Abstract
BACKGROUND
Hypoxic preconditioning (HPc) protects the neonatal brain in the setting of hypoxia-ischemia (HI). The mechanisms of protection may depend on activation of hypoxia inducible factor (HIF-1α). The present study sought to clarify the role of HIF-1α after HPc and HI.
METHODS
To induce HPc, HIF-1α knockout and wildtype mice were exposed to hypoxia at postnatal day 6. At day 7, the mice underwent HI. Brain injury was determined by histology. HIF-1α, downstream targets, and markers of cell death were measured by Western blot.
RESULTS
HPc protected the wildtype brain compared to wildtype without HPc, but did not protect the HIF-1α knockout brain. In wildtype, HIF-1α increased after hypoxia and after HI, but not with HPc. The HIF-1α knockout showed no change in HIF-1α after hypoxia, HI, or HPc/HI. After HI, spectrin 145/150 was higher in HIF-1α knockout, but after HPc/HI, it was higher in wildtype. LAMP2 was higher in wildtype early after HI, but not later. After HPc/HI, LAMP2 was higher in HIF-1α knockout.
CONCLUSION
These results indicate that HIF-1α is necessary for HPc protection in the neonatal brain, and may affect cell death after HI. Different death and repair mechanisms depend on the timing of HPc.
Undetectable under normoxic conditions, the transcription factor HIF-1α is activated and stabilized in response to hypoxia, suggesting that it has a role in response to oxidative injury, and in hypoxic preconditioning (HPc) protection (1). In the neonatal brain, a prior episode of hypoxia can attenuate injury from subsequent hypoxia-ischemia (HI) (2) and the protection afforded by HPc is long-lasting (3). Just as the mechanisms of neuronal cell death are complex (4), the mechanisms of HPc protection likely involve a complex cascade of cellular and molecular events. HPc has been shown to induce over 1000 genes in the P6 rat brain within the first 24 h, predominantly genes involved in apoptosis and brain development (5). Hypoxia induces many of the genes regulated by HIF-1α in the brain (6), and preconditioning with hypoxia induces HIF-1α and promotes cell survival in the subsequently hypoxic or ischemic brain (1). The role of HIF-1 and its target genes, whether beneficial or detrimental, depends on such factors as severity and type of insult and age of the animal (7).
Of particular interest among the large number of target genes of HIF-1 are vascular endothelial growth factor (VEGF) and erythropoietin (EPO) as these have been shown to be induced by hypoxia (8, 9) and to have protective properties in the brain under certain conditions (10), (11), (12), as well as to brain cells in vitro (13). VEGF administered intracerebroventricularly (ICV) to neonatal rats after HI can decrease the severity of injury, in a dose-dependent manner (14) and exogenous EPO shows beneficial affects, given either ICV (15) or systemically (12), after neonatal HI and stroke.
We have previously suggested that there is a protective role for HIF-1α in neonatal HI, since genetic reduction of HIF-1α worsens HI brain injury (16). In order to explore the role of HIF-1α in HPc, we used the same strain of mutant mice with a neuron-specific reduction of HIF-1α and subjected these mice to a period of hypoxia, as a preconditioning stimulus, prior to HI. We measured histopathological brain injury and downstream regulators (VEGF, ERK) and protein markers for cell death pathways [spectrin breakdown products for necrosis and apoptosis and lysosome-associated membrane protein (LAMP2) for autophagy] to better understand how brain injury is affected after HPc.
RESULTS
Histological Analysis for Degree of Injury
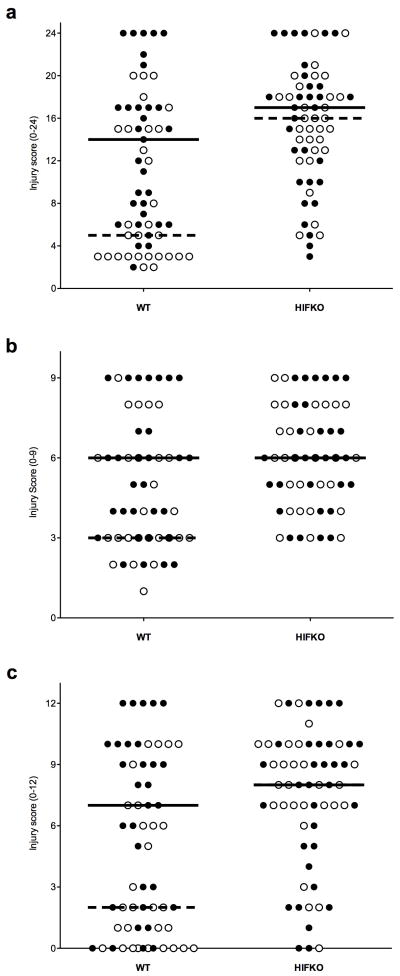
HPc was associated with significantly less histopathological damage in selectively vulnerable brain regions of the wildtype brain with HPc than wildtype without HPc (Figure 1 and Supplementary Figure 1 online). (Figure 1a: median scores, wildtype-HI = 14 (range = 2–24), wildtype-HPc/HI = 5 (range 2–20), p<0.01). The HIF-1α deficient brain, however, showed no protection with HPc (Figure 1a: median scores, HIF-1α knockout-HI = 17 (range 3–24), HIF-1α knockout-HPc/HI = 16 (range 5–24). Significant protection in the wildtype-HPc/HI mouse brain was seen both in the cortex (Figure 1b: median scores, WT-HI = 6 (range 2–9), wildtype-HPc/HI = 3 (range 1–9), p=0.01) and hippocampus (Figure 1c: median scores, wildtype-HI = 7 (range 0–12), wildtype-HPc/HI = 2 (range 0–10), p<0.001). The median score of the cortex for HIF-1α knockout-HI = 6 (range 3–9) and HIF-1α knockout-HPc/HI = 6 (range 3–9). The median score of the hippocampus for HIF-1α knockout-HI = 8 (range 0–12) and HIF-1α knockout-HPC/HI = 8 (range 0–12).
