Abstract
Under normal conditions, plasmacytoid dendritic cells (pDCs) are located in peripheral lymphoid organs or circulate in the blood, from where they can migrate to sites of infection or inflammation. In inflamed tissues, pDCs can be exposed to elevated levels of reactive oxygen species produced by inflammatory cells and we presume that oxidative stress could affect the cellular responses of pDCs to microenvironmental stimuli. To explore this possibility, human pDCs isolated from peripheral blood of healthy donors were treated with H2O2 and R837 (a Toll-like receptor 7 ligand), separately and in combination. Our results demonstrate that treatment with a low concentration (0.01 µM) of H2O2 resulted in only slight changes in the expression of CD40, CD80, CD86, and CD83; however, low-dose H2O2 markedly decreased the expression of HLA-DQ on pDCs. Exposure to H2O2 did not trigger the release of IL-6, TNF-α, IL-8, or IFN-α from pDCs. Although addition of H2O2 did not modify the capacity of pDCs to activate allogeneic IL-17- or IFN-γ-producing T cells, it significantly increased the ability of pDCs to stimulate IL-4-secreting T cells. Exposure of pDCs to H2O2 before cocultivation with naïve autologous T cells significantly lowered IL-10 production by T cells, but did not affect IL-17 release. It was also observed that H2O2-exposed pDCs provided stronger stimuli for Th2 than for Th1 differentiation upon autologous activation, compared to untreated pDCs, possibly because of elevated surface expression of OX40-L. Most importantly, when pDCs were stimulated with R837 in the presence of H2O2, decreased phenotypic activation, decreased chemokine and cytokine release, and impaired allo- and autostimulatory functions of pDCs were detected, indicating that pDCs exposed to oxidative stress in vivo may have an anti-inflammatory or tolerogenic role in regulating adaptive immune responses.
Keywords: Plasmacytoid dendritic cells, Oxidative stress, Inflammation, Immune regulation, Free radicals
Plasmacytoid dendritic cells (pDCs) are a unique and rare cell population of the immune system. Immature pDCs exhibit a spherical shape and are referred to as plasmacytoid predendritic cells. They are specialized for the direct recognition of viral and microbial infections by their selectively expressed endosomal nucleic acid-sensing Toll-like receptors (TLRs) such as TLR7 and TLR9 [1]. After detection of pathogen-derived nucleic acids, plasmacytoid predendritic cells secrete large amounts of type I interferons (IFNs) and other inflammatory cytokines involved in innate immune responses [2]. Upon activation, the shape of plasmacytoid predendritic cells changes to dendritic cell morphology. In this state, the expression of MHC class II and costimulatory molecules is up-regulated and pDCs are able to prime naïve CD4+ T lymphocytes [3]. Depending on the type of activation and maturation signals, pDCs have the ability to facilitate T helper (Th) 1, Th2, and regulatory T cell development [4–6]. Despite the fact that pDCs and conventional dendritic cells (DCs) may arise from common precursors through overlapping developmental pathways [7,8], pDCs display many features of lymphocytes and in their phenotypic and functional features they are explicitly distinct from conventional DCs. Plasmacytoid DCs lack expression of the myeloid markers such as CD11c, CD13, CD33, and mannose receptors; however, they express several lymphoid markers (CD2, CD5, and CD7), as well as transcripts for pre-T cell receptor a and immunoglobulin λ-like 14.2 [9,10]. Furthermore, expression of granzyme B and Spi-B, a lymphoid-restricted transcription factor, has also been reported in pDCs but not in conventional DCs [11]. Based on their tissue localization and migratory patterns, pDCs also appear different from conventional DCs but similar to lymphocytes [12]. After development in the bone marrow, pDCs are released into the blood circulation, and through high endothelial venules they can migrate from the bloodstream into secondary lymphoid organs and peripheral tissues [13]. Although it is difficult to detect pDCs under steady-state conditions in most peripheral tissues, their number increases dramatically in many tissues during inflammatory responses [14–16].
Several lines of evidence indicate that inflammation is associated with oxidative stress. The mechanisms described for this phenomenon include generation of reactive oxygen species (ROS) by inflammatory cells recruited to the infected and/or damaged tissues and ROS induced by exposure to environmental factors such as ozone, cigarette smoke or pollen grains [17,18]. Elevated levels of ROS can cause cellular injury in various ways; membrane lipids can be attacked leading to the formation of peroxide derivatives, protein side chains can be modified, and even peptide backbones can be broken, and finally, DNA can be damaged resulting in strand breaks or nucleotide modifications. In contrast to these harmful events, low concentrations of ROS are being recognized as essential participants of several signal transduction pathways [19] and ROS have been demonstrated to induce phenotypic and functional maturation of human monocyte-derived DCs [20,21].
It has been recently demonstrated that in addition to detection of infection and induction of adaptive immunity, pDCs have an important function in sensing tissue damage and initiating tissue repair [22]. During infiltration into inflamed tissues and recognition of nucleic acids released by injured cells, pDCs can be exposed to elevated levels of ROS. We hypothesized that these stimuli could affect the cellular responses of pDCs to signals from the surrounding microenvironment. Thus, in this study we have investigated how oxidative stress conditions can change the phenotype of pDCs and modulate their cytokine production and T-cell-polarizing capacity.
Materials and methods
Cell purification
Human pDCs
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll–Paque (GE Healthcare, Uppsala, Sweden) density gradient centrifugation of heparinized leukocyte-enriched buffy coats of healthy donors drawn at the Regional Blood Center of the Hungarian National Blood Transfusion Service (Debrecen, Hungary) in accordance with the written approval of the Director of the National Blood Transfusion Service and the Regional and Institutional Ethics Committee of the University of Debrecen, Medical and Health Science Center (Debrecen, Hungary). Plasmacytoid DCs were purified from PBMCs by negative selection using immunomagnetic cell separation kit (Miltenyi Biotec, Bergish Gladbach, Germany), according to the manufacturer's instructions. After separation on a VarioMACS magnet, the purity of BDCA2+BDCA4+CD123+ pDCs was >98%, as confirmed by flow cytometry.
Human monocytes
CD14+ monocytes were obtained from PBMCs by positive selection using immunomagnetic cell separation with anti-CD14 microbeads (Miltenyi Biotec), according to the manufacturer's instructions. After separation, 96 to 99% of the cells were CD14+ monocytes as measured by flow cytometry.
Human CD3+ pan-T cells
Anti-CD3 microbeads (Miltenyi Biotec) were used for the positive selection of CD3+ T cells from PBMCs, according to the manufacturer's instructions. Freshly isolated CD3+ T cells had a purity of at least 98% based on flow cytometric analysis.
Human naïve CD4+ T cells
Naïve CD4+CD45RA+CD45RO− T cells were isolated from PBMCs by magnetic depletion of CD4+CD45RO+ memory T cells and non-CD4+ T cells with the Naïve CD4+ T Cells Isolation Kit II (Miltenyi Biotec), according to the manufacturer's instructions. The homogeneity of untouched naïve T cells was >97%, as measured by flow cytometry.
Generation of conventional DCs from human CD14+ monocytes
Freshly isolated monocytes were cultured in 24-well tissue culture plates at a density of 2×106 cells/ml in RPMI 1640 medium (Sigma–Aldrich, St. Louis, MO, USA) supplemented with 2 mM l-glutamine (Sigma–Aldrich), 100 U/ml penicillin, 100 ng/ml streptomycin, 10% heat-inactivated fetal calf serum (FCS) (Invitrogen, Carlsbad, CA, USA), and 80 ng/ml granulocyte–macrophage colony-stimulating factor (Gentaur Molecular Products, Brussels, Belgium) and 100 ng/ml IL-4 (Peprotech EC, London, UK). On day 2, the same amounts of granulocyte – macrophage colony-stimulating factor and IL-4 were added to the cell cultures. More than 90% of the cells showed immature DC phenotype (DC-S1GN/CD209+, CD14low) and the percentage of CDla+ DCs varied among individuals (75–90%) on day 5 when they were used for experiments.
Assessment of cell viability
Isolated untouched pDCs and conventional DCs were seeded at a density of 5×105 cells/ml in RPMI 1640 medium containing 2 mM l-glutamine, 100 U/ml penicillin, 100 ng/ml streptomycin, and 10% heat-inactivated FCS (culture medium for pDCs was also supplemented with 50 ng/ml recombinant human IL-3; Peprotech) and treated with increasing concentrations (ranging from 0.01 to 10 µM) of H2O2 (Sigma–Aldrich) for 24 h. In control experiments, cells were pretreated with an antioxidant (30 mM N-acetylcysteine, NAC; Sigma–Aldrich) for 1 h and then cotreated with H2O2. Cell viability was determined by 7-aminoactinomycin-D (7-AAD; 10 µg/ml; Sigma–Aldrich) staining for 15 min immediately before flow cytometric analysis. Fluorescence intensities were measured by a FACSCalibur flow cytometer (BD Biosciences Immunocytometry Systems, Franklin Lakes, NJ, USA) and analysis of data was performed by FlowJo software (TreeStar, Ashland, OR, USA).
