Abstract
Background
Standardization of the hemoglobin A1c (A1c) assay has led to its increasing utilization as a screening tool for the diagnosis of prediabetes and type 2 diabetes in youth. However, significant A1c assay variability remains and has implications for clinical management.
Objective
To describe our center’s experiences with A1c results in youth and to evaluate inter-method differences and their clinical implications.
Subjects
75 youth (ages 10–18 years old), BMI ≥85th%ile participated.
Methods
72 participants had two A1c values performed on the same sample, one via immunoassay (DCA Vantage Analyzer, A1c1) and the other via high performance liquid chromatography (Bio-Rad Variant II, A1c2). 19 had A1c run on two immunoassay devices (A1c1 and Dimensions Vista, A1c3).
Results
Mean age of participants was 13.9 years, BMI% 97.89, 33% male, 16% white, 21% black, and 61% Hispanic. Mean A1c1 was 5.68%±0.38 vs. a mean A1c2 of 5.73%±0.39, p=0.049. Concordance in diabetes status between methods was achieved in 79% of subjects. 19 subjects with A1c3 results had testing performed an average of 22±9 days prior to A1c1. Mean A1c3 was 6.24% ±0.4, compared to a mean A1c1 of 5.74% ± 0.31, (p<0.0001). A1c1 was on average systematically −0.5±0.28 lower compared to A1c3. There was poor agreement in diabetes classification between A1c1 and A1c3, with a concordance in classification between methods of only 36.8%.
Conclusions
Clinically significant inter-method A1c variability exists that impacts patient classification and treatment recommendations. In the screening of obese youth for diabetes, A1c results should be interpreted with caution.
Keywords: Hemoglobin A1c, prediabetes, type 2 diabetes, obesity
Introduction
Standardization of hemoglobin A1c (A1c) methodologies by the National Glycohemoglobin Standardization Program (NGSP) to the Diabetes Control and Complications Trial (DCCT), which demonstrated direct relationships between A1c and diabetes outcomes, has promoted widespread use of A1c testing. In response, the American Diabetes Association (ADA) incorporated A1c into the diagnostic criteria for diabetes in 2010 (<5.7% normal, 5.7–6.4% prediabetes, ≥6.5% diabetes). (1) Despite lack of validated studies in pediatrics, these cut points have been extrapolated to youth, leading to increased A1c screening for diabetes by pediatricians (2, 3) and increased subspecialty referrals for abnormal A1c values. Our clinical experience suggested that abnormal A1c values obtained in outside hospitals were often normal when repeated at our institution. Our objective was to formally analyze differences between A1c results measured by multiple methodologies in a sample of overweight or obese adolescents.
Methods
Between March 2011 and December 2012, 75 overweight or obese participants were recruited from general pediatric clinics and referrals to the endocrine clinic at Children’s Hospital Colorado for a larger ongoing trial at this center. Inclusion criteria were ages 10–18 years, BMI≥85th%ile, and not on medications affecting glucose metabolism. A1c was obtained via immunoassay on a Siemens DCA Vantage Analyzer™ (Tarrytown, NY), A1c1, for all 75 participants. 72 (96%) participants also had an A1c performed on the same sample by high performance liquid chromatography (HPLC; Bio-Rad Variant II, Hercules, CA), A1c2. In addition, 19 (25%) participants also had A1c results obtained from the same outside hospital central lab operating a Siemens Dimension Vista® (Tarrytown, NY), A1c3. All three A1c devices are NGSP certified and have documented traceability to the DCCT reference method. The laboratory reference range for the A1c1 DCA Vantage Analyzer™ is 4.2–6.3%, with no distinction between normal and prediabetes. The reported reference ranges for the Bio-Rad Variant II, A1c2,and the Siemens Vista, A1c3, are identical to ADA cutpoints for defining normal glycemia, prediabetes, and diabetes. A1c1 and A1c3 are immunoassay devices that may be utilized as point-of-care (POC) analyzers but, in this report, are operated by central laboratories at large tertiary care hospitals with rigorous quality control.
