Abstract
The forelimbs of nonavian theropod dinosaurs have been the subject of considerable study and speculation due to their varied morphology and role in the evolution of flight. Although many studies on the functional morphology of a limb require an understanding of its musculature, comparatively little is known about the forelimb myology of theropods and other bipedal dinosaurs. Previous phylogenetically based myological reconstructions have been limited to the shoulder, restricting their utility in analyses of whole-limb function. The antebrachial and manual musculature in particular have remained largely unstudied due to uncertain muscular homologies in archosaurs. Through analysis of the musculature of extant taxa in a robust statistical framework, this study presents new hypotheses of homology for the distal limb musculature of archosaurs and provides the first complete reconstruction of dinosaurian forelimb musculature, including the antebrachial and intrinsic manual muscles. Data on the forelimb myology of a broad sample of extant birds, crocodylians, lizards, and turtles were analyzed using maximum likelihood ancestral state reconstruction and examined together with the osteology of the early theropod Tawa hallae from the Late Triassic of New Mexico to formulate a complete plesiomorphic myology for the theropod forelimb. Comparisons with previous reconstructions show that the shoulder musculature of basal theropods is more similar to that of basal ornithischians and sauropodomorphs than to that of dromaeosaurids. Greater development of the supracoracoideus and deltoideus musculature in theropods over other bipedal dinosaurs correlates with stronger movements of the forelimb at the shoulder and an emphasis on apprehension of relatively large prey. This emphasis is further supported by the morphology of the antebrachium and the intrinsic manual musculature, which exhibit a high degree of excursion and a robust morphology well-suited for powerful digital flexion. The forelimb myology of Tawa established here helps infer the ancestral conformation of the forelimb musculature and the osteological correlates of major muscle groups in early theropods. These data are critical for investigations addressing questions relating to the evolution of specialized forelimb function across Theropoda.
Keywords: Archosauria, functional morphology, myology, phylogenetic inference, Theropoda
Introduction
The forelimbs of nonavian theropod dinosaurs present complex functional problems for the reconstruction of behavior in extinct taxa. Their closest living relatives, crown-group crocodylians and birds, possess such radically different forelimb morphologies that at first glance they seem to have little in common, and neither has a great similarity to that of nonavian theropods. Most nonavian theropods also lack any extant analogs to forelimb function, as the only modern animals that do not use their forelimbs for locomotion are humans and terrestrial flightless birds. Nevertheless, the function of theropod forelimbs is a topic of extensive interest and speculation due in large part to the evolution of these forelimbs into instruments of flight. Recent studies on the evolution of theropod forelimbs have focused on the evolution of feathers and wing shape (e.g. Wang et al. 2011a), including the creation of aerodynamic models (Koehl et al. 2011), the developmental identity of the manual digits (Bever et al. 2011; Wang et al. 2011b), changes in forelimb proportions relating to flight (Dececchi & Larsson, 2009), and assessment of potential ranges of motion in the developing flight stroke (Gishlick, 2001). The myology of the forelimb and its importance in testing hypotheses of forelimb function, however, have been largely ignored.
Reconstructing the limb musculature of extinct tetrapods is one of the most fundamental steps in any analysis of the functional capability. The integrative phylogenetic and extrapolatory analysis (Bryant & Russell, 1992) and extant phylogenetic bracket (EPB; Witmer, 1995) methods have become the de facto toolkit for soft tissue reconstructions of extinct taxa (e.g. Carrano & Hutchinson, 2002; Jasinoski et al. 2006) because they analyze soft tissue data of the most closely related extant taxa in an explicit phylogenetic context. Among the few studies that have reconstructed forelimb musculature in dinosaurs, even fewer have been performed in an explicit phylogenetic context (Nicholls & Russell, 1985; Dilkes, 2000; Jasinoski et al. 2006; Langer et al. 2007; Maidment & Barrett, 2011). The musculature of the shoulder in theropods has been thoroughly documented (Jasinoski et al. 2006) but the musculature of the antebrachium and manus in a nonavian theropod has only been reconstructed using birds as the primary muscular model, thus lacking full phylogenetic context (Carpenter & Smith, 2001). Two studies have used phylogeny-based methods to reconstruct some antebrachial muscles in non-theropod dinosaurs (Dilkes, 2000; Langer et al. 2007) but both of these studies reconstructed only a few major muscles of the forearm and none of the manus. The muscles controlling the hand and digits in theropods present difficulties in their reconstruction due to the highly divergent manual morphologies of extant archosaurs, yet these muscles are some of the most important in determining the functional capabilities of the theropod forelimb. However, several recent studies on the development of the avian wrist and hand (Kundrát, 2009; Wang et al. 2011b) have made it possible to identify osteological homologs in this region and improved our ability to assess muscular morphology across Archosauria.
An interest in the evolution of flight has resulted in a primary focus on theropod taxa that are phylogenetically close to birds. Most previous reconstructions of theropod forelimb myology of any method have been performed in highly nested coelurosaurians (Nicholls & Russell, 1985; Carpenter & Smith, 2001; Jasinoski et al. 2006) but in general these taxa possess novel osteological features that can complicate muscular reconstruction, particularly in the antebrachium and manus. The reconstruction of the complete forelimb musculature in a phylogenetically early, plesiomorphic taxon establishes a ground state ancestral morphology that can be used in future muscular reconstructions as well as providing a starting point for the analysis of muscular and functional evolution of specialized theropod forelimbs across the entire clade.
The early theropod Tawa hallae from the Late Triassic Hayden Quarry of New Mexico (Nesbitt et al. 2009a) provides a nearly complete forelimb and pectoral girdle (lacking only the coracoid and furcula), allowing a full reconstruction of forelimb musculature. Tawa has been identified as the sister taxon to Neotheropoda, possessing a transitional morphology in the skull and postcranium intermediate between Neotheropoda and the most basal theropods (Nesbitt et al. 2009a). The forelimb shares apomorphic features with Herrerasaurus ischigualastensis and early neotheropods such as Coelophysis bauri and Dilophosaurus wetherelli, while retaining a plesiomorphically larger number of carpals (nine) than other theropods. This suite of features makes Tawa an ideal model for the reconstruction of the forelimb musculature in an early theropod.
Institutional abbreviations
AMNH, American Museum of Natural History, New York, NY, USA; GR, Ghost Ranch Ruth Hall Museum of Paleontology, Abiquiu, NM, USA; MPC, Mongolian Paleontological Collection, Ulaanbaatar, Mongolia; OUVC, Ohio University Vertebrate Collections, Athens, OH, USA; PVSJ, Museo de Ciencias Naturales, San Juan, Argentina; TMP, Royal Tyrrell Museum of Paleontology, Drumheller, AB, Canada.
Materials and methods
Data on muscle attachment sites in extant taxa were primarily obtained from published myological reports and supplemented with dissections of key taxa that are not represented in the literature. In total, data from the literature were collected for 41 avian species representing 26 family-level clades, four crocodylian species, six lepidosaurian species, and six testudine species (for a complete list of taxa and sources, see Supporting Information Table S1). Three additional avian taxa from the collection of Ohio University were dissected: Bubo virginianus (OUVC 10641), Caprimulgus carolinensis (OUVC 10642), and Megaceryle alcyon (OUVC 10643). Muscle data were also collected from two forelimbs of adult ostriches (Struthio camelus) obtained frozen from O.K. Corral Ostrich Farms (Oro Grande, CA, USA). Osteological features on the forelimb of Tawa hallae were assessed on all known forelimb material, which includes two previously described individuals (GR 241 and 242; Nesbitt et al. 2009a) and elements from larger individuals including a partial humerus (GR 359) and complete associated antebrachium (GR 360). Additionally, data collected on osteological features of other basal theropods such as Herrerasaurus (PVSJ 407, 373, 53), Sanjuansaurus (PVSJ 605), and Coelophysis (AMNH 7227, 7228, 7230, 7231, 7238; TMP 84.63.29, 84.63.30, 84.63.32, 84.63.33, 84.63.40, 84.63.50, 84.63.52) were used to create hypothetical reconstructions of coracoid attachment sites (not preserved in Tawa), and in cases where they provided osteological evidence for an otherwise equivocal origin or insertion.
Homologies of the muscles of the antebrachium and manus in archosaurs and other reptiles are not straightforward, and they are often not reported in the literature. A recent survey of reptile limb homologies with a broad taxonomic scope (Diogo & Abdala, 2010) provides a useful basis for many muscles but does not focus on archosaurs or the special problems presented by the avian manus. To address this, previous hypotheses of homology were concatenated from available sources including previous muscle reconstructions (Miner, 1925; Holmes, 1977; Dilkes, 2000), comparative anatomical reports (Howell, 1936; Haines, 1939, 1950; Straus, 1942; Meers, 2003), and developmental analyses (Sullivan, 1962). These hypotheses were critically appraised in light of the overall muscle morphology and novel dissections of the antebrachium and manus of the ostrich. Developmental studies of the carpus and metacarpus in birds (Kundrát, 2009) and crocodylians (Müller & Alberch, 1990; Buscalioni et al. 1997) were employed to assess muscle attachment site homologies in this highly modified region (Table 1). Homology hypotheses novel to this analysis are discussed below. In particular, the explicit homologies of the avian intrinsic manual musculature have not previously been proposed, and are summarized in Table 2. Terminology for muscles of the forelimb is not standardized and contributes to the confusion about homology, although an attempt to rectify this was made recently by Diogo & Abdala (2010). Their terminology is congruent with that of Jasinoski et al. (2006) and has been adopted in this study in most cases.
Table 1.
Homologies of the antebrachial musculature of archosaurs, lepidosaurs, and testudines.
| Muscle | Aves (Baumel et al. 1993) | Crocodylia (Meers, 2003) | Lepidosauria (Russell & Bauer, 2008) | Testudines (Walker, 1973) |
|---|---|---|---|---|
| Anconeus | Ectepicondylo-ulnaris | Flexor Ulnaris | Anconeus quartus | Extensor carpi ulnaris (part) |
| Extensor carpi ulnaris | Extensor carpi ulnaris | Absent | Extensor carpi ulnaris | Extensor carpi ulnaris (part) |
| Supinator | Supinator | Supinator | Supinator longus | Tractor radii |
| Extensor carpi radalis | Extensor carpi radialis | Extensor carpi radialis longus | Extensor carpi radialis superficialis | Extensor carpi radialis superficialis |
| Abductor radialis | Absent | Abductor radialis | Extensor carpi radialis intermedius and profundus | Extensor carpi radialis intermedius and profundus |
| Abductor pollicis longus | Extensor longus alulae | Extensor carpi radialis brevis | Supinator manus | Supinator manus |
| Extensor digitorum longus | Extensor digitorum communis | Extensor carpi ulnaris longus | Extensor digitorum longus | Extensor digitorum communis |
| Pronator teres | Pronator superficialis | Pronator teres | Pronator teres | Pronator teres |
| Pronator accessorius | Pronator profundus | Absent | Pronator accessorius | Absent |
| Pronator quadratus | Ulnometacarpalis ventralis | Pronator quadratus | Pronator profundus | Pronator profundus |
| Epitrochleoanconeus | Entepicondylo-ulnaris | Absent | Epitrochleo-anconeus | Flexor carpi ulnaris (part) |
| Flexor carpi ulnaris | Flexor carpi ulnaris | Flexor carpi ulnaris | Flexor carpi ulnaris | Flexor carpi ulnaris |
| Flexor digitorum longus superficialis | Flexor digitorum longus superficialis | Flexor digitorum longus pars humeralis | Flexor digitorum longus (humeral head) | Palmaris longus |
| Flexor digitorum longus profundus | Flexor digitorum longus profundus | Flexor digitorum longus pars ulnaris | Flexor digitorum longus (ulnar head) | Flexor digitorum longus |
Table 2.
Homologies of the avian intrinsic manual musculature with crocodylians, lepidosaurs, and testudines.
| Aves (Baumel et al. 1993) | Crocodylia (Meers, 2003) | Lepidosauria (Russell & Bauer, 2008) | Testudines (Walker, 1973) |
|---|---|---|---|
| Extensor longus digiti majorus pars proximalis | Extensor digitorum superficialis, digit II | Extensor digitorum brevis superficialis, digit II | Extensor digitorum brevis (part), digit II |
| Ulnimetacarpalis dorsalis | Extensor digitorum superficialis, digits III and/or IV | Extensor digitorum brevis superficialis, digits III and/or IV | Extensor digitorum brevis (part), digits III and/or IV |
| Extensor brevis alulae | Extensor digitorum profundus, digit I | Extensor digitorum brevis profundus, digit I | Extensor digitorum brevis (part), digit I |
| Extensor longus digiti majorus pars distalis | Extensor digitorum profundus, digit II | Extensor digitorum brevis profundus, digit II | Extensor digitorum brevis (part), digit II |
| Flexor alulae | Flexor digitorum brevis superificalis, digit I | Flexor digitorum brevis, digit I | Flexor brevis superficialis, digit I |
| Adductor alulae | Flexor digitorum brevis profundus, digit I | Lumbricals (part), digit I | Flexor brevis profundus, digit I |
| Abductor digiti majoris | Flexor digitorum brevis profundus, digit II | Lumbricals (part), digit II | Flexor brevis profundus, digit II |
| Flexor digiti minoris | Flexor digitorum brevis profundus, digit III | Lumbricals (part), digit III | Flexor brevis profundus, digit III |
| Abductor alulae | Abductor metacarpi I | Flexor digitorum brevis, digit I deep part | Abductor pollicis brevis |
| ‘Abductor digiti minimi’ (only present in Struthio) | Abductor metacarpi V | Abductor digiti quinti | Abductor digiti minimi |
Independent characters with discrete states were created for the locations of the origin and insertion for each muscle of the antebrachium and manus (for a complete list of characters and codings, see Supporting Information). Each taxon was coded for these characters and ancestral states at each node were reconstructed using maximum likelihood in the program mesquite (Maddison & Maddison, 2010) employing a consensus phylogeny (Fig. 1) built from recent morphological (Livezey & Zusi, 2007) and molecular (Jetz et al. 2012; tree was constructed based on the backbone from Hackett et al. 2008) avian phylogenies in combination with a recent lepidosaurian tree (Conrad, 2008), a recent total-evidence testudine tree (Sterli, 2010), and a review of crocodylian phylogenetics (Brochu, 2003) given the absence of phylogenetically unstable crocodylian taxa in the dataset. The phylogenetic placement of testudines is controversial (e.g. Werneburg & Sánchez-Villagra, 2009; Lyson et al. 2010, 2012; Crawford et al. 2012). Here they are placed outside of Sauria (Archosaurs + Lepidosaurs), but an arrangement of testudines as the sister to archosaurs does not substantially alter the results. Reconstructions were also tested on the independent molecular and morphological trees to assess their robustness to varying phylogenies. Proportional probabilities of the possible character states at the nodes surrounding Dinosauria (Supporting Information Table S2) were combined with observations of osteological correlates of muscle attachment sites in Tawa and used to create a map of the origin and insertion sites for each muscle. Reconstruction of the muscles crossing the shoulder utilized the results of Jasinoski et al. (2006) combined with observations of the osteological features of the scapula and humerus of Tawa and other early theropods.
Figure 1.
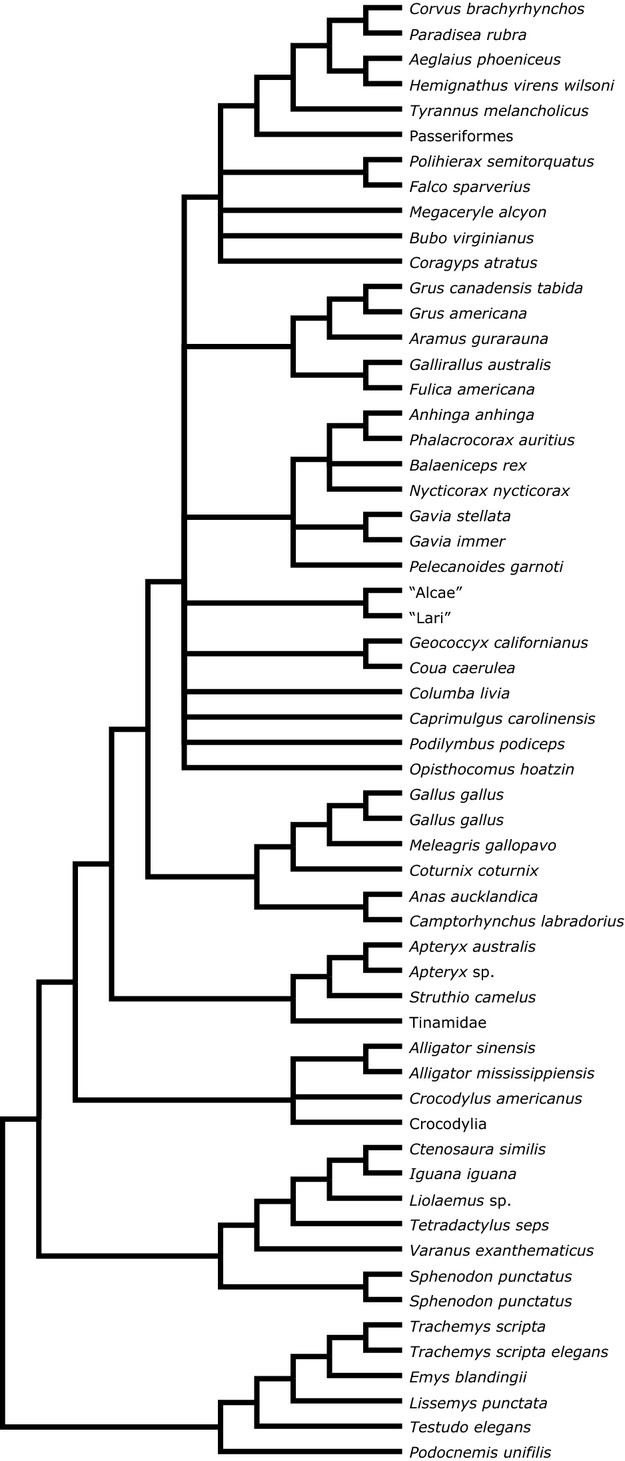
Consensus phylogeny of all extant taxa used in this analysis, based on the recent phylogenies of Livezey & Zusi (2007), Jetz et al. (2012), Conrad (2008), and Sterli (2010).
