Abstract
Background
Assessment and discussion of individual risk for breast cancer within the primary care setting are crucial to discussion of risk reduction and timely referral.
Methods
We conducted a randomized controlled trial of a multiethnic, multilingual sample of women ages 40 to 74 years from two primary care practices (one academic, one safety net) to test a breast cancer risk assessment and education intervention. Patients were randomly assigned to control or intervention group. All patients completed a baseline telephone survey and risk assessment (via telephone for controls, via tablet computer in clinic waiting room before visit for intervention). Intervention (BreastCARE) patients and their physicians received an individualized risk report to discuss during the visit. One-week follow-up telephone surveys with all patients assessed patient–physician discussion of family cancer history, personal breast cancer risk, high-risk clinics, and genetic counseling/testing.
Results
A total of 655 control and 580 intervention women completed the risk assessment and follow-up interview; 25% were high-risk by family history, Gail, or Breast Cancer Surveillance Consortium risk models. BreastCARE increased discussions of family cancer history [OR, 1.54; 95% confidence interval (CI), 1.25–1.91], personal breast cancer risk (OR, 4.15; 95% CI, 3.02–5.70), high-risk clinics (OR, 3.84; 95% CI, 2.13–6.95), and genetic counseling/testing (OR, 2.22; 95% CI, 1.34–3.68). Among high-risk women, all intervention effects were stronger.
Conclusions
An intervention combining an easy-to-use, quick risk assessment tool with patient-centered risk reports at the point of care can successfully promote discussion of breast cancer risk reduction between patients and primary care physicians, particularly for high-risk women.
Impact
Next steps include scaling and dissemination of BreastCARE with integration into electronic medical record systems.
Introduction
As identified by the U.S. National Comprehensive Cancer Network (NCCN; ref. 1), an alliance of leading cancer centers that promote clinical practice guidelines for use by patients, clinicians, and other health care decision-makers, effective use of risk reduction strategies is a necessary element of any comprehensive breast cancer program. Breast cancer risk reduction options include genetic counseling and testing for women at risk for hereditary breast cancer, chemoprevention, and lifestyle modifications (2–9).
Genetic counseling and testing offer the opportunity to identify women at high risk for hereditary breast and ovarian cancer because of BRCA1 and BRCA2 mutations (9). For these women, the risk of breast cancer is 5 times greater than for women without mutations (10,11). They may be offered early intervention through ovarian suppression, increased surveillance, or prophylactic surgery (9), which reduces their risk of breast cancer by 85% to 100% (12–14).
Among chemoprevention options, tamoxifen can reduce breast cancer risk by 50% more than 5 years for women with an estimated risk ≥1.67% (7,8), and benefits may persist for up to 10 years (7, 8). Raloxifene has been found to prevent breast cancer among postmenopausal women (7, 8). Although the efficacy of these medications for selected women has been demonstrated, they remain underused (15–17).
Identifying and targeting women who are most likely to benefit from a particular risk reduction approach will likely result in improvements in the uptake of breast cancer risk reduction strategies (18). This process requires assessing a woman’s risk factors to determine her individual estimates of risk, hereditary, and nonhereditary. Easily accessible models for risk identification in clinical practice include the Gail risk assessment model (19) and the Breast Cancer Surveillance Consortium (BCSC) model that incorporates mammographic breast density (20). A limited number of other tools [e.g., breast/ ovarian cancer genetics referral screening tool (RST); ref. 21], allow for easy screening for hereditary risk in the clinical setting to refer women for further assessment and possible genetic counseling/testing.
Primary care clinicians can play a critical role in assessing risk and initiating risk reduction options. However, in practice, the use of breast cancer risk assessment tools can be challenging in the context of the primary care setting (16, 22). There is evidence that less than 11% of health care professionals discuss genetic counseling for breast/ovarian cancer with their patients and less than 2% of patients are referred to genetic counseling or testing (23). Lack of time and lack of knowledge among primary care physicians are well-documented reasons for their failure to appropriately identify and refer high-risk women (24–26).
There is a dearth of information about primary care delivery models designed to systematically identify women at high risk for breast cancer and to offer discussion and appropriate referrals. To facilitate patient–physician discussion of breast cancer risk reduction options, we developed and tested a comprehensive Breast Cancer Assessment of Risk and Education (BreastCARE) intervention for women and their primary care physicians. Using a randomized controlled trial design, we evaluated its efficacy in primary care settings serving diverse populations. We hypothesized that frequency of patient-reported discussions with physicians about breast cancer risk, risk reduction options, and appropriate referrals, as well as electronic medical record (EMR) documentation of these outcomes, would be significantly higher in the intervention versus control group.
