Abstract
The macula flavae (MF), populated by vitamin A-storing stellate cells (SCs), are believed to play a fundamental role in development, maintenance and repair of the vocal fold (VF) mucosa; however, to date, they have mostly been examined in observational human cadaver studies. Here, we conducted an interspecies comparison of MF and SC phenotype, as well as vitamin A quantification and localization, in human, pig, dog, rabbit and rat VF mucosae. MF containing vitamin A-positive SCs were only identified in human and rat specimens. Pig, dog and rabbit VF mucosae contained no discernable MF, but rather exhibited preferential vitamin A localization to mucous (pig), serous (dog) or mixed (rabbit) glands. This glandular vitamin A storage corresponded to exceedingly high concentrations of retinol in pig and dog mucosae, and retinyl ester in dog mucosa. These findings have significant implications for the presumed role of the MF and SCs in VF biology, the nature of vitamin A storage within the VF mucosa, and the selection of an appropriate animal model for future experimental studies.
Keywords: glial fibrillary acidic protein, gold chloride, high-performance liquid chromatography, histology, larynx, macula flava, oil red O, retinol, retinyl ester
Introduction
Stellate cells (SCs) store the essential nutrient vitamin A (VA) and have been identified in multiple organs, including the liver, lung, pancreas and spleen (Wake, 1980; Nagy et al. 1997; Kim et al. 2009). SCs play an important role in the mobilization of VA for transport to target cells, where it is utilized in a variety of metabolic and transcriptional processes (Blomhoff et al. 1990). Following organ injury or damage, SCs transition from a quiescent to an activated myofibroblastic phenotype, characterized by the progressive release of VA stores, increased extracellular matrix (ECM) synthesis and the onset of fibrosis (Davis, 1988; Davis et al. 1996). Emerging evidence in the liver suggests that VA administration at the time of injury can attenuate hepatic SC activation and reduce fibrosis (de Freitas et al. 2003; Murakami et al. 2011).
Sato et al. (2001, 2003) first identified VA-storing SCs in the human vocal fold (VF). These vocal fold stellate cells (VFSCs), located in anatomic niches (termed macula flavae, MF) at the anterior and posterior boundaries of the mucosae, exhibit morphological differences when compared with VF fibroblasts, and have been hypothesized to drive ECM synthesis and functional maturation of the VF lamina propria (LP; Sato et al. 2010a,b2010b). Despite their potential importance, VFSCs have received little attention in experimental studies. Previous in vivo work showed a population of VA-storing VFSCs in rats (Tateya et al. 2006, 2008); however, there remains no direct comparison of MF appearance, VFSC phenotype or VA localization in humans and other species, and no quantitative analysis of the forms of VA stored within the VF mucosa of any species. Such data are needed to better evaluate the role of VA-storing VFSCs in development and maintenance of the VF mucosa, and to determine the most suitable animal model for experimental study of VF diseases involving altered VA storage or metabolism.
Here, we performed histological analyses of MF structure, presence of VFSCs and localization of VA in the VF mucosae of five mammalian species: human, pig, dog, rabbit and rat. We further conducted high-performance liquid chromatography (HPLC) to measure the concentration of VA stored in alcohol (retinol) and ester (retinyl ester) forms within the VF mucosae of all species. We purposefully selected the four non-human species based on their historical utility as experimental models for various VF biological and biomechanical applications (Thibeault et al. 2002; Tateya et al. 2005; Barker et al. 2006; Berke et al. 2007; Ge et al. 2009; Welham et al. 2009; Bless & Welham, 2010).
Materials and methods
Tissue procurement and preparation
We procured a total of 57 cadaver larynges: six larynges were obtained from three male and three female Caucasian humans aged 40–48 years; six larynges were obtained from female market pigs aged 6 months (body mass ∼ 100 kg); six larynges were obtained from male beagle dogs aged 4 months (body mass ∼ 10 kg); six larynges were obtained from female New Zealand white rabbits aged 4 months (body mass ∼ 5 kg); 33 larynges were obtained from Fischer 344 male rats aged 4 months (body mass ∼ 230 g). Human tissues were obtained within 24 h of death; animal tissues were harvested immediately following death.
Larynges intended for histology and immunohistochemistry (IHC; n = 3 per species) were dissected using surgical instruments and removed en bloc. Rabbit and rat larynges were embedded in optimal cutting temperature compound (Sakura Finetek, Tokyo, Japan), snap-frozen using liquid N2, and stored as whole organ blocks at −80 °C. Human, pig and dog VFs were further dissected from each larynx, embedded and snap-frozen as described above, and stored at −80 °C. Serial 5-μm sections were prepared in the axial plane using a cryostat.
Larynges intended for HPLC analysis of retinol and retinyl ester content (n = 3 human, pig, dog and rabbit; n = 30 rat) were dissected under a stereomicroscope to obtain bilateral VF mucosae containing all four MF and no visual evidence of thyroarytenoid muscle contamination. Based on pilot work examining HPLC-based retinol and retinyl ester detectability in VF samples (data not shown), bilateral human, pig, dog and rabbit VF mucosae were pooled for each individual larynx prior to HPLC, whereas bilateral rat VF mucosae were pooled across 10 larynges prior to HPLC.