The designation of levels of inference are as follows: Level I inference is assigned if the proportional probability of a particular character state is > 0.50 for both of the nodes immediately above and below Dinosauria (Aves and Archosauria, respectively). A Level II inference is assigned if only one of these nodes possesses a proportional probability > 0.50 for a character state. If neither node shows a proportional probability of > 0.50, this is designated as a Level III inference. In all cases, the ‘prime’ level (i.e. Level I′, II′, and III′) is assigned if osteological evidence that supports the character state is not present. Prime levels are ranked below non-primes of the same level, but are preferred over non-primes of a lower level (i.e. Level I′ is preferred over Level II). In this analysis, Level II inferences are minimally required to reconstruct a feature. It is important to note that especially in the case of the manus, osteological evidence is not limited to muscle scars, crests, and tubercles, but also to the overall morphology of the elements. Thus certain muscles are reconstructed with a plesiomorphic morphology because the carpus and manus of basal theropods bears a stronger osteological similarity to the manus of crocodylians, lepidosaurs, and testudines than that of birds. If the majority of the outgroup taxa share identical muscle morphologies, the plesiomorphic morphology is accepted as the most parsimonious to reconstruct in dinosaurian taxa.
Results
The following reconstruction is divided into two sections. The first contains a description of the morphology of the muscles of the shoulder and brachium based on a reappraisal of the shoulder reconstruction of Jasinoski et al. (2006), applied to the forelimb of Tawa. The second part is a novel reconstruction of the antebrachial and manual musculature in Tawa based on new data and analyses. In this section the proportional probabilities of the relevant nodes are given. Reconstructions for each forelimb element are given in Figs 5, and a left lateral view of the articulated forelimb is shown in Fig. 6. Comparisons with other muscular reconstructions are presented elsewhere (see Discussion).
Figure 5.
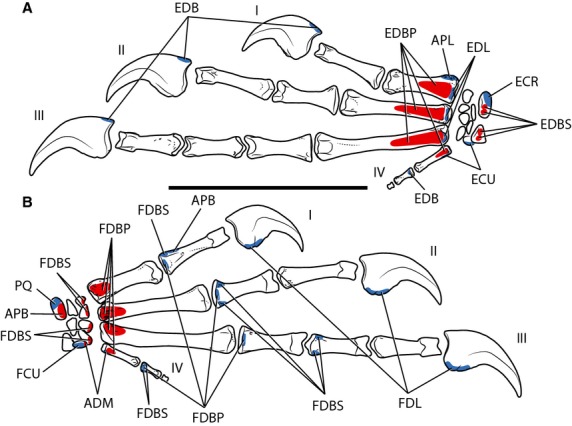
Myological reconstruction of the carpus and manus of Tawa hallae in dorsal (A) and ventral (B) views. Proposed muscle origins are indicated in red, proposed insertions are indicated in blue. APB, Abductor pollicis brevis; APL, Abductor pollicis longus; ADM, Abductor digiti minimi; ECR, Extensor carpi radialis; ECU, Extensor carpi ulnaris; EDB, Extensor digitorum brevis; EDBP, Extensor digitorum brevis profundus; EDBS, Extensor digitorum brevis superficialis; EDL, Extensor digitorum longus; FCU, Flexor carpi ulnaris; FDBP, Flexor digitorum brevis profundus; FDBS, Flexor digitorum brevis superficialis; FDL, Flexor digitorum longus; I, Digit I; II, Digit II; III, Digit III; IV, Digit IV; PQ, Pronator quadratus. Scale bar: 5 cm.
Figure 6.
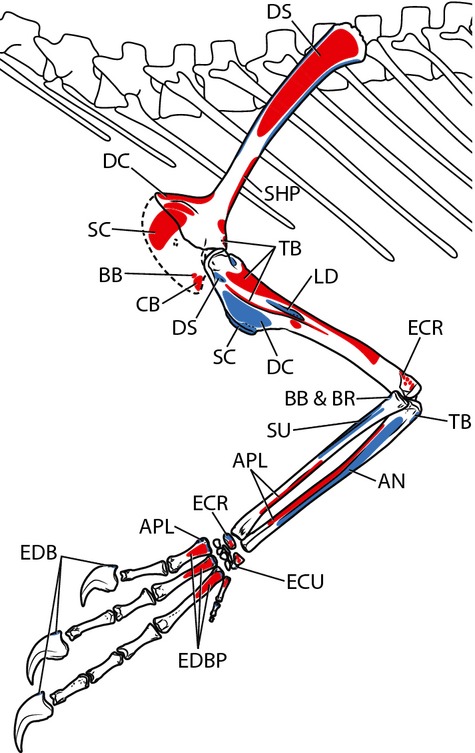
Myological reconstruction of the articulated pectoral girdle and forelimb of Tawa hallae in left lateral view with selected muscle attachment sites labeled. Proposed muscle origins are indicated in red, proposed insertions are indicated in blue. For muscle abbreviations see Figs 5 and text. Not to scale.
Pectoral and brachial musculature
Serratus superficialis (SS)
Serratus superficialis is phylogenetically unequivocally present in theropods, but the full extent of the origin is phylogenetically equivocal and not marked by osteological scars (Jasinoski et al. 2006). In both crocodylians and birds this broad, sheet-like muscle takes its origin from the lateral surfaces of the anterior dorsal ribs, extending to the cervical ribs in birds and some of the thoracic musculature in crocodylians (Jasinoski et al. 2006). In Tawa the origin is tentatively and conservatively reconstructed as arising from the lateral surfaces of the posteriormost cervical and anteriormost two to three dorsal ribs.
Based on a tubercle present in neognath birds and the oviraptorosaur Ingenia yanshini, Jasinoski et al. (2006) reconstructed this muscle as being composed of two separated divisions (cranial and caudal) at its insertion. The tubercle, located on the posteroventral surface of the scapular blade approximately one-third the way along the scapula from the proximal end, is the point of insertion of the cranial portion of this muscle. A scar in this area, varying in development from a simple tubercle to an elongate, rugose groove, is present in many coelurosaurian theropod taxa besides Ingenia but is absent in all non-tetanuran theropod taxa, including Herrerasaurus, Coelophysis, Sanjuansaurus, and Tawa. This lack of differentiation may indicate the retention of a single, elongate insertion along the posteroventral edge of the distal two-thirds of the scapular blade, as in crocodylians (Meers, 2003) and lepidosaurs (Russell & Bauer, 2008), and this morphology is reconstructed in Tawa (Fig. 2). With this morphology, the Serratus superficialis would have acted to retract and depress the scapula.
Figure 2.
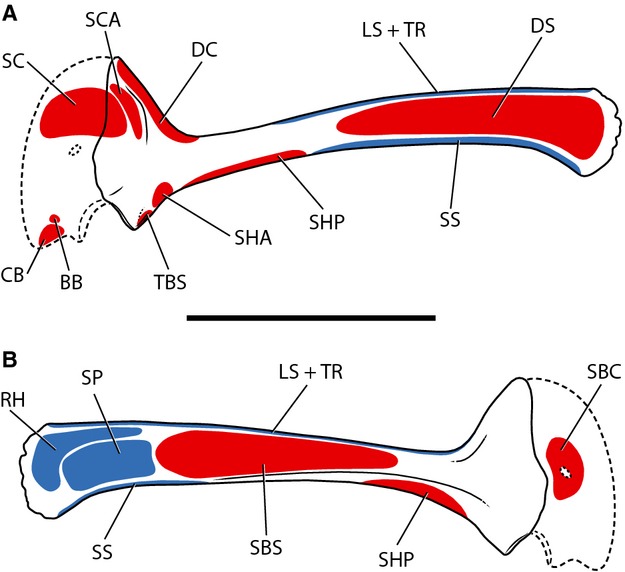
Myological reconstruction of the scapulocoracoid of Tawa hallae in lateral (A) and medial (B) views. Proposed muscle origins are indicated in red, proposed insertions are indicated in blue. BB, Biceps brachii; CB, Coracobrachialis; DC, Deltoideus clavicularis; DS, Deltoideus scapularis; LS, Levator scapulae; RH, Rhomboideus; SBC, Subcoracoideus; SBS, Subscapularis; SC, Supracoracoideus; SCA, Supracoracoideus accessorius; SHA, Scapulohumeralis anterior; SHP, Scapulohumeralis posterior; SP, Serratus profundus; SS, Serratus superficialis; TBS, Triceps brachii scapularis; TR, Trapezius. Scale bar: 5 cm.
Serratus profundus (SP)
As with Serratus superficialis, Serratus profundus is phylogenetically unequivocally present in theropods, although its origin is equivocal. It also originates from the anteriormost dorsal ribs in both birds and crocodylians but, unlike Serratus superficialis, it attaches close to the dorsal vertebrae and also takes its origin from the cervical and dorsal vertebrae in birds (Jasinoski et al. 2006). A likely origin for this muscle in Tawa would have been from the anteriormost dorsal ribs close to their articulation with the dorsal vertebrae.
The insertion of Serratus profundus is found on the medial surface of the distal end of the scapular blade in both crocodylians and birds (Jasinoski et al. 2006). There are no osteological signs on the scapula of Tawa that indicate the extent of the insertion of this muscle, but it is likely to have inserted over most of the distal half to one-third of the scapular blade (Fig. 2). With this morphology, Serratus profundus would have acted to protract the scapula.
Rhomboideus (RH)
The division of Rhomboideus into superficialis and profundus divisions is equivocal in theropods. A profundus division is only found in birds, and is reconstructed in dromaeosaurids by Jasinoski et al. (2006) on the basis of a likely subhorizontal position of the scapular blade in that clade. Ancestrally, in theropods the position of the scapular blade was more sharply angled (Senter, 2006) so, in the absence of any other osteological evidence, the profundus division is not reconstructed in Tawa. Rhomboideus has an equivocal origin on the body wall, which is dependent on the orientation of the scapular blade. Because Tawa likely possessed a scapular orientation somewhere in between that of birds (subhorizontal) and crocodylians (subvertical), it is possible that the origin of Rhomboideus was also intermediately located, attaching to both the fascia of the dorsal cervico-thoracic region and several neural spines of the posteriormost cervical and anteriormost dorsal vertebrae (Jasinoski et al. 2006).
Based on scapular orientation, the insertion of Rhomboideus in Tawa is reconstructed as attaching in a somewhat intermediate location on the anterior half of the distalmost portion of the medial scapular blade (Fig. 2). This differs from a more bird-like reconstruction along the anterior edge of the scapula provided by Jasinoski et al. (2006) in dromaeosaurids based on a subhorizontal orientation of the scapula. With this morphology, Rhomboideus would have acted to protract the scapula.
Levator scapulae (LS)
This muscle is not present in birds, and Jasinoski et al. (2006) did not reconstruct it as present in dromaeosaurids but noted that some non-coelurosaurian theropods possess muscle scars on the scapula that may correspond to the superficial part of this muscle. In crocodylians the superficial Levator scapulae inserts on the anterior edge of the scapular blade along most of its length posterior to the acromial expansion and sometimes leaves a scar in this region (Meers, 2003). An elongate sulcus or rugosity along the anterodorsal edge of the scapular blade can be found not only in ceratosaurs and tetanurans (Jasinoski et al. 2006) but also tyrannosaurids such as Tarbosaurus (MPC-D 107/2). This scar is not known from any early theropod, but its presence in more derived taxa provides a phylogenetic bracket to reconstruct this muscle (Fig. 2). The origin of Levator scapulae in nonavian theropods is equivocal but would most likely be from the cranial cervical ribs, as in crocodylians (Meers, 2003). With this morphology it would have acted as a rotator of the scapular blade, as well as a lateral flexor of the neck.
Trapezius (TR)
The presence of Trapezius in nonavian theropods follows the same pattern as Levator scapulae, although this muscle lacks any osteological correlates. If Levator scapulae and Trapezius are hypothesized to have been lost due to the reorientation of the scapular blade into a subhorizontal position in birds (following Jasinoski et al. 2006), they may be reconstructed in theropods that lack this scapular orientation (i.e. most non-maniraptorans). Given osteological evidence for the presence of Levator scapulae in nonavian theropods, the Trapezius is also reconstructed as present in these taxa.
The Trapezius is a broad, fan-shaped muscle and would have taken its origin from the median parts of the cervical and thoracodorsal fascia covering the axial musculature, as in crocodylians and lepidosaurs (Meers, 2003; Russell & Bauer, 2008). In these taxa, Trapezius inserts on the anterior edge of the acromion and acromial expansion of the scapula. In crocodylians the insertion of this muscle is often intermingled with the insertion of Levator scapulae (Meers, 2003). Because of this, Trapezius is reconstructed as inserting together with Levator scapulae in Tawa, but would primarily have been restricted to the proximal part of this insertion site (Fig. 2). With this morphology, the Trapezius would have acted to rotate the scapular blade, likely assisting in protraction of the forelimb, as in chameleons (Peterson, 1984).
Latissimus dorsi (LD)
This superficial muscle is composed of a broad, thin sheet in crocodylians and lepidosaurs with a long, linear origin arising from the neural spines of the last cervical vertebra to the sixth or seventh dorsal vertebra and/or the thoracodorsal fascia near the vertebral column in that area (Meers, 2003; Russell & Bauer, 2008). In birds this muscle is divided into two parts, but they are variably present across the clade and sometimes form an almost continuous sheet of muscle (George & Berger, 1966). As such, Latissimus dorsi is reconstructed as a single muscle in theropods (Jasinoski et al. 2006). Although the exact extent of the origin in theropods is equivocal, the muscle arises from the same general area in all taxa studied, and thus can be reconstructed as most likely originating from the neural spines or thoracodorsal fascia in the region of the first to fifth dorsal vertebrae.
A muscle scar for the insertion of Latissimus dorsi on the lateral side of the humerus posterior to the deltopectoral crest is present in crocodylians, birds, and lepidosaurs, and may be expressed as a rugose tubercle, crest, pit or linear sulcus (Meers, 2003; Jasinoski et al. 2006). The linear sulcus reported to be present in this region by Jasinoski et al. (2006) in dromaeosaurids and troodontids can be found in many theropods, including Tawa, and likely represents the insertion site of Latissimus dorsi in these taxa (Fig. 3). With this morphology, Latissimus dorsi would have acted to retract the humerus.
Figure 3.
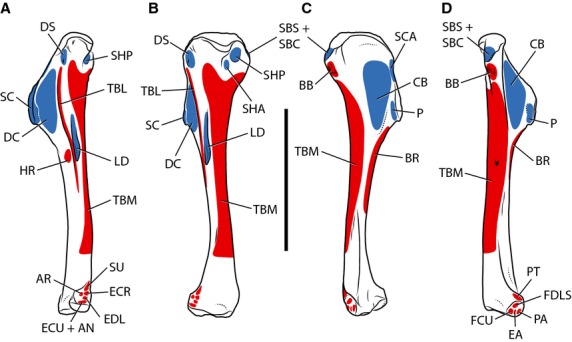
Myological reconstruction of the humerus of Tawa hallae in lateral (A), posterior (B), anterior (C), and medial (D) views. Proposed muscle origins are indicated in red, proposed insertions are indicated in blue. AN, Anconeus; AR, Abductor radialis; BB, Biceps brachii; BR, Brachialis; CB, Coracobrachialis; DC, Deltoideus clavicularis; DS, Deltoideus scapularis; EA, Epitrochleoanconeus; ECR, Extensor carpi radialis; ECU, Extensor carpi ulnaris; EDL, Extensor digitorum longus; FCU, Flexor carpi ulnaris; FDLS, Flexor digitorum longus superficialis; HR, Humeroradialis; LD, Latissimus dorsi; P, Pectoralis; PA, Pronator accessorius; PT, Pronator teres; SBC, Subcoracoideus; SBS, Subscapularis; SC, Supracoracoideus; SCA, Supracoracoideus accessorius; SHA, Scapulohumeralis anterior; SHP, Scapulohumeralis posterior; SU, Supinator; TBL, Triceps brachii longus; TBM, Triceps brachii medialis. Scale bar: 5 cm.
Pectoralis (P)
Pectoralis has a broad origin involving a variety of elements of the pectoral girdle in archosaurs and lepidosaurs, but they share a common area of origin on the ventral surface of the sternum. There are currently no sternal plates known for basal theropods, although it is presumed that the elements were present but cartilaginous in these taxa (Padian, 2004). Reconstructing additional areas of origin from the sternal ribs (as in crocodylians) or the coracoid (as in Struthio) requires a Level II′ inference, although unlike Jasinoski et al. (2006) this analysis does not eliminate an origin from the coracoid based on the presence of Coracobrachialis longus in this location (see below). Due to a lack of ossified and preserved elements in this area of the pectoral girdle, it is difficult to assign the exact boundaries of origin with any certainty.
The insertion of Pectoralis is unequivocally located on the medial surface of the deltopectoral crest. Unlike the condition in dromaeosaurids (Jasinoski et al. 2006), however, there is a scar for this insertion in Tawa expressed as a small, oblong depression on the medial surface of the deltopectoral crest near its tip (Fig. 3). This limited insertion area is similar to the insertion in crocodylians (Meers, 2003) and is less extensive than the insertion in birds, which extends over much of the medial surface of the deltopectoral crest (Jasinoski et al. 2006). The action of Pectoralis would have been to adduct and protract the humerus.