Materials and Methods
Study design
We conducted a randomized controlled trial of a multiethnic, multilingual sample of women ages 40 to 74 years who were scheduled for clinic visits at 2 primary care practices, randomized to BreastCARE or control. All participants completed baseline interviews by telephone followed by a breast cancer risk assessment by telephone (for control patients) or by tablet computer at the clinic before visit (for intervention patients). On average, 11 days after the clinic visit, all participants completed a follow-up telephone survey that assessed patient–physician discussion of the following: family cancer history, personal breast cancer risk, going to a high-risk clinic, and genetic counseling or testing. Six months post visit, we conducted a review of patients’ EMR for documentation of discussions of breast cancer risk, prevention, and risk reduction.
Setting and participants
The study was conducted between June 2011 and August 2012 (when recruitment was met) at 2 general medicine practices in the San Francisco Bay Area of California with ethnically and linguistically diverse patient panels, one in an academic medical center and the other in an academic safety-net setting. The safety-net practice provided health care to all, regardless of patients’ ability to pay for the services provided. Recruitment goals were based on sample size calculations assuming 80% power and α = 0.05 to detect significant differences in main effects between groups. Patients were eligible if they had an upcoming appointment at one of the participating sites; were female between the ages of 40 and 74; spoke English, Spanish, or Chinese (Cantonese or Mandarin); had no personal history of breast cancer; were able to complete a telephone interview; and their physicians did not object to their participation. Recruitment letters, including an opt-out postcard, were mailed to eligible patients inviting them to the study. One week after mailing, a BreastCARE recruiter called those who did not opt-out.
Before recruitment, all practicing physicians at the study clinics were provided with a description of the study and a passive consent form. They were also asked to review their initial patient panels to identify any women who would not be appropriate for the study (e.g., too clinically complex or the study might cause undue stress). In addition, at least 1 month before recruitment, the study team arranged Grand Rounds presentations at each site to provide physicians with up-to-date evidence on breast cancer risk and prevention.
Randomization and research procedures
At completion of a baseline telephone survey, participants were randomized based on random sequence codes designed by a statistician and stratified by race/ethnicity to ensure balance. Those randomized to the control group completed a breast cancer risk assessment by telephone. Intervention participants completed a tablet-based version of the same risk assessment at the clinic before their appointment. At no point were women apprised of their intervention status. After meeting with a research assistant at the practice, women signed a HIPAA form granting the study permission to review their EMR. Control patients continued on to their appointment, whereas intervention patients received BreastCARE. One to two weeks after the clinic visit, all participants were contacted for a follow-up telephone survey to assess study outcomes. Six months after visit, we completed a review of participants’ EMR. Interviewers and EMR abstractors were blinded to participants’ study assignment. The research protocol was reviewed and approved by the institutional review boards of both participating institutions.
Intervention
BreastCARE, available in English, Spanish, and traditional and simplified Chinese characters, consisted of a tablet-based patient risk assessment tool that generated individually tailored printouts for patients and their physicians. Patients were queried on breast cancer risk factors in a series of questions written at an eighth-grade reading level. The tablet connected wirelessly to a printer, and a research assistant handed the patient her printed individualized report (optimized for graphic appeal, using plain language) and a second report (optimized for rapid scanning by physicians) to be given to her physician who was thereby made aware of intervention status. The dual reports were designed to efficiently prompt patient–physician discussion of breast cancer risk.
A consensus panel of experts chose thresholds at which patients would be considered high-risk for each of the measures in our assessment. The rationale for all thresholds was to choose clinically actionable cut-points above which a woman should be referred for genetic counseling or high-risk evaluation for chemoprevention. For each woman identified as high-risk, the patient report stated that her risk was "higher than for other women [her] age" and suggested that she talk with her doctor. The patient message library included 30 potential messages based on risk factors, each in 3 languages, whereas the physician message library included 45 potential messages to account for all possible scenarios.
Measurements
Descriptive variables
Demographic and health information collected at baseline included age (40–49, 50–65, and 66–74 years), race/ethnicity (Asian/Pacific Islander, Black, Latina, non-Latina white, Native American or other), marital status (married/living with a partner vs. other), education (high school diploma or less, some college, college or higher), health insurance (any private, only public, no insurance), self-reported general health ("excellent/very good" vs. "good/fair/poor"; ref. 27), number of primary care visits in the past year (0–1, 2–3, 4+), and number of self-reported comorbidities (ref. 28; 0, 1–2, 3+).
Breast cancer risk assessment indicators included age at menarche, age at first birth, age at menopause, breast biopsy history, tamoxifen or raloxifene use, Ashkenazi Jewish ancestry, and family history of ovarian and breast cancer. For women who had a prior mammogram, the breast density from their most recent mammogram report was abstracted from the EMR.
