Abstract
This paper examined the relation of early environmental adversity associated with poverty to child resting or basal level of cortisol in a prospective longitudinal sample of 1,135 children seen at 7, 15, 24, 35, and 48 months of age. We found main effects for length of time in poverty, poor housing quality, African American ethnicity, and low positive caregiving behavior in which each was uniquely associated with an overall higher level of cortisol from age 7 months to 48 months. We also found that two aspects of the early environment in the context of poverty, adult exits from the home and perceived economic insufficiency, were related to salivary cortisol in a time dependent manner. The effect for the first of these, exits from the home, was consistent with the principle of allostatic load in which the effects of adversity on stress physiology accumulate over time. The effect for perceived economic insufficiency was one in which insufficiency was associated with higher levels of cortisol in infancy but with a typical but steeper decline in cortisol with age at subsequent time points.
It is well known that poverty has substantial effects on multiple aspects of children’s development, ranging from health to cognition to social-emotional development (Bradley & Corwyn, 2002; Brooks-Gunn & Duncan, 1994). Of more recent interest, however, is concern over the possible affects of poverty on stress physiology in children (Evans, 2003) and the idea that affects of poverty on stress physiology may be a primary mechanism through which poverty has widespread influence on child health and development (Blair, 2010; Shonkoff, Boyce, & McEwen, 2009). One framing of the question is to ask how poverty gets under the skin to influence biological processes that are highly relevant to physical health and mental well being. In addressing this question, the concepts of allostasis and allostatic load (McEwen, 2000) are invaluable as statements of the idea that experience can alter stress physiology in a cumulative fashion to provide short term benefits to physical and psychological functioning in unsupportive environments but that ultimately prove injurious to health and well being in the long term. The principle of allostasis refers to the idea that resting levels of stress hormones adapt or adjust to experience over time. Unlike physiological systems such as blood pressure, or body temperature, which are homeostatic systems, meaning that must be maintained within a relatively narrow range of values, resting levels of stress hormones have a relatively broad plausible range. When stress is chronic and ongoing, stress response systems including the hypothalamic-pituitary-adrenal (HPA) axis as well as the sympathetic adrenal system, are said to be under high allostatic load and to adapt by altering resting state set points to levels that are relatively high and therefore not flexibly regulated. Under supportive conditions, however, resting levels are in a moderate range and responsive to stimulation, exhibiting flexible up and down regulation in response to acute stress as needed (McEwen, 1998, 2000).
Although associations between chronic stress and alterations to physiological stress response systems are generally characterized by elevations, it is important to note, however, that resting levels of cortisol in particular have also been found to be altered downward as well as upward by chronic stress, such as seen in lower morning levels of cortisol and little change in cortisol across the day (a flat diurnal pattern) in children experiencing caregiving disruption and foster care (Tarullo & Gunnar, 2006). Similarly, it has been shown that children experiencing primarily physical abuse exhibit chronically low cortisol levels, while children experiencing extensive maltreatment exhibit chronically elevated cortisol, indicating that severe maltreatment can have distinct effects on stress response systems depending on maltreatment subtype (Cicchetti & Rogosh, 2009). The key implication here may be that alterations, whether upward or downward in resting levels, taking into account the circadian rhythm of cortisol, interfere with the flexible regulation of the hormone in response to acute stressors. The absence of flexible regulation of cortisol when needed might be one indication of the extent to which experience has become embedded in biology, that is, has gotten under the skin to influence behavior and psychological functioning in meaningful ways.
Relations between experience and stress physiology described by the principles of allostasis and allostatic load are of particular interest to developmental psychologists and psychopathologists given the role of stress response systems in the regulation of thought, emotion, and behavior. Stress response systems, including sympathetic, parasympathetic, and HPA are primary organizers of energy metabolism and associated attentional, emotional, and behavioral responses to stimulation (McEwen, 2008; Porges, 2001; Repetti, Taylor, & Seeman, 2002). In the realm of cognitive ability, for example, neuroendocrine hormones such as norepinephrine and cortisol potentiate either reactive or reflective responses to challenge depending on the amount of hormonal increase. Under conditions of acute stress, high level of stress hormone increase is associated with increased neural activity in subcortical brain areas associated with more reactive responses to stimulation, such as highly robust declarative memory formation, the so-called flashbulb memory phenomenon (Diamond, Campbell, Park, Halonen, & Zoladz, 2007). Under more moderate levels of stress arousal and stress hormone increase, however, stress hormones potentiate activity in brain areas, notably prefrontal cortex, associated with reflective responses to stimulation such as executive functions and the flexible use of attention and purposefully regulated emotional responses to stimulation (Arnten & Li, 2005).
The context of poverty and stress physiology
The principles of allostasis and allostatic load suggest that stress may be a primary mechanism through which poverty affects child development and increases risk for early developing psychopathology. Several studies have demonstrated that the context of poverty generally leads to elevated resting state levels in stress response systems, as seen in HPA as well as the sympathetic adrenal system. For example, Lupien and colleagues demonstrated that young children from low relative to high SES homes present higher morning levels of cortisol (Lupien, King, Meaney, & McEwen, 2001). Evans and colleagues (Evans, 2003) have demonstrated that a risk index composed of physical characteristics of the home, including crowding, housing quality, and noise levels as well as aspects of the psychosocial environment of families living in poverty was associated with elevated allostatic load in children as measured by an index of overnight levels of cortisol, epinephrine, norepinephrine, blood pressure, and body mass index.
Given the well demonstrated role of stress in disease processes (McEwen & Wingfield, 2003) and the demonstration that increased stress in the context of poverty is a primary contributor to SES gradients in physical and mental health, investigators have looked to stress processes in childhood as a possible early determinant of adult health status (Shonkoff, Boyce, & McEwen, 2009). Similarly, investigators have examined the extent to which the effects of poverty on stress physiology may be associated with problems with social-emotional and cognitive development in children that may be indicative of the developmental roots of problems with self-regulation and risk for early developing psychopathology (Cicchetti & Rogosh, 2009; Evans, 2003; Halligan, Herber, Goodyer, & Murray, 2006). For example, in a longitudinal sample of children in predominantly white, rural families followed by Evans and colleagues (Evans & Schamberg, 2009), length of time in poverty was associated with elevated allostatic load in early adolescence and allostatic load was found to account for the relation between years in poverty and deficits in an aspect of cognitive ability associated with reflective responses to stimulation, specifically the executive function of working memory.
Although the idea that poverty affects stress physiology in expected ways with discernable consequences for child health and development is theoretically coherent and has received growing empirical support, there are a number of questions and considerations for this area of research. Of particular interest are mediating and moderating mechanisms through which poverty might or might not become embedded in biology developmentally. Of specific interest is the extent to which early caregiving may be highly salient as a mediating mechanism through which stressors in the context of poverty affect child well being. Such an early caregiving mediation hypothesis has considerable support in animal models of early experience as well as in examinations of the intergenerational transmission of defensive responses in a number of species, including plants as well as insects, birds, and mammals (Cameron, Champagne, Parent, Fish, Ozaki-Kuroda, & Meaney, 2005).
The hypothesis that early caregiving functions as a primary source of information about the quality of the environment has considerable intuitive and empirical appeal for the study of child development. Parenting has been demonstrated to be less prototypically supportive and sensitive in the context of poverty and shown in longitudinal and experimental studies to be a primary mechanism through which poverty increases risk for poor child outcomes (Brooks-Gunn & Duncan, 1994; McLoyd, 1998). Evidence for this ranges from correlational findings indicating relations between variation in amount and type of experience and cognitive development (Hart & Risley, 1995) to experimental research demonstrating that alterations in parenting behavior through appropriate coaching models result in higher levels of prototypically sensitive caregiving that in turn lead to increases in child cognitive and social-emotional development relative to a control group (Landry, Smith, & Swank, 2006).