Subscapularis (SBS)
The origin of Subscapularis is unequivocally located on the medial surface of the scapular blade. As in dromaeosaurids (Jasinoski et al. 2006) and many other theropods, Tawa possesses a distinct ridge on the medial surface of the scapula that extends along the proximal half to two-thirds of the scapula. Jasinoski et al. (2006) noted that a ridge in a similar location defining the dorsal edge of the origin of Subscapularis is also present in Meleagris and used the ridge as evidence for an origin ventral to this ridge in dromaeosaurids. However, this ridge is also present in crocodylians (Meers, 2003) and the ventral fossa it creates is instead part of the site of origin of Scapulohumeralis posterior (see below). In Tawa this ridge is ventrally shifted from the midline and curves distally to meet the posteroventral edge of the scapula less than half-way along the scapular blade, resulting in an extremely reduced potential area of origin, whereas the flaring blade of the scapula provides an extensive surface for an origin more similar to that in crocodylians. It is possible that the origin of this muscle migrated ventrally to the medial ridge as the scapular orientation became more subhorizontal and bird-like in theropods (e.g. dromaeosaurids), but in Tawa it is reconstructed in a more dorsal location based on the reduced attachment area ventrally for this typically large muscle (Fig. 2).
The insertion site of this muscle is unequivocally located on the internal tuberosity of the humerus (Fig. 3), sharing an insertion tendon with Subcoracoideus (Jasinoski et al. 2006). Regardless of the exact location of the origin of Subscapularis, the primary action of this muscle would have been to retract and rotate the humerus.
Subcoracoideus (SBC)
Subcoracoideus is not an independent muscle in crocodylians and is instead fused to Subscapularis. In birds and lepidosaurs, however, it possesses a separate insertion on the medial surface of the coracoid, and thus it can be unequivocally reconstructed as distinct in theropods (Jasinoski et al. 2006). It is unknown how extensive the origin was in theropods, but in the absence of contrary evidence it is here reconstructed as in Jasinoski et al. (2006) as a small area covering the coracoid foramen (Fig. 2).
As mentioned above, Subcoracoideus shares a tendon of insertion with Subscapularis, which inserts on the internal tuberosity of the humerus (Fig. 3). With this morphology, Subcoracoideus would have adducted and laterally rotated the humerus.
Supracoracoideus (SC)
The extent of the origin of Supracoracoideus is variable, primarily arising from the coracoid but with attachments to the scapula in crocodylians and to the sternum in neognathous birds. Minimally it originated from the coracoid in theropods, potentially in the anterodorsal quadrant (Jasinoski et al. 2006). The subacromial depression of the scapula of nonavian theropods may represent the extension of the Supracoracoideus origin onto the scapula. This depression is usually continuous with the adjacent lateral surface of the coracoid, providing a broad, flat area for the origin of this muscle from both bones, as in crocodylians (Meers, 2003). Reconstruction of the Supracoracoideus accessorius muscle (see below) indicates that the subacromial depression may have housed the supracoracoideus complex of muscles, and it is reconstructed this way in Tawa (Fig. 2).
The area of insertion of Supracoracoideus is phylogenetically equivocal, inserting on the tip and nearby portion of the lateral surface of the deltopectoral crest in crocodylians, and on the posterior surface of the greater tubercle in birds. Jasinoski et al. (2006) reconstructed the insertion as in that of birds based on the presence of a rugose depression on the anterior surface of the greater tubercle in Velociraptor, but an insertion in this location is unlikely in earlier theropods. In neognathous birds Supracoracoideus is highly modified to provide elevation and rotation of the wing during upstroke (Poore et al. 1997), using an osteological structure of the scapulocoracoid called the triosseal canal that is not found in nonavian theropods. Without this specialized osteology, an insertion of Supracoracoideus on the greater tubercle in theropods would result in a nearly non-functional morphology. With an insertion on the tip of the deltopectoral crest, however, Supracoracoideus would retain its capabilities as a strong protractor of the humerus, as in crocodylians (Meers, 2003). Furthermore, the humerus of Tawa possesses a small oblong depression, located on the lateral surface of the deltopectoral crest immediately adjacent to its tip, that is consistent with this site of insertion and indicates the extent of the lateral excursion of the insertion (Fig. 3). With this morphology, Supracoracoideus would have acted as a protractor and slight abductor of the humerus.
Supracoracoideus accessorius (SCA)
Reconstruction of this muscle is based on a new hypothesis of homology presented here. The homology of the avian Deltoideus minor in crocodylians is controversial; typically it is regarded as a novel muscle in birds and thus lacking a homolog in crocodylians (Dilkes, 2000; Jasinoski et al. 2006) or homologized with the Deltoideus clavicularis (Diogo & Abdala, 2010). This confusion stems from conflicting evidence from different homology criteria. Embryologically, the Deltoideus minor is not a member of the deltoid group, although it arises from a similar area to the Deltoideus major in birds (Sullivan, 1962; see below). The avian Deltoideus minor is actually a derivative of the Supracoracoideus muscle mass, which is also closely related to the Coracobrachialis muscle mass in all reptiles (Romer, 1944; Sullivan, 1962). However, as pointed out by Sullivan (1962), the Deltoideus minor shares its innervation with the rest of the deltoid group via the Axillary nerve, but he argues that this innervation is derived from a common nerve trunk that originates at the triosseal canal. Topologically, the Deltoideus minor typically arises from the lateral surface of the acromion of the scapula, sometimes including the adjacent lateral coracoid, and inserts just distal to the proximal articular surface of the humerus, often along the proximal edge of the deltopectoral crest (Hudson & Lanzillotti, 1955; George & Berger, 1966; Jasinoski et al. 2006). This pattern of attachment and development almost exactly matches that of a small, semi-independent muscle in crocodylians. It is sometimes described as part of the Supracoracoideus; Dilkes (2000) labeled it as Supracoracoideus pars scapularis, and although Jasinoski et al. (2006) designate the Supracoracoideus complex as a single muscle, they describe a separate ‘M. supracoracoideus’ that does not share an origin or insertion with the other parts of Supracoracoideus (longus and intermedius) on the tip of the deltopectoral crest. Meers (2003) separated this muscle from the Supracoracoideus complex completely and called it the M. coracobrachialis brevis dorsalis. Despite the lack of support from the nerve supply, the homology hypothesis of Sullivan (1962) is used here based on support from the two homology criteria of development and topology. Future studies of the arrangement of the vasculature in relation to the muscles may be able to provide a fourth criterion to evaluate the homology of this muscle. This muscle is given the name Supracoracoideus accessorius based on its derivation from the Supracoracoideus group developmentally, but it is distinct from the other muscles of this group.
In nonavian theropods, the reconstruction of both the origin and insertion of this muscle are unequivocal. It would have originated from the subacromial depression of the scapula, possibly sharing this area with the Supracoracoideus (Fig. 2), and inserted on the proximal edge of the deltopectoral crest between the greater tubercle and the tip of the crest (Fig. 3). With this morphology the Supracoracoideus accessorius would have acted with the Supracoracoideus to protract and abduct the humerus.
Coracobrachialis (CB)
The origin of this muscle can be unequivocally reconstructed based on an origin from the posteroventral portion of the lateral surface of the coracoid in crocodylians and paleognathous birds and its position posterior to the origin of Biceps brachii in neognaths. As noted by Jasinoski et al. (2006), the posteroventral process of the coracoid in many theropods possesses a distinct subglenoid fossa that is the likely location for the origin of this muscle (Fig. 2).
The insertion site of this muscle is also phylogenetically unequivocal, located on the anterior surface of the humerus distal to the proximal articular surface and extending onto the medial surface of the deltopectoral crest. In many theropods, including Tawa, there is a broad, subtriangular depression in this area that covers most of the anterior surface of the humerus with a distally pointing apex that extends just distal to the end of the deltopectoral crest. This depression likely served as the insertion site of Coracobrachialis (Fig. 3). With this morphology, the primary action of this muscle would have been protraction of the humerus.
Coracobrachialis longus (CBL)
The presence of this muscle is phylogenetically equivocal, as it is not present in crocodylians. Although Jasinoski et al. (2006) reconstructed this muscle as unequivocally present based on the report of its presence in crocodylians by Nicholls & Russell (1985), they themselves did not find the muscle in any of their dissections, nor has the muscle or anything fitting its description been reported in any other discussion of crocodylian musculature (Romer, 1944; Holmes, 1977; Cong et al. 1998; Dilkes, 2000; Meers, 2003). In the face of this evidence, I regard the Coracobrachialis longus to be absent in crocodyliforms. Furthermore, the homology of the Coracobrachialis posterior of neognathous birds and the Coracobrachialis longus of lepidosaurs is uncertain. The muscle known as Coracobrachialis posterior in birds is a derivative of the Subcoracoideus muscle and part of the dorsal muscle mass (Sullivan, 1962), whereas the Coracobrachialis longus of lepidosaurs is related to the Biceps brachii and Supracoracoideus and is part of the ventral muscle mass (Romer, 1944). Thus the Coracobrachialis longus of lepidosaurs and the Coracobrachialis posterior of neognathous birds are not regarded as homologous, and reconstruction of Coracobrachialis longus in theropods becomes a Level II′ inference based on its status as a novel muscle in neognathous birds.
Scapulohumeralis posterior (SHP)
Scapulohumeralis posterior originates from the posteroventral part of the lateral surface of the scapular blade in both crocodylians and birds. The origin in birds is typically much more extensive distally than that of crocodylians, but in Struthio the origin is restricted to a narrow area along the posteroventral edge near the glenoid that closely matches the condition in crocodylians (Jasinoski et al. 2006). In crocodylians the origin of Scapulohumeralis posterior also wraps around the posteroventral edge of the scapula near the glenoid and inserts in the area ventral to the medial ridge of the scapula (Meers, 2003). Tawa possesses a similarly small area ventral to the medial ridge (see above), so the origin may have extended onto the medial surface in basal theropods as well (Fig. 2).
The insertion of Scapulohumeralis posterior is unequivocally on the posterior surface of the proximal humerus. Although it can be extensive in some crocodylians (Meers, 2003), a more restricted insertion on the posterior surface of the internal tuberosity, similar to the insertion area in birds, has also been reported (Jasinoski et al. 2006). Similar to that of some dromaeosaurids, the humerus of Tawa has an oval depression on the posterior surface of the internal tuberosity that may correspond to the insertion site of this muscle (Fig. 3). With this morphology, Scapulohumeralis posterior would have acted to retract the humerus.
Scapulohumeralis anterior (SHA)
Scapulohumeralis anterior is reconstructed in nonavian theropods based on its presence in birds (including tinamous) and lepidosaurs, although it has been lost in extant crocodylians and ratites (Jasinoski et al. 2006). In most lepidosaurs this muscle is composed of two parts, and the origin of this muscle in birds on the scapular blade near the glenoid cavity most closely matches with the short-fibered part of this muscle in lepidosaurs. The absence of the long-fibered part of this muscle in chameleons is related to increased humeral mobility relative to terrestrial lizards (Jasinoski et al. 2006), and it is likely that this is also the case in nonavian theropods. Jasinoski et al. (2006) assigned the origin of Scapulohumeralis anterior in dromaeosaurids to a small oval rugosity on the posteroventral portion of the scapular blade. No such scar exists among early theropods, but both Herrerasaurus (PVSJ 53) and Sanjuansaurus (PVSJ 605) possess a weak fossa on the posteroventral part of the scapular blade dorsal to the insertion area of Triceps brachii scapularis (see below) that may represent the area of origin for this muscle (Fig. 2).
The insertion of Scapulohumeralis anterior in birds and lepidosaurs is tendinous in a relatively small area on the posterior surface of the proximal end of the humerus, although it inserts farther laterally in lepidosaurs than in birds (Jasinoski et al. 2006). Unfortunately, there is no osteological correlate for the insertion of this muscle in nonavian theropods as there is in birds (i.e. the pneumatic fossa). In this study it is reconstructed as inserting just distal and lateral to the insertion of Scapulohumeralis posterior and medial to a ridge that extends down the posterior side of the proximal end of the humerus from the middle of the posteriorly projecting humeral head (Fig. 3). The action of Scapulohumeralis anterior would have primarily been to retract the humerus.
Deltoideus clavicularis (DC)
The reconstruction of Deltoideus clavicularis is not straightforward due to the morphology of its homolog in birds, Propatagialis. This homology is supported by the embryological origin of Propatagialis from the Deltoideus group musculature (Howell, 1937; Sullivan, 1962). Deltoideus clavicularis is not homologous with the avian Deltoideus minor as suggested by Diogo & Abdala (2010) (who erroneously ascribed this homology hypothesis to Dilkes, 2000) because Deltoideus minor is developmentally distinct from the rest of the Deltoideus musculature (see above; Sullivan, 1962). Meers (2003) suggested that Propatagialis is homologous to the crocodylian Humeroradialis, but there is no other published evidence for this hypothesis. Propatagialis is a highly modified muscle relating to the propatagium of the avian wing, and may consist of more than one belly or tendon of insertion (George & Berger, 1966). Crocodylians and some birds share a common area of origin on the scapula on or near the anterior edge of the acromion process, so I reconstruct the Deltoideus clavicularis as taking origin from the anterior edge of the acromion process and acromial expansion in early theropods. This differs from the reconstruction of Jasinoski et al. (2006), who placed the origin in the subacromial depression. The origin of Deltoideus clavicularis is nearly linear and restricted to the anterodorsal edge of the acromion process in all of the extant taxa studied, and I have found no evidence for the extension of this attachment site onto the lateral surface of the scapula ventral to the acromion process. Instead, the dorsal edge that bounds this depression likely represents the ventral extent of this muscle onto the scapula (Fig. 2). In birds, this muscle also originates from the dorsal surface of the furcula (clavicle), and this area of origin is also present in lepidosaurs (Jasinoski et al. 2006), suggesting that it has been independently lost in modern crocodylians. Although there is no furcula preserved in Tawa, furculae are known for many theropods including Coelophysis (Rinehart et al. 2007; Nesbitt et al. 2009b), and so the origin of Deltoideus clavicularis is reconstructed as extending onto the hypothetical furcula in this taxon.
The avian Propatagialis has a primary insertion in the region of the carpus in birds, which is highly modified from the state exhibited by its homolog in crocodylians and lepidosaurs. However, the fleshy belly itself extends only to the distal end of the deltopectoral crest in most birds, the rest of the length being composed of a long tendon (George & Berger, 1966). The insertion of Deltoideus clavicularis in crocodylians and in Sphenodon is broadly on the lateral surface of the deltopectoral crest (Dilkes, 2000; Meers, 2003), a location that is filled by the homolog of Deltoideus scapularis in birds (see below). The insertion of Deltoideus clavicularis is here reconstructed as occupying a relatively large area on the lateral surface of the deltopectoral crest, posterior to the insertion of the Supracoracoideus musculature. In many theropods, including Tawa, this area is set off from the humeral shaft by a low ridge, indicating the posterior extent of this muscle in these taxa (Fig. 3). With this morphology Deltoideus clavicularis would have acted to abduct and slightly protract the humerus.
Deltoideus scapularis (DS)
As with Deltoideus clavicularis, the avian homolog of Deltoideus scapularis is modified relative to its morphology in crocodylians and lepidosaurs. Its origin has shifted proximally from the primitive location of a broad area on the lateral surface of the distal half of the scapula to a location on the acromion process, near the origin of the Deltoideus clavicularis homolog (Jasinoski et al. 2006). Due to the specialized attachment of the Deltoideus clavicularis homolog on the carpus, Deltoideus scapularis assumes its functional role in birds. As a result, its action as an abductor of the humerus is diminished, but this is compensated by the development of Supracoracoideus. In basal nonavian theropods, where the primitive attachments of Deltoideus clavicularis are retained and Supracoracoideus is not modified to provide strong humeral abduction (see above), it is unlikely that the origin of Deltoideus scapularis would take the proximal position seen in birds. Furthermore, the broad, distally flaring scapula provides a large potential area of attachment for this muscle. Thus, this muscle is reconstructed as originating on the lateral surface of the distal end of the scapula (Fig. 2).
In crocodylians and Sphenodon, Deltoideus scapularis inserts in a small area on the posterior surface of the proximal end of the humerus, just distal to the greater tubercle (Dilkes, 2000). The insertion in birds is shifted distally, covering most of the lateral surface of the deltopectoral crest and in some cases extending down the humeral shaft to the ectepicondylar process (George & Berger, 1966). Following the reconstruction of the origin of this muscle as in crocodylians, the insertion is also reconstructed in the more primitive location. In Tawa there is a small, oval depression containing striations in this location that likely represents a scar for this muscle (Fig. 3). As reconstructed, Deltoideus scapularis would have acted to abduct and retract the humerus.
Triceps brachii (TB)
Although Triceps brachii can be unambiguously reconstructed, the number of heads that it possessed is equivocal phylogenetically. Birds and crocodylians both have the scapular and medial heads, but the coracoid head is vestigial in birds and the lateral head has been completely lost.
The origin of Triceps brachii caput scapulare (TBS) is conserved across archosaurs and lepidosaurs. It has a tendinous origin from a small area just posterodorsal to the scapular lip of the glenoid fossa, often associated with a scar in the form of a rugose tubercle (Jasinoski et al. 2006). A rugosity in this area is variably developed across Theropoda and, although no distinct tubercle appears in this location in Tawa, the area is lightly striated (Fig. 2).
Although Triceps brachii caput coracoideum (TBC) can be found in some neognathous birds, the muscle belly is extremely reduced and thought possibly to function as a mechanoreceptor in the wing (Vanden Berge & Zweers, 1993). As has been suggested for dromaeosaurids, Triceps brachii caput coracoideum may have already been vestigial or absent in basal theropods based on evidence from chameleons, in which this muscle has been lost to improve humeral mobility (Jasinoski et al. 2006).
Triceps brachii caput mediale (TBM) has a wide, fleshy origin on the posteromedial surface of the shaft of the humerus in both birds and crocodylians, although the exact boundaries of the origin are slightly variable. In both taxa the medial head of the triceps is bifid proximally, extending on either side of the insertion of Scapulohumeralis posterior (in crocodylians) and anterior (in birds; Jasinoski et al. 2006). It extends distally until the humeral shaft to flares and almost completely covers the humeral shaft, except at its anterolateral margin (Fig. 3). There are no muscle scars associated with the origin of Triceps brachii caput mediale in theropods.
