Syphilis infection was associated with HIV incidence in an HIV-prevention trial that randomized participants to once-daily emtricitabine/tenofovir (FTC/TDF) vs placebo. FTC/TDF had no effect on the association between incident syphilis and HIV acquisition; syphilis infection did not decrease FTC/TDF efficacy.
Keywords: chemoprophylaxis, HIV prevention, MSM, preexposure prophylaxis, syphilis
Abstract
Background. Syphilis infection may potentiate transmission of human immunodeficiency virus (HIV). We sought to determine the extent to which HIV acquisition was associated with syphilis infection within an HIV preexposure prophylaxis (PrEP) trial and whether emtricitabine/tenofovir (FTC/TDF) modified that association.
Methods. The Preexposure Prophylaxis Initiative (iPrEx) study randomly assigned 2499 HIV-seronegative men and transgender women who have sex with men (MSM) to receive oral daily FTC/TDF or placebo. Syphilis prevalence at screening and incidence during follow-up were measured. Hazard ratios for the effect of incident syphilis on HIV acquisition were calculated. The effect of FTC/TDF on incident syphilis and HIV acquisition was assessed.
Results. Of 2499 individuals, 360 (14.4%) had a positive rapid plasma reagin test at screening; 333 (92.5%) had a positive confirmatory test, which did not differ between the arms (FTC/TDF vs placebo, P = .81). The overall syphilis incidence during the trial was 7.3 cases per 100 person-years. There was no difference in syphilis incidence between the study arms (7.8 cases per 100 person-years for FTC/TDF vs 6.8 cases per 100 person-years for placebo, P = .304). HIV incidence varied by incident syphilis (2.8 cases per 100 person-years for no syphilis vs 8.0 cases per 100 person-years for incident syphilis), reflecting a hazard ratio of 2.6 (95% confidence interval, 1.6–4.4; P < .001). There was no evidence for interaction between randomization to the FTC/TDF arm and incident syphilis on HIV incidence.
Conclusions. In HIV-seronegative MSM, syphilis infection was associated with HIV acquisition in this PrEP trial; a syphilis diagnosis should prompt providers to offer PrEP unless otherwise contraindicated.
Approximately 10 million syphilis infections were diagnosed in 2008 according to the World Health Organization (WHO), many of which occurred in men who have sex with men or individuals coinfected with human immunodeficiency virus (HIV) [1]. The association of syphilis and HIV may be causal, as syphilis can facilitate HIV acquisition [2–8] via mucosal disruption, ulceration [9], or inflammation [10] and HIV transmission by increasing HIV RNA in blood and genital secretions [11]. Alternatively, the association could be due to increased sexual risk behaviors or participation in networks with high prevalence of HIV. Observational evidence from retrospective analyses suggests an association between syphilis and HIV acquisition [12], but few studies have assessed this association among a closed, prospective cohort of MSM with almost complete follow-up.
The Preexposure Prophylaxis Initiative (iPrEx) trial [13] and others [14–16] demonstrated the efficacy of once-daily oral emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) in preventing HIV acquisition. The iPrEx study prospectively followed a diverse cohort of men and transgender women who have sex with men (MSM) with frequent sexually transmitted infection (STI) and HIV testing and offers a unique opportunity to assess the relationship between syphilis and HIV acquisition in the context of FTC/TDF preexposure prophylaxis (PrEP) use. Syphilis provides an informative marker of heightened HIV risk and may be an important consideration in assessing the need for PrEP. It is also critical to determine whether syphilis decreases PrEP efficacy, which could result from compromising mucosal barriers, increasing HIV inoculum size [17], or decreasing PrEP adherence.
In the present study, we determined rates and correlates of prevalent and incident syphilis in the iPrEx study, the extent to which incident syphilis was associated with HIV acquisition, and whether that association varied by treatment group or by level of adherence (as measured by detected drug) among participants in the active arm.
