Abstract
4-Hydroxynonenal (4-HNE) is a lipid peroxidation end product generated in response to oxidative stress in the skin. Keratinocytes contain an array of antioxidant enzymes which protect against oxidative stress. In these studies, we characterized 4-HNE-induced changes in antioxidant expression in mouse keratinocytes. Treatment of primary mouse keratinocytes and PAM 212 keratinocytes with 4-HNE increased mRNA expression for heme oxygenase-1 (HO-1), catalase, NADPH:quinone oxidoreductase (NQO1) and glutathione S-transferase (GST) A1-2, GSTA3 and GSTA4. In both cell types, HO-1 was the most sensitive, increasing 86-98 fold within 6 h. Further characterization of the effects of 4-HNE on HO-1 demonstrated concentration- and time-dependent increases in mRNA and protein expression which were maximum after 6 h with 30 μM. 4-HNE stimulated keratinocyte Erk1/2, JNK and p38 MAP kinases, as well as PI3 kinase. Inhibition of these enzymes suppressed 4-HNE-induced HO-1 mRNA and protein expression. 4-HNE also activated Nrf2 by inducing its translocation to the nucleus. 4-HNE was markedly less effective in inducing HO-1 mRNA and protein in keratinocytes from Nrf2−/− mice, when compared to wild type mice, indicating that Nrf2 also regulates 4-HNE-induced signaling. Western blot analysis of caveolar membrane fractions isolated by sucrose density centrifugation demonstrated that 4-HNE-induced HO-1 is localized in keratinocyte caveolae. Treatment of the cells with methyl-β-cyclodextrin, which disrupts caveolar structure, suppressed 4-HNE-induced HO-1. These findings indicate that 4-HNE modulates expression of antioxidant enzymes in keratinocytes, and that this can occur by different mechanisms. Changes in expression of keratinocyte antioxidants may be important in protecting the skin from oxidative stress.
Keywords: heme oxygenase-1, caveolae, Nrf2, skin, antioxidants, stress proteins
Introduction
The skin is highly sensitive to oxidative stress induced environmental insults such as ultraviolet light, gamma radiation and various chemical toxicants (Isoir et al., 2006; Black et al., 2008b; Laskin et al., 2010). Oxidative stress is associated with the generation of excessive amounts of highly toxic intermediates including superoxide anion, hydrogen peroxide and hydroxyl radicals (Halliwell and Whiteman, 2004). These reactive oxygen species (ROS) can initiate lipid peroxidation, a process that generates α,β-unsaturated hydroxyalkenals (Niki, 2009). One of these electrophilic species is 4-hydroxynonenal (4-HNE), a relatively abundant aldehyde that forms Michael adducts with nucleophilic sites in DNA, lipids and proteins (LoPachin et al., 2009). 4-HNE is mutagenic and can disrupt cellular metabolic activity (Winczura et al., 2012). It has been identified in sun damaged skin (Tanaka et al., 2001; Hirao and Takahashi, 2005), radiation-induced dermatitis (Ning et al., 2012), and in skin treated with chemicals such as the sulfur mustard analog 2-chloroethyl ethyl sulfide (Tewari-Singh et al., 2012) and ozone (Valacchi et al., 2003).
It is well recognized that oxidative stress initiates an adaptive response involving upregulation of stress response genes and antioxidants important in protecting cells from injury (Davies, 2000). Driving this process is activation of signaling molecules and transcription factors that control expression of these genes. In keratinocytes, these include mitogen activated protein (MAP) kinases and phospho-inositide-3-kinase (PI3K)/Akt, as well as nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and NF-κB (Dhar et al., 2002; Keum et al., 2006; Marrot et al., 2008; Black et al., 2010; Haarmann-Stemmann et al., 2012). Molecules thought to be involved in mediating stress-induced alterations in adaptive response genes include lipid peroxidation products such as malondialdehyde, acrolein and 4-HNE (Pizzimenti et al., 2013). It should be noted that nitric oxide synthase is induced during oxidative stress (Piantadosi and Suliman, 2012) and nitric oxide generated reaction products such as peroxynitrite and various electrophilic nitrolipids can also upregulate oxidative stress responsive genes (Szabo et al., 2007; Freeman et al., 2008).
Earlier studies by our laboratory showed that in keratinocytes, oxidative stress induced by UVB light and chemical toxicants results in upregulation of adaptive response proteins (Black et al., 2008a). These include enzymes important in detoxifying or limiting the production of ROS such as superoxide dismutase (SOD), catalase and NAD(P)H quinone oxidoreductase 1 (NQO1) and the phase 2 enzymes heme oxygenase-1 (HO-1) and glutathione S-transferases (GST). The present studies were aimed at identifying antioxidants and stress proteins upregulated in mouse keratinocytes by 4-HNE and analyzing signaling pathways regulating this response. Coordinate regulation of expression of these adaptive response proteins is likely to be important in controlling oxidative stress and tissue injury following exposure of the skin to xenobiotics.
Materials and Methods
Materials
Mouse monoclonal 4-HNE antibody was from R&D Systems (Minneapolis, MN) and rabbit polyclonal HO-1 antibody was from Enzo Life Sciences (Farmingdale, NY). Rabbit polyclonal caveolin-1 antibody, rabbit polyclonal p38, phospho-p38, JNK, phospho-JNK, ERK1/2, phospho-ERK1/2, Akt and phospho-Akt antibodies were from Cell Signaling Technology (Beverly, MA). The DC (Detergent Compatible) protein assay kit was purchased from Bio-Rad Laboratories (Hercules, CA) and the Western Lightning enhanced chemiluminescence kit (ECL) from Perkin Elmer Life Sciences (Boston, MA). Reagents for MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) viability assays and M-MLV Reverse Transcriptase were from Promega (Madison, WI). SYBR Green Master Mix and other PCR reagents were purchased from Applied Biosystems (Foster City, CA). 4-HNE, PD 98059, SP600125 and wortmannin were from Calbiochem (La Jolla, CA). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were from Invitrogen Corp. (Carlsbad, CA). Mouse monoclonal β-actin antibody, SB203580, protease inhibitor cocktail (4-(2 aminoethyl)benzenesulfonyl fluoride, aprotinin, bestatin hydrochloride, N-(trans-epoxysuccinyl)-L-leucine 4-guanidinobutylamide, EDTA and leupeptin), methyl-β-cyclodextrin and all other chemicals were from Sigma-Aldrich (St. Louis, MO).
Cell culture and treatments
PAM 212 cells were obtained from Dr. Stuart Yuspa (National Institutes of Health) and maintained as previously described (Black et al., 2008b). Cells were cultured in DMEM containing 10% fetal bovine serum supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin. Primary mouse keratinocytes were isolated from the skin of newborn C57BL/6J wild type mice (The Jackson Laboratory, Bar Harbor, ME) or C57BL/6J Nrf2−/− mice (Khor et al., 2006; Khor et al., 2008) bred at the Rutgers University animal care facility. Keratinocytes were cultured following the procedure of Hager et al. (1999).
For experiments, keratinocytes were grown to 80~90% confluence in six well collagen IV-coated plates as previously described (Black et al., 2008a). Cells were then treated with vehicle or increasing concentrations of 4-HNE (1-100 μM). At these concentrations, cell viability of PAM 212 cells and primary keratinocytes at 6 h was greater than 98% and 90%, respectively. For kinase inhibition experiments, cells were pretreated with p38 MAP kinase inhibitor SB203580 (10 μM), JNK kinase inhibitor SP600125 (20 μM), ERK1/2 kinase inhibitor PD98059 (10 μM) or phosphatidylinositide 3-(PI3K) kinase inhibitor wortmannin (0.1 μM) for 3 h prior to treatment with 4-HNE or vehicle control. Cells were analyzed 6 h later for mRNA and protein expression by real time PCR and western blotting, respectively. For caveolae inhibition experiments, PAM 212 keratinocytes were pre-incubated with control or 5 mM of methyl-β-cyclodextrin for 3 h. After washing with PBS, cells were treated with 4-HNE for 6 h and analyzed for protein expression by western blotting.