Measurement of intracellular ROS levels
Freshly isolated untouched pDCs and conventional DCs were loaded with 50 µM 2′,7′-dihydrodichlorofluorescein diacetate (H2DCFDA; Invitrogen) at 37°C for 20 min. After excess fluorescent dye was removed, the cells were exposed to increasing concentrations of H2O2 for 2 h. Changes in DCF fluorescence intensity were detected on the FL1 (530±15 nm) channel using a BD FACSCalibur flow cytometer. Data were analyzed by FlowJo software.
Consumption of H2O2 in cell culture medium of pDCs and conventional DCs
Freshly isolated untouched pDCs and conventional DCs were seeded at a density of 5 × 105 cells/ml in RPMI 1640 medium containing 10% heat-inactivated FCS, allowed to equilibrate for 30 min, and then treated with 0.01 µM H2O2. Samples were withdrawn every 5 min to assay H2O2 content. To measure H2O2 levels, 50 µl of cell-free supernatant was mixed with 50 µl H2DCFDA (50 µM) and fluorescence intensity was assessed by a Synergy HT reader (Bio-Tek Instruments, Winooski, VT, USA) using a 485/20 excitation filter and a 528/20 emission filter. The level of H2O2 at time 0 was determined by taking a sample immediately after addition of H2O2 to the cells. For control experiments, H2O2 was added to cell-free medium and consumption of H2O2 was assayed as described above. The assay was linear over the concentration range from 20 nM to 156.25 pM (r2>0.99) with a minimum detectable limit of quantitation of 312.5 pM H2O2.
Stimulation of the cells
Freshly isolated pDCs were seeded at 1 × 105 cells/well in 96-well flat-bottom plates in RPMI 1640 medium supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 ng/ml streptomycin, 10% heat-inactivated FCS, and 50 ng/ml recombinant human IL-3. For stimulation, pDCs were treated with H2O2 at a final concentration of 0.01 µM and a TLR7 ligand (imiquimod, R837, 2.5 µg/ml; Invivogen, San Diego, CA, USA), separately and in combination. After treatments, cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 24 h and then supernatants were collected and stored at − 70°C until cytokine measurements.
Phenotypic analysis of pDCs by flow cytometry
Cell surface protein expression was analyzed by staining the cells with FITC-labeled human monoclonal antibodies (mABs) against CD40 (BD Pharmingen, San Diego, CA, USA) and CD80 (BioLegend, Uithoorn, The Netherlands); PE-labeled human mABs against CD86 (R&D Systems, Minneapolis, MN, USA), HLA-DQ (BioLegend), inducible costimulator ligand (1COS-L; eBioscience, Vienna, Austria), OX40-L, and programmed death ligand 1 (PDL-1); and PE-Cy5-labeled anti-CD83 mAb (BD Pharmingen). Isotype-matched control antibodies were obtained from BD Pharmingen. Fluorescence intensities were measured by FACSCalibur flow cytometer and analysis of data was performed by FlowJo software.
Measurement of cytokines produced by pDCs
ELISA kits (BD OptEIA; BD Biosciences, San Diego, CA, USA) were used to quantify IL-8 chemokine, as well as IL-6 and TNF-α cytokines. A human IFN-α ELISA kit was purchased from PBL InterferonSource (Piscataway, NJ, USA). Assays were performed according to the manufacturer's instructions. Absorbance measurements were performed with a Synergy HT reader at 450 nm.
Detection of cytokine secretion by CD3+ pan-T cells by means of enzyme-linked immunospot (ELISPOT)
ELISPOT was performed using human IL-17, IFN-γ, and IL-4 ELI-SPOT kits (eBioscience) according to the manufacturer’s instructions. CD3+ pan-T cells were cocultured with allogeneic pDCs pretreated with various agents as indicated above, at a ratio of 1:10 (pDC/T cell) in 48-well tissue culture plates. After 4 days, the cells were washed twice with phosphate-buffered saline (PBS; PAA Laboratories GmbH, Pasching, Austria) and plated in serum-free test medium (Cellular Technology Ltd. (C.T.L.), Bonn, Germany) at 1 × 105 cells/100 µl/well in polyvinylidene difluoride-coated 96-well Multiscreen Filter Plates (Millipore, Schwalbach, Germany) for 24 or 48 h. Plates were previously prepared by adding capture antibody and by blocking with RPMI 1640 medium supplemented with 10% heat-inactivated FCS. After cultivation, the cells were removed from the plates and detection antibody was added for 2 h. The plates were incubated for 45 min in the presence of avidin–horseradish peroxidase conjugate and finally, BD ELISPOT AEC (3-amino-9-ethylcarbazole) substrate (BD Biosciences) was added and color change was allowed to develop for 5–30 min at room temperature, followed by rinsing with distilled water. The plates were dried completely, and spots were read on an ImmunoScan analyzer using ImmunoSpot 4.0 software (C.T.L.).
Analysis of T cell polarization by intracellular cytokine staining and ELISA
Plasmacytoid DCs treated with H2O2 and TLR7 ligand, separately and in combination, for 24 h were washed once with PBS and then cocultured with autologous CD4+CD45RA+ naïve T cells (1×106 cells/well; pDC:T cell ratio, 1:10) in 48-well tissue culture plates in the presence of 2.5 µg/ml purified anti-human CD3 mAb (clone HIT3A.; BD Pharmingen). Six days after coculture, the cells were restimulated for 7 h with 2.5 µg/ml purified anti-human CD3 mAb, 100 ng/ml phorbol 12-myristate 13-acetate, and 1 µg/ml ionomycin (Sigma–Aldrich), in the presence of GolgiStop (BD Biosciences) protein transport inhibitor for the final 5 h. After restimulation, the cells were fixed and permeabilized with eBioscience Fixation/Per-meabilization buffer, according to the manufacturer’s instructions. The T cells were further incubated with FITC- and PE-conjugated mouse anti-lFN-γ/lL-4 mABs (clones 25723.11, 3010.211; BD Biosciences) and respective isotype controls from the same sources. Flow cytometry was performed using a FACSCalibur flow cytometer and data were analyzed by FlowJo software. From the supernatants of the cocultures, the levels of IL-10 (BD OptEIA; BD Biosciences) and IL-17 (eBioscience) cytokines secreted by T lymphocytes were measured by ELISA.
Statistical analysis
Data from the various treatment groups were analyzed by Student's paired t test or ANOVA, followed by Bonferroni's post hoc test for least-significant differences. Data analysis was performed with SPSS version 12.0 for Windows (SPSS, Inc., Chicago, IL, USA). Differences were considered statistically significant at P<0.05.
Results
Sensitivity of pDCs to oxidative stress
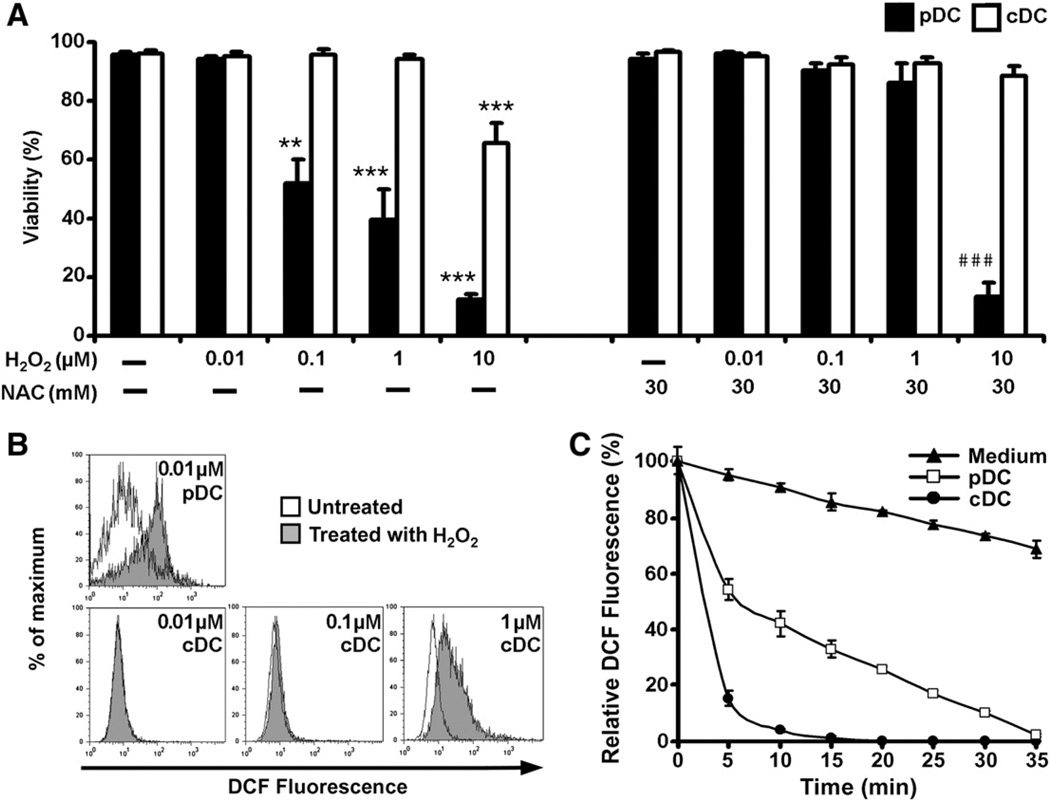
To investigate the sensitivity of pDCs and conventional DCs to oxidative stress, freshly isolated pDCs and monocyte-derived DCs were treated with increasing concentrations of H2O2 for 24 h and their viability was assessed by 7-AAD staining. Exposure to 0.1, 1, and 10 µM H2O2 resulted in a 48, 60, and 88% reduction in the viability of the pDCs, respectively, whereas the viability of conventional DCs significantly decreased only at a concentration of 10 µM (Fig. 1A). The LD50 value for H2O2 was 0.7 µM in pDCs, compared with 130 µM in conventional DCs. Pretreatment with an antioxidant (NAC) before the addition of H2O2 almost completely protected both pDCs and conventional DCs from H2O2-induced cell death, indicating that reductions in cell viability were mediated by ROS and not by other factors (Fig. 1A). It is worth noting that at the highest H2O2 concentration (10 µM), despite the presence of NAC, an 87% decrease in pDC viability was detected (Fig. 1A).
