Abstract
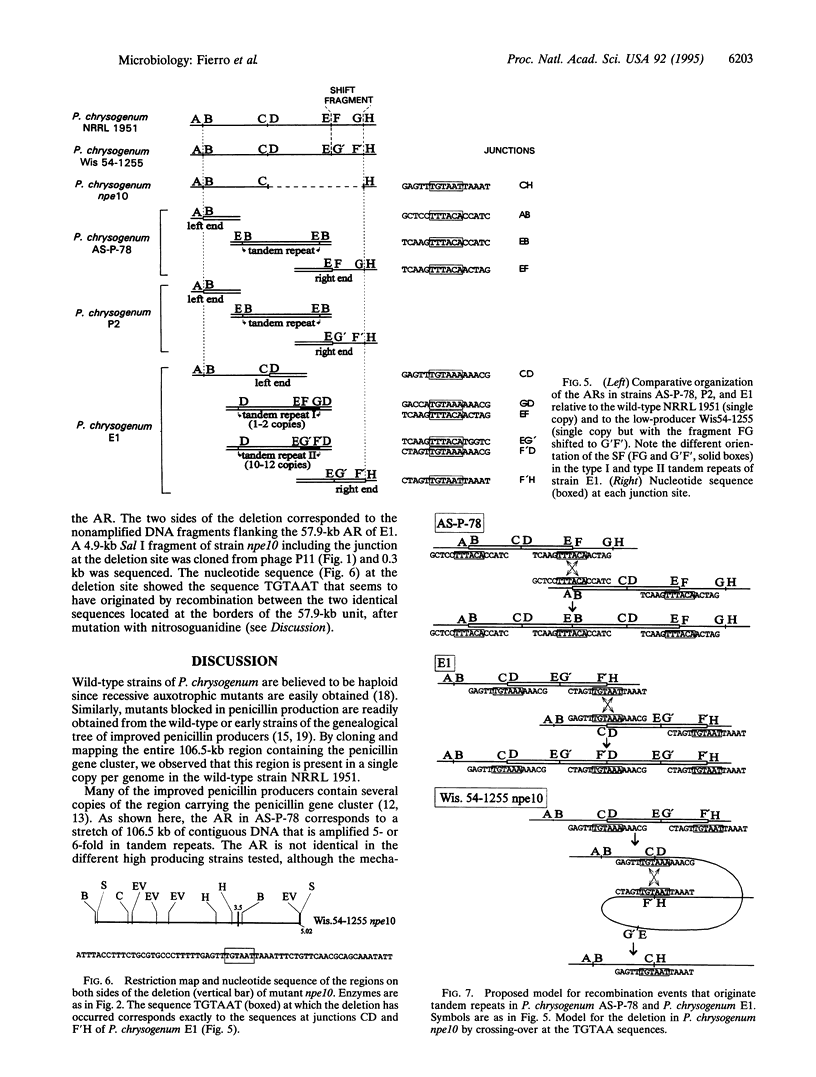
The penicillin biosynthetic genes (pcbAB, pcbC, penDE) of Penicillium chrysogenum AS-P-78 were located in a 106.5-kb DNA region that is amplified in tandem repeats (five or six copies) linked by conserved TTTACA sequences. The wild-type strains P. chrysogenum NRRL 1951 and Penicillium notatum ATCC 9478 (Fleming's isolate) contain a single copy of the 106.5-kb region. This region was bordered by the same TTTACA hexanucleotide found between tandem repeats in strain AS-P-78. A penicillin overproducer strain, P. chrysogenum E1, contains a large number of copies in tandem of a 57.9-kb DNA fragment, linked by the same hexanucleotide or its reverse complementary TGTAAA sequence. The deletion mutant P. chrysogenum npe10 showed a deletion of 57.9 kb that corresponds exactly to the DNA fragment that is amplified in E1. The conserved hexanucleotide sequence was reconstituted at the deletion site. The amplification has occurred within a single chromosome (chromosome I). The tandem reiteration and deletion appear to arise by mutation-induced site-specific recombination at the conserved hexanucleotide sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barredo J. L., Díez B., Alvarez E., Martín J. F. Large amplification of a 35-kb DNA fragment carrying two penicillin biosynthetic genes in high penicillin producing strains of Penicillium chrysogenum. Curr Genet. 1989 Dec;16(5-6):453–459. doi: 10.1007/BF00340725. [DOI] [PubMed] [Google Scholar]
- Burr K. W., Roper J. A., Relton J. Modification of chromosome instability in Aspergillus nidulans. J Gen Microbiol. 1982 Dec;128(12):2899–2907. doi: 10.1099/00221287-128-12-2899. [DOI] [PubMed] [Google Scholar]
- Cantoral J. M., Gutiérrez S., Fierro F., Gil-Espinosa S., van Liempt H., Martín J. F. Biochemical characterization and molecular genetics of nine mutants of Penicillium chrysogenum impaired in penicillin biosynthesis. J Biol Chem. 1993 Jan 5;268(1):737–744. [PubMed] [Google Scholar]
- Díez B., Gutiérrez S., Barredo J. L., van Solingen P., van der Voort L. H., Martín J. F. The cluster of penicillin biosynthetic genes. Identification and characterization of the pcbAB gene encoding the alpha-aminoadipyl-cysteinyl-valine synthetase and linkage to the pcbC and penDE genes. J Biol Chem. 1990 Sep 25;265(27):16358–16365. [PubMed] [Google Scholar]
- Fierro F., Gutiérrez S., Díez B., Martín J. F. Resolution of four large chromosomes in penicillin-producing filamentous fungi: the penicillin gene cluster is located on chromosome II (9.6 Mb) in Penicillium notatum and chromosome I (10.4 Mb) in Penicillium chrysogenum. Mol Gen Genet. 1993 Dec;241(5-6):573–578. doi: 10.1007/BF00279899. [DOI] [PubMed] [Google Scholar]