Statistical Analysis
Simple linear regression and Deming regression, which assumes measurement error in both X and Y, were used to explore the relationship between A1c1 vs. A1c2 and A1c1 vs. A1c3. Regression coefficients were reported as intercept ± SE and β ± SE and the regression equation for the lines of best fit were also reported. Multiple linear regression was used to adjust for time differences between A1c1 and A1c3. Bland-Altman plots, in which the difference in paired values is plotted against the mean of the paired values, explored the bias between A1c1 vs. A1c2 and A1c1 vs. A1c3. p<0.05 was considered significant. Paired t-tests were used to compare A1c types. Cohen’s kappa (k), a measure of inter-rater reliability used to compare two categorical methods of classification, was used to measure agreement in diabetes status. Fasting plasma glucose (FPG) and 2hour plasma glucose (2hr PG) after 75 g OGTT were available for these participants and concordance in diabetes classification between the different A1c assays with both FPG and 2hr PG were also calculated. Analyses were performed in SAS 9.3 (Cary, NC) and GraphPad Prism 5.0. The protocol was approved by the Colorado Multiple Institutional Review Board and appropriate consent and assent were obtained.
Results
Participants had a mean age of 13.9 years, mean BMI% 97.89, and were 33% male, 16% Non-Hispanic White (NHW), 21% African American (AA), and 61% Hispanic (H). Table 1 presents study population characteristics by gender and race/ethnicity. Of the N = 75 who had A1c1, N = 72 had A1c2 and N = 19 had A1c3 results. Thus these patients are subsets of the A1c1 data, however, there was no difference in distribution of sex or race/ethnicity for A1c2 and A1c3 compared to A1c1.
Table 1.
Characteristics of Study Population
| A1c1 (n=75) | A1c2 (n=72) | A1c3 (n=19) | |
|---|---|---|---|
| A1c (%, average ± SD) | 5.68 ± 0.38 | 5.73 ± 0.39 | 6.24 ± 0.4 |
| Age (years) | 13.9 ± 2.3 | 13.9 ± 2.3 | 14.3 ± 1.9 |
| Male | N = 25 (33%) | N = 23 (32%) | N = 5 (26.3%) |
| BMI % | 97.89 % | 97.89 ± 2.46 | 97.13 ± 2.85 |
| Race | |||
| Non-Hispanic White | 12 (16%) | 12 (17%) | 1 (5%) |
| African American | 16 (21%) | 16 (22%) | 5 (26%) |
| Hispanic | 46 (61%) | 43 (60%) | 13 (68%) |
| Other | 1 (1%) | 1 (1%) | --- |
Agreement between A1c1 and A1c2
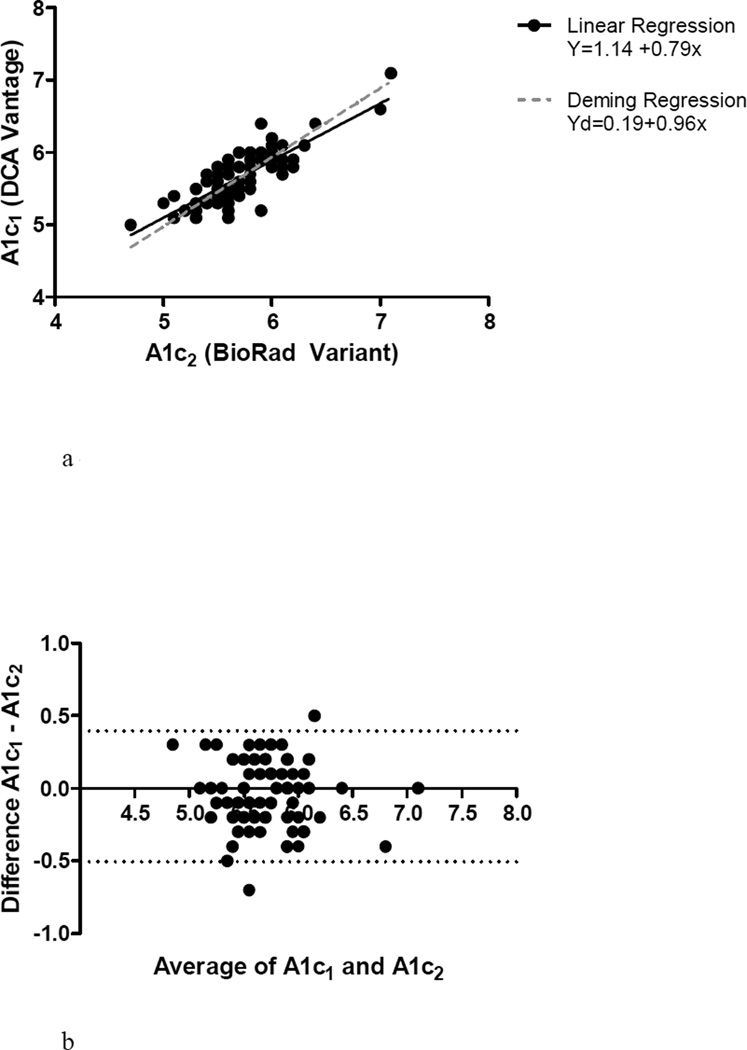
Mean A1c1 was 5.68%±0.38 compared to a mean A1c2 of 5.73%±0.39, p=0.049. As expected, there was a significant relationship between A1c1 and A1c2 (R2 = 0.67, p < 0.0001). Linear regression as depicted in Figure 1a demonstrates that A1c2 estimates were generally higher than A1c1. In the Bland Altman plot in Figure 1b, A1c1 was systematically 0.05±0.23 lower, compared to A1c2, p=0.049. There was reasonable agreement between A1c1 and A1c2 in diabetes status classification, (k = 0.60 (0.42–0.79)), with concordance in diabetes status achieved in 79% of subjects (Table 2a).