Histology and IHC
Cell and tissue morphology were examined using routine hematoxylin and eosin (H&E) staining. Cytoplasmic lipid droplets were examined using oil red O staining, as follows. Sections were fixed using 4% formaldehyde for 10 min, incubated with 60% oil red O solution (Newcomer, Middleton, WI, USA) for 15 min, washed three times with distilled water (5 min per wash), counterstained with hematoxylin, rinsed with distilled water, and mounted. VA localization was examined using gold chloride staining, as follows. Sections were fixed with 4% formaldehyde for 1 h, washed three times with distilled water (5 min per wash), incubated with 0.01% gold chloride solution (Newcomer) containing 0.01% HCl for 10 h in the dark, dehydrated using a graded ethanol series (70–100%), cleared using xylene, and mounted.
Human and rat MF were examined for VFSCs by immunostaining for the SC-specific marker glial fibrillary acidic protein (GFAP; Tateya et al. 2008; Sato et al. 2012). Sections were fixed using 4% paraformaldehyde for 10 min, washed three times with phosphate-buffered saline (PBS; 5 min per wash) and blocked with 5% fetal bovine serum in PBS for 30 min. Sections were incubated with antibody Cy3-conjugated mouse monoclonal anti-GFAP (1 : 400; C9205; Sigma-Aldrich, St Louis, MO, USA) for 1 h, washed with PBS and mounted. Laryngeal cartilage was used as a positive control tissue and showed chondrocyte-specific immunostain (Kasantikul & Shuangshoti, 1989; Vega et al. 1990).
Brightfield and fluorescent microscopy were performed using a microscope (E-600; Nikon, Melville, NY, USA) equipped with a digital microscopy camera (DP-71; Olympus, Center Valley, PA, USA). All images were collected with consistent exposure settings.
HPLC
Sample wet weight (ww) was recorded for later normalization of all HPLC data. Tissues were ground using ∼ 2 g sodium sulfate; 40 μL retinyl butyrate in methanol was added as an internal standard. Retinol and retinyl esters were extracted into 10 mL dichloromethane through Whatman #1 filter paper. Samples were dried with N2 gas and reconstituted in 60 μL of 3 : 1 methanol : dichloroethane (v : v). Forty microliters of each sample was injected into a HPLC system consisting of a Waters 1525 binary pump, Waters 2707 autosampler and Waters 2998 PDA detector running Empower 2 software (Milford, MA, USA). A Waters Symmetry C18 column (3.5 μm, 4.6 × 75 mm) was used in series with a Waters Resolve C18 column (5 μm, 3.9 × 300 mm). Compounds were separated using the following gradient: solvent A [85 : 15 acetonitrile : water (v : v) with 10 mmol ammonium acetate] was run at 1 mL min−1 for 15 min, then transitioned to 100% solvent B [70 : 20 : 10 acetonitrile : dichloroethane : methanol (v : v : v) with 10 mmol ammonium acetate] over 10 min, held at 100% solvent B for 20 min, then transitioned back to 100% solvent A over 5 min and equilibrated. Chromatograms were recorded at 325 nm, and concentrations of retinol and retinyl ester were calculated using curves derived from HPLC runs of purified standards.
Statistical analysis
Retinol and retinyl ester concentrations were expressed per g ww. Ratios of retinol : retinyl ester concentration were also calculated. Data were analyzed using one-way analysis of variance with log transformation to meet the equal variance assumptions of this approach. If the omnibus F-test revealed a significant difference, planned pair-wise comparisons (human vs. other species) were performed using Fisher's protected least significant difference method. A type I error rate of 0.05 was used. All P-values were two-sided.
Results
To enhance anatomic perspective and capture both anterior and posterior MF in a single histological section, we microdissected and embedded whole human, pig and dog VFs, and whole rabbit and rat larynges. All sections were cut in the axial plane. HPLC measurements were made using bilateral microdissected VF mucosae.
Human
Macula flavae were identified as ovoid, high-cell-density regions at the anterior and posterior poles of the VF mucosa, using H&E stain (Fig. 1a). Cells within the MF were positive for intracellular lipid droplets (oil red O stain), intracellular VA (gold chloride stain) and the SC-specific marker GFAP (Fig. 1b), consistent with previous characterization of the in vivo VFSC phenotype (Tateya et al. 2008; Sato et al. 2012). In contrast, cells in the LP region were uniformly oil red O−, gold chloride− and GFAP− (Fig. 1c). A small number of oil red O+ and gold chloride+ mixed glands were noted in sections from the inferior VF mucosa (data not shown).
Figure 1.