In two prior papers both of which analyzed data from the sample included in this paper, we examined the possibility that the deleterious effects of poverty on child cognitive development might be mediated to some extent through early caregiving effects on child stress physiology. In the more recent of these two (Blair, Granger, Willoughby, Mills-Koonce, Cox, Greenberg, Kivlighan, Fortunato, & the Family Life Project Investigators, in press) we demonstrated using structural equation modeling that the effect of poverty as indicated by income-to-need on parenting behavior for the most part accounted for effects of poverty on both executive function and IQ. As well, it was also shown that the relation between sensitive parenting and executive function was accounted for to some extent by the association between sensitive parenting and lower resting or basal levels of cortisol in children over the first two years. That is, higher level of prototypically sensitive parenting was associated with lower resting or basal level of cortisol in children at ages 7 through 24 months and lower level of cortisol was in turn associated with higher child executive function at age 3 years. This meditational path is consistent with evidence from a number of studies in animal models (e.g., Meaney, 2001) indicating the potentially central mediating role of early care as a primary source of information about the environment. These findings are also consistent with neurobiological studies demonstrating that prefrontal cortex, the seat of executive functions in the brain, is highly influenced by the physiological response to stress, exhibiting enhanced functioning under conditions of acute stress (Yeun, Liu, Karetsoreos, Feng, McEwen, & Yan, 2009) but demonstrating pronounced deficits in the context of chronic stress (Cerqueira, Mailliet, Almeida, Jay, & Sousa, 2007).
It is also important to note, however, that in the foregoing analysis, African American ethnicity, which was itself associated with lower positive parenting, remained a unique influence on child cortisol, even when controlling for poverty and parenting. Furthermore, in the first of the two prior papers from our research group with this sample, we examined child cortisol in separate models at ages 7 and 15 months and found that although parenting was a primary mediator of effects of poverty on cortisol, child temperament was also uniquely associated with cortisol levels (Blair, Granger, Kivlighan, Mills-Koonce, Willoughby, Greenberg, Hibel, Fortunato, & the Family Life Project Investigators, 2008). That is, this prior paper indicated that child characteristics including higher levels of attention, higher levels of distress to novelty, and lower levels of distress to limitations were associated with lower resting cortisol levels at child age 15 months and also with greater increase followed by decrease in cortisol in response to an emotion challenge.
Although the mediation of the effect of poverty on child outcomes through parenting and stress physiology has much to recommend it, it may be that a variety of responses to environmental adversity including the moderation of stress in the environment by caregiving and by child temperament is present. Such moderation of the relation between adversity and outcome is central to notions of resilience in development (Curtis & Cicchetti, 2003). In particular, it may be that the ability to regulate behavior in the context of risk is a key aspect of resilience and indicative of the process through which resilient outcomes are manifest by children facing adversity (Buckner, Mezzacappa, & Beardslee, 2003). In addition, of growing interest in the study of relations between adversity and outcome is the idea that temperamental characteristics of children may moderate the relation between stress associated with poverty and development. Prominent here are differential susceptibility models in which the effect of temperamental negativity in infancy on later child outcomes, particularly as indicated by measures of behavior problems, depends upon the quality of the caregiving environment. Specifically, children who are characterized by high levels of temperamental reactivity to stimulation in infancy exhibit poor developmental outcomes when in caregiving contexts of a low quality. In contexts that are of a higher quality, however, meaning that they are high in caregiver support and sensitivity, children characterized by high temperamental reactivity exhibit positive outcomes indicative of a high level of self-regulation ability (Belsky & Pluess, 2009). In this, it appears that temperamentally reactive children are maximally sensitive to the quality of the environment, such that they are more affected by environmental quality than their low reactivity counterparts in both good and bad ways, exhibiting worse outcomes in adverse rearing conditions but better outcomes in high quality environments (Belsky, 1997).
Testing the effects of experience on cortisol levels in early childhood
In this analysis we examine the possibility that experience over the first four years influences levels of cortisol in ways that have implications for psychological development. Specifically, we suggest that experience in early childhood acts to channel or canalize development and to increase or decrease risk for early developing psychopathology in part through effects on stress physiology. To test this possibility we examine resting or basal levels of salivary cortisol as measured longitudinally at four time points in early childhood, ages 7, 15, 24, and 48 months and test the general hypothesis that measures of adversity over the first four years will be associated with higher levels of cortisol in children. In particular we are interested in the extent to which cumulative adversity may be associated with increasingly higher levels of resting or basal levels of cortisol in children over time. Such a time by adversity interaction would indicate that adverse experience is acting on child stress physiology in a way that is consistent with conceptions of allostatically driven elevations in response to chronic stress. In contrast, main effects for measures of adversity on levels of cortisol at each time point might suggest that adversity prior to the first time point of data collection, 7 months of age, is having an enduring affect on child HPA axis, or that perhaps adversity is associated with pre-existing differences in HPA axis function in early childhood.
Furthermore, consistent with the idea that caregiving quality is a major source of influence or conduit through which information about the quality of the environment is transmitted to children, we also hypothesized that observed relations between adversity and child cortisol will be accounted for in part by relations between measures of early caregiving behavior and child cortisol. That is, consistent with animal models suggesting mediation of environmental quality through caregiving experience (Cameron et al., 2005), we expected that coefficients for adversity measures will be significantly reduced with the addition of measures of positive and negative caregiving behavior to regression equations predicting child cortisol. Furthermore, consistent with differential susceptibility models of child temperament we expected that measures of child temperamental negativity taken at the first data collection time point, age 7 months, will be associated with higher levels of child cortisol, indicating that physiological measures such as cortisol represent an intermediate marker or endophenotype of temperamental susceptibility to environmental influence. That is, in the differential susceptibility literature a number of studies (reviewed by Belsky & Pluess, 2009) have shown that measures of stress physiology, particularly those associated with the regulation of cardiac physiology, are related to temperamental negativity and interact with environmental quality in a for better and for worse fashion. Here we simply test the hypothesis that measures of temperamental reactivity are meaningfully related to stress response systems that may be acted on differentially by the environment. Specifically, we are interested in the extent to which relations of temperament to cortisol are distinct from or overlap with relations between measures of environmental adversity and cortisol. A high degree of overlap would suggest that the environment is acting to shape both temperament as well as underlying stress physiology while little or no overlap would suggest that child temperament represents a relatively distinct source of variation in child outcomes on which the environment can differentially act.
In the examination of environmental adversity associated with poverty we consider a number of variables. In terms of material hardship we include household income relative to household size quantified as income-to-need, as well as caregiver report of economic hardship both in terms of economic need, the amount of money available to families to meet needs, as well as perceptions of economic sufficiency, the extent to which caregivers feel that the family is able to adequately meet its needs with the money it has available to it. We also include a measure of housing quality as an index of the physical environments in which families are living. Material hardship may constitute sources of stress that occur as a result of the types of choices that families in poverty face. Lower income-to-need could increase stress through a variety of mechanisms such psychological and or physical distress resulting from problems paying bills or from inadequate nutrition associated with limited food choices. The physical characteristics of housing might also be associated with increased stress. Lower quality housing is typically noisy and less safe and frequently characterized by structural inadequacies that pose challenges for families leading to higher stress (Bradley & Corwyn, 2002; Evans, 2003).