Assessment of risk
To estimate objective risk for breast cancer, we used 3 measures: the RST (personal history of breast or ovarian cancer, Jewish ancestry, history of family breast and ovarian cancer in mother, sister, daughter, grandmother or aunt, and history of breast cancer in a male relative; c-statistic = 0.90; refs. 21 and 29), the Gail Model (personal history of breast cancer, age, age at first menstrual period, age at first birth, number of first-degree relatives with breast cancer, history of breast biopsy, and race/ethnicity; c-statistic = 0.67; refs. 19 and 30), and the BCSC (age, family history of breast cancer, prior breast biopsy, race/ethnicity, and breast density; c-statistic=0.66; ref. 20). Women were considered to be high risk if they met at least 1 of 3 mutually exclusive criteria, listed in order of priority: (i) family history based on RST (ref. 21; the RST was scored as positive if the patient scored 2 or more points with respect to the risk factors described above), (ii) BCSC score in top 5% of estimated 5-year risk for her age group when mammographic breast density data were available (20), or (iii) Gail score in the top 5% estimated 5-year risk for her age group (19). In addition, women between the ages of 40 and 50 were considered high risk if their Gail or BCSC score was ≥1.67 (31). All other women were classified as average risk.
The patient risk report for any woman identified as high risk (according to the RST, BCSC, or Gail models) stated that the woman’s risk was higher than for other women her age and suggested talking with her doctor about the matter. However, for those women in the intervention group meeting RST high-risk criteria, the physician report contained a message suggesting referral to genetic counseling, whereas for those meeting Gail/BCSC high-risk criteria, the physician report contained a message suggesting referral to a high-risk clinic.
Outcome variables
At 1-week follow-up, women were asked whether they discussed during the clinic visit (i) family cancer history, (ii) personal risk of breast cancer, (iii) referral to a high-risk breast clinic, and (iv) genetic counseling or testing.
At the 6-month EMR review, we extracted documentation of (i) breast cancer risk (positive family cancer history, high-risk status) and (ii) discussion of or referral for prevention or risk reduction (high-risk clinic, genetic counseling or testing, chemoprevention).
Statistical analysis
Baseline equivalence between intervention and control groups was assessed by comparing demographics and breast cancer risk factors. We used generalized estimating equations (GEE) regression to determine statistically significant differences between groups with respect to self-reported discussion with physician of family cancer history, breast cancer risk, high-risk clinic referral, and genetic counseling/testing, accounting for clustering of patients within physicians. We estimated odds ratios (OR) and 95% confidence intervals (CI) for women in the intervention group versus controls. Models adjusting for insurance status and physical activity were considered, but did not substantively differ from the unadjusted analyses and are therefore not reported.