METHODS
Participants and Specimens
The iPrEx study enrolled 2499 HIV-seronegative MSM to evaluate the safety and efficacy of once-daily oral FTC/TDF for HIV prevention [13]. Study visits were scheduled every 4 weeks after enrollment. The details of the primary study and visits have been described elsewhere [13]. In a subset of participants sampled from the active arm including longitudinal samples from HIV-positive cases with site-matched controls, longitudinal samples from participants enrolled in a bone mineral density substudy, and cross-sectional samples of participants at weeks 8 and 24, plasma was tested for the presence of FTC and TDF, and peripheral blood mononuclear cells (PBMCs) were tested for FTC triphosphate (FTC-TP) and tenofovir diphosphate (TFV-DP), as described elsewhere [18]. Diagnosis and treatment of symptomatic STIs occurred at every visit. At screening and at 24-week intervals during follow-up, participants were screened for asymptomatic urethritis, syphilis, and antibodies to herpes simplex virus type 2 (HSV-2), and they were examined for genital warts and ulcers. In addition, participants could present for an interim visit on an as-needed basis following a possible exposure, onset of new symptoms, or STI treatment. Partners of participants with a curable STI were offered evaluation and treatment.
Syphilis diagnoses were based on standard algorithms according to local guidelines or developed by normative bodies [19] and included an initial nonspecific test—either a rapid plasma reagin (RPR) test (bioMérieux, Marcy l'Etoile, France; Becton, Dickinson and Company, Franklin Lakes, New Jersey; Laborclin, Sao Paolo, Brazil; WAMA Diagnostica, Sao Paolo, Brazil) or Venereal Disease Research Laboratory (VDRL) test (Standard Diagnostics, Kyonggi-do, South Korea) performed at local sites. Specimens with a newly positive RPR or VDRL result were sent for confirmatory testing using fluorescent treponemal antibody-absorption (FTA-ABS) (Biokit, Barcelona, Spain; Fujirebio Inc, Tokyo, Japan; Zeus Scientific, Somerville, New Jersey; bioMérieux; WAMA Diagnostica).
Participants diagnosed with prevalent and incident syphilis (see definitions below) were treated in accordance with local guidelines, or if not available, according to the 2006 sexually transmitted disease guidelines of the Centers for Disease Control and Prevention (CDC) [19]. Evaluation and treatment were managed by the local treating physician under the supervision of the site investigator. Each incident case was also reviewed by a studywide clinical monitoring committee to ensure that appropriate guidance for clinical management was provided.
Definitions
At screening, we examined the number of participants with a positive RPR result and a positive confirmatory test, which was defined as syphilis prevalence. Patients newly diagnosed with syphilis were treated and their serological response was subsequently monitored. At follow-up, incident syphilis was defined as (1) a change in serology from negative to positive with confirmation by FTA-ABS, or (2) at least a 4-fold increase in serological titer from prior test with documentation of prior treatment appropriate for the stage of syphilis.
Person-time was calculated for participants with incident syphilis as time between enrollment and first episode and for HIV as the time between enrollment and first evidence of HIV infection. For participants without syphilis, person-time was calculated as years between enrollment and the date of last syphilis test. Those with confirmed syphilis at screening without clear documentation of prior effective treatment were treated and were excluded from the analysis of syphilis incidence, unless clear documentation of prior effective treatment was available.
Detected drug was defined as the detection of FTC or TDF in plasma or FTC-TP or TFV-DP in PBMCs regardless of level [18].
Statistical Methods
Baseline characteristics were compared by an unequal-variance t test for continuous variables and the Fisher exact test for categorical variables. Syphilis incidence was calculated as described above and the results were stratified by site, age, condomless insertive or condomless receptive anal intercourse in the past 3 months, reported STI in the past 6 months, HSV-2 positivity, and syphilis prevalence at screening. Predictors of incident syphilis were modeled using a stratified Cox proportional hazards approach.
HIV seroconversion rate was calculated as incident cases divided by person-years of observation and was stratified by incident syphilis. Hazard ratios for the effect of incident syphilis on HIV acquisition were calculated using a Cox proportional hazards model with incident syphilis as a time-dependent covariate stratified by study site. A bivariate model was evaluated for the effect of timing of incident syphilis on HIV seroconversion. A multivariate model that evaluated the association of incident syphilis and HIV seroconversion included study site, age, race, HSV-2 acquisition (as a time-dependent covariate), randomization group, condomless sex, and number of partners at screening and at follow-up; and a history of an STI, syphilis, HSV-2, HIV-positive partners, and circumcision status at screening. An interaction hypothesis was prespecified and tested for detected drug.