Over and above the material hardships imposed by income poverty, families may experience a range of interpersonal stressors that place family members (including children) under greater allostatic load. Past findings from clinical research suggest that exposure to high levels of affectively negative interpersonal interactions, alone or when combined with a high level of instability among caregivers, may be associated with children’s disrupted HPA axis functioning (Gunnar, Frenn, Wewerka, Van Ryzin, 2009). For example, children’s exposure to higher levels of adult anger and conflict in the home and to peer conflict in child care settings have been argued to disrupt children’s emotional and physiological reactivity, as indicated by compromised PNS functioning and higher RSA reactivity (Cummings, El-Sheikh, Kouros, & Buckhalt, 2009; Gunnar & Donzella, 2002). At the extreme end of the caregiving continuum, maltreatment experiences in early childhood have been found to be associated with both children’s and adults’ hypoactive HPA axis reactivity (e.g. suppressed baseline salivary and plasma cortisol and blunted ACTH responsiveness to laboratory stressors such as the Trier Social Stress Test) relative to similarly aged children and adults without maltreatment histories (Fisher Gunnar, Chamberlain, & Reid, 2000).
In the analyses presented below, we consider children’s cumulative exposure to single versus multiple episodes of instability in the number of adults in the household from early infancy to the preschool period as a rough proxy or indicator of interpersonal stress. While it may certainly be the case that some adults’ exits from some households signal emotionally positive transitions in the lives of families (e.g. a young adult goes off to college or moves out to start his or her own family), it is also probably the case that the departure of adults from at least some of the households in our sample may be preceded or accompanied by higher levels of negative emotional expression on the part of one or more household members (e.g. a relative or romantic partner moves out). If nothing else, we hypothesize that adults’ exits from the household represent a discriminable difference in the caregiving patterns experienced by the target infants in the sample, and for that reason alone may be justifiably considered as an interpersonal stressor.
In sum, past studies have not yet clarified what the trajectory of the development of HPA axis activity might be in response to chronic versus limited exposure to material and interpersonal stressors. This study offers us the opportunity to examine the role of chronicity, of the length of time of exposure to material and interpersonal stressors to both the intercept and slope of basal or resting level of cortisol, adjusted for time of day of data collection, as an indicator of the activity of the HPA axis stress response system.
Method
The Family Life Project (FLP) was designed to study young children and their families in two of the four major geographical areas of the United States with high poverty rates. Specifically, three counties in Eastern North Carolina and three counties in Central Pennsylvania were selected to be indicative of the Black South and Appalachia, respectively. The FLP adopted a developmental epidemiological design in which sampling procedures were employed to recruit a representative sample of 1,292 children whose families resided in one of the six counties at the time of the child’s birth. Low-income families in both states and African American families in NC were over-sampled (African American families were not over-sampled in PA because the target communities were at least 95% non-African American).
At both sites, recruitment occurred seven days per week over the 12-month recruitment period spanning September 15, 2003 through September 14, 2004 using a standardized script and screening protocol. The coverage rate was over 90% for all births that occurred to women in these counties in that one year period. In PA, families were recruited in person from three hospitals. These three hospitals represented a weighted probability sample (hospitals were sampled proportional to size within county) of seven total hospitals that delivered babies in the three target PA counties. PA hospitals were sampled because the number of babies born in all seven target hospitals far exceeded the number needed for purposes of the design. In NC, families were recruited in person and by phone. In-person recruitment occurred in all three of the hospitals that delivered babies in the target counties. Phone recruitment occurred for families who resided in target counties but delivered in non-target county hospitals. These families were located through systematic searches of the birth records located in the county courthouses of nearby counties.
FLP recruiters identified 5,471 (59% NC, 41% PA) women who gave birth to a child in the 12-month period. A total of 1,515 (28%) of all identified families were determined to be ineligible for participation for three primary reasons: not speaking English as the primary language in the home, residence in a non-target county, and intent to move within three years. Of the 2691 eligible families who agreed to the randomization process, 1,571 (58%) families were selected to participate using the sampling fractions that were continually updated from our data center. Of those families selected to participate in the study, 1,292 (82%) families completed a home visit at 2 months of child age, at which point they were formally enrolled in the study.
Procedures
Families were seen in home visits at child ages of approximately 7, 15, 24, 36, and 48 months. At time points other than 15 and 48 months, families were seen in two separate visits. All home visits for data collection were two or more hours in duration. During visits for data collection conducted at 7, 15, and 24 months, mothers completed questionnaires concerning family demographics and income, and engaged in a free play interaction (at 7 and 15 months) and an interactive puzzle completion task (at 24 months) with their child that was recorded with digital video for 10 minutes (Cox, Paley, Burchinal, & Payne, 1999; NICHD ECCRN, 1999). During the free play interaction mothers were given a standard set of toys and instructed to play with the child as they normally would if they had a little free time during the day. During the puzzle completion task, children were presented with 3 consecutive board puzzles that increased in difficulty. Mothers were instructed to interact and help their children with the puzzles as they saw necessary.
Near the conclusion of the home visit for data collection at 7, 15, and 24 months (usually the second visit at 7 months, usually the first visit at 24 months), at which time the data collectors had been in the home for at least one hour, children were presented with emotion challenge tasks designed to elicit emotional responding, including a mask presentation, barrier task, and arm restraint at 7 months, and a toy removal and mask presentation at 15 and 24 months. All procedures have been previously validated (Stifter & Braungart, 1995). To assess basal levels of cortisol and cortisol response to the emotion arousal, unstimulated whole saliva was collected using either cotton or hydrocellulose absorbent material and expressing sample into 2 ml cryogenic storage vials using a needleless syringe (cotton) or by centrifugation (hydrocellulose). Two prior studies have indicated no differences in cortisol concentrations associated with the two collection techniques (Granger, Kivlighan, Fortunato, Harmon, Hibel, Schwartz & Whembolua, 2007; Harmon, Granger, Hibel & Rumyantseva, 2007). Saliva was collected at baseline prior to the administration of the emotion challenge procedures and at 20 and 40 minutes post peak emotional arousal following exposure to the procedures. At 48 months, saliva was collected using the same methods as at the previous time points but was collected earlier in the home visit for data collection, with the first collection occurring on average after the data collectors had been in the home for only 15 to 20 minutes. At 48 months children were not administered the emotion challenge tasks. Instead, saliva samples were taken 20 minutes and 40 minutes post baseline while children were administered an executive function battery.
For this analysis, only the baseline cortisol measures adjusted for time of day of collection were used. Although the 20- and 40 minute measures of cortisol are available, we focus on baseline levels in order to most directly address hypotheses concerning processes of allostatic load and variation in typical level or set point for cortisol over the child’s first four years.
The characteristics of the sample, repeated interview schedule, length of each interview protocol (2–4 hours), and age of the infants required that in-home assessments were scheduled when families were available. Therefore, time of the day of the interview and saliva collection varied. Mean time of day of saliva sample collection was 13:04 hours (SD = 2.88) at age 7 months, 13:45 hours (SD = 2.94) at 15 months, 13:33 hours (SD = 3.20) at 24 months, and 13:25 hours (SD = 2:53) at 48 months. After collection, samples were immediately placed on ice, transported to interviewers homes and frozen (−20 °C). They were stored frozen until batched and shipped on dry-ice overnight to the Behavioral Endocrinology Laboratory at Penn State. Samples were then stored frozen at −80 °C until assay. On the day of testing, samples were brought to room temperature, centrifuged at 3,000 RPM for 15 minutes, and the clear top-phase of the sample was pipetted into appropriate test wells by robot (Genesis, Tecan).