Figure 1.
Hypoxic-ischemic brain injury scores. Filled circles represent brains with HI alone and open circles represent brains with HPc prior to HI. Solid horizontal line represents median score of mice receiving HI alone and dashed line represents median score of mice receiving HPc prior to HI. (a) Overall score (range 0–24). HPc protected the wildtype brain from subsequent HI. Wildtype-HI (median = 14, range 2–24) vs. wildtype-HPc/HI (median = 5, range 2–20), p=0.01. (b) Cortex (range 0–9). Wildtype-HI (median = 6 range 2–9) vs. wildtype-HPc/HI (median = 3 range 1–9) p=0.01. (c) Hippocampus (range 0–12). Wildtype-HI (median = 7 range 0–12) vs wildtype-HPc/HI (median = 2 range 0–10) p=0.001. The HIF-1α knockout brain was not protected by HPc. (a) HIF-1α knockout-HI (median = 17, range 3–24) vs HIF-1α knockout-HPc/HI (median = 16, range 5–24). (b) HIF-1α knockout-HI vs HIF-1α knockout-HPc/HI (both: median = 6 range 3–9). (c) HIF-1α knockout-HI vs HIF-1α knockout-HPc/HI (both: median = 8, range 0–12).
Animal numbers, mortality and sex of histology group
For determination of brain injury by histological analysis, the total number of wildtype mice was 123; 56 (46%) underwent HPc. The total number of HIF-1α knockout mice was 117; 53 (45%) underwent HPc (Table 1a). Mortality occurred both during and after experimental procedures (Table 1b). There were no differences in injury scores between male and female in each group (Table 1c).
Table 1.
Animal numbers, mortality and sex.
| aNumber of mice and mortality: n (%) | bManner of death: n (%) | cSex: n (median score) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Survived | Died | HPc | w/dam | hypoxia | post-tx | Male | Female | |
| WT-HI | 67 (28) | 32 (48) | 35 (52) | NA | 6 (17) | 22 (63) | 7 (20) | 17 (12) | 15 (15) |
| WT-HPc/HI | 56 (23) | 35 (63) | 21 (37) | 1 (5) | 5 (24) | 9 (43) | 6 (29) | 16 (8) | 19 ( 6) |
| KO HI | 64 (27) | 37 (58) | 27 (42) | NA | 4 (15) | 17 (63) | 6 (22) | 12 (15) | 25 (18) |
| KO HPc/HI | 53 (22) | 33 (62) | 20 (38) | 7 (35) | 3 (15) | 5 (25) | 5 (25) | 15 (15) | 18 (17) |
| TOTAL | 240 | 137 (57) | 103 (43) | ||||||
Total number of mice (n) and (%) per treatment group. Total number (n) and (%) that survived to perfusion at P12 or died prior to P12.
Manner of death for those that died before perfusion: during HPc; with dam after surgery; during post-surgery hypoxia treatment; after all treatments.
Total number of male and female mouse brains per experimental group scored for degree of injury and median score for each group.
Protein Expression
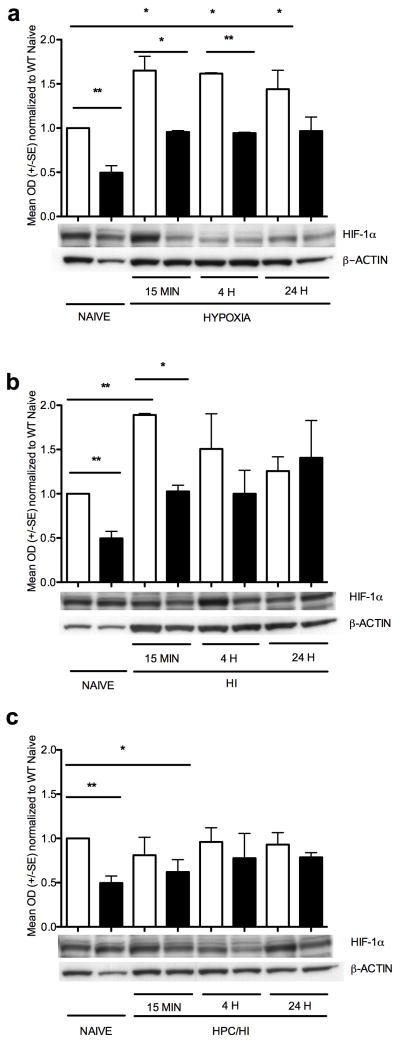
HIF-1α protein expression increased in wildtype but not in HIF-1α knockout cortex after HPc (Figure 2a). Wildtype-hypoxia is higher than wildtype naïve at 15 min (p<0.02), at 4 h (p<0.01) and at 24 h (p<0.05). HIF-1α protein expression was also higher in wildtype naïve compared to HIF-1α knockout naïve (Figure 2a, p<0.002) and in wildtype-hypoxia compared to HIF-1α knockout-hypoxia at 15 min (p<0.02) and at 4 h (p=0.001). By 24 h after hypoxia, however, HIF-1α expression in the wildtype has declined to levels similar to HIF-1α knockout (p<0.10). In mice subjected to HI (Figure 2b), HIF-1α protein expression increased in wildtype early (15 min) compared to wildtype naive (p<0.002) but was not changed significantly at later timepoints (4 h or 24 h). Wildtype is also higher than HIF-1α knockout at 15 min (p<0.02) but not at 4 h or 24 h. HPc suppresses subsequent expression of HIF-1α after HI (Figure 2c). Although no elevation of HIF-1α was observed in wildtype cortex of HPc/HI treated brains when compared to naïve, HIF-1α expression was decreased in the HIF-1α knockout cortex at 15 min compared to wildtype naïve (*p<0.05).