Figure 1.
a – Correlation between A1c1 and A1c2
b – Bland-Altman plot of the difference against the mean of A1c1 and A1c2 results
Table 2.
| a: A1c1 (DCA Vantage) vs A1c2 (Bio-Rad Variant II) – Classification by ADA Diagnostic Categories | |||
|---|---|---|---|
| Diabetes A1c1 (n=2) |
Prediabetes A1c1 (n=35) |
Normal A1c1 (n=35) |
|
| Diabetes A1c2 (n=2) | 2 | 0 | 0 |
| Prediabetes A1c2 (n=36) | 0 | 28 | 8 |
| Normal A1c2 (n=34) | 0 | 7 | 27 |
| b: A1c1 (DCA Vantage) vs A1c3 (Dimensions Vista) – Classification by ADA Diagnostic Categories | |||
|---|---|---|---|
| Diabetes A1c1 (n=0) |
Prediabetes A1c1 (n=12) |
Normal A1c1 (n=7) |
|
| Diabetes A1c3 (n=5) | 0 | 5 | 0 |
| Prediabetes A1c3 (n=14) | 0 | 7 | 7 |
| Normal A1c3 (n=0) | 0 | 0 | 0 |
Agreement between A1c1 and A1c3
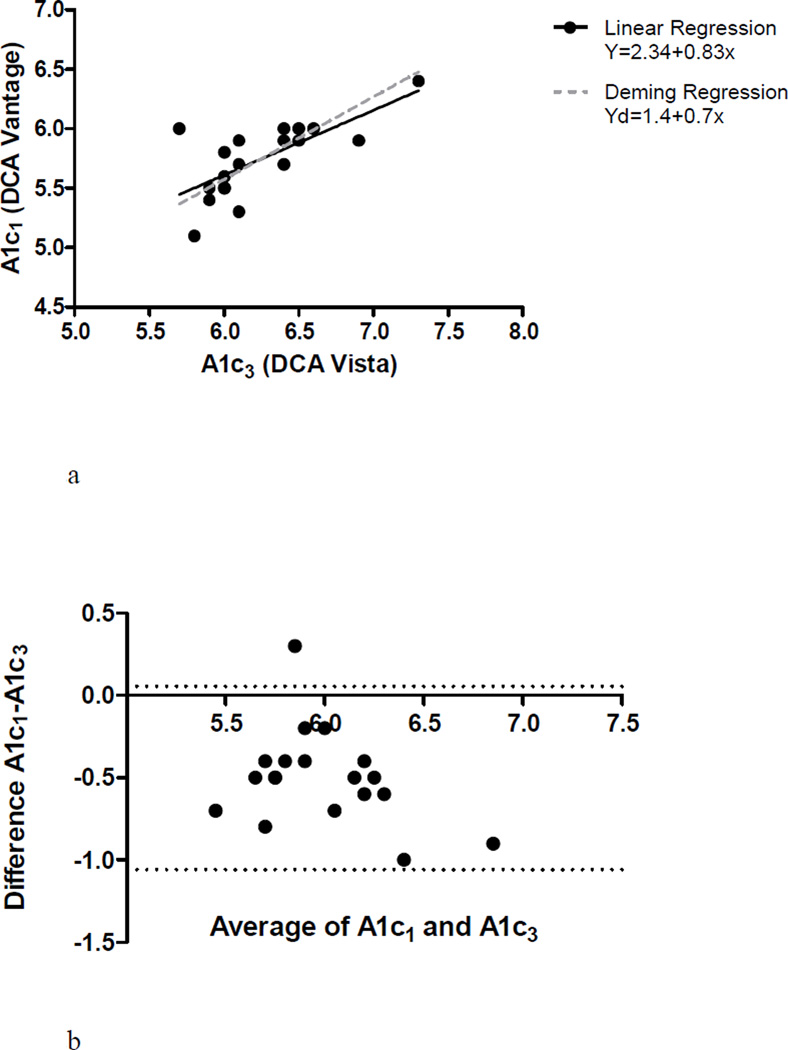
The 19 subjects with A1c3 results had testing performed an average of 22±9 days prior to A1c1. Mean A1c3 was 6.24%±0.4, compared to a mean A1c1, in the same 19 subjects, of 5.74% ± 0.31, (p<0.0001). There was a significant relationship between A1c1 and A1c3, R2 = 0.50 (p=0.0007) (Figure 2a) but the Bland Altman plot demonstrates that A1c1 was systematically lower than A1c3 by 0.50 ± 0.28%, (p<0.0001) (Figure 2b). There was little agreement in diabetes classification between A1c1 and A1c3, with a concordance of 36.8%, (k = −0.44 (−0.69-(−0.19))) (Table 2b).
Figure 2.
a – Correlation bewteen A1c1 and A1c3
b – Bland-Altman plot of the difference against the mean of A1c1 and A1c3 results
As African Americans (AA) have been reported to have a higher A1c for a given mean blood glucose, a subanalysis was performed removing AA subjects from the groups. When AA subjects were removed from the analysis, the A1c differences between methodologies were enhanced. With AA subjects removed from the comparison between A1c1 and A1c2, N = 56, kappa = 0.61 (0.41–0.82), and the A1c difference = −0.07 +/− 0.29, p = 0.03. For A1c1 versus A1c3, without AA subjects, N = 14 and the A1c difference = 0.61 +/− 0.19%, p < 0.0001. Kappa = −0.64, however the sample size was small.
To further explore the discrepancy in diabetes categorization between the subjects with both A1c1 and A1c2 and the subjects with both A1c1 and A1c3, we compared results to FPG and 2hr PG diabetes categories. 71 subjects with both A1c1 and A1c2 had FPG and 2hr PG data. For this group, the concordance between A1c1 and FPG = 48% and between A1c1 and 2hr PG = 52%. For A1c2, the concordance rate with FPG = 44% and with 2hr PG =58%. For the 19 subjects with both A1c1 and A1c3, A1c1 concordance rates with FPG = 42% and with 2hr PG 53%. Concordance in diabetes classification between A1c3 and FPG = 5% and between A1c3 and 2hr PG = 16%. (Tables 3a-h)