Fig. 1.
Sensitivity of pDCs and conventional DCs to oxidative stress. (A) Effect of exposure to H2O2 on the viability of pDCs and conventional DCs. Cells were treated with increasing concentrations of H2O2 for 24 h. In control experiments, cells were pretreated with an antioxidant, N-acetylcysteine, for 1 h and then cotreated with H2O2. After treatments, cells were stained with 7-AAD and the proportion of 7-AAD-negative (living) cells was assessed by flow cytometry. Percentages of viable cells are displayed. Data are presented as means ± SE of three individual experiments. **P<0.01, ***P<0.001 vs untreated control cells, ###P<0.001 vs cells pretreated with N-acetylcysteine. (B) Exposure to low-dose H2O2 (0.01 µM) increases intracellular ROS levels in pDCs, but not in conventional DCs. Cells were loaded with redox-sensitive H2DCFDA and treated with H2O2 for 2 h. Changes in intracellular DCF fluorescence were determined by flow cytometry. Results are representative of three independent experiments. (C) Consumption of H2O2 in cell culture medium of pDCs and conventional DCs. Cells were treated with 0.01 µM H2O2 and samples were withdrawn from the supernatant every 5 min to assay H2O2 content. To measure H2O2 levels, cell-free supernatant samples were mixed with H2DCFDA and fluorescence intensity was assessed by fluorimetry. Data are presented as means ± SE of three individual experiments. NAC, N-acetylcysteine; cDC, conventional DCs.
To study the effects of a low concentration of H2O2 on intracellular ROS levels, both pDCs and conventional DCs were loaded with redox-sensitive H2DCFDA and exposed to 0.01 µM H2O2. Flow cytometric analysis revealed that even this low concentration of H2O2 was able to induce a 3.7 ± 2.3-fold increase in median DCF fluorescence in pDCs compared to untreated control cells (Fig. 1B, top). In contrast, the same concentration of H2O2 did not trigger any changes in DCF fluorescence in conventional DCs and exposure of these cells to a 100-times higher H2O2 concentration (1 µM) was needed to elicit a notable increase in intracellular DCF signals (Fig. 1B, bottom).
Next, depletion of 0.01 µM H2O2 by cell-free medium and culture medium of conventional DCs and pDCs was analyzed by means of fluorimetry. In the cell-free medium, 31.4 ± 3.1% of H2O2 was eliminated by 35 min after H2O2 addition (Fig. 1C). In the culture medium of conventional DCs, rapid depletion of H2O2 was observed. By 20 min after H2O2 addition to the cell culture, H2O2 levels fell below the detection limit (Fig. 1C). However, in the medium of pDCs, H2O2 was degraded at a lower rate; its concentration decreased nearly to the detection limit by 35 min after its administration. These observations indicate that in comparison with conventional DCs, pDCs are more sensitive to cell death and oxidative stress induced by exogenous H2O2.
Phenotypic characterization of H2O2-treated pDCs
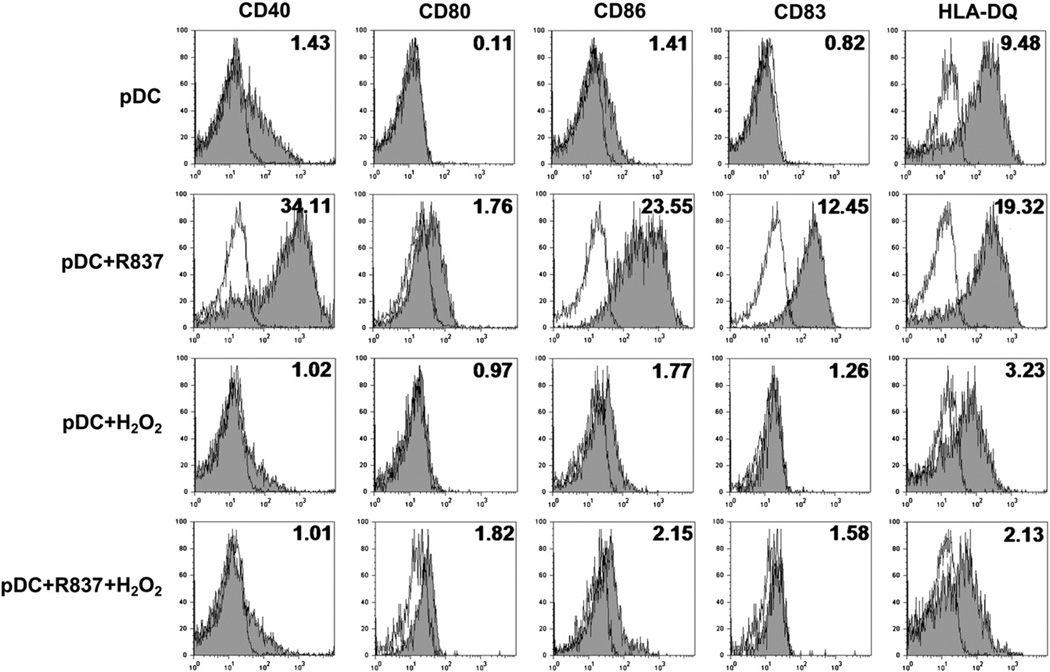
Based on the results described above, in all further experiments of our study 0.01 µM H2O2 was applied for treatments; this caused less than a 5% decline in the viability of pDCs. To assess the phenotypic changes in pDCs induced by exposure to a low concentration of exogenous H2O2, the expression of costimulatory molecules (CD40, CD80, and CD86); CD83, a specific maturation markeR; and the antigen-presenting molecule HLA-DQ was analyzed by means of flow cytometry. Treatment of pDCs with H2O2 resulted in minor changes in the expression of CD40, CD80, CD86, and CD83; however, it markedly decreased the expression of HLA-DQ (Fig. 2). In parallel experiments, pDCs were treated with R837, a synthetic TLR7 ligand, alone and in combination with H2O2. Stimulation of pDCs with R837 alone triggered remarkable increases in the expression of all cell-surface markers analyzed in our experiment (Fig. 2). When R837 was added together with H2O2, the expression of costimulatory molecules CD83 and HLA-DQ was close to that induced by H2O2 alone (Fig. 2). To exclude the possibility that oxidation of R837 by H2O2, leading to altered binding of R837 to TLR7, stands behind this observed phenomenon, various experimental settings were tested. In the next series of experiments, cells were treated with R837 and H2O2 spaced 30 min apart; this was performed with R837 exposure both preceding and after H2O2 treatment. We found that the levels of cell-surface molecules were identical to those measured after simultaneous administration of these activators (data not shown), suggesting that low-concentration H2O2 treatment does not trigger phenotypic maturation of pDCs; moreover, low-dose H2O2 down-regulates the maturation and activation program induced by TLR7-mediated signals, whereas it does not influence the TLR7–ligand interaction.
Fig. 2.
Phenotypic characteristics of pDCs exposed to H2O2. Freshly isolated pDCs were treated with 0.01 µM H2O2 and R837 (a synthetic TLR7 ligand), separately and in combination, for 24 h. Changes in the expression of cell-surface markers were analyzed using flow cytometry. Unfilled histograms indicate isotype controls and numbers indicate the relative fluorescence intensity, which is validated by the respective isotype-matched control for each mAb. Results are representative of four independent experiments.