Figure 2.
HIF-1α protein expression. Wildtype naïve have approximately twice the HIF-1α than HIF-1α knockout naïve (** p<0.002). (a) Mouse cortex after hypoxia only. In the wildtype mouse brain exposed to hypoxia, HIF-1 increased at 15 min (* p<0.02), at 4 h (* p<0.01) and at 24h (* p<0.05) compared to wildtype naïve. In addition, HIF-1α is elevated in the wildtype brain compared to HIF-1α knockout brain at 15 min (* p<0.02) and 4h (** p=0.001). HIF-1α does not change with hypoxia treatment in the HIF-1α knockout mice compared to HIF-1α knockout naïve at any time point. (b) Mouse cortex after HI. In the wildtype mouse brain exposed to HI, HIF-1α increased at 15 min compared to naïve (** p<0.002). In addition, HIF-1α is elevated in the wildtype brain compared to HIF-1α knockout brain at 15 min (* p<0.02). HIF-1α does not change after HI in the HIF-1α knockout cortex compared to naïve at any time point. (c) Mouse cortex after HPc and HI. HIF-1α does not change in the wildtype with HPc/HI. In the HIF-1α knockout, HIF-1α is decreased at 15 min compared to WT naïve (* p<0.05). White columns are wildtype. Black columns are HIF-1α knockout.
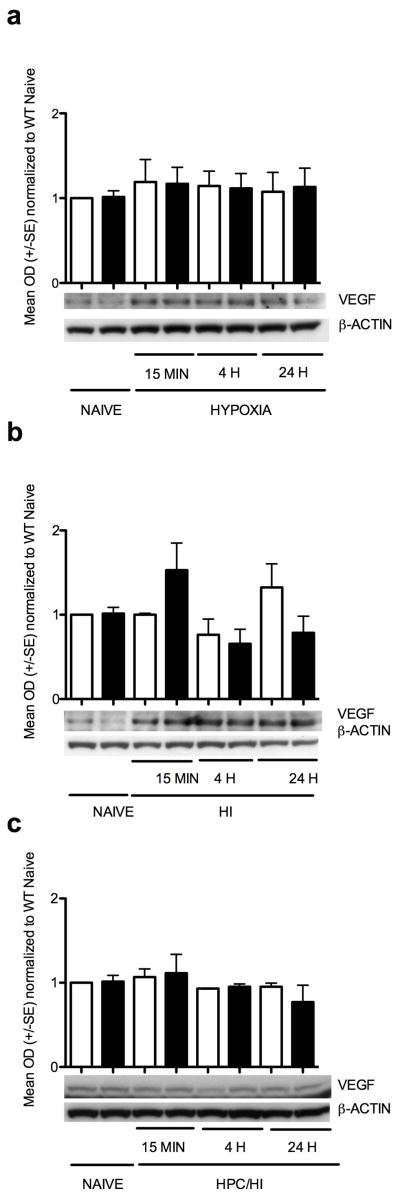
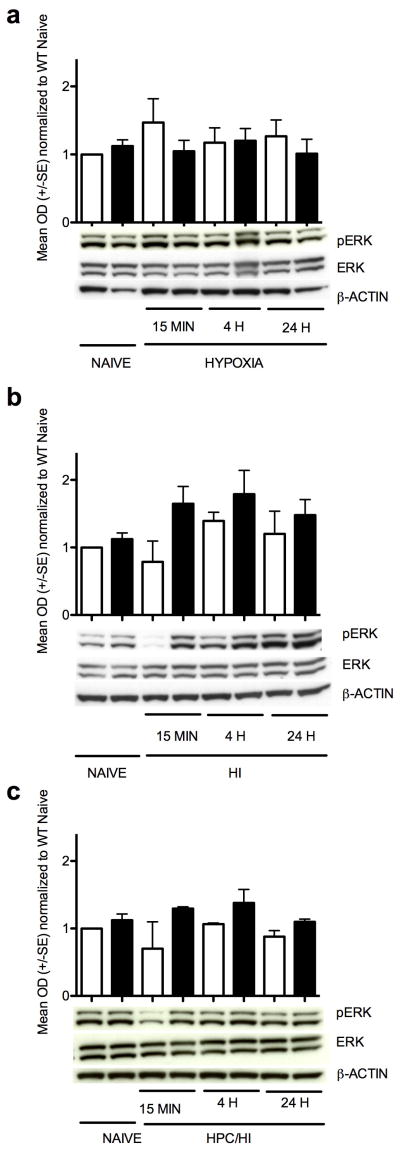
VEGF protein expression (Figure 3) was not significantly elevated in either the wildtype or HIF-1α knockout cortex by hypoxia, HI or HPc/HI. There were no significant differences in ERK activation, as shown by the pERK/ERK ratio, with hypoxia treatment alone, HI or HPc/HI (Figure 4).
Figure 3.
VEGF protein expression. (a) There were no changes in VEGF levels with hypoxia alone in either wildtype or HIF-1α knockout. (b) There also were no significant changes in VEGF levels after HI, although there was a trend toward an increase in the HIF-1α knockout 15 min after HI (p=0.11). (c) There was no change in VEGF expression in HPc/HI mouse cortex in either wildtype or HIF-1α knockout. White columns are wildtype. Black columns are HIF-1α knockout.
Figure 4.
ERK activation. Bar graph summarizes the ratio of pERK to ERK compared to wildtype naïve, there were no significant changes, (a) After hypoxia. There was a trend toward an increase in the wildtype cortex at 15 min (p=0.09). (b) After HI. There were trends toward an increase in HIFKO cortex at 15 min (p<0.14) and 4 h (p<0.09). There was a trend toward a decrease in wildtype at 15 min (p<0.06). (c) After HPc/HI. There was a trend toward a decrease in the wildtype cortex at 15 min, (p=0.06). White columns are wildtype. Black columns are HIF-1α knockout.
