Abstract
The neuroprotective role of Hsp72 has been demonstrated in several ischemic/stroke models to occur primarily through mediation of apoptotic pathways, and a number of heat shock proteins are upregulated in animal models capable of extended anoxic survival. In the present study, we investigated the role of Hsp72 on cell death and apoptotic regulators in one anoxia tolerant model system, the freshwater turtle Trachemys scripta. Since Hsp72 is known to regulate apoptosis through interactions with Bcl-2, we manipulated the levels of Hsp72 and Bcl-2 with siRNA in neuronally enriched primary cell cultures and examined downstream effects. The knockdown of either Hsp72 or Bcl-2 induced cell death during anoxia and reoxygenation. Knockdown of Bcl-2 resulted in increases in apoptotic markers and increased ROS levels 2-fold. However, significant knockdown of Hsp72 did not have any effect on the expression of key mitochondrial apoptotic regulators such as Cytochrome c and caspase-3. Hsp72 knockdown however significantly increased Apoptosis Inducing Factor in both anoxia and reoxygenation and resulted in a six-fold induction of hydrogen peroxide levels. These findings suggest that the neuroprotection offered by Hsp72 in the anoxia/reoxygenation tolerant turtle is through the mediation of ROS levels and not through modulation of caspase-dependent pathways.
Keywords: heat shock protein, apoptosis, anoxia, Trachemys scripta, reactive oxygen species
Introduction
It has proven difficult to distinguish pathological from adaptive strategies in mammalian models of anoxia or ischemia, as the mammalian brain is exquisitely sensitive to these insults, and pro-death and survival pathways are activated simultaneously. Because cellular function is similar between upper and lower vertebrates, potential therapeutic targets to enhance anoxic or reoxygenation survival may be investigated using model organisms that survive such conditions. Anoxia tolerance of at least several hours is known in Drosophila (Haddad, 2006), some frogs (Milton et al., 2003), and certain fish (Wilson et al., 2004; Smith R.W. et al. 2009; Stensløkken et al., 2010). Among the most anoxia-tolerant vertebrates are certain North American pond turtles, which withstand anoxia for days at room temperature to months at 3°C in a hypometabolic state (Jackson, 2000). Survival is a result of complex physiological and molecular adaptations that defend the turtle against the stress of oxygen deprivation (Milton and Prentice, 2007), with protective pathways robustly expressed (Krivoruchko and Storey, 2010). Adaptations to survive anoxia include suppression of excitatory amino acid release, increased Gamma-Aminobutyric Acid (GABA) and adenosine, and decreased membrane ion permeability, which together decrease electrical activity (reviewed in Lutz and Milton, 2004; Milton and Prentice, 2007). Freshwater turtles ameliorate reoxygenation damage via a combination of high antioxidant levels (Rice et al., 1995; Willmore and Storey, 1997a,b) and an innate suppression of reactive oxygen species (ROS) (Milton et al., 2007; Pamenter et al., 2007). It has been suggested that the ability to restrict oxidative stress may also contribute to the renowned longevity of certain vertebrates, including turtles (Lutz et al., 2003; Krivoruchko and Storey, 2010), and perhaps naked mole-rats (Andziak et al., 2006; Perez et al., 2009), though parrot lifespan does not appear to be related to significant differences in oxidative stress resistance compared to short-lived birds (Montgomery et al., 2012b).
At the molecular level, anoxia induced changes in the turtle include increases in heat shock proteins (HSPs) in several organs upon both anoxia and reoxygenation (Krivoruchko and Storey, 2010), including in the brain (Prentice et al. 2004; Kesaraju et al., 2009), along with increases in expression of the anti-apoptotic protein Bcl-2 (Haddad, 2007b; Kesaraju et al., 2009). HSPs are thought to enhance survival in part through an upregulation of protective mechanisms and/or by the suppression of pro-apoptotic pathways. One of the most studied stress proteins in mammalian models is Hsp72 (inducible Hsp70), due to its protective effects in cerebral ischemia (Kelly et al., 2001; van der Weerd et al., 2005; Giffard et al., 2008), neuronal cultures (Sato et al., 1996; Amin et al., 1996, Hoehn et al., 2001; Kelly et al., 2002), and in ischemic/reperfused myocardium (Corneleusen et al., 2003, Guisasola et al, 2006). The cytoprotective function of Hsp72 has been ascribed to its inhibitory effects on apoptotic and necrotic cell death pathways (Giffard et al, 2008), with the fate of cell survival decided by the equilibrium established between stress proteins and the apoptotic pathway of cell death (Beere, 2001), especially the ratio of the anti-apoptotic and pro-apoptotic proteins, Bcl-2 and Bax. Hsp72 has been implicated in the suppression of the translocation of Bax from the cytosol to the mitochondria (Stankiewicz et al., 2005), abrogating the release of Cytochrome c (Cyt c) from the mitochondria (Tsuchiya et al., 2003), and blocking formation of the apoptosome through interaction with Apaf 1 and through binding with caspases (Matsumori et al., 2006). Hsp72 also promotes cell survival by inhibiting the release of Apoptosis Inducing Factor (AIF) from the mitochondria and its translocation to the nucleus, where it leads to condensation of the chromatin and apoptosis independent of the action of caspases (Choudhury et al, 2011).
Due to its strong upregulation, Hsp72 is assumed to be protective in the anoxia tolerant turtle, however, its function and links to other molecular pathways in turtle neurons has not been previously investigated. Here we directly test the hypothesis that the innately high levels of Hsp72 and Bcl-2 offer neuroprotection during anoxia and reoxygenation in the turtle Trachemys scripta by manipulating their levels in neuronally enriched primary cell cultures. While the upregulation of Bcl-2 is required for preventing activation of apoptotic pathways during anoxia and reoxygenation in T. scripta, neuroprotection by Hsp72 appears to be associated instead with the maintenance of low levels of ROS.
2. Results
2.1. Increased expression of Bcl-2/Bax during anoxia and reoxygenation
Based on previous work showing that the Bcl-2:Bax ratio is critical to promote cell survival in mammalian models of ischemia/reperfusion (Qui et al., 2010; Li et al., 2012; reviewed in Ferrer and Planas, 2003; Chan, 2004), and the generally higher levels of Bcl-2 vs. Bax in anoxic turtles (Kesaraju et al., 2009; Nayak et al., 2011), we thus examined changes in Bcl-2, Bax, and cell survival following 4h anoxia and upon reoxygenation.
