Abstract
Biomaterials are essential to modern medicine as components of reconstructive implants, implantable sensors, and vehicles for localized drug delivery. Advances in biomaterials have led to progression from simply making implants that are nontoxic to making implants that are specifically designed to elicit particular functions within the host. The interaction of implants and the extracellular matrix during the foreign body response is a growing area of concern for the field of biomaterials, because it can lead to implant failure. Expression of matricellular proteins is modulated during the foreign body response and these proteins interact with biomaterials. The design of biomaterials to specifically alter the levels of matricellular proteins surrounding implants provides a new avenue for the design and fabrication of biomimetic biomaterials.
Keywords: Matricellular, extracellular matrix, biomaterials, foreign body response, biocompatible, decellularization
1. Introduction
The study of biomaterials began in the early nineteenth century when H.S. Levert first implanted a variety of materials into dogs to analyze the in vivo reaction and found metals to cause the least irritation (Levert, 1829). Of course, the study of biomaterials has advanced significantly since then leading to the creation of three major classes of modern biomaterials: bioinerts, biodegradables, and bioactive or biomimetic materials (Bryers et al., 2012; Cao and Hench, 1996; Hench, 1998; Shin et al., 2003). This review will discuss the role of the matricellular proteins in tissue-biomaterial interactions with a focus on the design of a new generation of biomimetic materials from matricellular proteins and their functional domains.
2. Biomaterials
Implantable materials have been useful for years as a way to create devices, replace tissues, deliver drugs, etc. A major goal of the field of biomaterials is to create bioinert materials - materials that are nontoxic and remain functional after implantation (Cao and Hench, 1996; Hench, 1998; Heness and Ben-Nissan, 2004). For example, many metals (steel, titanium, and cobalt- chromium alloys), ceramics (zirconia and alumina), silicone, and polyester are often considered bioinert because they are nontoxic and exhibit little tissue integration with the material (Cao and Hench, 1996; Hench, 1998; Heness and Ben-Nissan, 2004). However, the term bioinert is a misnomer because even these materials elicit a foreign body response (FBR) (Cao and Hench, 1996; Geetha et al., 2009; Heness and Ben-Nissan, 2004; Ratner, 2002).
Nearly all materials regardless of composition elicit a FBR, which is a unique inflammatory response and initiates with the rapid adsorption of proteins in random orientations and configurations (Figure 1) (Anderson et al., 2008; Ratner and Bryant, 2004; Ratner, 2002). Following protein adsorption, cells interact with the proteinaceous layer on the surface of the material leading to adhesion and activation (Anderson et al., 2008; Ratner and Bryant, 2004; Ratner, 2002). At the cellular level, the initial phase of the response is dominated by neutrophils and macrophages, similar to acute inflammation. After several days, macrophages undergo cell-cell fusion to form foreign body giant cells (FGBCs) (Anderson et al., 2008; Ratner and Bryant, 2004; Ratner, 2002; Xia and Triffitt, 2006). In addition to attacking the biomaterial surface, FBGCs and macrophages secrete factors that promote fibroblast migration and deposition of ECM, which leads to encapsulation of the implant by a largely avascular, fibrotic tissue. Consisting primarily of collagen, the collagenous capsule forms within 4 weeks and isolates the implant from the surrounding tissue (Anderson et al., 2008; Ratner and Bryant, 2004; Ratner, 2002). It is important to consider the unique alignment of collagen fibers in an orientation parallel to the implant surface and the striking paucity of blood vessels within the capsule. These differences distinguish the FBR from normal wound healing. In the latter, collagen organization is loose and there is an abundance of blood vessels. In some applications, such as implantable glucose sensors, the FBR often leads to device failure due to isolation of the sensing unit from the surrounding tissue and blood vessels. Therefore, tissue remodeling and blood vessel inhibition in the FBR has become a significant area of interest.
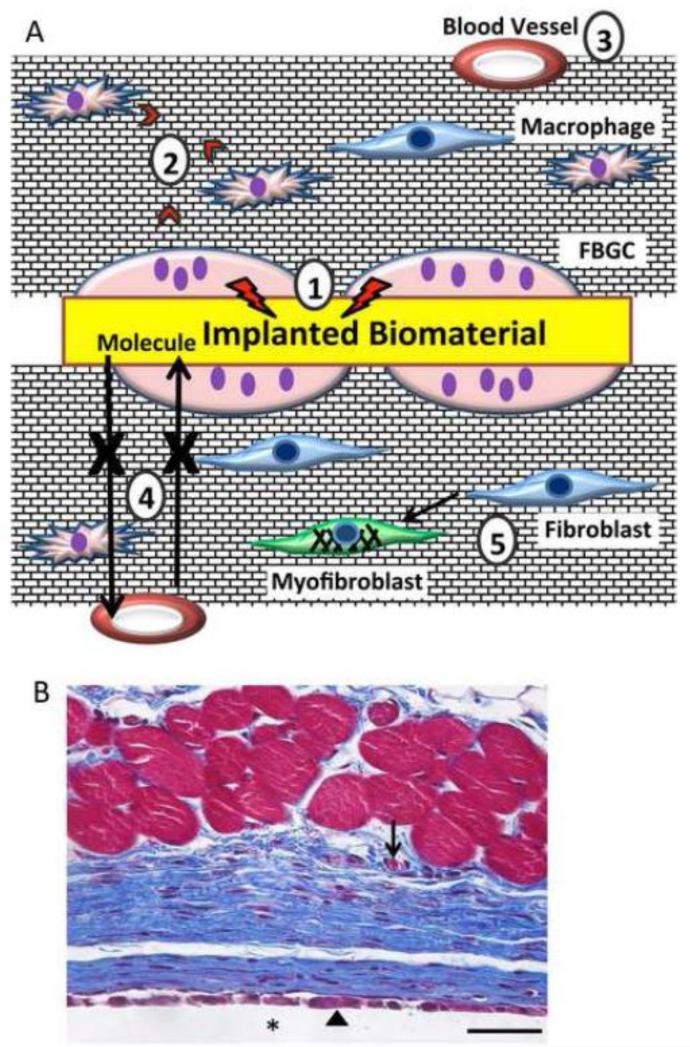
Figure 1.
