Abstract
Aims
Recently we found that mice bearing subcutaneous non-metastatic tumors exhibited elevated levels of two types of complex DNA damage, i.e., double-strand breaks and oxidatively-induced clustered DNA lesions in various tissues throughout the body, both adjacent to and distant from the tumor site. This DNA damage was dependent on CCL2, a cytokine involved in the recruitment and activation of macrophages, suggesting that this systemic DNA damage was mediated via tumor-induced chronic inflammatory responses involving cytokines, activation of macrophages, and consequent free radical production. If free radicals are involved, then a diet containing an antioxidant may decrease the distant DNA damage.
Results
Here we repeated our standard protocol in cohorts of two syngeneic tumor-bearing C57BL/6NCr mice that were on a Tempol-supplemented diet. We show that double-strand break and oxidatively-induced clustered DNA lesion levels were considerably decreased, about 2-3 fold, in the majority of tissues studied from the tumor-bearing mice fed the antioxidant Tempol compared to the control tumor-bearing mice. Similar results were also observed in nude mice suggesting that the Tempol effects are independent of functioning adaptive immunity.
Conclusions
This is the first in vivo study demonstrating the effect of a dietary antioxidant on abscopal DNA damage in tissues distant from a localized source of genotoxic stress. These findings may be important for understanding the mechanisms of genomic instability and carcinogenesis caused by chronic stress-induced systemic DNA damage and for developing preventative strategies.
Keywords: Tumor-bearing mice, DNA damage, antioxidants, Tempol, non-targeted effects
1. Introduction
Intercellular communication is mediated by substances released by damaged cells which then affect healthy cells. The radiation-induced bystander effect is one example of this phenomenon, where the released factors from irradiated cells may activate pathways in healthy ‘bystander’ cells leading to the induction of DNA damage [1; 2], increased genomic instability and decreased viability [3; 4]. The signal transduction from irradiated to bystander cells in vitro can occur through both cell media and gap junctions [1] and is reminiscent of the inflammatory response mediated by COX-2 related pathways, involving cytokines, growth factors, and membrane-permeable reactive oxygen and nitrogen species (ROS and RNS) [5; 6]. In addition to radiation-damaged cells, recent studies have reported that genetically unstable, senescent, and cancerous cells also can adversely affect their normal neighbors [7; 8; 9; 10], suggesting that the radiation-induced bystander effect is a specific instance of a much more general phenomenon of intercellular communication from damaged or abnormal cells to normal cells.
While these bystander-like phenomena have been well-documented in vitro, as have in vivo counterparts of the radiation-induced bystander (abscopal) effects [11; 12; 13], reports of other extensions of the more general phenomenon in vivo are not so abundant. An interesting example is that of animal tumors in a chronic inflammatory environment [14], with elevated levels of endogenous stress factors and ROS [15; 16], produced either directly by tumors, or indirectly via inflammatory responses, which can induce DNA damage in healthy neighboring cells [17].
While there are several methods for detecting ROS in vitro, they are difficult to monitor in vivo. All ROS detection methodologies have to overcome various limitations such as time, dye specificity, species specificity, and others [18; 19]. In our study with tumor-bearing mice, we employed two endpoints to monitor the effects of oxidative stress, the presence of two potentially lethal DNA lesions, bistranded oxidatively-induced clustered DNA lesions (OCDLs) [20; 21] and foci of phosphorylated histone H2AX (γ-H2AX), a surrogate marker of DNA double strand breaks (DSBs) [22; 23; 24]. Both biomarkers have been used to detect and monitor radiation- and cancer-related DNA damage in mouse and human tissues [25; 26; 27; 28]. While induction of γ-H2AX foci has been reported at non-DSB sites, such as dysfunctional telomeres [29] or in the absence of DNA damage [30], numerous studies related to the bystander effect have shown a direct link between DSBs and γ-H2AX foci [1; 2; 9; 31; 32]. In our recent study with mice implanted with localized tumors, we showed that the levels of these two types of complex DNA lesions were elevated in several distant tissues [26]. We also showed that the elevated levels of these lesions in distant tissues were mediated by inflammatory macrophages in a CCL2-dependent manner. The elevation of OCDLs and the participation of macrophages both point to ROS involvement in this distant DNA damage.
While ROS homeostasis can be maintained in unstressed healthy cells by a balance of the pathways that produce and destroy ROS, excessive ROS levels may be beyond the capacity of these endogenous systems to regulate. However, they can often be lowered by exogenous antioxidants such as Tempol, a cell-permeable superoxide dismutase mimetic and a free radical scavenger [33]. Belonging to nitroxide stable free radical family, Tempol is a promising agent for clinical use as an antioxidant and radioprotector [34]. It significantly reduces superoxide anion and peroxynitrite-associated inflammation, lowers blood pressure in a variety of animal models and also displays neuroprotective effects [35; 36; 37; 38; 39]. It has been found to be efficient in restoring mitochondrial and cardiac functions in tumour necrosis factor (TNF)α-induced oxidative stress and reducing cardiac hypertrophy in chronically hypoxic rats [40]. It reduces the incidence of hematopoietic neoplasms, increases the survival of irradiated mice [41] and topically protects mice against radiation-induced mucositis [42]. Preclinical studies in guinea pigs, and a Phase I clinical trial in patients receiving whole-brain radiotherapy, suggest that Tempol is effective in suppressing radiation-induced alopecia [43; 44; 45].
To test the hypothesis for an oxidative mechanism fueling these non-targeted effects in the organism, we examined whether an exogenous antioxidant treatment could lower systemic or abscopal oxidative DNA damage levels in tumor-bearing mice. For this reason we incorporated a well-known antioxidant Tempol, into the diets of several tumor-bearing mouse cohorts. Here we report that the local tumor-induced DSB and OCDL accumulation in normal tissues of tumor-bearing mice can be suppressed by feeding the mice a Tempol-supplemented diet. These findings show that oxidative stress pathways leading to elevated DSB and OCDL levels can be interrupted with exogenous antioxidants. Since these two lesions are often precursors to genomic instability and carcinogenesis, and it is estimated that as many as 20% of cancers may be due to chronic inflammatory conditions [14], these findings may have important implications for development of clinical strategies to mitigate chronic stress-induced systemic DNA damage.
2. Materials and Methods
2.1 Mice and Tumors
All necessary permits were obtained for the described study. The protocols were approved by the National Cancer Institute Animal Care and Use Committee. Six-week-old C57BL/6NCr (B6) and nude female mice were obtained from the Animal Production Area, National Cancer Institute (NCI)–Frederick. Cryopreserved murine B16 melanoma (MEL, host strain: B6) and Lewis lung carcinoma (LLC, host strain: B6) were obtained from the Division of Cancer Treatment and Diagnosis tumor repository, NCI-Frederick. One vial of cryopreserved tumor tissue was thawed according to the provided protocols. The tissue was minced into fragments of ∼8mm3 (2 × 2 × 2mm).
These tumor fragments were placed into an 11- to 13-gauge trocar. One fragment of each tumor was implanted s.c. into each of 3 B6 mice. These “donor” mice were subsequently used as the source of tumors grown in test animals, implanted using a similar procedure. B6 mice were implanted with both MEL and LLC tumors, and nude mice were implanted with the LLC tumor.
Bacon-flavored Tempol-supplemented chow (10 mg/g of chow) and control bacon-flavored chow were obtained from Bio-Serv, Frenchtown, NJ, USA. Mice were given the food one week prior to the experiment to acclimatize animals to the diet and during 16 days of the experiment. Body weights were taken at the feeding start point (∼ 7 days before the experiment), the first day of experiment (Day 0), and at euthanasia (Day 16).
Six cohorts of B6 mice were used: (1) “MEL”: 5 animals (fed control chow) were implanted with minced fragments of B16 tumor harvested from donor mice; (2) “LLC”: 5 animals (fed control chow) were implanted with minced fragments of LLC tumor harvested from donor mice; (3) “PBS”: 5 control animals (fed control chow) were subjected to a single s.c. injection of 0.05 mL sterile PBS (ie mock tumor implantation); (4) “MEL + Tempol”: 5 animals implanted with B16 tumor and fed Tempol chow; (5) “LLC + Tempol”: 5 animals implanted with LLC and fed Tempol chow; (6) “PBS + Tempol”: 5 control animals were subjected to PBS injection and fed Tempol chow. Four cohorts of nude mice were used: (1) “LLC”; (2) “PBS”; (3) “LLC + Tempol”; (4) “PBS + Tempol”.