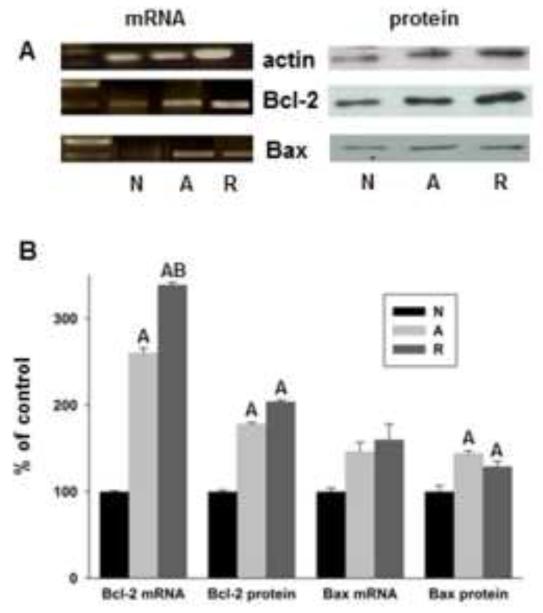
Both Bcl-2 and Bax mRNA and protein levels increase in 4h anoxic cells and upon anoxia/reoxygenation (4h/4h), but the increases in Bcl-2 transcription and translation are greater than changes in Bax (Fig. 1). Transcript levels of Bcl-2 as determined by Q-PCR showed significant 2.6-fold and 3.4-fold induction over basal during anoxia and reoxygenation, respectively, while Bax mRNA levels increased only 1.6-fold under the same conditions (not statistically significant). Protein changes were slightly lower, with Bcl-2 increasing 1.8-fold and 2-fold in anoxia and reoxygenation, but Bax increasing only1.4- and 1.3-fold over basal. The Bcl-2:Bax ratio thus increased 20% over 4h anoxia and 60% following 4h reoxygenation compared to normoxia.
Figure 1.
Bcl-2 mRNA and protein levels increase significantly in anoxia and anoxia/reoxygenation compared to Bax. (A) Representative RT-PCR gels and western blots from Bcl-2, Bax, and actin; Bcl-2 and Bax are normalized to actin for all data. N = normoxia, A = 4h anoxia, R = 4h anoxia+ 4h reoxygenation. (B) Induction of Bcl-2 and Bax mRNA and protein levels as determined by Q-PCR and western blot, respectively. A = significantly different from respective normoxic control, B = significantly different from respective anoxic control (p<0.05), Bax mRNA levels did not change significantly between conditions. Data are normalized to actin signal. N= 5/treatment.
2.2. Suppression of Bcl-2 during anoxia and reoxygenation increases cell death and activates caspase-3
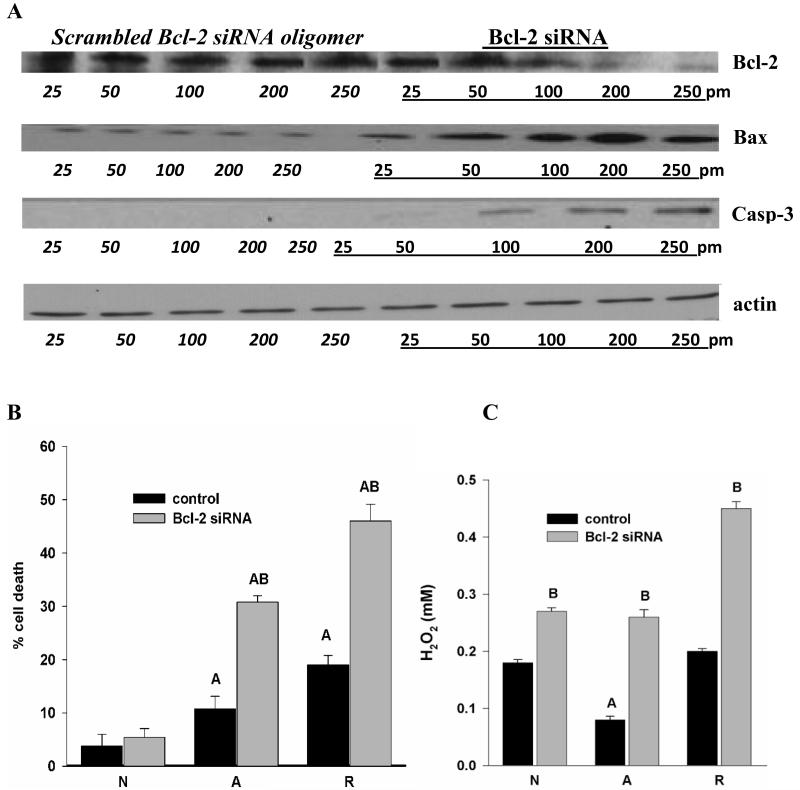
To determine if Bcl-2 is indeed protective during anoxia and reoxygenation, we used turtle-specific siRNA to knockdown Bcl-2. Transfections were performed using Stealth™ RNA in Lipofectamine-2000 (LF); controls were LF alone or LF plus scrambled siRNA sequence. Transfection of cells with scrambled (non-target) siRNA did not alter Bcl-2 or Bax levels, but the use of Bcl-2 specific siRNA resulted in a dose-dependent knockdown of Bcl-2 expression in 4h anoxic cells (Fig. 2A). At 250 pm siRNA, Bcl-2 decreased to a mean 38% of control and cell death increased significantly. Cell death increased from 11% to 31% in anoxic cultures and from 19% in anoxia/reoxygenation cells treated with scrambled siRNA oligomer to 46 % in cells with siRNA against Bcl-2 (Fig. 2B). Neither LF alone nor control siRNA increased cell death; cell death in cells treated with scrambled siRNA was not significantly different under any condition compared to levels of cell death in untreated cultures. The mean 62% decrease in Bcl-2 levels with siRNA treatment (250 pm) was accompanied by an increase in Bax to 238 ± 2% of basal (vs. 113 ± 11% of control in cells transfected with scrambled siRNA), and detectable levels of activated caspase-3 (Fig. 2A).
Figure 2.
(A) Representative western blots (of N=5/treatment) showing Bcl-2 Stealth™ siRNA decreases anoxia-induced induction of Bcl-2 in a dose dependent manner, correlated with increases in Bax and caspase-3. Actin levels are unchanged by treatment. Scrambled siRNA does not affect Bcl-2 expression. (B) Bcl-2 knockdown increases cell death (% propidium iodide positive cells), p<0.01. (C) Increased release of H202 relative to the respective scrambled siRNA controls. A = significantly different from normoxia, B = significantly different from control within treatment group.
Bcl-2 knockdown also resulted in a more than 2-fold increase in H2O2 release in transfected cells exposed to anoxia followed by 4h reoxygenation vs. control cells as determined by Amplex Red staining (Fig. 2C). H2O2 levels in untreated and scrambled siRNA cells, by contrast, return only to normoxic levels in untreated turtle neuronal cultures upon anoxia/reoxygenation, without the excess release characteristic of mammalian cells subjected to ischemia/reperfusion (Fig. 2C; Milton et al., 2007; Pamenter et al., 2007).