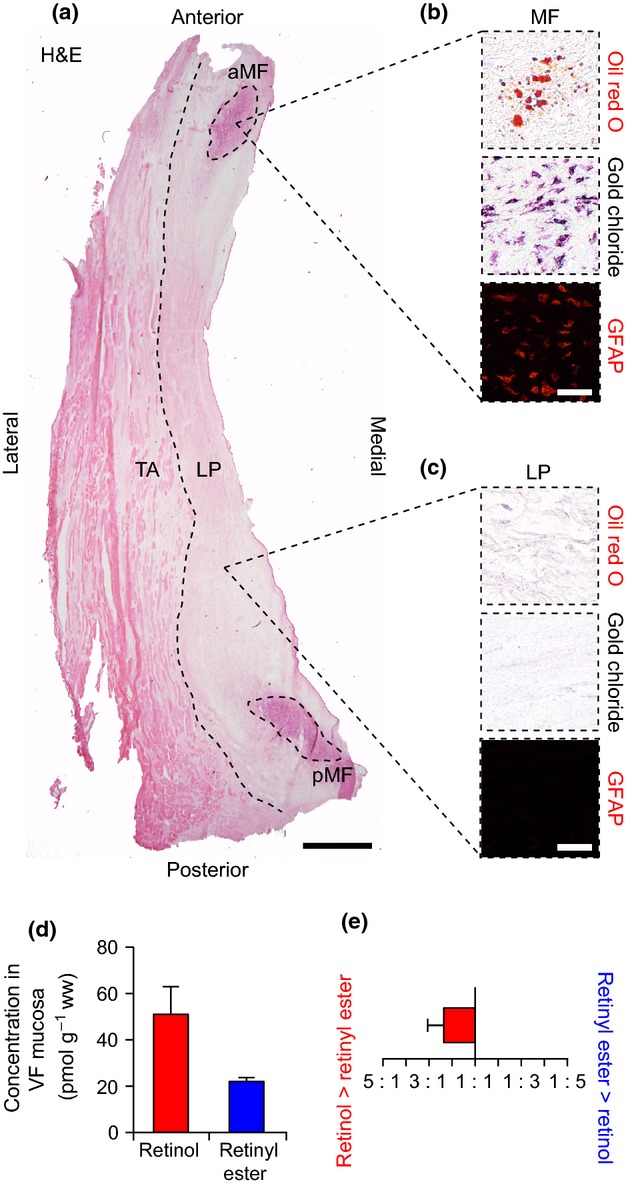
Analysis of histological features and vitamin A content in the human vocal fold (VF). (a) Hematoxylin and eosin (H&E)-stained axial section. The dashed black line shows the boundary between the lamina propria (LP) and thyroarytenoid muscle (TA). The dashed black ellipses show the anterior and posterior macula flavae (aMF, pMF). (b) Representative histological and immunostained sections showing oil red O+ (red), gold chloride+ (indigo/black) and glial fibrillary acidic protein (GFAP)+ (red) stellate cells within the MF. (c) Representative histological and immunostained sections showing oil red O−, gold chloride− and GFAP− cells within the LP. (d) Concentration of retinol and retinyl ester in human VF mucosa. (e) Ratio of retinol to retinyl ester concentration in human VF mucosa. Data in (d) and (e) are presented as mean ± SEM. Scale bar: 1 mm (a); 60 μm (b, c).
We detected both retinol and retinyl ester in human VF mucosa using HPLC (Fig. 1d). The mean ratio of retinol to retinyl ester concentration was 2.4 : 1 (Fig. 1e).
Pig
Macula flavae were not identified within the pig VF mucosa (Fig. 2a). We noted loosely organized high-cell-density regions in the tendons attaching the mucosa to the arytenoid and thyroid cartilages. The cells populating these tendons were oil red O− and gold chloride−. The mucosa was rich in oil red O+ and gold chloride+ mucous glands (Fig. 2b); non-glandular cells in the LP region were uniformly oil red O− and gold chloride− (Fig. 2c). As we observed no histological evidence of MF or VA-storing cells at the poles of the mucosa, we did not immunostain for GFAP. We detected retinol but no retinyl ester in pig VF mucosa using HPLC (Fig. 2d).
Figure 2.
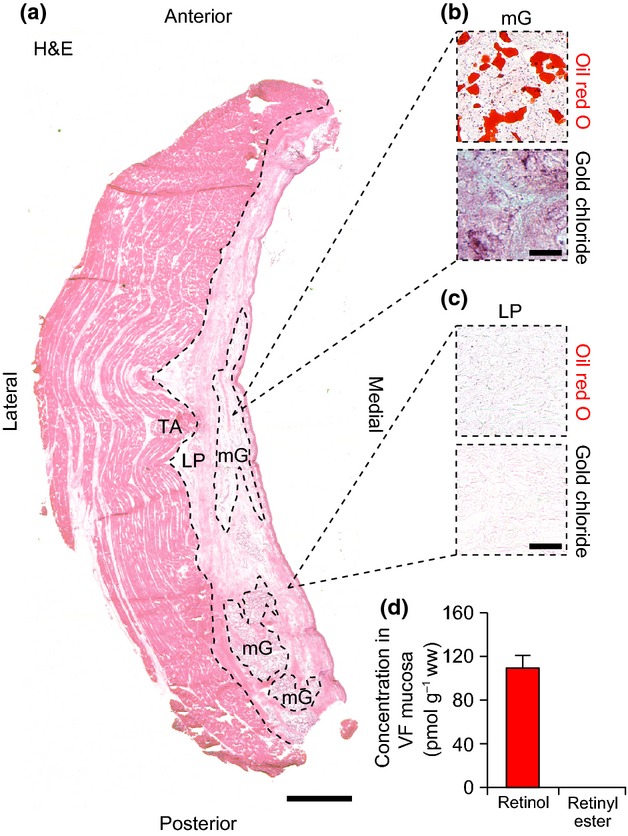
Analysis of histological features and vitamin A content in the pig vocal fold (VF). (a) Hematoxylin and eosin (H&E)-stained axial section. The dashed black line shows the boundary between the lamina propria (LP) and thyroarytenoid muscle (TA). MF were not identified within the LP. The dashed black ellipses show representative mucous glands (mG), the predominant gland type in the pig LP. (b) Representative histological sections showing oil red O+ (red) and gold chloride+ (indigo/black) mG tissue. (c) Representative histological sections showing oil red O− and gold chloride− non-glandular tissue within the LP. (d) Concentration of retinol in pig VF mucosa (mean ± SEM). Retinyl esters were not detected. Scale bar: 1 mm (a); 150 μm (b, c).
Dog
Macula flavae were not identified within the dog VF mucosa (Fig. 3a). We noted loosely organized high-cell-density regions in the tendons attaching the mucosa to the arytenoid and thyroid cartilages. The cells populating these tendons were oil red O− and gold chloride−. The mucosa contained oil red O+ and gold chloride+ serous glands (Fig. 3b); non-glandular cells in the LP region were uniformly oil red O− and gold chloride− (Fig. 3c). As we observed no histological evidence of MF or VA-storing cells at the poles of the mucosa, we did not immunostain for GFAP.