Overview of the foreign body response. A. Implantation of biomaterial into soft tissues elicits a unique inflammatory response leading to encapsulation by a largely avascular capsule consisting of dense collagenous matrix. A number of complications are encountered including: 1) FBGC form on the implant surface and can damage the implant; 2) FBGC and macrophages secrete pro-fibrotic factors; 3) blood vessels are generally excluded from the capsule; 4) the lack of vessels and the dense collagen arrangement limit diffusion of small molecules; and 5) fibroblasts can differentiate into myofibroblasts and contract the capsule. B. Representative image of the foreign body response to PDMS disk implanted subcutaneous (SC) in a mouse for 4 wk. Sections were stained with Masson's trichrome to visualize collagen deposition (blue color) in between the implant (*) and muscle fibers (red). Arrowhead and arrow indicate FBGC and blood vessel, respectively. Scale bar = 50 μm.
Biomimetic materials, or materials that seek to mimic the biology of the ECM to promote healing and integration into host tissues have garnered tremendous attention in recent years (Bryers et al., 2012; Causa et al., 2007; Ratner, 2001; Roach et al., 2007; Shin et al., 2003). Specifically, they are designed to actively influence protein adsorption (the first step of the FBR) and tissue interactions by controlling parameters such as material structure (on a micro/nano level), porosity, drug loading, and surface chemistry (Brodbeck et al., 2002; Bryers et al., 2012; Healy et al., 1996; Lan et al., 2005; Puleo and Nanci, 1999; Ratner, 2002, 2001; Roach et al., 2007; Shin et al., 2003). Commonly, biomimetic materials modify functional groups on the surface of a material or coat the material with ECM molecules (Brodbeck et al., 2002; Chen et al., 2013; Esch et al., 2011; Healy et al., 1996; Lan et al., 2005; Puleo and Nanci, 1999; Roach et al., 2007; Shin et al., 2003). Another thrust of engineering biomimetic materials is to create topographies that either elicit specific biological responses (such as microchannels) or mimic the structure of the ECM (Boudriot et al., 2006; Esch et al., 2011; Roach et al., 2007; Stevens and George, 2005).
Decellularized ECM represents a new class of biomimetic materials that has garnered significant attention in recent years. Tissues have been decellularized in a variety of ways including: chemical methods, enzymatic, physical, and more recently-induction of apoptosis (Bourget et al., 2012; Bourgine et al., 2013; Crapo et al., 2011; Gilbert et al., 2006; Song and Ott, 2011). The idea of creating decellularized ECM is to take tissue and remove all of its cellular and immunogenic components while retaining tissue architecture as well as (potentially) growth factors and cytokines that may be incorporated into the matrix. Many tissues throughout the body have been decellularized including blood vessels, lungs, liver, heart, skin, etc. (Bourget et al., 2012; Gilbert et al., 2006; Petersen et al., 2010; Reing et al., 2010; Song and Ott, 2011). These scaffolds are very attractive to the field of tissue engineering because they allow the retention of tissue architecture while eliminating immunogenic components and possibly minimizing the FBR.
Recently, the Badylak group has drawn attention to the bioinductive qualities of decellularized matrices and demonstrated that as they degrade, they release peptides from matricellular proteins (for example, peptides from thrombospondin (TSP) -1) that have a range of effects on the host tissue (Badylak, 2007). Additionally, recent work by the White group on decellularized human lung has probed the question of what ECM components remain after decellularization and found a variety of matricellular proteins including: periostin, tenascin-X, and tenascin-C (Booth et al., 2012). Therefore, it is important to consider the possibility that matricellular proteins that remain in the ECM could modulate cell functions and response to these materials (Figure 2). Consistent with this suggestion, we have shown that TSP2-null-derived decellularized ECM caused changes in cellular phenotype that were not rescued with administration of exogenous TSP-2, suggesting that changes in ECM architecture can influence cell function (Krady et al., 2008).
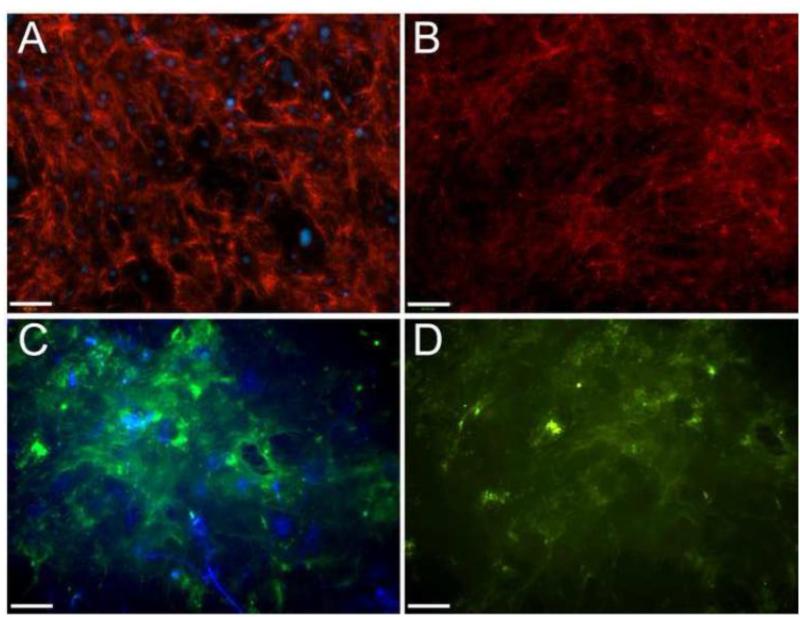
Figure 2.
ECM retention following decellularization. Dermal fibroblasts were cultured for 10 d in the presence of ascorbic acid to induce ECM production. Cultures were analyzed by immunohistochemistry for the deposition of fibronectin (red color in A, B) and TSP2 ( green color in C, D) prior (A, C) or following decellularization (B, D). Nuclei were counterstained with DAPI (blue). Retention of both proteins was observed following decellularization. Scale bar = 100 μm.
Clearly, there has been significant effort to modify materials such that they will elicit a minimal FBR; however, material based strategies must be developed independently for different materials and applications. Another method to limit the foreign body response, altering the biological response, could influence the FBR to most materials. Coating materials with matricellular proteins or specific DNA molecules that modulate the levels of matricellular proteins has shown promise as a method for biologically manipulating the host environment leading to reduced FBR (Bryers et al., 2012; Kyriakides et al., 2001; Ratner, 2002, 2001; Shin et al., 2003).