Jasinoski et al. (2006) did not reconstruct Triceps brachii caput laterale (TBL) as present in dromaeosaurids based on the lack of a clear lateral triceps ridge as is seen in crocodylians (Meers, 2003). However, a ridge in this area, used to define the posterior border of Deltoideus clavicularis (see above) is found in many other theropods, including Tawa, and likely represents the linear area of origin for this head of triceps (Fig. 3).
All three heads of Triceps brachii coalesce into a single tendon that inserts on the olecranon process of the ulna. Although the Tawa olecranon is short, it does have faint striations on its posterior surface, indicating the point of insertion of this muscle (Fig. 4). Triceps brachii would have acted as the primary extensor of the antebrachium, as well as contributing to the extension of the humerus.
Figure 4.
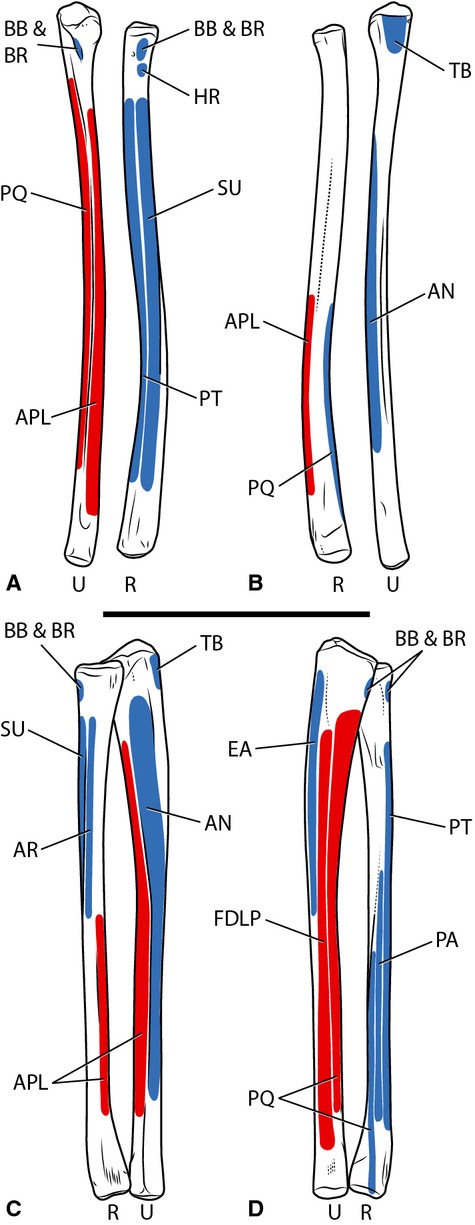
Myological reconstruction of the antebrachium of Tawa hallae in anterior (A), posterior (B), lateral (C), and medial (D) views. Proposed muscle origins are indicated in red, proposed insertions are indicated in blue. AN, Anconeus; APL, Abductor pollicis longus; AR, Abductor radialis; BB, Biceps brachii; BR, Brachialis; EA, Epitrochleoanconeus; FDLP, Flexor digitorum longus profundus; HR, Humeroradialis; PA, Pronator accessorius; PQ, Pronator quadratus; PT, Pronator teres; R, Radius; SU, Supinator; TB, Triceps brachii; U, Ulna. Scale bar: 5 cm.
Biceps brachii (BB)
The primary head of Biceps brachii, originating from the coracoid, was unequivocally present in nonavian theropods, but the presence of a secondary head originating from the humerus is ambiguous phylogenetically. Of the study taxa, only neognathous birds possess a humeral head of biceps; in reptiles that do have two heads, both heads typically arise from the coracoid, one tendinously and the other fleshily (Diogo & Abdala, 2010). The tendinous origin of biceps from the coracoid is typically located on a tubercle anterior to the glenoid fossa in both crocodylians and birds, and the coracoid tubercle of theropod dinosaurs has generally been accepted as the site of origin for this muscle. Although there is some debate, it seems likely that the assignment of this tubercle as the origin of Biceps brachii is correct (for a review see Jasinoski et al. 2006). Further evidence is provided by tracing evolutionary changes in the morphology of the coracohumeral/acrocoracohumeral ligament, which attaches very near the origin of Biceps brachii in both crocodylians and birds (Baier et al. 2007). Typically, early theropods do not have prominent or even distinct coracoid tubercles (e.g. Coelophysis, Syntarsus) but the attachment site in these taxa would likely have been located anterior to the glenoid and just dorsal to the subglenoid fossa (Fig. 2). The humeral head of biceps in birds takes its origin from a round area on the anterior surface of the internal tuberosity (Jasinoski et al. 2006), and the presence of the secondary attachment is supported in nonavian theropods by an oval, striated depression in this area in Tawa, as well as similar rugosities and depressions in many other theropods (Fig. 3).
Biceps brachii inserts on the proximal ends of the radius and ulna in birds and in lepidosaurs, where the pattern is highly consistent across taxa (Russell & Bauer, 2008). In crocodylians, it is typically described as only possessing a radial insertion (Cong et al. 1998; Meers, 2003; Jasinoski et al. 2006), although a secondary attachment to the ulna has been reported (Reese, 1915). Based on the outgroup bracket provided by lepidosaurs, an ulnar insertion for biceps is reconstructed in nonavian theropods. The insertion sites do not typically leave a distinct scar on either bone in the extant taxa, but in Tawa there is a slight bulge on the anterior edge of the ulna just distal to the articular surface that likely corresponds to this attachment (Fig. 4). The primary action of Biceps brachii would have been to flex the antebrachium.
Humeroradialis (HR)
The homology of the crocodylian Humeroradialis is uncertain and controversial. It is sometimes considered to be a neomorphic archosaurian muscle (Meers, 2003; Diogo & Abdala, 2010) but it has also been homologized with the muscle of the same name in Sphenodon (Romer, 1944). Both of these muscles appear to be embryological derivatives of the deltoid muscle mass, although Humeroradialis in Sphenodon may have a compound origin as evidenced by the dual innervation pattern of this muscle (Russell & Bauer, 2008). Its potential origin from the deltoideus musculature is likely the reason it has been homologized to Propatagialis (tensor propatagialis) in birds (Meers, 2003) but because these muscles share neither a common origin nor insertion, here Propatagialis is considered to be the homolog of Deltoideus clavicularis (see above). Sullivan (1962) identified a distal portion of the developing deltoid lobe in an early-stage chicken embryo as possibly a transitory vestige of Humeroradialis, but this portion is not retained in the adult.
The presence of Humeroradialis in nonavian theropods was inferred by Jasinoski et al. (2006) based on the presence of a rugose tuberosity distal to the deltopectoral crest on the lateral surface of the humeral shaft in maniraptorans, which corresponds to scars for this muscle found on the humeral shafts of crocodylians in this location. Unfortunately, a scar in this area is rare in more basal taxa, although a small rugosity anterior to the furrow for Latissimus dorsi is present in one specimen of Herrerasaurus (PVSJ 407) and may represent an origin scar for Humeroradialis (Fig. 3). The insertion of this muscle in crocodylians is marked by a distinct tubercle (Meers, 2003), and some nonavian theropods (e.g. Herrerasaurus, PVSJ 373) exhibit a small tubercle on the anterior surface of the radius near its proximal end. This tubercle likely represents the insertion Humeroradialis (Fig. 4). Because the theropod Humeroradialis is reconstructed here following the morphology seen in crocodylians, the ligamentous sling on the proximal radius that redirects the insertion tendon of this muscle at the elbow (Meers, 2003) is also reconstructed. The action of Humeroradialis as reconstructed would have been to flex the antebrachium.
Brachialis (BR)
In all birds, Brachialis originates from the Fossa musculus brachialis, an impression on the cranial surface of the distal end of the humerus just proximal to the condyles (Baumel et al. 1993). This contrasts with its elongate origin from the distal part of the deltopectoral crest extending along much of the anterolateral surface of the humeral shaft in crocodylians (Meers, 2003), lepidosaurs (Russell & Bauer, 2008), and turtles (Walker, 1973). The anterior intercondylar depression, present in many theropod dinosaurs, may be evidence for the distal migration of this muscle in nonavian theropods. However, this feature is absent or poorly developed in basal theropods such as Tawa and Herrerasaurus, indicating that they likely retained the more proximal origin of Brachialis (Fig. 3).
Brachialis inserts in common with Biceps brachii on the proximal ends of the radius and ulna in crocodylians and lepidosaurs, whereas it is restricted to the proximal end of the ulna in birds, leaving a distinct Impressio brachialis in most taxa (Baumel et al. 1993). There is no evidence of an anterior ulnar depression in theropods, so the Brachialis is reconstructed as inserting as in crocodylians (Fig. 4). With this morphology its action would have been to flex the forearm.
Antebrachial musculature
Anconeus (AN)
This muscle of the dorsal division originates on the ectepicondyle of the humerus and inserts on the anterolateral surface of the ulna. Its presence in nonavian theropods is phylogenetically unequivocal. In birds it is known as Ectepicondylo-ulnaris (Vanden Berge & Zweers, 1993), and Meers (2003) refers to it as Flexor ulnaris (Table 1). Developmentally, it is closely connected to Extensor carpi ulnaris, which it is fused to for all or part of its length in some taxa (Haines, 1939; Sullivan, 1962). It is present in turtles (Haines, 1939; Walker, 1973) and Sphenodon (Miner, 1925; Haines, 1939) but has been lost in squamates (Russell & Bauer, 2008).
The origin of Anconeus is the most distal on the ectepicondyle in all taxa studied, with the exception of those in which it shares a tendon of origin with Extensor carpi ulnaris. The fusion of the tendon with Extensor carpi ulnaris is ancestral for Aves, with a 0.820 proportional likelihood at the node at the base of the clade. Unfortunately, there is little resolution on this point on the other side of the tree because crocodylians lack Extensor carpi ulnaris, Anconeus is absent in squamates, it is almost entirely fused to Extensor carpi ulnaris in turtles (Haines, 1939; Walker, 1973; Abdala et al. 2008), and both states have been reported in Sphenodon (Miner, 1925; Haines, 1939), leaving the proportional likelihoods at exactly 0.50 at the base of the archosaur clade. Based on these likelihoods, I tentatively reconstruct the muscle as arising from the ectepicondyle along with ECU in basal theropods (Fig. 3). Regardless, the muscle possesses a very distally located origin that is closely associated with that of ECU.
Anconeus can be reconstructed unequivocally as inserting fleshily on the lateral surface of the ulna starting just distal to the proximal articular surface and extending for most of its length, with a proportional probability of near 1.0 for both nodes. In Tawa, a prominent ridge on the lateral surface of the ulna beginning at midshaft and extending to the distal end provides a distinct surface for the distal extent of Anconeus and separates its insertion from the origin of Abductor pollicis longus (Fig. 4). The action of Anconeus would have been to flex the forearm.
Extensor carpi ulnaris (ECU)
The homologies of this muscle in archosaurs are not straightforward due to the general uncertainty of the homology of some crocodylian extensor musculature. Crocodylians possess a dorsal division muscle that arises from the middle of the ectepicondyle and inserts on the base of metacarpal II, with variable extensions to the bases of metacarpals I, III, IV and the radiale (Ribbing, 1907; Haines, 1939; Cong et al. 1998; Meers, 2003). Although Meers (2003) identified this muscle as Extensor carpi ulnaris, other authors have homologized this muscle with Extensor digitorum longus [communis] (Ribbing, 1907; Haines, 1939; Cong et al. 1998), which inserts on the bases of the metacarpals in most tetrapods. Adding to the confusion, the insertion of Extensor carpi ulnaris in many neognathous birds has shifted to a process at the base of metacarpal II, hinting that this may be a derived feature among archosaurs if the crocodylian muscle is indeed ECU. However, in paleognaths the ECU inserts on the base of the lateralmost metacarpal (III), which is also one of the major insertions in lepidosaurs (see below). This distribution of states suggests that insertion on the lateralmost metacarpal, not metacarpal II, is the plesiomorphic state. In the absence of a developmental study on the forelimb musculature in crocodylians that could shed light on the affinities of the crocodylian muscle in question, I adopt the homology of earlier authors in assigning it to Extensor digitorum longus and coding ECU as absent in crocodylians.
As discussed above, the separation of the origins of Anconeus and Extensor carpi ulnaris is equivocal in theropods, though their close proximity even when separate does not allow for much variability in the reconstruction of their origins as the most distal muscles on the humeral ectepicondyle (Fig. 3). A secondary tendon of origin from the proximal ulna, as seen in some birds (George & Berger, 1966), is very unlikely (proportional probability of presence of 0.040). Extensor carpi ulnaris tends to insert to multiple areas around the carpus; in lepidosaurs, its insertion tendon attaches to both the pisiform and lateral edge of the lateralmost metacarpal (Russell & Bauer, 2008), although Varanus also has an attachment to the ulnare (Haines, 1939). In turtles, ECU inserts on the pisiform and the ulnare but not on the lateralmost metacarpal (Haines, 1939; Walker, 1973). Birds lack a pisiform and the ulnare of birds is not homologous to the ulnare of other tetrapods because it is a de novo ossification (Kundrát, 2009), so ECU in birds does not share any of these insertion points. As mentioned above, although ECU inserts at the base of metacarpal II in many neognaths, it inserts at the base of metacarpal III in paleognaths (Parker, 1891; Hudson et al. 1972; dissections) and there appears to be a reversal to insertion on metacarpal III in Passeriformes (proportional probability of 0.934; Hudson & Lanzillotti, 1955; Berger, 1956; George & Berger, 1966; Raikow, 1977; McKitrick, 1985). The proportional likelihoods at the base of Aves provide moderate support for insertion on metacarpal III, the lateralmost metacarpal (proportional probability of 0.650). Thus, insertion on the lateralmost metacarpal is unequivocal, but insertion on any carpals is phylogenetically equivocal. Because Tawa retains a full complement of carpals including a pisiform, I infer ECU to also insert on the pisiform as well as the lateralmost metacarpal, as in lepidosaurs (Fig. 5). Upon the loss of the pisiform in the theropod wrist, ECU likely lost that insertion but retained the insertion on the base of the lateralmost metacarpal, as seen in some birds. With these attachment points, the action of ECU would have been extension and abduction of the wrist, along with slight extension of the forearm.
Supinator (SU)
Supinator is a muscle of the dorsal division that originates on the ectepicondyle of the humerus and inserts on the shaft of the radius. In turtles, lepidosaurs, and crocodylians its origin is consistently the most proximal of the dorsal division muscles, often extending beyond the boundary of the ectepicondyle onto the shaft of the humerus (Haines, 1939). Alternately, in birds, Supinator has a much more distally located origin near that of Extensor digitorum longus, whereas the Extensor carpi radialis takes its place proximally, an arrangement that is consistently found across all of the bird taxa in this study. This leaves the proportional probabilities of the two states exactly opposite at the nodes surrounding Dinosauria. The avian conformation of Supinator and Extensor carpi radialis is an adaptation for the specialized automating musculoskeletal mechanisms of the wing (see below; Vazquez, 1994), so I tentatively reconstruct the origin of Supinator as the most proximal on the ectepicondyle in basal theropods (Fig. 3).
The insertion area of Supinator is located on the anterolateral surface of the radius for most of its length in all turtles, lepidosaurs, and crocodylians, and in all but a handful of derived avian species. Therefore, the insertion of Supinator in theropods can be unequivocally reconstructed on the anterolateral surface of the radius for greater than half its length (proportional probability of 0.999). The degree to which the insertion is oriented anteriorly or laterally on the shaft of the radius varies slightly and depends on the anatomical position of the bones, but both birds and crocodylians typically possess an almost entirely anteriorly located supinator insertion (George & Berger, 1966; Meers, 2003). Reconstruction of this location in basal theropods is supported by the flat anterior surface of the radius, bounded by low ridges running the length of the bone, seen in Tawa (Fig. 4). The action of Supinator in basal theropods would have been be to flex and supinate the forearm.
Extensor carpi radialis (ECR)
The origin of Extensor carpi radialis and its relationship to those of other dorsal division muscles is exactly the inverse of Supinator: in turtles, lepidosaurs, and crocodylians the origin is located between that of Supinator and Extensor digitorum longus, whereas in birds the origin is more proximally located than the other muscles arising from the ectepicondyle. This is taken to the extreme in some birds, which possess an anteriorly projecting Processus supracondylaris dorsalis onto which the ECR attaches (Baumel et al. 1993). The ECR is an important part of the automatic musculoskeletal mechanism for flexion and extension of the wrist and elbow in the avian wing, and the proximally shifted attachment of this muscle allows for slight extension of the elbow to fully extend the manus (Vazquez, 1994). As such, it is likely that this conformation of the origin evolved alongside the modification of the avian wrist, and was therefore not present in basal theropods. It is reconstructed here in a location similar to that of crocodylians, lepidosaurs, and turtles on the ectepicondyle (Fig. 3).
The insertion of ECR is phylogenetically equivocal because an insertion on the radiale as in lepidosaurs and crocodylians is not retained in birds, where it inserts on the carpometacarpus in the vicinity of the base of metacarpal I, no doubt due to the highly derived state of the avian wrist. The wrists of basal theropod dinosaurs such as Tawa possessed a plesiomorphic morphology that is more similar to those of lepidosaurs than either crocodylians or birds, so retention of the plesiomorphic insertion of ECR on the radiale is inferred here (Fig. 5). The action of the ECR in basal theropods would have been to extend and adduct the wrist as well as contribute to flexion of the forearm.