RESULTS
Baseline Characteristics, Predictors of Syphilis, and Syphilis Incidence
Of 2499 individuals, 360 (14.4%) had a positive RPR result at screening, of whom 333 (92.5%) had a positive confirmatory test, which did not differ between the arms (FTC/TDF vs placebo, P = .81). Fourteen had a false-positive RPR result and 13 had no confirmatory testing performed.
Individuals with prevalent syphilis infection at screening were less educated, were older, and reported having more sexual partners and more episodes of condomless anal intercourse in the previous 3 months. The overall syphilis incidence during the trial was 7.3 cases per 100 person-years, but varied by site, age, condomless insertive anal intercourse in the past 3 months, condomless receptive anal intercourse in the past 3 months, reported STI in the past 6 months, HSV-2 positivity, and syphilis prevalence at screening (Table 1). There was no difference in syphilis incidence between the study arms (7.8 cases per 100 person-years for FTC/TDF vs 6.8 cases per 100 person-years for placebo, P = .304).
Table 1.
Predictors of Onset of Incident Syphilis
| Predictor | Total No. | Incident Syphilis (n = 279) |
P Value | ||
|---|---|---|---|---|---|
| No. | Person-years | Ratea | |||
| Overall | 2499 | 279 | 3814 | 7.3 | |
| Site | <.001 | ||||
| Rio de Janeiro, Brazil (PRACA ONZE) | 94 | 9 | 82 | 10.9 | |
| Sao Paulo, Brazil (USP) | 76 | 7 | 67 | 10.4 | |
| Rio de Janeiro, Brazil (FIOCRUZ) | 200 | 26 | 194 | 13.4 | |
| Lima, Peru (IMPACTA) | 440 | 55 | 804 | 6.8 | |
| Lima, Peru (INMENSA) | 500 | 60 | 902 | 6.7 | |
| Iquitos, Peru (ACSA) | 460 | 72 | 825 | 8.7 | |
| Guayaquil, Ecuador (EQUIDAD) | 300 | 31 | 470 | 6.6 | |
| San Francisco, California (SFDPH) | 140 | 4 | 181 | 2.2 | |
| Boston, Massachusetts (Fenway Health) | 87 | 3 | 101 | 3.0 | |
| Cape Town, South Africa (DTHF) | 88 | 6 | 81 | 7.4 | |
| Chiang Mai, Thailand (RIHES) | 114 | 6 | 106 | 5.6 | |
| Education | .084 | ||||
| Less than secondary | 523 | 65 | 762 | 8.5 | |
| Completed secondary | 883 | 107 | 1372 | 7.8 | |
| Postsecondary | 1064 | 100 | 1627 | 6.1 | |
| No answer/missing | 29 | 7 | 54 | 13.0 | |
| Age at screening, y | .022 | ||||
| 18–24 | 1254 | 124 | 1936 | 6.4 | |
| 25–29 | 514 | 54 | 803 | 6.7 | |
| 30–39 | 473 | 72 | 728 | 9.9 | |
| ≥40 | 258 | 29 | 348 | 8.3 | |
| No. of partners at screening | .088 | ||||
| 1–3 | 702 | 49 | 960 | 5.1 | |
| 4–6 | 551 | 65 | 844 | 7.7 | |
| 7–17 | 624 | 77 | 990 | 7.8 | |
| >17 | 622 | 88 | 1021 | 8.6 | |
| Condomless insertive anal intercourse at screening | <.001 | ||||
| No | 1247 | 171 | 1900 | 9.0 | |
| 1 partner | 809 | 39 | 644 | 6.1 | |
| >1 partner | 443 | 69 | 1270 | 5.4 | |
| Condomless receptive anal intercourse at screening | <.001 | ||||