3. Matricellular Proteins
Matricellular proteins are a class of ECM proteins that primarily serve non-structural roles and regulate cell function by influencing cell adhesion, migration, proliferation, differentiation, and apoptosis (Bornstein and Sage, 2002; Bornstein, 2009; Bornstein et al., 2004; Murphy-Ullrich, 2001). Typically matricellular proteins are expressed at low levels in adult tissues, but are highly expressed in development and following injury or pathology (Alford and Hankenson, 2006; Bornstein and Sage, 2002; Kyriakides and Bornstein, 2003; Kyriakides and Maclauchlan, 2009). These proteins have both soluble and insoluble states, which allow them to differentially influence cells (Murphy-Ullrich, 2001). Additionally, matricellular proteins can interact with growth factors, for example TSP-1 binds to and activates latent TGF-β1 (Murphy-Ullrich et al., 1992; Schultz-Cherry and Murphy-Ullrich, 1993; Schultz and Wysocki, 2009).
Matricellular proteins are grouped together because of similar functions such as: promoting an intermediate cell adhesive state (which may encourage cell migration), interactions with growth factors, facilitating matrix-cell interactions (including binding to both ECM and cells), and bridging inorganic matter and the ECM (Bornstein and Sage, 2002; Murphy-Ullrich, 2001). Interestingly, most mice with genetic deletion of one or more matricellular proteins display a mild phenotype. In addition, they are composed of several different structural domains. These common structural domains include: EGF-like repeats that are common in the TSPs, SPARC, and the tenascins; the calcium binding domains common to TSPs, SPARC, and osteopontin; and the TSP type 1 repeats and von willebrand factor type C repeats common to the TSPs and CCNs (Adams and Lawler, 2004; Brekken and Sage, 2001; Frangogiannis, 2012; Giachelli and Steitz, 2000; Hsia and Schwarzbauer, 2005). (Table 1) Despite these common motifs, the structure of matricellular proteins is generally quite varied and it is instead their similar functions that unify them as a group.
Table 1.
Structural motifs in matricellular proteins.
| Matricellular Proteins | Common Protein Domains | ||||
|---|---|---|---|---|---|
| Ca2+ binding | EGF-like | RGD/Integrin Binding Domains | TSP type I repeats | VWF type C | |
| Thrombospondin-1/2 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Osteopontin | ✓ | ✓ | |||
| Tenascin-C | ✓ | ✓ | |||
| SPARC | ✓ | ✓ | ✓ | ||
| CCNs | ✓ | ✓ | ✓ | ||
| Periostin | ✓ | ||||
Matricellular proteins have been shown to play significant roles in the FBR and genetically modified mice have been particularly useful in elucidating their roles in this process. (Table 2) (Barker et al., 2005; Bornstein et al., 2004; Kyriakides and Bornstein, 2003; Kyriakides and Maclauchlan, 2009; Kyriakides et al., 1999; Puolakkainen et al., 2003, 2005). Specifically, mice that lack matricellular proteins display decreased capsule thickness or increased vascularity, both interesting phenotypes from the perspective of designing materials to minimize the FBR (Barker et al., 2005; Bornstein et al., 2004; Kyriakides and Bornstein, 2003; Kyriakides and Maclauchlan, 2009; Kyriakides et al., 1999; Puolakkainen et al., 2003, 2005). Moreover, reduction of TSP-1 or TSP-2 led to altered structural properties of the collagenous capsule as well as increased vascularity within the capsule (Kyriakides and Bornstein, 2003; Kyriakides and Maclauchlan, 2009; Kyriakides et al., 2001; Kyriakides et al., 1999; Reinecke et al., 2013). Interestingly, mice that lack SPARC display a decrease in capsule thickness with decreased vascularity (Barker et al., 2005; Kyriakides and Bornstein, 2003; Puolakkainen et al., 2003). Finally, osteopontin KO mice display an exaggerated FBR associated with increased numbers of FBGCs surrounding implants (Tsai et al., 2005).
Table 2.
Matricellular Proteins and the Foreign Body Response.
| Protein | Knockout Mouse FBR Phenotype | In Vivo Molecular Strategies | References |
|---|---|---|---|
| TSP-1 | Increased capsule vascularity. Irregular collagen fibrils. | Coated implants gene activated matrix expressing the N-terminal domain of TSP-1 resulted in increased capsular density | (Kyriakides and Bornstein, 2003; Sweetwyne et al., 2010) |
| TSP-2 | Greatly increased capsule vascularity Irregular collagen fibrils |
Coated implants in antisense gene-activated matrix to increase capsule vascularity | (Kyriakides et al., 2001; Kyriakides et al., 1999) |
| SPARC | Decreased capsule thickness and vascularity Small collagen fibrils |
(Puolakkainen et al., 2003) | |
| OPN | Increased FBGC formation | Coating implants in oriented OPN led to reduced capsule thickness OPN-gelatin mixtures promote repair of cavities and skull defects Coating titanium implants in OPN to increase osseointegration |
(Liu et al., 2008; Tsai et al., 2005; Xu et al., 2007; Goldberg et al., 2001; O'Toole et al., 2004) |
| Periostin | Periostin loaded gelfoam delivered after myocardial infarction reduced scar formation and improved cardiac function in rats | (Kühn et al., 2007) | |
| CCN1 | Coating decellularized vascular grafts in CCN1 promotes endothelialization and graft patency | (Bär et al., 2010; Spengler et al., 2013) |
Because of the importance of matricellular proteins in the biological response to implanted materials, a review of the major matricellular proteins follows. Each section will introduce some of the major functions of the protein and discuss its use in bioinspired materials design/synthesis.
3.1 Thrombospondins
The TSPs are a family of ECM glycoproteins consisting of five members, TSP 1-5. Only TSP-1 and -2 are considered for this review because of their interactions with biomaterials and their use in the design of biomimetic materials. For a comprehensive review of the entire thrombospondin family see Adams and Lawler (Adams and Lawler, 2004).
3.1.1 Thrombospondin-1
TSP-1 is a trimeric 420 kDa protein that consists of seven functional domains: N and C terminus globular domains, a coiled coil domain, a von Willebrand factor type c domain, type 1 repeats, type 2 repeats (EGF-like), and type 3 repeats (calcium binding) (Adams and Lawler, 2004; Lawler and Hynes, 1986). TSP-1 is a major component of platelets and can be readily isolated from human blood (Lawler, 2000). TSP-1 binds a variety of cell receptors including CD36, various integrins, CD47 (integrin associated protein), and LDL receptor related proteins as well as proteoglycans, and calcium (Adams and Lawler, 2004; Bornstein, 2009; Chen et al., 2000; Lawler, 2000). With its wide array of binding partners it is no surprise that TSP-1 is implicated in a variety of physiological functions including wound healing, angiogenesis, platelet aggregation, cell proliferation and migration, and influencing cellular interactions with growth factors (Adams and Lawler, 2004; Bornstein et al., 2004; Chen et al., 2000; Kyriakides and Maclauchlan, 2009; Lawler, 2000). TSP-1 null mice have been instrumental in assessing the functions of TSP-1 in vivo. For example, it was confirmed that TSP-1 activates latent TGF-β1 in vivo by combining studies of TSP-1 null mice and TGF- β1 null mice and that TSP-1 inhibits angiogenesis by studying cancer neovascularization (Chen et al., 2000; Crawford et al., 1998). TSP-1 null mice show slowed wound healing associated with reduced inflammation in the wounded area (Agah et al., 2002; Bornstein and Sage, 2002; Kyriakides and Bornstein, 2003).