2.3. Anoxia enhances the expression of Hsp72 in vitro
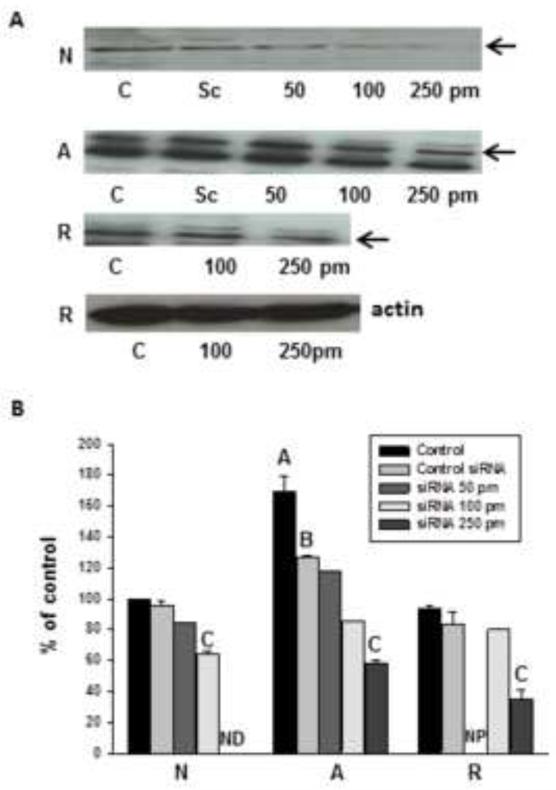
Anoxia (4h) increased the expression of Hsp72 in vitro, to 170 ± 8% of normoxic levels (Fig. 3), though mean levels following 4h reoxygenation returned nearly to basal. As Hsp72 is thought to affect Bcl-2 and Bax activity, and is strongly upregulated in the turtle brain in vivo (Kesaraju et al., 2009), we tested the hypothesis that Hsp72 protects turtle neurons by preventing activation of apoptotic pathways. We used specific siRNA designed against turtle Hsp72 to knock down constitutively high levels, and determined the effects on cell survival, levels of Bcl-2 and Bax, activated caspase-3, and AIF during anoxia and reoxygenation.
Figure 3.
HSP72 is upregulated in cultured anoxic turtle neurons; this induction can be abrogated with turtle sequence-specific siRNA against Hsp72. (A) Representative western blots showing control levels vs. siRNA treatment. Cells were titrated with no treatment (Control, C), scrambled non-target siRNA (Sc), and target siRNA levels ranging from 50 to 250 pm. Greater concentrations of siRNA were needed in anoxia and A+R (250pM) to achieve levels of reduction similar to controls (100pM). SiRNA treatment does not alter actin levels. Arrows indicate Hsp72 band as determined by mass compared to comparison ladder (PageRuler) (B) densitometric analysis of siRNA abrogation of anoxia induced increases in Hsp72. Groups are Normoxic controls (N), 4h anoxia (A), and 4h reoxygenation following 4h anoxia (R). ND = Not Detectable in most samples; NP = 50pm titration not performed, as there was no significant decrease at 100 pm.
2.4 Hsp72 is critical for cell survival
Specific siRNA transfection knocked down Hsp72 in both normoxic and anoxic cultures in a dose dependent manner; through these titration experiments we found that a higher concentration of siRNA was required to achieve an equivalent knockdown (40-60%) in anoxic and anoxic/reoxygenation cultures (250 pM) than in normoxic ones (100 pM) due to the greater upregulation of HSPs in anoxia (Fig. 3). At 100 pM, the siRNA decreased native expression in normoxia to 64 ± 1% of basal (vs. 96 ± 3% for scrambled siRNA, not detectable in most gels with 250 pM dose), while in anoxia the higher dose of siRNA knocked down Hsp72 to 34 ± 2% of anoxic untreated controls (100 pM dose no effect). While Lipofectamine alone did not affect Hsp72 expression, the scrambled siRNA did also decrease Hsp72 expression between 4% and 25% (significant only in anoxia, Fig. 3B).
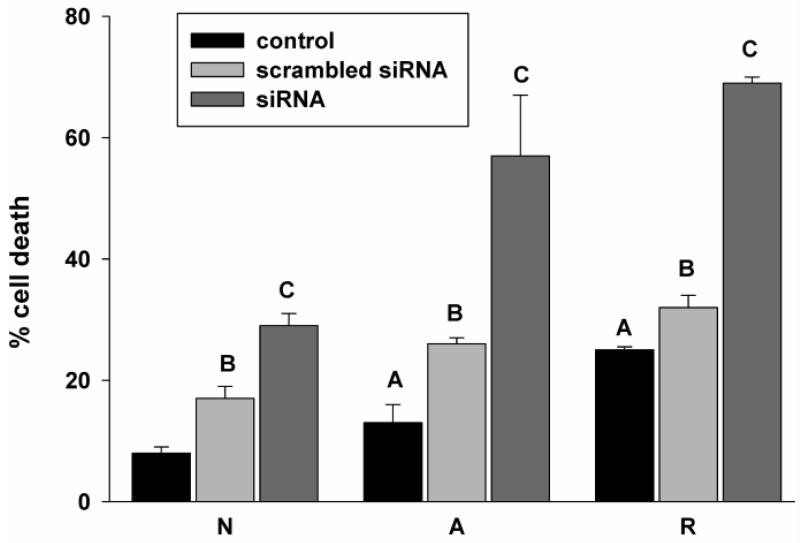
Detectable basal levels of Hsp72, and its strong and immediate upregulation (Kesaraju et al., 2009) suggest an important role in cell survival. Consistent with this hypothesis, we found that specific siRNA treatment significantly increased cell death (Fig. 4), even in normoxia (to 29 ± 3% versus 17 ± 2% for scrambled siRNA). And while 4h anoxia increased cell death in the group treated with the scrambled siRNA (to 26 ± 1% versus 13 ± 3% in anoxic controls), specific Hsp72 knockdown increased cell death more than four-fold compared to untreated anoxic cultures, to 57 ± 10% of total cells. Reoxygenation increased cell death from 32 ± 2% of total cells in siRNA controls to 69 ± 1% in knockdown cultures.
Figure 4.
siRNA knockdown of Hsp72 results in increased cell death in anoxia and reoxygenation. Cell death following siRNA treatment (% propidium iodide positive cells). Statistics: A = untreated controls significantly different from normoxic controls. B = significantly different from control within the same treatment group, C= significantly different from control and scrambled siRNA within the same treatment group, p<0.05.