Western blotting
Western blotting was performed as previously described (Zheng et al., 2013b). Briefly, cells were lysed by the addition of 300 μl SDS lysis buffer (10 mM Tris-base, pH 7.6, supplemented with 1% SDS and the protease inhibitor cocktail), transferred into 1.5 ml Eppendorf microcentrifuge tubes, sonicated on ice and centrifuged (300 × g, 10 min at 4° C). Total protein in supernatants was determined by the DC protein assay kit using bovine serum albumin as the standard. Lysates (15 μg protein/well) were electrophoresed on 10% SDS-polyacrylamide gels, transferred to nitrocellulose membranes and blocked in Tris buffer supplemented with 5% milk at room temperature. After 1 h, the blots were incubated overnight at 4°C with primary antibodies, washed with tTBS (Tris-buffered saline supplement with 0.1% Tween 20) and then incubated with horseradish peroxidase-conjugated secondary antibodies. After 1 h at room temperature, proteins were visualized by ECL chemiluminescence.
Real-time PCR
Total RNA was isolated from the cells using Tri Reagent (Sigma). cDNA was synthesized using M-MLV reverse transcriptase. The cDNA was diluted 1:10 in RNase-DNase-free water for PCR analysis. For each gene, a standard curve was created from serial dilutions of cDNA mixtures of all the samples. Real-time PCR was conducted on an ABI Prism 7900 Sequence Detection System using 96-well optical reaction plates. SYBR-Green was used for detection of the fluorescent signal and the standard curve method was used for relative quantitative analysis. The primer sequences for the genes were generated using Primer Express software (Applied Biosystems) and oligonucleotides synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The house keeping gene β-actin was used to normalize all values. The forward (5’-3’) and reverse (5’-3’) primers used are listed in Table 1.
Table 1.
Real-time PCR primer sequences.
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| β-actin | TCA CCC ACA CTG TGC CCA TCT ACG A | GGA TGC CAC AGG ATT CCA TAC CCA |
| HO-1 | ACC AGG GCA TCA AAA ACT TG | GCC CTG AAG CTT TTT GTC AG |
| GSTA1-2 | CAG AGT CCG GAA GAT TTG GA | CAA GGC AGT CTT GGC TTC TC |
| GSTA3 | GCA AGC CTT GCC AAG ATC AA | GGC AGG GAA GTA ACG GTT CC |
| GSTA4 | CCC TTG GTT GAA ATC GAT GG | GAG GAT GGC CCT GGT CTG T |
| NQO1 | ACG CCT GAG CCC AGA TAT TG | TCT GCA GCT TCC AGC TTC TTG |
| Catalase | ACC AGG GCA TCA AAA ACT TG | GCC CTG AAG CTT TTT GTC AG |
| GSTM1 | CCT ACA TGA AGA GTA GCC GCT ACA T | TAG TGA GTG CCC GTG TAG CAA |
| GSTP1 | CCT TGG CCG CTC TTT GG | GGC CTT CAC GTA GTC ATT CTT ACC |
| SOD | ACC AGT GCA GGA CCT CAT TTT AA | TCT CCA ACA TGC CTC TCT TCA TC |
Analysis of 4-HNE uptake and metabolism
4-HNE uptake and metabolism experiments were performed as described earlier (Siems et al., 1997). Briefly, keratinocytes were treated with 100 μM 4-HNE in 1.5 ml of serum-free culture medium. After 5-120 min, cells were washed and metabolism terminated by the addition of 1 ml of 70% perchloric acid in PBS. The cells were then removed from the dishes using a scrapper, transferred to 1.5 ml Eppendorf tubes and centrifuged at 3,000 g for 10 min at 4 °C. Supernatants were analyzed by HPLC (JASCO corporation, Tokyo, Japan) fitted with a JASCO 2075 plus UV detector and a Phenomenex 5μ C18 column (250 × 2.00 mm) using a mobile phase consisting of 70% 50 mM potassium phosphate buffer (pH 2.7) and 30% acetonitrile at a flow rate of 0.25 ml/min. 4-HNE (retention time = 20 min) was detected at 224 nm. In some experiments, 4-HNE metabolism was analyzed in cell lysates prepared by sonicating a suspension of 5 × 106 cells in 1 ml PBS on ice. Lysates were then centrifuged at 9,000 × g for 20 min at 4°C. Clear supernatants (15 μg protein/ml) were incubated with 100 μM 4-HNE in the absence or presence of 1 mM NADH or NADPH. Reactions were stopped by the addition of 0.1 ml 70% perchloric acid in PBS. Samples were then centrifuged (3,000 g for 10 min) and clear supernatants analyzed by HPLC.
Isolation of caveolae
Lipid rafts containing caveolae were prepared from PAM 212 cells as described by Smart et al. (1995). Briefly, treated cells were scraped into 5 ml ice-cold sucrose buffer (0.25 M sucrose, 1 mM EDTA, and 20 mM Tris, pH 7.8) and centrifuged at 1400 × g for 5 min at 4°C. Cell pellets were then suspended in 1 ml sucrose buffer and homogenized with 20 strokes in a Dounce homogenizer. Lysates were transferred to Eppendorf tubes and centrifuged for 10 min at 1000 × g at 4°C. Supernatants were collected and the homogenization process repeated with cell pellets. The supernatants were combined and then carefully layered on top of 8 ml of a 30% Percoll solution in sucrose buffer. The gradient was then centrifuged for 60 min at 84,000 × g in a Ti 70 rotor using an L7-55 Beckman ultracentrifuge (Brea, CA) to separate caveolae containing plasma membrane fractions. These were collected and stored at −70°C until analysis.
Statistical analysis
Data were evaluated using the two-way ANOVA. p < 0.05 was considered statistically significant.
Results
4-HNE metabolism in keratinocytes
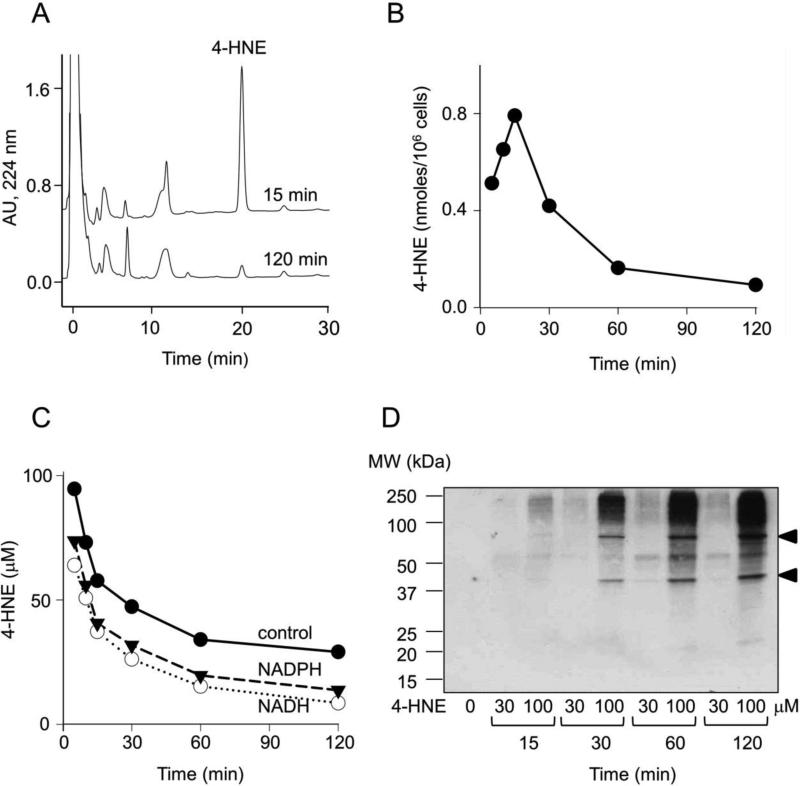
In initial experiments we analyzed the uptake and degradation of 4-HNE in PAM 212 keratinocytes. 4-HNE was found to accumulate in the cells within 15 min of treatment with 100 μM 4-HNE; rapid degradation of the reactive aldehyde was evident thereafter (Fig. 1, panels A and B). Using lysates from a human keratinocyte cell line, previous studies showed that 4-HNE was degraded in an NADH-dependent pathway (Aldini et al., 2003; Aldini et al., 2007). In contrast, we found that 4-HNE was rapidly degraded in PAM 212 cell lysates in the absence of pyridine nucleotides. However, both NADH and NADPH stimulated 4-HNE degradation (Fig. 1, panel C). The α,β-unsaturated bond of 4-HNE is known to form protein adducts by reacting with cysteine, histidine and lysine residues through Michael additions, processes thought to initiate the biological activity of reactive lipid peroxidation products (LoPachin et al., 2009). In intact keratinocytes, 4-HNE treatment resulted in the formation of 4-HNE-protein adducts (Fig. 1, panel D). Within 15-30 min of treatment with 100 μM 4-HNE, two prominent bands (Mr = 43,000 and 75,000) were evident in western blots. With increasing periods of time, additional proteins of higher and lower molecular weights were also modified by 4-HNE.