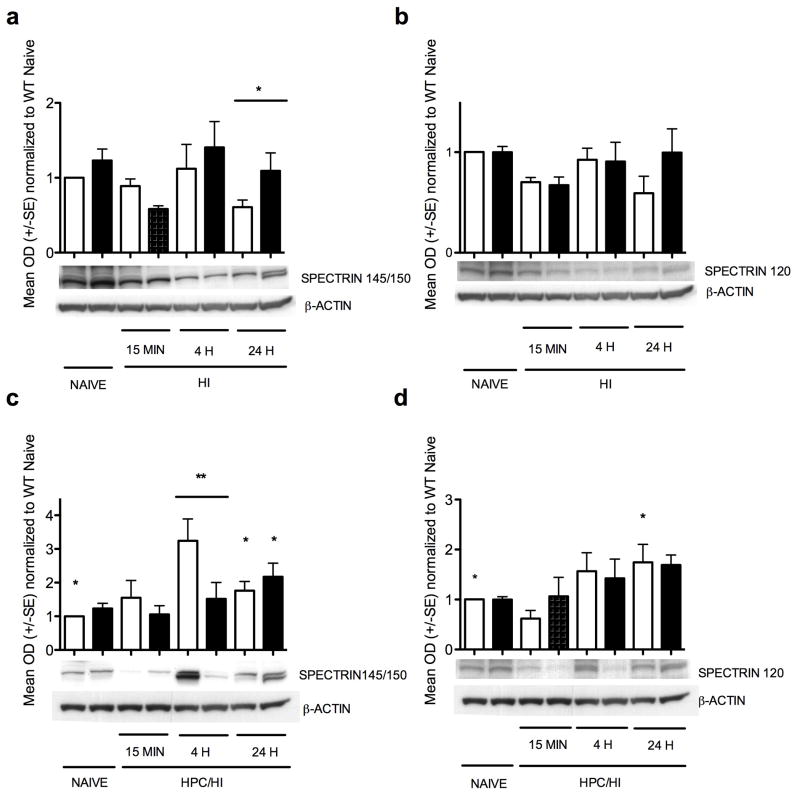
Spectrin was measured as a marker of the two major types of cell death (Figure 5). Necrotic cell death mediated via calpain is seen by the spectrin cleavage products at 145/150 kD, and apoptotic cell death mediated via caspase-3 is seen by the spectrin cleavage products at 120 kD. There were no changes with hypoxia alone (data not shown). After HI, however, spectrin 145/150 was higher at 24 h in HIF-1α knockout than wildtype (Figure 5a, p<0.04). Spectrin 120 was not significantly changed in either wildtype or HIF-1α knockout after HI, but the HIF-1α knockout cortex showed a trend toward more spectrin 120 than wildtype at 24 h (Figure 5b, p=0.08). After HPc/HI, spectrin 145/150 was 3-fold higher in wildtype than HIF-1α knockout at 4 h (Figure 5c, p=0.002). Also, both wildtype and HIF-1α knockout showed increased spectrin 145/150 at 24 h compared to wildtype naïve (Figure 5c, p<0.05). Spectrin 120 was only significantly higher in wildtype at 24 h after HPc/HI compared to wildtype naïve (Figure 5d, p<0.05).
Figure 5.
Spectrin protein expression. (a) Spectrin 145/150 after HI is higher in HIF-1α knockout than wildtype at 24 h (*p<0.04). (b) Spectrin 120 after HI. There were no significant changes in spectrin 120. (c) Spectrin 145/150 after HPc/HI is elevated 3-fold in wildtype compared to HIF-1α knockout at 4 h (** p=0.002), and is also elevated in both wildtype and HIF-1α knockout at 24 h compared to wildtype naïve (* p<0.05 for both). (d) Spectrin 120 after HPc/HI was elevated in wildtype at 24 h compared to wildtype naïve (*p<0.01). White columns are wildtype. Black columns are HIF-1α knockout.
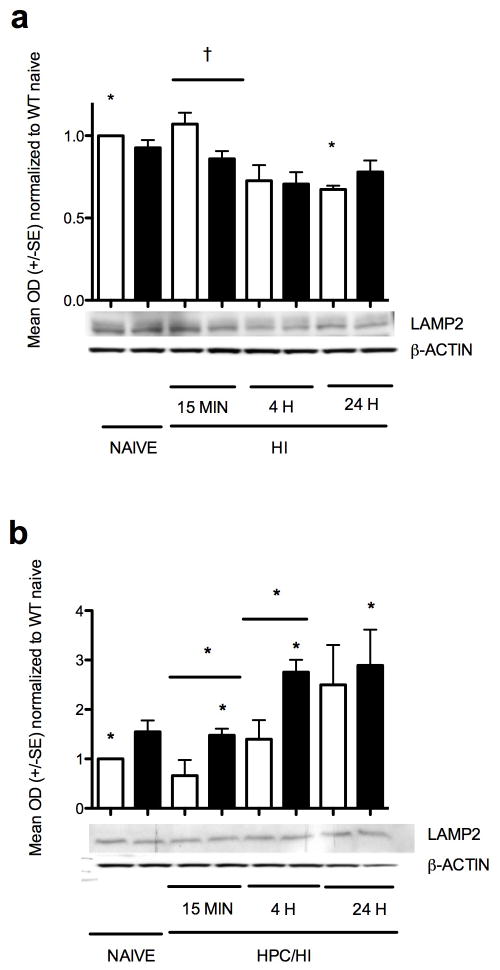
After HI, LAMP2, a marker of autophagy, was greater in wildtype compared to HIF-1α knockout at 15 min (Figure 6a, p<0.0002) but declined by 4 h and remained low at 24 h compared to wildtype naïve (p<0.02). After HPc/HI, LAMP2 was not significantly changed in wildtype compared to naïve. In the HIF-1α knockout, however, LAMP2 increased at 15 min (Figure 6b, p<0.03), again at 4 h (p<0.002) and remained elevated at 24 h (p<0.03). The HIF-1α knockout also had greater LAMP2 than corresponding wildtype with HPc/HI at 15 min (p<0.05, and 4 h (p<0.02) but not at 24 h.