Modulation of thrombospondin-1 expression and/or function by implanted biomaterials is an area of growing interest. To aid understanding of regions of the TSP-1, implants were coated with a gene activated matrix (GAM), which consisted of collagen gel laden with plasmid DNA expressing a GFP tagged N-terminal domain (NTD) of TSP-1. It was shown that this domain, which binds calreticulin and triggers focal adhesion disassembly, causes increased capsular density and promotes collagen deposition in vitro (Sweetwyne et al., 2010). It has been shown that upon degradation, implanted decellularized matrix can release peptides that contain the type-1 repeats of TSP-1, known to inhibit angiogenesis (Armstrong and Bornstein, 2003; Badylak, 2007). It is possible that this property could be useful in limiting undesired angiogenesis, however, it is likely that this consequence of ECM implantation is frequently overlooked. A similar discovery was that Choukroun's platelet-rich fibrin (PRF), a biomaterial that is isolated from blood and composed of leukocytes, platelets, and fibrin releases growth factors as well as large quantities of TSP-1 over a week-long period (even more TSP-1 than is initially in the material because the platelets continue to produce it) (Dohan Ehrenfest et al., 2009). While the growth factors from this material likely promote healing, the massive release of TSP-1 could inhibit angiogenesis and may confound results. Alginate nanoparticles have been created for sustained delivery of TSP-1 and TSP-1 peptides to inhibit tumor angiogenesis (Hartig et al., 2007). Saka and Bozkir created chitosan and Poly(ethylene imine) (PEI) hybrid polymeric nanoparticles coated in polyethylene glycol (PEG) as a gene delivery system to upregulate TSP-1 (Saka and Bozkir, 2012). The goal of the study was to use develop a method of using gene delivery to inhibit angiogenesis. The interactions of TSP-1 with TGF-β have been exploited by incubating the type 1 repeats of TSP-1 with TGF-β to protect the growth factor during encapsulation into a PEG hydrogel (McCall et al., 2011). Additionally, TSP-1 expression in vascular smooth muscle cells leading to fibroproliferative activation is stimulated by stainless steel ions in vitro (Pallero et al., 2010). Expression of TSP-1 in histology from resected stainless steel stents identifies the ions as a possible contributor to in-stent restenosis.
3.1.2 Thrombospondin-2
TSP-2 is similar to TSP-1 in that it is an extracellular trimeric glycoprotein with an analogous structure, but has unique functional characteristics (Adams and Lawler, 2004). TSP-2 has been shown to be important in collagen fibrillogenesis and matrix assembly, as TSP-2 null mice exhibit altered fibril size and morphology (Bornstein, 2009; Bornstein et al., 2000; Calabro et al., 2014; Kyriakides and Maclauchlan, 2009; Kyriakides et al., 1998). Abnormalities in collagen fibrils are due, in part, to increased MMP-2 and MMP-9 levels (Krady et al., 2008; Yang et al., 2001). TSP-2 null mice also show increased rates of wound healing and angiogenesis (Bornstein et al., 2000; Kyriakides and Bornstein, 2003; Kyriakides et al., 1999). Consistent with these findings, TSP-2 has been shown to decrease endothelial cell (EC) migration by limiting MMP activity and is known to be an inhibitor of angiogenesis (Armstrong and Bornstein, 2003; Bornstein and Sage, 2002; Bornstein et al., 2000; Streit et al., 1999). Another interesting phenotype of TSP-2 null mice, particularly for the subject of this review, is that they display a decreased foreign body response with altered encapsulation and increased vascularization in response to the implantation of biomaterials as well as cell grafts (Bornstein et al., 2004; Bryers et al., 2012; Kyriakides and Bornstein, 2003; Kyriakides and Maclauchlan, 2009; Kyriakides et al., 1999; Reinecke et al., 2013).
In an early study to modulate TSP-2 in the context of the FBR, implants were coated with a gene activated matrix (GAM) consisting of a collagen gel laden with plasmid DNA expressing antisense TSP-2 (Kyriakides et al., 2001). When these GAM-coated materials were implanted in mice, TSP-2 expression (near the implant) was inhibited for 4 weeks and there was a decrease in capsule thickness and increase in vascularity of surrounding tissue. Additionally, when TSP-2 sense DNA coated materials were implanted in TSP-2 null animals, the FBR phenotype was reversed (Kyriakides et al., 2001). A similar approach was used in a wound model to inject TSP-2 sense and antisense DNA in a pH sensitive polymer (Kyriakides et al., 2002). This study found efficient transfection of the cells in the wound bed and significant changes in neovascularization and altered matrix deposition in WT mice that received antisense TSP-2 (Kyriakides et al., 2002). Gene delivery to modulate TSP-2 expression in vivo holds promise for limiting the foreign body response to improve the lifetime of implants and altering wound healing to encourage desirable phenotypes.
Increasing TSP-2 expression has also been considered as a potential therapeutic approach. For example, a method of sustained delivery of TSP-2 in vivo is the implantation of cells that overexpress TSP-2. A study in which fibroblasts were made to overexpress the protein were seeded on biodegradable scaffolds and implanted in the peritoneal cavity of nude mice showed that the cells maintained high levels of expression and increased circulating levels of TSP-2 (Streit et al., 2002). These implants inhibited tumor growth and angiogenesis of several model tumors and showed twice the rate of apoptotic tumor cells as controls. TSP-2 overexpressing cell grafts hold promise as a cancer treatment because anti-angiogenic molecules are more effective when continuously perfused, but can be prohibitively expensive (Streit et al., 2002). Other groups are considering similar cellular generated TSP-2 therapies for endometriosis (Shubina et al., 2013).