Figure 3.
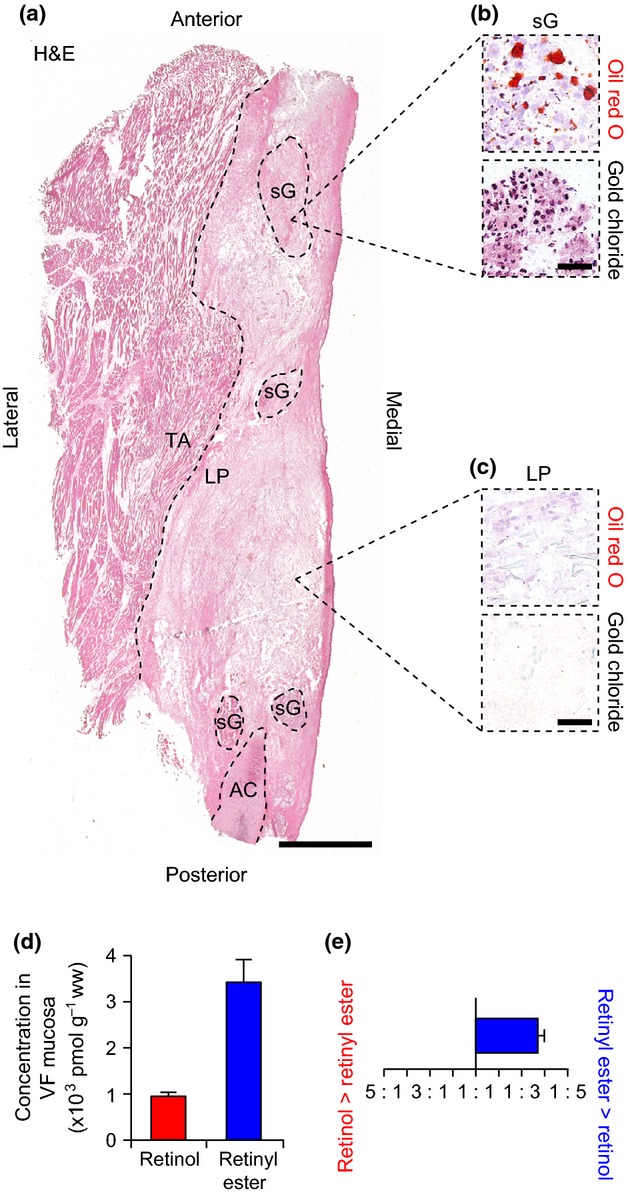
Analysis of histological features and vitamin A content in the dog vocal fold (VF). (a) Hematoxylin and eosin (H&E)-stained axial section. The dashed black lines show the boundary between the lamina propria (LP) and thyroarytenoid muscle (TA), and the anterior aspect of the arytenoid cartilage (AC). MF were not identified within the LP. The dashed black ellipses show representative serous glands (sG), the predominant gland type in the dog LP. (b) Representative histological sections showing oil red O+ (red) and gold chloride+ (indigo/black) sG tissue. (c) Representative histological sections showing oil red O− and gold chloride− non-glandular tissue within the LP. (d) Concentration of retinol and retinyl ester in dog VF mucosa. (e) Ratio of retinol to retinyl ester concentration in dog VF mucosa. Data in (d) and (e) are presented as mean ± SEM. Scale bar: 1 mm (a); 150 μm (b, c).
We detected both retinol and retinyl ester in dog VF mucosa using HPLC (Fig. 3d). The mean ratio of retinol to retinyl ester concentration was 1 : 3.7 (Fig. 3e).
Rabbit
Macula flavae were not identified within the rabbit VF mucosa (Fig. 4a). We noted loosely organized high-cell-density regions in the tendons attaching the anterior mucosa to the thyroid cartilage. The cells populating these tendons were oil red O− and gold chloride−. The mucosa contained oil red O− and gold chloride+ mixed glands (Fig. 4b); non-glandular cells in the LP region were uniformly oil red O− and gold chloride− (Fig. 4c). As we observed no histological evidence of MF or VA-storing cells at the poles of the mucosa, we did not immunostain for GFAP.
Figure 4.