Figure 6.
LAMP2 protein expression. (a) LAMP2 after HI was greater in wildtypecompared to HIF-1α knockout at 15 min (***p<0.0002) but declined by 4 h and remained low at 24 h compared to wildtype naïve (*p<0.02). (b) After HPc/HI, LAMP2 was not significantly changed in wildtype compared to naïve. In the HIF-1α knockout, however, LAMP2 increased at 15 min (*p<0.03), again at 4 h (*p<0.02) and remained elevated at 24 h (*p<0.03) compared to naive. The HIF-1α knockout also had greater LAMP2 than corresponding wildtype with HPc/HI at 15 min (*p<0.05, and 4 h (*p<0.02) but not at 24 h. White columns are wildtype. Black columns are HIF-1α knockout.
DISCUSSION
This study supports the hypothesis that HIF-1α plays an important role in the pathophysiology of neonatal HI brain injury and is a necessary component of the protective mechanisms involved in HPc. We have previously shown that HIF-1α knockout mice have more severe injury than their wildtype littermates after HI, suggesting a protective role for HIF-1α (16). Similarly, in this study, the HIF-1α knockout mice again have higher median injury scores than wildtype littermates. The finding that HPc attenuates HI injury in the wildtype brain, while the HIF-1α deficient brain is strikingly resistant to protection by HPc, suggests that HPc-induced stabilization of HIF-1α is an important factor in subsequent protection from HI injury.
In a study of adult stroke using the same strain of conditional, neuron-specific HIF-1α knockout mouse, investigators found increased brain injury in the HIF-1α deficient mice compared to wildtype (17). However, these investigators found that HPc was protective to both the WT and HIF-1α knockout adult mouse brain subjected to ischemia. This disparity from what we report in the neonatal brain suggests there are different mechanisms involved in preconditioning protection, at least in part, between immature and mature mice. This is not surprising given the selective vulnerability of the developing brain to injury, particularly injury associated with oxidative stress (18). This vulnerability may be due, in part, to the finding that the developing brain accumulates more of the oxidant H2O2 after HI than the adult mouse brain after HI (19). HPc is known to initiate activation of endogenous anti-oxidants in response to oxidative stress (18),(6) along with HIF-1α. It is therefore likely that H2O2 modulates the stabilization and transcriptional activation of HIF-1α to regulate a number of protective genes that are responsible not only for oxygen homeostasis but also for brain protection and repair after severe oxidative stress in neonatal hypoxia-ischemia.
Although overexpression of the endogenous anti-oxidant glutathione peroxidase-1 (GPx1) reduces HI injury (20), HPc reverses this protection (21). This reversal of protection with HPc is associated with an increase in H2O2 accumulation after HI in the GPx1 overexpressing mice to wildtype levels by 24 h. This suggests that while H2O2 at high concentrations is a mediator of cell death, it may also play a part in HPc protection at low levels, particularly during the early phase of the injury process. The accumulation of H2O2 in the neonatal brain exposed to HI as a consequence of incomplete deactivation by GPx1 (19), is toxic to neurons in high concentrations, but has also been associated with protection at lower levels (22). Exogenous H2O2, at low concentrations, acts as a preconditioning agent by inducing HIF-1α in neurons in vitro and conferring protection from subsequent ischemia (22). Micromolar levels of H2O2 in vitro can block HIF-1α degradation (23). Endogenous H2O2 produced by HPc in superoxide-dismutase transgenic neurons in vitro induces HIF-1α and is protective against subsequent extended hypoxia (24).
High levels of H2O2 are known to stimulate necrosis and apoptosis, whereas low levels induce an anti-apoptotic program (25). We recently showed that although HIF-1α expression in these GPx1 overexpressing mice was similar to wildtype after a period of hypoxia, but ERK1/2 activation was prevented (26). The wildtype mice had a transient increase in ERK at 30 min; not at 0 min or 6 h. Our observation that HIF-1α knockout mice do not benefit from HPc, therefore, may indicate aberrant H2O2 signaling as well as ERK1/2 activation in these animals. Although the ERK1/2 pathway has been suggested to be important for the regulation of HIF-1α after neonatal HI (27), we did not observe significant alterations in ERK signaling. This disparity may be due to the different background strain used in our previous study (CD1), since differences in a number of transcription factors, including HIF-1α and VEGF, have been shown between neonatal CD1 and C57Bl/6 mice (28). The interaction of HPc, HIF-1α and varying levels of H2O2 over time after injury merits further study.
Recently, it was shown in adult mice of a different strain of HIF-1α knockout than that used here, that wildtype mice that received HPc prior to a subsequent hypoxic episode showed improved brain tissue oxygenation along with upregulation of iNOS, while the HIF-1α knockout counterparts had no change in these parameters with identical hypoxia preconditioning (29). Also in adult mice, ischemic preconditioning of the heart has been shown to be lost in mice with a partial deficiency of HIF-1α, along with a reduction in mitochondrial ROS production (30), indicating a potential role for ROS in the mechanisms of ischemic Pc.