| No | 1485 | 71 | 1428 | 5.0 | |
| 1 partner | 814 | 47 | 623 | 7.5 | |
| >1 partner | 671 | 161 | 1763 | 9.1 | |
| Anal intercourse | .223 | ||||
| Insertive only | 645 | 20 | 336 | 6.0 | |
| Receptive | 1485 | 47 | 623 | 7.5 | |
| No condomless intercourse | 369 | 38 | 446 | 8.5 | |
| Reported STI in 6 months before screening | <.001 | ||||
| Yes | 652 | 127 | 1103 | 11.5 | |
| No | 1847 | 152 | 2711 | 5.6 | |
| Transactional sex reported at screening | .294 | ||||
| Yes | 1027 | 137 | 1689 | 8.1 | |
| No | 1472 | 142 | 2126 | 6.7 | |
| HSV-2–positive at screening | <.001 | ||||
| Yes | 892 | 159 | 1459 | 10.9 | |
| No | 1606 | 120 | 2355 | 5.1 | |
| Randomized treatment | .304 | ||||
| FTC/TDF | 1251 | 147 | 1886 | 7.8 | |
| Placebo | 1248 | 132 | 1928 | 6.8 | |
| Syphilis at screening | <.001 | ||||
| Positive | 333 | 103 | 507 | 20.3 | |
| Not positive | 2166 | 176 | 3307 | 5.3 | |
| Plasma drug levels at week 8 (n = 470 samples) | .49 | ||||
| Placebo | 1248 | 132 | 1928 | 6.8 | |
| Drug detected week 8 | 683b | 68b | 1024b | 6.7 | |
| No detected drug week 8 | 568b | 79b | 896b | 8.8 | |
P value comparing detected drug to no detected drug at week 8 = 0.33.
Abbreviations: ACSA, Asociación Civil Selva Amazónica; DTHF, The Desmond Tutu HIV Foundation; EQUIDAD, Fundación Ecuatoriana Equidad; FIOCRUZ, Fundação Oswaldo Cruz; FTC/TDF, emtricitabine/tenofovir; HSV-2, herpes simplex virus type 2; IMPACTA, Asociación Civil Impacta Salud y Educación; INMENSA, Investigaciones Médicas en Salud; PRACA ONZE, Projecto Praça Onze; RIHES, Research Institute for Health Sciences; SFDPH, San Francisco Department of Public Health; STI, sexually transmitted infection; USP, Universidade de São Paulo.
a Rate per 100 person-years. Excludes 22 patients who were confirmed to be syphilis positive at screening, but lack documentation of adequate treatment.
b Total participants, follow-up, and syphilis events estimated by sampling weights.
In a multivariate model that included race, randomization group, detected drug, number of partners at screening and follow-up, HSV status at screening and follow-up, condomless insertive anal intercourse at baseline and follow-up, condomless receptive anal intercourse at baseline and follow-up, history of an STI at screening, HIV-positive partners at screening, age, and circumcision status at screening, predictors of incident syphilis included having antibodies for HSV-2 at screening and at follow-up (P = .022 and P = .003, respectively), confirmed syphilis at screening (P < .001), condomless receptive anal intercourse (P = .006), and condomless insertive anal intercourse (P = .013). Partner number at screening (P = .769) and at follow-up (P = .767) was not predictive of incident syphilis.