Chemokine and cytokine production of pDCs in response to H2O2 treatment
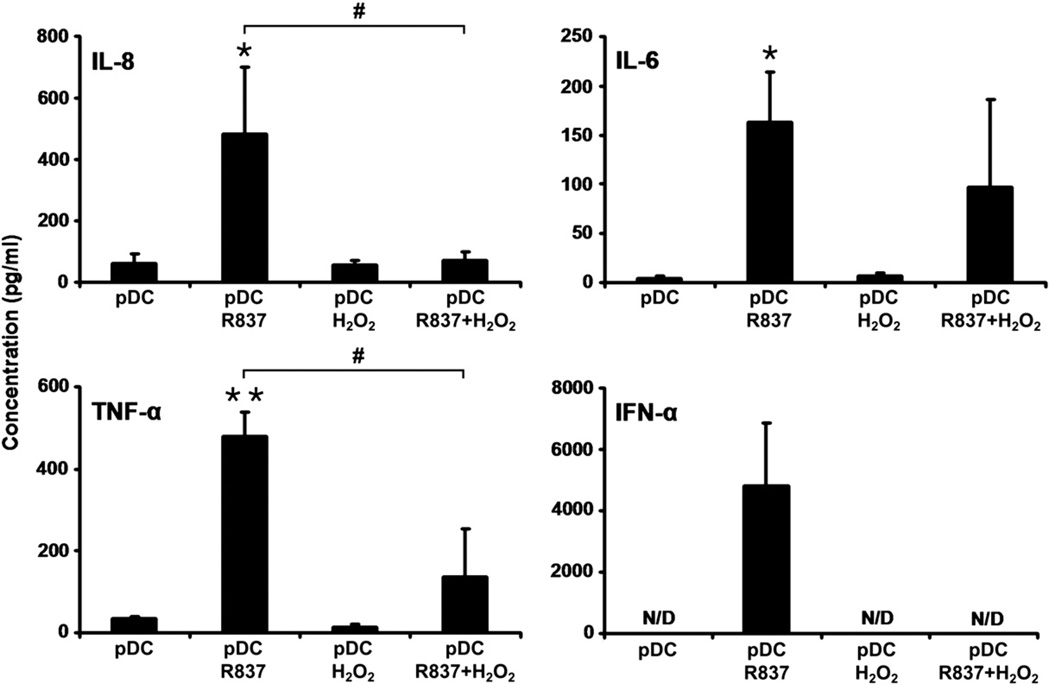
To assess the potential effects of low-dose H2O2 treatment on chemokine and cytokine release by pDCs, levels of IL-8, IL-6, TNF-α, and IFN-α were measured in the cell culture supernatant by ELISA. Twenty-four hours after administration of H2O2, the levels of IL-8, IL-6, and TNF-α produced by treated cells were similar to levels generated by untreated cells (Fig. 3). Neither pDCs exposed to H2O2 nor untreated cells released IFN-α into the culture supernatant (Fig. 3). However, treatment of pDCs with R837 induced significant elevations in the levels of IL-8 and proinflammatory cytokines and triggered the production of IFN-α (Fig. 3). Compared to treatment with R837 alone, simultaneous administration of R837 and H2O2 notably decreased IL-6 production, significantly lowered both IL-8 and TNF-α levels, and completely eliminated the release of IFN-α (Fig. 3). These observations indicate that exposure to low-dose H2O2 does not induce increased chemokine or cytokine release from pDCs; however, low-dose H2O2 markedly reduces the production of these mediators triggered by TLR7 activation.
Fig. 3.
Chemokine and cytokine production of H2O2-treated pDCs. H2O2 (0.01 µM) alone, R837 alone, or both together were added to cultured primary pDCs and the levels of IL-8, IL-6, TNF-α, and IFN-α were measured by means of ELISA in culture supernatants at 24 h after treatment Data are presented as means ±SE of three individual experiments. *P<0.05, **P<0.01 vs untreated control cells, #P<0.05 vs cells treated with R837 only. N/D, not detectable.
Effects of H2O2 treatment on the allostimulatory capacity of pDCs
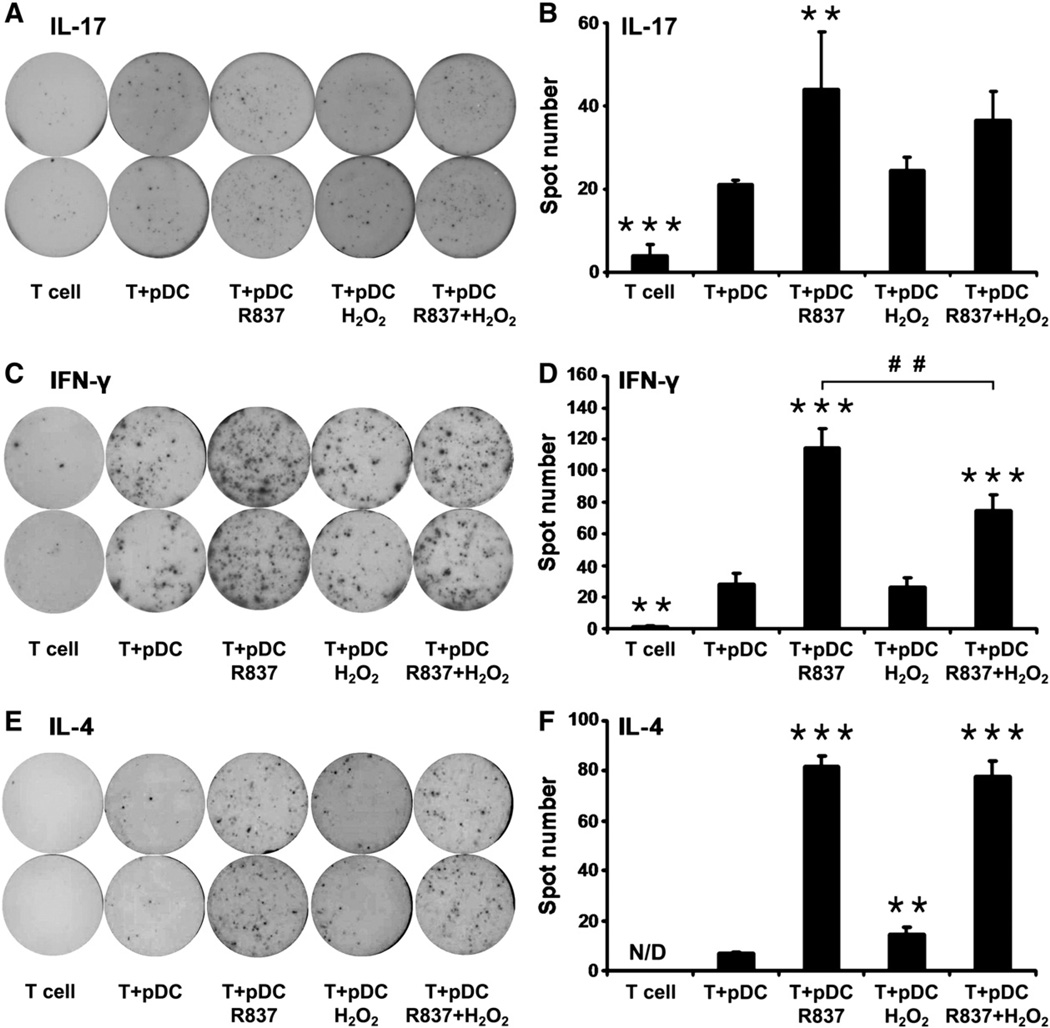
Next, to investigate their allostimulatory capacity, freshly isolated pDCs were exposed to H2O2 and R837, separately and in combination, for 24 h and cocultured with allogeneic CD3+ pan-T cells. Activation of CD3+ pan-T cells was assessed by IL-17, IFN-γ, and IL-4 ELISPOT assays. Addition of H2O2 to pDCs did not change their capacity to activate allogeneic IL-17- or IFN-γ-producing T cells (Figs. 4A – D). However, coculture of allogeneic CD3+ pan-T cells with pDCs treated with R837 alone significantly increased the frequency of both IL-17- and IFN-γ-producing T cells (Figs. 4A – D). Combined treatment with H2O2 and R837 caused only a slight decrease in the frequency of IL-17-secreting T cells (Figs. 4A and B); however, compared to only R837 treatment, combined treatment significantly impaired the ability of pDCs to induce IFN-γ secretion by pan-T cells (Figs. 4C and D). On the other hand, exposure of pDCs to H2O2 significantly increased their ability to stimulate allogeneic IL-4-secreting T cells (Figs. 4E and F). Treatment with only R837 also significantly enhanced the capacity of pDCs to induce activation of allogeneic IL-4-producing T cells (Figs. 4E and F). Compared to R837 treatment alone, simultaneous application of H2O2 and R837 did not alter the ability of pDCs to stimulate allogeneic T cells to produce IL-4 (Figs. 4E and F).
Fig. 4.
Effects of H2O2 treatment on the ability of pDCs to activate allogeneic T cells. Purified pDCs were treated with 0.01 µM H2O2 and R83 7, separately and in combination, for 24 h and then cocultured with allogeneic CD3+ pan-T cells. After 4 days of cocultivation, activation of CD3+ pan-T cells was assessed by IL-17, IFN-γ, and IL-4 ELISPOT assays. Spots indicate individual (A) IL-17-, (C) IFN-γ-, and (E) IL-4-producing T cells in representative assays from three independent experiments. (B, D, and F) Results are presented as means ±SE of three individual experiments. **P<0.01, ***P <0.001 vs T cells cocultured with untreated pDCs. ##P <0.01 vs T cells cocultured with R837-exposed pDCs. N/D, not detectable.