Despite its vulnerability, the immature brain may also have a unique ability for regeneration and repair. For example, immature (P9) rats show greater neurogenesis in response to HI than do juvenile (P21) rats (31). It has been shown that the target gene VEGF is induced by HPc and has a protective effect (11) (32) (14). However, we did not find significant alterations in expression of VEGF with HPc, suggesting that perhaps other HIF-1 target genes are involved in the protective mechanisms of HPc. Manipulation of the endogenous forms of VEGF for protection and repair merits further investigation (33). We have previously shown that both degree of injury and mortality is affected by the strain of mouse subjected to HI (34). C57Bl/6, the background strain used here, has high mortality while being somewhat resistant to injury compared to outbred strains. Li et al also found high mortality with chronic hypoxia in P7 and p21 mice of the same background strain (C57Bl/6) (28). For this study, we decreased the duration of hypoxia in order to limit mortality while providing a moderate degree of injury.
The finding that HIF-1α is more strongly upregulated by hypoxia alone than by HI has also been shown in neonatal rats, and cleaved caspase-3 expression was correspondingly lower in the hypoxia treated rats than the HI treated rats, suggesting that higher levels of HIF-1α may be associated with lower levels of apoptosis (33). This corresponds with our finding that spectrin 145/150 increased 4 h after HPc/HI in the wildtype brain, but not the HIF-1α knockout brain, along with no difference at 24 h, supporting the idea that there is a shift toward earlier cell death of a calpain-mediated nature in the wildtype, but not the HIF-1α knockout. In addition, spectrin 120 increased in wildtype at 24 h, suggesting a greater role for early, necrotic, calpain-specific cell death in the wildtype cortex with HPc/HI in addition to later, apoptotic cell death. It does not explain cell death in the HIF-1α knockout, however. Perhaps other cell death mechanisms are involved, such as autophagy.
Autophagy has long been considered as another mechanism of cell death since it is upregulated in response to injury, but recent reports suggest it may have a protective role in some circumstances (35). In neonatal HI, HPc has been shown to upregulate the autophagy marker Beclin-1 (36). However, our results with the autophagy marker LAMP2 indicating increased autophagy at 4 h and 24 h after HPc/HI in HIF-1α knockout but not in wildtype, may correspond with a switch of cell death phenotypes and the lack of HPc protection seen in the HIFKO. Again, the role of autophagy in neurodegeneration after neonatal HI is severity, time and region-specific and merits further investigation, especially with more autophagy markers.
It is well known that HPc not only affects neurons, but other brain cells, which may act to protect neurons. In particular, the HPc of astrocytes may be an important factor in neuroprotection under certain circumstances (37). Sen et al, for example, have shown that HPc induces the precocious maturation and differentiation of astrocytes, with a concomitant increase in their neuroprotective functions (38).
In summary, the hypoxic-ischemic neonatal HIF-1α knockout mouse brain is not protected by hypoxia preconditioning, supporting our hypothesis that activation and modulation of HIF-1α is a critical component in the protective mechanisms of HPc in the neonatal brain. A greater understanding of the mechanisms of preconditioning protection in the neonatal brain may lead to more efficacious therapies aimed at protecting the human neonatal brain from HI injury.
MATERIALS AND METHODS
Animal Procedures
All animal research was approved by the University of California San Francisco Institutional Animal Care and Use Committee and was performed with the highest standards of care under the National Institutes of Health guidelines. Mice with conditional neuron specific inactivation of HIF-1α were generated using Cre/Lox technology. Adult mice of this strain have previously been well characterized (17), (39). Briefly, the deletion was attained by breeding mice that have loxP-containing HIF-1α alleles with ‘R1ag#5’ line (40) mice expressing Cre recombinase under the control of the calcium/calmodulin-dependent kinase II promoter. The resulting litters produced mice with a forebrain predominant, neuron-specific deletion of HIF-1α (H HIF-1α knockout), as well as littermates without the deletion (WT). Genotyping was carried out using polymerase chain reaction (PCR) on tail DNA samples using standard methods. All mice negative by PCR for the Cre gene were considered wild type.
Hypoxic Stimulus
At postnatal day 6 (P6), pups were placed in chambers maintained at 37° C by a circulating water bath and subjected to 1 h of 8% oxygen (balance nitrogen). Naive pups were in a similar chamber exposed to room air or remained with the dam. Sham animals have been shown in previous experiments (16) to behave as the naïve so in an effort to avoid unnecessary sacrifice of large numbers of animals, shams were omitted.
Hypoxia-Ischemia and Histology
At P7, pups (n=240: 123 WT and 117 HIF-1α knockout) underwent the Vannucci procedure of neonatal HI (41), . Briefly, under isoflurane anesthesia, the right common carotid artery was permanently ligated. Following a 90-min recovery period with the dam, pups were exposed to hypoxia for 30 minutes (8% oxygen, balance nitrogen) while being maintained at 37° C. We have previously shown that the background mouse strain, C57Bl/6, while somewhat resistant to injury from HI, has high mortality after HI (34). For determination of degree of injury, 5 days after HI pups were perfused intracardiac with cold 4% paraformaldehyde in 0.1 M phosphate buffer; brains were removed and post-fixed overnight in the same fixative followed by cryoprotection with 30% sucrose. Brains were sectioned on a Vibratome (50 μm) and alternate sections collected for Nissl staining with Cresyl Violet and Perl’s iron stain to measure iron deposition. Brains were analyzed for degree of injury using a scoring system that employs both stains, as previously described (34), where 0=no injury and 24=severe injury with cystic infarction. With 30 minutes of hypoxia, we were able to limit mortality, while producing a moderate degree of injury in a majority of animals.
Western Blot Analysis
An additional cohort of animals (n=79: 41 WT and 38 HIF-1α knockout) underwent HPc and HI as described above and cortical brain samples were collected for determination of protein expression by Western blot for HIF-1α, VEGF, ERK 1/2 HIF-1α knockout cortices were collected from three treatment groups: HPc alone (n=3 at each timepoint), HI alone (n=3 for 15 min, n=3 for 4h, n=6 for 24 h) and HPc/HI (n=5 for 15 min, n=5 for 4 h, n=8 for WT 24 h and n=7 for HIFKO 24 h), as well as naïve WT (n=13) and naïve HIF-1α knockout (n=12).