2.5. Major apoptotic regulators are altered following Hsp72 knockdown
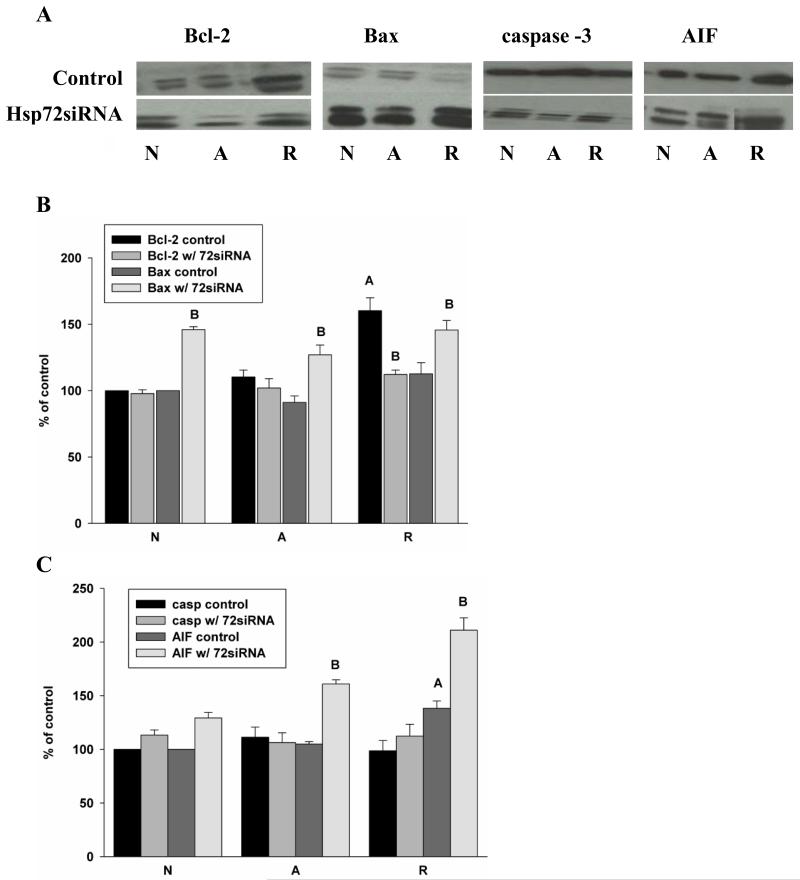
As Hsp72 knockdown increased cell death, we hypothesized that decreasing the expression of Hsp72 in the turtles would lower the expression of Bcl-2 and/or increase Bax levels. However, Hsp72 knockdown decreased anoxic Bcl-2 expression only slightly, by less than 8% over 4 hr anoxia. A greater and statistically significant decrease of 48% occurred upon reoxygenation vs. controls (Fig. 5A, B); Bax increased concurrently. The normoxic expression of Bax increased in Hsp72 knockdown samples to146 ± 2% over control; Bax levels were higher in knockdown cultures than in control siRNA treated samples in anoxia (127±7% of basal vs. 91 ± 5%) and reoxygenation (146 ±7% vs. 113 ± 8% in control). Thus the Bcl-2:Bax ratio decreased from 1.2 - 1.5 in control cultures to approximately 0.8 - 1.0 in siRNA treated cells. Unlike the direct knockdown of Bcl-2, however, which significantly increased levels of cleaved caspase-3, the smaller changes in Bcl-2 related to Hsp72 knockdown were not accompanied by increases in apoptotic factors downstream of the mitochondria. There was no elevation of cleaved caspase-3 (Fig. 5A, C) nor was there an increase in Cyt c in either whole cell extractions or in the cytosolic fraction, or any decrease in mitochondrial Cyt c (data not shown). AIF expression in untreated cultures was unchanged from control over 4h anoxia but increased significantly to 138 ± 7% of basal upon reoxygenation; Hsp72 knockdown increased AIF under all conditions, with significant increases in anoxia (to 161% of control) and upon reoxygenation (to 211% of basal).
Figure 5.
Knockdown of Hsp72 in turtle neuronal cultures results in altered Bcl-2:Bax ratios without increasing intrinsic apoptotic markers.. (A) Representative western blots of Bcl-2, Bax, caspase-3 and AIF in control (scrambled siRNA) and Hsp72 knockdown (specific siRNA) cultures. Densitometric analysis of N=5 cultures for (B) Bcl-2 and Bax, and (C) caspase-3 and AIF. Statistics: A = significantly different from normoxia, B = significantly different from control within treatment group, p<0.05.
2.4. Hsp72 aids in suppression of ROS release upon reoxygenation
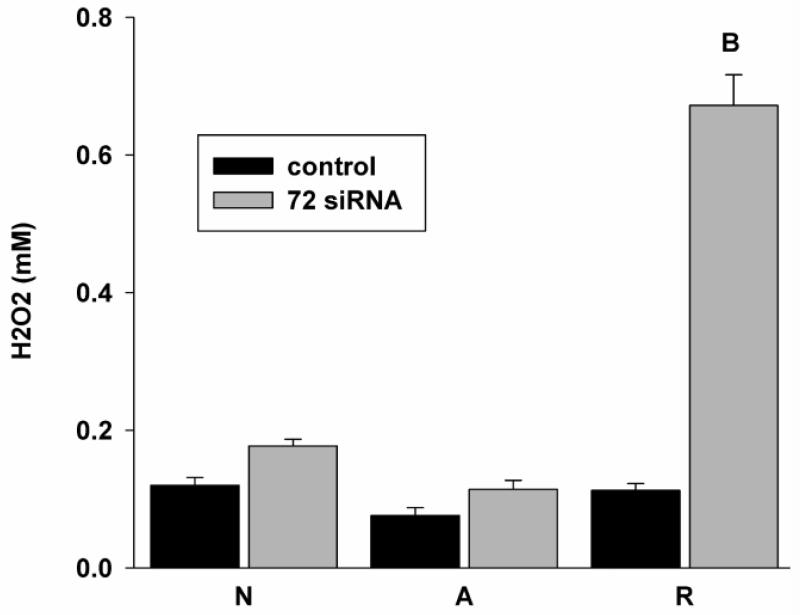
Several studies have shown that AIF release is mediated through ROS (Kang et al., 2004; Thayyullathil et al., 2008), while HSPs play a vital role in ameliorating ROS damage (Smolka et al., 2000) especially in neurodegenerative disorders (Calabrese et al., 2004), thus we also investigated the release of ROS (H2O2) following knockdown of Hsp72 in culture. In turtles, it has been shown that ROS levels fall by 4h anoxia, and increase upon reoxygenation only to basal, without the massive overproduction which occurs in mammalian cells following anoxia/ischemia (Milton et al., 2007; Pamenter et al., 2007); previous investigations in our laboratory have suggested that Hsp stabilization of the mitochondrial membrane may be responsible in part for this suppression of ROS production. And indeed, the greatest effect of Hsp72 knockdown was a six-fold increase in H2O2 release upon reoxygenation in siRNA treated cells (Fig. 6); ROS release increased in normoxic and anoxic knockdown cells as well, though not significantly.
Figure 6.
Hsp72 knockdown significantly induced H2O2 release during reoxygenation followed by anoxia. H2O2 concentration in the medium is assessed through amplex red assay. Statistics: A = control significantly different from normoxia, B = significantly different from control within treatment group, p<0.05.
3. Discussion
In earlier analyses of whole brain anoxic adaptations in the turtle we find constitutive preconditioning in normoxia involving Hypoxia Inducible Factor-1 (HIF-1), elevated HSPs (which stabilize HIF-1), and NFkB (Lutz and Milton, 2004) followed by activation of pathways in anoxia that enable cell survival. Utilizing primary neuronal cultures, we have shown these anoxia-triggered pathways are inherent to the turtle neurons, and include the activation of survival kinases, the suppression of apoptotic pathways (Nayak et al., 2011), and a critical modulation of ROS generation. By contrast, Hsp72 is expressed at minimal to undetectable levels under normal conditions in rodent models and is induced only after such insults as experimental ischemia by middle cerebral artery occlusion (Chen et al., 1996; Weinstein et al., 2004). In mammals, the expression of Hsp72 determines the delineation of the unsalvageable ischemic core and the salvageable penumbra in a cerebral infarction (Sharp et al., 2000; Weinstein et al., 2004), and is thus also a significant factor in preconditioning models (Sharp, 2000). Overexpression of Hsp72 through viral vectors or in transgenic animals also reduces infarct size and induces protective pathways (Rajdev et al., 2000; Hoehn et al., 2001; van der Weerd et al., 2005; Badin et al., 2006). Therefore, we had hypothesized that Hsp72 would be protective in the turtle through its interaction with pro-survival and pro-apoptotic pathways, as suggested by mammalian models.