T-cell-polarizing capacity of pDCs exposed to H2O2
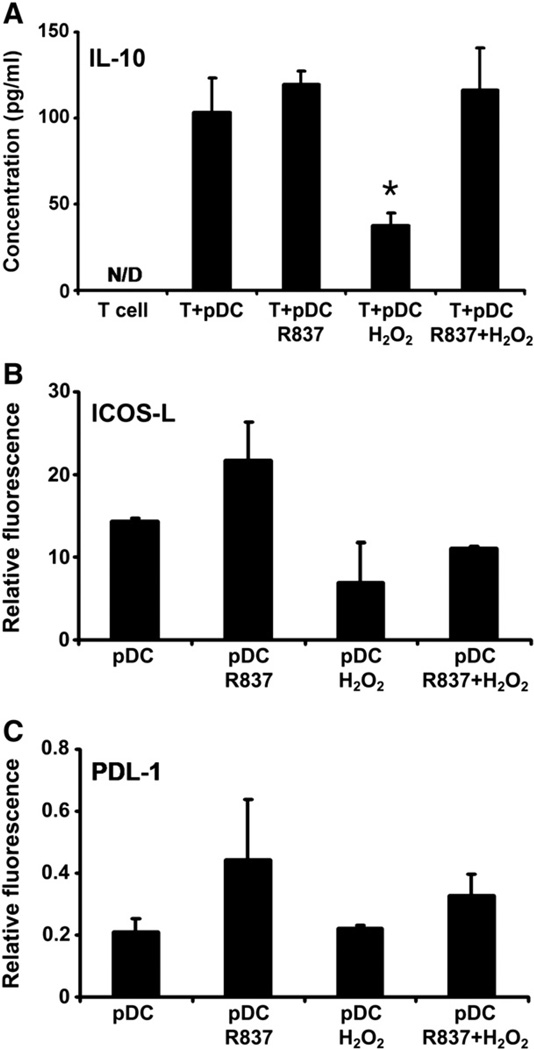
To assess the impact of H2O2 treatment on T cell polarization, pDCs were stimulated with H2O2 and R837, separately and in combination, for 24 h and then washed and cocultured for up to 6 days with naïve autologous CD4+CD45RA+ T cells. To investigate whether coculture with stimulated pDCs drives the differentiation of naïve autologous T cells toward IL-10-producing T lymphocytes, the amount of IL-10 in the culture supernatants was measured. Exposure of pDCs to H2O2 before cocultivation with naïve autologous T cells significantly lowered IL-10 production by T cells compared to those cocultured with untreated pDCs, pDCs treated with only R837, and pDCs stimulated with H2O2 and R837 in combination (Fig. 5A). To identify molecular mechanisms behind this phenomenon, the expression of ICOS-L and PDL-1 on pDCs was determined, because these molecules promote the formation of regulatory T cells [23,24], Treatment of pDCs with H2O2 markedly, but not significantly, lessened the expression of ICOS-L, but did not modify the expression of PDL-1 (Figs. 5B and C). Simultaneous administration of H2O2 lowered the potential of R837 to induce an increase in the expression of both ICOS-L and PDL-1 (Figs. 5B and C).
Fig. 5.
Effect of H2O2 treatment on the ability of pDCs to drive the differentiation of naïve autologous T lymphocytes toward IL-10-producing T cells. (A) Freshly isolated pDCs were treated with 0.01 µM H2O2 alone, R837 alone, or both together for 24 h and then washed and cocultured for up to 6 days with naïve autologous CD4+CD45RA+ T cells. Amounts of secreted IL-10 were determined in the culture supernatants by means of ELISA. (B and C) Expression of ICOS-L and PDL-1, promoters of the formation of regulatory T cells, on the surface of H2O2-exposed pDCs. Freshly isolated pDCs were stimulated with H2O2 and R837, separately and in combination, for 24 h and then stained for ICOS-L or PDL-1. Changes in the expression of tolerogenic cell-surface markers were analyzed using flow cytometry. Results are presented as means ± SE of three individual experiments. *P<0.05 vs T cells cocultured with untreated pDCs. N/D, not detectable.
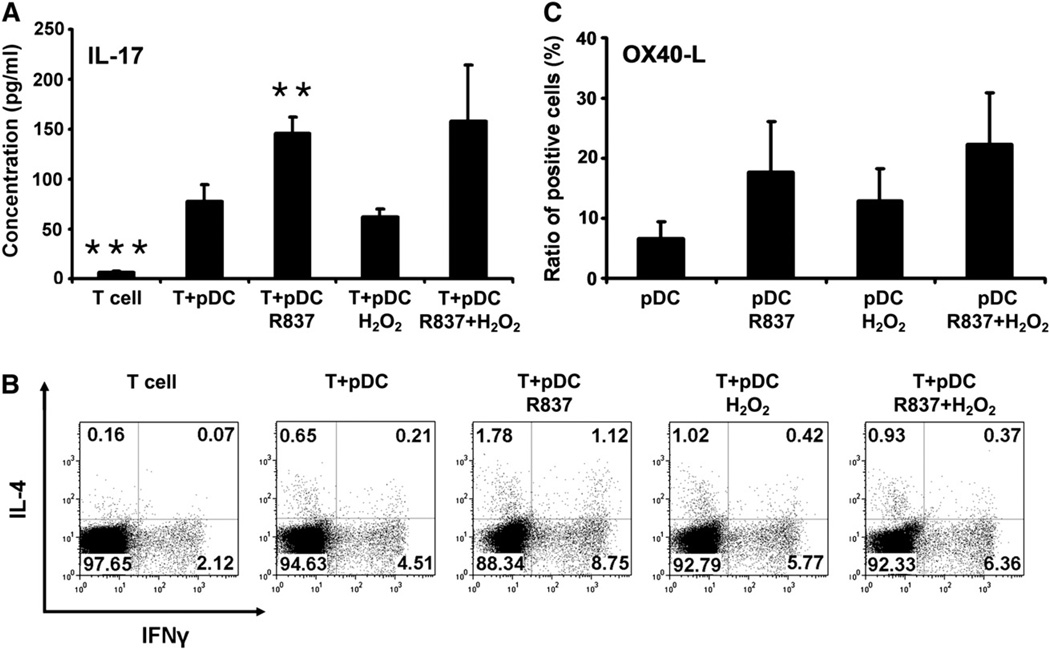
To investigate the development of Th17 cells in 6-day cocultures, levels of secreted IL-17 in the culture supernatants were measured. T cells primed with untreated pDCs secreted significantly higher levels of IL-17 than T cells cultured alone (Fig. 6A). Priming of T cells with H2O2-treated pDCs did not change their potential to produce IL-17 compared to T cells primed with untreated pDCs (Fig. 6A). In contrast, priming of T cells with pDCs exposed to R837, either alone or in combination with H2O2, led to a marked increase in IL-17 production (Fig. 6A). To define the frequency of Th1 and Th2 cells in the cocultures, intracellular staining for IFN-γ and IL-4 was performed. In T cell populations primed with untreated pDCs, 3.48 ± 1.03 and 0.59 ± 0.06% of the cells were positive for IFN-γ and IL-4 staining, respectively (Fig. 6B). Treatment of pDCs with H2O2 before coculturing with naïve autologous T cells induced a 1.16 ± 0.29-fold increase in the frequency of IFN-γ-positive T cells and a 1.61 ± 0.05-fold increase in the frequency of IL-4-positive T cells (Fig. 6B). When H2O2 and R837 were applied in combination to stimulate pDCs, a similar enhancement in the frequency of IFN-γ- and IL-4-positive T cells could be detected compared to priming with untreated pDCs (1.35±0.12- vs 1.27± 0.20-fold increase; Fig. 6B). Our data indicate that compared to untreated pDCs, H2O2-exposed pDCs provide stronger signals for Th2 than for Th1 stimulation upon both allogeneic and autologous activation (Figs. 4 and 6B) and thus the expression of OX40-L, which selectively regulates Th2 cell development [5], was assessed on pDCs. Treatment with H2O2 triggered an almost 2-fold (1.93 ±0.04) increase in the frequency of OX40-L-positive pDCs (Fig. 6C). Furthermore, simultaneous administration of H2O2 and R837 resulted in higher frequency of OX40-L-positive pDCs than R837 treatment alone (Fig. 6C).
Fig. 6.
Th-polarizing ability of pDCs exposed to H2O2. Freshly isolated pDCs were treated with 0.01 µM H2O2 and R837, separately and in combination for 24 h, and then washed and cocultured for up to 6 days with naïve autologous CD4+CD45RA+ T cells. (A) Th17-polarizing ability of H2O2-treated pDCs. To assess the development of Th17 cells in the cocultures, levels of secreted IL-17 were determined in the culture supernatants by ELISA. (B) Th1- and Th2-polarizing ability of H2O2-exposed pDCs. After 6 days of cocultivation, T cells were restimulated with anti-CD3 mAb, phorbol 12-myristate 13-acetate, and ionomycin, and intracellular IFN-γ and IL-4 staining was performed. The percentages of IFN-γ- and IL4-positive cells were analyzed by means of flow cytometry. The dot-plot diagrams represent the results from one of four individual experiments. (C) Expression of OX40-L, a regulator of Th2 cell development, on the surface of pDCs treated with H2O2. Freshly isolated pDCs were stimulated with H2O2 only, R837 only, or both together for 24 h and then stained for OX40-L. Changes in the frequency of OX40-L-positive cells were analyzed using flow cytometry. Results are presented as means ± SE of three individual experiments. **P<0.01, ***P<0.001 vs T cells cocultured with untreated pDCs.
