398-9995.2007.01307.x. [DOI] [PubMed] [Google Scholar]
- 8.Khaltaev N. WHO strategy for prevention and control of chronic obstructive pulmonary disease. Exp Lung Res. 2005;31(Suppl 1):55–56. [PubMed] [Google Scholar]
- 9.World Health Organization . WHO Strategy for Prevention and Control of Chronic Respiratory Diseases. Geneva: World Health Organization; 2001. [Accessed July 17, 2014]. Available from: http://www.who.int/respiratory/publications/WHO_MNC_CRA_02.1.pdf. [Google Scholar]
- 10.World Health Organization . Implementation of the WHO Strategy for Prevention and Control of Chronic Respiratory Diseases. Geneva: World Health Organization; 2002. [Accessed July 17, 2014]. Available from: http://www.who.int/respiratory/publications/WHO_MNC_CRA_02.2.pdf. [Google Scholar]
- 11.World Health Organization . Prevention and Control of Chronic Respiratory Diseases in Low and Middle-Income African Countries: A Preliminary Report. Geneva: World Health Organization; 2004. [Accessed July 17, 2014]. Available from: http://whqlibdoc.who.int/hq/2003/WHO_NMH_CRA_04.1.pdf. [Google Scholar]
- 12.World Health Organization . Prevention and Control of Chronic Respiratory Diseases at Country Level: Towards a Global Alliance against Chronic Respiratory Diseases. Geneva: World Health Organization; 2005. [Accessed July 17, 2014]. Available from: http://www.who.int/respiratory/publications/WHO_NMH_CHP_CPM_-CRA_05.1.pdf. [Google Scholar]
- 13.Silman AJ, Macfarlane GJ. Epidemiological Studies: A Practical Guide. 2nd ed. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 14.Allergic Rhinitis and its Impact on Asthma (ARIA) Management of Allergic Rhinitis and its Impact on Asthma. Pocket Guide. ARIA; 2007. [Accessed July 17, 2014]. Available from: http://www.whiar.org/docs/ARIA_PG_08_View_WM.pdf. [Google Scholar]
- 15.Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease; 2014. [Accessed July 17, 2014]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Mohammad Y, Shaaban R, Yassine F, et al. Executive summary of the multicenter survey on the prevalence and risk factors of chronic respiratory diseases in patients presenting to primary care centers and emergency rooms in Syria. J Thorac Dis. 2012;4(2):203–205. doi: 10.3978/j.issn.2072-1439.2011.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menezes AM, Perez-Padilla R, Jardim JR, et al. PLATINO Team Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;66(9500):1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 19.Martins P, Rosado-Pinto J, do Céu Teixeira M, et al. Under-report and underdiagnosis of chronic respiratory diseases in an African country. Allergy. 2009;64(7):1061–1067. doi: 10.1111/j.1398-9995.2009.01956.x. [DOI] [PubMed] [Google Scholar]
- 20.Bellevskiy A. GARD in Russia. Geneva: World Health Organization; [Accessed July 17, 2014]. Available from: http://www.who.int/gard/news_events/GARD%20in%20Russia.pdf. [Google Scholar]
- 21.Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD) Lancet. 2004;364(9434):613–620. doi: 10.1016/S0140-6736(04)16855-4. [DOI] [PubMed] [Google Scholar]
- 22.http://www.who.int [homepage on the Internet]. World Health Organization COPD predicted to be third leading cause of death in 2030. [Accessed July 17, 2014]. Available from: http://www.who.int/respiratory/copd/World_Health_Statistics_2008/en/
- 23.de Oca MM, Halbert RJ, Lopez MV, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J. 2012;40(1):28–36. doi: 10.1183/09031936.00141611. [DOI] [PubMed] [Google Scholar]
- 24.Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD prevalence estimates: what is the true burden of disease? Chest. 2003;123(5):1684–1692. doi: 10.1378/chest.123.5.1684. [DOI] [PubMed] [Google Scholar]
- 25.Lundbäck B, Lindberg A, Lindström M, et al. Not 15 but 50% of smokers develop COPD? – Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2003;97(2):115–122. doi: 10.1053/rmed.2003.1446. [DOI] [PubMed] [Google Scholar]
- 26.Siafakas NM, Vermeire P, Pride NB, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J. 1995;8(8):1398–1420. doi: 10.1183/09031936.95.08081398. [DOI] [PubMed] [Google Scholar]