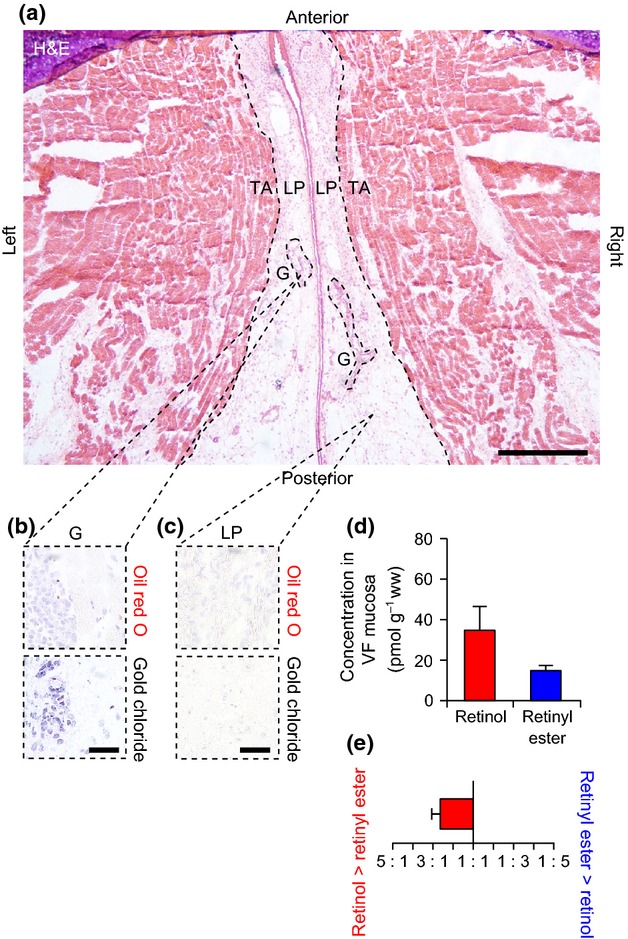
Analysis of histological features and vitamin A content in the rabbit vocal fold (VF). (a) Hematoxylin and eosin (H&E)-stained axial section showing the bilateral VFs. The dashed black lines show the boundaries between the lamina propria (LP) and thyroarytenoid muscle (TA). MF were not identified within the LP. The dashed black ellipses show representative glands (G), which were predominantly mixed glands comprised of both mucous and serous cells. (b) Representative histological sections showing oil red O− (red) and gold chloride+ (indigo/black) glandular tissue. (c) Representative histological sections showing oil red O− and gold chloride− non-glandular tissue within the LP. (d) Concentration of retinol and retinyl ester in rabbit VF mucosa. (e) Ratio of retinol to retinyl ester concentration in rabbit VF mucosa. Data in (d) and (e) are presented as mean ± SEM. Scale bar: 600 μm (a); 60 μm (b, c).
We detected both retinol and retinyl ester in rabbit VF mucosa using HPLC (Fig. 4d). The mean ratio of retinol to retinyl ester concentration was 2.6 : 1 (Fig. 4e).
Rat
Consistent with the human VF, MF were identified as ovoid, high-cell-density regions at the anterior and posterior poles of the rat VF mucosa (Fig. 5a). Further, the MF were populated by oil red O+, gold chloride+ and GFAP+ VFSCs (Fig. 5b). Cells in the LP region were uniformly oil red O−, gold chloride− and GFAP− (Fig. 5c). A small number of oil red O+ and gold chloride+ mixed glands were noted throughout the VF mucosa (data not shown).
Figure 5.
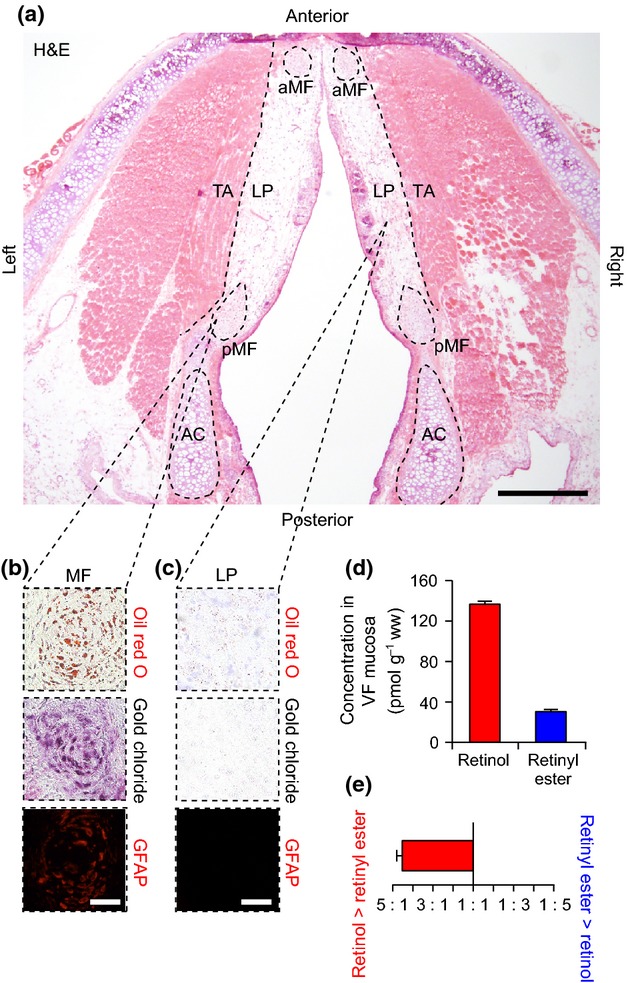
Analysis of histological features and vitamin A content in the rat vocal fold (VF). (a) Hematoxylin and eosin (H&E)-stained axial section showing the bilateral VFs. The dashed black lines show the boundary between the lamina propria (LP) and thyroarytenoid muscle (TA). The dashed black ellipses show the anterior and posterior macula flavae (aMF, pMF), and the arytenoid cartilages (AC). (b) Representative histological and immunostained sections showing oil red O+ (red), gold chloride+ (indigo/black) and glial fibrillary acidic protein (GFAP)+ (red) stellate cells within the MF. (c) Representative histological and immunostained sections showing oil red O−, gold chloride− and GFAP− cells within the LP. (d) Concentration of retinol and retinyl ester in rat VF mucosa. (e) Ratio of retinol to retinyl ester concentration in rat VF mucosa. Data in (d) and (e) are presented as mean ± SEM. Scale bar: 600 μm (a); 60 μm (b, c).
We detected both retinol and retinyl ester in rat VF mucosa using HPLC (Fig. 5d). The mean ratio of retinol to retinyl ester concentration was 4.5 : 1 (Fig. 5e).