Samples were snap frozen and stored at –80° C until use, at which point nuclear and cytoplasmic fractions were prepared from the injured cortices using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL) according to the manufacture’s protocol. Briefly, tissue was homogenized in 250 l ice-cold CER I buffer with protease and phosphatase inhibitors. After incubation with CER II buffer, the sample was centrifuged for 5 min at 16,000 x g at 4°C and the supernatant was saved as the cytoplasmic extract. The pellet was resuspended in 90 μl ice-cold NER buffer, vortexed and centrifuged at 16,000 x g at 4°C for 10 min. The supernatant was saved as the nuclear extract. The cytoplasmic and nuclear protein aliquots were stored at −80°C until use. Protein concentrations were measured by BCA assay (Pierce, Rockford, IL), using BSA as the standard.
Fifty μg of nuclear or cytoplasmic protein was applied to 4–12% Bis-Tris SDS polyacrylamide gels (Invitrogen, Carlsbad, CA) for electrophoresis and transferred to PVDF membranes (Bio-Rad, Hercules, CA). Membranes were blocked in 5% non-fat dry milk in TBS with 0.05 % Tween 20 for 1 h at room temperature. The membranes were probed with the following primary antibodies overnight at 4°C: HIF-1α (Novus Biologicals, Littleton, CO); VEGF (Abcam Inc., Cambridge, MA); ERK and phospho-ERK (pERK, Cell Signaling), spectrin (Millipore, Billerica, MA), LAMP2 (Abcam) and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Appropriate secondary horseradish peroxidase-conjugated antibodies (1:2000, Santa Cruz Biotechnology) were used and signal was visualized with enhanced chemiluminescence (Amersham, Buckinghamshire, UK). Image J software was used to measure the optical densities and areas of the protein signal on radiographic film after scanning. The optical density of the protein bands was normalized to β-actin. All experiments were repeated at least three times to ensure reproducibility of results.
Statistical Analysis
For histological scoring of brain injury, 2-way ANOVA with Bonferroni post-test was used to determine interaction based on genotype. Subsequently, significance was determined by 1-way ANOVA with Mann-Whitney test for multiple groups or t-test for two groups. For the Western blots, 1-way ANOVA with the Dunnett’s test or t-test was used. Significance was set at p<0.05. Analysis was done with Prism 5.0 (Graphpad Software Inc. San Diego, CA).
Supplementary Material
Acknowledgments
STATEMENT OF FINANCIAL SUPPORT
This work was funded by the National Institutes of Health (NIH), Washington, DC, US RO1 NS033997 (DMF).
We are grateful to Dr. Carrollee Barlowe for the provision of our initial HIFKO mice and Dr. Ioannis Dragatsis for the Cre-tg mice used to generate the mice used in these experiments; to Matthew J. Lam and Russell Fitzgerald for technical assistance and Frances J. Northington MD for insightful suggestions. We acknowledge T.K. McIntosh, MD for careful reading of the manuscript.
Footnotes
Disclosure: The authors certify that there are no potential perceived conflicts of interest or financial disclosures related to this work.
References
- 1.Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol. 2000;48:285–296. [PubMed] [Google Scholar]
- 2.Gidday JM, Fitzgibbons JC, Shah AR, Park TS. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosc Lett. 1994;168:221–224. doi: 10.1016/0304-3940(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 3.Gustavsson M, Anderson MF, Mallard C, Hagberg H. Hypoxic preconditioning confers long-term reduction of brain injury and improvement of neurological ability in immature rats. Pediatr Res. 2005;57:305–309. doi: 10.1203/01.PDR.0000151122.58665.70. [DOI] [PubMed] [Google Scholar]
- 4.Ikonomidou C, Kaindl AM. Neuronal death and oxidative stress in the developing brain. Antioxid Redox Signal. 2011;14(8):1535–50. doi: 10.1089/ars.2010.3581. [DOI] [PubMed] [Google Scholar]
- 5.Gustavsson M, Wilson MA, Mallard C, Rousset C, Johnston MV, Hagberg H. Global gene expression in the developing rat brain after hypoxic preconditioning: involvement of apoptotic mechanisms? Pediatr Res. 2007;61:444–450. doi: 10.1203/pdr.0b013e3180332be4. [DOI] [PubMed] [Google Scholar]
- 6.Ran R, Xu H, Lu A, Bernaudin M, Sharp FR. Hypoxia preconditioning in the brain. Dev Neurosci. 2005;27:87–92. doi: 10.1159/000085979. [DOI] [PubMed] [Google Scholar]
- 7.Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev. 2009;62:99–108. doi: 10.1016/j.brainresrev.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Calvert JW, Zhang JH. Neonatal hypoxia/ischemia is associated with decreased inflammatory mediators after erythropoietin administration. Stroke. 2005;36:1672–1678. doi: 10.1161/01.STR.0000173406.04891.8c. [DOI] [PubMed] [Google Scholar]
- 11.Laudenbach V, Fontaine RH, Medja F, et al. Neonatal hypoxic preconditioning involves vascular endothelial growth factor. Neurobiol Dis. 2007;26:243–252. doi: 10.1016/j.nbd.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci. 2009;31:403–411. doi: 10.1159/000232558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu PW, Beart PM, Jones NM. Preconditioning protects against oxidative injury involving hypoxia-inducible factor-1 and vascular endothelial growth factor in cultured astrocytes. Eur J Pharmacol. 2010;633:24–32. doi: 10.1016/j.ejphar.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Rhodes PG, Bhatt AJ. Neuroprotective effects of vascular endothelial growth factor following hypoxic ischemic brain injury in Neonatal Rats. Pediatr Res. 2008;64(4):370–4. doi: 10.1203/PDR.0b013e318180ebe6. [DOI] [PubMed] [Google Scholar]