In secondary analysis, we explored whether intervention effects differed according to race/ethnicity, practice site, patient education, age, or objective breast cancer risk by testing for interactions. No statistically significant interaction effects were observed by race/ethnicity, practice site, education, or age. Although we only observed a significant interaction effect by objective breast cancer risk for discussion of genetic counseling/testing (P < 0.001), we chose to present all results stratified by objective breast cancer risk to allow for separate discussion of findings for high- and average-risk women. We conducted all analyses in Stata Version 11.2 (32).
Results
Recruitment
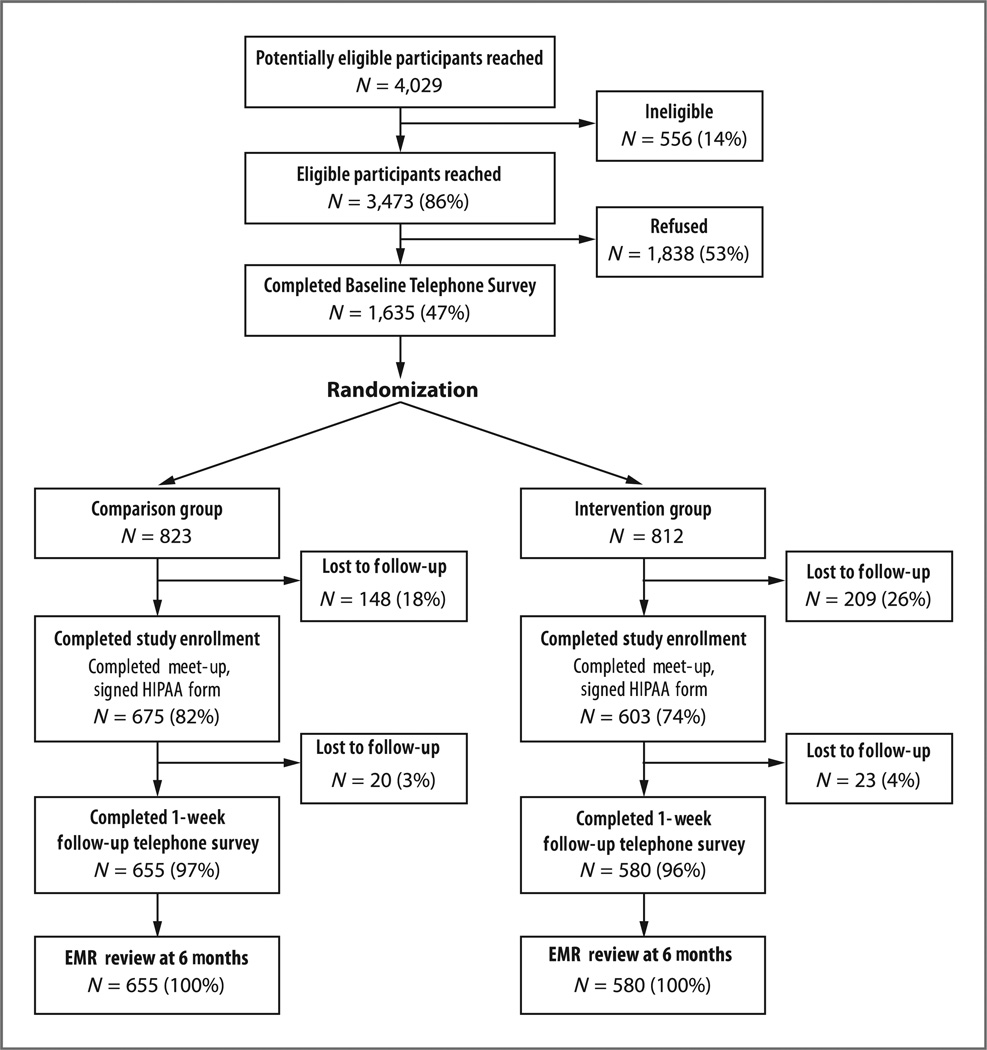
Among 1,635 women who completed the baseline telephone survey, 812 were randomized to the intervention group and 823 to control. Among randomized women, 74% of intervention patients and 82% of controls completed enrollment. More than 95% completed the 1-week follow-up telephone survey (Fig. 1).
Figure 1.
Flowchart of enrollment/exclusions for BreastCARE population, San Francisco, 2011 to 2012.
Description of study population
At baseline, intervention and control groups were well balanced with respect to demographic characteristics with good representation across racial/ethnic groups, including 13% who completed interviews in Spanish or Chinese (Table 1).
Table 1.
Baseline characteristics of participants by intervention and control groups, San Francisco, 2011 to 2012 (N = 1,235)
| Control group, n = 655 n (%) |
Intervention group, n = 580 n (%) |
P valuea | |
|---|---|---|---|
| Demographic characteristics | |||
| Age at diagnosis (categories) | |||
| <50 y | 163 (24.9) | 170 (29.3) | 0.331 |
| 50–65 y | 379 (57.9) | 313 (54.0) | |
| >65 y | 113 (17.3) | 97 (16.7) | |
| Race/ethnicity | |||
| Asian or Pacific Islander | 123 (18.8) | 105 (18.1) | 0.877 |
| Black or African American | 150 (22.9) | 125 (21.6) | |
| Latina | 144 (22.0) | 141 (24.3) | |
| Non-Latina White | 229 (35.0) | 202 (34.8) | |
| Native American or other | 9 (1.4) | 7 (1.2) | |
| Marital status | |||
| Married/living with a partner | 288 (44.3) | 261 (45.2) | 0.802 |
| Separated/divorced/widowed/never married | 362 (55.7) | 316 (54.8) | |
| Education | |||
| High school diploma or less | 216 (33.3) | 175 (30.4) | 0.200 |
| Some college | 155 (23.9) | 167 (29.1) | |
| College degree or higher | 278 (42.8) | 233 (40.5) | |
| Language of interview | |||
| English | 572 (87.3) | 507 (87.4) | 0.944 |
| Spanish or Chinese | 83 (12.7) | 73 (12.6) | |
| Health characteristics | |||
| Clinic site | |||
| Mount Zion Cancer Center | 435 (66.4) | 411 (70.9) | 0.135 |
| SF General Hospital | 220 (33.6) | 169 (29.1) | |
| Health insurance | |||
| Any private insurance | 291 (44.4) | 297 (51.2) | 0.022 |
| Only public insurance | 350 (53.4) | 265 (45.7) | |
| No insurance | 14 (2.1) | 18 (3.1) | |
| Primary care visits during last year | |||
| 0 to 1 | 176 (27.2) | 164 (28.6) | 0.097 |
| 2 to 3 | 211 (32.5) | 214 (37.4) | |
| 4+ | 261 (40.3) | 195 (34.0) | |
| Comorbid conditions | |||
| 0 | 45 (6.9) | 39 (6.7) | 0.979 |
| 1 to 2 | 256 (39.1) | 226 (39.0) | |
| 3+ | 354 (54.1) | 315 (54.3) | |
| Perception of health status | |||
| Excellent/very good | 211 (32.4) | 203 (35.2) | 0.399 |
| Less than excellent/very good | 441 (67.6) | 373 (64.8) | |
| Assessment of risk for breast cancer | |||
| Risk category for breast cancer | |||
| Average risk | 509 (77.7) | 419 (72.3) | 0.097 |
| High-risk, Gail/BCSC | 97 (14.8) | 101 (17.4) | |
| High-risk, RST ≥2 | 49 (7.5) | 60 (10.3) |
P values from GEE analyses accounting for clustering of observations by physician.