3.2 Secreted Protein, Acidic and Rich in Cysteine
Secreted Protein, Acidic and Rich in Cysteine (SPARC), also known as osteonectin, is a 32 kDa glycoprotein consisting of an acidic domain, follistatin-like domain, and calcium binding domain (Brekken and Sage, 2001). SPARC has been shown to contribute to a state of intermediate cell adhesion, induce osteoblast differentiation and survival, and influence the assembly of collagen I (Alford and Hankenson, 2006; Bornstein and Sage, 2002; Bradshaw, 2009; Murphy-Ullrich, 2001; Puolakkainen et al., 2003). SPARC interacts with and regulates the activity of several growth factors including PGDF, VEGF, and FGF-2 making SPARC an important factor in wound healing, angiogenesis, and fibrotic diseases (Brekken and Sage, 2001). Additionally, SPARC may influence the activity of TGF-β (Bradshaw, 2009). SPARC plays a role in intracellular signaling by binding integrin-linked kinase and promoting the formation of actin stress fibers as well as cell mediated assembly of fibronectin in the ECM (Barker et al., 2005a). SPARC is expressed during development and response to injury in a variety of tissues as well as in the pericellular matrix in adult bone tissue (Alford and Hankenson, 2006; Puolakkainen et al., 2003). SPARC null mice display smaller uniform collagen fibrils as well as less overall collagen content in the skin, heart , and adipose tissue (approximately half) (Bradshaw, 2009; Bradshaw et al., 2003). SPARC null mice display accelerated wound healing (for small wounds), early formation of cataracts, and increased heart failure after myocardial infarction (Bornstein and Sage, 2002; Kyriakides and Bornstein, 2003; Okamoto and Imanaka-Yoshida, 2012). Another important phenotype of SPARC null mice is that they show a decreased foreign body response with a thin capsule, but interestingly also a decrease in functional vessels (Puolakkainen et al., 2003). The decreased FBR is consistent with the decreased overall collagen content in these mice. SPARC is clearly important during wound healing and the FBR and studies have shown that the extracellular levels of SPARC may be regulated by its binding to the receptor, stabilin-1 on macrophages and being targeted for degradation (Kzhyshkowska et al., 2006). This pathway suggests a possible mechanism for which macrophages can control tissue remodeling and angiogenesis by manipulating matricellular proteins in the extracellular matrix.
Another matricellular protein, hevin, is homologous to SPARC with a longer acidic region at the amino terminus and may compensate for some of the functions of SPARC in SPARC null animals (Alford and Hankenson, 2006; Brekken and Sage, 2001). Hevin likely induces a state of intermediate cell adhesion, similarly to many matricellular proteins, and plays a role in cancer metastasis (Sullivan and Sage, 2004). In the FBR it was found that hevin tends to regulate inflammatory processes, playing an anti-inflammatory role, whereas SPARC regulates capsule formation. Additionally SPARC-hevin double null animals displayed capsules with dramatically increased vascularity, suggesting that the proteins are likely both involved in inhibiting angiogenesis and implicating both as possible therapeutic targets (Barker et al., 2005b).
Peptide sequences from SPARC have led to bioinspired material designs mainly for orthopedic implant purposes. When the glutamic acid peptide sequence from the C terminus of SPARC was functionalized with an acrylate group, it bound a hydrogel and the peptide sequence bound hydroxyapatite. This led to ionic bonds between nanoscale hydroxyapatite crystals and the hydrogel (instead of the typical dipole interactions) and significantly increased the shear modulus of the gel, creating a “bone-mimetic nanocomposite” (Sarvestani et al., 2008). Another group took a similar approach, using collagen-SPARC composites to cause nanocrystalline hydroxyapatite to bind collagen fibrils and orient along the axis of the fibril, mimicking in vivo structure (Liao et al., 2009). In vivo SPARC is cleaved by a variety of proteases leading to the inspiration for incorporating SPARC peptides into PEG hydrogels as sites for proteolysis by plasmin, MMP-1, and MMP-2 (Patterson and Hubbell, 2011). In this study, the investigators showed that incorporation of cleavable linkers allowed for faster degradation and cell proliferation as well as increased cell spreading. Even though these effects were not due to released SPARC peptides, the approach highlights the utility of incorporating features of proteins like SPARC to tune the degradability of engineered scaffolds. An additional consideration for future investigation is that SPARC-TSP-2-double-null mice exhibit reduced FBR and improved wound healing (when compared to either single KO or WT mice) (Puolakkainen et al., 2005). Therefore, it could be beneficial to manipulate the levels of both proteins in wound healing and the FBR.
3.3 Osteopontin
Osteopontin (OPN), also called bone sialoprotein 1 (BSP-1), is an acidic ECM protein that has been shown to have a role in bone mineralization, wound healing, angiogenesis, cell adhesion, differentiation, tumor metastasis, and the foreign body response (Giachelli and Steitz, 2000; Wai and Kuo, 2008). Osteopontin has a variety of binding sites including: heparin, collagen, fibronectin, various integrins, hydroxyapatite crystals, and calcium ions (Giachelli and Steitz, 2000). Osteopontin expression is increased in several pathologies including fibrosis, atherosclerosis, tumor metastasis, etc. (Frangogiannis, 2012; Giachelli and Steitz, 2000; Wai and Kuo, 2008). In healthy human tissue, osteopontin is expressed in the kidney, epithelial tissues, and is highly expressed in bone (Giachelli and Steitz, 2000). In fact, osteopontin is one of the main proteins in decellularized/demineralized bone matrix and is one of the reasons the matrix retains an osteoinductive capacity (Decup et al., 2000). Interestingly, the presence of OPN leads to decreased FBGC formation and therefore a diminished FBR (Tsai et al., 2005). OPN's role in wound healing/fibrosis, osteoblast adhesion, and bone mineralization has led to an increased interest in using it in combination with biomaterials to limit the FBR and/or promote bone regeneration.
Dental implants have provided an avenue for exploration of matricellular molecules incorporated into biomaterials since 2000 (Decup et al., 2000; Ratner, 2001). Decup et al combined OPN with gelatin and implanted the mixture into the exposed pulp in cavities that they had drilled into the teeth (Decup et al., 2000). Subsequently, they filled the remainder of the cavity with liquid cement. The results of this study showed that repair was first slower with the OPN – gelatin material, but healing was improved at later stages. Later, the same group replicated these results and also found that the osteopontin-gelatin mixture outperformed other osseoinductive proteins and that there was little inflammatory reaction to the OPN-gelatin (Goldberg et al., 2001). Overall OPN laden gelatin (under a protective layer of cement) has proven to induce more complete and ideal healing of cavities than standard dental treatments (Decup et al., 2000; Goldberg et al., 2009, 2008, 2001; Ratner, 2001). Wang et al showed OPN-gelatin caused increased regeneration when implanted in defects of the skull, but demonstrated that OPN in a collagen carrier performed much better (Xu et al., 2007).They showed that gelatin allowed for much faster release of the OPN, but that collagen-OPN blends healed significantly faster than gelatin-OPN or collagen or gelatin alone.