Syphilis Effect on HIV Acquisition
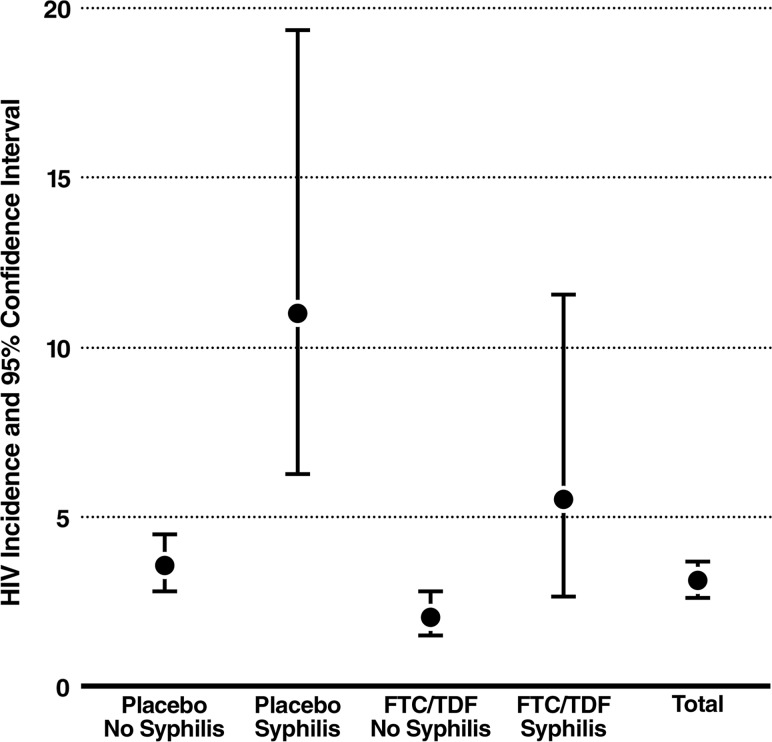
There were 129 incident HIV infections (excluding 2 participants who were censored for having syphilis at screening without evidence for effective treatment). HIV incidence varied by incident syphilis (2.8 cases per 100 person-years for no syphilis vs 8.0 cases per 100 person-years for incident syphilis), giving a hazard ratio (HR) of 2.6 (95% confidence interval [CI], 1.6–4.4; P < .001) (Figure 1). In the bivariate model, HIV incidence also varied by timing of incident syphilis with respect to HIV seroconversion (11.8 cases per 100 person-years for incident syphilis ≤90 days from HIV seroconversion vs 6.2 cases per 100 person-years for incident syphilis >90 days from HIV seroconversion), giving an HR of 3.8 (95% CI, 1.9–7.6; P < .001) for syphilis ≤90 days vs 2.0 (95% CI, 1.0–4.0; P = .040) for incident syphilis >90 days. These associations remained significant but did not differ from each other significantly in a multivariate model that included study site, race, randomization group, syphilis at screening, HSV-2 at screening, HSV-2 acquisition (as a time-dependent covariate), condomless sex (at screening and at follow-up), number of partners at screening and at follow-up, history of an STI at screening, HIV-positive partners at screening, age, and circumcision status (HR for syphilis ≤90 days, 3.4 [95% CI, 1.7–7.0; P = .001] vs >90 days, 2.1 [95% CI, 1.0–4.2; P = .035]; P = .30 for the interaction). A similar association of incident syphilis on HIV acquisition was seen among participants without syphilis at screening and also within each study arm.
Figure 1.
Human immunodeficiency virus (HIV) incidence according to incident syphilis and treatment arm. Abbreviation: FTC/TDF, emtricitabine/tenofovir.
Evaluation of Interactions of Incident Syphilis, FTC/TDF, and HIV Acquisition
There was no evidence for interaction of syphilis on FTC/TDF's efficacy. In the modified intent-to-treat analysis (mITT), FTC/TDF efficacy was 40% (95% CI, 11%–59%) in the absence of incident syphilis (70 placebo, 40 FTC/TDF), whereas mITT efficacy was 47% (95% CI, <0% to 79%) in the presence of incident syphilis (12 placebo, 7 FTC/TDF) (P = .98) (Figure 1).
Within the active arm, there was no evidence for interaction of detected drug on the effect of incident syphilis on HIV incidence. The risk reduction (relative to participants randomized to the placebo arm and without incident syphilis) for HIV acquisition among participants randomized to the active arm with no drug detected was 26% among those with incident syphilis and 11% among those without incident syphilis (P = .71 for the interaction), whereas the risk reduction for HIV acquisition among participants randomized to the active arm with detected drug was 79% among participants with incident syphilis and 88% among those without incident syphilis (P = .64 for the interaction).
DISCUSSION
In this large randomized placebo-controlled clinical trial of oral FTC/TDF for PrEP, HIV acquisition was strongly associated with incident syphilis, and syphilis did not attenuate the protective benefit of antiretroviral chemoprophylaxis against HIV. Syphilis infection was highly prevalent at screening (13.3%). Syphilis incidence during the study period was 7.3 cases per 100 person-years overall, but the rate varied by study site, age, condomless anal intercourse, recent STI, HSV-2 serostatus, and syphilis prevalence at screening. The independent effect of syphilis on HIV acquisition was affirmed in both study arms and with multivariable analyses that controlled for known predictors of HIV and other STIs. Predictors of syphilis incidence were similar to predictors of syphilis prevalence and did not include randomization group or detectable drug. Even when cases of incident syphilis that coincided with HIV diagnosis were excluded (to exclude HIV occurring prior to syphilis in between study visits), the independent effect of syphilis on HIV acquisition was still affirmed.
