Abstract
Trimethylamine-N-oxide (TMAO) levels in blood predict future risk for major adverse cardiac events including myocardial infarction, stroke and death. Thus, the rapid determination of circulating TMAO concentration is of clinical interest. Here we report a method to measure TMAO in biological matrices by stable isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) with lower and upper limits of quantification of 0.05 and >200 µM, respectively. Spike and recovery studies demonstrate an accuracy at low (0.5 µM), mid (5 µM) and high (100 µM) levels of 98.2%, 97.3% and 101.6%, respectively. Additional assay performance metrics include intra-day and inter-day coefficients of variance of < 6.4% and < 9.9%, respectively, across the range of TMAO levels. Stability studies reveal TMAO in plasma is stable both during storage at −80 °C for 5 years and to multiple freeze thaw cycles. Fasting plasma normal range studies among apparently healthy subjects (n=349) shows a range of 0.73 – 126 µM, median (interquartile range) levels of 3.45 (2.25–5.79) µM, and increasing values with age. The LC/MS/MS based assay reported should be of value for further studies evaluating TMAO as a risk marker and for examining the effect of dietary, pharmacologic and environmental factors on TMAO levels.
Trimethylamine-N-oxide (TMAO) is a gut microbiota-dependent generated metabolite of certain dietary trimethylamine containing compounds, including choline, phosphatidylcholine (lecithin) and carnitine [1–2]. Several recent clinical studies demonstrate that systemic levels of trimethylamine-N-oxide (TMAO) are independently associated with cardiovascular risks, including incident risks for myocardial infarction, stroke and death [1–3]. Of interest, TMAO appears to be more than simply a biomarker of risk since it is mechanistically linked to development of atherosclerosis and cardiovascular disease through changes elicited in cholesterol and bile acid metabolism in multiple compartments [1–2]. Thus, development of a rapid and accurate method for the quantification of circulating levels of TMAO is of clinical interest.
Several methods for quantifying TMAO in biological matrices have been reported. Early gas chromatography-mass spectrometry (GC/MS) studies employed a complicated multi-step derivatization protocol that first reduced TMAO to the gas trimethylamine (TMA), which was then derivatized with 2,2,2-trichloroethyl chloroformate and detected as N,N-dimethyl-2,2,2-trichloroethyl carbamate [4]. In addition to being labor intensive, this method has multiple limitations, including the high volatility of TMA complicating its quantification, and the fact that the method does not easily discriminate between TMA, dimethylamine and TMAO since all give the same product. Thus, TMAO levels with this common assay method are calculated as the difference in product formed in samples run twice before and then following reduction. More recent studies have reported TMAO analyses in urine samples employing fast atom bombardment mass spectrometry or electrospray quadrupole time-of-flight mass spectrometry [5–6]. While specific, critical assay performance metrics were not reported, and the mass spectrometry platforms employed are not widely used as routine analytical instruments in diagnostic laboratories. Additional reports have used either non-suppressed ion chromatography with determination of organic nitrogen, or NMR, for the determination of blood TMAO [7–8]. The utility of these platforms for the high throughput demands of a clinical diagnostic TMAO test have not yet been assessed. We and others have reported TMAO quantification in human and animal plasma, serum or urine by LC/MS/MS without derivatization [1–3, 9]. However, detailed methodology amenable for implantation of an assay as a high throughput clinical diagnostic test has not yet been reported. In the present report we describe a rapid, sensitive and accurate approach for the quantification of TMAO in biological matrices using stable isotope dilution liquid chromatography with on-line electrospray ionization tandem mass spectrometry (LC/MS/MS).
Materials and methods
Reagents
Deuterated trimethylamine-N-oxide (d9-TMAO) was purchased from Cambridge Isotope Laboratories (Catalog# DLM-4779-1, Andover, MA). All other reagents were purchased from either Sigma-Aldrich (St. Louis, MO) or Fisher Scientific Chemicals (Pittsburg, PA) unless otherwise stated.
Research subjects
Samples and associated clinical data were collected from fasting subjects undergoing community health screens. All subjects gave written informed consent and the Institutional Review Board of the Cleveland Clinic approved all study protocols.
Sample processing
Samples (20 µl plasma or serum) were aliquoted to a 1.5 ml eppendorf tube and mixed with 80 µl of 10 µM internal standard comprised of d9-TMAO in methanol. Protein in the samples was precipitated by vortexing for 1 minute and then the supernatant was recovered following centrifugation at 20,000×g, 4°C for 10 minutes. In general, when analyzing TMAO levels, we run 3 different quality control (QC) samples with TMAO concentrations ranging between 0.25 µM and 20 µM in duplicate before each sample batch of less than 30 samples. TMAO concentrations from the batch were acceptable when the accuracy of the values determined from each QC sample were within 100 ±10% of their expected values and the intra-batch CVs for the same QC samples were all less than 10%.