Abductor radialis (AR)
The nomenclature of this muscle is confusing and varied (Table 1) due to its developmental origin in the extensor group of muscles but its lack of function as an extensor. It originates on the humeral ectepicondyle in close proximity to the origin of Extensor carpi radialis and its affinity with this muscle has led to its designation in many publications as Extensor carpi radialis intermedius and/or profundus (e.g. Russell & Bauer, 2008), despite the fact that it has no action on the carpus. It also has been referred to as Extensor antebrachii radialis (Diogo & Abdala, 2010) but this is misleading because it implies that the muscle is an extensor of the antebrachium. I adopt the terminology of Meers (2003), who describes the action of the muscle for most tetrapods in which it is present. Although this muscle possesses two parts in lepidosaurs and some turtles (Haines, 1939; Walker, 1973; Russell & Bauer, 2008), it has only one belly in crocodylians (Meers, 2003). In birds, ECR sometimes possesses a second head at its origin that joins the main belly not long after origin (George & Berger, 1966); although it does not attach to the radius, it is likely that this head represents a remnant of Abductor radialis, which has itself been referred to as a division of ECR in other taxa. This, along with the presence of only a single belly in crocodylians, indicates a general reduction of this muscle in archosaurs, and results in a phylogenetically unequivocal origin of a single belly in close proximity to ECR on the ectepicondyle (Fig. 3). The insertion of Abductor radialis remains equivocal due to its fusion distally to ECR in birds. If it was not fused in basal theropods, it likely would have inserted on the proximal half of the lateral surface of the radius (Fig. 4), where it would have a stabilizing function similar to that in crocodylians (Meers, 2003). The action of Abductor radialis would have been to abduct and slightly flex the forearm.
Abductor pollicis longus (APL)
This muscle is another that has been given many very different names in the literature (Table 1); for theropods I have adopted one of the more common designations, which describes one of the primary actions of this muscle. The origin of APL is phylogenetically unequivocal and is synapomorphic for Archosauria. In lepidosaurs and turtles the muscle arises only from the shaft of the ulna (Haines, 1939; Russell & Bauer, 2008), but crocodylians and birds both possess a second head of origin from the shaft of the radius, making the muscle bipennate (George & Berger, 1966; Meers, 2003). This has been reversed in Passeriformes (Swinebroad, 1954; Hudson & Lanzillotti, 1955; Berger, 1956; George & Berger, 1966; Raikow, 1977), but the radial head is present in all other birds studied. The proportional probability of presence of the radial head at the Archosaur node is 0.955, thus the APL unequivocally originated from the facing surfaces of the radius and ulna in Tawa (Fig. 4).
Although birds possess the derived origin of APL, they retain the plesiomorphic insertion site on the medial side of the base of metacarpal I, as in lepidosaurs and turtles (Walker, 1973; Russell & Bauer, 2008). Abductor pollicis longus (Extensor longus alulae) in birds inserts on the extensor process of the carpometacarpus, which is developmentally part of metacarpal I (Kundrát, 2009). This insertion is not shared by crocodylians, in which the insertion tendon attaches to the radiale (Haines, 1939; Meers, 2003). Phylogenetic inference strongly suggests that this is a derived state within the clade, with a proportional likelihood of 0.980 at the base of Archosauria in favor of insertion on metacarpal I. Additionally, metacarpal I of Tawa, Herrerasaurus, and other basal theropods possesses a medial flange at the base that likely represents an insertion site similar to the extensor process in birds (Fig. 5). With these attachments, the action of APL in basal theropods would have been extension and abduction of the wrist, and abduction of the first digit.
Extensor digitorum longus (EDL)
The origin of Extensor digitorum longus exhibits little variation in relation to the other muscles originating on the ectepicondyle of the humerus. In almost all taxa studied, it originates from approximately the middle of the ectepicondyle, between the origins of Extensor carpi ulnaris and Extensor carpi radialis or Supinator (proportional probability of near 1.0 at all nodes), and so it can unequivocally be reconstructed in this location in basal theropods (Fig. 3). Its insertion, however, is less straightforward. Possibly representing the plesiomorphic tetrapod condition (Haines, 1939), EDL inserts on the base of all five metacarpals in all of the turtle taxa studied with the exception of Lissemys (Shah & Patel, 1964), but insertion on the fifth digit is lost in all lepidosaurs and archosaurs (proportional probability of 0.995). All lepidosaurs and turtles possess insertion tendons for metacarpal IV, and attachment to this digit has also been reported in Alligator mississippiensis (see Reese, 1915; Haines, 1939). A similar pattern exists for attachment to digit III, except in this case an insertion on metacarpal III has also been reported for Crocodylus acutus (see Ribbing, 1907). An insertion at the base of metacarpal II is invariably present in all turtles, lepidosaurs, and crocodylians, whereas insertion on the base of metacarpal I is only present in turtles (except Lissemys; Shah & Patel, 1964), Sphenodon (Miner, 1925; Haines, 1939), and Crocodylus acutus (see Meers, 2003). In the highly modified manus of birds, EDL inserts on both digits I and II, but on the base of phalanx I of these digits rather than the metacarpal. Phylogenetically, insertion on digits I and II is unequivocally supported, but other attachments remain equivocal. The manus of Tawa contains three functional digits and a highly reduced digit IV, thus functional inference supports insertion on metacarpal III as in lepidosaurs and some crocodylians (Fig. 5). Because of the small size of digit IV, it is likely that the insertion on metacarpal IV was already lost in early theropods. The action of EDL would have been to extend the wrist.
Pronator teres (PT)
Pronator teres is present in all taxa used in this study. Its origin is consistently the most proximally located of all the ventral division muscles. In some neognaths such as Charadriiformes and Anatidae, the origin has migrated proximal to the borders of the entepicondyle (Hudson et al. 1969; Zusi & Bentz, 1978; Livezey, 1990) and the ancestral state slightly favors this reconstruction at the base of Neognathae (posterior probability of 0.528). Pronator teres arises from the entepicondyle itself in paleognaths, dropping the posterior probability of the proximal insertion to 0.082 at the base of Aves. Thus, phylogenetically its origin is unequivocally located on the entepicondyle in theropod dinosaurs; the ridge and small anterior projection at the proximal extent of the entepicondyle in Tawa probably represent the anteroproximal border of the origin (Fig. 3).
Pronator teres has an elongate, narrow insertion on the anteromedial surface of the radius to varying extents in the taxa surveyed here. In turtles and most lepidosaurs, it inserts on less than half of the radius distally, though it has been reported to insert on the radius for most of its length in a variety of squamate taxa including Varanus and Ctenosaura (Straus, 1942; Haines, 1950; Russell & Bauer, 2008). This long insertion is also present in all of the crocodylians and paleognathous birds studied, as well as some unrelated neognaths. A derived insertion on less than half of the radius proximally is present in many neognaths and is reconstructed as the most likely ancestral state in this clade (posterior probability of 0.677), although there is no clear pattern to its evolution. Phylogenetically, the insertion of Pronator teres is unequivocally reconstructed in a line along the anteromedial shaft of the radius for greater than half of its overall length (posterior probability at both Aves and Archosauria nodes of 0.815). This is supported by the morphology of the radius in Tawa, which features a distinct anteromedial surface defined by ridges running the length of the radius (Fig. 4). The action of Pronator teres would have been to flex the forearm and pronate the antebrachium.
Pronator accessorius (PA)
Pronator accessorius is absent in crocodylians, Sphenodon, and paleognaths except for tinamous (Hudson et al. 1972), but present in squamates, turtles, and all neognaths studied, thus its presence is reconstructed as unequivocal. Its origin is consistently located more distally than that of Pronator teres, at the distal end of the entepicondyle near the origin of Flexor digitorum longus superficialis, and it is reconstructed with this morphology in Tawa (Fig. 3).
The narrow insertion along the medial side of the radius is variable across the tree. In turtles it is consistently located distally for less than half of the length of the radius, and this state is present in some squamates such as Varanus and Tetradactylus (Haines, 1950; Berger-Dell'Mour, 1983) where it is hypothesized to be primitive (Russell & Bauer, 2008). An insertion on the proximal end of the radius for less than half its length is present in some other lepidosaurs, including Iguana and Liolaemus, and a few neognaths (George & Berger, 1966; Abdala & Moro, 2006; Russell & Bauer, 2008), but the majority of birds have an insertion that extends for the most of the length of the radius. This distribution of character states causes the long insertion to be reconstructed at the base of Aves (posterior probability of 0.950), and the restricted distal insertion to be reconstructed at the lepidosaur + archosaur node (posterior probability of 0.878). There are unfortunately no osteological signals on the radius of Tawa to indicate the extent of the insertion of this muscle in basal theropods. To account for this uncertainty, I tentatively reconstruct the insertion on the distal end of the radius for slightly over half its length (Fig. 4). Pronator accessorius would have acted to flex and pronate the antebrachium.
Pronator quadratus (PQ)
Pronator quadratus of crocodylians, lepidosaurs, and turtles is likely homologous to the Ulnimetacarpalis ventralis of birds (Sullivan, 1962), although this is not obvious due to the derived insertion site of Ulnimetacarpalis ventralis on the base of the carpometacarpus in most birds. This muscle originates from a line along the ventral/medial surface of the ulna in all taxa studied, but the proximal extent of its origin is variable. In crocodylians, lepidosaurs, and most turtles, Pronator quadratus arises from more than half of the length of the ulna (Walker, 1973; Meers, 2003; Russell & Bauer, 2008), whereas in birds it is typically restricted to the distal half or less (George & Berger, 1966). An elongate origin is found in the clade containing passeriforms and raptors as well as a few other birds, leaving a reduced distal origin in birds at a 0.834 posterior probability. The posterior probability of an elongate origin at the Archosauria node is 0.756, making the reconstruction of the proximal extent of this muscle equivocal. Given the distally displaced insertion of this muscle in birds, a distally shifted origin is not unexpected. It is unlikely that basal theropods possessed the derived avian morphology of the insertion (see below), so this muscle is reconstructed in Tawa with a proximally extensive origin covering most of the length of the ulna (Fig. 4).
The insertion of this muscle in taxa other than birds is consistently on the ulnar-facing side of the ventral radius (Meers, 2003; Russell & Bauer, 2008). However, in some lepidosaurs and all turtles this insertion extends to the ventral surface of the carpals (Straus, 1942; Haines, 1950; Walker, 1973; Berger-Dell'Mour, 1983), which is consistent with the insertion of this muscle onto the base of the carpometacarpus in birds. This attachment can be unambiguously reconstructed in basal theropods (posterior probability of its presence at the base of Archosauria of 0.783), having been secondarily lost in modern crocodylians. The retention of the radial attachment of Pronator quadratus in nonavian theropods is equivocal phylogenetically, but it was likely present because its absence in birds is a derived state relating to the evolution of the avian wing. Dissections of Struthio revealed a double insertion of this muscle onto the distal end of the radius and the base of the carpometacarpus. Although this does not affect the equivocal results from the ancestral state reconstruction, it provides some further evidence that Pronator quadratus in nonavian theropods retained the radial insertion (Figs 4 and 5). With this morphology, the primary action of Pronator quadratus would have been to pronate the antebrachium and manus.
Epitrochleoanconeus (EA)
This muscle, known as Entepicondylo-ulnaris in birds, is only present in turtles, lepidosaurs, galloanseriform birds, Apteryx, and tinamous. It is the mirror in the flexor compartment of Anconeus, arising from the entepicondyle of the humerus and inserting on the ventral surface of the ulna. In Apteryx it is largely fused to Flexor carpi ulnaris, and often cannot be distinguished from this muscle (McGowan, 1982). This is also true of most turtles, in which Epitrochleoanconeus is usually described as the deep or medial part of Flexor carpi ulnaris (Shah & Patel, 1964; Walker, 1973; Abdala et al. 2008). Reconstruction of its presence in nonavian theropods is phylogenetically unequivocal (posterior probability of presence at the Archosauria node of 0.863, presence at the base of Aves of 0.903), although the morphology of its origin is not. In turtles and lepidosaurs, this muscle is in close proximity to the origin of Flexor carpi ulnaris, sometimes arising from the same tendon, although an origin just proximal to that of Flexor carpi ulnaris is typical in lepidosaurs (Russell & Bauer, 2008). In birds this muscle typically takes its origin from the tendon of Pronator accessorius, except in Apteryx, where the muscle is not differentiated from Flexor carpi ulnaris (George & Berger, 1966). The posterior probability slightly favors the derived state at the base of Aves (0.527), so reconstructing the origin in either state is a Level II′ inference. I tentatively reconstruct the origin in Tawa to be located between the origins of Flexor carpi ulnaris and Pronator accessorius on the entepicondyle, similar to the morphology in lepidosaurs, which may represent an intermediate morphology between the two alternate states (Fig. 3).
The extent of the insertion of Epitrochleoanconeus on the ventral/medial surface of the ulna is unequivocally restricted to its proximal half. In birds (except Apteryx) and most lepidosaurs, it inserts on only the proximal one-quarter to one-half of the ulna, whereas in turtles and Apteryx it inserts on the majority of the length of the ulna; in Sphenodon and Tetradactylus it inserts only on the distal half (Miner, 1925). The proportional probabilities moderately favor the proximally restricted insertion (0.876 at the base of Aves, 0.698 at the Archosauria + Lepidosauria node), so it is reconstructed in this location in basal theropods as well (Fig. 4). Epitrochleoanconeus would have acted to flex the antebrachium.
Flexor carpi ulnaris (FCU)
This muscle is found in every study taxon and has a relatively consistent morphology. Its tendon of origin is always the most distally located on the humeral entepicondyle, arising from its posterior aspect just above the distal articular surface. It is sometimes composed of multiple parts with separate origins in lepidosaurs (Straus, 1942; Abdala & Moro, 2006) but these do not seem to be related to the smaller second belly present in many birds, which possesses a novel attachment to the base of the secondary flight feathers (George & Berger, 1966). Thus, FCU in Tawa is reconstructed as arising from a single tendon on the posterodistal aspect of the entepicondyle (Fig. 3).
The insertion of FCU is phylogenetically equivocal due to the modified avian wrist. In crocodylians and most lepidosaurs, FCU has a single tendinous insertion on the pisiform, which is joined by a secondary tendon inserting on the ulnare in most turtles and Varanus (Haines, 1950; Shah & Patel, 1964; Walker, 1973). The insertion in birds is also on the ulnare but, as mentioned above, this bone is neomorphic in birds and not homologous to the tetrapod ulnare, which disappears during development (Kundrát, 2009). With the loss of the two primary attachment areas, the insertion of this muscle would have shifted to the neomorphic avian ‘pseudoulnare’ to maintain its functional role. Because Tawa retains a full complement of carpals, including an ossified pisiform, I reconstruct FCU as attaching primarily to the pisiform, with a possible additional attachment to the (true) ulnare (Fig. 5). It is unknown when the pseudoulnare replaced the ulnare in the theropod wrist; Kundrát (2009) interpreted the ulnare of Archaeopteryx to be the avian pseudoulnare. Regardless, pseudoulnare is the functional analog of the ulnare and, as such, the shift in its identity does not change the functional role of FCU. The primary actions of this muscle would have been to flex and adduct the wrist.
Flexor digitorum longus (FDL)
Flexor digitorum longus is composed of two main parts, Flexor digitorum longus superficialis (FDLS), which originates on the humerus, and profundus (FDLP), which originates from the antebrachium. Both parts coalesce into a single set of tendons for insertion in the manus, so they are treated under one heading here.
Although FDLS is absent in ratites (McGowan, 1982; dissections), it is present in all other birds including tinamous (Hudson et al. 1972), as well as in crocodylians. Thus, it is unambiguously reconstructed as present in nonavian theropods. It arises from a single tendinous origin on the entepicondyle of the humerus, sandwiched between the origins of Flexor carpi ulnaris and Pronator teres, in nearly all taxa studied; it is therefore reconstructed in this location in Tawa (Fig. 3). A second head of origin from the entepicondyle, as in some squamates (Straus, 1942; Russell & Bauer, 2008), or the ulna, as in a few bird taxa (Fisher & Goodman, 1955; Fitzgerald, 1969), are rare occurrences and are not likely to have been present in nonavian theropods (posterior probabilities of their absences of nearly 1.0 at both nodes).
An origin of FDLP from the ulna is present in all study taxa and thus phylogenetically unequivocal. FDLP arises from the ventral/medial surface of the ulna along most of its length; in crocodylians and lepidosaurs its extent is roughly equivalent with that of the origin of Pronator quadratus (Cong et al. 1998; Meers, 2003; Russell & Bauer, 2008), whereas in birds the two origins do not overlap and the distal extent of FDLP is limited by the proximal extent of PQ (George & Berger, 1966). The limited origin in birds may be related to the reduction of the FDL musculature as a result of the reduction of the digits in extant birds. Tawa retains a four-fingered hand, so the FDLP is reconstructed here with a full insertion, as is seen in crocodylians and lepidosaurs (Fig. 4).
As mentioned before, the tendons of insertion of FDLS fuse with those of FDLP in all outgroup taxa and some neognathous birds. Although this state is present in less than half of the bird taxa sampled, the distribution is such that it is reconstructed as the most likely state at the base of Aves (posterior probability of 0.885). The joined tendons insert on the ventral surface of the terminal phalanx of all digits in lepidosaurs and turtles (Walker, 1973; Russell & Bauer, 2008). In crocodylians, which have somewhat reduced, non-ungual-bearing manual digits IV and V, the tendinous slips to these digits are typically lost (Ribbing, 1907; Meers, 2003) and a slip to digit IV has only been reported once (Cong et al. 1998). In the modified avian manus, the tendon of FDL typically inserts only onto the major digit, which is identified as digit II (Bever et al. 2011), although there are several notable exceptions. A tendinous slip to digit I, the alula, is found in tinamous (Hudson et al. 1972), Struthio (dissections), Opisthocomus (Hudson & Lanzillotti, 1964), Balaeniceps (Vanden Berge, 1970), Coturnix (Fitzgerald, 1969), and Bubo (dissections). The retention of this slip in some neognaths is possibly functionally linked. The young of Opisthocomus, the Hoatzin, retain a functional, clawed first digit that is used in climbing trees prior to fledging, potentially requiring flexor capacity in the first digit beyond that of most birds (Young, 1888; Beddard, 1889; Shufeldt, 1918). Despite the low number of avian taxa exhibiting this character, a slip to the first digit is reconstructed as plesiomorphic at the base of Aves (posterior probability of 0.882). An insertion slip to the third digit is found in all outgroup taxa but is not found in birds, with the exception of Struthio, where a small tendon to digit III was found in one dissected specimen. This does not appreciably affect the posterior probability of the presence of this slip at the base of Aves (0.043), making the reconstruction of it in basal theropods phylogenetically equivocal. However, the manus of Tawa possesses a well-developed, functional third digit that likely would have retained its insertion slip from FDL. The fourth digit of Tawa is very reduced, so this digit probably lacked a tendinous slip, as in crocodylians (Fig. 5). FDL would have acted to flex the digits and the wrist in basal theropods.