Growth of tumors was monitored at least twice during the course of the experiment. Mice of all ten cohorts were euthanized with CO2 when grafted tumors in the test cohorts reached a volume of ∼200 mg (L = 7 mm, W = 5 mm). Stomach, duodenum, colon, rectum, liver, kidney, lung, ovary, spleen, brain, tumor mass and skin proximal to the tumor were harvested, and for stomach, duodenum, colon and rectum, fresh tissue samples were “touch-printed” to a slide surface for the γ-H2AX assay, as described below. All tissues were then frozen and fixed by formaldehyde exposure, and paraffin-embedded. Pathology reports were prepared for all organs of test and control mice.
2.2 γ-H2AX Immunostaining and Analysis
Touch-print specimens were prepared by gentle pressing stomach, duodenum, colon and rectum tissues against a microscopic slide surface. The samples were air-dried, fixed in 2% para-formaldehyde for 20 min at room temperature, permeabilized with 1% Triton X-100, and processed for γ-H2AX immunostaining, as previously described [26; 46]. Primary rabbit polyclonal anti-γ-H2AX antibody (Novus Biologicals) and secondary goat anti-rabbit Alexa-488-conjugated IgG (Invitrogen) were used. Nuclei were stained with propidium iodide. Laser scanning confocal microscopy was performed with a Nikon PCM 2000 (Nikon, Inc.). γ-H2AX foci were counted in three randomly selected microscopic fields per data point (at least 300-500 cells per mouse) using FociCounter software (http://focicounter.sourceforge.net/index.html) [47].
2.3 Isolation and Processing of Mouse DNA and OCDL Detection
The High Pure PCR Template Kit (Roche) was used for isolation of DNA from the tissues as previously described [48; 49]. To minimize oxidation artifacts during DNA isolation, all buffers were freshly prepared, autoclaved, purged with argon, and supplemented with 50 μM phenyl-tert-butyl nitrone, a free radical scavenger (Sigma-Aldrich) [50]
Detection of OCDLs was performed as analytically described in [25]. Briefly, DNA isolated from the tissues was digested with human BER repair enzymes APE1 and OGG1, and E. coli EndoIII (New England Biolabs). The specific enzymatic DNA damage probes detect a variety of DNA lesions covering a wide spectrum of oxidatively-generated DNA damage. Specifically APE1 detects abasic DNA lesions, OGG1 primarily oxidized purines and EndoIII will cleave mainly oxidized pyrimidines [51]. For both glycosylases, OGG1 and EndoIII, the minor detection of some abasic sites cannot be excluded. Forty ng of isolated DNA was mixed with 7 μL of the appropriate enzyme reaction buffer and left on ice for 30 min. The enzyme buffers used were: APE1 buffer (50mM potassium acetate, 20mM Tris acetate, 10mM magnesium acetate, pH7.9), OGG1 buffer (50 mM NaCl, 10 mM MgCl2, 10 mM Tris-HCl, pH 7.9), and E. coli EndoIII buffer (20 mM Tris-HCl, 1 mM EDTA, pH 8.0). In each case, repair enzyme was added in order to achieve optimum cleavage i.e. APE1 and OGG1: 2 enzyme units and EndoIII: 2 enzyme units [40]. For each enzyme-treated sample, a corresponding non-enzyme containing sample was also run as a control following the same steps. An adaptation of constant field gel electrophoresis was used along with quantitative electronic imaging and number average length analysis [48]. Electronic images for each gel lane were processed using QuantiScan (BioSoft). DNA standards (λ-HindIII Digest) were used to obtain the corresponding dispersion curve with Origin 6.1 (OriginLab). The number average lengths (Ln) for each sample were calculated using the equations described in [52]. The frequencies of OCDLs were measured based on the Ln values of the enzyme-treated sample (+ lane) and the accompanying control sample (− lane).
2.4 Statistical Analysis
The paired Student's t test was used to evaluate the differences between control and MEL or LLC cohorts, and between MEL or LLC and MEL+Tempol or LLC+Tempol cohorts of mice (P<0.001, P<0.01 and P<0.05).
3. Results
3.1 Tempol effect on animals
Since ROS-induced DNA damage may occur in neighboring or distant normal tissues in tumor-bearing mice, we hypothesized that treatment with an antioxidant such as Tempol may ameliorate the level of the consequential oxidatively-induced DNA damage. The protocol used previously [26] was followed closely to enable direct comparison of the results (Figure 1). Six cohorts of five B6 mice each were used, two cohorts were implanted with syngeneic B16 melanoma (MEL) cells, two with syngeneic Lewis lung carcinoma (LLC) cells and two control cohorts with mock tumor inoculation with PBS. One cohort of each pair was placed on a diet of Tempol-supplemented chow 7 days before tumor implantation to ensure that the Tempol was present in sufficient concentration to be effective at the time of DNA damage induction [34]. No significant effect of Tempol diet on tumor growth has been detected. The weight of Tempol-supplemented mice was modestly decreased (∼15% for control and ∼25% for tumor-bearing cohorts at day 16) compared to the mice on Tempol-free diet (Sup. Figure 1a). Weight loss without toxicity in Tempol-fed mice has been previously reported upon switching mice to Tempol chow; however, in most mouse strains the weight differential is observed after 6-10 weeks after switching the diet [41; 53]. It should be noted that mice on Tempol-supplemented diets exhibit the same food consumption as mice on control diets [41]. All Tempol-supplemented animals were active and healthy, therefore the weight loss in this study cannot be considered as a negative effect implying toxicity.
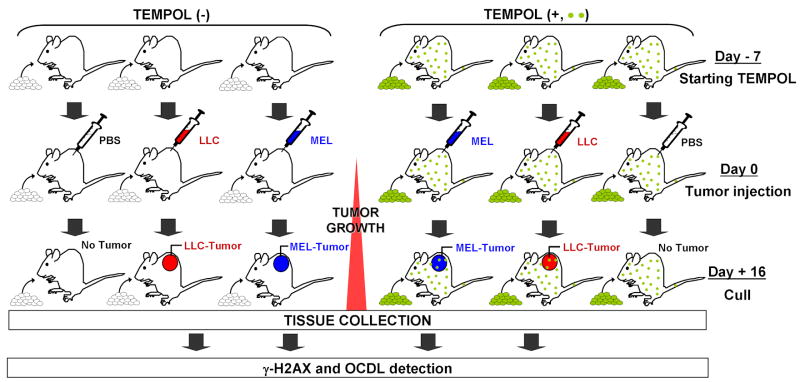
Figure 1. Experimental scheme.
B6 female age-matched control mice were compared with mice s.c. implanted with either of two tumors (B16 melanoma, MEL. or Lewis lung carcinoma, LLC), in two experimental arms: mice on Tempol-free diet, Tempol (−), or on Tempol-added diet, Tempol (+), (which started 7 days prior to the tumor injection). Six 5-animal cohorts of mice, two injected with PBS (control), and 4 test tumor-bearing cohorts, were analyzed for each experiment. When the tumors approached 200 mg (∼day 16 of tumor growth), the animals were culled and various organs were examined. Samples of stomach, duodenum, colon, rectum, lung, spleen, ovary, adjacent skin, brain, liver, and tumor, were taken for analysis. All samples were analyzed by immunofluorescence confocal microscopy, using γ-H2AX foci formation to identify DSBs. OCDLs were measured in tissue samples using human and bacterial lesion-specific repair enzymes as damage probes coupled with constant-field gel electrophoresis of the enzyme-treated DNA.
3.2 Tumor-induced DSB levels can be ameliorated by Tempol treatment
We previously found that gastro-intestinal tract (GIT) organs, such as stomach, duodenum, colon and rectum were particularly vulnerable to DNA DSB induction in mice implanted with an early stage non-metastatic tumor. This vulnerability may arise in highly proliferative tissues such as in the GIT, because a single-strand DNA lesion, oxidatively-generated or otherwise, with the potential to become a single-strand break can then become a DSB when it interacts with a replication fork [26; 54; 55].