- Fogel S., Welch J. W. Tandem gene amplification mediates copper resistance in yeast. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5342–5346. doi: 10.1073/pnas.79.17.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez S., Velasco J., Fernandez F. J., Martín J. F. The cefG gene of Cephalosporium acremonium is linked to the cefEF gene and encodes a deacetylcephalosporin C acetyltransferase closely related to homoserine O-acetyltransferase. J Bacteriol. 1992 May;174(9):3056–3064. doi: 10.1128/jb.174.9.3056-3064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49:727–764. doi: 10.1146/annurev.bi.49.070180.003455. [DOI] [PubMed] [Google Scholar]
- Newmeyer D., Galeazzi D. R. The Instability of Neurospora Duplication Dp(IL-->IR)H4250 , and Its Genetic Control. Genetics. 1977 Mar;85(3):461–487. doi: 10.1093/genetics/85.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nga B. H., Roper J. A. Quantitative intrachromosomal changes arising at mitosis in Aspergillus nidulans. Genetics. 1968 Feb;58(2):193–209. doi: 10.1093/genetics/58.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normansell P. J., Normansell I. D., Holt G. Genetic and biochemical studies of mutants of Penicillium chrysogenum impaired in penicillin production. J Gen Microbiol. 1979 May;112(1):113–126. doi: 10.1099/00221287-112-1-113. [DOI] [PubMed] [Google Scholar]
- Parag Y., Roper J. A. Genetic control of chromosome instability in Aspergillus nidulans as a mean for gene amplification in eukaryotic microorganisms. Mol Gen Genet. 1975 Oct 22;140(4):275–287. doi: 10.1007/BF00267319. [DOI] [PubMed] [Google Scholar]
- Sexton C. E., Roper J. A. Spontaneous duplications and transpositions of a large chromosome segment in Aspergillus nidulans. J Gen Microbiol. 1984 Mar;130(3):583–595. doi: 10.1099/00221287-130-3-583. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Bull J. H., Edwards J., Turner G. Amplification of the isopenicillin N synthetase gene in a strain of Penicillium chrysogenum producing high levels of penicillin. Mol Gen Genet. 1989 Apr;216(2-3):492–497. doi: 10.1007/BF00334395. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Burnham M. K., Bull J. H., Hodgson J. E., Ward J. M., Browne P., Brown J., Barton B., Earl A. J., Turner G. Beta-lactam antibiotic biosynthetic genes have been conserved in clusters in prokaryotes and eukaryotes. EMBO J. 1990 Mar;9(3):741–747. doi: 10.1002/j.1460-2075.1990.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J. D., Paquin C. E., Kaneko K., Williamson V. M. Resistance to antimycin A in yeast by amplification of ADH4 on a linear, 42 kb palindromic plasmid. Cell. 1986 Sep 12;46(6):857–863. doi: 10.1016/0092-8674(86)90067-x. [DOI] [PubMed] [Google Scholar]
- Welch J. W., Fogel S., Cathala G., Karin M. Industrial yeasts display tandem gene iteration at the CUP1 region. Mol Cell Biol. 1983 Aug;3(8):1353–1361. doi: 10.1128/mcb.3.8.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]