Intrinsic manual musculature
The homologies of the muscles of the manus in birds are difficult and somewhat speculative (e.g. Diogo & Abdala, 2010). The highly modified avian manus has undergone extensive fusion of metacarpal elements and reduction in number and size of phalanges, resulting in the reduction and loss of much of the intrinsic manual musculature. Nevertheless, these muscles control the independent movements of the manual digits, and reconstructing them in nonavian theropods, even tentatively, is an important step in assessing the functional capabilities of their forelimbs. The hypotheses of homology used in this study are summarized in Table 2.
Extensores digitores breves (EDB)
Tetrapods have two layers of intrinsic extensor musculature, Extensor digitores breves superficiales (EDBS) and profundi (EDBP). Crocodylians, lepidosaurs, and turtles all have EDB musculature that arises by way of separate muscle bellies from the proximal carpals (EDBS) and the metacarpals (EDBP). The superficialis and profundus bellies for each digit coalesce into a single tendon of insertion that attaches to the dorsal surface of the proximal end of each terminal phalanx in crocodylians and lepidosaurs (Meers, 2003; Russell & Bauer, 2008), although it only extends to more proximal phalanges in most turtles (Shah & Patel, 1964; Walker, 1973). Crocodylians have a somewhat unusual arrangement of the EDBS origin sites, in which they are spread across the proximal carpals instead of being confined to the ulnare, intermedium, and/or distal ulna as in turtles and lepidosaurs (Haines, 1939). Thus although the origins of the lateralmost divisions are relatively conserved in the outgroup taxa, the medial divisions do not have consistent sites of origin.
In birds, the digital extensors attach to the first and second digits and consist of Extensor brevis alulae and Extensor longus digit majorus, which has both proximal and distal parts (Vanden Berge & Zweers, 1993). Additionally, the robust Ulnimetacarpalis dorsalis is likely a short extensor and not a homolog of Abductor digiti minimi (see below). Extensor brevis alulae originates from the dorsal surface of the extensor process of the avian carpometacarpus, which corresponds embryologically to the base of the first metacarpal (Kundrát, 2009). This is consistent with the origin of EDBP of digit I in all other taxa; a secondary origin from the adjacent surface of metacarpal II, seen primarily in ‘gruiform’ birds (e.g. Fisher & Goodman, 1955), has been reported in Alligator and Sphenodon (Haines, 1939), but is not likely to have been present in basal theropods (posterior probabilities of its absence are nearly 1.0 at both Aves and Archosauria nodes). There is little evidence for a superficial division of EDB to digit I in birds; however, a second, more proximal belly arising from the radiale has been reported in Geococcyx (Berger, 1954) and a similar belly arising from the distal end of the radius and radiale has also been found in Struthio (dissections). Although the radial attachment of these bellies may have been secondarily gained in birds, they may also indicate that the arrangement of the EDBS musculature in crocodylians may be a shared derived feature for Archosauria.
Extensor longus digiti majorus likely corresponds to the EDB divisions of digit II despite its proximally shifted origins. This muscle contains both proximal (superficialis) and distal (profundus) portions that join to form a single tendon that inserts on the dorsal surface of the distal phalanx of digit II. The proximal belly arises from the ulnar surface of the radius in all birds, although the length of the belly varies from nearly the entire length of the radius to being restricted to the distal third (George & Berger, 1966). This creates an entirely ambiguous character reconstruction at the archosaurian node but the origin is still most closely aligned with that of crocodylians, from the radiale (Meers, 2003), than with an origin from the ulnare, intermedium or distal ulna, as in lepidosaurs and turtles. When present, the distal belly of this muscle arises from various structures near the radiale in birds but it is restricted to the dorsal surface of metacarpal II in some neognaths and in all paleognaths (Hudson et al. 1972; dissections). As with Extensor brevis alulae, this morphology is congruent with that of the EDBP belly of the corresponding digit in crocodylians and lepidosaurs. In these taxa the origin often extends onto the base of metacarpal I as well (Haines, 1939; Meers, 2003) and this may also be the case in birds when it arises from the base of the carpometacarpus, where it is impossible to delineate the borders of the metacarpals in most taxa.
Ulnimetacarpalis dorsalis has been suggested to be either a homolog for Abductor digiti minimi or a member of the short manual extensors (Diogo & Abdala, 2010). I consider the latter hypothesis to be more strongly supported. This muscle is embryologically derived from the dorsal division of the manual muscles and is thus closely related to the extensor musculature of the manus (Sullivan, 1962), whereas Abductor digiti minimi is usually described as being in close association with the flexor musculature (Russell & Bauer, 2008; see below). Its proximally displaced origin from the distal end of the ulna suggests that it pertains to the EDBS musculature but, unlike other EDB musculature, it only extends to insert on the lateral surface of metacarpal III (George & Berger, 1966). Although it would seem to correspond to EDB of digit III, embryologically it is associated with the primordium of digit IV, which is resorbed later in development (Sullivan, 1962; Kundrát, 2009). EDBS of digit IV has been reported as arising from the distal end of the ulna in crocodylians (Ribbing, 1907), as has EDBS of digit V (Ribbing, 1907; Haines, 1939; Meers, 2003). Although EDBS of digits IV and V both retain a distally located insertion on the terminal phalanges, the insertion of EDBP in digit V is shifted to metacarpal V in crocodylians, in which this digit is reduced (Meers, 2003). Whether this muscle pertains to the EDB slips of digit III, IV, or V, its proximal insertion on the shaft of metacarpal III may be a result of the reduction and/or loss of these three digits in birds.
The general similarities of the extensor musculature of the first two to three digits in birds to the organization of the crocodylian musculature suggests that the morphology of the EDB musculature in basal theropods was similar to that of the crocodylian manus. It is unlikely that Tawa lacked EDB divisions to either the large third digit or the reduced fourth digit, because small bellies of EDB are still found in the reduced fifth digit of modern crocodylians as well as in the reduced digits of some lepidosaurs (Berger-Dell'Mour, 1983; Meers, 2003). However, the EDBP belly to digit IV may have exhibited an insertion on the metacarpal rather than the terminal phalanx, as in crocodylians and possibly birds (Meers, 2003). The areas of origin of EDBS in early theropods are somewhat equivocal but, based on evidence from the avian manus, I reconstruct the divisions for digits I and II as arising from the dorsal surface of the radiale and the divisions for digits III and IV as arising from the dorsal surface of the ulnare in Tawa (Fig. 5). Origins for the EDBP divisions are more conserved; in all taxa the bellies associated with each digit arise from their metacarpals, and extend onto the base of the metacarpal medial to it in most crocodylians, lepidosaurs, and possibly in some birds. Thus, this morphology is reconstructed for EDBP for all digits in Tawa (Fig. 5). The insertion of this musculature can unequivocally be reconstructed as on the dorsal surface of the proximal end of the distal phalanges (unguals). In Tawa, an oval, lightly striated area on the dorsal surface of all three manual unguals likely represents the insertion area (Fig. 5). In Tawa, EDB would have acted to extend the metacarpophalangeal and interphalangeal joints of the digits.
Flexores digitores breves (FDB)
Like the extensors, the short flexor musculature of the hand is divided into superficialis and profundus layers but, unlike the extensors, these two layers maintain separate insertions on the phalanges. Additionally, many tetrapods possess an assortment of smaller muscles related to FDB that vary between and sometimes within clades. These muscles are sometimes called Flexor digitorum brevis intermedius or Contrahentes digitorum and typically originate on the medial side of the carpus and insert on the fourth and/or fifth digits. Due to their variable nature, the reconstruction of these muscles would be highly speculative in extinct taxa. Furthermore, these muscles may have been lost in the manus of Tawa, as they have in lizards that exhibit a similar pattern of reduction of digits four and five (Berger-Dell'Mour, 1983). Thus, in this reconstruction I will focus on the two major layers of this muscle group, FDBS and FDBP.
The only likely avian homolog for FDBS is Flexor alulae, which arises from the base of the carpometacarpus and the tendon of FDL. This is nearly identical to the origin of FDBS in crocodylians, where it arises from the distal carpals and the tendon of FDL (Meers, 2003). In lepidosaurs and turtles, the bellies of FDBS take their origin entirely from the annular ligament covering the carpals, but this ligament is absent in extant birds and crocodylians due to the modified wrists in both taxa. The wrist of Tawa was more similar in morphology to that of a lepidosaur than to those of either modern birds or crocodylians, and it is likely that the annular ligament was retained in it and other basal theropods with unmodified wrists. This would allow the FDBS divisions to originate on this ligament in the basal taxa, shifting to an origin from the distal carpals upon later modification of the theropod wrist. Although Tawa possessed a plesiomorphic carpus, I have reconstructed origins for the FDBS muscles on the distal carpals, which may also be an archosaurian synapomorphy (Fig. 5).
The distal tendons of the FDBS muscles bifurcate to allow passage of the tendons of FDL and then either rejoin to form a single tendon of insertion (lepidosaurs; Russell & Bauer, 2008) or insert separately on either side of the flexor processes of the first phalanx of digits I and II, and the second phalanx of digit III, as in crocodylians and turtles (Walker, 1973; Meers, 2003). The exception to this is FDBS of digit I, which does not have a bifid tendon in lepidosaurs or birds, and inserts simply on the ventromedial side of the base of the first phalanx of digit I. The reconstruction of this morphology is favored at the base of Archosauria (posterior probability of 0.786), so it is reconstructed that way in Tawa (Fig. 5).
The bellies of FDBP variously arise from the distal carpals and the ventral surfaces of the metacarpals and insert near FDBP on the flexor process of the first phalanx in crocodylians, lepidosaurs, and turtles. In birds, each digit of the manus has retained its division of FDBP: Adductor alulae, lying just deep to Flexor alulae, is the probable homolog of FDBP to digit I; Abductor digiti majoris is the probable homolog of FDBP to digit II; and Flexor digiti minoris is the probable homolog of FDBP to digit III. Adductor alulae has a very similar origin and insertion to that of Flexor alulae, and in some birds they are difficult to distinguish, but its insertion is slightly medial and distal to that of the Flexor alulae on the carpometacarpus. Abductor digiti majoris has its origin on the base of the carpometacarpus and the ventral surface of the shaft of metacarpal II, and it inserts on the base of the proximal phalanx of digit II. Flexor digiti minoris is variably developed and is only weakly present in some birds, but it has a large fleshy belly in Struthio. It takes its origin from the distal half of metacarpal III and inserts tendinously on the base of the proximal phalanx of digit III. In lizards and turtles the origins of the FDBP divisions are typically restricted to the distal carpal row (Walker, 1973; Russell & Bauer, 2008), whereas crocodylians exhibit origins involving both the distal carpals and the metacarpals of each digit (Meers, 2003). An origin from the metacarpals may be an archosaurian synapomorphy, but it also may be convergent due to the modified wrists of modern archosaurs, as in the origin of FDBS. In this case it is most parsimonious to reconstruct the origin of these muscles from both the distal carpals and the metacarpals, as in crocodylians, which is an intermediate state between that of lepidosaurs and that of birds (Fig. 5). The insertion of FDBP is highly conserved, with a single attachment to the ventral surface of the flexor process of the first phalanx of each digit in nearly every taxon studied. Thus it is reconstructed to have retained this attachment in the manus of Tawa, where it would have inserted between the two distal tendons of FDBS (Fig. 5). This group of muscles would have been primarily responsible for flexing the metacarpophalangeal joints of each digit.
Abductor pollicis brevis (APB)
The likely homolog of APB in the avian manus is Abductor alulae, which is located on the anteroventral side of the manus. The origin of this muscle is from the area of the carpus on the radial side, but the exact points of origin exhibit a high degree of variability. It arises from the ventral surface of the radiale in all crocodylians, most lepidosaurs, and one turtle (Lissemys; Shah & Patel, 1964). Other reported origins have been from the distal carpals in Trachemys (Walker, 1973) and Tetradactylus (Berger-Dell'Mour, 1983), from the distal radius in Podocnemis and Trachemys (Abdala et al. 2008), from the base of the carpometacarpus in some birds (e.g. Fisher & Goodman, 1955), and from the tendon of ECR in most birds (George & Berger, 1966). An accessory attachment to the distal radius has been found in dissections to be variably present in Struthio. Because it is present in most lepidosaurs as well as in crocodylians, origin from the radiale is likely the plesiomorphic condition, retained in crocodylians despite their modified wrists, and is the most likely character state at the base of Archosauria (posterior probability of 0.881). Although it remains phylogenetically equivocal, this morphology is reconstructed in Tawa because it is unlikely that basal theropods exhibited the derived avian morphology (Fig. 5).
The insertion of APB is unequivocally located on the medial surface of the first phalanx of digit I near its base (posterior probability of 0.837 at Archosauria and 0.993 at Aves). This muscle has a more proximal attachment in crocodylians (Meers, 2003), Sphenodon (Miner, 1925), and Liolaemus (Abdala & Moro, 2006), inserting on the lateral aspect of the first metacarpal, but the distal attachment is found in turtles, other lepidosaurs, and all birds. Thus, this insertion site is reconstructed in Tawa as well (Fig. 5). Abductor pollicis brevis would have acted to abduct the first digit.
Abductor digiti minimi (ADM)
Abductor digiti minimi of crocodylians, lizards, and turtles originates from the distal edge of the pisiform and inserts on the lateral surface of the lateralmost metacarpal (in crocodylians; Meers, 2003) or the ventrolateral surface of the first phalanx (in lepidosaurs and turtles; Russell & Bauer, 2008). It has been suggested that the avian homolog of this muscle is Ulnimetacarpalis dorsalis (Diogo & Abdala, 2010) but this hypothesis was rejected based on the embryological differences between the two muscles (see above, in Extensores digitores breves (EDB)). ADM is ventrally located in the hand, and in some cases has been described as part of Flexor carpi ulnaris, or arising from its tendon of insertion (Günther, 1867; Russell & Bauer, 2008). No similar muscle has ever been previously described in the avian manus, but a muscle fitting this description was found in both specimens of Struthio that I dissected. This muscle is well developed, originating from the ventral surface of the pseudoulnare and the insertion tendon of Flexor carpi ulnaris and inserting on the ventrolateral surface of metacarpal III. In birds, muscles attaching to the pisiform have shifted their attachments to the pseudoulnare (see above, in Flexor carpi ulnaris (FCU)), so in this case an origin from the pseudoulnare is considered homologous to the origin from the pisiform in other taxa. The insertion on the lateralmost metacarpal is similar to the condition in crocodylians and may be related to reduction of the lateral digits in extant archosaurs. In Tawa, this muscle would have originated from the pisiform, which was still present, and likely inserted on the metacarpal of the reduced digit IV (Fig. 5). With this morphology, it would have acted to abduct the fourth digit and the manus.
Lumbricales (L)
Although there seem to be no homologs of these muscles present in the avian manus, Lumbricales to at least digits II through IV are present in all other taxa studied. However, the number and exact insertion of the slips of this muscle are extremely variable. In all cases they arise from the tendons of Flexor digitorum longus in the manus and/or the palmar aponeurosis, although they may insert on all digits (Haines, 1950; Walker, 1973; Abdala & Moro, 2006), or only digits II through IV (Meers, 2003; Russell & Bauer, 2008), and on either the metacarpophalangeal joint (Meers, 2003; Abdala & Moro, 2006) or the proximal interphalangeal joint (Haines, 1950; Russell & Bauer, 2008). Thus, although it is likely that Lumbricales were present in the manus of Tawa given their presence in all taxa with ‘normal’ manual morphology, reconstruction of their exact morphology is considered too speculative in the present study. The lumbricals would have acted to extend the metacarpophalangeal and possibly the proximal interphalangeal joints of the digits.
Interossei (IO)
All birds possess two muscles that are named ‘Interosseus’, but their homology to the Interossei of other tetrapods is uncertain based on their attachments and development (Sullivan, 1962). Even among tetrapods, the homology of the muscles variously called Interossei, Intermetacarpales, Dorsometacarpales, and Contrahentes digitorum is not clear (Howell, 1936). As with Lumbricales, Interossei were almost certainly present in the manus of Tawa given their presence in some form in the manus of all other tetrapods, but there is no consistency in their number or morphology across the studied taxa, making them impossible to reconstruct with any confidence. Furthermore, there are no osteological correlates that correspond to the possible attachment locations in the metacarpals of Tawa. In crocodylians, each digit is served by an abductor (dorsal) and adductor (ventral) that originate from the proximal end of the adjacent metacarpal and insert on the distal metacarpal, joint capsule, and/or proximal phalanx of that digit (Cong et al. 1998; Meers, 2003). This arrangement is not unlike that of the human hand, allowing for independent adduction and abduction of each digit. If the Interossei of early theropods possessed a crocodylian arrangement, these muscles would have provided more control over the movements of the individual manual digits.