Measures
Salivary cortisol
All samples were assayed for salivary cortisol using a highly-sensitive enzyme immunoassay US FDA 510k cleared for use as an in vitro diagnostic measure of adrenal function (Salimetrics, State College, PA). The test used 25 µl of saliva (for singlet determinations), had a range of sensitivity from .007 to 1.8 µg/dl, and average intra-and inter-assay coefficients of variation of less than 10% and 15%. All samples were assayed in duplicate. The criterion for repeat testing was variation between duplicates greater than 20%, and the average of the duplicates was used in all analyses. The cortisol distributions were subject to log transformation to correct positive skew. Outliers greater than 3 standard deviations from the mean were treated as missing (n = 15, 16, 17, and 8 at 7, 15, 24, and 48 months, respectively.) Time of day of saliva collection was significantly related to cortisol level at each time point (r = −.25 on average). We also examined child temperature, time since eating, time since sleeping, and use of medications (e.g., acetaminophen) as influences on child cortisol levels at 7 and 24 months (data not available at 15 and 48 months.) Small significant relations of time since eating and time since sleeping with cortisol at 7 months were accounted for by adjustment for time of day of saliva collection (Hibel, Granger, Kivlighan, Blair & the FLP Investigators, 2006). .
Parenting
Mother-child interactions in the free play at 7 and 15 months and in the structured interaction at 24 months were coded to assess levels of mothers’ sensitivity, detachment, intrusiveness, positive regard, negative regard, and animation in interacting with the child. Ratings for each code were made on a 1–5 scale at 7 and 15 months and a 1–7 scale at 24 months, with one being not at all characteristic and five (or seven) being highly characteristic. Factor analyses conducted with an oblique rotation (i.e., Promax) at each time point indicated distinct positive and negative dimensions of parenting. Maternal positive parenting included five maternal characteristics: sensitivity, detachment (reverse-scored), positive regard (e.g., positive feelings expressed toward child), animation (level of energy), and stimulation for development (appropriate level of scaffolding of activities with child). Maternal negative parenting included two maternal characteristics: intrusiveness and negative regard (level of harsh, negative feelings expressed toward child). Inter-rater reliability was determined by calculating the intra-class correlation (ICC) for ratings made by two coders to approximately 30% of the tapes randomly drawn at the infant and toddler assessments. ICCs were .85 – .91 for positive parenting and .72 −.86 across 7, 15, and 24 month assessments.
Income-to-need was calculated as the estimated total household income divided by the federal poverty threshold for 2005 adjusted for number of persons in the home. Family transitions into and out of poverty were calculated as 1 versus 0 for each assessment period using federally recommended thresholds of income-to-needs ratio less than or equal to 1.0 defined as “poor” and coded as 1 while families whose income fell above 1.0 were coded as “nonpoor” for that assessment period and were given a score of 0. Chronicity of time spent in poverty from 15 to 48 months was calculated by summing the number of times families were categorized as poor over those 4 assessment periods.
Economic need and economic sufficiency were reported by the primary caregiver at the 7, 15, 24 and 35 month assessments using the Economic Strain Questionnaire (Conger & Elder, 1994) a six item measure. Two items assess economic need (the extent to which the family has difficulty paying bills and runs out of money each month) and four items assess economic sufficiency (the extent to which the family feels it is able to adequately meet its needs for housing, clothing, food, and medical care.)
Adult exits and entrances from the household
The primary caregiver reported on the number of adults in the home at each data collection time point and changes, both entrances and exits of adults from the home were compiled from these datda.
Housing quality
Families’ exposure to substandard housing quality was assessed via observer reports on 4 items tapping the cleanliness of the home, the number of rooms in the home, the safety of the building’s interior, and safety of the area outside the building on a 0–4 Likert-type scale, with higher scores indicating higher housing quality. Average scores across all 4 items (reversed) were calculated to indicate low housing quality at four time points (6 months, 24 months, 35 months and 48 months).
Child temperament
Temperamental reactivity at age 7 months was assessed by the Infant Behavior Record (IBR, Bayley, 1969) as adapted for use by Stifter and Corey (2001) and completed independently by both home visitors. The IBR was applied to infant behavior observed globally across the entire home visit. The IBR scales included sociability, positive affect, attention, activity level, reactivity, and irritability. The summed mean score of the two data collectors’ ratings across both visits were used. Alphas ranged from .70 (irritability) to .88 (attention).
Child emotionality
Assessments of peak emotional arousal in response to three emotional challenge tasks at 7 months were completed via independent coders’ ratings of low, moderate and high negative reactivity using Better Coding Approach software (Danville, Pennsylvania). A composite score for negative reactivity for each task was created by summing the seconds of low, moderate, and high negative reactivity and then calculating the proportion by dividing the sum of all negative reactivity scores by the total time of the task. Coders were trained to achieve at least .75 (Cohen’s k) reliability on the reactivity coding. Subsequent interrater reliability was calculated on 15% of cases using kappa coefficients, resulting in a kappa of .94 for the masks task, .89 for the barrier task, and .86 for the arm restraint task.
Data analysis
Total sample size recruited at study entry was 1,292 with 1,204 children seen at age 7 months, 1,169 at 15 months, 1,144 at 24 months, 1,123 at 36 months, and 1,066 at 48 months. Children missing cortisol at all time points or all but one time point were excluded from the sample, yielding an analysis data set of n = 1,135. To assess possible differential attrition in the sample at each time point we examined a number of variables for which we had complete information collected at child age of approximately 2 months including state of residence, race, sex, child age at the 2 month follow-up, an income screen, total number of household members, number of children in the household, and primary caregiver age, education, marital status, and employment. Few variables indicated differences between families who were present and those who were missing at each time point. For example, at 15 months, no variables differentiated participants who were missing from those who were present. At 24 months, missing participants were more likely have been older at the 2 month follow-up, to have resided in North Carolina, and to have a primary caregiver who was employed. To avoid bias in estimates associated with missing data, we imputed data using the MI procedure in SAS. We imputed 20 analysis data sets and recombined regression estimates using the MIANALYZE procedure in SAS, yielding efficiency in the estimates of greater than 99%.
Results
Descriptive statistics for each of the variables in the analysis are presented in Table 1. The table indicates that families were on average below the poverty level at 2 out of 5 time points during the child’s first four years with 112 families reporting income-to-need at or below the poverty line at all 5 time points and 377 families never reporting household income-to-need at or below the poverty threshold. Families reported moderate levels of financial hardship and economic strain. Ratings of families’ housing quality were generally high. On average there were four persons in the household over the child’s first four years and 355 families had one adult enter the home during that time and 77 families had two adults enter the home. In contrast, 363 families had one adult exit the home and 114 families experienced two adults exiting the home.
Table 1.
Descriptive statistics for variables in the analysis.
| Variable | N | Mean | Std Dev |
|---|---|---|---|
| Chronic poverty | 948 | 1.71 | 1.81 |
| Economic need | 1131 | 2.06 | 0.53 |
| Economic sufficiency | 1131 | 2.55 | 0.84 |
| Housing quality | 1133 | 3.01 | 0.46 |
| Number of adults | 1135 | 4.38 | 1.22 |
| Maternal education | 1017 | 15.11 | 2.62 |
| Positive parenting | 1128 | 2.86 | 0.70 |
| Negative parenting | 1128 | 2.37 | 0.61 |
| Infant temperament | 1099 | 52.29 | 7.36 |
| Peak emotionality | 979 | 0.49 | 0.35 |
We first examined change in typical or resting salivary cortisol levels in children over the first four years controlling for time of day at each data collection time point (7, 15, 24, and 48 months) and including linear and polynomial terms for time. In the first model we included terms for length of time in poverty, mean level of housing quality, mean level of perceived economic strain (whether or not the family has enough money), mean level of perceived economic sufficiency (whether or not the family is able to sufficiently meet its needs with the money that is has), and total number of adult exits and entrances in the home. Terms for maternal education, child ethnicity and state of residence, and mean number of adults in the household were included as covariates. For each variable we initially included interactions with the linear and polynomial terms for time of assessment. Coefficients from these interactions making significant contributions to the model were retained.