Discussion
Previous studies have provided evidence that pDCs accumulate in inflamed peripheral tissues, suggesting that pDCs might be involved in the establishment and maintenance of inflammatory processes [14–16]. Furthermore, pDCs have also been found in many solid tumors, including head and neck cancer, breast cancer, ovarian cancer, lung cancer, and skin tumors (reviewed in [25]). In the target tissue, pDCs can be exposed to ROS induced by environmental factors or produced by granulocytes and macrophages. In this study, we have investigated the effects of H2O2, a precursor of the highly reactive peroxyl radical, on the phenotypic characteristics and functions of pDCs.
First, we have observed that pDCs are more sensitive to cell death and oxidative stress induced by exogenous H2O2 than monocyte-derived DCs. These findings are in line with previous observations that when separated fractions of human PBMCs were exposed to various doses of H2O2, there was significant DNA damage in cells of lymphoid origin, such as CD4+ and CD8+ T cells, NK cells, and B cells, whereas monocytes had low sensitivity to H2O2 [26]. The ability of conventional DCs to survive in a highly oxidant environment is probably due to their elevated peroxiredoxin production [27]. Our observation that monocyte-derived DCs are more efficient at degrading exogenous H2O2 than pDCs can be explained by high catalase and surface thiol expression by the former cell type [28]. In control experiments we observed that at a higher H2O2 concentration (10 µM), despite the presence of excess NAC, the viability of pDCs significantly decreased. It is widely accepted that NAC can scavenge various forms of oxygen radicals, and it also serves as a precursor for the biosynthesis of glutathione. However, there are references in the literature implicating NAC and other thiols in the oxidative damage of biological systems both in vitro [29–31] and in vivo [32,33], depending on the experimental conditions, such as the presence or absence of transition metal ions. It was demonstrated that NAC in millimolar concentrations increased the extent of H2O2-induced oxidative lipid and DNA damage when copper or iron ions were involved. It is proposed that NAC imparts a pro-oxidant function by means of the reduction of transition metal ions to catalytic states capable of reacting with H2O2 to generate hydroxyl radicals. The released hydroxyl radicals attack macromolecules before they are scavenged by NAC [29–31]. Cell culture medium contains micromolar concentrations of both copper [34] and iron [35] ions and thus creates feasible conditions for NAC to enhance metal ion- or H2O2-mediated oxidative actions. Our results indicate that 10 µM H2O2 and the pro-oxidant effect of NAC are able to overwhelm the antioxidant defense mechanisms of pDCs but not those of conventional DCs.
Furthermore, H2O2 seems to be an activating signal for conventional DCs, as it induces an up-regulation of several DC surface markers involved in the activation of naïve T cells, including HLA-DQ and HLA-DR and the costimulatory molecules CD40 and CD86 [20]. Moreover, it has also been observed that H2O2-treated monocyte-derived DCs acquire an enhanced ability to promote autologous T cell proliferation compared to untreated DCs [20]. In contrast, pDCs displaying several lymphoid features responded to H2O2 treatment in radically different ways. Exposure of pDCs to H2O2 did not lead to significantly increased expression of either antigen-presenting or costimulatory molecules. Additionally, H2O2 treatment blocked the activation of pDCs induced by a TLR7 ligand. It is worth noting that in addition to the different phenotypic and functional characteristics of the two major DC subsets, another factor may also be involved in the two distinct observed phenomena, namely, conventional DCs were treated with a high (300 µM) concentration of H2O2 [20], whereas pDCs were exposed to 0.01 µM H2O2 in our experiments.
Investigating chemokine and cytokine release by H2O2-treated pDCs, we have found that exposure to low-dose H2O2 did not increase the production of IL-8, IL-6, TNF-α, or IFN-α. In addition, treatment with H2O2 notably inhibited the production of these mediators triggered by a TLR7-mediated stimulus. It is known that the expression of IL-8, IL-6, and TNF-α is regulated by NF-κB [36]. It has been shown that cytokine production of activated/memory T cells is reduced after exposure to micromolar levels of H2O2 and the impaired cytokine expression induced by H2O2 correlates with a block in NF-κB activation [37]. Based on these observations, we propose that blockage and/or the disturbed balance of the NF-κB- and IRF-mediated activation pathways might be the underlying mechanism for the impaired IL-8, IL-6, and TNF-α production in pDCs treated with a combination of TLR7 ligand and H2O2. In a murine model, it has been found that aged pDCs exhibit an impaired ability to produce IFN-α, because of a decreased up-regulation of IRF7, a key adaptor molecule in the type I IFN signaling pathway during TLR9 activation [38]. Other adaptor molecules upstream of IRF7 were not altered by aging under experimental circumstances. It has also been noted that aged pDCs have increased intracellular levels of ROS both at rest and during TLR9 activation compared to young pDCs. Importantly, reduction of age-induced oxidative stress in pDCs led to augmented IFN-α production upon TLR9 activation [38]. As TLR7 and TLR9 appear to trigger very similar signaling pathways with similar functional outcomes, we hypothesize that not only in murine but also in human pDCs the impaired up-regulation of IRF7 is the pivotal event in blocking TLR7/9-induced IFN-α production under oxidative stress conditions. However, further studies are needed to prove this hypothesis.
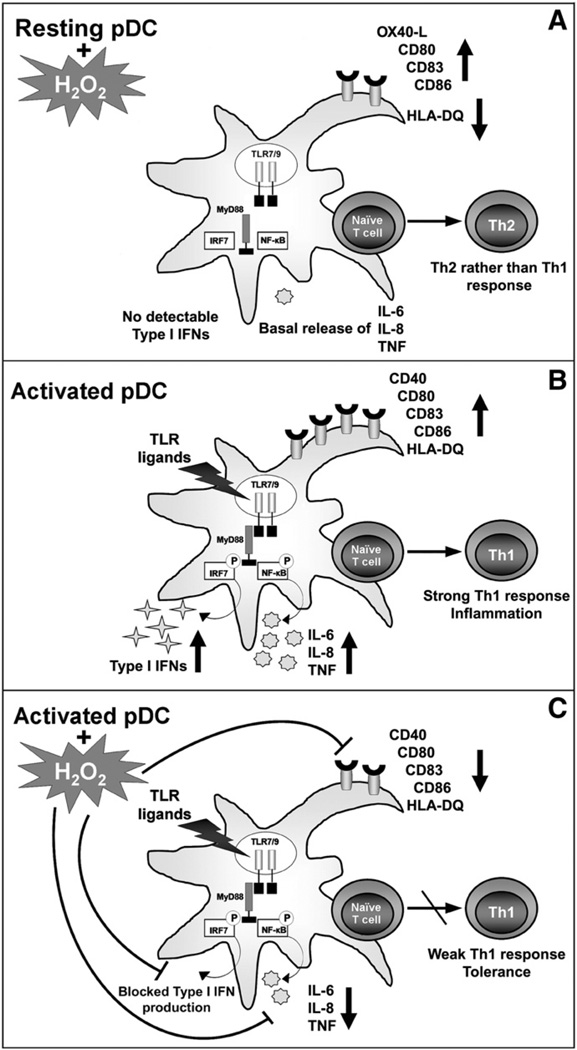
Although there is evidence for distinct and complementary roles of conventional DCs and pDCs in regulating T-cell-mediated immunity, both DC subsets show wide functional plasticity in determining T cell responses. Conventional DCs at an immature stage have the ability to prime naïve T cells toward IL-10-producing T lymphocytes, whereas pDCs at a mature stage seem to have an intrinsic capacity to perform this priming activity [24]. Maturing pDCs but not conventional DCs express high levels of ICOS-L, a key molecule in driving the generation of IL-10-producing T cells [24]. Our finding that treatment of pDCs with H2O2 markedly lessens the expression of ICOS-L, leading to impaired capacity of pDCs to induce IL-10-producing T cells, further confirms the inhibitory effect of H2O2 on pDCs. A recent study has demonstrated that pDCs are able to promote Th17 differentiation under certain circumstances [39]. We have also observed that priming of naïve T cells with TLR7-stimulated pDCs triggers a marked increase in IL-17 production and that the presence of H2O2 during TLR7 ligation does not influence this phenomenon. Interestingly, although exposure of pDCs to H2O2 alone significantly decreases their ability to promote differentiation of IL-10-producing T cells, it does not affect the Th17-priming capacity of pDCs. Regarding Th1/Th2 polarization, H2O2-exposed pDCs provide stronger signals for Th2 than for Th1 stimulation during both allogeneic and autologous activation. Elevated expression of OX40-L on pDCs may be responsible for this phenomenon, as we observed that treatment with H2O2 almost doubled the frequency of OX40-L-positive pDCs and it has been previously demonstrated that pDCs prime Th2 cells through OX40-L-dependent mechanisms [5]. Importantly, when pDCs were stimulated with R837 in the presence of H2O2, decreased phenotypic activation, lower chemokine and cytokine release, as well as impaired alio- and autostimulatory capacities of pDCs could be detected, suggesting that during in vivo circumstances pDCs exposed to oxidative stress have an anti-inflammatory or a tolerogenic role in regulating adaptive immune responses (Fig. 7).