Table 3.
| a: A1c1 vs FPG – Classification by ADA Diagnostic Categories | |||
|---|---|---|---|
| Diabetes FPG (n=0) |
Prediabetes FPG (n=5) |
Normal FPG n=66) |
|
| Diabetes A1c1 (n=2) | 0 | 1 | 1 |
| Prediabetes A1c1 (n=36) | 0 | 3 | 33 |
| Normal A1c1 (n=33) | 0 | 1 | 32 |
| b: A1c1 vs OGTT – Classification by ADA Diagnostic Categories | |||
|---|---|---|---|
| Diabetes OGTT (n=1) |
Prediabetes OGTT (n=20) |
Normal OGTT n=48) |
|
| Diabetes A1c1 (n=2) | 1 | 1 | 0 |
| Prediabetes A1c1 (n=36) | 2 | 11 | 23 |
| Normal A1c1 (n=33) | 0 | 8 | 25 |
| c - A1c2 vs FPG – Classification by ADA Diagnostic Categories | |||
|---|---|---|---|
| Diabetes FPG (n=0) |
Prediabetes FPG (n=5) |
Normal FPG (n=66) |
|
| Diabetes A1c2 (n=2) | 0 | 1 | 1 |
| Prediabetes A1c2 (n=35) | 0 | 4 | 31 |
| Normal A1c2 (n=34) | 0 | 0 | 34 |
| d: A1c2 vs OGTT – Classification by ADA Diagnostic Categories | |||
|---|---|---|---|
| Diabetes OGTT (n=3) |
Prediabetes OGTT (n=20) |
Normal OGTT n=48) |
|
| Diabetes A1c2 (n=2) | 1 | 1 | 0 |
| Prediabetes A1c2 (n=34) | 2 | 12 | 20 |
| Normal A1c2 (n=35) | 0 | 7 | 28 |
| e: A1c1 vs FPG - Classification by ADA Diagnostic Categories | |||
|---|---|---|---|
| Normal FPG (n=18) |
Prediabetes FPG (n=1) | Diabetes FPG (n=0) |
|
| Normal A1c1 (n=7) | 7 | 0 | 0 |
| Prediabetes A1c1 (n=12) | 11 | 1 | 0 |
| Diabetes A1c1 (n=0) | 0 | 0 | 0 |
| f: A1c1 vs 2hr PG - Classification by ADA Diagnostic Categories | |||
|---|---|---|---|
| Normal 2hr PG (n=14) |
Prediabetes 2hr PG (n=5) |
Diabetes 2hr PG (n=0) |
|
| Normal A1c1 (n=7) | 6 | 1 | 0 |
| Prediabetes A1c1 (n=12) | 8 | 4 | 0 |
| Diabetes A1c1 (n=0) | 0 | 0 | 0 |
| g: A1c3 vs FPG - Classification by ADA Diagnostic Categories | |||
|---|---|---|---|
| FPG nl | FPG preDM | FPG DM | |
| A1c3 nl | 0 | 0 | 0 |
| A1c3 preDM | 13 | 1 | 0 |
| A1c3 DM | 5 | 0 | 0 |
| h: A1c3 vs 2hr PG - Classification by ADA Diagnostic Categories | |||
|---|---|---|---|
| Normal 2hr PG (n=14) |
Prediabetes 2hr PG (n=5) |
Diabetes 2hr PG (n=0) |
|
| Normal A1c3 (n=0) | 0 | 0 | 0 |
| Prediabetes A1c3 (n=14) | 11 | 3 | 0 |
| Diabetes A1c3 (n=5) | 3 | 2 | 0 |
There were no observations for FPG in the Diabetes category so Cohen’s Kappa could not be calculated. The concordance rate was 48%
Cohen’s Kappa = 0.12 (−0.08–0.31) which means there is no agreement. The Concordance rate was 52%.
There were no observations for FPG in the Diabetes category so Cohen’s Kappa could not be calculated. The concordance rate was 44%
Cohen’s Kappa = 0.20 (0.005–0.40) which means there is no agreement. The Concordance rate was 58%.
There were no observations for FPG in the Diabetes category so Cohen’s Kappa could not be calculated. The Concordance rate was 42%.