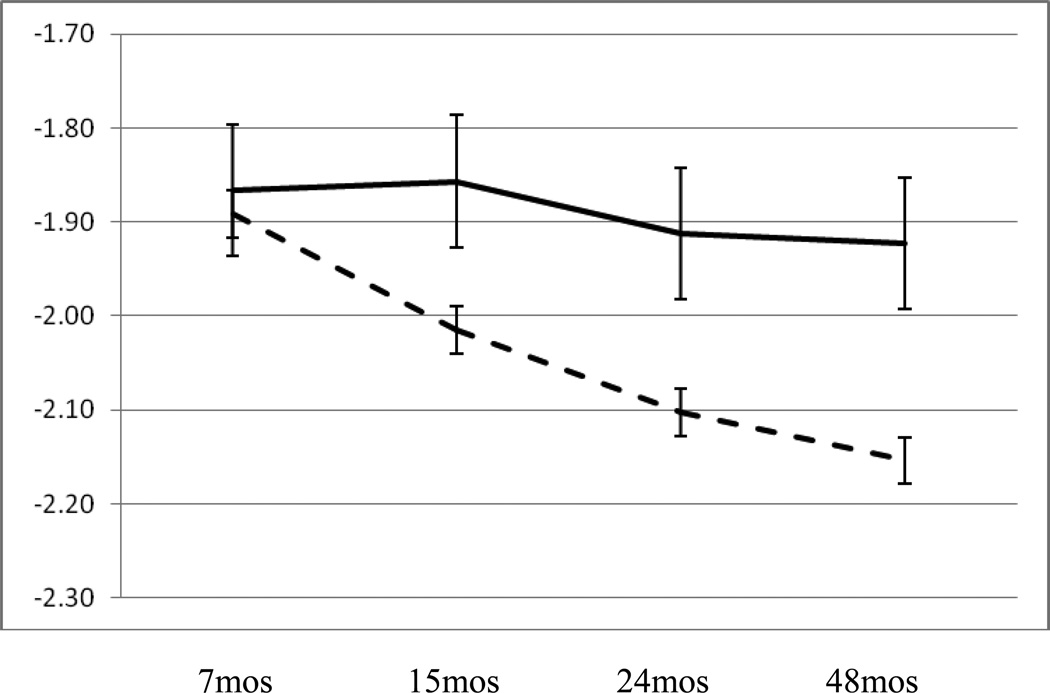
Results presented in Model A in Table 2 indicated main effects for African-American ethnicity, b = .20, se = .03, p < .001, length of time in poverty, b = −.02, se = .009, p < .05, and housing quality, b = −.11, se = .03, p < .01, such that longer time in poverty and poorer housing quality were both uniquely associated with an overall higher level of cortisol during the child’s first four years. None of these variables interacted with either the linear or polynomial terms for time. Results did indicate, however, an effect for the interaction of number of adult exits from the home and time, b = .08, se = .03, p < .05. Examination of this effect, presented in Figure 1, indicated that children in families experiencing two or more adult exits from the home over the first four years had higher levels of cortisol at time points following seven months of age than did children in families experiencing one or no adult exits. Interestingly this effect is present when controlling for the number of adult entrances into the home over the first four years as well as the overall number of adults in the household. The marginal negative effect for number of adults in the household indicates that a greater number of adults in the household tended to be associated with an overall lower level of child cortisol across the first four years, suggesting that the effect for number of exits from the household is not merely a function of a greater number of adults present.
Table 2.
Regression coefficients and standard errors for the mixed model analysis predicting cortisol level at child age 7, 15, 24, and 48 months.
| Model A | Model B | Model C | ||||
|---|---|---|---|---|---|---|
| Variable | B | SE | B | SE | B | SE |
| Intercept | −0.609 | 0.29 | −0.587 | 0.30 | −0.673 | 0.315 |
| Time | −0.003 | 0.23 | −0.003 | 0.23 | −0.003 | 0.228 |
| Time*Time | −0.007 | 0.05 | −0.007 | 0.05 | −0.007 | 0.045 |
| PA White vs NC White | 0.040 | 0.03 | 0.043 | 0.03 | 0.049 | 0.034 |
| NC Black vs NC White | 0.202*** | 0.03 | 0.181*** | 0.03 | 0.181*** | 0.034 |
| Time of day 7 mos | 0.001*** | 0.000 | 0.000*** | 0.000 | 0.000*** | 0.000 |
| Time of day 15 mos | −0.012** | 0.004 | −0.012** | 0.004 | −0.012* | 0.004 |
| Time of day 24 mos | −0.011* | 0.004 | −0.011* | 0.004 | −0.010* | 0.004 |
| Time of day 48 mos | −0.017*** | 0.004 | −0.017*** | 0.004 | −0.017* | 0.004 |
| Time in poverty | −0.020* | 0.009 | −0.022* | 0.009 | −0.022* | 0.009 |
| Adult exits from home | −0.160 | 0.09 | −0.158 | 0.092 | −0.152 | 0.092 |
| Adult exits * time | 0.077* | 0.03 | 0.077* | 0.033 | 0.077* | 0.033 |
| Adult entrances | 0.016 | 0.02 | 0.012 | 0.022 | 0.010 | 0.022 |
| Maternal education | −0.008 | 0.006 | −0.004 | 0.006 | −0.003 | 0.006 |
| Housing Quality | −0.106** | 0.03 | −0.091** | 0.033 | −0.095** | 0.033 |
| Economic need | −0.217 | 0.10 | −0.218 | 0.097 | −0.219 | 0.097 |
| Time*economic need | 0.173 | 0.089 | 0.173 | 0.089 | 0.173 | 0.089 |
| Time*time*econ need | −0.034 | 0.02 | −0.034 | 0.018 | −0.034 | 0.018 |
| Economic sufficiency | 0.352* | 0.15 | 0.352* | 0.154 | 0.351* | 0.154 |
| Time*econ sufficiency | −0.307* | 0.14 | −0.307* | 0.139 | −0.307* | 0.139 |
| Time*time*econ suff | 0.055* | 0.03 | 0.055* | 0.027 | 0.055* | 0.027 |
| Number of adults | −0.018 | 0.01 | −0.017 | 0.010 | −0.015 | 0.010 |
| Positive parenting | −0.048* | 0.021 | −0.050* | 0.021 | ||
| Negative parenting | 0.011 | 0.022 | 0.009 | 0.022 | ||
| Peak emotionality | −0.089* | 0.036 | ||||
| Infant temperament | 0.002 | 0.002 | ||||
p < .05
p < .01
p < .001
Figure 1.
Relation between resting cortisol at 7, 15, 24, and 48 months of age and number of adult exits from the home; solid line = 2 or more exits, dashed line = 0 or 1 exits.