“Touch-print” preparations, a standard source of cytopathological material, have been used on our and other laboratories for immunofluorescence staining [26; 56; 57]. Touch-prints of the four test GIT tissues from mice in all six cohorts were stained for γ-H2AX as a measure of DSB levels (Figure 2A).
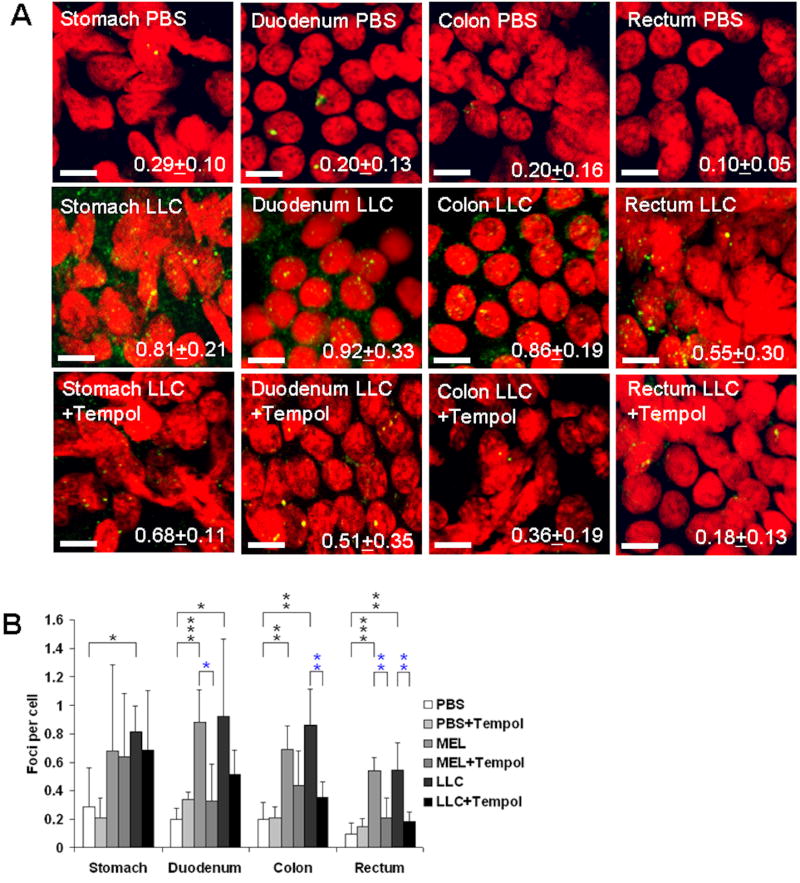
Figure 2. Tempol inhibits DSB formation in GIT tissues of tumor-bearing mice.
(A) Representative confocal images. γ-H2AX-stained touch-print preparations of stomach, duodenum, colon and rectum of control (PBS-injected) and LLC-bearing mice, without Tempol treatment, and with Tempol treatment, are shown (green, γ-H2AX; red, DNA stained with propidium iodide). The images demonstrate elevated γ-H2AX foci levels in duodenum, colon and rectum tissues of tumor-bearing mice and inhibition of this increase in Tempol-fed animals. Average numbers of γ-H2AX foci per cell + standard deviation are shown in the right lower corner. Magnification, 400×. Scale bars, 5 pm. (B) Summary of results. Automatic counts of γ-H2AX foci in touch-print preparations of stomach, duodenum, colon and rectum of control (PBS-injected) and tumor-bearing mice, without Tempol treatment, and with Tempol treatment. Error bars, standard deviations in groups of mice (N=5). * (p<0.05); ** (p<0.01); *** (p<0.001). Black asterisks, statistical significance between tumors and PBS; blue asterisks, statistical significance between tumors, and tumors+Tempol. There was also significant difference in duodenum between PBS and PBS+Tempol cohorts (p<0.05). Scale bars = 5μm.
In agreement with previous findings, the tumor-bearing cohorts not fed Tempol exhibited elevated levels of γ-H2AX foci in all four tissues compared to the tumor-free cohorts (Figures 2B). The Tempol-fed tumor-bearing cohorts exhibited decreased levels of γ-H2AX foci in three of the tissues, duodenum (1.8-2.7 fold), colon (1.6-2.4 fold) and rectum (2.6-3 fold) for both MEL and LLC tumors compared to the no-Tempol cohorts. However, the reductions were not statistically significant in stomach tissues, which may be attributed to their chronic over-exposure to Tempol via local topical delivery from gastric contents. In two cases, of the duodenum of the LLC-bearing, and the colon of the MEL-bearing cohorts, the decreased γ-H2AX foci levels in the Tempol-fed relative to the Tempol-free cohorts did not reach statistical significance.
3.3 Tumor-induced OCDL levels can be ameliorated by Tempol treatment
Three types of OCDL lesions, abasic sites, oxidized pyrimidines, and oxidized purines cleaved by APE1, EndoIII and hOGG1 respectively were measured in the normal tissues and tumors of all six cohorts (Figure 3). Representative images of constant-field gel electrophoresis gels are shown in Sup. Figure 2.
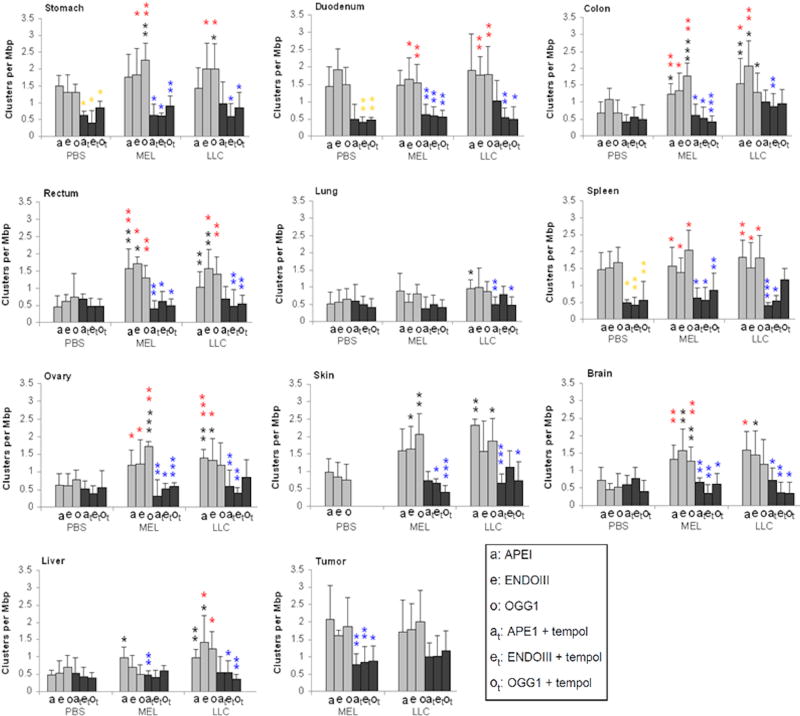
Figure 3. Tempol inhibits OCDL formation in normal and tumor tissues of tumor-bearing B6 mice.
Three types of oxidative lesions, APE1 (“a”, abasic sites), EndoIII (“e”, oxidized pyrimidines), and hOGG1 (“o”, oxidized purines) were measured in normal tissues and tumors of 6 cohorts of mice, control (PBS-injected), MEL- and LLC-bearing mice without Tempol treatment and with Tempol treatment. Results are grouped by normal organs and tumors examined. Error bars are standard deviations in groups of mice (N=5). Asterisks indicate statistical significance of the difference in lesion incidence between pairs of particular tissues. Gold asterisks, statistically significant difference between PBS and PBS+Tempol; black asterisks, statistically significant difference between tumors and PBS; blue asterisks, statistically significant difference between tumors and tumors+Tempol; red asterisks, statistically significant difference between tumors and PBS+Tempol. For the PBS+Tempol versus PBS, the reduction of APE1 lesions by Tempol exposure is only significant for stomach and spleen. * (p<0.05); ** (p<0.01); *** (p<0.001).