Another combination of OPN and biomaterials of interest is coating titanium femoral implants with osteopontin. Orthopedic implants wear and corrode over time, which can cause the implant to loosen and even fail. Coatings such as hydroxyapatite and RGD peptides cause the bone to integrate better with surrounding tissue. Because OPN is osteoinductive and contains RGD sequences, it was hypothesized that it could outperform other coatings (O'Toole et al., 2004). Titanium wires coated with an osteopontin-gelatin solution implanted into rat femurs did lead to increased osteoinduction, but this was not associated with stronger bonds to the bone (O'Toole et al., 2004).
Non-specific protein adsorption to the surface of the implant is one of the first events in the FBR and the randomness of this adsorption may be the cause of subsequent events (Ratner, 2002, 2001). In an attempt to present a surface that would minimize the FBR, Liu et al functionalized a polymer to have a charge over its entire surface and then placed the polymer into osteopontin solutions to allow OPN to adsorb in a more controlled fashion (the charge will control the orientation of the OPN molecule) (Pan et al., 2008). When implanted in vivo the positively charged polymer coated with OPN showed increased cell spreading after 7 days and a foreign body capsule that was significantly thinner after 4 weeks, suggesting that controlling OPN adsorption and orientation can be a successful strategy for minimizing the FBR (Pan et al., 2008). Additionally, OPN antisense oligodeoxynucleotides loaded into Pluronic gel promote wound closure at early time points and reduce wound fibrosis and scarring at later time points (Mori et al., 2008). Wounds that had antisense OPN delivered to them showed decreased leukocyte recruitment and more angiogenesis (Mori et al., 2008).
Osteopontin in combination with biomaterials has also proved to be useful for studies in vitro. In 1997 it was discovered that a 15 amino acid sequence from osteopontin (containing the RGD domain) increased cell adhesion, focal adhesion formation, and cell adhesion strength to quartz as compared to control surfaces (a peptide containing RGE sequence and a clean quartz surface) (Rezania et al., 1997). Recently it was found that coating surfaces with osteopontin promoted endothelial cell progenitor adhesion and spreading with dose dependency in vitro and may prove to be a useful system for the functionalization of vascular grafts to be endothelialized in situ (Yuan et al., 2013). Another group sought to make a protein that would both strongly bind collagen and hydroxyapatite to treat bone defects by making a chimeric protein containing a glutamic acid rich sequence from OPN (that nucleates hydroxyapatite crystals) and a segment from decorin known to have a high affinity for collagen (Hunter et al., 2001). This chimeric protein successfully bound collagen and served as a nucleation site for hydroxyapatite crystals and is potentially useful because in collagen gels, it encouraged mineralization along the length of the collagen fibrils (instead of random crystal formation as seen in poly-L-glutamic acid controls) (Hunter et al., 2001).
OPN segments have also been immobilized onto gold in various patterns to influence cell adhesion and shape as well as response to growth factors (Mieszawska and Kaplan, 2010; Mitchell et al., 2010). Functionalizing biomaterials with osteopontin has proven to be a useful technique for influencing cell behavior, limiting the FBR to implants, and encouraging tissue regeneration, particularly for orthopedic implants.
3.4 Periostin
Periostin is a 90 kDa protein that can be divided into seven domains: a secretory peptide, one EMI domain, four FAS1 domains, and a hydrophobic C-terminus (Kudo et al., 2007). Periostin is expressed in connective tissues, cardiac tissues during development and after damage, and the wound bed (Hamilton, 2008; Norris et al., 2009, 2012). Periostin belongs to the fasciclin gene family and shares many residues with FAS1 in Drosophila (Hamilton, 2008; Norris et al., 2009, 2012). Periostin is involved in cell adhesion, MSC differentiation, cardiac development, wound healing, collagen fibrillogenesis, atherosclerosis, cancer metastasis, and possibly hypertrophic cardiomyopathy (Hamilton, 2008; Hixson et al., 2011; Kudo et al., 2007; Norris et al., 2009, 2012; Seidman and Seidman, 2011). Although periostin null mouse pups are phenotypically normal, these mice grow more slowly and exhibit higher mortality than WT (Frangogiannis, 2012). Periostin is implicated heavily in cancer metastasis and embryogenesis because it stimulates cell migration and differentiation after the epithelial-mesenchymal transition (EMT) (Lindsley et al., 2007; Ruan et al., 2009). Periostin has received significant attention recently because of the possible influence it may have on the cardiomyocyte cell cycle (Kühn et al., 2007; Lorts et al., 2009; Matsui et al., 2010; Polizzotti et al., 2012). The effects of periostin on cardiac cell cycle remain controversial because although several groups report that it causes cardiomyocytes to proliferate after injury, one report shows that periostin overexpressing and periostin null mice show no changes in cardiac cell proliferation or cardiac regeneration after injury, when compared to wild type (Lorts et al., 2009).
Because of the effects that periostin may have on stimulating cardiomyocytes to reenter the cell cycle, combinations of periostin and biomaterials are of interest in treating injured heart tissue, particular after myocardial infarction. Specifically, a group showed that periostin can cause differentiated adult cardiomyocytes to enter the cell cycle and begin replication, and demonstrated that periostin loaded gelfoam delivered after myocardial infarction reduced scar formation and improved cardiac function in rats (Kühn et al., 2007). A follow up paper demonstrated that when periostin loaded gelfoam was injected into the pericardial cavity of pigs, a fibrin rich hydrogel spontaneously encapsulated the material, creating a drug-eluting hydrogel that prolongs periostin release and forms in situ (Polizzotti et al., 2012). The use of periostin loaded biomaterials for cardiac regeneration is still a controversial topic and in need of further research.
3.5 CCNs
The CCN family of proteins is involved in a variety of processes from development to inflammation, wound healing, and cancer (Chen and Lau, 2009). There are 6 members of the CCN family, but only CCN1 and CCN2 have been considered in the context of biomaterials. CCN2 (also called connective tissue growth factor) is thought to increase ECM production and is both a downstream target of and co-factor for TGF-β (Grotendorst, 1997; Perbal, 2004; Liu, Thompson, & Leask, 2014). Both CCN1 and 2 promote angiogenesis and endothelial cell survival (Perbal, 2004). Both CCN1 and CCN2 are known to interact with a variety of integrins and have been shown to regulate cell adhesion and migration (Chen and Lau, 2009). CCN1 also regulates proliferation, apoptosis, and the effects of TNFα. The CCNs have very similar structures and contain domains similar to those of many other matricellular proteins: a secretory peptide, four insulin-like growth factor binding domains, a von willebrand factor type C repeat, a TSP type 1 repeat, and a C-terminal domain (Frangogiannis, 2012).