Fig. 7.
An overview of the modulatory effects of low-dose H2O2 on the functions of pDCs. (A) Exposure of resting pDCs to 0.01 µM H2O2 results in a slight increase in the expression of costimulatory molecules (CD80 and CD86); CD83, a specific maturation markeR.; and OX40-L, a selective regulator of Th2 cell development; however, it markedly decreases the expression of the antigen-presenting molecule HLA-DQ. Low-dose H2O2 does not trigger production of type I IFNs or modify the basal proinflammatory cytokine and chemokine release by pDCs. Furthermore, H2O2-exposed pDCs without TLR-mediated activation provide stronger signals for Th2 than for Th1 stimulation. (B) Human pDCs selectively express both TLR7 and TLR9 localized in the endoplasmic reticulum. TLR7 and TLR9 recognize RNA and DNA fragments of viruses and bacteria, respectively, and initiate a signal transduction pathway through a key adaptor molecule, MyD88. MyD88-mediated signals activate IRF7 and NF-κB, leading to secretion of type I IFNs, proinflammatory cytokines, and chemokines. Ligation of TLR receptors also leads to high expression levels of costimulatory molecules, as well as CD83 and HLA-DQ. In this activation state pDCs are able to induce a strong Th1 response upon interaction with naïve T cells. (C) In contrast, when pDCs are stimulated with TLR ligands in the presence of low-dose H2O2, decreased cytokine and chemokine release as well as lowered expression of cell-surface molecules can be detected, indicating that pDCs exposed to ROS in vivo may have an anti-inflammatory or a tolerogenic phenotype.
Human pDCs represent only 0.1–0.6% of PBMCs [40], and therefore limited numbers of these cells can be isolated from blood of healthy volunteers for in vitro experiments. This leads to a significant technical restriction of pDC studies. Indeed, much less information on the functions of pDCs in human immune responses is available compared with the more abundant conventional DCs. Our in vitro finding that pDCs are highly sensitive to oxidative stress raises the question how pDCs can be long-lived in vivo if they cannot withstand elevated levels of ROS, which occur during infection and inflammation. The main difficulty in interpreting our results is the lack of a proper method to measure the exact amount of reactive radicals the pDCs have to face in situ [41]. Another issue that should be considered is that although infection and inflammation are characterized by increased production of ROS, levels of enzymatic and nonenzymatic antioxidants are also elevated in affected tissues to balance the harmful effects of oxidation products [42–44]. Furthermore, inflammation enhances vascular permeability, allowing plasma antioxidant substances to move into inflamed tissues [45–48]. Thus, in inflamed tissues pDCs are exposed to a net effect of both pro-oxidant and antioxidant factors. The fact that pDCs can be identified in inflamed tissues proves that these cells can survive oxidative stress in vivo. However, our findings indicate that pDCs may have lower intracellular antioxidant capacity than conventional DCs. Additionally, it seems that pDCs release (or express on their surface) lower amounts of antioxidant factors than conventional DCs do. Although the molecular mechanisms behind our findings need further investigations, our in vitro observations may explain why pDCs respond to oxidative stress in vivo in radically different ways compared to conventional DCs.
Indeed, it has been reported that in a murine model of allergic airway inflammation, which is associated with oxidative stress [18], pDCs perform anti-inflammatory activities irrespective of their maturation state [23]. In solid tumors, in which tumor-derived macrophages [49] and granulocytes [50] secrete H2O2, pDCs are present in a nonactivated state and are associated with the development of an immunosuppressive microenvironment [25]. Furthermore, oxidative stress is implicated as a pathogenic factor in a number of viral infections [51–53]. A lack of dietary antioxidants leading to nutritionally induced oxidative stress, or exposure to air pollutants, including diesel exhaust and cigarette smoke, resulting in oxidative stress in the airways, is thought to be associated with an increase in severity from and susceptibility to viral infections [54,55]. The iron-catalyzed Haber–Weiss reaction generates hydroxyl radicals, which are by far the most reactive oxygen radicals, thus iron-overload-mediated oxidative stress may also contribute to viral pathogenicity [53,56]. Although oxidative stress may have profound effects on several antiviral mechanisms, including effector functions of activated Th1 and Th2 lymphocytes [57], and also on pathogens [54], our findings raise the possibility that impaired pDC functions are responsible, at least in part, for exacerbation of symptoms in viral infections associated with oxidative stress. In accordance with our observations, a recent study has demonstrated that cigarette smoke, which is known to induce oxidative stress, suppresses TLR7-mediated responses to virus infection in pDCs [58].
In conclusion, here we provide evidence that human pDCs respond to oxidative stress in a manner similar to that of cells of lymphoid origin. The inhibitory effects of H2O2 on TLR-induced phenotypic activation, cytokine production, and the T-cell-polarizing capacity of pDCs may be involved in the development and maintenance of their anti-inflammatory or tolerogenic properties observed in several pathologic conditions.
Acknowledgments
This work was supported by the Hungarian Scientific Research Fund (K-73347 to A.B. and NK-72937 to E.R.), the U.S. National Institute of Environmental Health Science (RO1-ES018948 to IB.), the U.S. National Institute of Allergic and Infectious Diseases (AI062885-01 to IB.), and the TAMOP 4.2.1/B-09/1/KONV-2010-0007 project (to A.B. and E.R.). The project is cofinanced by the European Union and the European Social Fund.
Abbreviations
- 7-AAD
7-aminoactinomycin-D
- DC
dendritic cell
- ELISPOT
enzyme-linked immunospot
- FCS
fetal calf serum
- H2DCFDA
2′,7′-dihydrodichlorofluorescein diacetate
- ICOS-L
inducible costimulator ligand
- IFN
interferon
- mAb
monoclonal antibody
- NAC
N-acetylcysteine, pDC, plasmacytoid dendritic cell
- PBMC
peripheral blood mononuclear cell
- PDL-1
programmed death ligand 1
- ROS
reactive oxygen species
- Th
helper T cell
- TLR
Toll-like receptor
References
- 1.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent Th1 polarization. Nat. Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 5.Ito T, Amakawa R, Inaba M, Hori T, Ota M, Nakamura K, Takebayashi M, Miyaji M, Yoshimura T, Inaba K, Fukuhara S. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J. Immunol. 2004;172:4253–4259. doi: 10.4049/jimmunol.172.7.4253. [DOI] [PubMed] [Google Scholar]
- 6.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 7.Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O’Keeffe M, Bahlo M, Papenfuss A, Kwak JY, Wu L, Shortman K. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 8.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M−CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 9.Spits H, Couwenberg F, Bakker AQ, Weijer K, Uittenbogaart CH. Id2 and Id3 inhibit development of CD34(+) stem cells into predendritic cell (pre-DC)2 but not into pre-DC1: evidence for a lymphoid origin of pre-DC2. J. Exp. Med. 2000;192:1775–1784. doi: 10.1084/jem.192.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendriss-Vermare N, Barthelemy C, Durand I, Bruand C, Dezutter-Dambuyant C, Moulian N, Berrih-Aknin S, Caux C, Trinchieri G, Briere F. Human thymus contains IFN-alpha-producing CD11 c(−), myeloid CD11 c(+), and mature interdig-itating dendritic cells. J. Clin. Invest. 2001;107:835–844. doi: 10.1172/JCI11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rissoan MC, Duhen T, Bridon JM, Bendriss-Vermare N, Peronne C, de Saint Vis B, Briere F, Bates EE. Subtractive hybridization reveals the expression of immunoglobulin-like transcript 7, Eph-B1, granzyme B, and 3 novel transcripts in human plasmacytoid dendritic cells. Blood. 2002;100:3295–3303. doi: 10.1182/blood-2002-02-0638. [DOI] [PubMed] [Google Scholar]
- 12.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 13.Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, Narumi S, Morikawa S, Ezaki T, Lu B, Gerard C, Ishikawa S, Matsushima K. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 2004;16:915–928. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- 14.Jahnsen FL, Lund-Johansen F, Dunne JF, Farkas L, Haye R, Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 2000;165:4062–4068. doi: 10.4049/jimmunol.165.7.4062. [DOI] [PubMed] [Google Scholar]
- 15.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerlini G, Mariotti G, Bianchi B, Pimpinelli N. Massive recruitment of type I interferon producing plasmacytoid dendritic cells in varicella skin lesions. J. Invest. Dermatol. 2006;126:507–509. doi: 10.1038/sj.jid.5700052. [DOI] [PubMed] [Google Scholar]