High rates of STIs in other studies of MSM have been consistently associated with incident HIV [12, 20–28]. The importance of such findings as they relate to the current study is highlighted by current estimates of high syphilis prevalence among MSM. Among countries included in iPrEx with available data, WHO has estimated the following prevalence of syphilis among MSM: Brazil, 13.4% (2010); Ecuador, 6.5% (2010); and Thailand, 21.6% (2008) [29]. According to the CDC 2011 estimate, the prevalence of syphilis among men visiting CDC Sexually Transmitted Disease Surveillance Network clinics in the United States was 2.6% among HIV-negative MSM and 10.1% among HIV-positive MSM [30]. Our data reflecting a study screening prevalence of syphilis of 13.3% are therefore consistent with global estimates.
The strong independent effect of incident syphilis on HIV acquisition in this PrEP study suggests the importance of timely diagnosis and treatment of syphilis among at-risk MSM to decrease HIV incidence; additionally, a new syphilis diagnosis offers a key opportunity for PrEP initiation, given the increased risk for HIV infection among individuals infected with syphilis. HIV-uninfected individuals who present with new syphilis infections should be offered PrEP, unless otherwise contraindicated, in addition to immediate syphilis treatment, and treatment for their sexual partners.
Furthermore, we found that FTC/TDF had no effect on the association between incident syphilis and incident HIV. Concern has been raised that antiretroviral PrEP may lead to increases in condomless anal sex as a form of risk compensation in an era of therapeutic optimism [31–33], which may in turn increase the overall burden of STIs among MSM. Importantly, within the iPrEx trial, sexual practices reported by participants trended toward safety over time, even among those who perceived they were on the active arm and believed that the active arm would be effective [34]. Syphilis incidence decreased, moreover, providing both self-reported and objective laboratory evidence against risk compensation [34]. Although syphilis incidence was high, it declined overall and in both treatment arms during iPrEx follow-up. In the current analysis, we found that the efficacy of FTC/TDF is not affected by syphilis infection.
Study limitations include an inherent underestimation of syphilis cases because participants were censored at the time of first incident infection and our routine syphilis surveillance occurred at study screening and every 24 weeks only. Our study population was composed of MSM and may not be generalizable to other populations such as heterosexuals or those outside of a clinical trial setting. The effect of PrEP on syphilis and HIV incidence demonstrated in this study must be interpreted in the context of a randomized clinical trial; the results of demonstration projects are needed to more definitively evaluate the relationship between syphilis and HIV incidence with open-label PrEP use. Furthermore, a larger sample size would provide more power to definitely address whether syphilis infection affects PrEP efficacy.
The results of this study demonstrate the striking, increased HIV incidence associated with incident syphilis infection, an effect independent of other predictors and unaltered by study arm. Timely diagnosis and treatment of syphilis infections is the mainstay of epidemic control in the absence of a vaccine, and may decrease transmission of HIV [35]. A syphilis infection should trigger a recommendation to start PrEP unless contraindications are present. Creatinine clearance <60 mL/minute and preexisting HIV infection are the only contraindications listed in the US Food and Drug Administration–approved information from the manufacturer [36]. The CDC recommends that PrEP be prioritized toward individuals at “very high risk for HIV acquisition” [37, 38] and here we demonstrate that individuals with syphilis fulfill that criterion. A recent secondary analysis of iPrEx data demonstrated a population-attributable fraction (PAF) for syphilis of approximately 10% with a number needed to treat of approximately 10 to prevent 1 HIV infection. Higher PAFs were represented by report of condomless receptive anal intercourse (PAF = 64%) with a partner of unknown HIV infection status, and reporting >5 sex partners (PAF = 13%) [39]. Clinicians should prioritize PrEP for individuals at highest risk, including those infected with syphilis.