Figure 1. Metabolism of 4-HNE in PAM 212 keratinocytes.
Cells were treated with 30 or 100 μM 4-HNE for increasing periods of time up to 120 min. For analysis of 4-HNE, cells were washed and 4-HNE extracted as described in the Materials and Methods. Panel A. Representative HPLC tracing of 4-HNE extracted from cells 15 min and 120 min post treatment with 100 μM 4-HNE. Panel B. Time course of 4-HNE accumulation and degradation in cells treated with 100 μM 4-HNE. Panel C. Time-course of 4-HNE metabolism in S9 fractions of mouse keratinocytes. S9 fractions of PAM 212 cells were incubated with 100 μM 4-HNE in the absence and presence of 1 mM NADH or NADPH. At the indicated times, 4-HNE was extracted and quantified by HPLC. Panel D. Time-dependent formation of 4-HNE-protein adducts in intact cells treated with control, 30, or 100 μM 4-HNE. For this analysis, treated cells were lysed and analyzed by Western blotting using a monoclonal antibody to 4-HNE. Arrowheads show the appearance of modified proteins with molecular weights of 43 and 75 kDa.
4-HNE induces antioxidant proteins
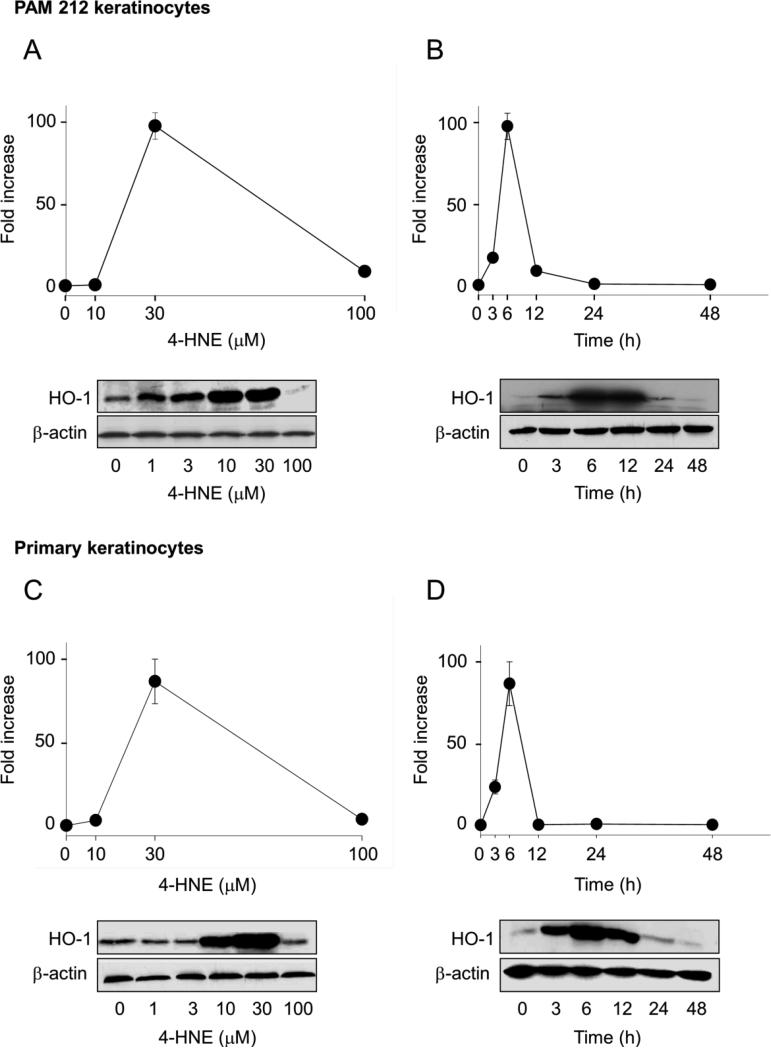
Earlier studies showed that stress resulting from the reaction of lipid peroxidation products with cellular components upregulates oxidative stress-related genes including HO-1(Basu-Modak et al., 1996). Consistent with these findings, we observed that 4-HNE readily induced HO-1 mRNA and protein in both PAM 212 cells and primary keratinocytes from wild type mice, as determined by real time PCR and Western blotting, respectively (Table 2 and Fig. 2). The effects of 4-HNE were concentration- and time-dependent. In both cell types, maximal increases in HO-1 mRNA expression (approximately 85-90-fold) were noted with 30 μM 4-HNE after 6 h. Increases in protein expression were maximal with 10-30 μM 4-HNE after 6-12 h (Fig. 2). We next determined if 4-HNE upregulated other antioxidants in the cells. We found that 4-HNE (30 μM) induced expression of mRNA for NQO1, as well as the glutathione S-transferases, GSTA1-2, GSTA3 and GSTA4 (Table 2). Whereas increased expression of NQO-1 was 4-5 fold after 6 h and 24 h incubation with 4-HNE, in both PAM 212 cells and primary keratinocytes, increases in GSTA1-2 (30-60-fold) were only observed after 24 h. 4-HNE also upregulated GSTA3 and GSTA4 approximately 2-8 fold. Catalase was also upregulated by 4-HNE approximately 3 fold, but only in primary keratinocytes after 24 h. 4-HNE had no significant effects on SOD, GSTM1 or GSTP1.
Table 2.
Effects of 4-HNE on antioxidant and stress-related gene expression
| Gene | PAM 212 keratinocytes1 | Primary keratinocytes | ||
|---|---|---|---|---|
| 6 h | 24 h | 6 h | 24 h | |
| Fold increase | ||||
| HO-1 | 97.7 ± 8.2* | 9.7 ± 0.3* | 86.3 ± 13.3* | 1.7 ± 0.2 |
| NQO1 | 5.0 ± 0.8* | 4.5 ± 1.4* | 5.1 ± 0.8* | 4.8 ± 0.5* |
| SOD | 1.9 ± 0.7 | 1.5 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.2 |
| Catalase | 1.5 ± 0.2 | 1.0 ± 0.0 | 1.5 ± 0.3 | 2.8 ± 0.3* |
| GSTA1-2 | 3.9 ± 0.9* | 63.1 ± 15.8* | 3.0 ± 0.6* | 30.6 ± 3.0* |
| GSTA 3 | 2.9 ± 0.5* | 1.0 ± 0.3 | 2.6 ± 0.2* | 4.1 ± 0.2* |
| GSTA 4 | 8.5 ± 0.3* | 1.4 ± 0.4 | 2.8 ± 1.0 | 2.6 ± 0.3* |
| GSTP1 | 1.7 ± 0.4 | 2.4 ± 0.6 | 0.9 ± 0.2 | 2.4 ± 0.4 |
| GSTM1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.3 | 1.9 ± 0.2 |
Keratinocytes were treated with vehicle control or 30 μM 4-HNE.
mRNA was extracted 6 h and 24 h later and analyzed for gene expression by real time PCR. Data are presented as fold change relative to control. Each value represents the mean ± SE (n = 3). Asterisks show values that are significantly different from control (p < 0.05).
Figure 2. Effects of 4-HNE on HO-1 expression in keratinocytes.
PAM 212 cells (panels A and B) or primary cultures of mouse keratinocytes (panels C and D) were treated with increasing concentrations of 4-HNE for 6 h (panels A and C) or with 30 μM 4-HNE for increasing periods of time (panels B and D). Cells were then analyzed for HO-1 mRNA and protein expression by real time PCR and Western blotting, respectively. In each panel, the upper figures show HO-1 mRNA expression, while the lower panels show Western blots for HO-1 protein expression. Expression of β-actin is shown in each of the western blots as a control. Data for mRNA are expressed as fold increase relative to control. Bars represent the mean ± SE (n = 3).