Interestingly, the control (without tumors) Tempol-fed mice usually had the lower levels of damage (0.37-0.86 clusters per Mbp) than their no-Tempol counterparts (0.44-1.92 clusters per Mbp). The differences were not statistically significant for all tissues sampled, but background levels of damage were generally significantly reduced by Tempol for stomach, duodenum and spleen.
Comparison of OCDL levels in tissues taken from tumor-bearing and tumor-free mice showed that there was no significant tumor-induced OCDL damage due to the presence of a tumor in stomach, duodenum and spleen taken from Tempol-free mice, However, the majority of tissues, such as colon, rectum, ovary, brain, and liver exhibited increased OCDL levels in tumor-bearing mice relative to controls, indicating that a localized tumor may lead to elevated levels of complex DNA damage in distant tissues (Figure 3).
More specifically, the presence of either tumor increased the oxidative stress-associated DNA damage by a factor 1.1-3.5, as presented in Sup. Figure 3A and B, where the ratios of OCDL formation in normal tissues of MEL and LLC-bearing mice vs. control mice are shown. There are several exceptions for example in the MEL-bearing mice. There is no OCDL increase in duodenum for all 3 lesions, in spleen for APE1 and ENDOIII, in lung for OGG1 lesions, and for LLC-bearing mice, in stomach for APE1, in duodenum for ENDOIII, and in spleen for OGG1. These are 10 exceptional combinations compared to 27 combinations that confirmed to the increased damage trend.
OCDL levels for the experiment that involved LLC tended to be higher compared to B16 melanoma. Comparison between the average OCDL values for each organ gives a ratio of 0.8-1.4 for OCDL(LLC)/OCDL(B16) with the lowest ratio (∼0.8) being detected for brain rectum and skin. The highest ratio (∼1.4) has been detected for the liver. These differences may reflect many pathophysiological variations including the degree of the induced inflammatory response and organ antioxidant capacity.
Chronic exposure to Tempol reduced OCDL levels in all examined tissues, on average 2-fold, with a higher reduction for APE1-abasic and EndoIII-oxypyrimidine sites (Figures 3 and Sup Figure 3A, B). In addition,, Tempol was efficient in reducing OCDL levels within the tumor mass (1.8-fold decrease for LLC and 2-fold decrease for B16 on average for the different OCDL types) (Figure 3).
3.4 Effect of suppressed immune system environment on DNA damage inhibition by Tempol
To study if the suppressed immune system influences systemic oxidative damage induction and prevention, we repeated our standard protocol using LLC-implanted nude mice, which have a deteriorated or absent thymus, resulting in a compromised immune system due to a greatly reduced number of T cells [58]. Similar to B6 mice, the feeding schedule temporarily interrupted weight gain in nude mice (∼7% for control and ∼5% for tumor-bearing mice at day 16) (Sup. Figure 1B). We assessed OCDL levels in cohorts of nude mice on Tempol-supplemented and Tempol-free diet (Figure 4).
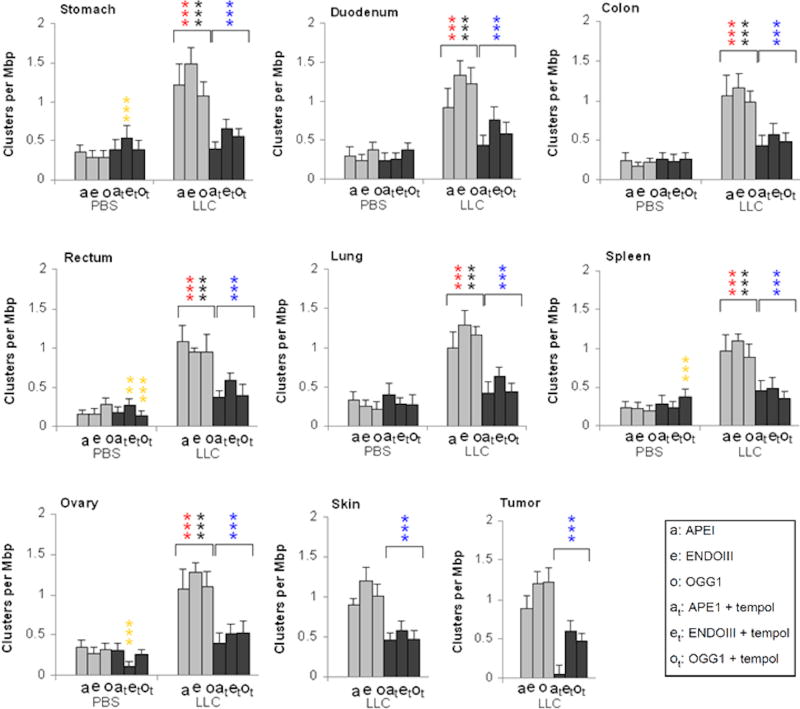
Figure 4. Effect of the compromised immune system (nude mice) on systemic oxidative DNA damage and its inhibition by Tempol.
Three types of oxidative lesions, APE1, EndoIII, and hOGG1 (oxidized purines) were measured in normal tissues and tumors of 4 cohorts of mice, control (PBS-injected) and LLC-bearing mice without Tempol treatment and with Tempol treatment. Results are grouped by normal organs examined. Error bars, standard deviations in groups of mice (N=5). Gold asterisks, statistically significant difference between PBS and PBS+Tempol; black asterisks, statistically significant difference between tumors and PBS; blue asterisks, statistically significant difference between tumors and tumors+Tempol; red asterisks, statistically significant difference between tumors and PBS+Tempol. For the PBS+Tempol versus PBS, the reduction of APE1 lesions by Tempol exposure is only significant for stomach and spleen. *** (p<0.001).
Tissues taken from control nude mice (Figure 4, PBS, light grey bars) uniformly displayed low OCDL levels (0.12-0.37 cluster per Mbp), even in the stomach, duodenum, and spleen, which had unexplained elevated levels in the B6 mice (Figure 3), suggesting this may have been an issue with the particular batches of B6 mice used in these experiments or an enhanced effect of the immune-suppression in reducing the endogenous (background) levels of oxidative DNA lesion. A Tempol diet appeared to have little if any effect on these levels.
In tissues from tumor-bearing nude mice not fed Tempol, the OCDL levels were elevated ∼3 fold relative to the tumor free mice (Figure 4, light grey bars). In comparison, these tissues taken from Tempol-fed tumor-bearing nude mice exhibited significant, ∼2-2.8 fold, reductions in these OCDL levels (Figures 4 and Sup. Figure 3B). Thus, the tumor-bearing cohort of nude mice had a clearer effect of systemic DNA damage induction and Tempol treatment than in B6 mice, in which, due to higher endogenous OCDL levels, the phenomenon may be sometimes masked or hindered.
4. Discussion
High oxidative stress and inflammation have been connected with transformation of normal cells and tissues to a malignant phenotype [14]. Sustained oxidative stress is a hallmark of cancer, driving DNA damage and genetic instability, and shaping the tumor microenvironment by promoting angiogenesis and immune evasion [59; 60; 61]. However, many questions still remain regarding the impact of tumor on neighboring or distant tissues. We have shown previously that the presence of a tumor affects the whole organism by creating oxidative stress which produces systemic DNA damage, i.e. OCDLs and DSBs in a wide range of normal tissues throughout the body [26]. Here we presented evidence that systemic oxidative stress can be reduced by treatment with a dietary antioxidant; feeding tumor-bearing mice with food supplemented with the antioxidant Tempol during the course of the implanted tumor growth, dramatically reduced DNA damage levels in a wide range of normal tissues.
There is a cellular dynamic balance between the production of various ROS (by mitochondria, NADPH oxidases, NO synthase among others) and intracellular antioxidants of enzymatic, (superoxide dismutase (SOD), catalase and glutathione peroxidase (GTPx)) and of non-enzymatic nature (radical scavengers like vitamin E and various thiols) [62]. In the case of cancer cells, a significant imbalance between ROS/RNS and antioxidant production may explain findings associating tumor growth and a state of high oxidative stress. Changes in pH or oxygenation levels of tumor tissues can significantly interfere with several DNA repair pathways including those for base excision repair (BER), which repairs oxidatively damaged DNA (like 8-oxo-7,8-dihydro-2′-deoxyguanosine) and homologous recombination (HR), which repairs DNA DSBs. These changes diminish the ability of cancer cells to deal efficiently with DNA damage, leading to accumulation of mutations, chromosomal instability and metastasis [63].