Discussion
The reconstruction of soft tissues in extinct animals is inherently subject to uncertainty. Although some of the muscles investigated in this study can be reconstructed with a good deal of confidence, others possess much greater ambiguity. In muscles presenting substantial uncertainty, I have hypothesized attachment sites based on osteological clues, and occasionally used extrapolatory functional inference (e.g. Jasinoski et al. 2006) based on the topology of unambiguous muscles and functional similarities to extant taxa. This particular method runs the risk of circularity if ambiguous muscles are then relied upon to assess function of the limb, and I have avoided utilizing ambiguous muscles when discussing functional implications (see below). Perhaps the region with the greatest uncertainty in theropods is the carpus and manus, which exhibits substantial osteological shifts that were accompanied by the acquisition of novel attachments of some muscles on the line to birds. In many cases within this study, the plesiomorphic arrangement of the manual muscles was given priority due to the morphology of the manus in Tawa, but it should be noted that the plesiomorphic morphology of the carpus as seen in Tawa only characterizes the basal-most members of the clade. The possibility that avian-like modifications to the muscles attaching to the carpus occurred early in Theropoda must be considered carefully in future reconstructions of other members of this clade. The exact boundaries of the muscle attachment sites are often unclear, particularly in cases of large, fleshy muscles. In this study, the attachment sites are delineated (e.g. Fig. 2) based on osteological features that have bounded them, but in cases where few hard boundaries exist, they have been estimated based on potential bounding by other muscles. Thus, these muscle boundaries should not be used for precise calculations of muscle size and shape without being subjected to substantial sensitivity analyses. In general, care should be taken when using any muscle reconstruction for further functional analyses to ensure that ambiguous muscles are not being relied upon for support of functional hypotheses.
Comparisons with previous reconstructions
Among the few studies that have reconstructed the forelimb musculature in extinct archosaurs, the majority focuses on a small subset of large muscles crossing the glenohumeral joint and do not assess the smaller muscles of the forelimb, including those of the antebrachium and manus. The major deviations of the shoulder musculature of this reconstruction from that of Jasinoski et al. (2006) have been detailed above, but mostly concern the origins of the supracoracoideus musculature, the absence of Coracobrachialis longus, and the origins and insertions of the deltoideus musculature (Fig. 7). The configuration of the deltoid musculature proposed here is consistently displayed in other recent reconstructions (Dilkes, 2000; Langer et al. 2007; Maidment & Barrett, 2011) and, although Langer et al. (2007) also reconstruct the Coracobrachialis longus as present, most other workers agree with Romer (1944) that its absence is plesiomorphic among archosaurs (Dilkes, 2000; Meers, 2003; Maidment & Barrett, 2011). A broad origin of Supracoracoideus from the anterolateral surface of the coracoid extending onto the scapula in the subacromial depression is reconstructed by both Dilkes (2000) and Langer et al. (2007), and an origin crossing the scapulocoracoid suture is also hypothesized by Maidment & Barrett (2011), although with a much more dorsally restricted attachment (Fig. 7).
Figure 7.
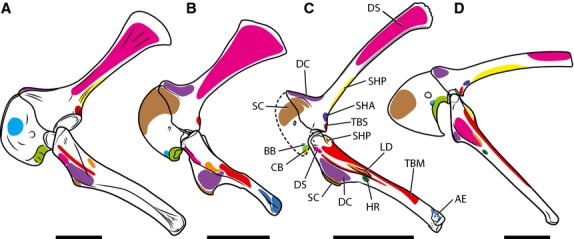
Comparison of published myological reconstructions of the shoulder in a generalized basal ornithischian (A, adapted from Maidment & Barrett, 2011), the basal sauropodomorph Saturnalia (B, adapted from Langer et al. 2007), the basal theropod Tawa (C), and the dromaeosaurid Saurornitholestes (D, adapted from Jasinoski et al. 2006 and TMP 88.121.39). Muscles are labeled on Tawa and represented in the same color on other taxa. AE, antebrachial extensors; BB, Biceps brachii; CB, Coracobrachialis; DC, Deltoideus clavicularis; DS, Deltoideus scapularis; HR, Humeroradialis; LD, Latissimus dorsi; SC, Supracoracoideus; SHA, Scapulohumeralis anterior; SHP, Scapulohumeralis posterior; TBM, Triceps brachii medialis; TBS, Triceps brachii caput scapulare. Scale bars: 5 cm.
The humeral origin(s) of Triceps brachii is more controversial. The presence of two humeral heads (TBM and TBL) as reconstructed here is equivocal phylogenetically, and most authors choose to reconstruct only one humeral head in dinosaurs (Dilkes, 2000; Jasinoski et al. 2006; Langer et al. 2007; Maidment & Barrett, 2011). However, reconstructions for both heads are Level II inferences and thus equally parsimonious, and a distinct ridge on the posterior surface of the humerus in basal theropods provides osteological evidence of a separate origin of TBL (see above). Whether the humeral heads of triceps were fused in dinosaurs or not, both crocodylians and birds exhibit a wide fleshy origin of the humeral head(s) of triceps, and thus it is likely that the origin in dinosaurs was not restricted to a small area, as some authors have proposed (Fig. 7; Langer et al. 2007; Maidment & Barrett, 2011). Langer et al. (2007) reconstruct Brachialis as originating distally from the anterior intercondylar depression, and this feature may correspond to the Fossa musculus brachialis of birds (Baumel et al. 1993), but it is typically not present as a distinct impression in basal theropods or basal ornithischians (Maidment & Barrett, 2011). Although the extent of the distal excursion of the origin of Brachialis is equivocal and a more distally placed origin is possible (Maidment & Barrett, 2011), the potential for a more proximally placed origin, as in crocodylians (Meers, 2003) and as reconstructed by Jasinoski et al. (2006), would result in a longer moment arm for this muscle and thus a greater mechanical advantage.
Distally on the humerus, the origins of the muscles attaching to the entepicondyle and the ectepicondyle have only been reconstructed individually in two studies (Carpenter & Smith, 2001; Langer et al. 2007), neither of which reconstruct all possible muscles. Though consisting of only four muscles, the arrangement in Carpenter & Smith's (2001) avian-based reconstruction is generally congruent with the current study, as is the arrangement of the extensor (ectepicondylar) muscles of Langer et al. (2007). Although a joined origin of Extensor carpi ulnaris and Supinator is not supported phylogenetically, it is possible that these two muscles originated in close proximity to each other, given the phylogenetic uncertainty in their proximodistal arrangement. Additionally, it is unlikely that the origin of Flexor carpi ulnaris was located proximal to the origin of Flexor digitorum longus, as has been proposed by Langer et al. (2007), because this muscle consistently has the most distal origin on the entepicondyle in all extant taxa studied.
Of the muscle attachment sites on the antebrachium, the insertion of Biceps brachii is one of the few that is typically reconstructed. Most authors agree on a dual insertion on both the radius and ulna (Dilkes, 2000; Jasinoski et al. 2006; Langer et al. 2007) and in some cases small rugosities and tubercles have been identified on both bones that may correspond to the insertion site in this area (Carpenter & Smith, 2001; Langer et al. 2007). A more distally located tubercle on the anterior surface of the radius, as exhibited in Herrerasaurus (Sereno, 1993), likely does not represent the insertion of Biceps brachii or Brachialis but instead that of Humeroradialis, which possesses an insertion site marked by a large tubercle and located distal to those of Biceps brachii and Brachialis in crocodylians (Meers, 2003).
Langer et al.'s (2007) reconstructions of ‘Flexor ulnaris’ (here Anconeus) and Pronator quadratus are congruent with those of this study, but an insertion of Flexor carpi ulnaris onto the medial surface of the proximal ulna is not supported. An accessory ulnar origin of Flexor carpi ulnaris has been reported for some lepidosaurs (Russell & Bauer, 2008) but it is not commonly or consistently present within a single genus. Carpenter & Smith (2001), who based their reconstruction primarily on birds, also reconstructed an attachment to the ulna but again there is little evidence for this. In neognathous birds, Flexor carpi ulnaris passes through a ligamentous structure called the humeroulnar pulley, which attaches to the proximal end of the ulna, but it does not take origin from the ulna or the humeroulnar pulley and thus has no attachment to the proximal end of the ulna (George & Berger, 1966). The insertion extends onto the distal end of the ulna in Apteryx (McGowan, 1982), but not in Struthio or in tinamous (Hudson et al. 1972; dissections). Most differences in the reconstruction of the antebrachial musculature of this study and that of Dilkes (2000) are related to the presence of the radiale and ulnare, which have been lost in Maiasaura but retained in Tawa, and the derived morphology of the manus in the former taxon.
Evolutionary and functional implications
Reconstruction of the shoulder musculature in a basal theropod allows for direct comparison with recent muscular reconstructions of the basal members of other dinosaurian clades as well as with more derived theropods. Although many ornithischians and sauropodomorphs reevolved quadrupedality, the most basal members of both clades were bipedal and retained a similar forelimb morphology to that of basal theropods (Maidment & Barrett, 2011). As such, the overall arrangement of the shoulder musculature in basal ornithischians (Maidment & Barrett, 2011) and basal sauropodomorphs (Langer et al. 2007) is remarkably similar to that of basal theropods in both the relative development of various muscle groups and their potential actions (Fig. 7). In each clade the scapulocoracoid and proximal end of the humerus exhibit large, well-developed attachment sites for all major muscle groups of the shoulder. The humeri of basal saurischians generally exhibit larger, more anteriorly protruding deltopectoral crests than those of basal ornithischians. This provides a longer moment arm for the Supracoracoideus musculature, increasing its mechanical advantage for protracting the humerus and resulting in stronger flexion of the shoulder in basal saurischians. An expanded deltopectoral crest also enlarges the potential area for the insertion of Deltoideus clavicularis, indicating a potentially greater capacity for abduction in basal saurischians.
Basal theropods differ from basal sauropodomorphs and basal ornithischians in possessing relatively longer scapular blades, placing the origin of Deltoideus scapularis farther from the glenohumeral joint and thus slightly increasing the torque provided by the muscle for extension of the humerus. The more distal insertion of Latissimus dorsi on the humerus, lengthening its lever arm, reinforces the emphasis on extension of the humerus in early theropods. The accentuation of humeral extension in early theropods over the morphology seen in early sauropodomorphs and ornithischians may reflect the role of the forelimb in predation. A large struggling prey item would exert a flexor moment on the shoulder, which would need to be counteracted by powerful extension. Although this would have been important for a carnivorous early theropod like Tawa, basal ornithischians and sauropodomorphs are usually inferred to be herbivorous to omnivorous (Barrett, 2000; Barrett et al. 2011) and likely would not have been hunting large prey. The similarities of the rest of the forelimb musculature between the basal taxa indicate that they likely shared many other possible functions, such as manipulation of small prey items, grooming, or intraspecific interactions.
Reorientation of the scapulocoracoid in derived maniraptorans to a more bird-like position (Jasinoski et al. 2006) caused many functional changes in the shoulder musculature relative to the basal condition. The large, sheet-like muscles attaching to the scapular blade are responsible for scapular protraction, retraction, and overall rotation, and have an important role in increasing the anteroposterior excursion of the entire forelimb in crocodylians (Meers, 2003) and especially in arboreal lizards such as chameleons and anoles (Peterson, 1973). Levator scapulae and Serratus profundus are active during retraction of the forelimb, pulling the distal end of the scapula anteriorly and thus moving the coracoid posteriorly, whereas Serratus superficialis, acting on the distal end of the scapula in the opposite direction, is active during protraction of the forelimb (Peterson, 1973). Trapezius also assists in protraction of the forelimb through its fibers that insert near the acromion of the scapula, thus pulling the proximal end of the scapula anteriorly (Meers, 2003). This mechanism was likely also in place in basal theropods, allowing a greater anterior reach of the forelimb than has been previously described when considering only the range of motion of the glenohumeral joint (e.g. Carpenter, 2002). However, both Trapezius and Levator scapulae have been lost in birds, and the horizontal orientation of the scapula has altered the functions of Serratus muscles to assist in respiration (superficialis) and stabilization of the scapula and movements related to gliding (profundus; Fisher, 1946). A subhorizontal scapular orientation in dromaeosaurids, as interpreted by Jasinoski et al. (2006), would result in a similar reduction of the rotational capability of the scapula and subsequent reduction in the anterior excursion of the forelimb in these taxa.
Despite extreme modification of the distal segments of the forelimb in birds, there is a large amount of conservation in the muscles attaching to the antebrachium among archosaurs. Birds retain a full complement of pronator and supinator muscles, and their development is potentially related to the amount of nonsteady flight in which a bird engages (Dial, 1992b). In this capacity, these muscles possess some ability to pronate and supinate the distal segments of the wing (Dial, 1992a), although the specifics of their function and mechanism are not well understood. It has been proposed that the morphology of the radius of theropods limits the degree of pronation and/or supination of the forearm (e.g. Carpenter, 2002), but rotation of the forearm on its long axis to some degree also occurs in lepidosaurs (Landsmeer, 1983) and crocodylians (Baier & Gatesy, 2013), so it is likely that the pronators and supinator of the forearm in basal theropods possessed some pronatory and supinatory capabilities along with their roles as flexors of the forearm.
The carpus of basal dinosaurs exhibits the morphology of neither extant birds nor extant crocodylians, instead bearing a closer overall resemblance with that of lepidosaurs, but it quickly became modified in the theropod lineage. In particular, the loss of the pisiform early in theropod evolution necessitated the shift of the attachments of several antebrachial muscles to other elements. In birds, the insertion of FCU and the origin of ADM have both shifted from the pisiform to the neomorphic ‘pseudoulnare’ (not homologous to the ulnare of other tetrapods; Kundrát, 2009); it is unknown when this structure evolved, but these muscles probably attached to the ulnare in theropods that lack a pisiform, regardless of homology. These new attachments to a nearby bone do not change the function of these muscles, both of which would be active during ulnar deviation of the manus. Although the osteology of the avian manus is highly modified, many of the intrinsic manual muscles can be considered to retain their plesiomorphic attachments when the development of the carpometacarpus is considered (Kundrát, 2009). Additionally, the newly dissected, well-developed manual musculature of the ostrich, which revealed the presence of Abductor digiti minimi, further elucidates the plesiomorphic morphology of the intrinsic manual muscles. Evidence for well-developed Abductor pollicis longus muscles in basal theropods indicates that digit I had some independence from the other digits of the hand, but close articulation of the metacarpals likely precluded any true opposition of the theropod thumb. The manual unguals of basal theropods typically exhibit a very large flexor tubercle but no distinct extensor tubercle or process, indicating that, whereas digital flexion was important in these taxa, extension and especially hyperextension of the phalanges and unguals was less so.
Concluding remarks
This study provides the first full reconstruction of the forelimb musculature in a dinosaur, resulting in a more complete picture of each muscle and how these muscles work together. The inclusion of a phylogenetically broad sample of extant taxa and a phylogenetic ancestral state reconstruction in this analysis allowed for the unequivocal reconstruction of many distal forelimb muscles that have been previously deemed too uncertain to reconstruct. Although these muscles have been dismissed as secondary in investigations of locomotor function (e.g. Maidment & Barrett, 2011), they have great importance when considering function of the forelimbs in bipedal theropods, including hypotheses of grasping and predatory behavior. Furthermore, some antebrachial muscles have an important role in the automating musculoskeletal mechanism of avian flight (Vazquez, 1994), and an analysis of the changes in their distal attachments may elucidate when this mechanism evolved in the avian lineage. Hypotheses of theropod forelimb function have previously been tested primarily through range of motion studies (Carpenter, 2002; Senter & Robins, 2005) that do not consider the potential contribution by the musculature and the potential restrictions that it may impose on forelimb movement. The myology provided by this reconstruction allows for further testing of functional hypotheses using techniques such as three-dimensional modeling of muscle moment arms (e.g. Hutchinson et al. 2005).
Nonavian theropods exhibit a diverse range of forelimb morphologies from highly reduced to extremely elongate but we still understand very little about their evolution and function. This study provides the basis for future investigations of forelimb function in derived theropod taxa by providing a foundation for muscle reconstructions in individual taxa and enabling analysis of the sequential changes in the forelimb musculature along their evolutionary trajectories.
Acknowledgments
I thank D. Krause, A. Turner, B. Demes, and S. Gatesy for their help and support in the development of this study and for comments on early drafts of the manuscript. Comments from J. Hutchinson and an anonymous reviewer, and the editorial assistance of J. Clarke, greatly improved the clarity of this paper. I also thank A. Turner, S. Nesbitt, R. Irmis, and N. Smith for the opportunity to work on the Tawa forelimb material. R. Martinez (PVSJ), D. Henderson (RTMP), B. Strilisky (RTMP), M. Norell (AMNH), and C. Mehling (AMNH) generously provided access to specimens in their care. Thanks to P. O'Connor for providing the forelimbs of OUVC avian specimens for dissection, and to A. Pritchard for assistance with ostrich dissections. I thank J. McCartney for support and feedback during this project as well as comments on the manuscript. Funding for this project was provided by a National Science Foundation Graduate Research Fellowship, a National Science Foundation Doctoral Dissertation Improvement Grant (DEB 111036), and by the Doris O. and Samuel P. Welles Research Fund of the UCMP.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. List of extant taxa scored for analysis, with myological references.
Table S2. Proportional likelihoods of each character state at the nodes Aves, Archosauria, and Archosauria + Lepidosauria based on maximum likelihood ancestral state reconstruction using the consensus phylogeny.
Data S1. List of myological characters used in this analysis.
Data S2. Data matrix of character scores used in this analysis.
Data S3. Supplemental references.