Distribution of breast cancer risk was similar between groups. The majority of women (75%) were at average risk. Twenty-five percent were identified as high-risk, 9% with hereditary risk, and 16% because of their BCSC or Gail score.
Discussion with physicians
Compared with controls, women in the intervention group were significantly more likely to report having discussed with their physician family cancer history (40% intervention vs. 31% usual care; P < 0.001), personal breast cancer risk (41% vs. 15%; P< 0.001), going to a high-risk clinic (7% vs. 2%; P < 0.001), and genetic counseling/ testing (8% vs. 4%; P = 0.002; Table 2). When restricted to high-risk women, results were similar (Table 3).
Table 2.
Patient–physician discussion of breast cancer risk and associated referrals and lifestyle behaviors, BreastCARE intervention versus control (N = 1,235)
| Control group n (%) 655 |
Intervention group n (%) 580 |
P valuea | |
|---|---|---|---|
| Did you talk with your doctor about…? | |||
| Your family’s cancer history | 196 (30.5) | 231 (40.4) | <0.001 |
| Your risk of getting breast cancer | 94 (14.6) | 233 (40.7) | <0.001 |
| Going to a special high-risk clinic | 12 (1.9) | 39 (6.8) | <0.001 |
| Getting genetic counseling or testing | 23 (3.6) | 43 (7.5) | 0.002 |
P values from GEE analyses accounting for clustering of observations by physician.
Table 3.
Patient–physician discussion of breast cancer risk and associated referrals and lifestyle behaviors, BreastCARE intervention versus control, for high risk participants only (N = 307)
| Control group n (%) 146 |
Intervention group n (%) 161 |
P valuea | |
|---|---|---|---|
| Did you talk with your doctor about…? | |||
| Your family’s cancer history | 50 (34.7) | 84 (52.5) | 0.001 |
| Your risk of getting breast cancer | 26 (18.1) | 81 (50.6) | <0.001 |
| Going to a special high-risk clinic | 6 (4.1) | 30 (18.8) | <0.001 |
| Getting genetic counseling or testing | 6 (4.1) | 33 (20.8) | <0.001 |
P values from GEE analyses accounting for clustering of observations by physician.
Electronic medical record
Based on the EMR, greater percentages of women in the intervention group had documentation of family cancer history (10.2% vs. 5.5%; P = 0.006) and high-risk status (5.3% vs. 0.2%; P < 0.001; results not shown in table). Greater percentages also had documented discussion, referral, or completed visit to genetic counseling/testing (3.3% vs. 0.9%; P = 0.005), and of discussion of chemo-prevention (1.0% vs. 0%; P < 0.001). Most of the risk and risk reduction discussions and referrals (>85%) occurred among high-risk women.
GEE analysis
Women in the intervention group were more likely than controls to report discussions with their physician about family cancer history (OR, 1.54; 95% CI, 1.25–1.91), personal breast cancer risk (OR, 4.15; 95% CI, 3.02–5.70), going to a high-risk clinic (OR, 3.84; 95% CI, 2.13–6.95), and genetic counseling or testing (OR, 2.22; 95% CI, 1.34–3.68; Table 4).
Table 4.
Odds of patient–physician discussion of breast cancer risk and associated referrals and lifestyle behaviors, BreastCARE intervention versus control (N = 1,235)a
| Total population aOR (95% CI) |
High-risk women aOR (95% CI) |
Average-risk women aOR (95% CI) |
|
|---|---|---|---|
| Discussed breast cancer risk | |||
| Discussed family cancer history | 1.54 (1.25–1.91); P < 0.001 | 2.07 (1.34–3.20); P = 0.001 | 1.34 (1.03–1.73); P = 0.027 |
| Discussed breast cancer risk | 4.15 (3.02–5.70); P < 0.001 | 4.78 (2.90–7.89); P < 0.001 | 3.87 (2.63–5.69); P < 0.001 |
| Discussed going to high-risk clinic | 3.84 (2.13–6.95); P < 0.001 | 5.32 (0.21–12.8); P < 0.001 | 1.84 (0.66–5.18); P = 0.246 |
| Discussed genetic counseling | 2.22 (1.34–3.68); P = 0.002 | 5.99 (2.69–13.3); P < 0.001 | 0.71 (0.31–1.59); P = 0.401 |
Referent category = control group; analyses account for clustering of patients by physician.