There were no observations for 2hr PG in the Diabetes category so Cohen’s Kappa could not be calculated. The Concordance rate was 53%
There were no observations for FPG in the Diabetes category so Cohen’s Kappa could not be calculated. The Concordance rate was 5%
There were no observations for 2hr PG in the Diabetes category so Cohen’s Kappa could not be calculated. The Concordance rate was 16%
Discussion
In this report, we demonstrate clinically important intra-individual A1c variability. When subjects had an A1c repeated on the same sample using two different NGSP certified methodologies, there was strong correlation between the two absolute values, but substantial discordance when the results were interpreted as categorical variables to determine diabetes classification. Even more importantly, testing performed on the same patient by the same assay methodology, but in different institutions, was systematically different and concordance in classification of diabetes status was poor. While lifestyle modifications between the two tests could explain the observed change in part, this explanation is unlikely to account for the consistency of the 0.5% lowering of A1c nor the degree of change noted within an average of only 3 weeks.
20 years ago when the DCCT and UKPDS first demonstrated a direct relationship between glycemic control and risk for diabetes complications, lack of comparability among A1c methods prevented implementation of A1c-based guidelines for diabetes screening and management. As a result of the efforts of the NGSP, standardization has greatly reduced variability among A1c methodologies leading to incorporation of A1c as an approved method for diabetes screening. However, it is important for providers utilizing A1c as a diagnostic tool to be aware of the clinically significant variability that remains, despite national standardization. Even prior to incorporation of A1c into the diagnostic guidelines, variability among DCCT aligned A1c assays was described (4–6). In 2012, allowed variability was +/−7% meaning that for a reference A1c of 6.5%, the allowed A1c for NGSP/DCCT certification ranged between 6.0% and 7.0%. These limits of acceptability are continuing to improve and in 2013 decreased to +/− 6%. However, while some degree of A1c measurement variability is acceptable for monitoring of glycemic control on a single device in a patient with known diabetes, even the current standards of allowed variability become an important problem for screening and diagnosis when strict categorical ADA cut-points are applied and the allowable certified range around a patient’s value on differing devices runs from non-diabetic to diabetic.