3.5.1 CCN1
CCN1 has potent pro-angiogenic effects and has been shown to promote endothelial cell survival (Chen and Lau, 2009; Perbal, 2004). In an attempt to encourage the formation of a continuous endothelial cell coating on the lumen of decellularized vascular matrices, Bär et al coated their decellularized vessels with CCN1 (Bär et al., 2010). Twice as many endothelial cells adhered to vessels coated with CCN1 and showed increased metabolic activity in these cells (possibly due to increased cell viability) (Bär et al., 2010). Ravi et al created an elastin-like protein polymer that contained a sequence from CCN1 that is known to interact with the αvβ3 integrin of endothelial cells, in an attempt to promote endothelialization of the polymer (Ravi et al., 2012). They found increased HUVEC adhesion to this polymer (when compared to untreated plates), increased cell migration, and low levels of ICAM-1 and E-selectin expression, demonstrating a quiescent endothelium (Ravi et al., 2012). A more recent paper examined the effects of CCN1 coated decellularized carotid arteries when implanted in vivo in sheep and showed that CCN1 promoted the formation of a continuous endothelial layer and increased SMC invasion into the decellularized matrix when compared to uncoated decellularized arteries (Spengler et al., 2013). CCN1 coatings are a promising method for promoting endothelialization and maintaining vascular graft patency.
3.5.2 CCN2
Percutaneous implants require adequate wound healing in the area surrounding the implant to maximize success. To increase the healing surrounding titanium implants, Wei et al functionalized an implant surface with CCN2-loaded titania nanotubes to promote fibroblast activity and dermal healing surrounding the implant (Wei et al., 2012). In vitro these implants released most of their CCN2 within 2 hours but promoted fibroblast adhesion and rapid formation of actin stress fibers that became denser over time, suggesting increased fibroblast activity (Wei et al., 2012). It is unclear whether these benefits would remain if the devices were implanted in vivo, but the idea of drug loading the nanotopography of a metallic implant with matricellular proteins holds promise.
3.6 Tenascin-C
Tenascin-C (TNC) is a hexameric protein with each subunit approximately 200 kDa. It modulates cell adhesion, particularly by regulating cell-fibronectin interactions (Bornstein and Sage, 2002; Hsia and Schwarzbauer, 2005). TNC has multiple domains including: its N terminal domains, a fibrinogen-like C terminus, epidermal growth factor repeats, and fibronectin type III repeats which bind to integrins (Chiquet-Ehrismann, 2004; Erickson, 1993; Hsia and Schwarzbauer, 2005; Leahy et al., 1992). Tenascin-C is highly expressed during development and in areas of tissue formation (after injury, during angiogenesis, or tissue remodeling), and at low levels in adult tendons (Alford and Hankenson, 2006; Bornstein and Sage, 2002; Hsia and Schwarzbauer, 2005). Tenascin-C null mice have neurological changes resulting in altered behavior, as well as altered healing in models of skin and bone injuries (Alford and Hankenson, 2006; Bornstein and Sage, 2002; Kyriakides and Bornstein, 2003).
Fibronectin type III domains of TNC have been incorporated into elastic hydrogels as binding sites for cells to mimic the in vivo architecture (Lv et al., 2013). The domains from TNC promoted cell adhesion and spreading in these hydrogels, which could be tuned to have varying mechanical properties (Lv et al., 2013). Another group modified polyamide electrospun nanofibers to display tenascin-C peptides for neuronal cell culture (Ahmed et al., 2005). This unique approach combined a biomimetic ECM architecture with peptides that promoted cell attachment, neurite generation, and neurite extension, ultimately leading to neurons that had longer and more branched neurites than control materials (Ahmed et al., 2005). In addition, it was discovered that TNC-coated tissue culture treated plates promoted MSC survival in the presence of death signals (Rodrigues et al., 2013).
4. Conclusions and Future Directions
Matricellular proteins are an important class of ECM proteins that influence a variety of cell processes. Their diverse functions make them an important consideration when using materials such as decellularized ECM derived from tissues or cells in vitro. Moreover, matricellular proteins have functions that range from influencing ECM production to inhibiting angiogenesis and promoting adhesion. Therefore, biomaterials that contain matricellular proteins or their domains or DNA to influence their expression are emerging as a new generation of biomimetic materials. These materials may be key to promoting tissue regeneration and reducing the foreign body response to biomaterials (Bryers et al., 2012).
As the number of materials that incorporate ECM components increases, it will be important to consider manipulation of the micro- and nano-architecture as well as matricellular protein content to achieve the desired effect. Additionally, double-null mice have shown that matricellular proteins can have synergestic effects (Agah et al., 2002; Barker et al., 2005b; Puolakkainen et al., 2005). Investigating combinations of multiple matricellular proteins or domains from these proteins will be an important area for further research. Ultimately, as knowledge of the mechanisms of action of matricellular proteins is elucidated, it will lead to improved design of materials to promote healing, reduce the FBR, and achieve greater successes in tissue engineering.
Highlights.
Matricellular proteins participate in the foreign body response to biomaterials.
Modulation of matricellular proteins or their components can alter the FBR.
Biomimetic materials can incorporate matricellular proteins or their components.
Matricellular proteins retained in decellularized ECM influence cell function.