Oxidatively-induced DNA and protein damage has also been shown to increase in normal tissues and cells bordering tumors [64; 65], such as cancer-associated fibroblasts (CAFs). Most ROS have a short half-life restricting their effects to local damage, but tumor cells have also been shown to release a relatively stable H2O2 [66], which can directly affect CAFs, or induce the production of other ROS [67]. This secretion induces a feedback bystander effect on adjacent cancer cells, leading to increased DNA damage, genomic instability and aneuploidy [67; 68]. However, induction of oxidative damage in tissues distant from a tumor site requires a different mechanism, perhaps through the blood or the lymphatic system. Since it takes only seconds for blood to circulate throughout the body of a mouse [69], chemicals released from a tumor into the blood would be distributed to distant organs, assuming that blood did not contain compounds to neutralize them. Chemicals released from the tumor may be more stable ROS species, or any of a variety of inflammatory factors. In many cases, pro-inflammatory cytokines with pleiotropic downstream effects, like TNFα or CCL2, have been implicated in the induction of elevated oxidative stress and damage to DNA and proteins [26; 70; 71]. Interestingly, CCL2 has recently been shown to be a mediator in cross-talk between breast cancer cells and CAFs [71]. In a previous study, we have also demonstrated a role for CCL2 in the activation of tumor-associated and/or local macrophages that can induce oxidative DNA damage in a variety of normal tissues [26].
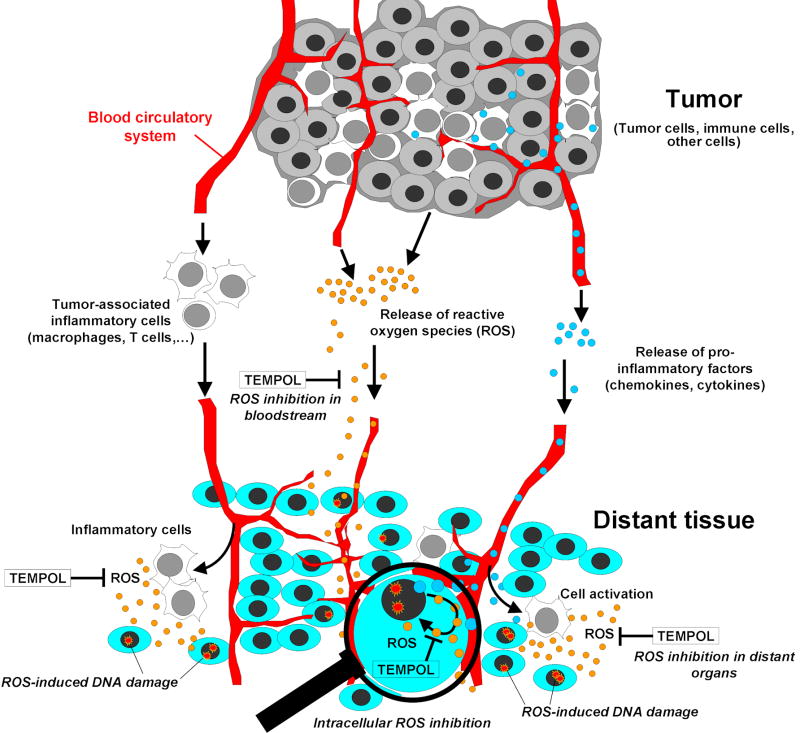
Figure 5 presents a model that can explain our findings. When oxidative stress overwhelms cellular protective systems, as in the case of a tumor-bearing organism, oxidative injury to DNA, lipids and proteins can occur. In our previous reports [54; 55] we suggested three possible contributing mechanisms to ROS production and resulting oxidative injury in tumor-bearing animals: (i) direct release of ROS by the tumor; (ii) involvement of pro-inflammatory cytokines such as CCL2, which result in ROS release by activation of macrophages at distant sites, or (iii) activation of inflammatory macrophages resident in tumors which can then migrate to distant sites releasing ROS [72].
Figure 5. Three suggested pathways for systemic effects of a tumor growing in an organism, and inhibition by Tempol.
Tumor growth can induce complex DNA damage in adjacent and distant sites via different contributing mechanisms as shown. The determining factors in this case are the distance from the tumor, endogenous anti-oxidant and repair ability of the tissue, and the immune status. Exogenous antioxidants (i.e., Tempol) can suppress the ROS action to normal tissues adjacent to and distant from a tumor, regardless the mechanism involved. The use of magnifying glass illustrates that in addition to ROS inhibition in extracellular compartments, including the bloodstream, Tempol may inhibit cytokines-induced ROS production within the cells.
Dietary antioxidants can help inhibit ROS production to prevent accumulation of oxidative injury. MRI imaging with Tempol in mice indicates that it distributes to all tissues, including brain and bone marrow [73; 74]. Indeed, we found that Tempol decreased oxidative DNA damage in normal tissues both neighboring to and distant from injected tumors, suggesting that it can efficiently inhibit the systemic DNA damage independently of the mechanism involved in the damage production. Here, we did not study if Tempol affects macrophage function or recruitment to distant tissues, or CCL2 expression. Based on the current evidence, Tempol is not expected to influence directly CCL2 levels or macrophage infiltration [75; 76; 77]. Rather, it may contribute to an attenuated inflammatory response indirectly, by decreasing the redox status due to the presence of a tumor (including the whole organism carrying a tumor) [77; 78]. Because Tempol is cell permeable [79], the inhibition of ROS production may happen in both extracellular (i.e., bloodstream, extracellular spaces) and intracellular compartments (Figure 5). Thus, Tempol may be able to suppress the direct formation of cytokines-induced ROS within the cells. The final outcome may depend on several important factors such as the type of tumor, the distance from the tumor, the overall repair capacity of the tissues, and the organism's immune status. We can not rule out that the weight reduction in B6 mice could have contributed to the effect. Yet, it is interesting to note that while weight loss is not significant in tempol-fed nude mice, tempol has still a considerable effect on reducing DNA damage. Finally, studying chemoprevention in cancer-prone mice, Erker et al. [80], pointed to another possible mechanism of action for Tempol. In this study, the authors suggested a direct effect of Tempol on p53 and cell cycle, leading to cell cycle delay that may result in a decrease in chromosomal translocations. It should be noted that because replicating cells are highly sensitive to the bystander effect [81], the concept of Tempol inhibiting DSB formation by delaying cell cycle is interesting and may be relevant to our findings.
This study is the first report of in vivo inhibition of systemic complex DNA damage in normal tissues by exogenous antioxidants; independent of the mechanism involved, it is considered positive. Complex DNA lesions (DSBs and OCDLs) are repair resistant [82], and can result into the development of topologically constrained cells or groups of cells with high levels of mutations and/or chromosomal instability, especially in sites of high proliferation [83; 84]. Therefore, chronic elevated ROS levels underlying this complex DNA damage formation, can promote loss of homeostasis and human pathogenesis [85]. Evidence exists that oxidative stress plays a role in carcinogenesis and a wide variety of aging-related disorders such as some neurological diseases, diabetes, autoimmune, reproductive disorders, cardiovascular and musculoskeletal diseases, digestive tract dysfunction, and others (reviewed in [55; 85]). There is a close bond between induction of different types of localized stresses (radiation, tumor growth etc.) and oxidative stress via inflammation [86]. Therefore, our results should be viewed under the possible synergy of multiple factors, such as replication, environmental stress and generation of oxidative stress topically or throughout the whole organism.
Thus, tempol antioxidant therapy can suppress the oxidative load to normal tissues in the organism, and could potentially reduce the initiation of chronic inflammatory diseases and secondary carcinogenesis, occurring in some cases many years after the initial tumor appearance. As it has recently been shown by Dickey et al [87], treatment of rats carrying breast tumors with cardiotoxic anti-tumor agent doxorubicin resulted in tumor reduction and cardiomyopathy. Addition of cardioprotectant dexrazoxane and a Tempol derivative Mito-T (4) ameliorated cardiomyopathy without altering the antitumor activity.