The problem of inconsistent categorization of diabetes classification is not unique to A1c. Poor reproducibility of fasting plasma glucose (FPG) and 2 hour plasma glucose (2hrPG) have previously been demonstrated (7–11). In a study by Libman et al, only 30% of children with impaired glucose tolerance (IGT) on an initial oral glucose tolerance test (OGTT) had IGT during a second OGTT performed within 2 weeks. The percent positive agreement between the two OGTTs in this study for impaired fasting glucose (IFG) and IGT was low at 22.2 and 27.3% respectively. Another study on the prevalence of dysglycemia found that in a population of 2501 adolescents averaging 14.3 yrs of age, 175 had IFG on an initial screen, but only 11 (6%) of these participants had IFG upon repeat testing (11).
The validity of A1c as a screening or diagnostic tool in youth has also been questioned. Several studies have found poor sensitivity of A1c for detecting diabetes when compared to FPG and 2hrPG (12–14). In the data presented here, concordance rates in diabetes categorization between the different A1c assays with FPG and 2hrPG were generated. A1c3 demonstrated the lowest concordance with FPG and 2hrPG, although overall concordance rates of A1c assays to FPG and 2hrPG were poor. However, although OGTT is considered the gold-standard for diagnosing diabetes, we must bear in mind that OGTT, FPG, and A1c cut-points have all been extrapolated from adult populations to identify the A1c at which microvascular complications (largely retinopathy) are evident. None of these criteria have been validated in youth and the implications of these cut-points for diagnosing prediabetes and diabetes in youth are unclear. Given these unknowns, the argument has been made that the A1c may be no less valid than the OGTT in children (15, 16). Regardless of the controversy over the validity of A1c as a diagnostic tool in youth, A1c has become increasingly utilized by general practitioners as a screening tool over the OGTT due to the theoretical advantages of the A1c test as an index of chronic glycemia, lack of dependence on fasting, and greater convenience of sampling (2, 3), and further studies to understand the implications of A1c and OGTT in predicting glucose abnormalities in children are necessary.
It should be noted that the ADA has approved only traditional central lab methodologies, and not POC devices, for the diagnosis of diabetes. However, the list of NGSP certified A1c devices (17) includes numerous POC assays that have been shown to demonstrate acceptable precision in relation to DCCT standards when operated and maintained by a reliable lab. Concerns arise over the precision of POC devices because they are not required to participate in ongoing proficiency testing. Additionally, POC devices with a coefficient of variation (CV) ≥3% may have clinically significant analytic errors that are not reliably detected by clinical laboratories despite routine calibration (4). With an analytical CV of 3%, for example, a measured A1c of 6% may have an uncertainty of measurement ranging from 5.6–6.4% (18) and this may lead devices to increasingly underestimate or overestimate A1c relative to NGSP references, leading to large inter-method differences over time (4, 5, 19). On the other hand, POC methods have advantages over traditional central lab techniques including a faster turnaround time, ease of operation, and ability to handle high-volume testing. With improved standardization, many central labs have abandoned traditional high-performance liquid chromatography techniques in favor of these more rapid POC assays. Thus, given the increasing adoption of POC assays by large laboratories, the methodology can no longer simply be determined based on location of test performance (back-office versus central lab) and is usually not obvious to the general practitioner ordering the A1c screening test.
There are limitations to the results presented here. The population with A1c3 was a convenience sample of obese youth with available results for comparison, rather than a pre-defined group of subjects with A1c run by different devices on the same sample. We thus limited the analysis to individuals with A1c3 values from the same hospital laboratory obtained within 1 month of study date. In addition, A1c represents average glucose levels in the preceding 3 months due to red cell lifespans of 60–120 days. However, A1c is weighted towards the most recent 3–4 weeks (20), which may have influenced the assay differences noted. Participants who were made aware of a recent abnormal A1c may also have instituted lifestyle changes that could have lowered A1c within this short time frame. Nevertheless, the A1c differences described here are consistent with results that would be expected based on the known variances of these devices.
Additionally, despite the small sample size, the statistically significant discrepancy reported here shows that further studies are needed to understand the implications of A1c.