Notes
Acknowledgments. The following are members of the iPrEx Study Team who contributed to this work: Linda-Gail Bekker, Susan Buchbinder, Martin Casapia, Suwat Chariyalertsak, Juan Guanira, Esper Kallas, Javier Lama, Kenneth Mayer, Orlando Montoya, Mauro Schechter, Valdiléa Veloso.
We thank the study participants for their dedication to HIV prevention; the community advisory boards; the members of the Division of Acquired Immunodeficiency Syndrome (DAIDS) multinational independent data and safety monitoring board; Megha Mehrotra for data quality assurance and John Carroll for providing original versions of the table and figure (Gladstone Institutes); and Brian Postle of DF/Net Research for help in database management and data transfer.
Author contributions. M. M. S. led the manuscript development and drafted the manuscript. D. V. G., K. H. M., M. M. S., A. Y. L., and R. M. G. contributed to the data analysis and interpretation. K. H. M., A. Y. L., V. M., J. V. G., S. C., T. F., and R. M. G. contributed to site leadership, study oversight, study coordination, and manuscript development.
Financial support. This work was supported by DAIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (cooperative agreement UO1 AI64002 to R. M. G.) and by the Bill & Melinda Gates Foundation. Study drugs were donated by Gilead Sciences. Support for some specimen handling came from a grant from DAIDS (RO1 AI062333 to R. M. G.) and by the J. David Gladstone Institutes. Some infrastructure support at the University of California, San Francisco, was provided by a grant from the National Institutes of Health (UL1 RR024131).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization . Global incidence and prevalence of selected curable sexually transmitted infections—2008. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- 2.Sexton J, Garnett G, Rottingen JA. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sex Transm Dis. 2005;32:351–7. doi: 10.1097/01.olq.0000154504.54686.d1. [DOI] [PubMed] [Google Scholar]
- 3.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickerson MC, Johnston J, Delea TE, White A, Andrews E. The causal role for genital ulcer disease as a risk factor for transmission of human immunodeficiency virus. An application of the Bradford Hill criteria. Sex Transm Dis. 1996;23:429–40. doi: 10.1097/00007435-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Clottey C, Dallabetta G. Sexually transmitted diseases and human immunodeficiency virus. Epidemiologic synergy? Infect Dis Clin North Am. 1993;7:753–70. [PubMed] [Google Scholar]
- 6.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 7.Mertens TE, Hayes RJ, Smith PG. Epidemiological methods to study the interaction between HIV infection and other sexually transmitted diseases. AIDS. 1990;4:57–65. doi: 10.1097/00002030-199001000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Cameron DW, Padian NS. Sexual transmission of HIV and the epidemiology of other sexually transmitted diseases. AIDS. 1990;4(suppl 1):S99–103. [PubMed] [Google Scholar]
- 9.Fox J, Fidler S. Sexual transmission of HIV-1. Antiviral Res. 2010;85:276–85. doi: 10.1016/j.antiviral.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:305–10. doi: 10.1097/COH.0b013e32833a8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarzebowski W, Caumes E, Dupin N, et al. Effect of early syphilis infection on plasma viral load and CD4 cell count in human immunodeficiency virus-infected men: results from the FHDH-ANRS CO4 cohort. Arch Intern Med. 2012;172:1237–43. doi: 10.1001/archinternmed.2012.2706. [DOI] [PubMed] [Google Scholar]
- 12.Buchacz K, Klausner JD, Kerndt PR, et al. HIV incidence among men diagnosed with early syphilis in Atlanta, San Francisco, and Los Angeles, 2004 to 2005. J Acquir Immune Defic Syndr. 2008;47:234–40. [PubMed] [Google Scholar]
- 13.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 16.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 17.Duwal S, Schutte C, von Kleist M. Pharmacokinetics and pharmacodynamics of the reverse transcriptase inhibitor tenofovir and prophylactic efficacy against HIV-1 infection. PLoS One. 2012;7:e40382. doi: 10.1371/journal.pone.0040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra25. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2006. Atlanta, GA: CDC; 2006. [Google Scholar]