Signaling pathways regulating 4-HNE-induced alterations in HO-1 expression
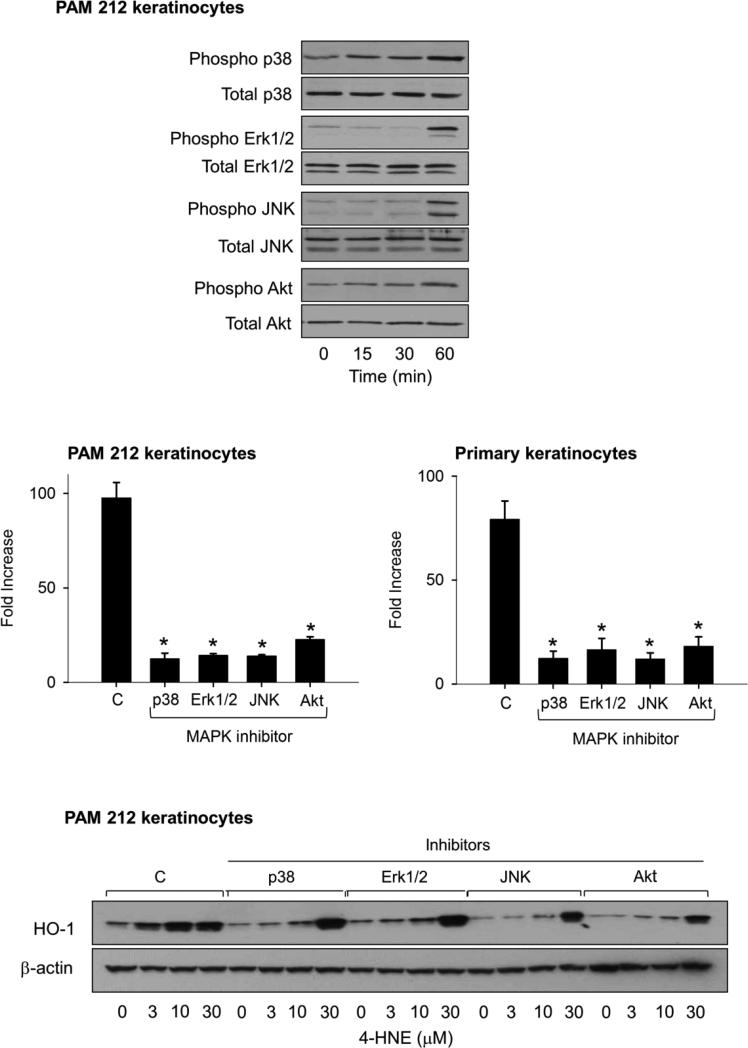
MAP kinase and PI3K/Akt signaling are known to be important in regulating antioxidant expression. We next characterized these signaling pathways in 4-HNE treated PAM 212 keratinocytes. 4-HNE was found to activate p38, Erk1/2, JNK MAP kinase activity, as well as Akt in the cells as measured by phosphorylation of these proteins (Fig. 3, upper panel). No significant changes were apparent in total expression of the MAP kinases or Akt. We next used inhibitors of the MAP kinases and PI3 kinase to evaluate their role in regulating HO-1 expression. SB203580, a p38 kinase inhibitor, SP600125, a JNK inhibitor, PD98059, an ERK1/2 kinase inhibitor and wortmanin, a PI3K inhibitor, were found to suppress 4-HNE-induced HO-1 mRNA and protein expression (Fig. 3, center and lower panels). The highest concentration of 4-HNE (30 μM) was able to overcome inhibition of HO-1 protein expression by the kinase inhibitors.
Figure 3. Role of MAP kinase and PI3K/Akt signaling in 4-HNE-induced HO-1 expression in keratinocytes.
Upper panel. Effects of 4-HNE on expression of MAP kinases and PI3K/Akt. PAM 212 keratinocytes were treated with vehicle control or 30 μM 4-HNE for 15, 30 or 60 min. Cell lysates were prepared and analyzed for total and phosphorylated p38, JNK, Erk1/2, or PI3K/Akt by western blotting. Center panel. Effects of MAP kinase and PI3K/Akt inhibitors on 4-HNE-induced HO-1 mRNA expression. PAM 212 cells and primary mouse keratinocytes were pre-incubated for 3 h with inhibitors of p38 kinase (SB203580, 10 μM), JNK (SP600125, 20 μM), Erk1/2 (PD98059, 10 μM) or PI3K (wortmannin, 0.1 μM) and then with 30 μM 4-HNE for additional 6 h. Expression of HO-1 mRNA was analyzed by real-time PCR and is expressed as fold-increase relative to vehicle control. Each bar represents the mean ± SE (n = 3). *Significantly different from control (p < 0.05). Lower panel. Effects of MAP kinase and PI3K/Akt inhibitors on 4-HNE-induced HO-1 protein expression in PAM 212 keratinocytes. Total cell lysates were prepared from control and treated cells and analyzed for HO-1 protein expression by Western blotting.
Role of caveolae in 4-HNE-induced expression of HO-1
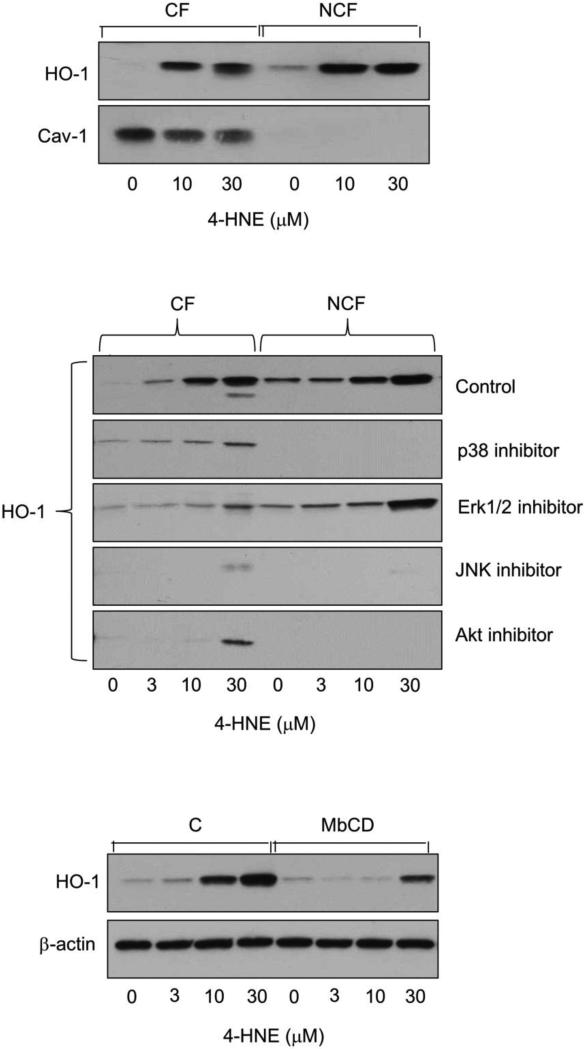
We previously showed that plasma membrane caveolae play a role in the regulation of electrophilic nitro fatty acid-induced expression of stress proteins and antioxidants in keratinocytes (Zheng et al., 2013a). In further studies we determined if caveolae regulate 4-HNE-induced expression of HO-1. Caveolar fractions, but not non-caveolar fractions of PAM 212 keratinocytes, were found to contain caveolin-1, the major structural protein in caveolae (Fig. 4, upper panel). 4-HNE upregulated HO-1 expression in both caveolar and non-caveolar fractions of the cells. The MAP kinase and Akt inhibitors were effective in suppressing expression of HO-1 in both caveolar and non-caveolar fractions (Fig. 4, center panel). Inhibitors of JNK and p38 were the most effective in blocking caveolar HO-1 expression. Treatment of keratinocytes with methyl-β-cyclodextrin, which depletes cholesterol and disrupts caveolae, similarly reduced 4-HNE-induced HO-1 expression (Fig. 4, lower panel).
Figure 4. Localization of 4-HNE-induced HO-1 in caveolae.