When these analyses were restricted to high-risk women, those in the intervention group were significantly more likely than controls to discuss family cancer history with their physician (OR,2.07; 95% CI, 1.34–3.20),personal breast cancer risk (OR, 4.78; 95% CI, 2.90–7.89), going to a high-risk clinic (OR, 5.32; 95% CI, 2.21–12.8), and genetic counseling or testing (OR, 5.99; 95% CI, 2.69–13.3).
Discussion
The BreastCARE intervention had positive effects on each of the 4 main outcomes (discussion of family cancer history, of personal breast cancer risk, of going to a high-risk clinic, and of genetic counseling/testing), primarily among those most in need of risk reduction recommendations—women at high-risk for breast cancer. Self-reported results were confirmed by data from EMR reviews, giving further credence to our findings.
Overall, our results are consistent with and extend findings from a small observational study in a primary care setting, in which greater proportions of participants reported having discussed tamoxifen and genetic counseling after using a computerized program that matched their objective risk with recommendations, compared with baseline (33). However, another study evaluating a web-based risk appraisal tool in the primary care setting was not associated with increased discussion of family history (34). Although risk estimates for that study were sent to electronic health records for clinicians to view, they included those for several different types of cancers (as well as heart disease, diabetes, and stroke), therefore, any single preventive recommendation based on these risk estimates may have been diluted, contributing to the lack of significant effect.
Our study is distinct from other breast cancer risk assessment studies using online risk assessments in several ways. We were able to successfully recruit a racially and ethnically diverse sample that was nearly two-thirds non-white. Online assessments tend to discourage participation among those with limited Internet access (35); however, our participants completed the tablet-based risk assessment at the point of care without difficulty and satisfaction with BreastCARE was high among both patients and physicians (results will be published separately). Our assessment and messages were available in 3 languages, calibrated to an eighth-grade reading level, which may have facilitated patients’ understanding and contributed to the intervention’s success. Our intervention was implemented during regular operation of busy primary care practices without creating interruption in patient flow or physician productivity. BreastCARE introduced new data that were practical, relevant to patient care, and enhanced discussions on prevention. The tablet-based approach in the waiting room, which on average took 5 minutes to complete, saved physicians the time-consuming task of evaluating risk and identifying those for whom it was clinically important to spend time on risk discussions.
We were surprised at the significant proportion of participants who scored as high-risk for breast cancer by either Gail or BCSC models. Possibly, more high-risk women chose to participate because they knew the study related to breast cancer. However, despite this relatively large proportion of high-risk women and evidence that chemoprevention with selective estrogen receptor modulators (SERM) reduces breast cancer risk in this population, virtually none of the participating women were offered this treatment. Although physician barriers to prescribing SERMs are well documented (15, 16), a previous study reported that a majority of women would consider SERMS if they were at high risk and this option was offered by their physicians (36). The September 2013 update to the US Preventive Services Task Force recommendation on use of breast cancer chemoprevention recommends that "clinicians engage in shared, informed decision making with women who are at increased risk for breast cancer about medications to reduce their risk" (37). Such a recommendation is predicated on exactly the type of risk assessment and information sharing between women and their physicians that BreastCARE was successful at promoting. However, even with appropriate risk assessment, our results suggest that physicians may need more education and additional tools to easily weigh the benefits and harms for an individual woman before they feel comfortable prescribing SERMs, even to a high-risk patient.
Strengths of this study include the use of a randomized design and the high follow-up rate. The ethnic and linguistic diversity of our sample and the universal impact across these groups support the potential acceptability of Breast-CARE among diverse populations. As an additional strength, primary care practices are the main source of preventive care, and therefore, an optimal environment for a breast cancer prevention and risk reduction intervention. Furthermore, BreastCARE is easily exportable and could be incorporated into EMR systems in future adaptations.
There were several limitations to our study. BreastCARE was implemented in one academic practice and in one safety-net setting, so the findings may not be generalizable to other settings or to areas outside of San Francisco. Also, we assessed only discussion of risk and risk reduction, not whether women actually attended genetic counseling or took chemoprevention. Furthermore, although there was a large absolute difference between groups in discussion of individual breast cancer risk, the absolute differences in discussion of family cancer history and genetic counseling or testing, while statistically significant, were small. The majority of information was also derived from self-report, which may result in an over- or underestimation of discussion. However, similar results were obtained from the EMR examination, which supported the self-reported outcomes. Finally, although our study demonstrated a greater rate of patient–physician discussion of breast cancer risk among those receiving the BreastCARE intervention, a sizable fraction of patients in the intervention group did not have discussions of risk with their physicians. We acknowledge that to increase the proportion of patients who discuss their personal risk with their physician, the intervention would need to increase in intensity and include a physician component that specifically addresses physician-level barriers to discussion of risk.