While our results show that the Hsp72 increases are indeed protective in anoxic turtle neurons as well as those re-exposed to oxygen, this protection does not appear to be primarily through interactions with the intrinsic apoptotic pathway. Mammalian ischemic studies have shown the interaction of Hsp72 at several points upstream and downstream of mitochondria in the apoptotic pathway in preventing cell death, including increased Bcl-2 (Kelly et al., 2002, Yenari et al., 2005), and decreased Bax activation (Cheng et al., 2011). However, while Hsp72 knockdown significantly increased cell death and altered Bax levels in this study, unlike the larger effect of direct suppression of Bcl-2, Hsp72 knockdown apparently did not impact Bcl-2:Bax ratios sufficiently to trigger apoptosis through Cyt c release and caspase activation.
In T. scripta, the greater anoxia-induced increase in Bcl-2 compared to Bax also contrasts sharply with the mammalian brain, where oxygen depletion is associated with decreased Bcl-2 levels and increases in Bax and activated caspase-3 (Feldenberg et al., 1999; Zovein et al., 2004; Li et al., 2012). In the turtle in vivo, Bcl-2 and Bax change concurrently in anoxia, maintaining a relatively constant Bcl-2:Bax ratio greater than one despite initial decreases in both proteins (Kesaraju et al., 2009). In vitro, both proteins increase in anoxia, but with a greater increase in Bcl-2 (this study; Nayak et al., 2011). This contrasts dramatically with mammalian models of cellular stress, where Bax increases as much as 12-fold and significantly decreases Bcl-2:Bax ratios (Zhu et al., 2010) and increases apoptosis (Li et al., 2010). Bcl-2 knockdown, however, directly decreased Bcl-2:Bax ratios and allowed caspase activation, shifting cells away from survival and towards apoptosis. In vivo, neither procaspase 3 nor cleaved caspase-3 levels increased even over 24h anoxia (Kesaraju et al., 2009), showing the extraordinary resistance of turtle neurons to apoptosis even in extended anoxia.
Thus the turtle neurons clearly possess the machinery for apoptosis, but activation is suppressed in anoxia and reoxygenation. This suppression, though, does not appear to be due to the elevation of Hsp72. And while we did see increases in AIF in the cells treated with Hsp72 siRNA, there was still no accompanying activation of caspase-3 or loss of mitochondrial Cyt c. Similar increases in AIF in vivo during anoxia (to 181% of normoxia) did not result in any translocation to the nucleus or apparent increases in neuronal death, nor were there further increases upon reoxygenation (Kesaraju et al., 2009), suggesting that the AIF levels observed here are not likely to be the main cause of cell death. The 6-fold increase in ROS release upon reoxygenation, then, is likely the primary cause of cell death, though as there are no increases in Cyt c release or caspase-3 activation, the mechanism of cell death is apparently through pathways independent of mitochondrial-triggered apoptosis.
We conclude that turtle neurons do indeed possess apoptotic machinery, but that compensatory molecular pathways strongly inhibit execution of the apoptotic pathway and promote cell survival. The levels of Bcl-2 vs. Bax, while reduced compared to controls, may still be sufficient to overcome any direct effects of Hsp72 knockdown on apoptotic activation; these mechanisms, however, are unable to prevent ROS increases and subsequent cell death in reoxygenated cells in which Hsp72 levels are significantly decreased. In mammalian studies, overexpression of Hsp72 prevents the release of ROS in astrocytes (Ouyang et al., 2006, Voloboueva et al., 2008, Xu et al., 2011) as well as increases the activity of SOD (Suzuki et al., 2002). Hsp72 also suppressed cell death and caspase-3 and -9 activation induced by H2O2 in Schwann cells, and increased Bcl-2 expression (Luo et al., 2012).
Interestingly, ROS production is also known to increase with age (Sohal and Sohal, 1991; Lee and Wei, 2012), while antioxidant capacities decrease (Feuers, 1998; Vinokur et al., 2009). The free radical theory of aging states that the inevitable generation of ROS from the mitochondrial respiratory chain causes oxidative damage to proteins, lipids, and DNA (Harman, 1956), and the buildup of such damaged macromolecules has been implicated in a wide variety of diseases and age-related disorders. The ability to suppress ROS production and thus avoid oxidative damage may then also be linked to the reputation of turtles for aging with “negligible senescence” (Miller, 2001; Congdon et al., 2001; Lutz et al., 2003; Krivoruchko and Storey, 2010). Low levels of mitochondrial free radicals are correlated with longer lives in some homeotherms (Lambert et al., 2007), though not in the long-lived parrots (Montgomery et al., 2012a); and increased Hsp72 expression is associated with decreased oxidative damage in aging rats (Murlasits et al., 2006) and humans (Simar et al., 2007). While the free radical theory of aging is thus still a matter of controversy (Hekimi et al., 2011), clearly a vertebrate model of exceptional longevity that presents enhanced protection against ROS production and damage, and presents an anoxia-regulated HSP induction in vivo, could be of considerable value to elucidate the links between neuronal resistance to oxidative damage and longevity.
4. Experimental Procedure
Experiments conducted were approved by the Florida Atlantic University Institutional Animal Care and Use Committee.
4.1.Development of enriched neuronal cell culture
We prepared neuronal enriched cultures from juvenile turtle brains by modification of Brewer’s (1997) method of density gradient isolation of neurons, as previously described (Milton et al., 2007; Nayak et al., 2011).
4.2.RNA isolation and RT-PCR
Total RNA was isolated from the turtle brain and from cell cultures using Trizol reagent following the manufacturer’s protocol. The RNA sample was treated with DNase (Invitrogen, Eugene, OR) and RNA was quantified using UV spectrophotometry; 1 μg of total RNA was used for RT-PCR. Reverse transcription was performed at 50°C for 55 min using 5 units Superscript III, 125 pM forward and reverse primers (IDT DNA), 1X First strand synthesis buffer, 0.1 mM DTT, and 2 units of RNAse OUT ribonuclease inhibitor. All the reagents for RT-PCR were obtained from Invitrogen, (Eugene, OR) To detect Hsp72, PCR was performed using 100 ng of template, 200μM dNTP, 50 pM Forward and Reverse primer, 1X PCR buffer with 1.5 mM Mg and 5 U Taq polymerase. All PCR reagents were obtained from Fisher Scientific (Pittsburgh, PA). The amplification cycle consisted of 5 min initial denaturation at 94°C followed by 35 cycles of 94°C for 1min; 58°C for 1 min and 72°C for 1min. Degenerate primers were made to detect Hsp72; Forward Primer: 5′ GTGTAGAAGTCGATGCCCTCA 3′ and Reverse primer: 5′ AANGAGCCCAGCGCNCCC3′ where “N” could be A/G/C/T with a 300 bp PCR product.