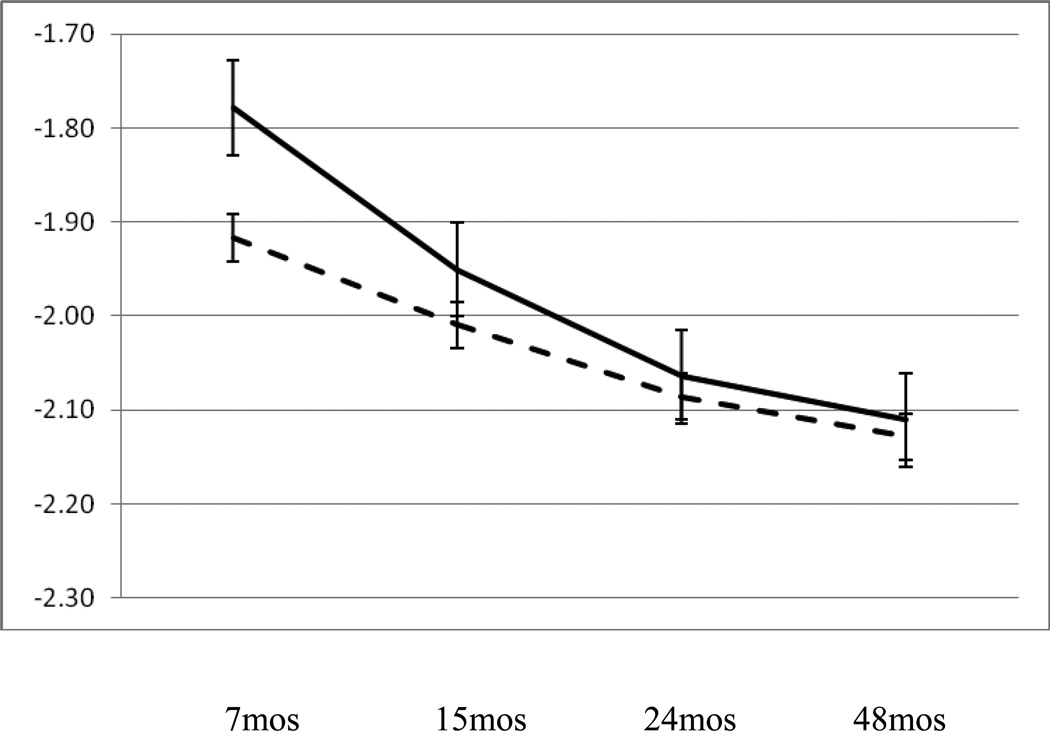
The model presented in Table 2 also indicated a significant polynomial term for the interaction of perceived economic sufficiency with time, b = .06, se = .03, p < .05. This effect graphed in Figure 2 indicated that children in families reporting low perceived economic sufficiency had higher basal cortisol values at age 7 months and exhibited a steeper decline between 7 and 24 months followed by a flatter trajectory toward 48 month levels than did children in families reporting higher perceived sufficiency.
Figure 2.
Relation between resting cortisol at 7, 15, 24, and 48 months of age and perceived economic sufficiency; solid line = highest 25% of perceived insufficiency, dashed line = lower 75% perceived insufficiency.
Mediation of environmental risk by parenting
To examine the possibility that early caregiving mediates the associations between environmental risks and child cortisol presented in Model B in Table 2, we next included terms for mean levels of observed positive and negative parenting over the child’s first two years. Results indicated a main effect for observed positive, b = − .05, se = .02, p < .05, but not negative parenting and no interaction of either parenting variable with the linear and polynomial terms for time. As seen in Table 2, inclusion of the main effect for positive parenting in the regression equation had little effect on the environmental risk variables. Coefficients for these variables changed minimally if at all, suggesting that the effects of environmental risks on child cortisol over the first four years are distinct from observed parenting as measured in this study. The two terms in the model demonstrating some change with the inclusion of positive parenting, however, were African American ethnicity as indicated by the coefficient for the comparison of the North Carolina African American and White samples, and housing quality. Changes to these coefficients were relatively small, however, indicating that only approximately 10% of the effect of each variable on child cortisol levels over the child’s first four years was accounted for by observed positive parenting.
Moderation of environmental risk by parenting
Next we examined the possibility that observed positive parenting would moderate some of the effect of environmental risk on child cortisol. The inclusion of terms for the interaction of positive parenting with chronicity of poverty, housing quality, adult exists from the home, and economic sufficiency indicated no significant interactions.
The role of child temperament
We then examined child temperamental reactivity and observed emotional reactivity at age 7 months as influences on child cortisol levels over the first four years. As seen in Model C in Table 2, inclusion of terms for the temperament composite as reported by the data collectors using the modified version of the Bayley IBR rating scale and the observed peak emotional reactivity to the emotion challenge tasks indicated a main effect for the latter variable only. Neither variable interacted with the linear or polynomial terms for time. The finding for emotional reactivity indicated that children who were observed to be more emotionally reactive to the tasks had lower rather than higher levels of cortisol over the first four years, indicating that greater emotional reactivity at this age is a positive characteristic of children in relation to change in cortisol levels. The observation of the unique effect for this variable when controlling for environmental risk and parenting suggests that higher reactivity to emotional stimulation in infancy may indicate of some aspect of prenatal or early postnatal experience leading to higher emotional reactivity and lower levels of cortisol.
Discussion
To our knowledge this is the first study of its kind to examine change in cortisol in early childhood using a prospective longitudinal design from infancy to age four years in which participants were representatively sampled from predominantly low-income and nonurban communities. As such, the findings tended to confirm the expectation that early adversity in the context of poverty would be associated with increased allostatic load as indicated by higher levels of resting or basal cortisol in early childhood. Our analysis indicated that both the material and psychosocial aspects of poverty are relevant to understanding how poverty might get under the skin to influence development. Specifically several aspects of poverty over the child’s first four years, including length of time in poverty, housing quality, perceived economic insufficiency, and adult exits from the home were each uniquely associated with higher resting levels of cortisol in children. In particular we were interested in the extent to which the results would indicate a pattern of increasing allostatic load in which chronic adversity cumulatively leads to elevations in stress physiology. Results from our analysis did indicate that this pattern was present for children in homes in which two or more adult exits occurred. Specifically, resting cortisol levels at 7 months of age for children in homes that experienced two or more adult exits over the first four years were no different from those of children in homes with one or no exits. At subsequent time points, however, the difference in cortisol levels associated with exits increased as a function of time such that the difference in cortisol levels at age 48 months associated with this variable was substantial. This trend in cortisol associated with exits from the home was driven by the fact that children in homes with one or no exits exhibited a normative or typical decline in average resting cortisol levels in early childhood. The finding here that this pattern of typical or normative change is absent for children experiencing a high number of exits suggests a risk related alteration of the set point of the HPA axis as reflected in salivary cortisol levels.
In addition to the finding of an effect of exits from the home on cortisol levels over time, our regression models also indicated that the effect of perceived economic insufficiency varied as a function of time in a polynomial fashion. This pattern was distinct from that for exits in that cortisol levels were higher at age 7 months for children in homes in which caregivers reported greater difficulty making ends meet but the decline in resting cortisol for these children was steeper between 7 and 24 months leading to no difference in cortisol at 48 months associated with perceived insufficiency. It may be that this effect indicates that stress associated with economic insufficiency is most acute in the infancy period when caregivers are faced with the care of a newborn and the dependence of the child on the caregiver is very high. It may also be indicative of the idea that caregiver concerns and anxiety related to insufficiency is most disruptive to the parent–child relationship at earlier rather than later time points.
Main effects but not interactions with time were observed for the chronicity of poverty, housing quality, African American ethnicity and observed prototypically sensitive caregiving behavior. Each of these variables was uniquely associated with cortisol in early childhood. Children facing chronic poverty, living in lower quality housing, of African American ethnicity, and receiving lower levels of positive parenting were found to have consistently elevated cortisol levels in early childhood that persist at least through age 4 years.
Possible mediation of risk through caregiving
In this analysis we were particularly interested in the extent to which caregiving might mediate the association among risk variables and child cortisol. This interest was motivated by evidence from a variety of species suggesting that caregiving behavior serves as a major conduit of information about the environment, working through stress hormones to increase or decrease defensive, i.e., reactive responses to environmental contingencies (Cameron et al., 2005). In environments that are inhospitable and unpredictable, types of caregiving that are less sensitive and more directive would be expected to increase resting levels of stress hormones and to potentiate more reactive responses to stimulation. In contrast, more prototypically sensitive types of care would be expected to lead to low resting levels and more flexible up and down regulation of stress hormones associated with more well regulated responses to stimulation. In our analysis, however, we found no evidence to suggest that either positive or negative aspects of caregiving behavior were mediating relations between risk and child cortisol in a way that is consistent with models of context, caregiving, and stress physiology in animal models. As well, unlike the findings of Evans et al. (2007) in which caregiving was shown to moderate an association between psychosocial risk and allostatic load in a sample of children in early adolescence, we found no evidence of the moderation of risk by positive caregiving behavior in early childhood.