- 15.Aydin A, Genc K, Akhisaroglu M, Yorukoglu K, Gokmen N, Gonullu E. Erythropoietin exerts neuroprotective effect in neonatal rat model of hypoxic- ischemic brain injury. Brain and Dev. 2003;25:494–498. doi: 10.1016/s0387-7604(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 16.Sheldon RA, Osredkar D, Lee CL, Jiang X, Mu D, Ferriero DM. HIF-1 alpha-deficient mice have increased brain injury after neonatal hypoxia-ischemia. Dev Neurosci. 2009;31:452–458. doi: 10.1159/000232563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferriero DM. Neonatal brain injury. N Engl JMed. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 19.Lafemina MJ, Sheldon RA, Ferriero DM. Acute hypoxia-ischemia results in hydrogen peroxide accumulation in neonatal but not adult mouse brain. Pediatr Res. 2006;59:680–683. doi: 10.1203/01.pdr.0000214891.35363.6a. [DOI] [PubMed] [Google Scholar]
- 20.Sheldon RA, Jiang X, Francisco C, et al. Manipulation of antioxidant pathways in neonatal murine brain. PediatrRes. 2004;56:656–662. doi: 10.1203/01.PDR.0000139413.27864.50. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon RA, Aminoff A, Lee CL, Christen S, Ferriero DM. Hypoxic preconditioning reverses protection after neonatal hypoxia-ischemia in glutathione peroxidase transgenic murine brain. Pediatr Res. 2007;61(6):666–70. doi: 10.1203/pdr.0b013e318053664c. [DOI] [PubMed] [Google Scholar]
- 22.Chang S, Jiang X, Zhao C, Lee C, Ferriero DM. Exogenous low dose hydrogen peroxide increases hypoxia-inducible factor-1alpha protein expression and induces preconditioning protection against ischemia in primary cortical neurons. Neurosci Lett. 2008;441:134–138. doi: 10.1016/j.neulet.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millonig G, Hegedusch S, Becker L, Seitz HK, Schuppan D, Mueller S. Hypoxia-inducible factor 1 alpha under rapid enzymatic hypoxia: cells sense decrements of oxygen but not hypoxia per se. Free Radic Biol Med. 2009;46:182–191. doi: 10.1016/j.freeradbiomed.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 24.Liu M, Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J Cereb Blood Flow Metab. 2005;25:939–948. doi: 10.1038/sj.jcbfm.9600085. [DOI] [PubMed] [Google Scholar]
- 25.Blomgren K, Hagberg H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic Biol Med. 2006;40:388–397. doi: 10.1016/j.freeradbiomed.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 26.Autheman D, Sheldon RA, Chaudhuri N, et al. Glutathione peroxidase overexpression causes aberrant ERK activation in neonatal mouse cortex after hypoxic preconditioning. Pediatr Res. 2006;72:568–575. doi: 10.1038/pr.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Xiong Y, Qu Y, et al. The requirement of extracellular signal-related protein kinase pathway in the activation of hypoxia inducible factor 1 alpha in the developing rat brain after hypoxia-ischemia. Acta Neuropathol. 2008;115:297–303. doi: 10.1007/s00401-008-0339-5. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Michaud M, Stewart W, Schwartz M, Madri JA. Modeling the neurovascular niche: murine strain differences mimic the range of responses to chronic hypoxia in the premature newborn. J Neurosci Res. 2008;86:1227–1242. doi: 10.1002/jnr.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taie S, Ono J, Iwanaga Y, et al. Hypoxia-inducible factor-1 alpha has a key role in hypoxic preconditioning. J Clin Neurosci. 2009;16:1056–1060. doi: 10.1016/j.jocn.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Cai Z, Zhong H, Bosch-Marce M, et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res. 2008;77:463–470. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- 31.Zhu C, Qiu L, Wang X, et al. Age-dependent regenerative responses in the striatum and cortex after hypoxia-ischemia. J Cereb Blood Flow Metab. 2009;29:342–354. doi: 10.1038/jcbfm.2008.124. [DOI] [PubMed] [Google Scholar]
- 32.Feng Y, Rhodes PG, Bhatt AJ. Hypoxic preconditioning provides neuroprotection and increases vascular endothelial growth factor A, preserves the phosphorylation of Akt-Ser-473 and diminishes the increase in caspase-3 activity in neonatal rat hypoxic-ischemic model. Brain Res. 2010;1325:1–9. doi: 10.1016/j.brainres.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Qu Y, Li J, Xiong Y, Mao M, Mu D. Relationship between HIF-1alpha expression and neuronal apoptosis in neonatal rats with hypoxia-ischemia brain injury. Brain Res. 2007;1180:133–139. doi: 10.1016/j.brainres.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 34.Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Research. 1998;810:114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- 35.Xu F, Gu JH, Qin ZH. Neuronal autophagy in cerebral ischemia. Neurosci Bull. 2012;28:658–666. doi: 10.1007/s12264-012-1268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 37.Vangeison G, Rempe DA. The Janus-faced effects of hypoxia on astrocyte function. Neuroscientist. 2009;15:579–588. doi: 10.1177/1073858409332405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sen E, Basu A, Willing LB, et al. Pre-conditioning induces the precocious differentiation of neonatal astrocytes to enhance their neuroprotective properties. ASN Neuro. 2011;326(3):e00062. doi: 10.1042/AN20100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helton R, Cui J, Scheel JR, et al. Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci. 2005;25:4099–4107. doi: 10.1523/JNEUROSCI.4555-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dragatsis I, Zeitlin S. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis. 2000;26:133–135. doi: 10.1002/(sici)1526-968x(200002)26:2<133::aid-gene10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 41.Rice E, Jr, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic- ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.