Interspecies comparison
Histological findings and quantitative HPLC data are summarized for all species in Table 1. Rats, the only non-human species with clearly defined MF and VA-storing VFSCs, shared the most histological features with humans. Retinol concentrations in pig, dog and rat VF mucosae were significantly higher than in human VF mucosa (P < 0.05), whereas retinol concentration in rabbit VF mucosa was most similar to human tissue (P > 0.05). Retinyl ester concentration in dog VF mucosa was significantly higher, and retinyl ester concentration in rabbit VF mucosa was significantly lower than human VF mucosa (P < 0.05); retinyl ester concentration in rat VF mucosa was most similar to human tissue (P > 0.05). Ratios of retinol : retinyl ester concentration were significantly different in dog and rat VF mucosae compared with human VF mucosa (P < 0.05), whereas the ratio in rabbit VF mucosa was most similar to human tissue (P > 0.05).
Table 1.
Interspecies comparison of vocal fold histological features and vitamin A content.
| Human | Pig | Dog | Rabbit | Rat | |
|---|---|---|---|---|---|
| Histological feature | |||||
| Cell-dense MF | + | − | − | − | + |
| VFSC population | + | − | − | − | + |
| Lipid droplet location (oil red O) | MF; G | G | G | − | MF; G |
| Vitamin A location (gold chloride) | MF; G | G | G | G | MF; G |
| Vitamin A concentration | |||||
| Retinol** | 50.8 ± 20.6 | 109.3 ± 19.7* | 958 ± 165* | 33.9 ± 2.1 | 135.2 ± 5.9* |
| Retinyl ester** | 22.4 ± 2.9 | − | 3400 ± 850* | 13.7 ± 4.8* | 30.1 ± 2.9 |
| Retinol : retinyl ester*** | 2.4 : 1 | − | 1 : 3.7* | 2.6 : 1 | 4.5 : 1* |
G, gland; MF, macula flava; VFSC, vocal fold stellate cell; +, detected; −, not detected.
P < 0.05 compared with human.
pmol g−1 ww (mean ± SD).
Mean of individual sample ratios.
In addition to exhibiting consistently high retinol and retinyl ester concentrations compared with all other species, dog VF mucosa was the only tissue with preferential VA storage in retinyl ester form (Table 1; Fig. 3e).
Discussion
The MF and their constituent VFSCs are purported to play a fundamental role in development, maintenance and repair of the VF mucosa; however, to date, they have mostly been examined in human cadaver studies (Sato et al. 2010a,b2010b). Our dataset represents the first interspecies comparison of MF and VFSC appearance, VA localization, and HPLC-based VA quantification in the VF mucosa. Interestingly, MF and VA-storing VFSCs were only identified in human and rat specimens. In both species, VFSCs were the primary repository of VA. In contrast, pig, dog and rabbit VF mucosae contained no discernable MF, but exhibited preferential VA localization to mucous (pig), serous (dog) or mixed (rabbit) glands. This glandular VA storage corresponded to exceedingly high concentrations of retinol in pig and dog VF mucosae, and retinyl ester in dog VF mucosa. These findings have significant implications for the presumed role of the MF and VFSCs in VF biology, the nature of VA storage within the VF mucosa, and the selection of an appropriate animal model for future experimental studies.
Our inability to detect MF and their characteristic VA-storing VFSCs in pig, dog and rabbit VF mucosae suggests that either: (i) VFSCs are not present in all species and therefore are not critical to functional development of the VF mucosa, as previously claimed (Sato et al. 2010a,b2010b); or (ii) VFSCs are present in these species but exhibit significant differences in immunophenotype, localization pattern and/or VA storage capacity, perhaps due to interspecies variation in mucosal or biomechanical microenvironment. Such phenotypic differences have been reported for hepatic SCs in the dog, which, while present, are negative for the classic markers GFAP, vimentin and desmin (Ijzer et al. 2006). Our observations in the pig suggest that previously reported VFSC data generated using cultured cells harvested from the anterior/posterior pig vocal ligament may not closely represent VA-storing human VFSCs harvested from the MF niche, despite the pig cells' capacity for VA uptake and transdifferentiation in vitro (Fuja et al. 2005, 2006). Additional experimental work is needed to further characterize the phenotype of these cells under biologically relevant conditions.
It is well established that retinol and retinyl ester concentrations are both organ and species specific (Wright & Hall, 1979; Schäffer et al. 2010). In our dataset, despite marked differences in VA localization on histology, rabbit and rat VF mucosae contained HPLC-measured VA concentrations that were most similar to human VF mucosa. Ratios of retinol : retinyl ester concentration were closely aligned in rabbit and human VF mucosae; however, as noted above, the rabbit does not appear to contain MF or the VA-storing VFSCs that are characteristic of the human VF. These histological features are clearly shared by the rat, which additionally has plasma retinol transport capacity similar to that reported in humans. Plasma retinol is transported to extrahepatic organs via retinol-binding protein (RBP), predominantly in complex with transthyretin (holo-transthyretin RBP), but also in free form (holo-free RBP; Blomhoff et al. 1990). Multiple analyses of RBP-mediated plasma retinol transport indicate that circulating holo-transthyretin RBP and holo-free RBP concentrations are equivalent in rats and humans (Muto et al. 1973; Burri et al. 1993). Further, retinyl esters can also be distributed to extrahepatic organs postprandially by chylomicra (Ross & Li, 2007; Sun et al. 2008).