Upper panel. Distribution of HO-1 in caveolar fractions (CF) and non-caveolar fractions (NCF) of PAM 212 cells. Keratinocytes were treated with 0, 10 or 30 μM 4-HNE. After 6 h, caveolar and non-caveolar fractions were prepared and analyzed for HO-1 and caveolin-1 (Cav-1) protein expression by western blotting. Center panel. Effects of kinase inhibitors on expression of HO-1 in caveolar and non-caveolar fractions of PAM 212 cells. Keratinocytes were pre-incubated with MAP kinase and PI3K inhibitors and then 4-HNE (0, 3, 10 or 30 μM) as described in the legend to Figure 3. After an additional 6 h, caveolar and non-caveolar fractions were prepared and analyzed for HO-1 protein by western blotting. Lower panel. Effects of cholesterol depletion on HO-1 expression. Cells were pretreated with 5 mM methyl-β-cyclodextrin (MbCD) or control (C) for 3 h and then with 4-HNE for 6 h. Total cell lysates were then prepared and analyzed for HO-1 protein expression by western blotting.
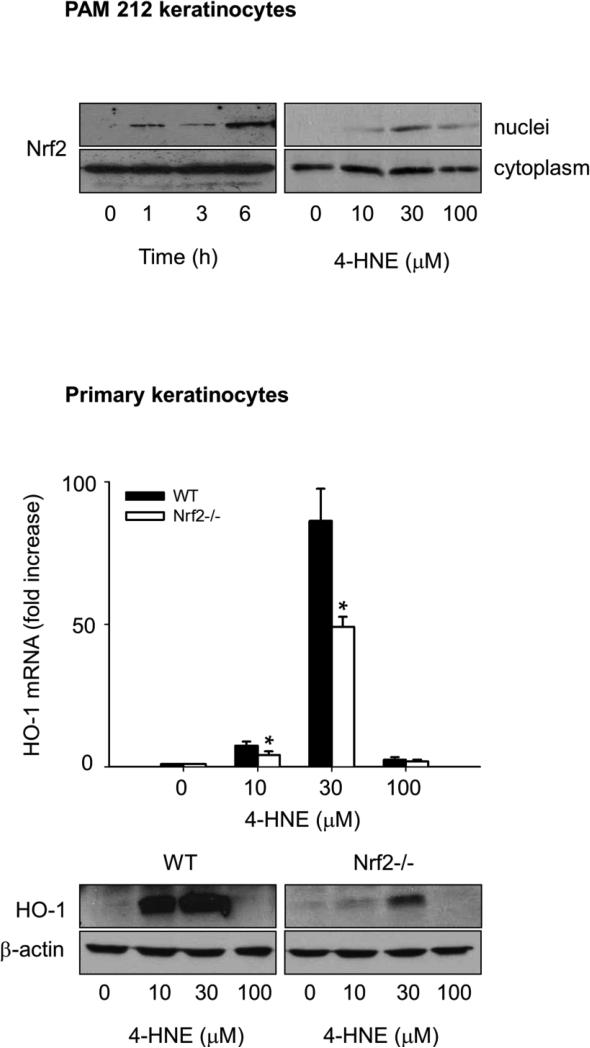
Role of Nrf2 in 4-HNE-induced expression of HO-1
The transcription factor Nrf2 is known to play a role in the regulation of expression of antioxidants in response to 4-HNE (Huang et al., 2012). Nrf2 forms a complex with Keap1, which functions to retain Nrf2 in the cytoplasm (Li and Kong, 2009). Binding of 4-HNE to Keap1 allows Nrf2 to translocate to the nucleus where it regulates antioxidant gene expression (Levonen et al., 2007; Kobayashi et al., 2009). In PAM 212 cells, 4-HNE was found to stimulate nuclear localization of Nrf2 in a concentration- and time-dependent manner (Fig. 5, upper panel). To assess the role of Nrf2 in 4-HNE-induced expression of HO-1, we used keratinocytes from Nrf2−/− mice. We found that 4-HNE was significantly less effective in upregulating HO-1 mRNA expression in keratinocytes from Nrf2−/− mice than keratinocytes from wild type mice (Fig 5, lower panel).
Figure 5. Role of Nrf2 in 4-HNE-induced HO-1 expression in mouse keratinocytes.
Keratinocytes were incubated with control or 4-HNE and analyzed for Nrf2 or HO-1 expression. Upper panel. Effects of 4-HNE on nuclear localization of Nrf2. PAM 212 cells were treated with 30 μM 4-HNE for 0, 1, 3 and 6 h or 0, 10, 30 and 100 μM 4-HNE for 3 h. Nuclear and cytoplasmic fractions of the cells were then prepared and Nrf2 expression analyzed by western blotting. Lower panel. Effects of Nrf2 expression on 4-HNE-induced HO-1 expression. Primary keratinocytes from wild type and Nrf2−/− mice were treated with 0, 10, 30 and 100 μM 4-HNE for 6 h and expression of HO-1 mRNA (upper panel) and protein (lower panel) analyzed by real-time PCR and Western blotting, respectively, as described in the Materials and Methods. HO-1 mRNA expression is presented as fold-increase relative to control. Each bar represents the mean ± SE (n = 3).
Discussion
4-HNE is a highly reactive aldehyde that can modify cellular components and induce cytotoxicity. Metabolic degradation of 4-HNE limits its cytotoxic activity and protects against tissue injury (O'Brien et al., 2005). The present studies demonstrate that 4-HNE is readily taken up by mouse keratinocytes; intracellular levels were maximal within 15 min of treatment, declining rapidly thereafter. Decreases in intracellular keratinocyte 4-HNE are consistent with its metabolism. In this regard, earlier studies showed both oxidative and reductive metabolism of 4-HNE in human keratinocytes, as well as the formation of GSH reaction products (Aldini et al., 2003; Aldini et al., 2007). A number of enzymes have been identified that metabolize 4-HNE, including alcohol dehydrogenase, aldehyde dehydrogenase, aldo-keto reductases, cytochrome P450's and glutathione S-transferases (Hartley et al., 1995; Srivastava et al., 2000; Amunom et al., 2011). Glutathione S-transferases conjugate 4-HNE to glutathione, a process that promotes its extracellular transport (Tjalkens et al., 1999). Glutathione conjugates and related metabolites generated by enzymatic oxidation/reduction of these conjugates have been identified in culture medium from human keratinocytes treated with 4-HNE (Aldini et al., 2007). Similarly, we observed rapid degradation of 4-HNE in lysates of mouse keratinocytes. Of note, this process was stimulated by both NADH and NADPH. These pyridine nucleotides are cofactors for enzymes that degrade 4-HNE including alcohol dehydrogenase and aldehyde dehydrogenase which have been identified in human keratinocytes (Alary et al., 2003; Aldini et al., 2003). A considerable fraction of 4-HNE metabolism in mouse keratinocytes was found to be pyridine nucleotide-independent. Enzymes mediating this process in keratinocytes remain to be determined.
We also found that 4-HNE formed protein adducts in keratinocytes which was maximal 30 and 60 min post treatment. This occurred despite rapid degradation of 4-HNE. It has previously been reported that approximately 3-6% of 4-HNE administered to rat hepatocytes forms protein adducts (Siems and Grune, 2003); similar levels of 4-HNE protein adducts have been noted in human keratinocytes (Tanaka et al., 2001). Although many 4-HNE modified proteins have been identified and characterized in different tissues (Petersen and Doorn, 2004; Jacobs and Marnett, 2010), including several involved in the regulation of signal transduction (Nakashima et al., 2003), the identity of these proteins in mouse keratinocytes and their role in mediating adaptive responses remains to be determined.
Treatment of both primary keratinocytes and PAM 212 keratinocytes with 4-HNE was associated with upregulation of key stress proteins including HO-1, NQO-1, catalase, GSTA1-2, GSTA3 and GSTA4, which play important roles in antioxidant defense. Most prominent were changes in expression of HO-1 and GSTA1-2. HO-1 is the rate limiting enzyme in the degradation of heme; it confers protection against oxidative stress by inhibiting the formation of reactive oxygen species and suppressing inflammation (Gozzelino et al., 2010). GST's such as GSTA1-2 are important in the conjugation of electrophilic compounds to reduced glutathione (Board and Menon, 2013). Three major GST gene families, alpha (GSTA), mu (GSTM) and pi (GSTP) have been identified and each has preferred substrates. GSTA enzymes, and to a lesser extent, GSTP enzymes, remove lipid peroxidation products including 4-HNE (Hayes et al., 2005). Our observation that there were marked increases in GSTA1-2 are consistent with the identification of 4-HNE-glutathione conjugates and related metabolites in human keratinocytes (Aldini et al., 2003). They are in accord with findings that oxidative stressors in the skin such as UVB, as well as electrophilic nitrolipids formed during nitrosative stress, upregulate keratinocyte GSTA1-2 (Black et al., 2008b; Zheng et al., 2013b). In contrast, GSTM1 and GSTP1, which remove DNA and protein oxidation products (Hayes et al., 2005), were unaffected by 4-HNE suggesting that they do not play a role in protecting the cells against this reactive aldehyde. Of note were our findings that following 4-HNE treatment of keratinocytes, the time for maximal induction of HO-1 expression was 6 h, while maximal induction of GSTA1-2 was 24 h. These differences likely represent distinct signaling pathways and/or transcription factors for these genes that are activated by 4-HNE. Increases in GSTA3 and GSTA4 were remarkably less relative to GSTA1-2, and were variable with respect to maximal induction times. This is consistent with findings that distinct signaling pathways control expression of the different GSTA isoforms (Hayes et al., 2005).