Some of the results presented in this study are at variance with previous experiments where the tumor induced 1.5-3 fold increase in OCDL levels for GIT tissues [26]. However, despite we did not find tumor presence-dependent OCDL increase in duodenum and stomach, two other GIT tissues, colon and rectum, as well as ovary, brain, and liver exhibited similar changes in the present study compared to previous work. In addition to the reduction of oxidative damage in normal tissues of Tempol-fed tumor-bearing mice, we also showed that Tempol significantly decreased the baseline level of oxidative damage in the tumors. While there is risk that anti-oxidant treatment may interfere with the tumor response to therapy, a previous study showed that Tempol had no significant effect on the level or type of cell death induced by chemotherapeutic drugs on Burkitt lymphoma cells [88]. In addition, Tempol was suggested to be a chemopreventive agent reducing the incidence of radiation-induced second malignancies after a course of radiation therapy [41]. It suppresses lymphoma and increases longevity in the cancer-prone ATM-deficient mice [89]. Effect of antioxidants, such as vitamin E, C, and multivitamins, on cancer incidence are currently under investigation in several large-scale clinical trials (www.cancer.gov/cancertopics/factsheet/prevention/antioxidants). Generally, ROS regulation by decreasing oxidative stress with antioxidants is a proven anti-inflammatory and anti-cancer therapy strategy [90; 91], and our observation could possibly have an impact in cancer therapy development and/or help chemoprevention in humans with cancer-prone syndromes.
Supplementary Material
Highlights.
Detection of Complex DNA Damage In Vivo
Non-Targeted Effects In Vivo
Antioxidant Treatment Reduced Complex DNA Levels in the Organism
Tumor Growth Can Induce Systemic Effects Like Clustered DNA Damage in Distant Tissues
Acknowledgments
We are grateful to the Laboratory Animal Sciences Program and Pathology Histotechnology Laboratory staff (National Cancer Institute-Frederick) for help with animal maintenance and histological analysis. We thank Roger Martin, Peter MacCallum Cancer Centre, for his advice and comments on the manuscript. This study was partly supported by the NIH Intramural Program, by A.G.'s funding from East Carolina University, EU grant MC-CIG-303514, COST Action CM1201 ‘Biomimetic Radical Chemistry’, Hellenic National Strategic Reference Framework (NSRF) – Research Funding Program: THALES (Grant number MIS 37946), and by O.M.'s Australian National Health and Medical Research Council (NHMRC) grant number 1027558.
Footnotes
Conflict of Interest statement: All authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sokolov MV, Smilenov LB, Hall EJ, Panyutin IG, Bonner WM, Sedelnikova OA. Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene. 2005;24:7257–7265. doi: 10.1038/sj.onc.1208886. [DOI] [PubMed] [Google Scholar]
- 2.Sedelnikova OA, Nakamura A, Kovalchuk O, Koturbash I, Mitchell SA, Marino SA, Brenner DJ, Bonner WM. DNA double-strand breaks form in bystander cells after microbeam irradiation of three-dimensional human tissue models. Cancer Research. 2007;67:4295–4302. doi: 10.1158/0008-5472.CAN-06-4442. [DOI] [PubMed] [Google Scholar]
- 3.Prise KM, O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. NatureReviews Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little JB. Cellular radiation effects and the bystander response. Mutation research. 2006;597:113–118. doi: 10.1016/j.mrfmmm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, Hei TK. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14641–14646. doi: 10.1073/pnas.0505473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao C, Folkard M, Prise KM. Role of TGF-beta1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene. 2008;27:434–440. doi: 10.1038/sj.onc.1210653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagar S, Morgan WF. The death-inducing effect and genomic instability. Radiation research. 2005;163:316–323. doi: 10.1667/rr3312. [DOI] [PubMed] [Google Scholar]
- 8.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickey JS, Baird BJ, Redon CE, Sokolov MV, Sedelnikova OA, Bonner WM. Intercellular communication of cellular stress monitored by gamma-H2AX induction. Carcinogenesis. 2009;30:1686–1695. doi: 10.1093/carcin/bgp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokolov MV, Dickey JS, Bonner WM, Sedelnikova OA. gamma-H2AX in bystander cells: not just a radiation-triggered event, a cellular response to stress mediated by intercellular communication. Cell Cycle. 2007;6:2210–2212. doi: 10.4161/cc.6.18.4682. [DOI] [PubMed] [Google Scholar]
- 11.Koturbash I, Loree J, Kutanzi K, Koganow C, Pogribny I, Kovalchuk O. In vivo bystander effect: cranial X-irradiation leads to elevated DNA damage, altered cellular proliferation and apoptosis, and increased p53 levels in shielded spleen. International Journal of Radiation Oncology, Biology, Physics. 2008;70:554–562. doi: 10.1016/j.ijrobp.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Koturbash I, Rugo RE, Hendricks CA, Loree J, Thibault B, Kutanzi K, Pogribny I, Yanch JC, Engelward BP, Kovalchuk O. Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene. 2006;25:4267–4275. doi: 10.1038/sj.onc.1209467. [DOI] [PubMed] [Google Scholar]
- 13.Mancuso M, Pasquali E, Leonardi S, Tanori M, Rebessi S, Di Majo V, Pazzaglia S, Toni MP, Pimpinella M, Covelli V, Saran A. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12445–12450. doi: 10.1073/pnas.0804186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 15.Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. American journal of physiology. Cell Physiology. 2003;285:C353–369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 16.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 17.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nature reviews Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Xing D, Luo S, Yang L, Chen Q. Quantitative measurement of reactive oxygen species in vivo by utilizing a novel method: chemiluminescence with an internal fluorescence as reference. Journal of Biomedical Optics. 2010;15:027006. doi: 10.1117/1.3368688. [DOI] [PubMed] [Google Scholar]
- 19.Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nature Genetics. 2008;40:356–361. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- 20.Georgakilas AG. Processing of DNA damage clusters in human cells: current status of knowledge. Molecular Bio Systems. 2008;4:30–35. doi: 10.1039/b713178j. [DOI] [PubMed] [Google Scholar]
- 21.Georgakilas A. Detection of clustered DNA lesions: Biological and clinical applications. World journal of biological chemistry. 2011;2:173–176. doi: 10.4331/wjbc.v2.i7.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedelnikova OA, Pilch DR, Redon C, Bonner WM. Histone H2AX in DNA damage and repair. Cancer Biology & Therapy. 2003;2:233–235. doi: 10.4161/cbt.2.3.373. [DOI] [PubMed] [Google Scholar]