- 17.Riedl MA, Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr. Opin. Allergy Clin. Immunol. 2008;8:49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- 18.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 20.Rutault K, Alderman C, Chain BM, Katz DR. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic. Biol. Med. 1999;26:232–238. doi: 10.1016/s0891-5849(98)00194-4. [DOI] [PubMed] [Google Scholar]
- 21.Csillag A, Boldogh I, Pazmandi K, Magyarics Z, Gogolak P, Sur S, Rajnavolgyi E, Bacsi A. Pollen-induced oxidative stress influences both innate and adaptive immune responses via altering dendritic cell functions. J. Immunol. 2010;184:2377–2385. doi: 10.4049/jimmunol.0803938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, Arai N, Gallo RL, Digiovanni J, Gilliet M. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J. Exp. Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kool M, van Nimwegen M, Willart MA, Muskens F, Boon L, Smit JJ, Coyle A, Clausen BE, Hoogsteden HC, Lambrecht BN, Hammad H. An antiinflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J. Immunol. 2009;183:1074–1082. doi: 10.4049/jimmunol.0900471. [DOI] [PubMed] [Google Scholar]
- 24.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lande R, Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann. N. Y. Acad. Sci. 2010;1183:89–103. doi: 10.1111/j.1749-6632.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- 26.Weng H, Lu Y, Weng Z, Morimoto K. Differential DNA damage induced by H2O2 and bleomycin in subpopulations of human white blood cells. Mutat. Res. 2008;652:46–53. doi: 10.1016/j.mrgentox.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Rivollier A, Perrin-Cocon L, Luche S, Diemer H, Strub JM, Hanau D, van Dorsselaer A, Lotteau V, Rabourdin-Combe C, Rabilloud T, Servet-Delprat C. High expression of antioxidant proteins in dendritic cells: possible implications in atherosclerosis. Mol. Cell. Proteomics. 2006;5:726–736. doi: 10.1074/mcp.M500262-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Thoren FB, Betten A, Romero AI, Hellstrand K. Antioxidative properties of myeloid dendritic cells: protection of T cells and NK cells from oxygen radical-induced inactivation and apoptosis. J. Immunol. 2007;179:21–25. doi: 10.4049/jimmunol.179.1.21. [DOI] [PubMed] [Google Scholar]
- 29.Oikawa S, Yamada K, Yamashita N, Tada-Oikawa S, Kawanishi S. N-acetylcysteine , a cancer chemopreventive agent, causes oxidative damage to cellular and isolated DNA. Carcinogenesis. 1999;20:1485–1490. doi: 10.1093/carcin/20.8.1485. [DOI] [PubMed] [Google Scholar]
- 30.Sagrista ML, Garcia AE, Africa De Madariaga M, Mora M. Antioxidant and pro-oxidant effect of the thiolic compounds N-acetyl-L-cysteine and glutathione against free radical-induced lipid peroxidation. Free Radic. Res. 2002;36:329–340. doi: 10.1080/10715760290019354. [DOI] [PubMed] [Google Scholar]
- 31.Su M, Yang Y, Yang G. Quantitative measurement of hydroxyl radical induced DNA double-strand breaks and the effect of N-acetyl-L-cysteine. FEBS Lett. 2006;580:4136–4142. doi: 10.1016/j.febslet.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 32.Wang AL, Wang JP, Wang H, Chen YH, Zhao L, Wang LS, Wei W, Xu DX. A dual effect of N-acetylcysteine on acute ethanol-induced liver damage in mice. Hepatol. Res. 2006;34:199–206. doi: 10.1016/j.hepres.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin C and N-acetylcysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic. Biol. Med. 2001;31:745–753. doi: 10.1016/s0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 34.Freedman JH, Weiner RJ, Peisach J. Resistance to copper toxicity of cultured hepatoma cells: characterization of resistant cell lines. J. Biol. Chem. 1986;261:11840–11848. [PubMed] [Google Scholar]
- 35.Kakuta K, Orino K, Yamamoto S, Watanabe K. High levels of ferritin and its iron in fetal bovine serum. Comp. Biochem. Physiol. A Physiol. 1997;118:165–169. doi: 10.1016/s0300-9629(96)00403-3. [DOI] [PubMed] [Google Scholar]
- 36.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 37.Malmberg KJ, Arulampalam V, Ichihara F, Petersson M, Seki K, Andersson T, Lenkei R, Masucci G, Pettersson S, Kiessling R. Inhibition of activated/memory (CD45RO(+)) T cells by oxidative stress associated with block of NF-kappaB activation. J. Immunol. 2001;167:2595–2601. doi: 10.4049/jimmunol.167.5.2595. [DOI] [PubMed] [Google Scholar]
- 38.Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J. Immunol. 2008;181:6747–6756. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu CF, Peng WM, Oldenburg J, Hoch J, Bieber T, Limmer A, Hartmann G, Barchet W, Eis-Hubinger AM, Novak N. Human plasmacytoid dendritic cells support Th17 cell effector function in response to TLR7 ligation. J. Immunol. 2010;184:1159–1167. doi: 10.4049/jimmunol.0901706. [DOI] [PubMed] [Google Scholar]
- 40.Magyarics Z, Csillag A, Pazmandi K, Rajnavolgyi E, Bacsi A. Identification of plasmacytoid pre-dendritic cells by one-color flow cytometry for phenotype screening. Cytometry A. 2008;73:254–258. doi: 10.1002/cyto.a.20529. [DOI] [PubMed] [Google Scholar]
- 41.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tkaczyk J, Vizek M. Oxidative stress in the lung tissue—sources of reactive oxygen species and antioxidant defence. Prague Med. Rep. 2007;108:105–114. [PubMed] [Google Scholar]
- 43.Kohen R, Gati I. Skin low molecular weight antioxidants and their role in aging and in oxidative stress. Toxicology. 2000;148:149–157. doi: 10.1016/s0300-483x(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 44.Kiroglu AF, Noyan T, Oger M, Kara T. Oxidants and antioxidants in tonsillar and adenoidal tissue in chronic adenotonsillitis and adenotonsillar hypertrophy in children. Int.J. Pediatr. Otorhinolaryngol. 2006;70:35–38. doi: 10.1016/j.ijporl.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Cross CE, van der Vliet A, O’Neill CA, Louie S, Halliwell B. Oxidants, antioxidants, and respiratory tract lining fluids. Environ. Health Perspect. 1994;102(Suppl. 10):185–191. doi: 10.1289/ehp.94102s10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polidori MC, Stahl W, Eichler O, Niestroj I, Sies H. Profiles of antioxidants in human plasma. Free Radic. Biol. Med. 2001;30:456–462. doi: 10.1016/s0891-5849(00)00345-2. [DOI] [PubMed] [Google Scholar]
- 47.Roche M, Rondeau P, Singh NR, Tamils E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582:1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 48.Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Atherosclerosis. 2000:131–139. doi: 10.1016/s0021-9150(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 49.Kono K, Salazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, Nakazawa T, Anderson P, Kiessling R. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell- and natural killer cell-mediated cytotoxicity. Eur.J. Immunol. 1996;26:1308–1313. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 50.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 51.Schwarz KB. Oxidative stress during viral infection: a review. Free Radic. Biol. Med. 1996;21:641–649. doi: 10.1016/0891-5849(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 52.Israel N, Gougerot-Pocidalo M. A Oxidative stress in human immunodeficiency virus infection. Cell. Mol. Life Sci. 1997;53:864–870. doi: 10.1007/s000180050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi J, Ou JH. Mechanisms of liver injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G847–G851. doi: 10.1152/ajpgi.00522.2005. [DOI] [PubMed] [Google Scholar]
- 54.Beck MA, Handy J, Levander OA. The role of oxidative stress in viral infections. Ann. N. Y. Acad. Sci. 2000;917:906–912. doi: 10.1111/j.1749-6632.2000.tb05456.x. [DOI] [PubMed] [Google Scholar]
- 55.Gowdy KM, Krantz QT, King C, Boykin E, Jaspers I, Linak WP, Gilmour MI. Role of oxidative stress on diesel-enhanced influenza infection in mice. Part. Fibre Toxicol. 2010;7:34. doi: 10.1186/1743-8977-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savarino A, Pescarmona GP, Boelaert JR. Iron metabolism and HIV infection: reciprocal interactions with potentially harmful consequences? Cell Biochem. Funct. 1999;17:279–287. doi: 10.1002/(SICI)1099-0844(199912)17:4<279::AID-CBF833>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 57.Frossi B, De Carli M, Piemonte M, Pucillo C. Oxidative microenvironment exerts an opposite regulatory effect on cytokine production by Th1 and Th2 cells. Mol. Immunol. 2008;45:58–64. doi: 10.1016/j.molimm.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Castro SM, Chakraborty K, Guerrero-Plata A. Cigarette smoke suppresses TLR-7 stimulation in response to virus infection in plasmacytoid dendritic cells. Toxicol. In Vitro. 2010;25:1106–1113. doi: 10.1016/j.tiv.2011.03.011. [DOI] [PubMed] [Google Scholar]