Retinol and retinyl ester concentrations in pig and dog VF mucosae were notably different than concentrations in human tissue, with pig mucosa containing ∼ twofold greater retinol and no detectable retinyl ester, and dog mucosa containing ∼ 20-fold greater retinol and ∼ 150-fold greater retinyl ester. Our histological data suggest that these differences may be due to preferential glandular VA storage in these species. While we observed a small amount of VA storage within mixed glands (containing both serous and mucous cells) in human, rabbit and rat specimens, VA was exclusively localized to mucous glands in pig VF mucosa, and to serous glands in dog VF mucosa. Pure serous glands were unique to the dog and, consistent with our HPLC-based quantification, these glands exhibited the highest density of gold chloride+ cells of all VF tissue regions examined across all species. There are no reports directly characterizing the VA storage capacity of serous glands in vivo; however, recent work in mouse has shown a relationship between systemic VA deficiency, serous cell hypotrophy and submucosal gland hyposecretion in the subglottis (Kim et al. 2012). A further possible contributor to the high concentration of VA in dog VF mucosa is superior retinol transport to extrahepatic organs. Compared with humans, dogs have significantly greater plasma concentrations of holo-transthyretin RBP and holo-free RBP, as well as significant non-RBP-mediated retinol trafficking by serum albumin and lipoproteins (Burri et al. 1993).
Conclusions
Our data suggest that MF and VA-storing VFSCs are not conserved across the VF mucosae of all mammalian species but, consistent with prior reports, exist in humans and rats (Sato et al. 2001, 2003; Tateya et al. 2006, 2008). Further, VA storage within the VF mucosa of different species varies significantly with respect to overall VA concentration, relative abundance of retinol and retinyl ester forms, and preferential localization to VFSCs or glands. Overall, given their histological features, primary VA storage within VFSCs, roughly similar retinol and retinyl ester concentrations, and documented serum retinol transport capacity (Muto et al. 1973; Burri et al. 1993), rats appear to offer the most appropriate in vivo experimental system (of the non-human species studied here) for modeling the role of VA and VFSCs in human VF biology. Additional experimental research is needed to unravel the role of VA-storing VFSCs in important biological contexts such as developmental morphogenesis and wound healing.
Acknowledgments
The authors gratefully acknowledge Zhen Chang, Teri Enters and McLean Gunderson for assistance with tissue procurement, and Glen E. Leverson for statistical consultation.
Competing interests
The authors declare no competing financial interests.
Author contributions
Y.T., D.M.B. and N.V.W. conceived and designed the study. Y.T. conducted all primary tissue dissection, processing and histology. N.R. and C.R.D. performed all HPLC assays and associated analyses. S.A.T. directed the HPLC experiments. Y.K. assisted with histological analyses. Y.T., D.M.B. and N.V.W. wrote the manuscript. All authors reviewed and approved the final version.
Funding
This work was funded by grants from the National Institute on Deafness and other Communication Disorders of the National Institutes of Health (grant numbers R01 DC004428 and R01 DC010777).
References
- Barker E, Haverson K, Stokes CR, et al. The larynx as an immunological organ: immunological architecture in the pig as a large animal model. Clin Exp Immunol. 2006;143:6–14. doi: 10.1111/j.1365-2249.2005.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke GS, Neubauer J, Berry DA, et al. Ex vivo perfused larynx model of phonation: preliminary study. Ann Otol Rhinol Laryngol. 2007;116:866–870. doi: 10.1177/000348940711601113. [DOI] [PubMed] [Google Scholar]
- Bless DM, Welham NV. Characterization of vocal fold scar formation, prophylaxis, and treatment using animal models. Curr Opin Otolaryngol Head Neck Surg. 2010;18:481–486. doi: 10.1097/MOO.0b013e3283407d87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomhoff R, Green MH, Berg T, et al. Transport and storage of vitamin A. Science. 1990;250:399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- Burri BJ, Neidlinger TR, Zwick H. Comparison of the properties and concentrations of the isoforms of retinol-binding protein in animals and human beings. Am J Vet Res. 1993;54:1213–1220. [PubMed] [Google Scholar]
- Davis BH. Transforming growth factor-β responsiveness is modulated by the extracellular collagen matrix during hepatic ito cell culture. J Cell Physiol. 1988;136:547–553. doi: 10.1002/jcp.1041360323. [DOI] [PubMed] [Google Scholar]
- Davis BH, Chen A, Beno DWA. Raf and mitogen-activated protein kinase regulate stellate cell collagen gene expression. J Biol Chem. 1996;271:11 039–11 042. doi: 10.1074/jbc.271.19.11039. [DOI] [PubMed] [Google Scholar]
- de Freitas S, Jr, Bustorff-Silva JM, Castro e Silva O, Jr, et al. Retinyl-palmitate reduces liver fibrosis induced by biliary obstruction in rats. Hepatogastroenterology. 2003;50:146–150. [PubMed] [Google Scholar]
- Fuja TJ, Probst-Fuja MN, Titze IR. Transdifferentiation of vocal-fold stellate cells and all-trans retinol-induced deactivation. Cell Tissue Res. 2005;322:417–424. doi: 10.1007/s00441-005-0028-9. [DOI] [PubMed] [Google Scholar]