- 23.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. gammaH2AX and cancer. Nature reviews Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickey JS, Redon CE, Nakamura AJ, Baird BJ, Sedelnikova OA, Bonner WM. H2AX: functional roles and potential applications. Chromosoma. 2009;118:683–692. doi: 10.1007/s00412-009-0234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowsheen S, Wukovich RL, Aziz K, Kalogerinis PT, Richardson CC, Panayiotidis MI, Bonner WM, Sedelnikova OA, Georgakilas AG. Accumulation of oxidatively induced clustered DNA lesions in human tumor tissues. Mutation Research. 2009;674:131–136. doi: 10.1016/j.mrgentox.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redon CE, Dickey JS, Nakamura AJ, Kareva IG, Naf D, Nowsheen S, Kryston TB, Bonner WM, Georgakilas AG, Sedelnikova OA. Tumors induce complex DNA damage in distant proliferative tissues in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17992–17997. doi: 10.1073/pnas.1008260107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivashkevich A, Redon CE, Nakamura AJ, Martin RF, Martin OA. Use of the gamma-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Letters. 2012;327:123–133. doi: 10.1016/j.canlet.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baird BJ, Dickey JS, Nakamura AJ, Redon CE, Parekh P, Griko YV, Aziz K, Georgakilas AG, Bonner WM, Martin OA. Hypothermia postpones DNA damage repair in irradiated cells and protects against cell killing. Mutation Research. 2011;711:142–149. doi: 10.1016/j.mrfmmm.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura AJ, Chiang YJ, Hathcock KS, Horikawa I, Sedelnikova OA, Hodes RJ, Bonner WM. Both telomeric and non-telomeric DNA damage are determinants of mammalian cellular senescence. Epigenetics Chromatin. 2008;1:6. doi: 10.1186/1756-8935-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soutoglou E, Misteli T. Activation of the cellular DNA damage response in the absence of DNA lesions. Science. 2008;320:1507–1510. doi: 10.1126/science.1159051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burdak-Rothkamm S, Rothkamm K, Prise KM. ATM acts downstream of ATR in the DNA damage response signaling of bystander cells. Cancer research. 2008;68:7059–7065. doi: 10.1158/0008-5472.CAN-08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanot M, Hoarau J, Carriere M, Angulo JF, Khodja H. Membrane-dependent bystander effect contributes to amplification of the response to alpha-particle irradiation in targeted and nontargeted cells. International Journal of Radiation Oncology, Biology, Physics. 2009;75:1247–1253. doi: 10.1016/j.ijrobp.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, Mitchell JB. The chemistry and biology of nitroxide compounds. Free Radical Biology & Medicine. 2007;42:1632–1650. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist. 15:360–371. doi: 10.1634/theoncologist.2009-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hahn SM, Mitchell JB, Shacter E. Tempol inhibits neutrophil and hydrogen peroxide-mediated DNA damage. Free Radical Biology & Medicine. 1997;23:879–884. doi: 10.1016/s0891-5849(97)00079-8. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Fink GD, Galligan JJ. Tempol lowers blood pressure and sympathetic nerve activity but not vascular O2- in DOCA-salt rats. Hypertension. 2004;43:329–334. doi: 10.1161/01.HYP.0000112304.26158.5c. [DOI] [PubMed] [Google Scholar]
- 37.Elmedal B, de Dam MY, Mulvany MJ, Simonsen U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. British Journal of Pharmacology. 2004;141:105–113. doi: 10.1038/sj.bjp.0705580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipman T, Tabakman R, Lazarovici P. Neuroprotective effects of the stable nitroxide compound Tempol on 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in the Nerve Growth Factor-differentiated model of pheochromocytoma PC12 cells. European Journal of Pharmacology. 2006;549:50–57. doi: 10.1016/j.ejphar.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Rak R, Chao DL, Pluta RM, Mitchell JB, Oldfield EH, Watson JC. Neuroprotection by the stable nitroxide Tempol during reperfusion in a rat model of transient focal ischemia. Journal of Neurosurgery. 2000;92:646–651. doi: 10.3171/jns.2000.92.4.0646. [DOI] [PubMed] [Google Scholar]
- 40.Mariappan N, Soorappan RN, Haque M, Sriramula S, Francis J. TNF-alpha-induced mitochondrial oxidative stress and cardiac dysfunction: restoration by superoxide dismutase mimetic Tempol. American journal of physiology Heart and Circulatory Physiology. 2007;293:H2726–2737. doi: 10.1152/ajpheart.00376.2007. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell JB, Anver MR, Sowers AL, Rosenberg PS, Figueroa M, Thetford A, Krishna MC, Albert PS, Cook JA. The antioxidant tempol reduces carcinogenesis and enhances survival in mice when administered after nonlethal total body radiation. Cancer Research. 2012;72:4846–4855. doi: 10.1158/0008-5472.CAN-12-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cotrim AP, Yoshikawa M, Sunshine AN, Zheng C, Sowers AL, Thetford AD, Cook JA, Mitchell JB, Baum BJ. Pharmacological protection from radiation +/− cisplatin-induced oral mucositis. International Journal of Radiation Oncology, Biology, Physics. 2012;83:1284–1290. doi: 10.1016/j.ijrobp.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuscela D, Coffin D, Lupton GP, Cook JA, Krishna MC, Bonner RF, Mitchell JB. Protection from radiation-induced alopecia with topical application of nitroxides: fractionated studies. The Cancer Journal from Scientific American. 1996;2:273–278. [PubMed] [Google Scholar]
- 44.Goffman T, Cuscela D, Glass J, Hahn S, Krishna CM, Lupton G, Mitchell JB. Topical application of nitroxide protects radiation-induced alopecia in guinea pigs. International Journal of Radiation Oncology, Biology, Physics. 1992;22:803–806. doi: 10.1016/0360-3016(92)90528-p. [DOI] [PubMed] [Google Scholar]
- 45.Metz JM, Smith D, Mick R, Lustig R, Mitchell J, Cherakuri M, Glatstein E, Hahn SM. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:6411–6417. doi: 10.1158/1078-0432.CCR-04-0658. [DOI] [PubMed] [Google Scholar]
- 46.Sedelnikova OA, Horikawa I, Redon C, Nakamura A, Zimonjic DB, Popescu NC, Bonner WM. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell. 2008;7:89–100. doi: 10.1111/j.1474-9726.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- 47.Jucha A, Wegierek-Ciuk A, Koza Z, Lisowska H, Wojcik A, Wojewodzka M, Lankoff A. FociCounter: a freely available PC programme for quantitative and qualitative analysis of gamma-H2AX foci. Mutation Research. 2009 doi: 10.1016/j.mrgentox.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Gollapalle E, Wang R, Adetolu R, Tsao D, Francisco D, Sigounas G, Georgakilas AG. Detection of oxidative clustered DNA lesions in X-irradiated mouse skin tissues and human MCF-7 breast cancer cells. Radiation Research. 2007;167:207–216. doi: 10.1667/rr0659.1. [DOI] [PubMed] [Google Scholar]
- 49.Sutherland BM, Bennett PV, Sidorkina O, Laval J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:103–108. doi: 10.1073/pnas.97.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holt SM, Georgakilas AG. Detection of complex DNA damage in gamma-irradiated acute lymphoblastic leukemia Pre-b NALM-6 cells. Radiation Research. 2007;168:527–534. doi: 10.1667/RR0974.1. [DOI] [PubMed] [Google Scholar]
- 51.Hada M, Georgakilas AG. Formation of clustered DNA damage after high-LET irradiation: a review. Journal of Radiation Research. 2008;49:203–210. doi: 10.1269/jrr.07123. [DOI] [PubMed] [Google Scholar]
- 52.Sutherland BM, Georgakilas AG, Bennett PV, Laval J, Sutherland JC. Quantifying clustered DNA damage induction and repair by gel electrophoresis, electronic imaging and number average length analysis. Mutation Research. 2003;531:93–107. doi: 10.1016/j.mrfmmm.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell JB, Xavier S, DeLuca AM, Sowers AL, Cook JA, Krishna MC, Hahn SM, Russo A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radical Biology & Medicine. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 54.Martin OA, Redon CE, Dickey JS, Nakamura AJ, Bonner WM. Para-inflammation mediates systemic DNA damage in response to tumor growth. Communicative & Integrative Biology. 2011;4:78–81. doi: 10.4161/cib.4.1.13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin OA, Redon CE, Nakamura AJ, Dickey JS, Georgakilas AG, Bonner WM. Systemic DNA damage related to cancer. Cancer Research. 2011;71:3437–3441. doi: 10.1158/0008-5472.CAN-10-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 57.Tamminga J, Koturbash I, Baker M, Kutanzi K, Kathiria P, Pogribny IP, Sutherland RJ, Kovalchuk O. Paternal cranial irradiation induces distant bystander DNA damage in the germline and leads to epigenetic alterations in the offspring. Cell Cycle. 2008;7:1238–1245. doi: 10.4161/cc.7.9.5806. [DOI] [PubMed] [Google Scholar]
- 58.Fogh J, Giovanella BCe. The Nude Mouse in Experimental and Clinical Research. Academic Press. 1982;2 [Google Scholar]
- 59.Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS letters. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 60.Pani G, Galeotti T, Chiarugi P. Metastasis: cancer cell's escape from oxidative stress. Cancer Metastasis Reviews. 29:351–378. doi: 10.1007/s10555-010-9225-4. [DOI] [PubMed] [Google Scholar]