LC/MS/MS
Supernatants (10 µl) were analyzed by injection onto a silica column (4.6 × 250 mm, 5 μm Luna silica; Cat# 00G-4274-E0, Phenomenex, Torrance, Ca) at a flow rate of 0.8 ml min−1 using a 4 LC-20AD Shimadazu pump system, SIL-HTC autosampler and dual column switching valve system (2 Rheodyne 6 port automated valves, Cat# MXP7900, IDEX Health & Science, Oak Harbor, WA) (Levison et al. 2013) interfaced with an API 4000 Q-TRAP mass spectrometer (AB SCIEX, Framingham, MA). A discontinuous gradient was generated to resolve the analytes by mixing solvent A (0.1% propanoic acid in water) with solvent B (0.1% acetic acid in methanol) at different ratios starting from 2% B linearly to 15% B over 10 min, then linearly to 100% B over 2.5 min, then hold for 3 min and then back to 2% B. TMAO and d9-TMAO were monitored using electrospray ionization in positive-ion mode with multiple reaction monitoring (MRM) of precursor and characteristic product ion transitions of m/z 76 → 58 amu and 85 → 66 amu, respectively. The parameters for the ion monitoring were as follows: Spray voltage, 4.5 kV; Curtain gas, 15; GS1, 60; GS2, 50; CAD gas, medium; DP, 60; CE, 25.0 volts for TMAO and 28.3 volts for d9-TMAO; CXP, 10; EP,10. Nitrogen (99.95% purity) was used as the only gas. Various concentrations of non-isotopically labeled TMAO standard were spiked into control plasma to prepare the calibration curves for quantification of TMAO. The internal standard d9-TMAO was used for quantification as well as to calculate recovery rate of TMAO (which was 99±1% based upon separate control studies).
Precision, accuracy, limit of quantitation and linearity
Four replicates were performed on a single day to establish the intra-day coefficient of variation (CV) for 6 different pooled plasma samples with different concentrations of TMAO. The inter-day CV was determined by assaying aliquots of these pooled samples daily over a span of more than 20 days. Carry-over between injections was not observed. Accuracy is expressed as the ratio of the TMAO concentration measured to the TMAO concentration added to a plasma sample that was dialysed against 1000 volumes of 0.89% NaCl for 3 times. The lower limit of detection (LLOD) was defined as the lowest concentration of TMAO spiked into the dialysed plasma on-column generating a signal-to-noise ratio of 3. The lower limit of quantification (LLOQ) was determined by reducing the concentration of standard solution gradually to the dialysed plasma and is expressed as the lowest concentration yielding a signal-to-noise ratio of 10. No upper limit of quantification (ULOQ) was found, with linear responses noted in serially diluted samples spiked with increasing the concentration of standard solution as long as the diluted sample (concentration, peak area ratio of TMAO/d9-TMAO) was still situated on the standard curve. To determine assay linearity, a standard curve over the 0.01–200 µM concentration range was checked for linearity by linear regression fit. The linear range was defined as the region of the standard curve where the difference between calculated TMAO concentration and standard TMAO concentration was less than 15%.
Results
Optimization of parameters for mass spectrometric assay of TMAO and d9-TMAO
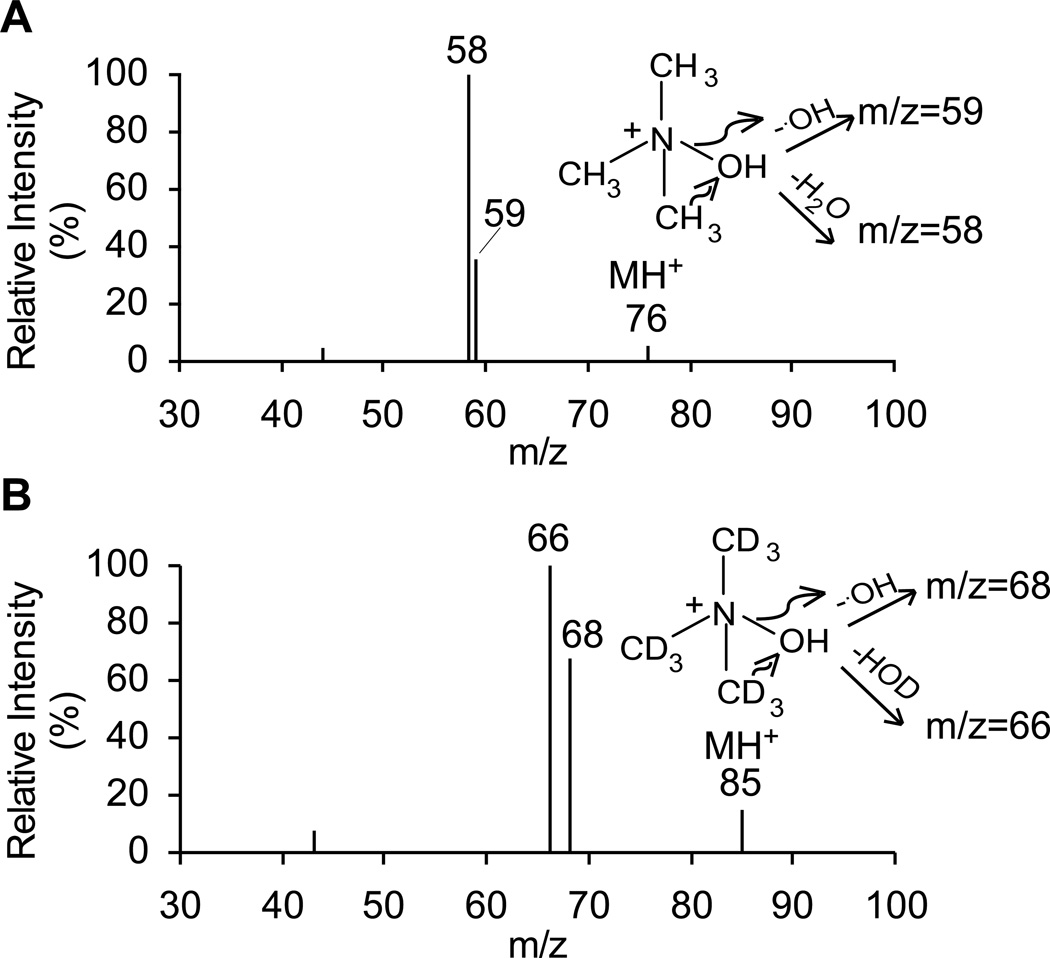
In order to determine the TMAO concentration in human blood, we first optimized the parameters for TMAO and its isotope labeled internal standard, d9-TMAO, by direct infusion of these two standards. Shown in Fig. 1A-B, are the collision induced dissociation (CID) mass spectra of TMAO and d9-TMAO, which are fragmented with collision energy of 25 electron volts. The product ions are 58 and 59 amu for TMAO, which are due to loss of water and hydroxyl group, respectively, as are the product ions for d9-TMAO at 66 and 68 amu. The intensity ratios of the two product ions are different between TMAO and d9-TMAO due to the difference in bond energy between C and H and C and D (deuterium). We therefore quantified TMAO and d9-TMAO in the MRM mode using the precursor to product transitions 76→58 amu and 85→66 amu, respectively. Among all the instrument parameters, collision energy (CE) was the most critical one.