Elevated cortisol levels in African American children
Two variables that did share some variance in child cortisol with positive parenting were African American ethnicity and housing quality, however, this overlap was small. Although findings for poverty, housing, and parenting behavior are somewhat expected, the finding in this sample that cortisol is elevated in African American children relative to their white counterparts even when controlling for multiple aspects of risk is somewhat surprising and alarming. The finding for African American ethnicity may indicate an enduring intergenerational effect of social injustice on stress physiology levels in African Americans such as that hypothesized to be associated with intractable disparities in birth and health outcomes (Kuzawa & Sweet, 2009; Lu & Halfon, 2003). We have noted this pronounced effect for African American ethnicity in prior analyses of child cortisol in the Family Life Project data set and note here that it is robust even when including a wider variety of aspects of risk associated with poverty than those we have examined previously (Blair et al., 2008, in press). We also note that the effect observed here in which positive parenting partially mediated the association between Africans American ethnicity and cortisol was also observed in an analysis using structural equation modeling to examine the relation of poverty to basal cortisol levels at 7, 15, and 24 months and executive functioning at age 36 months (Blair et al., in press). In that analysis, the mediated effect for African American ethnicity through positive parenting on cortisol was similarly small even in a latent variable framework, however, the effect for African American ethnicity on executive function and IQ was completely mediated through a combination of cortisol, parenting, and household risk variables.
Temperament and emotional reactivity in infancy
In terms of differential susceptibility theory our findings indicated that our measure of child temperamental negativity was not a unique indicator of child salivary cortisol levels in a way that would suggest cortisol might act as an intermediate indicator of higher versus lower susceptibility to environmental influence. When we examined the effect for the data collectors’ reports of child temperamental irritability to the regression equation predicting cortisol, this variable was not uniquely related either to child cortisol levels overall or to change in cortisol levels over time. It may be that irritability at later time points is associated with cortisol levels but the important point for this analysis is that the early measure of temperament, which is presumed to be most indicative of the aspect of child behavior acted on differentially by the environment, was not related to cortisol over and above the measures of environmental quality. In sum, it appears that environmental quality may be activity shaping cortisol levels in children in a way consistent with the theory of allostasis, rather than cortisol levels representing a somewhat unique aspect of child functioning that could promote or impede development in a for better and for worse manner in high versus low quality environments as predicted by the differential susceptibility theory.
Conclusions, implications, and limitations
In conclusion, in this paper we examined the extent to which early environmental adversity might be shaping underlying stress physiology in ways that have implications for child development and longer term risk for psychopathology as suggested by the principles of allostasis and allostatic load. We found that two aspects of the early environment in the context of poverty, adult exits from the home and perceived economic insufficiency, were related to salivary cortisol in a time dependent manner. The effect for the first of these, exits from the home, was consistent with a process of allostasis in which the effects of adversity accumulated over time. The effect for the second, perceived insufficiency was somewhat different in that children in families reporting a high level of insufficiency exhibited higher levels of cortisol in infancy but then exhibited a typical but steeper decline in typical or resting cortisol levels at subsequent time points. In contrast to the interaction of each of these variables with time, we found main effects for length of time in poverty, poor housing quality, African American ethnicity, and low positive caregiving behavior in which each was uniquely associated with an overall higher level of cortisol from age 7 months to 48 months. The absence of an interaction between time and each of these variables may indicate that each represents a pre-existing influence on cortisol or that they reflect aspects of experience that occurred in the prenatal or early postnatal (prior to age 7 months) environment to increase cortisol levels in early childhood.
One implication of our findings for the study of developing psychopathology concerns the indication in an increasing number of studies that elevated basal cortisol levels are associated with risk for internalizing disorders (Lopez-Duran, Kovacs, & George, 2009). Longitudinal studies with children have tended to demonstrate associations between elevated cortisol and internalizing disorders and low levels of cortisol and externalizing disorders (Gunnar & Vasquez, 2006). Given a generally high correlation between externalizing and internalizing types of behaviors in childhood, however, elevated cortisol levels may be a nonspecific indicator of risk for later symptom severity leading either to internalizing or externalizing disorders depending on the types of experiences encountered in middle childhood and early adolescence (Shirtcliff & Essex, 2008). Dysregulation of the HPA axis is characteristic of a range of mental health problems and as such our findings suggest that the aspects of the home environment in combination with child temperament may lead through processes of allostatic load to increases in levels of basal cortisol that render the individual more susceptible to the types of experiences in the family, with peers, and in school and community contexts that precipitate the development of psychopathology, whether of an internalizing or externalizing variety.
Although our results provide a somewhat unique prospective look at levels of salivary cortisol in early childhood with implications for the development of psychopathology, our analysis is limited in certain respects. One of these limitations concerns the fact that our study design was such that we examined cortisol for each individual at each time point using only one saliva collection and that time of day of data collection varied for individuals in the sample. This aspect of our cortisol data is potentially problematic in that cortisol follows a pronounced diurnal pattern in which resting levels are high in the morning and low in the evening. Therefore, the extent to which time of day of sample collection might have varied systematically with risk in our sample is a potential threat to the validity of our findings. Furthermore, given that we were collecting data in the home with young children, it is possible that the presence of the data collector in the home, a stranger, may have differentially influenced cortisol levels in children, with more temperamentally anxious children demonstrating a reactive response to the stranger’s presence. Three aspects of our data collection, however, help to alleviate concerns relating to these points. The first is that although time of day of data collection did vary in the sample, the majority of families participating in the study were seen between the hours of 10am and 4pm, a time of relatively gradual diurnal change in cortisol. Secondly, although higher income families were somewhat more likely to be seen later in the day than lower income families, raising potential concerns about the artificial inflation of the association between cortisol and risk, the association of income with time of day of data collection was very small and we controlled for time of day of data collection in all of our analyses. Thirdly, for all but the last time point in our study, saliva samples were collected late in the home visit for data collection, at which point the data collectors had been in the home for at least one hour, providing ample time for any effect of the presence of the stranger in the home on child cortisol levels to dissipate (Fernald & Gunnar, 2009).
A second limitation to our study concerns the fact that we have only salivary cortisol as an indicator of allostatic load of environmental risk on stress physiology. Although salivary cortisol is an excellent marker for the impact of environmental stress and has been used in many studies, it is limited in certain respects. The HPA is a complex system for which levels of cortisol detectable in saliva are merely an endpoint of a dynamic process. As valuable as salivary cortisol has been as a marker of alterations to stress physiology, however, in the examination of allostatic load it is desirable to have multiple indicators of stress physiology, including measures of the sympathetic-adrenal system. For example, the measurement of stress physiology in the research of Evans and colleagues (e.g., Evans et al., 2007) included measures of overnight urinary levels of catecholamines as well as cortisol. A further welcome addition to the measurement of allostatic load in children would be measures of cardiac physiology, both respiratory sinus arrhythmia as well as the pre-ejection period, and to have markers of inflammatory immune system processes. Although it was not feasible in the design of this study to include multiple measures of stress physiology and immune system processes due to constraints on data collection and participant burden, we hope that in subsequent data collections with this sample and in other studies to have more extensive measurement of allostatic load.