In summary, BreastCARE demonstrated that an intervention combining an easy-to use, quick risk assessment tool with patient-centered risk reports at the point of care can successfully promote discussion of breast cancer risk reduction between patients and their primary care physicians. It represents a promising approach to stimulating and enhancing discussions for high-risk women across race/ethnic groups and in diverse primary care delivery settings. Next steps include scaling and dissemination of the intervention with integration into EMR systems, as well as incorporating additional tools to assist high-risk patients and their physicians in weighing individual risks and benefits of treatment with SERMs.
Acknowledgments
All authors have made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, and the drafting or critical revision of the article for important intellectual content, and all have provided final approval of the version to be published. Drs. C.P. Kaplan and J. Livaudais-Toman had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Grant Support
This research was funded by the California Breast Cancer Research Program (150B-0158; C.P. Kaplan) and S.G. Komen for the Cure (KG090504; C.P. Kaplan). Both trials were recorded under 1 NCT identifier based on recommendations from NIH ClinicalTrials.gov. Research efforts were also supported by grant no. P30-AG15272 under the Resource Centers for Minority Aging Research program of the National Institute on Aging. The funding organizations were not involved in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: C.P. Kaplan, J. Livaudais-Toman, J.A. Tice, K. Kerlikowske, S.E. Gregorich, E.J. Pérez-Stable, R.J. Pasick, J. Quinn, L.S. Karliner
Development of methodology: C.P. Kaplan, J.A. Tice, K. Kerlikowske, S.E. Gregorich, R.J. Pasick, J. Quinn, L.S. Karliner
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): C.P. Kaplan, A. Chen, J. Quinn
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): C.P. Kaplan, J. Livaudais-Toman, K. Kerlikowske, S.E. Gregorich, J. Quinn, L.S. Karliner
Writing, review, and/or revision of the manuscript: C.P. Kaplan, J. Livaudais-Toman, J.A. Tice, K. Kerlikowske, S.E. Gregorich, E.J. Pérez-Stable, R.J. Pasick, A. Chen, J. Quinn, L.S. Karliner
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): C.P. Kaplan, J. Livaudais-Toman, J. Quinn
Study supervision: C.P. Kaplan, J. Quinn
Trial Registration: ClinicalTrials.gov identifier NCT01830933.
References
- 1.NCCN Guidelines® and Derivative Information Products: User Guide. National Comprehensive Cancer Network; 2013. [Google Scholar]
- 2.Peplonska B, Lissowska J, Hartman TJ, Szeszenia-Dabrowska N, Blair A, Zatonski W, et al. Adulthood lifetime physical activity and breast cancer. Epidemiology. 2008;19:226–236. doi: 10.1097/EDE.0b013e3181633bfb. [DOI] [PubMed] [Google Scholar]
- 3.Silvera SA, Jain M, Howe GR, Miller AB, Rohan TE. Energy balance and breast cancer risk: a prospective cohort study. Breast Cancer Res Treat. 2006;97:97–106. doi: 10.1007/s10549-005-9098-3. [DOI] [PubMed] [Google Scholar]
- 4.Zhang SM, Lee IM, Manson JE, Cook NR, Willett WC, Buring JE. Alcohol consumption and breast cancer risk in the Women's Health Study. Am J Epidemiol. 2007;165:667–676. doi: 10.1093/aje/kwk054. [DOI] [PubMed] [Google Scholar]
- 5.Physical activity and good nutrition: Essential elements to prevent chronic diseases and obesity at a glance. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, United States Department of Health and Human Services; 2003. [Google Scholar]
- 6.Healthy People 2020. Washington, DC: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 7.Chlebowski RT, Col N, Winer EP, Collyar DE, Cummings SR, Vogel VG3rd, et al. American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase inhibition. J Clin Oncol. 2002;20:3328–3343. doi: 10.1200/JCO.2002.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Kinsinger LS, Harris R, Woolf SH, Sox HC, Lohr KN. Chemoprevention of breast cancer: a summary of the evidence for the U.S Preventive Services Task Force. Ann Intern Med. 2002;137:59–69. doi: 10.7326/0003-4819-137-1-200207020-00017. [DOI] [PubMed] [Google Scholar]
- 9.Salhab M, Bismohun S, Mokbel K. Risk-reducing strategies for women carrying BRCA1/2 mutations with a focus on prophylactic surgery. BMC Womens Health. 2010;10:28. doi: 10.1186/1472-6874-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann LC, Sellers TA, Schaid DJ, Frank TS, Soderberg CL, Sitta DL, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. 2001;93:1633–1637. doi: 10.1093/jnci/93.21.1633. [DOI] [PubMed] [Google Scholar]
- 13.Meijers-Heijboer H, van Geel B, van Putten WL, Henzen-Logmans SC, Seynaeve C, Menke-Pluymers MB, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:159–164. doi: 10.1056/NEJM200107193450301. [DOI] [PubMed] [Google Scholar]