Fig. 1.
Collision induced dissociation (CID) mass spectra of trimethylamine-N-oxide (TMAO, Panel A) and trimethylamine-N-oxide-d9 (d9-TMAO, Panel B). Proposed pathways for formation of the fragment ions are also shown.
Validation of the LC/MS/MS assay for TMAO in human plasma
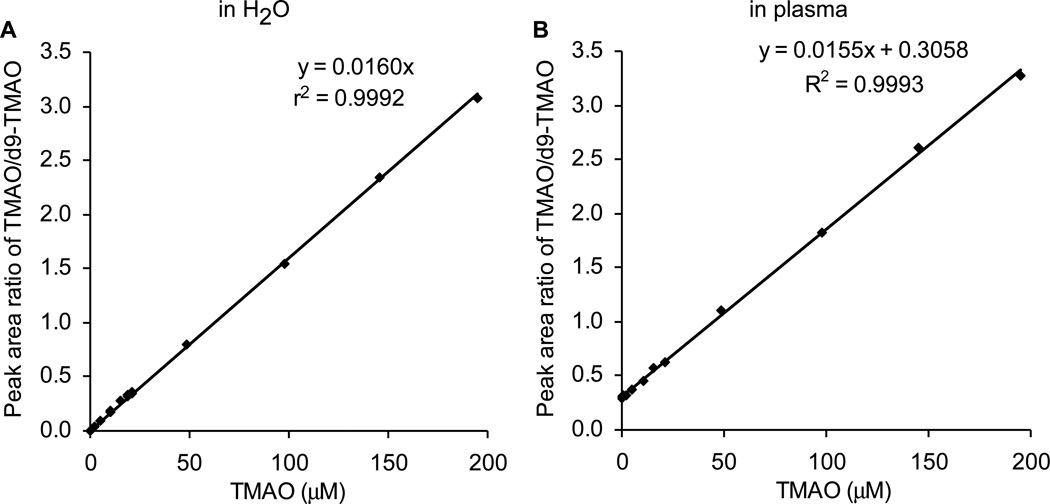
Fig. 2A-B shows the selected ion extraction LC chromatograms of TMAO and d9-TMAO standards (in buffer) in positive MRM mode. Fig. 2C-D shows the LC chromatograms extracted in plasma after spiking with d9-TMAO internal standard in methanol followed by precipitation to remove protein. TMAO and d9-TMAO have nearly the same retention times in the LC chromatograms. The data acquisition window is 6 minutes, and began after an initial 4.5 minutes of column eluent diversion to waste. To increase sample throughput, we routinely use 2 multiplexed binary pump HPLC system equipped with separate columns and automated column valve system [10] (Fig. 3), allowing for data acquisition only during a narrower time window from each column. The current column and gradient presented is what we typically use, and allows for an ~11 minute per sample run time, while also allowing multiple additional biogenic amines and amino acids to simultaneously be quantified in multiple reaction monitoring mode [11]. If only quantifying TMAO, we increase throughput per sample to ~5 minutes by using a shorter column and gradient (not shown). Following data acquisition, peak areas of TMAO and d9-TMAO were determined using Analyst 1.4.1 software and the peak area ratio is used to quantify TMAO. Using a series of concentrations of TMAO standard mixed with a fixed amount of d9-TMAO internal standard spiked or non-spiked into plasma, we prepared calibration curves, which are shown in Fig. 4A and B. The Y axis intercept in the Fig. 4B (non-dialyzed plasma) corresponds to the endogenous TMAO concentration, which can be calculated as intercept/slope. The slopes of the two standard curves showed a difference of ≤3.2%. By spiking a series of concentrations of TMAO standards into 8 different plasma samples to prepare the standard curves, we got a slope (mean ±SD) of 0.0156±0.0003, suggesting that using different spiked plasmas to generate the calibration curves for TMAO gives an error of ≤ 2.0%. These data also indicate that in either EDTA plasma (shown) or serum (not shown) the matrix effect for quantifying TMAO by the method described is negligible.
Fig. 2.
Detection of TMAO in biological matrices. Extracted ion LC chromatograms from multiple reaction monitoring in positive ion mode of TMAO (A), d9-TMAO (B) standards and plasma (C and D), spiked with 4 volumes of internal standard, d9-TMAO, at a concentration of 10 µM in methanol. The precursor-to-product transitions were 76→58 amu, 85→66 amu, respectively. Note, prior to data collection, the eluent was diverted to waste for the initial 4.5 minutes of the gradient. The concentrations of TMAO in standard (A) and plasma (C) were 20.0 and 56.9 µM, respectively.