Acknowledgements
We thank Nina Kristofik for providing images. We would also like to thank our colleagues in the Vascular Biology and Therapeutics Program for helpful discussions. Our research cited in this review was funded by NIH (GM072194, HL107205). This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1122492. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Abbreviations
- FBR
foreign body response
- FBGCs
foreign body giant cells
- TSP
thrombospondin
- CD36
cluster of differentiation 36
- LDL
low density lipoprotein
- ECM
extracellular matrix
- PEI
poly(ethylene imine)
- EC
endothelial cell
- PEG
polyethylene glycol
- GAM
gene-activated matrix
- WT
wild type
- SPARC
Secreted Protein, Acidic and Rich in Cysteine
- MMP
matrix metalloproteinase
- OPN
osteopontin
- BSP
bone sialoprotein
- RGD
Arginine-Glycine-Aspartic acid
- HUVEC
human umbilical vein endothelial cells
- TNC
tenascin-C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JC, Lawler J. The thrombospondins. Int. J. Biochem. Cell Biol. 2004;36:961–8. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am. J. Pathol. 2002;161:831–9. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed I, Liu H-Y, Mamiya PC, Ponery AS, Babu AN, Weik T, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces covalently modified with tenascin-C-derived peptides enhance neuronal growth in vitro. J. Biomed. Mater. Res. A. 2005;76:851–60. doi: 10.1002/jbm.a.30587. [DOI] [PubMed] [Google Scholar]
- Alford AI, Hankenson KD. Matricellular proteins: Extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–57. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin. Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003;22:63–71. doi: 10.1016/s0945-053x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–93. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Bär A, Dorfman SE, Fischer P, Hilfiker-Kleiner D, Cebotari S, Tudorache I, Suprunov M, Haverich A, Hilfiker A. The pro-angiogenic factor CCN1 enhances the reendothelialization of biological vascularized matrices in vitro. Cardiovasc. Res. 2010;85:806–13. doi: 10.1093/cvr/cvp370. [DOI] [PubMed] [Google Scholar]
- Barker TH, Baneyx G, Cardó-Vila M, Workman GA, Weaver M, Menon PM, Dedhar S, Rempel SA, Arap W, Pasqualini R, Vogel V, Sage EH. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J. Biol. Chem. 2005a;280:36483–93. doi: 10.1074/jbc.M504663200. [DOI] [PubMed] [Google Scholar]
- Barker TH, Framson P, Puolakkainen PA, Reed M, Funk SE, Sage EH. Matricellular Homologs in the Foreign Body Response Together Diminish Angiogenesis. Am. J. Pathol. 2005b;166:923–933. doi: 10.1016/S0002-9440(10)62312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 2012;186:866–76. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Matricellular proteins: an overview. J. Cell Commun. Signal. 2009;3:163–5. doi: 10.1007/s12079-009-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int. J. Biochem. Cell Biol. 2004;36:1115–25. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Kyriakides TR, Yang Z, Armstrong LC, Birk DE. Thrombospondin 2 modulates collagen fibrillogenesis and angiogenesis. J. Investig. Dermatol. Symp. Proc. 2000;5:61–6. doi: 10.1046/j.1087-0024.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr. Opin. Cell Biol. 2002;14:608–16. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- Boudriot U, Dersch R, Greiner A, Wendorff JH. Electrospinning approaches toward scaffold engineering--a brief overview. Artif. Organs. 2006;30:785–92. doi: 10.1111/j.1525-1594.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- Bourget J, Gauvin R, Larouche D, Lavoie A, Labbé R, Auger FA, Germain L. Human fibroblast-derived ECM as a scaffold for vascular tissue engineering. Biomaterials. 2012;33:9205–13. doi: 10.1016/j.biomaterials.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Bourgine PE, Pippenger BE, Todorov A, Tchang L, Martin I. Tissue decellularization by activation of programmed cell death. Biomaterials. 2013;34:6099–108. doi: 10.1016/j.biomaterials.2013.04.058. [DOI] [PubMed] [Google Scholar]
- Bradshaw AD. The role of SPARC in extracellular matrix assembly. J. Cell Commun. Signal. 2009;3:239–46. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Helene Sage E. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J. Invest. Dermatol. 2003;120:949–55. doi: 10.1046/j.1523-1747.2003.12241.x. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- Brodbeck WG, Patel J, Voskerician G, Christenson E, Shive MS, Nakayama Y, Matsuda T, Ziats NP, Anderson JM. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10287–92. doi: 10.1073/pnas.162124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryers JD, Giachelli CM, Ratner BD. Engineering biomaterials to integrate and heal: the biocompatibility paradigm shifts. Biotechnol. Bioeng. 2012;109:1898–911. doi: 10.1002/bit.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro NE, Kristofik NJ, Kyriakides TR. Thrombospondin-2 and extracellular matrix assembly. Biochim. Biophys. Acta. 2014:1–7. doi: 10.1016/j.bbagen.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Hench LL. Bioactive Materials. Ceram. Int. 1996;8842:493–507. [Google Scholar]
- Causa F, Netti P. a, Ambrosio L. A multi-functional scaffold for tissue regeneration: the need to engineer a tissue analogue. Biomaterials. 2007;28:5093–9. doi: 10.1016/j.biomaterials.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Chen AK, Delrio FW, Peterson AW, Chung K-H, Bhadiraju K, Plant AL. Cell spreading and proliferation in response to the composition and mechanics of engineered fibrillar extracellular matrices. Biotechnol. Bioeng. 2013;110:2731–41. doi: 10.1002/bit.24921. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int. J. Biochem. Cell Biol. 2009;41:771–83. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol. 2000;19:597–614. doi: 10.1016/s0945-053x(00)00107-4. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. Molecules in focus: Tenascins. Int. J. Biochem. Cell Biol. 2004;36:986–990. doi: 10.1016/j.biocel.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–70. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Decup F, Six N, Palmier B, Buch D, Lasfargues JJ, Salih E, Goldberg M. Bone sialoprotein-induced reparative dentinogenesis in the pulp of rat's molar. Clin. Oral Investig. 2000;4:110–9. doi: 10.1007/s007840050126. [DOI] [PubMed] [Google Scholar]
- Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27:63–9. doi: 10.1080/08977190802636713. [DOI] [PubMed] [Google Scholar]
- Erickson HP. Tenascin-C, tenascin-R and tenascin-X: a family of talented proteins in search of functions. Curr. Opin. Cell Biol. 1993;5:869–876. doi: 10.1016/0955-0674(93)90037-q. [DOI] [PubMed] [Google Scholar]
- Esch MB, Post DJ, Shuler ML, Stokol T. Characterization of in vitro endothelial linings grown within microfluidic channels. Tissue Eng. Part A. 2011;17:2965–71. doi: 10.1089/ten.tea.2010.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol. Rev. 2012;92:635–88. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha M, Singh a. K., Asokamani R, Gogia AK. Ti based biomaterials, the ultimate choice for orthopaedic implants – A review. Prog. Mater. Sci. 2009;54:397–425. [Google Scholar]
- Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–22. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. J. Biomed. Mater. Res. Part A. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Lacerda-Pinheiro S, Priam F, Jegat N, Six N, Bonnefoix M, Septier D, Chaussain-Miller C, Veis A, Denbesten P, Poliard A. Matricellular molecules and odontoblast progenitors as tools for dentin repair and regeneration. Clin. