Abstract
Purpose
To develop and evaluate a method for volumetric contrast-enhanced MR imaging of the liver, with high spatial and temporal resolutions, for combined dynamic imaging and MR angiography using a single injection of contrast.
Methods
An interleaved variable density (IVD) undersampling pattern was implemented in combination with a real-time-triggered, time-resolved, dual-echo 3D spoiled gradient echo sequence. Parallel imaging autocalibration lines were acquired only once during the first time-frame. Imaging was performed in ten subjects with focal nodular hyperplasia (FNH) and compared with their clinical MRI. The angiographic phase of the proposed method was compared to a dedicated MR angiogram acquired during a second injection of contrast.
Results
A total of 21 FNH, 3 cavernous hemangiomas, and 109 arterial segments were visualized in 10 subjects. The temporally-resolved images depicted the characteristic arterial enhancement pattern of the lesions with a 4 s update rate. Images were graded as having significantly higher quality compared to the clinical MRI. Angiograms produced from the IVD method provided non-inferior diagnostic assessment compared to the dedicated MRA.
Conclusion
Using an undersampled IVD imaging method, we have demonstrated the feasibility of obtaining high spatial and temporal resolution dynamic contrast-enhanced imaging and simultaneous MRA of the liver.
Keywords: DCE liver perfusion, T1 weighted liver, IVD, Liver MRA
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer deaths worldwide (1). The liver is also a common site of metastatic disease of other primary malignancies as well as a host of other lesions including focal nodular hyperplasia (FNH), hemangiomas, cysts and hepatic adenomas that often warrant evaluation. T1-weighted gadolinium-enhanced MRI has been shown to be sensitive and specific for detection of HCC (2–4). After injection of a gadolinium-based contrast agent (GBCA), a typical HCC lesion rapidly enhances (“wash-in”). It subsequently becomes hypo-intense (“wash-out”) relative to the liver parenchyma. Rapidly updated temporal information that can capture this dynamic behavior aids in lesion characterization. Further, it has been shown that multiple time-frames can improve the confidence of lesion detection by accounting for unpredictability in tumor pathophysiology and variations in delivery of the GBCA agent (5). Inclusion of patients on the liver transplant list requires a limited tumor burden (6,7). Therefore, high spatial resolution imaging is important not only for tumor detection, but also for accurate size measurements of tumors. Moreover, emerging anti-angiogenic agents have demonstrated some survival benefit in patients with advanced HCC (8). These agents disrupt neoangiogenesis of HCC but may not lead to measurable changes in tumor size measured using conventional criteria (9). For this reason, quantitative or semi-quantitative perfusion methods (10) that can measure tumor blood supply are attractive for determining whether a tumor has responded to these agents. Finally, accurate depiction of vascular anatomy is essential prior to transplantation or transarterial chemoembolization (TACE) since hepatic arterial anatomy is highly variable within the patient population. For this reason, an MR angiogram (MRA) is routinely acquired using a dedicated MRA sequence and an additional injection of GBCA administered after acquisition of the contrast-enhanced MRI perfusion images that are used to evaluate liver parenchyma and lesions.
Clinical assessment of the liver typically includes volumetric, breath-held and fat-suppressed T1-weighted imaging covering the entire liver (11). After acquisition of pre-contrast T1-weighted images, a GBCA is injected and a late-arterial phase image (timed using real-time triggering or a timing bolus) is acquired. This acquisition is followed by imaging during the portal-venous phase at approximately 70 seconds after the injection and during a delayed-venous phase at approximately 5 minutes after the injection. Each of these image sets is obtained during a separate 15–25 second-long breath-holding period. The pharmacokinetics of the GBCA in the liver parenchyma and the lesions evolve rapidly during this time, particularly during the arterial phase. Since only one arterial phase image is typically acquired, the change in GBCA concentration during this acquisition is effectively averaged. Depiction of this pharmacokinetic behavior through improved temporal resolution would improve the characterization of lesions and possibly the detection of small lesions.
There are challenges that need to be addressed in order to achieve the goal of high-resolution time-resolved liver imaging. i) The compromise between spatial and temporal resolution needs to be considered. Several groups have developed approaches to address this trade-off for dynamic contrast-enhanced (DCE) liver imaging (12–15). ii) The unpredictability in arrival time of GBCA to the liver must also be accounted for. This time is variable between individuals due to factors such as cardiac output. Variability in cardiac output is particularly problematic in patients with concomitant cirrhosis and portal hypertension (16). iii) Suppression of fat signal is also important. Bright fat signal is distracting and can lead to residual motion, ghosting and parallel imaging artifacts. In routine clinical imaging, fat signal is typically suppressed using fat-selective RF pulses (17). In this work, data-driven (auto-calibrating k-space-based) parallel imaging (18,19), Cartesian undersampled acquisition and view-sharing reconstruction was used to address (i), a monitoring volume with real-time triggering (SmartPrep) was used to address (ii) and a dual-echo fat-water separation Dixon method (20,21) was used in order to address (iii).
FNH is a common arterially-enhancing benign lesion that is often discovered incidentally (22). FNH lesions have a characteristic rapid contrast enhancement (23) and therefore, are an excellent model for testing the feasibility and performance of time-resolved contrast-enhanced imaging methods.
In this work, technical developments leading to a novel approach for high spatial and temporal resolution imaging of the liver are described. The proposed method is prospectively validated in subjects with known FNH. This approach combines interleaved variable density (IVD) sampling, data-driven parallel imaging, 2-point fat-water separation and real-time triggering to produce high resolution, time-resolved images with update times of four seconds. We will also demonstrate that angiographic phase images obtained using this approach are an accurate substitute for conventional dedicated MRA, without the need for an extra injection of GBCA.
Methods
MRI Pulse Sequence
Three-dimensional volumetric coverage of the liver was achieved using a spoiled gradient recalled (SPGR) bipolar dual-echo pulse sequence with an IVD undersampling pattern (24). The ky-kz plane was pseudo-randomly undersampled across five time-frames. The density of sampled readout lines decreases with increasing distance from the center of k-space. An effective undersampling factor of 3.0 was achieved by using IVD undersampling.
Data-driven parallel imaging (19) was used to accelerate the acquisition further by a factor of R = 2 (S/I) × 2 (A/P). Figure 1a shows the sampling pattern for the pre-contrast mask image set, with parallel imaging acceleration only. Auto-calibration signal (ACS) lines needed for parallel imaging reconstruction of the mask image set spanned an area of size 24 × 24 at the center of k-space. A variety of strategies exist for acquiring the ACS lines needed for calibration of the parallel imaging kernel for the time-frame data sets, as shown in Figures 1b, c and d. Figure 1b shows the sampling pattern for multiple time-frame data sets when the ACS lines were acquired in a separate breath-hold (i.e. during a pre-contrast acquisition). This pattern leads to maximum acceleration of the sampling of the time-frame data sets and the shortest breath-hold duration. However, this approach is susceptible to parallel imaging errors if the anatomy is not accurately reproduced between the two breath-holds used to collect calibration data and the time-resolved data. Figure 1c shows the sampling pattern for multiple time-frames when ACS lines are acquired every time-frame and used only to reconstruct the frame in which they were acquired. Using the typical imaging parameters in the proposed protocol (TR = 3.9 ms), each set of calibration lines adds an additional 1.7 seconds to the length of the breath-hold for every time-frame in which they are acquired. With ACS lines acquired in each of the five time-frame data sets using the proposed protocol, the breath-hold interval is extended by 8.6 seconds, which may lead to breath-hold times that are longer than clinically-acceptable times or alternatively spatial resolution that is lower than clinically-acceptable values.
FIG 1.
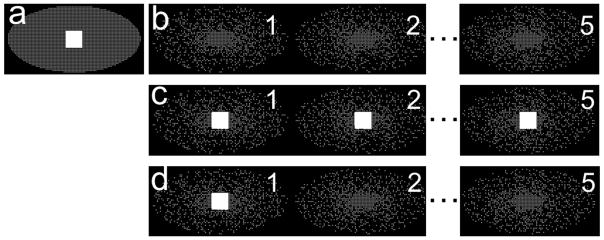
Data-driven parallel imaging calibration lines can be acquired only once in a time-series to shorten the breath-holding time. a) Sampling pattern (shown in ky-kz plane) accelerated for parallel imaging (factor of 2 × 2) for a pre-contrast frame, with ACS lines needed for parallel imaging kernel calibration in the central region of k-space. Acquisition of this pattern requires a 16 s breath-hold. b) IVD sampling pattern combined with parallel imaging acceleration for time-resolved imaging, with no ACS lines (uses the pre-contrast kernel from (a)) requires a 22 s breath-hold. c) Sampling pattern with ACS lines acquired within each time-frame requires a significantly longer scan time (32 s). d) Proposed sampling pattern with ACS lines only acquired during the first time-frame and requires a 24 s breath-hold. The parallel imaging kernel is calibrated only once within the breath-hold and the same kernel weights are used for all the time-frames.
Figure 1d is the proposed sampling method used in this work. In this sampling pattern, ACS lines are acquired in the first time-frame only and the same synthesis kernel is then used for all subsequent time-frames in the same breath-hold. This acquisition is expected to be robust to breath-hold misregistration errors. Moreover, it also uses the imaging time efficiently leading to only a total of 1.7 seconds of extra breath-hold time compared to an extra 8.6 seconds required for the method in Figure 1b.
Using the pattern shown in Figure 1d, an overall acceleration factor of 16.8 was achieved after combining the IVD undersampling, parallel imaging and also skipping acquisition of the corners of k-space (25), which is possible since the dual-echo Dixon fat-suppression method does not require sequential k-space filling.
Sampled k-space lines in each time-frame were acquired in an edge-center-edge order (26). In this approach, acquisition begins at the edge of k-space, advances towards the center following an elliptical centric trajectory and then recedes back out to the edge for each time-frame to minimize gradient transient effects when transitioning from one time-frame to the next.
Imaging parameters for the dynamic IVD sequence included: axial slab excitation, FOV = 38 cm (R/L) × 34 cm (A/P) × 26 cm (S/I), with 320 × 202 × 100 matrix size for true spatial resolution of 1.2 mm (R/L) × 1.7 mm (A/P) × 2.6 mm (S/I), interpolated to 0.7 mm × 0.7 mm × 1.3 mm through zero-filling. Other parameters included TR/TE1/TE2 = 3.9/1.2/2.3 ms, flip angle = 15°, bandwidth = ± 166.7 kHz and R = 2 × 2 data-driven parallel imaging acceleration (ARC, GE Healthcare, Waukesha, WI) for a total scan time (i.e. breath-hold duration) of 24 seconds for all 5 time-frames.
Subjects
Ten subjects (3 male, 7 female, average age 43 ± 11 years) with known FNH from prior clinical MRI exams were recruited for this prospective, HIPAA-compliant and IRB-approved study. Informed written consent was obtained in accordance with the IRB protocol. Subjects were scanned on a 3.0T clinical MRI scanner (Discovery MR750, GE Healthcare, Waukesha, WI) using a 32-channel torso array coil (Neocoil, Pewaukee, WI). A schematic overview of the imaging protocol is shown in Figure 2a.
FIG 2.
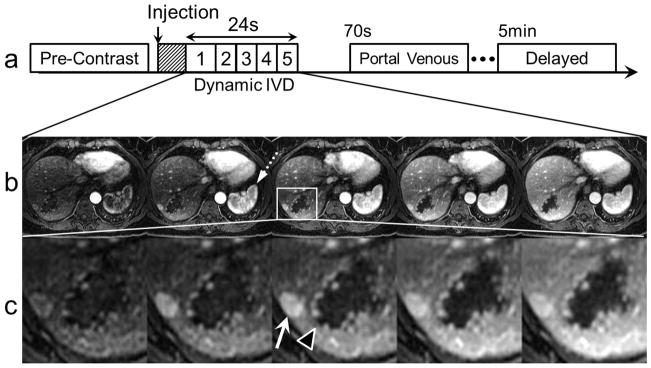
An imaging strategy using the proposed IVD undersampled acquisition can depict contrast dynamics in different types of liver lesions during a breath-hold. a) Proposed dynamic IVD liver protocol: hashed box denotes real-time triggering used to synchronize the arrival of GBCA with the 3D acquisition. b) Uptake of GBCA in an FNH and a cavernous hemangioma. Also note the uptake of GBCA in the liver parenchyma and spleen (dotted arrow). c) Magnified views depict a small FNH (white arrow) and a cavernous hemangioma (black arrow head) with high resolution, showing a subtle central scar in the FNH and the discontinuous, peripheral foci of nodular enhancement that coalesced over time.
Injection Protocol
For the IVD dynamic scan, a single 0.1 mmol/kg dose of gadobenate dimeglumine (MultiHance, Bracco Diagnostics, Monroe, NJ) equivalent to an average of 15.4 mL of GBCA was administered at a rate of 2 mL/s followed by 50 mL of saline flush injected at the same rate. The scan was initiated 7 seconds after the bolus was detected in the right ventricular outflow tract using a monitoring volume (SmartPrep).
Image Reconstruction
Automated reconstruction was performed on the scanner (Intel Xeon dual core 3 GHz processor with 8 GB of memory) using a nearest-temporal-neighbor view-sharing technique (24). All data acquired during the entire breath-holding period were included in the view-sharing window. The highest spatial frequency lines were shared by all five time-frame images since these were the most undersampled lines, while the lines representing lower spatial frequencies were shared among a smaller number of frames. Lines in an elliptical region at the center of k-space (with axes spanning a region of approximately 14 × 28 lines) were acquired for each time-frame and were not shared. The temporal window for reconstruction of each time-frame was 24 seconds at the widest for spatial frequencies corresponding to the outer edges of k-space and 1.5 seconds at the narrowest for spatial frequencies corresponding to the central region of k-space.
Separation of fat and water was performed using an algorithm based on the 2-point Dixon method (20) applied to data acquired using a dual-echo, bipolar gradient readout with out-of-phase and in-phase data acquired respectively at echo times TE1/TE2 = 1.2/2.3 ms (21).
To speed up the parallel imaging reconstruction time, direct virtual coil (DVC) reconstruction was used for the data-driven parallel imaging calibration and synthesis (27). Use of this method can speed up the reconstruction of time-resolved volumetric data by a factor of approximately 12 (28).
Dedicated MRA
Approximately 15 minutes after the completion of the IVD scan, a second injection of GBCA (same agent, dose, rate and flush as the first injection) was administered and a conventional, dedicated clinical MRA scan was performed. A real-time fluoro-triggering was used to coincide the start of the acquisition with arrival of contrast agent in the abdominal aorta (29). MRA imaging parameters for the 3D coronal SPGR included: coronal slab excitation, FOV = 38 cm (S/I) × 30 cm (R/L) × 29 cm (A/P), 256 × 154 × 160 matrix, for true spatial resolution of 1.5 mm (S/I) × 2.0 mm (R/L) × 1.8 mm (A/P) interpolated to 0.7 mm × 0.7 mm × 0.9 mm through zero filling. Other parameters included TR/TE = 3.6/1.2 ms, flip angle = 28°, bandwidth = ± 83.3 kHz and R = 2 × 2 data-driven parallel imaging acceleration (19), for a total acquisition time of 20 seconds.
Clinical MRI
All subjects had a prior clinical MRI study at our institution, and had a radiological diagnosis of FNH. Of the prior 10 clinical studies examined, 8 were acquired at 1.5 T (Signa HDxt, GE Healthcare, Waukesha, WI) using an 8-channel phased array torso coil (GE Healthcare, Waukesha, WI). Clinical imaging was performed with a 3D T1-weighted SPGR acquisition using spectrally-selective inversion-recovery fat suppression and the following imaging parameters: axial slab excitation, FOV = 36 (R/L) × 27 cm (A/P) × 22 cm (S/I), 256 × 144 × 44 matrix, for true spatial resolution of 1.4 mm (R/L) × 1.9 mm (A/P) × 5 mm (S/I), interpolated to 0.7 mm × 0.7 mm × 2.5 mm through zero filling. Other parameters included TR/TE = 4.1/1.8 ms, flip angle = 12°, bandwidth = ± 62.5 kHz and R = 2 × 2 data-driven parallel imaging acceleration. The other 2 studies from the 10 clinical studies examined were acquired at 3.0 T (Discovery MR750, GE Healthcare, Waukesha, WI) using a 32-channel phased array body coil (Neocoil, Pewaukee, WI) and the following image parameters: axial slab excitation, FOV = 36 (R/L) × 32 cm (A/P) × 25 cm (S/I), 320 × 170 × 80 matrix, for true spatial resolution of 1.1 mm (R/L) × 1.9 mm (A/P) × 3.2 mm (S/I), interpolated to 0.7 mm × 0.7 mm × 1.6 mm through zero filling. Other parameters included TR/TE = 4.5/2.1 ms, flip angle = 15°, bandwidth = ± 83.3 kHz and R = 2 × 2 data-driven parallel imaging acceleration. In all clinical exams, acquisition was timed using a monitoring volume.
All clinical MRI exams used 0.05 mmol/kg of gadoxetic acid (Eovist, Bayer Schering Pharma, Berlin-Wedding, Germany) administered at 2 mL/s with a 30 mL saline flush injected at the same rate. Clinical diagnosis of FNH was based on the unequivocal characteristic radiological findings that included lesion iso-intensity on pre-contrast T1-weighted images, iso- or slight hyper-intensity on T2-weighted images, characteristic nodular border, vigorous and uniform arterial-phase enhancement, near iso-intensity during the portal venous phase and iso- or hyper-intensity on delayed hepatobiliary phase gadoxetic-acid-enhanced MRI. A central scar is not necessary to make a radiological diagnosis of FNH, but when present was considered a highly-supportive feature.
Image Analysis
The overall image quality for each of the five dynamic IVD time-frame images for all 10 subjects was graded independently by two experienced abdominal radiologists with 14 and 6 years of experience. Grades were based on a five-point scale (4: excellent image quality without any artifact, 3: good image quality with minimal artifact, 2: fair image quality with moderate artifact, 1: poor image quality with severe artifact hindering diagnosis, 0: non-diagnostic). The late-arterial phase images from the prior clinical MRI acquired at our institution were graded on the same scale. Prior imaging was done from four years to two months (average 27 months) before the research scan. The readers were also asked to select which of the two image sets (dynamic IVD or clinical) they preferred for overall image quality and diagnostic confidence.
Both readers also independently evaluated 11 mesenteric and hepatic arterial segments: celiac, splenic, left gastric, common hepatic, gastroduodenal, proper hepatic, right hepatic, left hepatic, superior mesenteric, right renal and left renal arteries. The evaluation was based on the time-frame identified as the angiographic phase of the dynamic IVD series. Each reader chose the best arterial time-frame among the five time-frames to evaluate the arteries prior to grading. Both readers graded the arterial segment visibility on a five-point scale (4: excellent visibility – excellent contrast against the background structures, 3: good visibility – sufficient contrast against the background structures, 2: moderate visibility – moderate enhancement or blurring, 1: poor visibility – low signal intensity and/or marked blurring, 0: not visible due to poor image quality). These grades were then compared to those obtained from images acquired using the dedicated MRA scans.
Circular regions-of-interest (ROIs) were placed in the FNH lesions and in the liver parenchyma for all subjects. Lesion ROIs were placed to cover as much of the lesion as possible (without extending outside the lesion), while ROIs of the liver were placed to avoid large vessels. Relative contrast was calculated as follows:
| [1] |
where SFNH is the mean signal from the ROI placed within the lesion, and SLiver is the mean signal from the ROI placed in the liver parenchyma.
Statistical Analysis
A two-sided Wilcoxon signed-rank test with a significance level of 0.05 was used to compare the dynamic IVD images with those acquired with prior clinical MRI to find the superior method.
A one-sided Wilcoxon test was applied to the arterial segment visibility grades obtained from both the IVD angiographic phase images and the dedicated MRA images to show non-inferiority of the arterial segment visibility in the dynamic IVD images. The grades for the arterial segment visibility were categorized as sufficient (grades 3 and 4) and insufficient (grades 0–2) to compare the clinical utility of the MRA images obtained from the IVD sequence with the dedicated MRA using a McNemar’s test.
Results
In the 10 subjects imaged, dynamic IVD images were successfully acquired with no technical difficulties. The on-line reconstruction time for the entire series was approximately 5 minutes in all cases as recorded in a reconstruction log file. A total of 24 enhancing lesions were identified, including 21 FNH and 3 cavernous hemangiomas. One subject had a missing splenic artery due to prior splenectomy resulting in a total of 109 graded arterial segments evaluated for MR angiography.
In all subjects, 5 time-frame image sets were acquired using the dynamic IVD method within a single 24-second breath-hold interval during the arterial phase of GBCA passage. Figures 2b,c show representative examples of dynamic IVD images acquired from one of the subjects with both an FNH and a cavernous hemangioma.
Figure 3 shows images from a second subject with two FNH lesions clearly visible (only 3 of 5 time frames shown). Magnified views demonstrate how the high spatial resolution of this method can depict the characteristic fine lobular borders of FNH. Note the FNH lesions take up the GBCA in the earliest time-frames while normal parts of liver parenchyma show a slower uptake, enhancing only in the later time-frames of the arterial phase.
FIG 3.
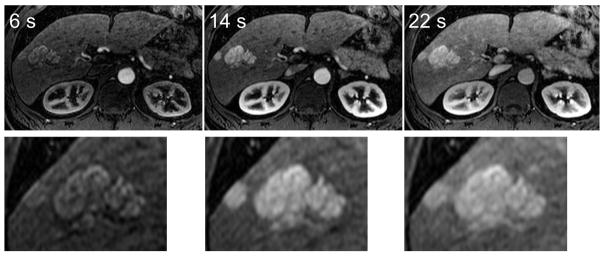
FNH lesions can be seen with high resolution using the IVD method. Three out of five time-resolved images are shown for brevity. In this subject, images depicting two FNH lesions that were acquired at 6 seconds, 14 seconds, and 22 seconds after the start of the scan are shown. Magnified views depict the fine lobular edge features of the lesions, as well as the rapid enhancement pattern in these lesions.
Both readers identified the first time-frame as the best angiographic phase in all subjects. In contrast, the subjective peak enhancement that provided the clearest visualization of the FNH was found in the first phase in 2 cases, the second phase in 6 cases and the third phase in 2 cases.
Figure 4a shows curves representing the mean signal intensity in ROIs placed on an FNH lesion, the portal vein, the aorta and the liver in the time-frame images shown in Figure 2b. Figure 4b shows the relative contrast between the FNH and the liver in each time-frame obtained using the data in Figure 4a. In this case the maximum lesion/liver relative contrast occurred during the second time-frame. Across all 10 subjects, the maximum lesion/liver relative contrast occurred during the first to third time-frames.
FIG 4.
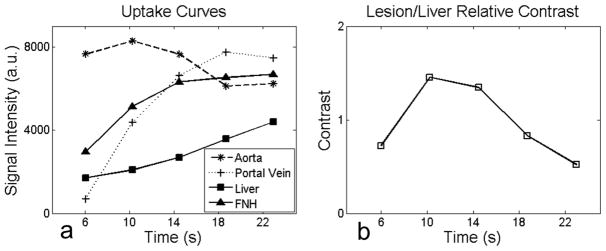
High temporal resolution imaging enables the capture of rapid contrast changes between the lesions, liver parenchyma and blood vessels. A time-frame with maximum lesion/liver contrast is identifiable. a) A characteristic signal intensity curve shows the change of intensity due to uptake of the GBCA in the liver from ROIs placed on the aorta, portal vein, liver parenchyma and the FNH in a subject. b) Relative lesion/liver contrast curve across multiple time-frames from the same subject. The second time-frame in this example has the maximum lesion/liver relative contrast. This temporal information is not available in clinical MRI since only one image is typically acquired within a breath-hold. The horizontal axis shows the time each frame was completed relative to the start of data acquisition.
Figure 5a shows an axial image from the second of five time-frames acquired from one of the subjects. This time-frame yielded the maximum lesion/liver contrast. Figure 5b shows a coronal reformat of the same time-frame image clearly depicting the lesion in this orientation too, including the central scar. Full cranial-caudal coverage of the liver (26 cm) was achieved with this protocol, allowing full visualization of the adrenal glands, kidneys, pancreas and spleen in all subjects.
FIG 5.
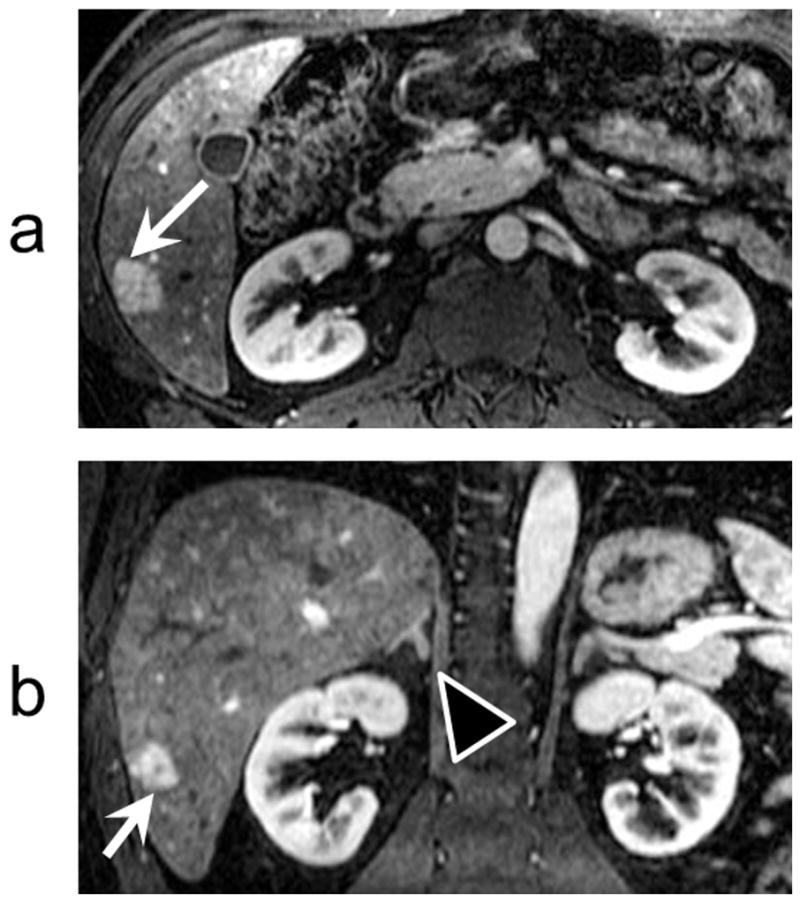
IVD images were acquired with near-isotropic spatial resolution (1.2 mm × 1.7 mm × 2.6 mm). High spatial resolution enables assessment of the liver lesions in different planes. a) A cropped axial time-frame image from a subject with an FNH (white arrows). b) A cropped coronal reformat of the image in (a). Note the central scar in the FNH lesion in both the axial and coronal planes. Also note the excellent depiction of the right adrenal gland (black arrow head) and renal cortex. The full S/I coverage is 26 cm.
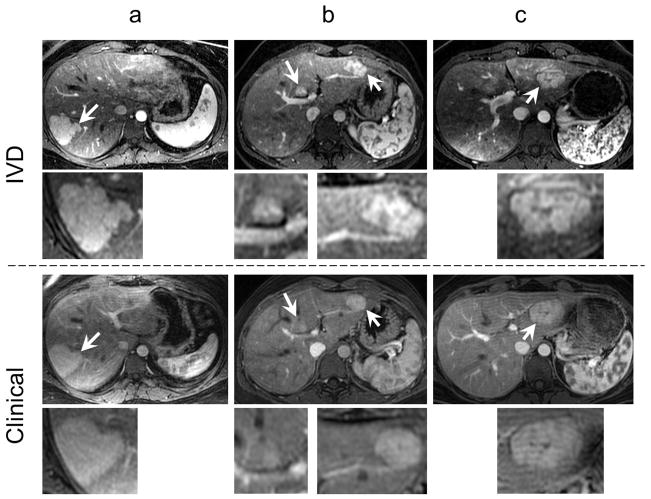
Figure 6 compares the maximum lesion contrast time-frame from the dynamic IVD scan with the clinical image in three different subjects. Note the improved contrast and spatial resolution in the IVD images.
FIG 6.
The IVD images show superior quality compared to the clinical MR images. The top two rows show a late-arterial phase time-frame from the IVD series with magnified views of the lesions while the bottom two rows show the single late-arterial phase image from the clinical MRI protocol, in three different subjects with magnified views of the same lesions. Arrows point to the FNH lesions. Note the improved spatial resolution and lesion conspicuity in the IVD images relative to the clinical MR images.
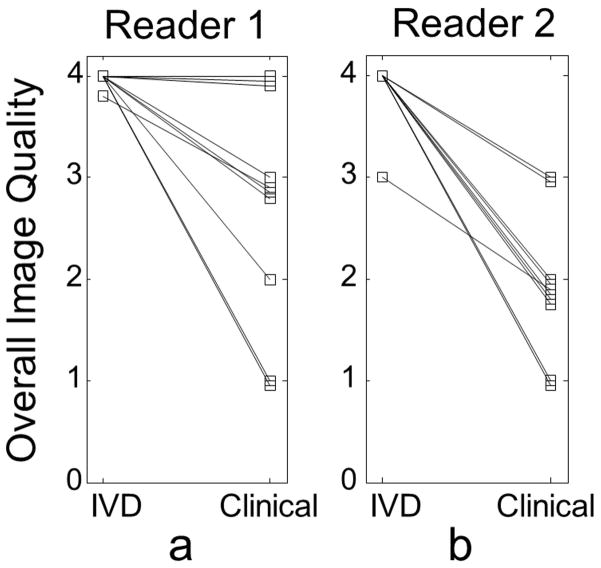
Figure 7 plots the grades obtained from the dynamic IVD images by reader 1 (a) (mean 4.0 ± 0.1) and reader 2 (b) (mean 3.9 ± 0.3) compared with the grades obtained from the clinical MR images by reader 1 (mean 2.8 ± 1.1) and reader 2 (mean 2.0 ± 0.7). The grades for the images acquired using the dynamic IVD technique were significantly higher for both readers with p = 0.02 for reader 1 and p < 0.01 for reader 2. Reasons cited by the readers for lower grades included: truncation or ringing artifacts seen at the edges, artifacts due to peristalsis movement, imperfect fat suppression, and early or late timing of the scans. In two of the cases, clinical images were graded “poor”, because the artifacts appeared on the liver and were considered to inhibit appropriate clinical assessment.
FIG 7.
Two experienced radiologists graded the overall quality of the IVD images as significantly higher than the prior clinical MR images. Both readers independently graded each time-frame of the IVD method (five time-frames leading to five grades). These grades were averaged and compared with the grade given to the clinical MR image. Note the improvement achieved using the IVD method. Since there was a great deal of overlap in the scores for the 10 subjects, to aid with the visualization, a very small bias was added to the grades when multiple subjects had the same image quality grades, in order to distinguish them on the plot.
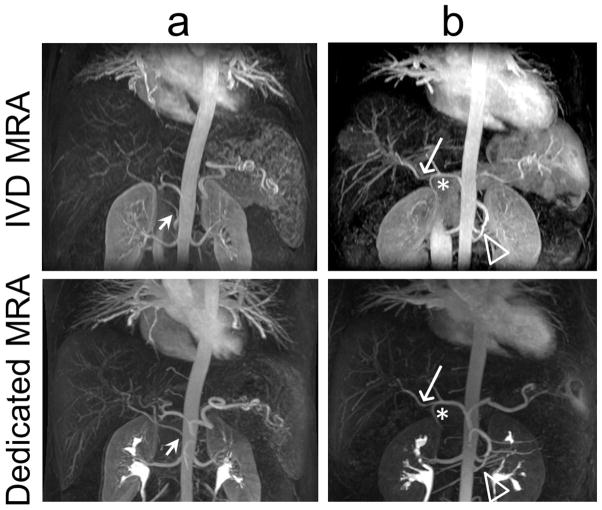
Figure 8 shows a limited-slab coronal MIP of the first time-frame of the IVD series of images acquired in two of the subjects (Figure 8a, b). Subject (a) has a replaced right hepatic artery from the superior mesenteric artery that is clearly visible on the IVD and conventional MRA images. There is comparable quality between the two images. Subject (b) also has a variant of the right hepatic artery (long arrow) taking off prior of the gastroduodenal artery (asterisk). A variant of two left renal arteries (hollow arrowhead) is also visible in this subject in both the IVD MRA and the dedicated MRA images. Note the residual contrast in the renal collecting system on the dedicated MRA images from the first injection used to acquire the dynamic images.
FIG 8.
High quality MR angiograms were obtained from the dynamic IVD time-series in all subjects. a) A limited-slab coronal MIP of a subject shows a vascular variation with a replaced right hepatic artery from the superior mesenteric artery (SMA) visible both in the IVD MRA and in the conventional dedicated MRA (short arrow). b) In another subject, clear delineation of a variant of the right hepatic artery (long arrow) arising directly from the common hepatic artery prior to the take-off of the gastroduodenal artery (asterisk) was seen. Also clearly depicted is the variant of two left renal arteries (hollow arrowhead). Bright signal in the renal pelvis in the dedicated MRA images is from the previous injection of GBCA.
Table 1 summarizes the arterial visibility grades for the dynamic IVD method compared to the dedicated MRA method. Reader 1 found all arterial segments sufficiently visible (grades 4 excellent or 3 good visibility) with the IVD method and found one arterial segment that was sufficiently visible with the IVD method but not with the dedicated MRA method. Reader 2 found 105 of 109 segments sufficiently visible both in the dynamic IVD method and the dedicated MRA method and a total of 4 segments insufficiently visible in the IVD MRA method of which 1 segment was not sufficiently visible in the dedicated MRA method either. No significant difference was found between the two methods using a McNemar’s test.
Table 1.
Reader 1 and reader 2, respectively, scored 100% and 96% of graded arterial segments as having excellent to good visibility in the angiographic phase of the IVD images. Reader 1 identified no segments that exhibited insufficient visibility in the IVD MRA. Reader 2 identified only 4 segments with insufficient visibility in the IVD MRA, of which one segment was also not sufficiently visible in the dedicated MRA.
| Reader 1 | Reader 2 | ||||
|---|---|---|---|---|---|
| dynamic IVD | dynamic IVD | ||||
| sufficient visibility | insufficient visibility | sufficient visibility | insufficient visibility | ||
| Clinical | sufficient visibility | 108 | 0 | 105 | 3 |
| insufficient visibility | 1 | 0 | 0 | 1 | |
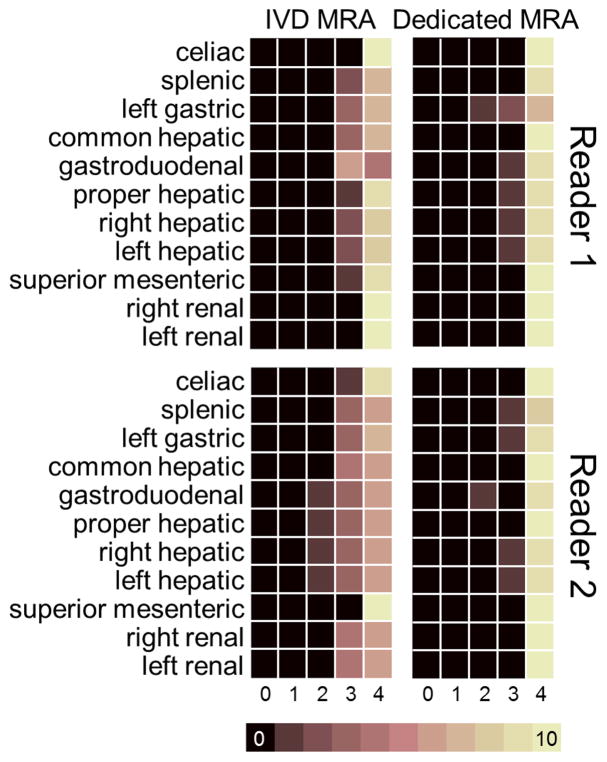
Figure 9 shows a color map of the artery segment visibility grades obtained from the images acquired using the dynamic IVD method compared to the grades obtained from the images acquired using the dedicated MRA method. Grades assigned to the arterial segments in the images acquired using the IVD method by reader 1 (mean 3.82 ± 0.39) and reader 2 (mean 3.64 ± 0.55) were non-inferior to those assigned to the arterial segments in the images acquired using the dedicated MRA method by reader 1 (mean 3.93 ± 0.30) and reader 2 (mean 3.94 ± 0.27), with a statistically significant non-inferiority (p = 0.01 and p = 0.05, respectively) in the one-sided Wilcoxon signed-rank test.
FIG 9.
MR angiograms obtained from the IVD method depicted nearly all of the graded arterial segments as having high visibility in all subjects. Visibility of 109 arterial vessel segments in the 10 subjects was graded on a five-point scale. Color maps show the frequency of each score (maximum possible was 10, corresponding to the number of subjects) for all 109 arterial segments in the angiographic phase of the IVD scan (left column) and in the dedicated MRA scan (right column) for reader 1 (top row) and reader 2 (bottom row).
Both readers graded fat-water separation quality of all IVD images as excellent or good. Only minor fat-water swaps were visible outside the liver at the edges of the FOV.
Discussion
In this work, we described the development and evaluation of a dynamic IVD undersampled Cartesian acquisition strategy. This method was successfully used to acquire and reconstruct multiple contrast-enhanced T1-weighted images of the abdomen with high spatial and temporal resolution. The acquisition was done during the intravenous injection of GBCA in a single breath-hold. The images were used to visualize 21 FNH, 3 cavernous hemangiomas, and 109 arterial segments in a group of 10 subjects. Further, the arterial phase image of the IVD acquisition demonstrated very similar performance to the dedicated MRA demonstrating the feasibility of avoiding the need for an additional MRA and contrast injection.
Saranathan et al. (13) recently introduced an undersampled Cartesian sampling approach for liver imaging (DISCO). The DISCO method combined parallel imaging with a view-sharing strategy based on an elliptical centric subsampling of the k-space periphery, alternating with the central region of k-space. Further, acquisition of central k-space lines were repeated for each time-frame. There are important differences between the DISCO approach and our approach. In our work, we used a different sampling strategy (IVD) and k-space ordering (edge-center-edge) and also employed a new stratgey for acquiring parallel imaging ACS lines (first time-frame only). Time saved by avoiding repeated acquisition of ACS lines can be used to enhance the temporal resolution by about 25% or alternatively increase spatial resolution or S/I coverage (26 cm in our method). Further, we have validated that the arterial phase of the IVD method is equivalent to abdominal MRA for visualization of abdominal arterial segments. We also employed a real-time triggering algorithm to ensure consistent timing of the bolus. Using a standard delay of 15 seconds between injection of the contrast agent and the start of image acquisition, the DISCO method was shown to capture an arterial phase in only 88% of cases (30). Although further clinical studies would be needed to demonstrate the performance of the proposed IVD method with regard to consistently capturing the late arterial phase, it would be reasonable to expect that real-time triggering would likely improve capture of the late arterial phase. A study by Agrawal et al. (31) showed that use of real-time triggering improves bolus timing when spiral dynamic acquisition is used.
In our study, ACS readout lines were acquired only once during the breath-hold interval at the center of the first time-frame. Calibration lines contain implicit information about the coil sensitivity maps (32,33). Therefore, despite the change in image contrast, a synthesis kernel calibrated on data from one time-frame can be used on data for other time-frames as long as there is no significant spatial misregistration between frames acquired during the breath-hold. While misregistration of the liver can be significant between breath-holds, the displacement within a breath-hold interval is very small (34). For this reason, it is essential to acquire the calibration lines within the same breath-hold, but it is not necessary to repeatedly acquire these lines for each time-frame. An internal calibration strategy with ACS lines acquired in every time-frame would be more robust to motion within the breath-hold and is not susceptible to breath-hold reproducibility artifacts, but would require an unacceptably long breath-hold time (32 seconds for the protocol used in this work) or trade-offs in spatial resolution. Reconstruction time also increases significantly with repeated parallel imaging calibration in each time-frame.
In addition, we chose a specific k-space ordering strategy, progressing from the edge to the center and back to the edge of k-space along an elliptical centric trajectory. We found this approach was effective at avoiding artifacts caused by rapid changes of gradients, which can occur when transitioning from the edge of k-space in one time-frame to the center of k-space in a subsequent time-frame.
By synchronizing the dynamic IVD acquisition with the arrival of GBCA in the liver, our method uses the breath-hold period efficiently. Real-time triggering of the dynamic IVD method enables acquisition of an angiographic phase, defined as a frame when only arteries are enhanced before the contrast agent enhances the portal vein or liver parenchyma substantially. With accurate timing and high spatial resolution, angiographic evaluation of the arterial anatomy was easily performed without the need for a dedicated MRA. High temporal resolution also provides separate earlier and later phase images and allows identification of a time-frame when the contrast between the lesion(s) of interest and the surrounding liver parenchyma is maximized.
There are several limitations to this technical development and feasibility study. Although this is a prospective study, the subjects were limited to those with FNH, one of the many types of clinically relevant liver lesions. These subjects were chosen because FNH are arterially-enhancing lesions with rapid uniform enhancement, similar to HCC and other arterially-enhancing lesions. For this reason, FNH are excellent lesions in which to test time-resolved contrast-enhanced MRI methods. Future validation in other focal liver lesions such as HCC, hepatic adenoma, metastases and hemangiomas will be needed. Another limitation is that the field strength and imaging coil were different for the clinical exams (1.5 T, 8-channel) compared with the IVD exams (3T, 32-channel) and may have contributed to differences in image quality. The high spatial and temporal resolution of the IVD exams was simply not achievable using the available clinical software. Further, the clinical imaging was performed using a different contrast agent that was administered at a different concentration relative to the IVD imaging. The clinical imaging was performed using 0.05 mmol/kg of gadoxetic acid per our standard of care. We consider this a minor limitation, since it has been demonstrated previously that arterial-phase enhancement using 0.05 mmol/kg of gadoxetic acid is almost identical to the enhancement achieved using 0.1 mmol/kg of gadobenate dimeglumine at 3.0 T (35). In addition, the dedicated MRA in this study was performed after the IVD acquisition, which could impair the quality of the dedicated MRA due to the presence of residual circulating contrast agent. The acquisition of a dedicated MRA with a second injection after the dynamic T1-weighted imaging is similar to the current clinical protocol at our institution for patients evaluated for potential transplantation. A final limitation of this study is that it is possible, although unlikely, that the FNH lesions could have grown or regressed during the time interval between performing the clinical and research studies (36). Prospective comparative studies will be needed to ascertain the value of time-resolved imaging in aiding the diagnosis of patients with other focal liver lesions such as HCC.
Generating quantitative perfusion maps is beyond the scope of this work but imaging with combined high spatial and temporal resolution is a first step towards parametric perfusion mapping of the liver. More mathematically-complex image reconstruction techniques including methods based on HYPR/HYCR algorithms (24,37,38), iterative combined compressed sensing and parallel imaging (39), or other k-t methods (40,41) can also be used to further enhance image quality. However, their clinical utilization has to be weighed carefully against i) potential artifacts, ii) the added benefits over view-sharing and iii) increases in reconstruction time.
In conclusion, we have developed a fat-suppressed, time-resolved, 3D, contrast-enhanced, T1-weighted MRI method that provides simultaneous high spatial and high temporal resolution. This method demonstrates great potential for clinical arterial-phase imaging of the liver. This method allows for visualization of rapidly-changing pharmacokinetics in arterially-enhancing lesions such as FNH and cavernous hemangiomas and holds excellent promise for detection and characterization of other arterially-enhancing focal liver lesions. Finally, the combination of high spatial and temporal resolution may facilitate simultaneous acquisition of MR angiograms, avoiding the need for a dedicated MRA and second injection of contrast.
Acknowledgments
Funding for this work was provided by NIH grant RC1EB010384 and the R&D committee of University of Wisconsin Department of Radiology. The authors would like to acknowledge Alejandro Munoz del Rio for helpful discussions on the statistical analysis and Deborah Gawin, Jenelle Fuller and Sara John for help with recruiting and scanning of human subjects. We also wish to thank GE Healthcare for their support.
References
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular Carcinoma Incidence, Mortality, and Survival Trends in the United States From 1975 to 2005. Journal of Clinical Oncology. 2009;27(9):1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrel M, Llovet JM, Ayuso C, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: An explant correlation. Hepatology. 2003;38(4):1034–1042. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 3.Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, Duca P. Accuracy of Ultrasonography, Spiral CT, Magnetic Resonance, and Alpha-Fetoprotein in Diagnosing Hepatocellular Carcinoma: A Systematic Review. Am J Gastroenterol. 2006;101(3):513–523. doi: 10.1111/j.1572-0241.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 4.Abdullah SS, Pialat JB, Wiart M, Duboeuf F, Mabrut J-Y, Bancel B, Rode A, Ducerf C, Baulieux J, Berthezene Y. Characterization of hepatocellular carcinoma and colorectal liver metastasis by means of perfusion MRI. Journal of Magnetic Resonance Imaging. 2008;28(2):390–395. doi: 10.1002/jmri.21429. [DOI] [PubMed] [Google Scholar]
- 5.Bashir MR, Gupta RT, Davenport MS, Allen BC, Jaffe TA, Ho LM, Boll DT, Merkle EM. Hepatocellular carcinoma in a North American population: Does hepatobiliary MR imaging with Gd-EOB-DTPA improve sensitivity and confidence for diagnosis? Journal of Magnetic Resonance Imaging. 2013;37(2):398–406. doi: 10.1002/jmri.23818. [DOI] [PubMed] [Google Scholar]
- 6.Freeman RB. Transplantation for hepatocellular carcinoma: The Milan criteria and beyond. Liver Transplantation. 2006;12(S2):S8–S13. doi: 10.1002/lt.20936. [DOI] [PubMed] [Google Scholar]
- 7.Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: Comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transplantation. 2002;8(9):765–774. doi: 10.1053/jlts.2002.34892. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in Advanced Hepatocellular Carcinoma. New England Journal of Medicine. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1. 1) European Journal of Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Materne R, Smith AM, Peeters F, Dehoux JP, Keyeux A, Horsmans Y, Van Beers BE. Assessment of hepatic perfusion parameters with dynamic MRI. Magnetic Resonance in Medicine. 2002;47(1):135–142. doi: 10.1002/mrm.10045. [DOI] [PubMed] [Google Scholar]
- 11.Rofsky NM, Lee VS, Laub G, Pollack MA, Krinsky GA, Thomasson D, Ambrosino MM, Weinreb JC. Abdominal MR Imaging with a Volumetric Interpolated Breath-hold Examination1. Radiology. 1999;212(3):876–884. doi: 10.1148/radiology.212.3.r99se34876. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky EK, Bultman EM, Johnson KM, Horng DE, Schelman WR, Block WF, Reeder SB. High-spatial and high-temporal resolution dynamic contrast-enhanced perfusion imaging of the liver with time-resolved three-dimensional radial MRI. Magnetic Resonance in Medicine. 2013 doi: 10.1002/mrm.24727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saranathan M, Rettmann DW, Hargreaves BA, Clarke SE, Vasanawala SS. DIfferential subsampling with cartesian ordering (DISCO): A high spatio-temporal resolution dixon imaging sequence for multiphasic contrast enhanced abdominal imaging. Journal of Magnetic Resonance Imaging. 2012;35(6):1484–1492. doi: 10.1002/jmri.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B, Spincemaille P, Chen G, Agrawal M, Nguyen TD, Prince MR, Wang Y. Fast 3D contrast enhanced MRI of the liver using temporal resolution acceleration with constrained evolution reconstruction. Magnetic Resonance in Medicine. 2013;69(2):370–381. doi: 10.1002/mrm.24253. [DOI] [PubMed] [Google Scholar]
- 15.Chandarana H, Block K, Winfeld M, Lala S, Mazori D, Giuffrida E, Babb J, Milla S. Free-breathing contrast-enhanced T1-weighted gradient-echo imaging with radial k-space sampling for paediatric abdominopelvic MRI. European Radiology. 2013:1–7. doi: 10.1007/s00330-013-3026-4. [DOI] [PubMed] [Google Scholar]
- 16.Lee SS. Cardiac abnormalities in liver cirrhosis. The Western journal of medicine. 1989;151(5):530–535. [PMC free article] [PubMed] [Google Scholar]
- 17.Foo TK, Sawyer AM, Faulkner WH, Mills DG. Inversion in the steady state: contrast optimization and reduced imaging time with fast three-dimensional inversion-recovery-prepared GRE pulse sequences. Radiology. 1994;191(1):85–90. doi: 10.1148/radiology.191.1.8134602. [DOI] [PubMed] [Google Scholar]
- 18.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magnetic Resonance in Medicine. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 19.Brau ACS, Beatty PJ, Skare S, Bammer R. Comparison of reconstruction accuracy and efficiency among autocalibrating data-driven parallel imaging methods. Magnetic Resonance in Medicine. 2008;59(2):382–395. doi: 10.1002/mrm.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J. Breath-hold water and fat imaging using a dual-echo two-point dixon technique with an efficient and robust phase-correction algorithm. Magnetic Resonance in Medicine. 2004;52(2):415–419. doi: 10.1002/mrm.20146. [DOI] [PubMed] [Google Scholar]
- 21.Ma J, Slavens Z, Sun W, Bayram E, Estowski L, Hwang K-P, Akao J, Vu AT. Linear phase-error correction for improved water and fat separation in dual-echo dixon techniques. Magnetic Resonance in Medicine. 2008;60(5):1250–1255. doi: 10.1002/mrm.21747. [DOI] [PubMed] [Google Scholar]
- 22.Chun Hsee L, McCall JL, Koea JB. Focal nodular hyperplasia: what are the indications for resection? HPB. 2005;7(4):298–302. doi: 10.1080/13651820500273624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grazioli L, Bondioni MP, Haradome H, Motosugi U, Tinti R, Frittoli B, Gambarini S, Donato F, Colagrande S. Hepatocellular Adenoma and Focal Nodular Hyperplasia: Value of Gadoxetic Acid–enhanced MR Imaging in Differential Diagnosis. Radiology. 2012;262(2):520–529. doi: 10.1148/radiol.11101742. [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Busse RF, Holmes JH, Beatty PJ, Brittain JH, Francois CJ, Reeder SB, Du J, Korosec FR. Interleaved variable density sampling with a constrained parallel imaging reconstruction for dynamic contrast-enhanced MR angiography. Magnetic Resonance in Medicine. 2011;66(2):428–436. doi: 10.1002/mrm.22814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein MA, Fain SB, Riederer SJ. Effect of windowing and zero-filled reconstruction of MRI data on spatial resolution and acquisition strategy. Journal of Magnetic Resonance Imaging. 2001;14(3):270–280. doi: 10.1002/jmri.1183. [DOI] [PubMed] [Google Scholar]
- 26.Lim RP, Shapiro M, Wang EY, et al. 3D Time-Resolved MR Angiography (MRA) of the Carotid Arteries with Time-Resolved Imaging with Stochastic Trajectories: Comparison with 3D Contrast-Enhanced Bolus-Chase MRA and 3D Time-Of-Flight MRA. American Journal of Neuroradiology. 2008;29(10):1847–1854. doi: 10.3174/ajnr.A1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beatty PJ, Chang S, Holmes JH, Wang K, Brau ACS, Reeder SB, Brittain JH. Design of k-space channel combination kernels and integration with parallel imaging. Magnetic Resonance in Medicine. 2013 doi: 10.1002/mrm.24883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang K, Beatty PJ, Nagle SK, Reeder SB, Holmes JH, Rahimi MS, Bell LC, Korosec FR, Brittain JH. Application of direct virtual coil to dynamic contrast-enhanced MRI and MR angiography with data-driven parallel imaging. Magnetic Resonance in Medicine. 2013 doi: 10.1002/mrm.24686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lum DP, Busse RF, Francois CJ, Brau AC, Beatty PJ, Huff J, Brittain JH, Reeder SB. Increased volume of coverage for abdominal contrast-enhanced MR angiography with two-dimensional autocalibrating parallel imaging: Initial experience at 3. 0 Tesla. Journal of Magnetic Resonance Imaging. 2009;30(5):1093–1100. doi: 10.1002/jmri.21964. [DOI] [PubMed] [Google Scholar]
- 30.Hope TA, Saranathan M, Petkovska I, Hargreaves BA, Herfkens RJ, Vasanawala SS. Improvement of gadoxetate arterial phase capture with a high spatio-temporal resolution multiphase three-dimensional SPGR-dixon sequence. Journal of Magnetic Resonance Imaging. 2013;38(4):938–945. doi: 10.1002/jmri.24048. [DOI] [PubMed] [Google Scholar]
- 31.Agrawal MD, Spincemaille P, Mennitt KW, Xu B, Wang Y, Dutruel SP, Prince MR. Improved hepatic arterial phase MRI with 3-second temporal resolution. Journal of Magnetic Resonance Imaging. 2013;37(5):1129–1136. doi: 10.1002/jmri.23920. [DOI] [PubMed] [Google Scholar]
- 32.Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magnetic Resonance in Medicine. 2013 doi: 10.1002/mrm.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griswold MA, Blaimer M, Breuer F, Heidemann RM, Mueller M, Jakob PM. Parallel magnetic resonance imaging using the GRAPPA operator formalism. Magnetic Resonance in Medicine. 2005;54(6):1553–1556. doi: 10.1002/mrm.20722. [DOI] [PubMed] [Google Scholar]
- 34.Bultman E, Horng D, Brodsky EK, Johnson KM, Block WF, Reeder SB. Simple Motion Correction for Hepatic DCE-MRI: Registration of Sequential Breath Holds in 3D Radial Time-Resolved Scans; 2012 May; Melbourne, Australia. p. 165. [Google Scholar]
- 35.Frydrychowicz A, Nagle SK, D’Souza SL, Vigen KK, Reeder SB. Optimized high-resolution contrast-enhanced hepatobiliary imaging at 3 tesla: A cross-over comparison of gadobenate dimeglumine and gadoxetic acid. Journal of Magnetic Resonance Imaging. 2011;34(3):585–594. doi: 10.1002/jmri.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Carlis L, Pirotta V, Rondinara GF, et al. Hepatic adenoma and focal nodular hyperplasia: Diagnosis and criteria for treatment. Liver Transplantation and Surgery. 1997;3(2):160–165. doi: 10.1002/lt.500030209. [DOI] [PubMed] [Google Scholar]
- 37.Johnson KM, Velikina J, Wu Y, Kecskemeti S, Wieben O, Mistretta CA. Improved waveform fidelity using local HYPR reconstruction (HYPR LR) Magnetic Resonance in Medicine. 2008;59(3):456–462. doi: 10.1002/mrm.21505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busse R, Pineda A, Wang K, Holmes J, Brittain J, Korosec F. Time-Resolved Imaging with Multiplicative Algebraic Reconstruction Technique (MART): An Application of HYPR Principles for Variable Density Cartesian Acquisitions; 2009 April; Honolulu. p. 2834. [Google Scholar]
- 39.Lustig M, Alley M, Vasanawala S, Donoho D, Pauly J. L1 SPIR-iT: Autocalibrating Parallel Imaging Compressed Sensing; 2009 April; Honolulu. p. 379. [Google Scholar]
- 40.Tsao J, Boesiger P, Pruessmann KP. k-t BLAST and k-t SENSE: Dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magnetic Resonance in Medicine. 2003;50(5):1031–1042. doi: 10.1002/mrm.10611. [DOI] [PubMed] [Google Scholar]
- 41.Huang F, Akao J, Vijayakumar S, Duensing GR, Limkeman M. k-t GRAPPA: A k-space implementation for dynamic MRI with high reduction factor. Magnetic Resonance in Medicine. 2005;54(5):1172–1184. doi: 10.1002/mrm.20641. [DOI] [PubMed] [Google Scholar]