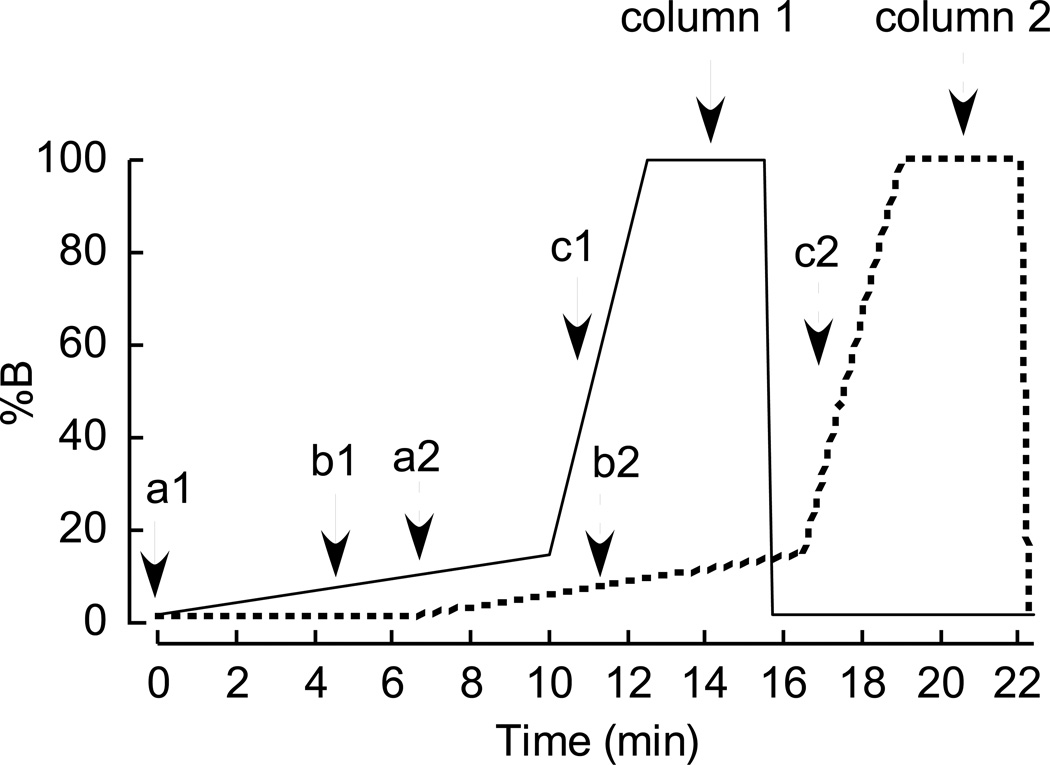
Fig. 3.
Multiplex binary gradient elution profile for quantitation of trimethylamine-N-oxide (TMAO) by LC/MS/MS. Two different solvents, A, 0.1% propanoic acid in water; B, 0.1% acetic acid in methanol, were used to generate the gradient. a1 and a2, time point for sample injection on column 1 and 2 respectively; b1 and b2, time point for start of data collection on column 1 and 2 respectively; c1, c2, time point for data collection to end and column 1 and 2 to be switched to waste, respectively.
Fig. 4.
Standard curves for LC/ESI/MS/MS analysis of TMAO. 80 µl 10 µM d9-TMAO internal standard in methanol was added to 20 µl different concentrations of TMAO standard (A) or 20 µl control plasma spiked with different concentrations of TMAO standard followed by spin-down to remove precipitated protein. Analyses were performed using electrospray ionization in positive-ion mode with multiple reaction monitoring of precursor and characteristic product ions. The transitions monitored were mass-to-charge ratio (m/z): m/z 76 → 58 amu for TMAO; and m/z 85 → 66 amu for d9-TMAO. Standard curves of TMAO were generated by plotting peak area ratio versus the concentration in water or spiked into plasma.
Assay performance metrics
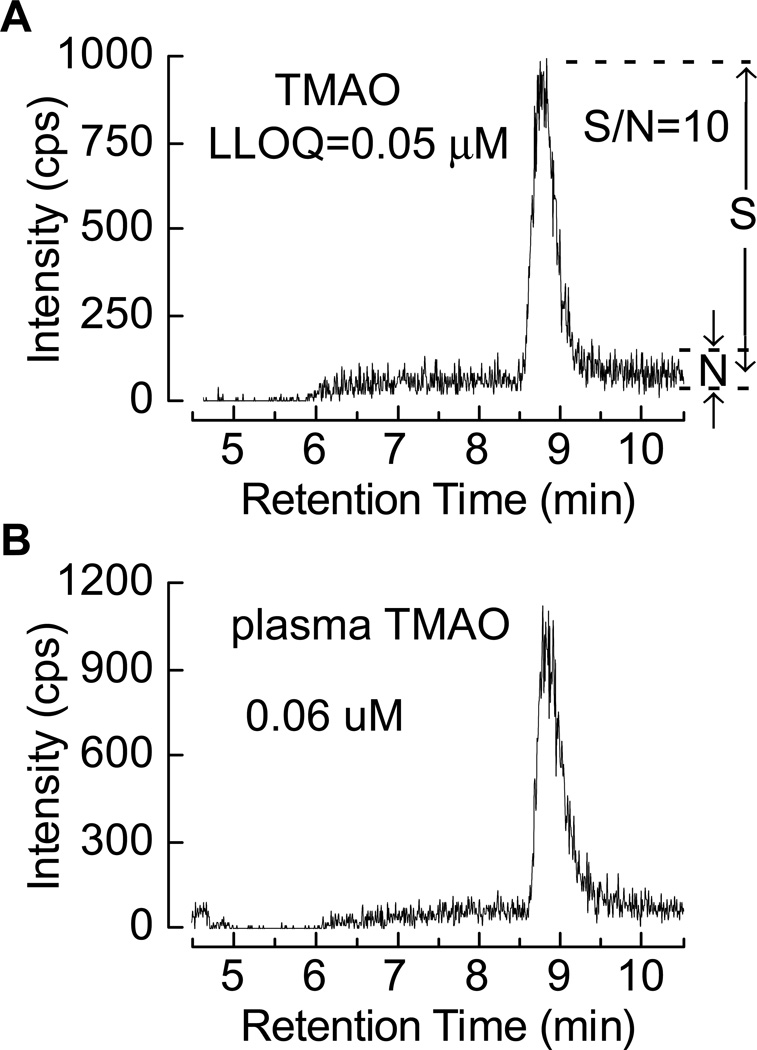
To examine assay precision, TMAO concentration was measured in 6 different pooled plasma samples with different TMAO levels, on multiple different days. Table 1 shows the intra-day and inter-day CV%s for TMAO observed, all of which were less than 6.4 and 9.9, respectively, regardless of the TMAO level. The pooled plasma TMAO concentrations used were as low as 0.82 µM and as high as 102.4 µM, whose range embraces 99% subjects (see below). Additional assay performance metrics including accuracy across a span of different concentration of TMAO (all ≥95%), LLOD, LLOQ and ULOQ are given in Table 2. The LC chromatogram of the TMAO standard spiked into the dialyzed plasma with a concentration at LLOQ is shown in Fig. 5A. Fig. 5B shows the LC chromatogram of TMAO in human plasma with the lowest concentration (0.06 µM) we have ever measured among more than 4000 subjects. The assay was found to be linear across the range examined (0.1–200 µM). Dilution of plasma samples containing higher TMAO levels (the highest endogenous level yet observed is 4.8 mM) with water gave an accuracy of greater than 98%.
Table 1.
Precision of TMAO concentrations measured in 6 pooled plasma samples with different levels over a span of more than 20 days.
| Pooled plasma | mean ± SD | Intra-day CV% | Inter-day CV% |
|---|---|---|---|
| 1 | 0.82 ± 0.05 | 6.4 | 5.8 |
| 2 | 1.68 ± 0.10 | 4.1 | 9.9 |
| 3 | 2.95 ± 0.11 | 3.1 | 5.8 |
| 4 | 2.44 ± 0.09 | 2.7 | 6.1 |
| 5 | 66.8 ± 2.1 | 2.1 | 5.2 |
| 6 | 102.4 ± 3.3 | 1.3 | 6.2 |
The calculated mean, SD, intra-day CV% and inter-day CV% are given. For each of the 6 different plasma samples, a cluster of 4 determinations is presented as they were run on eight separate days (32 determinations total for each pooled plasma sample).
Table 2.
Characteristics of the method for TMAO determination by LC/MS/MS
| Characteristic | value | |
|---|---|---|
| LLOD | 0.02 μM | |
| LLOQ | 0.05 μM | |
| ULOQ | > 200 μM | |
| Accuracy (%) | 0.5 μM | 98.2 |
| 2 μM | 97.1 | |
| 5 μM | 97.3 | |
| 100 μM | 101.6 | |
| 200 μM | 97.4 | |
Lower Limit of Detection (LLOD) = 3:1 signal to noise,
Lower Limit of Quantitation (LLOQ) = 10:1 signal to noise,
Upper Limit of Quantitation ULOQ = greater than highest standard investigated
Accuracy = ratio of the TMAO concentration measured to the TMAO concentration added to a dialyzed plasma sample
Fig. 5.
(A) Extracted ion LC chromatogram at the LLOQ from multiple reaction monitoring in the positive mode of the 76 amu to 58 amu precursor to product ions from a 0.05 µM TMAO standard spiked to dialyzed plasma, note this gives a signal-to-noise ratio (S/N) of 10 : 1. (B) Extracted ion LC chromatogram recorded as above of the plasma TMAO level at the lowest concentration (0.06 µM) among the more than 4,000 human samples analyzed.
Normal range of fasting plasma TMAO in healthy subjects
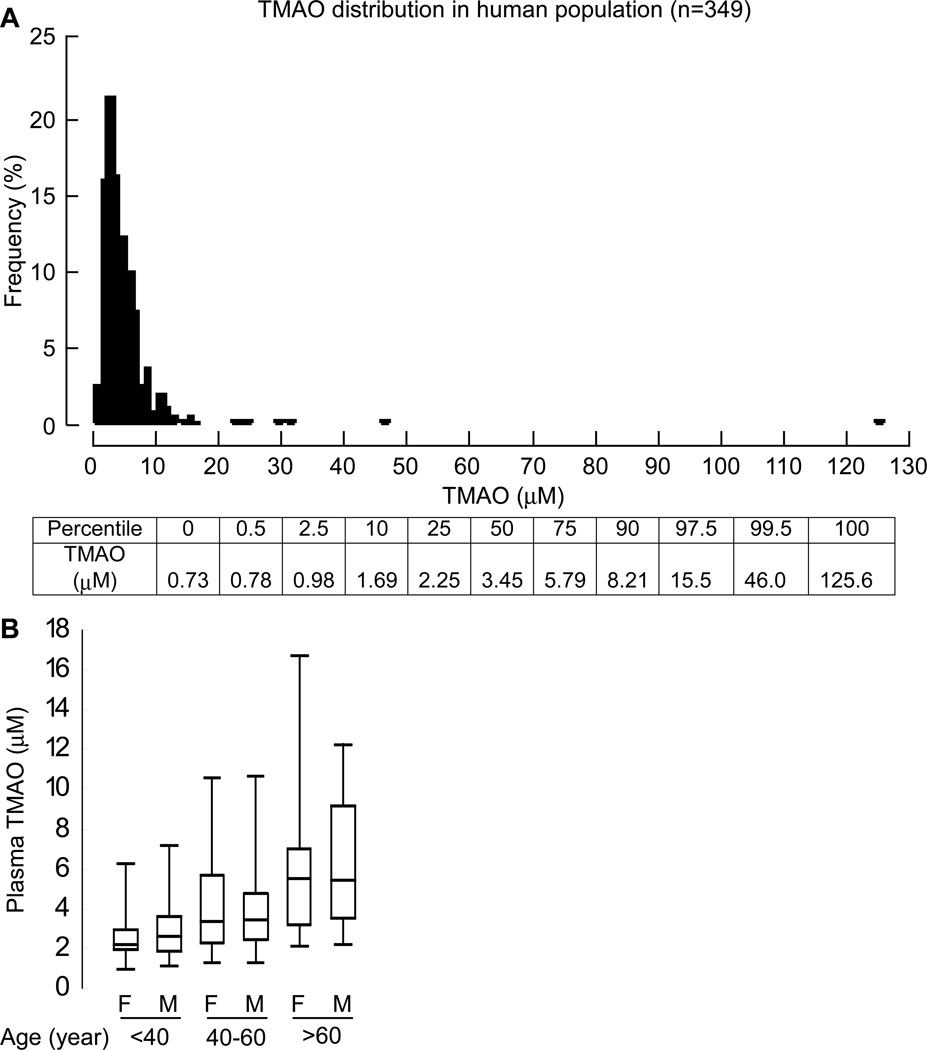
To assess the normal range of fasting (≥12 h) TMAO levels, apparently healthy subjects (n=349) undergoing routine health screens in the community were examined. Fig. 6A shows the plasma TMAO concentration distribution, and Table 3 describes the clinical and laboratory characteristics observed. Within the healthy cohort, 5% demonstrated mild hypertension and 5% of them were current smokers. The median (interquartile range) levels of TMAO noted was 3.45 (2.25 – 5.79) µM. Male and female TMAO levels showed no significant differences, though TMAO levels did show increases with age (Fig. 6B).
Fig. 6.
(A) Distribution of TMAO concentrations in healthy volunteers (n=349). Lower bar gives the concentrations of TMAO at each percentile. (B) Box whisker plot showing distribution of the TMAO concentration with respect to age and gender in the above population (n=349). The population was segregated by age and gender as shown on lower axis. Horizontal in middle of box = TMAO concentration at median value, top of box at 75th percentile, bottom of box at 25th percentile, top of whisker at 97.5 percentile and bottom of whisker at 2.5 percentile in the specified population.
Table 3.
Clinical and laboratory characteristics of healthy controls
| Characteristic | All participants (n=349) |
|---|---|
| Age-yr | 54±16 |
| Male sex-% | 33 |
| Median body-mass index (interquartile range) | 25 (23–29) |
| Hypertension-% | 5 |
| Current or former smoker-% | 5 |
| Median cholesterol (interquartile range)-mg/dl Low-density lipoprotein High-density lipoprotein |
117 (95–142) 53 (45–64) |
| Median triglycerides (interquartile range)-mg/dl | 102 (73–154) |
| Median lipoprotein (interquartile range)-mg/dl B A1 A2 |
82 (67–98) 150 (132–171) 38 (34–43) |
| Median TMAO (interquartile range)-μM | 3.4 (2.3–5.8) |
All subjects gave written informed consent and the Institutional Review Board of the Cleveland Clinic approved all study protocols. Fasting blood lipid profiles were measured on the Abbott ARCHITECT platform (Abbott Diagnostics, Abbot Park, IL).
Stability studies
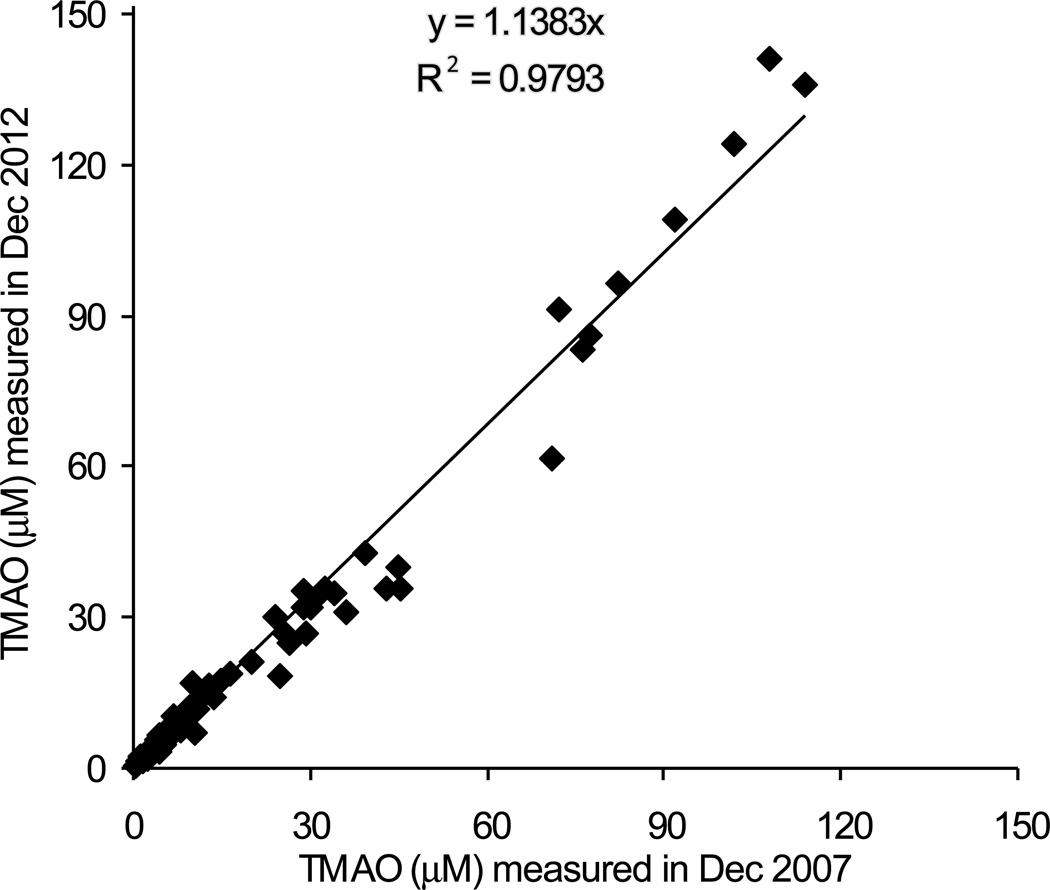
In order to investigate the stability of TMAO in plasma during storage, we measured TMAO concentration in a collection of samples in which initial levels were determined using stable isotope dilution LC/MS/MS analyses over 5 years ago, and for which samples had been maintained at −80 °C. Fig. 7 shows results of the comparison in TMAO levels between the two time points, and reveals a high correlation (r2=0.98) with slope and Y intercept very close to 1 and 0, respectively, indicating TMAO is stable during storage at −80 °C. Separate studies examining the stability of TMAO through multiple freeze thaw cycles showed TMAO levels do not significantly change, with inter-cycle CV% less than 10 (Table 4).
Fig. 7.
The stability of TMAO in frozen plasma samples after long term storage (5 years at −80 °C). The same set of 124 human plasma samples (randomly selected from Genebank, a large (n = 10,000) and well-characterized tissue repository with longitudinal data from sequential consenting subjects undergoing elective diagnostic left heart catheterization) was measured in Dec 2007 and in Dec 2012 by LC/MS/MS. The plasma samples were collected between Nov 5, 2001 and April 5, 2005, and kept frozen at −80 °C until assay. All plasma samples were defrosted at least 2 times.
Table 4.
Evaluation of three QC plasma TMAO concentrations which were subjected to 5 freeze and thaw cycles.
| QC Plasma |
Plasma TMAO (μM) | CV% | |||||
|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Inter- cycle |
Intra- cycle |
|
| 1 | 1.61 1.68 |
1.77 1.77 |
1.78 1.71 |
1.75 1.74 |
1.71 1.80 |
4.1 |
2.6 |
| 2 | 2.47 2.38 |
2.71 2.60 |
2.99 2.77 |
2.58 2.65 |
2.66 2.58 |
8.8 |
3.3 |
| 3 | 15.2 14.9 |
16.2 15.4 |
16.3 15.2 |
15.1 15.9 |
15.3 15.3 |
2.9 |
3.4 |
Plasma were frozen at −80 °C in dry ice box and thawed to room temperature. The same vial of plasma was subjected to five freeze and thaw cycles. TMAO in each sample at each cycle was measured in duplicate.
Discussion
Herein we present an LC/MS/MS method for the measurement of TMAO levels in biological matrices with high accuracy, precision and throughput that is amenable to the demands of clinical diagnostic practice. Using multiplexed HPLC with column switching, shorter column and further optimizing of the column gradients, we have adapted the stable isotope dilution LC/MS/MS assay described to permit a between sample acquisition time of 5 minutes (averaged) without affecting assay results or the column longevity. With a LLOQ of 0.05 µM, which is well below the lowest TMAO level observed among the >300 healthy volunteers examined, a highly accurate TMAO level was able to be determined in every subject examined. The linear relationship observed across the large dynamic range explored in the standard curve (up to 200 µM) is suitable for the majority of samples in human population studies. In our experience, which cumulatively includes analyses of over 4,000 individuals (both healthy subjects and cardiovascular disease patients), the lowest plasma TMAO level observed thus far has been 0.06 µM (Fig. 5B), which is higher than the LLOQ, and the maximum level observed has been 4.8 mM. In the present studies, which report for the first time the normal range of fasting plasma TMAO levels among apparently healthy adult subjects, plasma TMAO levels at the 0.5 percentile level cutoff were 0.78 µM and at the 99.5 percentile cutoff were 46.0 µM. Previously, we measured plasma TMAO levels in a large patient population that consisted of 4,007 sequential subjects with largely preserved renal function undergoing elective diagnostic cardiac evaluations (3). In those studies, we examined the relationship between TMAO levels and risk for both prevalent cardiovascular disease and incident risk for myocardial infarction, stroke or death within the 3 years following sample collection and observed a hazard ratio for highest vs. lowest TMAO quartile of 2.54 fold (95% confidence interval, 1.96 to 3.28; P<0.001) [3]. Of note, the extremes of TMAO levels observed within this cardiology clinic cohort are similar (i.e. the 0.5 percentile was 0.63 µM and the 99.5 percentile was 77.2 µM) to those observed in the healthy volunteers reported in the present study. Therefore, most of the plasma TMAO levels that will likely be observed in subjects will be situated within the linearity range, 0.1–200 µM, developed for the present assay. The results of the stability studies shown for TMAO in plasma during storage at −80 °C indicate that analyses of TMAO in archival specimens of long duration will be a reliable indicator of the TMAO levels. Although TMAO can work as electron acceptor in bacterium [12–13], its oxidative potential is very limited. Thus, it is perhaps not surprising that the present study demonstrates negligible impact of multiple freeze – thaw cycles on TMAO levels, attesting to the stability of this analyte in sterile complex biological matrices. Thus, TMAO does not readily undergo spontaneous degradation, and alternatively, trimethylamine containing precursors like choline, phosphatidylcholine and carnitine do not readily contribute to the formation of TMAO during storage. The accuracy, precision and high throughput nature of the presently described LC/MS/MS assay should greatly facilitate the exploration of the clinical utility of this unique and interesting biomarker of human health and disease.
Acknowledgement
SLH is supported by National Institutes of Health (NIH) and Office of Dietary Supplement grant R01 HL103866, and NIH grant P20 HL113452. SLH is also partially supported by a gift from the Leonard Krieger Fund. ZW is partially supported by an American Heart Association Scientist Development Grant (12SDG12050473). Mass spectrometry instrumentation used is housed in a facility partially supported by a Center of Innovation Award with AB SCIEX.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
Drs. Hazen, Wang, and Levison report being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Hazen reports having been paid as a consultant for the following companies: AstraZeneca Pharmaceuticals LP, BG Medicine Inc., Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., Pfizer Inc., Proctor & Gamble, and Takeda. Dr. Hazen reports receiving research funds from Cleveland Heart Lab, Liposcience Inc., Proctor & Gamble, and Takeda. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics and therapeutics for the companies shown below: Cleveland Heart Lab., Frantz Biomarkers, LLC, Liposcience Inc. Drs. Levison and Wang report having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Liposcience Inc. and Proctor & Gamble.
References
- 1.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of Lcarnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.daCosta KA, Vrbanac JJ, Zeisel SH. The measurement of dimethylamine, trimethylamine, and trimethylamine N-oxide using capillary gas chromatography-mass spectrometry. Anal. Biochem. 1990;187:234–239. doi: 10.1016/0003-2697(90)90449-j. [DOI] [PubMed] [Google Scholar]
- 5.Mamer OA, Choiniere L, Treacy EP. Measurement of trimethylamine and trimethylamine Noxide independently in urine by fast atom bombardment mass spectrometry. Anal. Biochem. 1999;276:144–149. doi: 10.1006/abio.1999.4351. [DOI] [PubMed] [Google Scholar]
- 6.Mamer OA, Choiniere L, Lesimple A. Measurement of urinary trimethylamine and trimethylamime oxide by direct infusion electrospray quadrupole time-of-flight mass spectrometry. Anal Biochem. 2010;406:80–82. doi: 10.1016/j.ab.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 7.Erupe ME, Liberman-Martin A, Silva PJ, Malloy QG, Yonis N, Cocker DR, 3rd, Purvis-Roberts KL. Determination of methylamines and trimethylamine-N-oxide in particulate matter by nonsuppressed ion chromatography. J. Chromatogr. 2010;A1217:2070–2073. doi: 10.1016/j.chroma.2010.01.066. [DOI] [PubMed] [Google Scholar]
- 8.Bell JD, Lee JA, Lee HA, Sadler PJ, Wilkie DR, Woodham RH. Nuclear magnetic resonance studies of blood plasma and urine from subjects with chronic renal failure: identification of trimethylamine-N-oxide. Biochim. Biophys. Acta. 1991;1096:101–107. doi: 10.1016/0925-4439(91)90046-c. [DOI] [PubMed] [Google Scholar]
- 9.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levison BS, Zhang R, Wang Z, Fu X, DiDonato JA, Hazen SL. Quantification of fatty acid oxidation products using online high-performance liquid chromatography tandem mass spectrometry. Free Radic Biol. Med. 2013;59:2–13. doi: 10.1016/j.freeradbiomed.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Tang WH, Cho L, Brennan DM, Hazen SL. Targeted metabolomic evaluation of arginine methylation and cardiovascular risks: potential mechanisms beyond nitric oxide synthase inhibition. Arterioscler. Thromb. Vasc. Biol. 2009;29:1383–1391. doi: 10.1161/ATVBAHA.109.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strom AR, Olafsen JA, Larsen H. Trimethylamine oxide: a terminal electron acceptor in anaerobic respiration of bacteria. J. Gen. Microbiol. 1979;112:315–320. doi: 10.1099/00221287-112-2-315. [DOI] [PubMed] [Google Scholar]
- 13.Stenberg E, Styrvold OB, Strom AR. Trimethylamine oxide respiration in Proteus sp. strain NTHC153: electron transfer-dependent phosphorylation and L-serine transport. J. Bacteriol. 1982;149:22–28. doi: 10.1128/jb.149.1.22-28.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]