In summary, there is persistent variability in current A1c methodologies that can lead to potential clinical pitfalls related to interpretation of a continuous variable in a categorical manner. Since a diagnosis of diabetes is dependent on A1c results that change category with a difference of 0.1%, the noted variability means that individuals with A1cs in the upper normal to mildly elevated range will be inconsistently categorized when testing is performed on different devices. Discrepant categorization leads to inconsistent diagnoses, which may confuse providers and families, lead to under- or over-treatment, and result in patient and parental anxiety and psychological stress. There are also implications of assay variability for the recruitment of patients into pediatric diabetes clinical trials, where A1c inclusion and exclusion criteria affect selection of potential participants to be screened. Finally, the intraassay variation identified here also raises caveats regarding the use of A1c, not only in screening, but for diabetes management, where caution should be exercised when comparing A1c ‘trends’ between different assays or devices.
We face an epidemic of obesity with rising rates of type 2 diabetes in youth. However, given the low prevalence of type 2 diabetes, even among high risk youth, reliable, efficient and cost-effective approaches to screening adolescents and detecting those at highest risk for progression to disease are needed. Measurement of A1c offers a number of potential advantages as a screening approach. However, variation in methodologies leads to inconsistent classification of and possible under- or over-treatment depending on the assay used or institution at which the assay is run. This variability in A1c results underscores the need for repeated measurements over time, consideration of a combination of screening methods such as A1c and FPG to reduce the bias inherent in a single test, and the employment of clinical judgment to determine the appropriate course of management when screening each patient. Accordingly, the ADA recommendations require two repeated measures of A1c or plasma glucose meeting diabetes criteria or signs and symptoms of diabetes prior to making a diagnosis of diabetes. Until we have a clearer understanding of the A1c cut points and their implications in pediatrics, and until the precision of A1c assays is improved, A1c values obtained for screening purposes in obese youth should be interpreted with caution.
Acknowledgments
Christine L. Chan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This study was supported by grants from the Colorado Clinical Translational Sciences Institute (CCTSI) NIH/NCATS Colorado CTSI, UL1 TR000154 and The Genentech Center for Clinical Research in Endocrinology, 1210-F05.
Abbreviations
- A1c
Hemoglobin A1c
- NGSP
National Glycohemoglobin Standardization Program
- DCCT
Diabetes Control and Complications Trial
- ADA
American Diabetes Association
- HPLC
High performance liquid chromatography
- POC
Point-of-care
- OGTT
Oral glucose tolerance test
- FPG
Fasting plasma glucose
- IFG
Impaired fasting glucose
- IGT
Impaired glucose tolerance
Footnotes
The authors have no relevant disclosures or conflicts of interest.
Contributor Information
Christine L. Chan, Children’s Hospital Colorado and University of Colorado Denver, 13123 E. 16th Avenue, B265, Aurora, CO, USA, 80045, Phone: 720-777-6128, Fax: 720-777-7301, Christinel.chan@childrenscolorado.org.
Kim McFann, University of Colorado Denver, 13001 E. 17th Place, B119, Aurora, CO, USA, 80045, Kim.McFann@ucdenver.edu.
Lindsey Newnes, Children’s Hospital Colorado, 13123 E. 16th Avenue, B265, Aurora, CO, USA, 80045, Lindsey.newnes@childrenscolorado.org.
Kristen J. Nadeau, Children’s Hospital Colorado and University of Colorado Denver, 13123 E. 16th Avenue, B265, Aurora, CO, USA, 80045, Kristen.nadeau@childrenscolorado.org.
Philip S. Zeitler, Children’s Hospital Colorado and University of Colorado Denver, 13123 E. 16th Avenue, B265, Aurora, CO, USA, 80045, Philip.zeitler@childrenscolorado.org.
Megan Kelsey, Children’s Hospital Colorado and University of Colorado Denver, 13123 E. 16th Avenue, B265, Aurora, CO, USA, 80045, Megan.Kelsey@childrenscolorado.org.