Thirdly, a further somewhat general but no less pressing limitation to our findings concerns the fact that the study is correlational and can provide no causal evidence of the association between environmental adversity and cortisol levels in children. Recent experimental evaluation of an intervention for children experiencing severe caregiving disruption associated with foster care (Fisher, Stoolmiller, Gunnar, & Burraston, 2007) and quasi-experimental evaluation of an income redistribution intervention in low-income communities in Mexico (Fernald & Gunnar, 2009) indicated that changes in the caregiving environment and in the financial status of homes have meaningful effects on child cortisol levels. The evaluation of the foster care intervention indicated that therapeutic treatment to increase caregiving sensitivity for children experience multiple placements resulted in an increasingly typical diurnal pattern in cortisol for children in the treated group. Similarly, the evaluation of the income redistribution program indicated that children in homes in a community receiving income supplements had lower levels of cortisol adjusted for time of day and for the presence of the data collectors in the home than did children in a carefully selected comparison community.
Findings such as these help to bolster confidence in the associations between environmental adversity and child cortisol reported here and suggest that efforts to alleviate poverty and improve the experiences of children in poverty can have meaningful benefits to child health and well being. Longitudinal associations among disruptions in early caregiving, altered levels of cortisol, and increased risk for psychological disorders suggest a process through which early life experiences get under the skin to increase risk for mental health disorders. Our analysis, which is one of the first to examine prospective longitudinal data on this process, suggests that efforts to alleviate stress associated with poverty and that include the measurement of allostatic load as an aspect of the evaluation of such efforts are likely to provide valuable data with which to inform social policy directed at fostering the well being of children in poverty. In particular, these data suggest the value of programs to enhance caregiving sensitivity as one primary mechanism through which to beneficially influence child development. For example, using a randomized design, Landry and colleagues have shown that training to assist parents in interacting in a developmentally sensitive manner with infants and toddlers through interactive coaching and provision of targeted examples results in higher levels of communicative behavior and complex play in infants and lower levels of negative affect (Landry, Smith, & Swank, 2006). Associations between the psychosocial characteristics of the home and child levels of cortisol that are independent of parenting influences, however, also suggest the need for services that target family economic well-being and and household quality, directly. Programs that alleviate poverty (including the Earned Income Tax Credit) and that buffer families from some of poverty’s most deleterious sequelae (such as Section 8 housing choice vouchers) would be expected to have clear salutary benefits for low-income children’s trajectories of regulation and allostasis, over time (Eamon, Wu & Zhang, 2009).
Acknowledgements
We would like to thank the many families and research assistants that made this study possible. Support for this research was provided by the National Institute of Child Health and Human Development grants R01 HD51502 and P01 HD39667 with co-funding from the National Institute on Drug Abuse.
References
- Arnsten AF, Li BM. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biological Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Bayley N. Manual for Bayley scales of in/ant development. New York: Psychological Corporation; 1969. [Google Scholar]
- Belsky J. Theory testing, effect-size evaluation, and differential susceptibility to rearing influence: The case of mothering and attachment. Child Development. 1997;68(4):598–600. [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Blair C. Stress and the development of self-regulation in context. Child Development Perspectives. 2010;4:181–188. doi: 10.1111/j.1750-8606.2010.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Diamond A. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Development and Psychopathology. 2008;20(3):899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger DA, Kivlighan KT, Mills-Koonce R, Willoughby M, Greenberg MT, Hibel L, Fortunato C the Family Life Project Investigators. Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology. 2008;44:1095–1109. doi: 10.1037/0012-1649.44.4.1095. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger D, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, Kivlighan K, Fortunato C the FLP Investigators. Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development. in press doi: 10.1111/j.1467-8624.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Socioeconomic status and child development. Annual Review of Psychology. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Duncan G. Economic deprivation and early childhood development. Child Development. 1994;65:296–318. [PubMed] [Google Scholar]
- Buckner JC, Mezzacappa E, Beardslee WR. Characteristics of resilient youths living in poverty: The role of self-regulatory processes. Development and Psychopathology. 2003;15(1):139–162. doi: 10.1017/s0954579403000087. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29(4–5):843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. The Journal of Neuroscience. 2007;27(11):2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Adaptive coping under conditions of extreme stress: Multilevel influences on the determinants of resilience in maltreated children. New Directions for Child and Adolescent Development. 2009;2009(124):47–59. doi: 10.1002/cd.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD, Elder GH. Families in troubled times: Adapting to change in rural America. NY: Aldine de Gruyter; 1994. [Google Scholar]
- Cox M, Paley B, Burchinal M, Payne C. Marital perceptions and interactions across the transition to parenthood. Journal of Marriage and the Family. 1999;61:611–625. [Google Scholar]
- Cummings EM, El-Sheikh M, Kouros CD, Buckhalt JA. Children and violence: The role of children's regulation in the marital aggression-child adjustment link. Clinical Child and Family Psychology Review. 2009;12(1):3–15. doi: 10.1007/s10567-009-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis WJ, Cicchetti D. Moving research on resilience into the 21st century: Theoretical and methodological considerations in examining the biological contributors to resilience. Development and Psychopathology. 2003;15(3):773–810. doi: 10.1017/s0954579403000373. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the yerkes-dodson law. Neural Plasticity. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamon MK, Wu C, Zhang S. Effectiveness and limitations of the earned income tax credit for reducing child poverty in the United States. Children and Youth Services Review l. 2009;31:919–926. [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39(5):924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald LC, Gunnar MR. Poverty-alleviation program participation and salivary cortisol in very low-income children. Social Science & Medicine (1982) 2009;68(12):2180–2189. doi: 10.1016/j.socscimed.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: Impact on children's behavior, neuroendocrine activity, and foster parent functioning. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(11):1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(8–10):892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology & Behavior. 2007;92(4):583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10-12-year-old children. Psychoneuroendocrinology. 2009;34(1):62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55(4):376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Harmon AG, Hibel LC, Rumyantseva O, Granger DA. Measuring salivary cortisol in studies of child development: Watch out--what goes in may not come out of saliva collection devices. Developmental Psychobiology. 2007;49(5):495–500. doi: 10.1002/dev.20231. [DOI] [PubMed] [Google Scholar]
- Hart Risley. Meaningful differences in the everyday experience of young American children. Baltimore, MD: Brookes; 1995. [Google Scholar]
- Hibel LC, Granger DA, Kivlighan KT, Blair C the Family Life Project Investigators. Individual differences in salivary cortisol: Relation to common over-the-counter and prescription medications in infants and their mothers. Hormones and Behavior. 2006;50:293–300. doi: 10.1016/j.yhbeh.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: Developmental origins of US racial disparities in cardiovascular health. American Journal of Human Biology : The Official Journal of the Human Biology Council. 2009;21(1):2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR. Responsive parenting: Establishing early foundations for social, communication, and independent problem-solving skills. Developmental Psychology. 2006;42(4):627–642. doi: 10.1037/0012-1649.42.4.627. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran N, Kovacs M, George C. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: A life-course perspective. Maternal and Child Health Journal. 2003;7(1):13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biological Psychiatry. 2000;48(10):976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. The New England Journal of Medicine. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 2000;886(1–2):172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. The American Psychologist. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24(1):1161. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network [NICHD ECCRN] Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Developmental Psychology. 1999;35(5):1297–1310. doi: 10.1037//0012-1649.35.5.1297. [DOI] [PubMed] [Google Scholar]
- Porges S. The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–366. [PubMed] [Google Scholar]
- Shirtcliff E, Essex M. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA : The Journal of the American Medical Association. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Braungart JM. The regulation of negative reactivity in infancy: Function and development. Developmental Psychology. 1995;31(3):448–55. [Google Scholar]
- Stifter C, Corey J. Vagal regulation and observed social behavior in infancy. Social Development. 2001;10:189–201. [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences, USA. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]