- 27.Lindberg A, Bjerg A, Rönmark E, Larsson LG, Lundbäck B. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking. Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2006;100(2):264–272. doi: 10.1016/j.rmed.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 28.Lindberg A, Larsson LG, Muellerova H, Rönmark E, Lundbäck B. Up-to-date on mortality in COPD – report from the OLIN COPD study. BMC Pulm Med. 2012;12:1. doi: 10.1186/1471-2466-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viegi G, Pedreschi M, Pistelli F, et al. Prevalence of airways obstruction in a general population: European Respiratory Society vs American Thoracic Society definition. Chest. 2000;117(5 Suppl 2):339S–345S. doi: 10.1378/chest.117.5_suppl_2.339s. [DOI] [PubMed] [Google Scholar]
- 30.Celli BR, Halbert RJ, Isonaka S, Schau B. Population impact of different definitions of airway obstruction. Eur Respir J. 2003;22(2):268–273. doi: 10.1183/09031936.03.00075102. [DOI] [PubMed] [Google Scholar]
- 31.Bateman ED, Bousquet J, Busse WW, et al. Stability of asthma control with regular treatment: an analysis of the Gaining Optimal Asthma controL (GOAL) study. Allergy. 2008;63(7):932–938. doi: 10.1111/j.1398-9995.2008.01724.x. [DOI] [PubMed] [Google Scholar]
- 32.Buist AS1, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 33.Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet. 1998;351(9105):773–780. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- 34.Friedman M, Serby CW, Menjoge SS, Wilson JD, Hilleman DE, Witek TJ., Jr Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest. 1999;115(3):635–641. doi: 10.1378/chest.115.3.635. [DOI] [PubMed] [Google Scholar]
- 35.Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 36.Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 37.Corhay JL, Vincken W, Schlesser M, Bossuyt P, Imschoot J. Chronic bronchitis in COPD patients is associated with increased risk of exacerbations: a cross-sectional multicentre study. Int J Clin Pract. 2013;67(12):1294–1301. doi: 10.1111/ijcp.12248. [DOI] [PubMed] [Google Scholar]
- 38.Cerveri I, Accordini S, Verlato G, et al. Variations in the prevalence across countries of chronic bronchitis and smoking habits in young adults. Eur Respir J. 2001;18(1):85–92. doi: 10.1183/09031936.01.00087101. [DOI] [PubMed] [Google Scholar]
- 39.Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma; 2014. [Accessed July 17, 2014]. Available from: http://www.ginasthma.org/local/uploads/files/GINA_Report_March13.pdf. [Google Scholar]
- 40.Braman SS. The global burden of asthma. Chest. 2006;130(Suppl 1):4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 41.Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control Study. Am J Respir Crit Care Med. 2004;170(8):836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 42.Brogger J, Bakke P, Eide GE, Johansen B, Andersen A, Gulsvik A. Long-term changes in adult asthma prevalence. Eur Respir J. 2003;21(3):468–472. doi: 10.1183/09031936.03.00056103. [DOI] [PubMed] [Google Scholar]
- 43.Pawankar R, Bunnag C, Khaltaev N, Bousquet J. Allergic rhinitis and its impact on asthma in Asia Pacific and the ARIA Update 2008. World Allergy Organ J. 2012;5(Suppl 3):S212–S217. doi: 10.1097/WOX.0b013e318201d831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janson C, Anto J, Burney P, et al. The European Community Respiratory Health Survey: what are the main results so far? European Community Respiratory Health Survey II. Eur Respir J. 2001;18(3):598–611. doi: 10.1183/09031936.01.00205801. [DOI] [PubMed] [Google Scholar]
- 45.Gaugris S, Sazonov-Kocevar V, Thomas M. Burden of concomitant allergic rhinitis in adults with asthma. J Asthma. 2006;43(1):1–7. doi: 10.1080/02770900500446823. [DOI] [PubMed] [Google Scholar]
- 46.Fineman SM. The burden of allergic rhinitis: beyond dollars and cents. Ann Allergy Asthma Immunol. 2002;88(4 Suppl 1):2–7. doi: 10.1016/s1081-1206(10)62022-4. [DOI] [PubMed] [Google Scholar]
- 47.Schoenwetter WF, Dupclay L, Jr, Appajosyula S, Botteman MF, Pashos CL. Economic impact and quality-of life burden of allergic rhinitis. Curr Med Res Opin. 2004;20(3):305–317. doi: 10.1185/030079903125003053. [DOI] [PubMed] [Google Scholar]
- 48.Spencer S, Jones PW, GLOBE Study Group Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax. 2003;58(7):589–593. doi: 10.1136/thorax.58.7.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez Varela MV, Montes de Oca M, Halbert RJ, et al. Sex-related differences in COPD in five Latin American cities: the PLATINO study. Eur Respir J. 2010;36(5):1034–1041. doi: 10.1183/09031936.00165409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Burden of major respiratory diseases: chronic respiratory diseases core questionnaire.