References
- Abdala V, Moro S. Comparative myology of the forelimb of Liolaemus sand lizards (Liolaemidae) Acta Zool. 2006;87:1–12. [Google Scholar]
- Abdala V, Manzano AS, Herrel A. The distal forelimb musculature in aquatic and terrestrial turtles: phylogeny or environmental constraints? J Anat. 2008;213:159–172. doi: 10.1111/j.1469-7580.2008.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier DB, Gatesy SM. Three-dimensional skeletal kinematics of the shoulder girdle and forelimb in walking Alligator. J Anat. 2013;223:462–473. doi: 10.1111/joa.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier DB, Gatesy SM, Jenkins FA. A critical ligamentous mechanism in the evolution of avian flight. Nature. 2007;445:307–310. doi: 10.1038/nature05435. [DOI] [PubMed] [Google Scholar]
- Barrett PM. Prosauropods and iguanas: speculation on the diets of extinct reptiles. In: Sues H-D, editor. Evolution of Herbivory in Terrestrial Vertebrates: Perspectives from the Fossil Record. Cambridge: Cambridge University Press; 2000. pp. 42–78. [Google Scholar]
- Barrett PM, Butler RJ, Nesbitt SJ. The roles of herbivory and omnivory in early dinosaur evolution. Earth Environ Sci Trans R Soc Edinb. 2011;101:383–396. [Google Scholar]
- Baumel JJ, King AS, Breazile JE, et al. Handbook of Avian Anatomy: Nomina Anatomica Avium. 2nd edn. Cambridge: Nuttall Ornithological Club; 1993. p. 779. [Google Scholar]
- Beddard FE. Contributions to the anatomy of the Hoatzin (Opisthocomus cristatus), with particular reference to the structure of the wing in the young. Ibis. 1889;31:283–293. [Google Scholar]
- Berger AJ. The myology of the pectoral appendage of three genera of American cuckoos. Misc Publ Mus Zool Univ Mich. 1954;85:1–35. [Google Scholar]
- Berger AJ. On the anatomy of the Red Bird of Paradise, with comparative remarks on the Corvidae. Auk. 1956;73:427–446. [Google Scholar]
- Berger-Dell'Mour HAE. Der Übergang von Echse zu Schleiche in der Gattung Tetradactylus Merrem. Zool Jahrb Abt Anat Ontogenie Tiere. 1983;110:1–152. [Google Scholar]
- Bever GS, Gauthier JA, Wagner GP. Finding the frame shift: digit loss, developmental variability, and the origin of the avian hand. Evol Dev. 2011;13:269–279. doi: 10.1111/j.1525-142X.2011.00478.x. [DOI] [PubMed] [Google Scholar]
- Brochu CA. Osteology of Tyrannosaurus rex: insights from a nearly complete skeleton and high-resolution computed tomographic analysis of the skull. J Vertebr Paleontol. 2003;22:1–138. [Google Scholar]
- Bryant HN, Russell AP. The role of phylogenetic analysis in the inference of unpreserved attributes of extinct taxa. Philos Trans Biol Sci. 1992;337:405–418. [Google Scholar]
- Buscalioni AD, Ortega F, Rasskin-Gutman D, et al. Loss of carpal elements in crocodilian limb evolution: morphogenetic model corroborated by palaeobiological data. Biol J Linn Soc. 1997;62:133–144. [Google Scholar]
- Carpenter K. Forelimb biomechanics of nonavian theropod dinosaurs in predation. Senckenb Lethaea. 2002;82:59–76. [Google Scholar]
- Carpenter K, Smith M. Forelimb osteology and biomechanics of Tyrannosaurus rex. In: Tanke D, Carpenter K, editors. Mesozoic Vertebrate Life. Bloomington: Indiana University Press; 2001. pp. 90–116. [Google Scholar]
- Carrano MT, Hutchinson JR. Pelvic and hindlimb musculature of Tyrannosaurus rex (Dinosauria: Theropoda) J Morphol. 2002;253:207–228. doi: 10.1002/jmor.10018. [DOI] [PubMed] [Google Scholar]
- Cong L, Hou L, Wu X-C, et al. The Gross Anatomy of Alligator sinensis Fauvel. Beijing: Science Press; 1998. [Google Scholar]
- Conrad JL. Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bull Am Mus Nat Hist. 2008;310:1–182. [Google Scholar]
- Crawford NG, Faircloth BC, McCormack JE, et al. More than 1000 ultraconserved elements provide evidence that turtles are the sister group of archosaurs. Biol Lett. 2012;8:783–786. doi: 10.1098/rsbl.2012.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dececchi TA, Larsson HCE. Patristic evolutionary rates suggest a punctuated pattern in forelimb evolution before and after the origin of birds. Paleobiology. 2009;35:1–12. [Google Scholar]
- Dial KP. Activity patterns of the wing muscles of the pigeon (Columba livia) during different modes of flight. J Exp Zool. 1992a;262:357–373. [Google Scholar]
- Dial KP. Avian forelimb muscles and nonsteady flight: can birds fly without using the muscles in their wings? Auk. 1992b;109:874–885. [Google Scholar]
- Dilkes DW. Appendicular myology of the hadrosaurian dinosaur Maiasaura peeblesorum from the Late Cretaceous (Campanian) of Montana. Trans R Soc Edinb Earth Sci. 2000;90:87–125. [Google Scholar]
- Diogo R, Abdala V. Muscles of Vertebrates: Comparative Anatomy, Evolution, Homologies and Development. Enfield, NH: Science Publishers; 2010. [Google Scholar]
- Fisher HI. Adaptation and comparative anatomy of the locomotor apparatus of New World vultures. Am Midl Nat. 1946;35:545–727. [Google Scholar]
- Fisher HI, Goodman DC. 1955. pp. 1–127. The myology of the Whooping Crane, Grus americana. Illinois Biological Monograph 24.
- Fitzgerald TC. The Coturnix Quail: Anatomy and Histology. Des Moines: The Iowa State University Press; 1969. [Google Scholar]
- George JC, Berger AJ. Avian Myology. New York: Academic Press; 1966. [Google Scholar]
- Gishlick AD. The function of the manus and forelimb of Deinonychus antirrhopus and its importance for the origin of avian flight. In: Gauthier JA, Gall LF, editors. New Perspectives on the Origin and Early Evolution of Birds: Proceedings. New Haven: Peabody Museum of Natural History, Yale University; 2001. pp. 301–318. [Google Scholar]
- Günther A. Contribution to the anatomy of HatteriaRhynchocephalus, Owen) Philos Trans R Soc Lond. 1867;157:595–629. [Google Scholar]
- Hackett SJ, Kimball RT, Reddy S, et al. A phylogenomic study of birds reveals their evolutionary history. Science. 2008;320:1763–1768. doi: 10.1126/science.1157704. [DOI] [PubMed] [Google Scholar]
- Haines RW. A revision of the extensor muscles of the forearm in tetrapods. J Anat. 1939;73:211–233. [PMC free article] [PubMed] [Google Scholar]
- Haines RW. The flexor muscles of the forearm and hand in lizards and mammals. J Anat. 1950;84:13–29. [PMC free article] [PubMed] [Google Scholar]
- Holmes R. The osteology and musculature of the pectoral limb of small captorhinids. J Morphol. 1977;152:101–140. doi: 10.1002/jmor.1051520107. [DOI] [PubMed] [Google Scholar]
- Howell AB. Phylogeny of the distal musculature of the pectoral appendage. J Morphol. 1936;60:287–315. [Google Scholar]
- Howell AB. Morphogenesis of the shoulder architecture: Aves. Auk. 1937;54:364–375. [Google Scholar]
- Hudson GE, Lanzillotti PJ. Gross anatomy of the wing muscles in the Family Corvidae. Am Midl Nat. 1955;53:1–44. [Google Scholar]
- Hudson GE, Lanzillotti PJ. Muscles of the pectoral limb in galliform birds. Am Midl Nat. 1964;71:1–113. [Google Scholar]
- Hudson GE, Hoff KM, Vanden Berge JC, et al. A numerical study of the wing and leg muscles of Lari and Alcae. Ibis. 1969;111:459–524. [Google Scholar]
- Hudson GE, Schreiweis DO, Wang SYC, et al. A numerical study of the wing and leg muscles of Tinamous (Tinamidae) Northwest Sci. 1972;46:207–255. [Google Scholar]
- Hutchinson JR, Anderson FC, Blemker SS, et al. Analysis of hindlimb muscle moment arms in Tyrannosaurus rex using a three-dimensional musculoskeletal computer model: implications for stance, gait and speed. Paleobiology. 2005;31:676–701. [Google Scholar]
- Jasinoski SC, Russell AP, Currie PJ. An integrative phylogenetic and extrapolatory approach to the reconstruction of dromaeosaur (Theropoda: Eumaniraptora) shoulder musculature. Zool J Linn Soc. 2006;146:301–344. [Google Scholar]
- Jetz W, Thomas GH, Joy JB, et al. The global diversity of birds in space and time. Nature. 2012;491:444–448. doi: 10.1038/nature11631. [DOI] [PubMed] [Google Scholar]
- Koehl MAR, Evangelista D, Yang K. Using physical models to study the gliding performance of extinct animals. Integr Comp Biol. 2011;51:1002–1018. doi: 10.1093/icb/icr112. [DOI] [PubMed] [Google Scholar]
- Kundrát M. Primary chondrification foci in the wing basipodium of Struthio camelus with comments on interpretation of autopodial elements in Crocodilia and Aves. J Exp Zool. 2009;312B:30–41. doi: 10.1002/jez.b.21240. [DOI] [PubMed] [Google Scholar]
- Landsmeer JMF. Mechanism of forearm rotation in Varanus exanthematicus. J Morphol. 1983;175:119–130. doi: 10.1002/jmor.1051750202. [DOI] [PubMed] [Google Scholar]
- Langer MC, Franca MAG, Gabriel S. The pectoral girdle and forelimb anatomy of the stem-sauropodomorph Saturnalia tupiniquim (Upper Triassic, Brazil) Spec Pap Palaeontol. 2007;77:113–137. [Google Scholar]
- Livezey BC. Evolutionary morphology of flightlessness in the Aukland Islands Teal. Condor. 1990;92:639–673. [Google Scholar]
- Livezey BC, Zusi RL. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and discussion. Zool J Linn Soc. 2007;149:1–95. doi: 10.1111/j.1096-3642.2006.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyson TR, Bever GS, Bhullar B-AS, et al. Transitional fossils and the origin of turtles. Biol Lett. 2010;6:830–833. doi: 10.1098/rsbl.2010.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyson TR, Sperling EA, Heimberg AM, et al. MicroRNAs support a turtle + lizard clade. Biol Lett. 2012;8:104–107. doi: 10.1098/rsbl.2011.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. 2010. Mesquite: a modular system for evolutionary analysis http://mesquiteproject.org.
- Maidment SCR, Barrett PM. The locomotor musculature of basal ornithischian dinosaurs. J Vertebr Paleontol. 2011;31:1265–1291. [Google Scholar]
- McGowan C. The wing musculature of the Brown kiwi Apteryx australis mantelli and its bearing on ratite affinities. J Zool. 1982;197:173–219. [Google Scholar]
- McKitrick MC. Myology of the pectoral appendage in kingbirds (Tyrannus) and their allies. Condor. 1985;87:402–417. [Google Scholar]
- Meers MB. Crocodylian forelimb musculature and its relevance to Archosauria. Anat Rec A. 2003;274A:891–916. doi: 10.1002/ar.a.10097. [DOI] [PubMed] [Google Scholar]
- Miner RW. The pectoral limb of Eryops and other primitive tetrapods. Bull Am Mus Nat Hist. 1925;51:145–312. [Google Scholar]
- Müller GB, Alberch P. Ontogeny of the limb skeleton in Alligator mississippiensis: developmental invariance and change in the evolution of archosaur limbs. J Morphol. 1990;203:151–164. doi: 10.1002/jmor.1052030204. [DOI] [PubMed] [Google Scholar]
- Nesbitt SJ, Smith ND, Irmis RB, et al. A complete skeleton of a Late Triassic saurischian and the early evolution of dinosaurs. Science. 2009a;326:1530–1533. doi: 10.1126/science.1180350. [DOI] [PubMed] [Google Scholar]
- Nesbitt SJ, Turner AH, Spaulding M, et al. The theropod furcula. J Morphol. 2009b;270:856–879. doi: 10.1002/jmor.10724. [DOI] [PubMed] [Google Scholar]
- Nicholls EL, Russell AP. Structure and function of the pectoral girdle and forelimb of Struthiomimus altus (Theropoda: Ornithomimidae) Palaeontology. 1985;28:643–677. [Google Scholar]
- Padian K. Basal Avialae. In: Weishampel DB, Dodson P, Osmolska H, editors. The Dinosauria. 2nd edn. Berkeley: University of California Press; 2004. pp. 210–231. [Google Scholar]
- Parker TJ. Observations on the anatomy and development of Apteryx. Philos Trans R Soc B. 1891;182:25–134. [Google Scholar]
- Peterson JA. Adaptation for Arboreal Locomotion in the Shoulder Region of Lizards. University of Chicago; 1973. PhD Thesis. [Google Scholar]
- Peterson JA. The locomotion of Chamaeleo (Reptilia: Sauria) with particular reference to the forelimb. J Zool. 1984;202:1–42. [Google Scholar]
- Poore SO, Sanchez-Haiman A, Goslow GE. Wing upstroke and the evolution of flapping flight. Nature. 1997;387:799–802. [Google Scholar]
- Raikow RJ. Pectoral appendage myology of the Hawaiian honeycreepers (Drepanididae) Auk. 1977;94:331–342. [Google Scholar]
- Reese AM. The Alligator and its Allies. New York: The Knickerbocker Press; 1915. [Google Scholar]
- Ribbing L. Die distale armmuskulatur der Amphibien, Reptilien und Säugetiere. Zool Jahrb Abt Anat Ontogenie Tiere. 1907;23:587–682. [Google Scholar]
- Rinehart LF, Lucas SG, Hunt AP. Furculae in the Late Triassic theropod dinosaur Coelophysis bauri. Palaeontol Z. 2007;81:174–180. [Google Scholar]
- Romer AS. The development of tetrapod limb musculature – the shoulder region of Lacerta. J Morphol. 1944;74:1–41. [Google Scholar]
- Russell AP, Bauer AM. The appendicular locomotor apparatus of Sphenodon and normal-limbed squamates. In: Gans C, Gaunt AS, Adler K, editors. Biology of the Reptilia 24, Morphology 1. Ithaca, NY: Society for the Study of Amphibians and Reptiles; 2008. pp. 1–466. [Google Scholar]
- Senter P. Scapular orientation in theropods and basal birds, and the origin of flapping flight. Acta Palaeontol Pol. 2006;51:305–313. [Google Scholar]
- Senter P, Robins JH. Range of motion in the forelimb of the theropod dinosaur Acrocanthosaurus atokensis, and implications for predatory behavior. J Zool. 2005;266:307–318. [Google Scholar]
- Sereno PC. The pectoral girdle and forelimb of the basal theropod Herrerasaurus ischigualastensis. J Vertebr Paleontol. 1993;13:425–450. [Google Scholar]
- Shah RV, Patel VB. Myology of the chelonian pectoral appendage. J Anim Morphol Physiol. 1964;11:58–84. [Google Scholar]
- Shufeldt RW. Notes on the osteology of the young of the Hoatzin (Opisthocomus cristatus) and other points on its morphology. J Morphol. 1918;31:599–606. [Google Scholar]
- Sterli J. Phylogenetic relationships among extinct and extant turtles: the position of Pleurodira and the effects of fossils on rooting crown-group turtles. Contrib Zool. 2010;79:93–106. [Google Scholar]
- Straus WL. The homologies of the forearm flexors: urodeles, lizards, mammals. Am J Anat. 1942;70:281–316. [Google Scholar]
- Sullivan GE. Anatomy and embryology of the wing musculature of the domestic fowl (Gallus. Aust J Zool. 1962;10:458–518. [Google Scholar]
- Swinebroad J. A comparative study of the wing myology of certain passerines. Am Midl Nat. 1954;51:488–514. [Google Scholar]
- Vanden Berge JC. A comparative study of the appendicular musculature of the Order Ciconiiformes. Am Midl Nat. 1970;84:289–364. [Google Scholar]
- Vanden Berge JC, Zweers GA. Myologia. In: Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC, editors. Handbook of Avian Anatomy: Nomina Anatomica Avium. Cambridge: Nuttall Ornithological Club; 1993. pp. 189–247. [Google Scholar]
- Vazquez RJ. The automating skeletal and muscular mechanisms of the avian wing (Aves) Zoomorphology. 1994;114:59–71. [Google Scholar]
- Walker JWF. The locomotor apparatus of testudines. In: Gans C, Parsons TS, editors. Biology of the Reptilia 4. New York: Academic Press; 1973. pp. 1–100. [Google Scholar]
- Wang X, Nudds RL, Dyke GJ. The primary feather lengths of early birds with respect to avian wing shape evolution. J Evol Biol. 2011a;24:1226–1231. doi: 10.1111/j.1420-9101.2011.02253.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Young RL, Xue H, et al. Transcriptomic analysis of avian digits reveals conserved and derived digit identities in birds. Nature. 2011b;477:583–587. doi: 10.1038/nature10391. [DOI] [PubMed] [Google Scholar]
- Werneburg I, Sánchez-Villagra MR. Timing of organogenesis support basal position of turtles in the amniote tree of life. BMC Evol Biol. 2009;9:82. doi: 10.1186/1471-2148-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer LM. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In: Thomason JJ, editor. Functional Morphology in Vertebrate Paleontology. Cambridge: Cambridge University Press; 1995. pp. 9–33. [Google Scholar]
- Young CG. On the habits and anatomy of Opisthocomus cristatus, Illig. Notes Leyden Museum. 1888;10:169–175. [Google Scholar]
- Zusi RL, Bentz GD. The appendicular myology of the Labrador Duck (Camptorhynchus labradorius. Condor. 1978;80:407–418. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of extant taxa scored for analysis, with myological references.
Table S2. Proportional likelihoods of each character state at the nodes Aves, Archosauria, and Archosauria + Lepidosauria based on maximum likelihood ancestral state reconstruction using the consensus phylogeny.
Data S1. List of myological characters used in this analysis.
Data S2. Data matrix of character scores used in this analysis.
Data S3. Supplemental references.