Complementary DNA was synthesized from total RNA using primers specific for Bcl-2, and actin. The PCR using Taq polymerase for Bcl-2 comprised denaturation for 7 min, 94°C, PCR: 40 cycles (1 min, 94°C; 45 s, 55°C; 1.0 min, 72°C), followed by elongation: 10 min, 72°C. For actin, 30 cycles (1 min 94°C; 45 s, 55°C; 1.5 min 72°C) followed by elongation: 10 min 72°C. Primers specific to turtle Bcl-2 cDNA were designed from a partial cDNA sequence that was obtained previously by RT-PCR analysis of turtle brain mRNA using degenerate primers from mouse and zebrafish. The primers employed for PCR were the following: turtle brain specific: Bcl-2 primers 5′-GGTGCCACC- TGTGGTCCACCTG- 3− (forward) and 5′-CTTCACTTGTGGCCCAGATAGG-3′(reverse); actin primers: 5′-CAC CAACTGGGACGACATGG-3′ (forward) and 5′-GTCGGCCAGCTCGTAGCTCT-3′ (reverse). PCR products were separated by gel electrophoresis, visualized by ethidium bromide and photographed using a digital camera for quantification using National Institute of Health Image J 1.60 software. For semi-quantitative measurement of transcript levels, RT-PCR signal intensities were calculated as a ratio of levels of PCR products amplified from turtle actin cDNAs.
For all the reactions actin was run as an internal control, as it does not change under these experimental conditions (Prentice et al., 2004). All the data were normalized to actin and expressed as percent control.
4.3. Q-PCR
Following reverse transcription as described earlier a 95 °C incubation for 10 min, forty cycles of PCR (95 °C/15 s; 60 °C/1 m), were then performed on an ABI Prism 7900HT Sequence Detection System with 1 l of the RT reaction, 100 um PCR primers (Actin, Bax and Bcl-2), and SYBR Green PCR Master Mix in 20-μl reactions. Threshold cycles (CT) for three replicate reactions were determined using Sequence Detection System software (version 2.0, release 4) and relative transcript abundance calculated following normalization with actin PCR amplicon. Amplification of only a single species was verified by a dissociation curve for each reaction.
4.4. siRNA design and transfection
Target siRNA against turtle Hsp72 was identified by using the siRNA design tool of Invitrogen/Molecular Probes (Eugene, OR) from the turtle Hsp72 specific sequence. Specific siRNA sequences against Hsp72 and the scrambled control are as follows: 5′ UGUCCCGCUUGUGCUUGCGCUUGAA3′;5′UGUCGCGUUCGUGUUCGCUUCGGAA3′. All the controls in the siRNA experiments were performed using scrambled siRNA. Transfection was performed using Lipofectamine-2000 as per the manufacturer’s protocol. The amount of siRNA was optimized for different experimental conditions. Titration experiments (Figure 3) showed that a higher concentration of siRNA was required to achieve an equivalent knockdown (40-60%) in anoxic and anoxic/reoxygenation cultures (250 pM) than in normoxic ones (100 pM) due to the greater upregulation of HSPs in anoxia. Protein /cell lysate was harvested from the transfected cell cultures for further analysis. Transfection efficiency of lipofectamine was analyzed using GFP. Cells were transfected with 1 ug GFP plasmid following a similar protocol as experimental samples.
Specific siRNA against turtle Bcl-2 was designed using the Invitrogen/Molecular Probes (Eugene, OR) siRNA design tool from the turtle Bcl-2 specific sequence (ref: NG_009361) and cells transfected (250 pmol) as above.
4.5. Cell viability assay
Propidium iodide (PI) staining was used to obtain the ratio of dead cells to total cells for each treatment, including in the cell cultures transfected with siRNA against Hsp72 and the control siRNA. The ratio of live to dead cells ratio was obtained by using 1.5 μM of PI (Molecular Probes) where the dying cells take up the red stain. Cells were viewed under confocal microscope and at least 5 fields were counted per each experimental condition.
4.6. Hydrogen peroxide (H2O2) release assay
Although the turtle is able to survive repeated anoxia/reoxygenation events, turtle neurons were found susceptible to extrinsic stress of hydrogen peroxide (Milton et al., 2007). Hydrogen peroxide (H2O2) by itself is inactive but it can diffuse freely across the cell membrane and can transform into the highly reactive hydroxyl radical (Cui et al., 2004). The extent of H2O2 released into the extracellular medium of the control and knockdown cultures was measured by using Amplex Red assay kit (Molecular Probes) utilizing the manufacturer’s protocol. A mix of horseradish peroxidase (HRP) and the fluorescent Amplex Red was added to 50 μl of the culture medium of each condition and the fluorescence emitted (on reduction of H2O2) was read using a microplate reader at 560 nm.
4.7. Western blot analysis
The primary antibodies used were Hsp72 (Stressgen, Victoria, BC, Canada); Bcl-2 (Chemicon, Temecula, CA); Bax (Santa Cruz Biotechnology, Santa Cruz, CA); Cyt C (Abcam Inc, Boston, MA) Caspase 3 (Cell Signaling Technology, Danvers, MA); AIF (Stressgen); pJNK(Cell Signaling Technology); and pERK (Cell Signaling Technology). All primary antibodies except for Hsp72 (1:4000) were used 1:1000 dilution.
Total cell protein was extracted using RIPA cell lysis buffer (0.15 M Nacl, 5 mM EDTA pH 8, 1% Triton X100, 10 mM Tris-Cl pH 7.4; with added 5 M DTT, 100 mM PMSF, 5 M mercaptoethanol diluted 1:1000) and 10 μg of the protein was loaded on a 12% SDS-PAGE gel. The gel was transferred onto a nitrocellulose membrane (Hybond ECL, Amersham Biosciences, Piscataway, NJ) for 1 h at 0.3 mA. The membrane was blocked in 5% milk for 1 h and then incubated with the respective primary antibody overnight at 4°C in 2% milk. The blot was washed 3X in TBS-T and incubated with respective secondary antibody (Southern Biotech #4050, Birmingham, AL) at room temperature for 2 h and after 3X rinses, the bands were visualized by ECL chemiluminescence (Amersham Biosciences). The band intensity for the band at the correct molecular weight was measured using NIH ImageJ software; secondary bands are likely post-transcriptional modifications and were not quantified.
4.8. Separation of mitochondrial and cytosolic fractions
Mitochondrial and cytosolic fractions were isolated to analyze the levels of Cyt c using Cytochrome C release apoptosis assay kit based on manufacturer’s instructions(Calbiochem, Gibbstown, NJ). Briefly, cells that were grown to confluence were first washed with PBS and then the cells were scraped using a sterile cell scraper. The cells were then centrifuged at 600g for 10 min at 4°C. The pelleted cells were suspended into the cytosolic buffer and incubated for 10 min on ice. The cells were then homogenized using a glass douncer and the homogenate centrifuged at 700 g for 10 min at 4°C. The supernatant was collected as the cytosolic fraction and the pellet suspended in the mitochondrial extraction buffer.
Statistical analysis was performed using the SPSS statistical package. Results were analyzed using ANOVA (Analysis of variance) with post-hoc analysis and the data were expressed as Mean ± SE. p<0.05 was considered statistically significant.
Highlights.
We investigated the role of Hsp72 on cell death in the anoxia tolerant turtle neuron.
We manipulated the levels of Hsp72 and Bcl-2 through siRNA in vitro.
Knockdown of Hsp72 or Bcl-2 induced cell death during anoxia and upon reoxygenation.
Knockdown of Hsp72 did not alter key apoptotic regulators, but increased ROS.
Hsp72 neuroprotection in this model organism may thus be via modulation of ROS.