- 20.Aynalem G, Smith L, Bemis C, Taylor M, Hawkins K, Kerndt P. Commercial sex venues: a closer look at their impact on the syphilis and HIV epidemics among men who have sex with men. Sex Transm Infect. 2006;82:439–43. doi: 10.1136/sti.2006.020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53:537–43. doi: 10.1097/QAI.0b013e3181c3ef29. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Wu Z, Detels R, et al. HIV/STD prevalence among men who have sex with men in Chengdu, China and associated risk factors for HIV infection. J Acquir Immune Defic Syndr. 2010;53(suppl 1):S74–80. doi: 10.1097/QAI.0b013e3181c7dd16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hope-Rapp E, Anyfantakis V, Fouere S, et al. Etiology of genital ulcer disease. A prospective study of 278 cases seen in an STD clinic in Paris. Sex Transm Dis. 2010;37:153–8. doi: 10.1097/OLQ.0b013e3181bf5a98. [DOI] [PubMed] [Google Scholar]
- 24.Huhn GD, McIntyre AF, Broad JM, et al. Factors associated with newly diagnosed HIV among persons with concomitant sexually transmitted diseases. Sex Transm Dis. 2008;35:731–7. doi: 10.1097/OLQ.0b013e31817f97a0. [DOI] [PubMed] [Google Scholar]
- 25.Katz KA, Lee MA, Gray T, Marcus JL, Pierce EF. Repeat syphilis among men who have sex with men—San Diego County, 2004–2009. Sex Transm Dis. 2011;38:349–52. doi: 10.1097/OLQ.0b013e3181fe650b. [DOI] [PubMed] [Google Scholar]
- 26.Scott KC, Philip S, Ahrens K, Kent CK, Klausner JD. High prevalence of gonococcal and chlamydial infection in men who have sex with men with newly diagnosed HIV infection: an opportunity for same-day presumptive treatment. J Acquir Immune Defic Syndr. 2008;48:109–12. doi: 10.1097/QAI.0b013e318165dc0b. [DOI] [PubMed] [Google Scholar]
- 27.Torrone EA, Bertolli J, Li J, et al. Increased HIV and primary and secondary syphilis diagnoses among young men—United States, 2004–2008. J Acquir Immune Defic Syndr. 2011;58:328–35. doi: 10.1097/QAI.0b013e31822e1075. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson D, Rutherford G. Population-based interventions for reducing sexually transmitted infections, including HIV infection. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD001220. CD001220. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Data on other STIs: men who have sex with men (MSM) with active syphilis by country. Available at: http://apps.who.int/gho/data/node.main.A1361STI. Accessed 14 April 2014. [Google Scholar]
- 30.Centers for Disease Control and Prevention. STDs in men who have sex with men. Available at: http://www.cdc.gov/std/stats11/msm.htm. Accessed 14 April 2014. [Google Scholar]
- 31.Pinkerton SD. Sexual risk compensation and HIV/STD transmission: empirical evidence and theoretical considerations. Risk Anal. 2001;21:727–36. doi: 10.1111/0272-4332.214146. [DOI] [PubMed] [Google Scholar]
- 32.Cassell MM, Halperin DT, Shelton JD, Stanton D. Risk compensation: the Achilles’ heel of innovations in HIV prevention? BMJ. 2006;332:605–7. doi: 10.1136/bmj.332.7541.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blower SM, McLean AR. Prophylactic vaccines, risk behavior change, and the probability of eradicating HIV in San Francisco. Science. 1994;265:1451–4. doi: 10.1126/science.8073289. [DOI] [PubMed] [Google Scholar]
- 34.Marcus JL, Glidden DV, Mayer KH, et al. No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis. PLoS One. 2013;8:e81997. doi: 10.1371/journal.pone.0081997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1–110. [PubMed] [Google Scholar]
- 36.Gilead Sciences. Highlights of prescribing information. Available at: http://www.gilead.com/approximately/media/Files/pdfs/medicines/hiv/truvada/truvada_pi.PDF. Accessed 14 April 2014. [Google Scholar]
- 37.Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586–9. [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65–8. [PubMed] [Google Scholar]
- 39.Buchbinder SP, Glidden DV, Liu AY, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014;14:468–75. doi: 10.1016/S1473-3099(14)70025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]