- Fuja TJ, Probst-Fuja MN, Titze IR. Changes in expression of extracellular matrix genes, fibrogenic factors, and actin cytoskeletal organization in retinol treated and untreated vocal fold stellate cells. Matrix Biol. 2006;25:59–67. doi: 10.1016/j.matbio.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Ge PJ, French LC, Ohno T, et al. Model of evoked rabbit phonation. Ann Otol Rhinol Laryngol. 2009;118:51–55. doi: 10.1177/000348940911800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijzer J, Roskams T, Molenbeek RF, et al. Morphological characterisation of portal myofibroblasts and hepatic stellate cells in the normal dog liver. Comp Hepatol. 2006;5:7. doi: 10.1186/1476-5926-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasantikul V, Shuangshoti S. Positivity to glial fibrillary acidic protein in bone, cartilage, and chordoma. J Surg Oncol. 1989;41:22–26. doi: 10.1002/jso.2930410109. [DOI] [PubMed] [Google Scholar]
- Kim N, Yoo W, Lee J, et al. Formation of vitamin A lipid droplets in pancreatic stellate cells requires albumin. Gut. 2009;58:1382–1390. doi: 10.1136/gut.2008.170233. [DOI] [PubMed] [Google Scholar]
- Kim SC, Lee HJ, Joo J-H, et al. Vitamin A deficiency induces fluid hyposecretion from the airway submucosal glands of mice. J Nutr. 2012;142:739–743. doi: 10.3945/jn.111.154047. [DOI] [PubMed] [Google Scholar]
- Murakami K-I, Kaji T, Shimono R, et al. Therapeutic effects of vitamin A on experimental cholestatic rats with hepatic fibrosis. Pediatr Surg Int. 2011;27:863–870. doi: 10.1007/s00383-011-2853-0. [DOI] [PubMed] [Google Scholar]
- Muto Y, Smith FR, Goodman DS. Comparative studies of retinol transport in plasma. J Lipid Res. 1973;14:525–532. [PubMed] [Google Scholar]
- Nagy NE, Holven KB, Roos N, et al. Storage of vitamin A in extrahepatic stellate cells in normal rats. J Lipid Res. 1997;38:645–658. [PubMed] [Google Scholar]
- Ross AC, Li N-Q. Lung retinyl ester is low in young adult rats fed a vitamin A deficient diet after weaning, despite neonatal vitamin A supplementation and maintenance of normal plasma retinol. J Nutr. 2007;137:2213–2218. doi: 10.1093/jn/137.10.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Hirano M, Nakashima T. Stellate cells in the human vocal fold. Ann Otol Rhinol Laryngol. 2001;110:319–325. doi: 10.1177/000348940111000405. [DOI] [PubMed] [Google Scholar]
- Sato K, Hirano M, Nakashima T. Vitamin A-storing stellate cells in the human vocal fold. Acta Otolaryngol. 2003;123:106–110. doi: 10.1080/0036554021000028077. [DOI] [PubMed] [Google Scholar]
- Sato K, Umeno H, Nakashima T. Functional histology of the macula flava in the human vocal fold – Part 1: its role in the adult vocal fold. Folia Phoniatr Logop. 2010a;62:178–184. doi: 10.1159/000314261. [DOI] [PubMed] [Google Scholar]
- Sato K, Umeno H, Nakashima T. Functional histology of the macula flava in the human vocal fold – Part 2: its role in the growth and development of the vocal fold. Folia Phoniatr Logop. 2010b;62:263–270. doi: 10.1159/000316962. [DOI] [PubMed] [Google Scholar]
- Sato K, Umeno H, Nakashima T. Vocal fold stem cells and their niche in the human vocal fold. Ann Otol Rhinol Laryngol. 2012;121:798–803. doi: 10.1177/000348941212101205. [DOI] [PubMed] [Google Scholar]
- Schäffer MW, Roy SS, Mukherjee S, et al. Qualitative and quantitative analysis of retinol, retinyl esters, tocopherols and selected carotenoids out of various internal organs form different species by HPLC. Anal Methods. 2010;2:1320–1332. doi: 10.1039/c0ay00288g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Surles RL, Tanumihardjo SA. Vitamin A concentrations in piglet extrahepatic tissues respond differently ten days after vitamin A treatment. J Nutr. 2008;138:1101–1106. doi: 10.1093/jn/138.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateya T, Tateya I, Sohn JH, et al. Histologic characterization of rat vocal fold scarring. Ann Otol Rhinol Laryngol. 2005;114:183–191. doi: 10.1177/000348940511400303. [DOI] [PubMed] [Google Scholar]
- Tateya T, Tateya I, Muñoz-Del-Río A, et al. Postnatal development of rat vocal folds. Ann Otol Rhinol Laryngol. 2006;115:215–224. doi: 10.1177/000348940611500310. [DOI] [PubMed] [Google Scholar]
- Tateya T, Tateya I, Surles RL, et al. Roles of vitamin A and macula flava in maintaining vocal folds. Ann Otol Rhinol Laryngol. 2008;117:65–73. doi: 10.1177/000348940811700113. [DOI] [PubMed] [Google Scholar]
- Thibeault SL, Gray SD, Bless DM, et al. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- Vega JA, Del Valle ME, Alvarez-Mendez JC, et al. Expression of glial fibrillary acidic protein-like and S-100 protein-like immunoreactivities on cartilages of the rat vestibulum nasi. Post-natal development changes. Arch Anat Histol Embryol. 1990;73:59–66. [PubMed] [Google Scholar]
- Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol. 1980;66:303–353. doi: 10.1016/s0074-7696(08)61977-4. [DOI] [PubMed] [Google Scholar]
- Welham NV, Montequin DW, Tateya I, et al. A rat excised larynx model of vocal fold scar. J Speech Lang Hear Res. 2009;52:1008–1020. doi: 10.1044/1092-4388(2009/08-0049). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KE, Hall RC. Association between plasma and liver vitamin A levels in the calf; weanling pig, rabbit and rat; and adult goat fed fixed intakes of vitamin A. J Nutr. 1979;109:1063–1072. doi: 10.1093/jn/109.6.1063. [DOI] [PubMed] [Google Scholar]