Acknowledgements
This work was funded by an AHA grant and NIH grant 1R15AG033374-01 to SLM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin V, Cumming DV, Latchman DS. Over-expression of heat shock protein 70 protects neuronal cells against both thermal and ischaemic stress but with different efficiencies. Neurosci. Lett. 1996;206:45–48. doi: 10.1016/0304-3940(96)12421-6. [DOI] [PubMed] [Google Scholar]
- Andziak B, et al. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;6:463–71. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Badin RA, et al. Neuroprotective effects of virally delivered HSPs in experimental stroke. J Cereb. Blood Flow Metab. 2006;26:371–381. doi: 10.1038/sj.jcbfm.9600190. [DOI] [PubMed] [Google Scholar]
- Beere HM. Stressed to death: regulation of apoptotic signaling pathways by the heat shock proteins. Sci. Stke. Re1. 2001 doi: 10.1126/stke.2001.93.re1. [DOI] [PubMed] [Google Scholar]
- Calabrese V, et al. Redox regulation in neurodegeneration and longevity: role of the heme oxygenase and HSP70 systems in brain stress tolerance. Antioxid. Redox Signal. 2004;6:895–913. doi: 10.1089/ars.2004.6.895. [DOI] [PubMed] [Google Scholar]
- Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem. Res. 2004;29:1943–49. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Stress proteins and tolerance to focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1996;16:566–577. doi: 10.1097/00004647-199607000-00006. [DOI] [PubMed] [Google Scholar]
- Cheng L, et al. Modulation of Cellular Hsp72 Levels in Undifferentiated and Neuron-Like SH-SY5Y Cells Determines Resistance to Staurosporine-Induced Apoptosis. PLoS ONE. 2011;6:e24473. doi: 10.1371/journal.pone.0024473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S, et al. Role of AIF in cardiac apoptosis in hyoertrophic cardiomyocytes from Dahl salt-sensitive rats. Cardiovasc. Res. 2010;85:28–37. doi: 10.1093/cvr/cvp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon JD, et al. Hypothesis of aging in along-lived vertebrate, Blanding’s turtle (Emydoidea blandingii) Exp. Gerentol. 2001;36:813–827. doi: 10.1016/s0531-5565(00)00242-4. [DOI] [PubMed] [Google Scholar]
- Cornelussen RN, et al. Proteins involved in salvage of the myocardium. Adv. Exp. Med. Biol. 2003;543:277–291. doi: 10.1007/978-1-4419-8997-0_20. [DOI] [PubMed] [Google Scholar]
- Feldenberg LR, et al. Partial ATP depletion induces Fas- and caspase-mediated apoptosis in MDCK cells. Am. J. Physiol. 1999;276:F837–46. doi: 10.1152/ajprenal.1999.276.6.F837. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J. Neuropathol. Exp. Neurol. 2003;62:329–39. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- Feuers R,J. The effects of dietary restriction on mitochondrial dysfunction in aging. Ann. NY Acad. Sci. 1998;854:192–201. doi: 10.1111/j.1749-6632.1998.tb09902.x. [DOI] [PubMed] [Google Scholar]
- Giffard RG, et al. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthes. 2008;109:339–348. doi: 10.1097/ALN.0b013e31817f4ce0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisasola MC, et al. Heat shock proteins, end effectors of myocardium ischemic preconditioning? Cell Stress Chaperones. 2006;11:250–258. doi: 10.1379/CSC-181R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad GG. Tolerance to low O2: lessons from invertebrate genetic models. Exp. Physiol. 2006;91:277–82. doi: 10.1113/expphysiol.2005.030767. [DOI] [PubMed] [Google Scholar]
- Haddad JJ. The role of Bax/Bcl-2 and pro-caspase peptides in hypoxia/reperfusion-dependent regulation of MAPK(ERK): discordant proteomic effect of MAPK(p38) Protein Pept. Lett. 2007;14:361–71. doi: 10.2174/092986607780363925. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn B, et al. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J. Cereb. Blood Flow Metab. 2001;21:1303–1309. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Jackson DC. Living without oxygen: lessons from the freshwater turtle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000;125:299–315. doi: 10.1016/s1095-6433(00)00160-4. [DOI] [PubMed] [Google Scholar]
- Kelly S, et al. Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of Bcl-2. Ann. Neurol. 2002;52:160–167. doi: 10.1002/ana.10264. [DOI] [PubMed] [Google Scholar]
- Kesaraju S, et al. Modulation of stress proteins and apoptotic regulators in the anoxia tolerant turtle brain. J. Neurochem. 2009;109:1413–1426. doi: 10.1111/j.1471-4159.2009.06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivoruchko A, Storey KB. Forever young: mechanisms of natural anoxia tolerance and potential links to longevity. Oxid. Med. Cell Longev. 2010;3:186–98. doi: 10.4161/oxim.3.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AJ, et al. Low rates of hydrogen peroxide by isolated heart mitochondria associate with long maimum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–18. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Lee HC, Wei YH. Mitochondria and aging. Adv. Exp. Med. Biol. 2012;42:311–327. doi: 10.1007/978-94-007-2869-1_14. [DOI] [PubMed] [Google Scholar]
- Li XM, et al. Single-prolonged stress induced mitochondrial-dependent apoptosis in hippocampus in the rat model of post-traumatic stress disorder. J. Chem. Neuroanat. 2010;40:248–55. doi: 10.1016/j.jchemneu.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Li Z, et al. Resveratrol attenuates brain damage in a rat model of focal cerebral ischemia via up-regulation of hippocampal Bcl-2. Brain Res. 2012;23:116–24. doi: 10.1016/j.brainres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Luo X, et al. Extracellular heat shock protein 72 protects schwann cells from hydrogen peroxide-induced apoptosis. J. Neurosci. Res. 2012;90:1261–9. doi: 10.1002/jnr.22810. [DOI] [PubMed] [Google Scholar]
- Lutz PL, Milton SL. Negotiating brain anoxia survival in the turtle. J. Exp. Biol. 2004;207:3141–3147. doi: 10.1242/jeb.01056. [DOI] [PubMed] [Google Scholar]
- Lutz PL, Prentice HM, Milton SL. Is turtle longevity linked to enhancd mechanisms for surviving brain anoxia and reoxygenation. Exp. Gerontol. 2003;38:797–800. doi: 10.1016/s0531-5565(03)00111-6. [DOI] [PubMed] [Google Scholar]
- Matsumori Y, et al. Reduction of caspase-8 and -9 cleavage is associated with increased c-FLIP and increased binding of Apaf-1 and Hsp70 after neonatal hypoxic/ischemic injury in mice overexpressing Hsp70. Stroke. 2006;37:507–512. doi: 10.1161/01.STR.0000199057.00365.20. [DOI] [PubMed] [Google Scholar]
- Miller JK. Escaping senescence: demographic data from the three-toed box turtle(Terrapene Carolina triunguis) Exp. Gerontol. 2001;36:829–832. doi: 10.1016/s0531-5565(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Milton SL, Prentice HM. Beyond anoxia: the physiology of metabolic downregulation and recovery in the anoxia-tolerant turtle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007;147:277–290. doi: 10.1016/j.cbpa.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton SL, Manual L, Lutz PL. Slow death in the leopard frog Rana pipiens: neurotransmitters an anoxia tolerance. J. Exp. Biol. 2003;206:4021–4028. doi: 10.1242/jeb.00647. [DOI] [PubMed] [Google Scholar]
- Milton SL, et al. Suppression of reactive oxygen species production enhances neuronal survival in vitro and in vivo in the anoxia-tolerant turtle Trachemys scripta. J. Neurochem. 2007;101:993–1001. doi: 10.1111/j.1471-4159.2007.04466.x. [DOI] [PubMed] [Google Scholar]
- Montgomery MK, Hulbert AJ, Buttemer WA. Does the oxidative stress theory of aging explain longevity differences in birds? I. Mitochondrial ROS production. Exp. Gerontol. 2012;47:203–10. doi: 10.1016/j.exger.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Montgomery MK, Buttemer WA, Hulbert AJ. Does the oxidative stress theory of aging explain longevity differences in birds? II. Antioxidant systems and oxidative damage. Exp. Gerontol. 2012;47:211–22. doi: 10.1016/j.exger.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Murlasits Z, et al. Resistance training increases heat shock protein levels in skeletal muscle of young and old rats. Exp. Gerentol. 2006;41:398–406. doi: 10.1016/j.exger.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Nayak G, Prentice HM, Milton SL. Neuroprotective signaling pathways are modulated by adenosine in the anoxia tolerant turtle. J. Cereb. Blood Flow Metab. 2011;31:467–75. doi: 10.1038/jcbfm.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, et al. Overexpression of inducible heat shock protein 70 and its mutants in astrocytes is associated with maintenance of mitochondrial physiology during glucose deprivation stress. Cell Stress Chaper. 2006;11:180–186. doi: 10.1379/CSC-182R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamenter ME, Richards MD, Buck LT. Anoxia-induced changes in reactive oxygen species and cyclic nucleotides in the painted turtle. J. Comp. Physiol. [B] 2007;177:473–481. doi: 10.1007/s00360-007-0145-8. [DOI] [PubMed] [Google Scholar]
- Pérez VI, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc. Natl. Acad. Sci. U S A. 2009;106:3059–64. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice HM, et al. The upregulation of cognate and inducible heat shock proteins in the anoxic turtle brain. J. Cereb. Blood Flow Metab. 2004;24:826–828. doi: 10.1097/01.WCB.0000126565.27130.79. [DOI] [PubMed] [Google Scholar]
- Qiu W, et al. A glycine site-specific NMDA receptor antagonist protects retina ganglion cells from ischemic injury by modulating apoptotic cascades. J. Cell. Physiol. 2010;223:819–26. doi: 10.1002/jcp.22118. [DOI] [PubMed] [Google Scholar]
- Rajdev S, et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann. Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- Rice ME, Lee EJ, Choy Y. High levels of ascorbic acid, not glutathione, in the CNS of anoxia-tolerant reptiles contrasted with levels in anoxia-intolerant species. J. Neurochem. 1995;64:1790–1799. doi: 10.1046/j.1471-4159.1995.64041790.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Saito H, Matsuki N. HSP70 is essential to the neuroprotective effect of heat-shock. Brain Res. 1996;740:117–123. doi: 10.1016/s0006-8993(96)00846-3. [DOI] [PubMed] [Google Scholar]
- Sharp FR, et al. Multiple molecular penumbras after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2000;20:1011–1032. doi: 10.1097/00004647-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Simar D, et al. Physical activity modulates heat shock protein-72 expression and limits oxidative damage accumulation in ahealthy elderly population aged 60-90 years. J. Gerentol. A Biol. Sci. Med. Sci. 2007;62:1413–9. doi: 10.1093/gerona/62.12.1413. [DOI] [PubMed] [Google Scholar]
- Smith RW, et al. Proteomic changes in the crucian carp brain during exposure to anoxia. Proteomics. 2009;9(8):2217–29. doi: 10.1002/pmic.200800662. [DOI] [PubMed] [Google Scholar]
- Smolka MB, et al. HSP72 as a complementary protection against oxidative stress induced by exercise in the soleus muscle of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1539–1545. doi: 10.1152/ajpregu.2000.279.5.R1539. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Sohal BH. Hydrogen peroxide release by mitochondria increases during aging. Mech. Ageing Dev. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-w. [DOI] [PubMed] [Google Scholar]
- Stankiewicz AR, et al. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J. Biol. Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- Stensløkken KO, et al. Expression of heat shock proteins in anoxic crucian carp (Carassius carassius): support for cold as a preparatory cue for anoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298(6):R1499–508. doi: 10.1152/ajpregu.00675.2009. [DOI] [PubMed] [Google Scholar]
- Suzuki K, et al. Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation. 2002;106:I270–276. [PubMed] [Google Scholar]
- Thayyullathil F, et al. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Rad. Biol. Med. 2008;45:1403–12. doi: 10.1016/j.freeradbiomed.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Tsuchiy D, et al. Overexpression of rat heat shock protein 70 is associated with reduction of early mitochondrial cytochrome C release and subsequent DNA fragmentation after permanent focal ischemia. J. Cereb. Blood Flow Metab. 2003;23:718–727. doi: 10.1097/01.WCB.0000054756.97390.F7. [DOI] [PubMed] [Google Scholar]
- van der Weerd L, et al. Neuroprotective effects of HSP70 overexpression after cerebral ischaemia--an MRI study. Exp. Neurol. 2005;195:257–266. doi: 10.1016/j.expneurol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Vinokur V, et al. Methionine -centered redox cycle in organs of aero-digestive tract of young and old rats. Biogerentol. 2009;10:43–52. doi: 10.1007/s10522-008-9152-8. [DOI] [PubMed] [Google Scholar]
- Voloboueva LA, et al. Overexpression of mitochondrial Hsp70/Hsp75 protects astrocytes against ischemic injury in vitro. J. Cereb. Blood Flow Metab. 2008;28:1009–16. doi: 10.1038/sj.jcbfm.9600600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein PR, Hong S, Sharp FR. Molecular identification of the ischemic penumbra. Stroke. 2004;35:2666–2670. doi: 10.1161/01.STR.0000144052.10644.ed. [DOI] [PubMed] [Google Scholar]
- Willmore WG, Storey KB. Antioxidant systems and anoxia tolerance in a freshwater turtle Trachemys scripta elegans. Mol. Cell. Biochem. 1997;170:177–185. doi: 10.1023/a:1006817806010. [DOI] [PubMed] [Google Scholar]
- Wilson PJ, et al. Discordance between genetic structure and morphological, ecological, and physiological adaptation in Lake Magadi tilapia. Physiol. Biochem. Zool. 2004;77:537–55. doi: 10.1086/422054. [DOI] [PubMed] [Google Scholar]
- Xu L, et al. Heat shock protein 72 (Hsp72) improves long term recovery after focal cerebral ischemia in mice. Neurosci. Lett. 2011;488:279–82. doi: 10.1016/j.neulet.2010.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, et al. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann. NY Acad. Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- Zhu JR, et al. Protective effects of ginsenoside Rb(3) on oxygen and glucose deprivation-induced ischemic injury in PC12 cells. Acta Pharmacol. Sin. 2010;31:273–80. doi: 10.1038/aps.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein A, et al. Postnatal hypoxic-ischemic brain injury alters mechanisms mediating neuronal glucose transport. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R273–82. doi: 10.1152/ajpregu.00160.2003. [DOI] [PubMed] [Google Scholar]