- 14.Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van 't Veer L, Garber JE, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004;22:1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong K, Quistberg DA, Micco E, Domchek S, Guerra C. Prescription of tamoxifen for breast cancer prevention by primary care physicians. Arch Intern Med. 2006;166:2260–2265. doi: 10.1001/archinte.166.20.2260. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan CP, Haas JS, Perez-Stable EJ, Des Jarlais G, Gregorich SE. Factors affecting breast cancer risk reduction practices among California physicians. Prev Med. 2005;41:7–15. doi: 10.1016/j.ypmed.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan CP, Haas JS, Perez-Stable EJ, Gregorich SE, Somkin C, Des Jarlais G, et al. Breast cancer risk reduction options: awareness, discussion, and use among women from four ethnic groups. Cancer Epidemiol Biomarkers Prev. 2006;15:162–166. doi: 10.1158/1055-9965.EPI-04-0758. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Ann Intern Med. 2005;143:355–361. doi: 10.7326/0003-4819-143-5-200509060-00011. [DOI] [PubMed] [Google Scholar]
- 19.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 20.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellcross CA, Lemke AA, Pape LS, Tess AL, Meisner LT. Evaluation of a breast/ovarian cancer genetics referral screening tool in a mammography population. Genet Med. 2009;11:783–789. doi: 10.1097/GIM.0b013e3181b9b04a. [DOI] [PubMed] [Google Scholar]
- 22.Suther S, Goodson P. Barriers to the provision of genetic services by primary care physicians: a systematic review of the literature. Genet Med. 2003;5:70–76. doi: 10.1097/01.GIM.0000055201.16487.61. [DOI] [PubMed] [Google Scholar]
- 23.Levy DE, Garber JE, Shields AE. Guidelines for genetic risk assessment of hereditary breast and ovarian cancer: early disagreements and low utilization. J Gen Intern Med. 2009;24:822–828. doi: 10.1007/s11606-009-1009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke W, Acheson L, Botkin J, Bridges K, Davis A, Evans J, et al. Genetics in primary care: a USA faculty development initiative. Community Genet. 2002;5:138–146. doi: 10.1159/000065165. [DOI] [PubMed] [Google Scholar]
- 25.Hayflick SJ, Eiff MP. Role of primary care providers in the delivery of genetics services. Community Genet. 1998;1:18–22. doi: 10.1159/000016131. [DOI] [PubMed] [Google Scholar]
- 26.Lapham EV, Kozma C, Weiss JO, Benkendorf JL, Wilson MA. The gap between practice and genetics education of health professionals: HuGEM survey results. Genet Med. 2000;2:226–231. doi: 10.1097/00125817-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Kosinski M, Keller SD. How to score the SF-12 physical and mental health summary scales. 2nd ed. Boston: The Health Institute, New England Medical Center; 1995. [Google Scholar]
- 28.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 29.Bellcross CA. Breast Cancer Genetics Referral Screening Tool (B-RST) Peachtree Solutions, LLC; 2013. [Google Scholar]
- 30.Tice JA, Cummings SR, Ziv E, Kerlikowske K. Mammographic breast density and the Gail model for breast cancer risk prediction in a screening population. Breast Cancer Res Treat. 2005;94:115–122. doi: 10.1007/s10549-005-5152-4. [DOI] [PubMed] [Google Scholar]
- 31.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 32.STATA Statistics/Data Analysis version 11.2. College Station, TX: Statacorp; 2009. [Google Scholar]
- 33.Skinner CS, Rawl SM, Moser BK, Buchanan AH, Scott LL, Champion VL, et al. Impact of the Cancer Risk Intake System on patient-clinician discussions of tamoxifen, genetic counseling, and colonoscopy. J Gen Intern Med. 2005;20:360–365. doi: 10.1111/j.1525-1497.2005.40115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baer HJ, Schneider LI, Colditz GA, Dart H, Andry A, Williams DH, et al. Use of a web-based risk appraisal tool for assessing family history and lifestyle factors in primary care. J Gen Intern Med. 2013;28:817–824. doi: 10.1007/s11606-013-2338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruffin MTt, Nease DE, Jr., Sen A, Pace WD, Wang C, Acheson LS, et al. Effect of preventive messages tailored to family history on health behaviors: the Family Healthware Impact Trial. Ann Fam Med. 2011;9:3–11. doi: 10.1370/afm.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan CP, Kim SE, Wong ST, Sawaya GF, Walsh JM, Perez-Stable EJ. Willingness to use tamoxifen to prevent breast cancer among diverse women. Breast Cancer Res Treat. 2012;133:357–366. doi: 10.1007/s10549-012-1960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medications for risk reduction of primary breast cancer in women. Rockville, MD: United States Preventive Services Task Force; 2013. [Google Scholar]