- 61.Poschke I, Mougiakakos D, Kiessling R. Camouflage and sabotage: tumor escape from the immune system. Cancer immunology, immunotherapy: CII. 2011;60:1161–1171. doi: 10.1007/s00262-011-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cabral LA, Lima CF, Coutinho de Oliveira ML, Brandao AA, Almeida JD. Oral exfoliative cytology for the diagnosis of paracoccidoidomycosis in a patient with human immunodeficiency virus: a case report. Acta Cytologica. 2010;54:1127–1129. doi: 10.1159/000325256. [DOI] [PubMed] [Google Scholar]
- 63.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nature reviews Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 64.Jungst C, Cheng B, Gehrke R, Schmitz V, Nischalke HD, Ramakers J, Schramel P, Schirmacher P, Sauerbruch T, Caselmann WH. Oxidative damage is increased in human liver tissue adjacent to hepatocellular carcinoma. Hepatology. 2004;39:1663–1672. doi: 10.1002/hep.20241. [DOI] [PubMed] [Google Scholar]
- 65.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutation Research. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 66.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Research. 1991;51:794–798. [PubMed] [Google Scholar]
- 67.Martinez-Outschoorn UE, Lin Z, Trimmer C, Flomenberg N, Wang C, Pavlides S, Pestell RG, Howell A, Sotgia F, Lisanti MP. Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: implications for PET imaging of human tumors. Cell Cycle. 2011;10:2504–2520. doi: 10.4161/cc.10.15.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer KM, Lin Z, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Knudsen ES, Sotgia F, Lisanti MP. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 9:3256–3276. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parzy E, Miraux S, Franconi JM, Thiaudiere E. In vivo quantification of blood velocity in mouse carotid and pulmonary arteries by ECG-triggered 3D time-resolved magnetic resonance angiography. NMR in Biomedicine. 2009;22:532–537. doi: 10.1002/nbm.1365. [DOI] [PubMed] [Google Scholar]
- 70.Westbrook AM, Wei B, Hacke K, Xia M, Braun J, Schiestl RH. The role of tumour necrosis factor-alpha and tumour necrosis factor receptor signalling in inflammation-associated systemic genotoxicity. Mutagenesis. 2012;27:77–86. doi: 10.1093/mutage/ger063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, Luu T, Li AX, Wu X, Ye W, Chen S, Zhou W, Yu Y, Wang YZ, Ren X, Li H, Scherle P, Kuroki Y, Wang SE. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Research. 2012;72:2768–2779. doi: 10.1158/0008-5472.CAN-11-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. Journal of Leukocyte Biology. 2006;80:705–713. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- 73.Davis RM, Matsumoto S, Bernardo M, Sowers A, Matsumoto K, Krishna MC, Mitchell JB. Magnetic resonance imaging of organic contrast agents in mice: capturing the whole-body redox landscape. Free Radical Biology & Medicine. 2011;50:459–468. doi: 10.1016/j.freeradbiomed.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davis RM, Sowers AL, DeGraff W, Bernardo M, Thetford A, Krishna MC, Mitchell JB. A novel nitroxide is an effective brain redox imaging contrast agent and in vivo radioprotector. Free Radical Biology & Medicine. 2011;51:780–790. doi: 10.1016/j.freeradbiomed.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinaud F, Loufrani L, Toutain B, Lambert D, Vandekerckhove L, Henrion D, Baufreton C. In vitro protection of vascular function from oxidative stress and inflammation by pulsatility in resistance arteries. The Journal of Thoracic and Cardiovascular Surgery. 2011;142:1254–1262. doi: 10.1016/j.jtcvs.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 76.Renna NF, Lembo C, Diez E, Miatello RM. Role of Renin-Angiotensin system and oxidative stress on vascular inflammation in insulin resistence model. International Journal of Hypertension. 2013;2013:420979. doi: 10.1155/2013/420979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuhako MH, Augusto O, Linares E, Chadi G, Giorgio S, Pereira CA. Tempol ameliorates murine viral encephalomyelitis by preserving the blood-brain barrier, reducing viral load, and lessening inflammation. Free Radical Biology & Medicine. 2010;48:704–712. doi: 10.1016/j.freeradbiomed.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Knight SF, Yuan J, Roy S, Imig JD. Simvastatin and tempol protect against endothelial dysfunction and renal injury in a model of obesity and hypertension. American journal of physiology Renal Physiology. 2010;298:F86–94. doi: 10.1152/ajprenal.00351.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamada J, Yoshimura S, Yamakawa H, Sawada M, Nakagawa M, Hara S, Kaku Y, Iwama T, Naganawa T, Banno Y, Nakashima S, Sakai N. Cell permeable ROS scavengers, Tiron and Tempol, rescue PC12 cell death caused by pyrogallol or hypoxia/reoxygenation. Neuroscience Research. 2003;45:1–8. doi: 10.1016/s0168-0102(02)00196-7. [DOI] [PubMed] [Google Scholar]
- 80.Erker L, Schubert R, Yakushiji H, Barlow C, Larson D, Mitchell JB, Wynshaw-Boris A. Cancer chemoprevention by the antioxidant tempol acts partially via the p53 tumor suppressor. Human Molecular Genetics. 2005;14:1699–1708. doi: 10.1093/hmg/ddi181. [DOI] [PubMed] [Google Scholar]
- 81.Dickey JS, Baird BJ, Redon CE, Avdoshina V, Palchik G, Wu J, Kondratyev A, Bonner WM, Martin OA. Susceptibility to bystander DNA damage is influenced by replication and transcriptional activity. Nucleic Acids Research. 2012;40:10274–10286. doi: 10.1093/nar/gks795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Georgakilas AG, O'Neill P, Stewart RD. Induction and Repair of Clustered DNA Lesions: What Do We Know So Far? Radiation Research. 2013 doi: 10.1667/RR3041.1. [DOI] [PubMed] [Google Scholar]
- 83.Hair JM, Terzoudi GI, Hatzi VI, Lehockey KA, Srivastava D, Wang W, Pantelias GE, Georgakilas AG. BRCA1 role in the mitigation of radiotoxicity and chromosomal instability through repair of clustered DNA lesions. Chemico-biological Interactions. 2010;188:350–358. doi: 10.1016/j.cbi.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 84.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nature reviews Molecular Cell Biology. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 85.Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM. Role of oxidatively induced DNA lesions in human pathogenesis. Mutation Research. 2010;704:152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sprung CN, Ivashkevich A, Forrester HB, Redon CE, Georgakilas A, Martin OA. Oxidative DNA damage caused by inflammation may link to stress-induced non-targeted effects. Cancer Letters. 2013 doi: 10.1016/j.canlet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dickey JS, Gonzalez Y, Aryal B, Mog S, Nakamura AJ, Redon CE, Baxa U, Rosen E, Cheng G, Zielonka J, Parekh P, Mason KP, Joseph J, Kalyanaraman B, Bonner W, Herman E, Shacter E, Rao VA. Mito-Tempol and Dexrazoxane Exhibit Cardioprotective and Chemotherapeutic Effects through Specific Protein Oxidation and Autophagy in a Syngeneic Breast Tumor Preclinical Model. PloS One. 2013;8:e70575. doi: 10.1371/journal.pone.0070575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shacter E, Williams JA, Hinson RM, Senturker S, Lee YJ. Oxidative stress interferes with cancer chemotherapy: inhibition of lymphoma cell apoptosis and phagocytosis. Blood. 2000;96:307–313. [PubMed] [Google Scholar]
- 89.Reliene R, Schiestl RH. Antioxidants suppress lymphoma and increase longevity in Atm-deficient mice. The Journal of Nutrition. 2007;137:229S–232S. doi: 10.1093/jn/137.1.229S. [DOI] [PubMed] [Google Scholar]
- 90.Nowsheen S, Aziz K, Kryston TB, Ferguson NF, Georgakilas A. The interplay between inflammation and oxidative stress in carcinogenesis. Current Molecular Medicine. 2012;12:672–680. doi: 10.2174/156652412800792642. [DOI] [PubMed] [Google Scholar]
- 91.Zhang QS, Eaton L, Snyder ER, Houghtaling S, Mitchell JB, Finegold M, Van Waes C, Grompe M. Tempol protects against oxidative damage and delays epithelial tumor onset in Fanconi anemia mice. Cancer Research. 2008;68:1601–1608. doi: 10.1158/0008-5472.CAN-07-5186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.