References
- 1.International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Love-Osborne KA, Sheeder J, Svircev A, Chan C, Zeitler P, Nadeau KJ. Use of glycosylated hemoglobin increases diabetes screening for at-risk adolescents in primary care settings. Pediatr Diabetes. 2013 doi: 10.1111/pedi.12037. [DOI] [PubMed] [Google Scholar]
- 3.Lee JM, Eason A, Nelson C, Kazzi NG, Cowan AE, Tarini BA. Screening Practices for Identifying Type 2 Diabetes in Adolescents. J Adolesc Health. 2013 doi: 10.1016/j.jadohealth.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes EW, Ersahin C, Augustine GJ, Charnogursky GA, Gryzbac M, Murrell JV, et al. Analytic bias among certified methods for the measurement of hemoglobin A1c: a cause for concern? Am J Clin Pathol. 2008;129(4):540–547. doi: 10.1309/U3GPPTCBP1VLL8AW. [DOI] [PubMed] [Google Scholar]
- 5.Petersen JR, Omoruyi FO, Mohammad AA, Shea TJ, Okorodudu AO, Ju H. Hemoglobin A1c: assessment of three POC analyzers relative to a central laboratory method. Clin Chim Acta. 2010;411(23–24):2062–2066. doi: 10.1016/j.cca.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Alcala H, Ruiz-Arguelles A, Cedillo-Carvallo B. Effect of the method to measure levels of glycated hemoglobin on individual clinical decisions: comparison of an immunoassay with high-performance liquid chromatography. Am J Clin Pathol. 2009;132(3):332–335. doi: 10.1309/AJCPIWRO1ST6HCIY. [DOI] [PubMed] [Google Scholar]
- 7.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93(11):4231–4237. doi: 10.1210/jc.2008-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roman R, Zeitler PS. Oral glucose tolerance testing in asymptomatic obese children: more questions than answers. J Clin Endocrinol Metab. 2008;93(11):4228–4230. doi: 10.1210/jc.2008-1993. [DOI] [PubMed] [Google Scholar]
- 9.Kleber M, Lass N, Papcke S, Wabitsch M, Reinehr T. One-year follow-up of untreated obese white children and adolescents with impaired glucose tolerance: high conversion rate to normal glucose tolerance. Diabet Med. 2010;27(5):516–521. doi: 10.1111/j.1464-5491.2010.02991.x. [DOI] [PubMed] [Google Scholar]
- 10.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167(14):1545–1551. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- 11.Dolan LM, Bean J, D'Alessio D, Cohen RM, Morrison JA, Goodman E, et al. Frequency of abnormal carbohydrate metabolism and diabetes in a population-based screening of adolescents. J Pediatr. 2005;146(6):751–758. doi: 10.1016/j.jpeds.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 12.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr. 2011;158(6):947–952. e1–e3. doi: 10.1016/j.jpeds.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care. 2011;34(6):1306–1311. doi: 10.2337/dc10-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JM, Gebremariam A, Wu EL, LaRose J, Gurney JG. Evaluation of nonfasting tests to screen for childhood and adolescent dysglycemia. Diabetes Care. 2011;34(12):2597–2602. doi: 10.2337/dc11-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapadia CR. Are the ADA hemoglobin A(1c) criteria relevant for the diagnosis of type 2 diabetes in youth? Curr Diab Rep. 2013;13(1):51–55. doi: 10.1007/s11892-012-0343-y. [DOI] [PubMed] [Google Scholar]
- 16.Chan CLMK, Nadeau K, Newnes L, Zeitler P, Kelsey M. The Relationship between A1c, 2hr Plasma Glucose, and Continuous Glucose Monitoring-Determined Glycemic Patterns in Obese Adolescents. Abstract and Poster presented at 2013 American Diabetes Association 73rd Scientific Sessions; Chicago, IL. 2013. [Google Scholar]
- 17.(NGSP) NGSP. College of American Pathologists (CAP) Survey Data 2012 [March 18, 2013] Available from: http://www.ngsp.org/CAP/CAP12b.pdf. [Google Scholar]
- 18.White GH, Farrance I. Uncertainty of measurement in quantitative medical testing: a laboratory implementation guide. Clin Biochem Rev. 2004;25(4):S1–S24. [PMC free article] [PubMed] [Google Scholar]
- 19.Lippi G, Targher G. Haemoglobin A1c and diagnosis of diabetes. Not ready for the prime time? Ann Clin Biochem. 2012;49(Pt 5):508. doi: 10.1258/acb.2012.012026. [DOI] [PubMed] [Google Scholar]
- 20.Sidorenkov G, Haaijer-Ruskamp FM, de Zeeuw D, Denig P. A longitudinal study examining adherence to guidelines in diabetes care according to different definitions of adequacy and timeliness. PloS one. 2011;6(9):e24278. doi: 10.1371/journal.pone.0024278. [DOI] [PMC free article] [PubMed] [Google Scholar]