4-HNE was also found to modulate expression of NQO1 and catalase. Whereas increases in NQO1 were evident after 6 h and 24 h in both keratinocyte populations, only a small increase in catalase was observed in primary keratinocytes after 24 h. NQO1 functions as an antioxidant by catalyzing the two-electron reduction of quinones and related electrochemically active compounds, an enzymatic process that limits formation of reactive oxygen species resulting from chemical redox cycling. NQO1 also directly scavenges superoxide anion (Siegel et al., 2004). Catalase is important in degrading hydrogen peroxide and increases in expression of this enzyme, as well as NQO1 are important in limiting oxidative stress. Mechanisms mediating selective increases in catalase in primary keratinocytes require further investigation.
A question remains as to the mechanism by which 4-HNE upregulates expression of adaptive response proteins in keratinocytes. Several signaling molecules have been identified that control their expression, most notably, MAP kinases and PI3K/Akt (Yang et al., 2003; Usatyuk and Natarajan, 2004). We previously demonstrated that 4-HNE activates both of these signal transduction pathways in corneal epithelial cells (Zheng et al., 2013b); herein we report that 4-HNE has similar effects on keratinocytes. The fact that inhibitors of these enzymes suppressed 4-HNE-induced expression of HO-1 in keratinocytes demonstrates their functional significance of these cells. Generally similar findings on signaling pathways mediating the actions of 4-HNE have been reported in rat and human liver cells and bovine lung microvascular endothelial cells (Moneypenny and Gallagher, 2005; Usatyuk et al., 2006; Sampey et al., 2007). The mechanism by which MAP kinase signaling cascades are activated by 4-HNE is not known. In human bronchial epithelial cells, 4-HNE down modulates the protein-tyrosine phosphatase SH2 domain containing phosphatase-1 (SHP-1) which negatively regulates JNK activity (Rinna and Forman, 2008). This may be important in the action of 4-HNE in mouse keratinocytes. Recent studies have also demonstrated that activation of PI3K/Akt signaling by 4-HNE in hepatocytes occurs via modification and inhibition of PTEN, a regulatory protein that suppresses Akt activity (Shearn et al., 2011). A similar pathway may be involved in 4-HNE-induced PI3K/Akt signaling in keratinocytes.
Nrf2/Keap-1 is an electrophilic stress signaling pathway active in keratinocytes that has been reported to regulate expression of adaptive response genes including HO-1, NQO1 and various GST's (Marrot et al., 2008; Piao et al., 2012). Earlier studies have shown that electrophilic substrates, including 4-HNE, modulate Nrf2 and antioxidant expression (Chen et al., 2005; Zhang and Forman, 2009). In the skin, Nrf2 is thought to be important in controlling inflammation during wound healing, responses to photodamage, and chemical carcinogenesis (Braun et al., 2002; Xu et al., 2006; Schafer et al., 2010; Saw et al., 2011). Our studies demonstrate that 4-HNE activates Nrf2 in keratinocytes, as measured by nuclear localization of the protein; moreover, this process is coordinate with induction of HO-1. Findings that loss of Nrf2 markedly reduces 4-HNE-induced HO-1 expression indicate that the Nrf2/Keap-1 mediates, at least in part, the stress response pathway in keratinocytes. These data also indicate that 4-HNE utilizes more than one pathway to control HO-1 induction.
Caveolae are specialized flask-shaped glycosphingolipid-containing membrane structures controlling many cellular functions including vesicular transport, cholesterol homeostasis, inflammation, apoptosis and proliferation (Razani et al., 2002). The major structural component of caveolae is caveolin-1, a protein known to be expressed in basal keratinocytes in mouse and human skin (Capozza et al., 2003; Sando et al., 2003). Caveolin-1 controls epidermal proliferation and differentiation and aberrant caveolin-1 expression is common in diseases such as systemic sclerosis and psoriasis (Campbell and Gumbleton, 2000; Manetti et al., 2012). We found that 4-HNE-induced HO-1 was localized in both caveolar and non-caveolar fractions of keratinocytes. Previous studies have described compartmentalization of HO-1 in caveolae in rat pulmonary artery endothelial cells and mouse mesangial cells induced by endotoxin and in mouse keratinocytes treated with electrophilic nitrofatty acids (Jung et al., 2003; Kim et al., 2004; Zheng et al., 2013b). The function of caveolar localized HO-1 is unknown. Caveolin-1 is known to directly interact with HO-1 and modulate its activity (Taira et al., 2011). Caveolin-1 also interacts with caveolar toll-like receptor-4 (TLR4), an endotoxin receptor important in negatively regulating proinflammatory signaling including cytokine production (Wang et al., 2009; Mirza et al., 2010). These investigators further showed that this process is enhanced when HO-1 traffics to caveolae. It is possible that caveolar-associated HO-1 also contributes to the antiinflammatory activity of this antioxidant in keratinocytes by suppressing proinflammatory signaling. Cytoplasmic HO-1 resides in the endoplasmic reticulum where it presumably functions as an antioxidant. Of note was our observation that MAP kinases and PI3K/Akt are not selective and suppress localization of HO-1 in both caveolar and cytoplasmic fractions of keratinocytes. These data are consistent with studies showing that p38 MAP kinase signaling can control endotoxin-induced production of HO-1 and its localization in mouse macrophage caveolae (Wang et al., 2006). Taken together, these data suggest that MAP kinase and PI3K/Akt signaling are important in the control of the synthesis of HO-1, but not trafficking of this antioxidant to caveolae.
A novel aspect of our work is the observation that not only is HO-1 localized in keratinocyte caveolae, but it was also regulated by these structures. Thus, disruption of caveolae by cholesterol depletion suppressed 4-HNE-induced HO-1 expression. These results are in accord with our previous studies showing that disruption of caveolae in mouse keratinocytes reduced 9- and 10-nitrooleic acid-induced expression of HO-1, hsp 27 and hsp70 (Zheng et al., 2013a). These data provide additional support for the idea that caveolae are important in negatively controlling inflammation. The factors that contribute to caveolae regulation of antioxidant expression in keratinocytes are not known. It is possible that 4-HNE initiates signaling via caveolae. In this regard, many growth factors and cytokines initiate signaling via caveolae-associated receptors and these may be targets for 4-HNE (Pike, 2005; Harvey and Calaghan, 2012)
In summary, our findings support the idea that 4-HNE functions as a signaling molecule in keratinocytes, upregulating adaptive response genes that are crucial for protecting cells against oxidative stress. Multiple signaling pathways controlling expression of antioxidants in keratinocytes were identified including MAP kinase and PI3K signaling and activation of Nrf2. It is well recognized that signaling pathways in addition to these have been identified that control expression of genes such as HO-1; thus, it is likely that more than one pathway is involved in regulating keratinocyte antioxidant expression in response to 4-HNE. An emerging literature also implicates stress protein trafficking to caveolae as a control point in inflammation (Chidlow and Sessa, 2010). Our data support this idea as electrophilic species generated during oxidative stress are able to drive antioxidants into caveolae. Further studies are needed to more precisely understand the role of adaptive response genes and caveolae in protecting the skin against oxidative stress.
Highlights.
Lipid peroxidation generates 4-hydroxynonenal, a reactive aldehyde.
4-HNE induces antioxidant proteins in mouse keratinocytes.
Induction of antioxidant proteins is regulated via MAP kinases, Nrf2 and caveolae.
4-HNE is an effective signaling molecule in keratinocytes.
Acknowledgements
This work was supported by National Institutes of Health grants U54AR055073, ES004738, CA132624 and P30ES005022
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Alary J, Gueraud F, Cravedi JP. Fate of 4-hydroxynonenal in vivo: disposition and metabolic pathways. Mol Aspects Med. 2003;24:177–187. doi: 10.1016/s0098-2997(03)00012-8. [DOI] [PubMed] [Google Scholar]
- Aldini G, Granata P, Marinello C, Beretta G, Carini M, Facino RM. Effects of UVB radiation on 4-hydroxy-2-trans-nonenal metabolism and toxicity in human keratinocytes. Chem Res Toxicol. 2007;20:416–423. doi: 10.1021/tx0601657. [DOI] [PubMed] [Google Scholar]
- Aldini G, Granata P, Orioli M, Santaniello E, Carini M. Detoxification of 4- hydroxynonenal (HNE) in keratinocytes: characterization of conjugated metabolites by liquid chromatography/electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2003;38:1160–1168. doi: 10.1002/jms.533. [DOI] [PubMed] [Google Scholar]
- Amunom I, Dieter LJ, Tamasi V, Cai J, Conklin DJ, Srivastava S, Martin MV, Guengerich FP, Prough RA. Cytochromes P450 catalyze the reduction of alpha,beta-unsaturated aldehydes. Chemical research in toxicology. 2011;24:1223–1230. doi: 10.1021/tx200080b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu-Modak S, Luscher P, Tyrrell RM. Lipid metabolite involvement in the activation of the human heme oxygenase-1 gene. Free Radic Biol Med. 1996;20:887–897. doi: 10.1016/0891-5849(95)02182-5. [DOI] [PubMed] [Google Scholar]
- Black AT, Gray JP, Shakarjian MP, Laskin DL, Heck DE, Laskin JD. Distinct effects of ultraviolet B light on antioxidant expression in undifferentiated and differentiated mouse keratinocytes. Carcinogenesis. 2008a;29:219–225. doi: 10.1093/carcin/bgm242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AT, Gray JP, Shakarjian MP, Laskin DL, Heck DE, Laskin JD. Increased oxidative stress and antioxidant expression in mouse keratinocytes following exposure to paraquat. Toxicol Appl Pharmacol. 2008b;231:384–392. doi: 10.1016/j.taap.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AT, Joseph LB, Casillas RP, Heck DE, Gerecke DR, Sinko PJ, Laskin DL, Laskin JD. Role of MAP kinases in regulating expression of antioxidants and inflammatory mediators in mouse keratinocytes following exposure to the half mustard, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol. 2010;245:352–360. doi: 10.1016/j.taap.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board PG, Menon D. Glutathione transferases, regulators of cellular metabolism and physiology. Biochim Biophys Acta. 2013;1830:3267–3288. doi: 10.1016/j.bbagen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, Werner S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol. 2002;22:5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, Gumbleton M. Aberrant caveolin-1 expression in psoriasis: a signalling hypothesis. IUBMB Life. 2000;50:361–364. doi: 10.1080/713803750. [DOI] [PubMed] [Google Scholar]
- Capozza F, Williams TM, Schubert W, McClain S, Bouzahzah B, Sotgia F, Lisanti MP. Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation. Am J Pathol. 2003;162:2029–2039. doi: 10.1016/S0002-9440(10)64335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Saito Y, Yoshida Y, Sekine A, Noguchi N, Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- Chidlow JH, Jr., Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86:219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- Dhar A, Young MR, Colburn NH. The role of AP-1, NF-kappaB and ROS/NOS in skin carcinogenesis: the JB6 model is predictive. Mol Cell Biochem 234. 2002;235:185–193. [PubMed] [Google Scholar]
- Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- Haarmann-Stemmann T, Abel J, Fritsche E, Krutmann J. The AhR-Nrf2 pathway in keratinocytes: on the road to chemoprevention? J Invest Dermatol. 2012;132:7–9. doi: 10.1038/jid.2011.359. [DOI] [PubMed] [Google Scholar]
- Hager B, Bickenbach JR, Fleckman P. Long-term culture of murine epidermal keratinocytes. J Invest Dermatol. 1999;112:971–976. doi: 10.1046/j.1523-1747.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? British journal of pharmacology. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley DP, Ruth JA, Petersen DR. The hepatocellular metabolism of 4-hydroxynonenal by alcohol dehydrogenase, aldehyde dehydrogenase, and glutathione S-transferase. Arch Biochem Biophys. 1995;316:197–205. doi: 10.1006/abbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- Harvey RD, Calaghan SC. Caveolae create local signalling domains through their distinct protein content, lipid profile and morphology. Journal of molecular and cellular cardiology. 2012;52:366–375. doi: 10.1016/j.yjmcc.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hirao T, Takahashi M. Carbonylation of cornified envelopes in the stratum corneum. FEBS Lett. 2005;579:6870–6874. doi: 10.1016/j.febslet.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li W, Kong AN. Anti-oxidative stress regulator NF-E2-related factor 2 mediates the adaptive induction of antioxidant and detoxifying enzymes by lipid peroxidation metabolite 4-hydroxynonenal. Cell Biosci. 2012;2:40. doi: 10.1186/2045-3701-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoir M, Buard V, Gasser P, Voisin P, Lati E, Benderitter M. Human keratinocyte radiosensitivity is linked to redox modulation. J Dermatol Sci. 2006;41:55–65. doi: 10.1016/j.jdermsci.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Jacobs AT, Marnett LJ. Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc Chem Res. 2010;43:673–683. doi: 10.1021/ar900286y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung NH, Kim HP, Kim BR, Cha SH, Kim GA, Ha H, Na YE, Cha YN. Evidence for heme oxygenase-1 association with caveolin-1 and -2 in mouse mesangial cells. IUBMB Life. 2003;55:525–532. doi: 10.1080/15216540310001620968. [DOI] [PubMed] [Google Scholar]
- Keum YS, Yu S, Chang PP, Yuan X, Kim JH, Xu C, Han J, Agarwal A, Kong AN. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006;66:8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- Khor TO, Huang MT, Prawan A, Liu Y, Hao X, Yu S, Cheung WK, Chan JY, Reddy BS, Yang CS, Kong AN. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev Res (Phila) 2008;1:187–191. doi: 10.1158/1940-6207.CAPR-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HP, Wang X, Galbiati F, Ryter SW, Choi AM. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J. 2004;18:1080–1089. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin JD, Black AT, Jan YH, Sinko PJ, Heindel ND, Sunil V, Heck DE, Laskin DL. Oxidants and antioxidants in sulfur mustard-induced injury. Ann N Y Acad Sci. 2010;1203:92–100. doi: 10.1111/j.1749-6632.2010.05605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levonen AL, Inkala M, Heikura T, Jauhiainen S, Jyrkkanen HK, Kansanen E, Maatta K, Romppanen E, Turunen P, Rutanen J, Yla-Herttuala S. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler Thromb Vasc Biol. 2007;27:741–747. doi: 10.1161/01.ATV.0000258868.80079.4d. [DOI] [PubMed] [Google Scholar]
- Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Gavin T, Petersen DR, Barber DS. Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation. Chem Res Toxicol. 2009;22:1499–1508. doi: 10.1021/tx900147g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti M, Allanore Y, Saad M, Fatini C, Cohignac V, Guiducci S, Romano E, Airo P, Caramaschi P, Tinazzi I, Riccieri V, Della Rossa A, Abbate R, Caporali R, Cuomo G, Valesini G, Dieude P, Hachulla E, Cracowski JL, Tiev K, Letenneur L, Amouyel P, Lambert JC, Chiocchia G, Martinez M, Ibba-Manneschi L, Matucci-Cerinic M. Evidence for caveolin-1 as a new susceptibility gene regulating tissue fibrosis in systemic sclerosis. Ann Rheum Dis. 2012;71:1034–1041. doi: 10.1136/annrheumdis-2011-200986. [DOI] [PubMed] [Google Scholar]
- Marrot L, Jones C, Perez P, Meunier JR. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008;21:79–88. doi: 10.1111/j.1755-148X.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- Mirza MK, Yuan J, Gao XP, Garrean S, Brovkovych V, Malik AB, Tiruppathi C, Zhao YY. Caveolin-1 deficiency dampens Toll-like receptor 4 signaling through eNOS activation. Am J Pathol. 2010;176:2344–2351. doi: 10.2353/ajpath.2010.091088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneypenny CG, Gallagher EP. 4-Hydroxynonenal inhibits cell proliferation and alters differentiation pathways in human fetal liver hematopoietic stem cells. Biochem Pharmacol. 2005;69:105–112. doi: 10.1016/j.bcp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Nakashima I, Liu W, Akhand AA, Takeda K, Kawamoto Y, Kato M, Suzuki H. 4-hydroxynonenal triggers multistep signal transduction cascades for suppression of cellular functions. Mol Aspects Med. 2003;24:231–238. doi: 10.1016/s0098-2997(03)00018-9. [DOI] [PubMed] [Google Scholar]
- Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Ning S, Budas GR, Churchill EN, Chen CH, Knox SJ, Mochly-Rosen D. Mitigation of radiation-induced dermatitis by activation of aldehyde dehydrogenase 2 using topical alda-1 in mice. Radiat Res. 2012;178:69–74. doi: 10.1667/rr2861.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Suliman HB. Redox regulation of mitochondrial biogenesis. Free Radic Biol Med. 2012;53:2043–2053. doi: 10.1016/j.freeradbiomed.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao MS, Park JJ, Choi JY, Lee DH, Yun SJ, Lee JB, Lee SC. Nrf2-dependent and Nrf2-independent induction of phase 2 detoxifying and antioxidant enzymes during keratinocyte differentiation. Arch Dermatol Res. 2012;304:387–395. doi: 10.1007/s00403-012-1215-7. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta. 2005;1746:260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Pizzimenti S, Ciamporcero E, Daga M, Pettazzoni P, Arcaro A, Cetrangolo G, Minelli R, Dianzani C, Lepore A, Gentile F, Barrera G. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol. 2013;4:242. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- Rinna A, Forman HJ. SHP-1 inhibition by 4-hydroxynonenal activates Jun N-terminal kinase and glutamate cysteine ligase. Am J Respir Cell Mol Biol. 2008;39:97–104. doi: 10.1165/rcmb.2007-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampey BP, Stewart BJ, Petersen DR. Ethanol-induced modulation of hepatocellular extracellular signal-regulated kinase-1/2 activity via 4-hydroxynonenal. J Biol Chem. 2007;282:1925–1937. doi: 10.1074/jbc.M610602200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando GN, Zhu H, Weis JM, Richman JT, Wertz PW, Madison KC. Caveolin expression and localization in human keratinocytes suggest a role in lamellar granule biogenesis. J Invest Dermatol. 2003;120:531–541. doi: 10.1046/j.1523-1747.2003.12051.x. [DOI] [PubMed] [Google Scholar]
- Saw CL, Huang MT, Liu Y, Khor TO, Conney AH, Kong AN. Impact of Nrf2 on UVB-induced skin inflammation/photoprotection and photoprotective effect of sulforaphane. Mol Carcinog. 2011;50:479–486. doi: 10.1002/mc.20725. [DOI] [PubMed] [Google Scholar]
- Schafer M, Dutsch S, auf dem Keller U, Navid F, Schwarz A, Johnson DA, Johnson JA, Werner S. Nrf2 establishes a glutathione-mediated gradient of UVB cytoprotection in the epidermis. Genes Dev. 2010;24:1045–1058. doi: 10.1101/gad.568810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearn CT, Smathers RL, Stewart BJ, Fritz KS, Galligan JJ, Hail N, Jr., Petersen DR. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibition by 4-hydroxynonenal leads to increased Akt activation in hepatocytes. Mol Pharmacol. 2011;79:941–952. doi: 10.1124/mol.110.069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Molecular pharmacology. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- Siems W, Grune T. Intracellular metabolism of 4-hydroxynonenal. Mol Aspects Med. 2003;24:167–175. doi: 10.1016/s0098-2997(03)00011-6. [DOI] [PubMed] [Google Scholar]
- Siems WG, Zollner H, Grune T, Esterbauer H. Metabolic fate of 4-hydroxynonenal in hepatocytes: 1,4-dihydroxynonene is not the main product. J Lipid Res. 1997;38:612–622. [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Dixit BL, Cai J, Sharma S, Hurst HE, Bhatnagar A, Srivastava SK. Metabolism of lipid peroxidation product, 4-hydroxynonenal (HNE) in rat erythrocytes: role of aldose reductase. Free Radic Biol Med. 2000;29:642–651. doi: 10.1016/s0891-5849(00)00351-8. [DOI] [PubMed] [Google Scholar]
- Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- Taira J, Sugishima M, Kida Y, Oda E, Noguchi M, Higashimoto Y. Caveolin-1 is a competitive inhibitor of heme oxygenase-1 (HO-1) with heme: identification of a minimum sequence in caveolin-1 for binding to HO-1. Biochemistry. 2011;50:6824–6831. doi: 10.1021/bi200601t. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Tajima S, Ishibashi A, Uchida K, Shigematsu T. Immunohistochemical detection of lipid peroxidation products, protein-bound acrolein and 4-hydroxynonenal protein adducts, in actinic elastosis of photodamaged skin. Arch Dermatol Res. 2001;293:363–367. doi: 10.1007/s004030100239. [DOI] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Inturi S, Agarwal C, White CW, Agarwal R. Silibinin attenuates sulfur mustard analog-induced skin injury by targeting multiple pathways connecting oxidative stress and inflammation. PLoS One. 2012;7:e46149. doi: 10.1371/journal.pone.0046149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalkens RB, Cook LW, Petersen DR. Formation and export of the glutathione conjugate of 4-hydroxy-2, 3-E-nonenal (4-HNE) in hepatoma cells. Arch Biochem Biophys. 1999;361:113–119. doi: 10.1006/abbi.1998.0946. [DOI] [PubMed] [Google Scholar]
- Usatyuk PV, Natarajan V. Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J Biol Chem. 2004;279:11789–11797. doi: 10.1074/jbc.M311184200. [DOI] [PubMed] [Google Scholar]
- Usatyuk PV, Parinandi NL, Natarajan V. Redox regulation of 4-hydroxy-2-nonenal- mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J Biol Chem. 2006;281:35554–35566. doi: 10.1074/jbc.M607305200. [DOI] [PubMed] [Google Scholar]
- Valacchi G, Pagnin E, Okamoto T, Corbacho AM, Olano E, Davis PA, van der Vliet A, Packer L, Cross CE. Induction of stress proteins and MMP-9 by 0.8 ppm of ozone in murine skin. Biochem Biophys Res Commun. 2003;305:741–746. doi: 10.1016/s0006-291x(03)00812-x. [DOI] [PubMed] [Google Scholar]
- Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- Wang XM, Kim HP, Song R, Choi AM. Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am J Respir Cell Mol Biol. 2006;34:434–442. doi: 10.1165/rcmb.2005-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winczura A, Zdzalik D, Tudek B. Damage of DNA and proteins by major lipid peroxidation products in genome stability. Free Radic Res. 2012;46:442–459. doi: 10.3109/10715762.2012.658516. [DOI] [PubMed] [Google Scholar]
- Xu C, Huang MT, Shen G, Yuan X, Lin W, Khor TO, Conney AH, Kong AN. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol. 2003;50:319–336. [PubMed] [Google Scholar]
- Zhang H, Forman HJ. Signaling pathways involved in phase II gene induction by alpha, beta-unsaturated aldehydes. Toxicol Ind Health. 2009;25:269–278. doi: 10.1177/0748233709102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Heck DE, Black AT, Gow A, Laskin DL, Laskin JD. Regulation of keratinocyte expression of stress proteins and antioxidants by the electrophilic nitrofatty acids 9- and 10-nitrooleic acid. Free Radic Biol Med. 2013a doi: 10.1016/j.freeradbiomed.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Po I, Mishin V, Black AT, Heck DE, Laskin DL, Sinko PJ, Gerecke DR, Gordon MK, Laskin JD. The generation of 4-hydroxynonenal, an electrophilic lipid peroxidation end product, in rabbit cornea organ cultures treated with UVB light and nitrogen mustard. Toxicol Appl Pharmacol. 2013b;272:345–355. doi: 10.1016/j.taap.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]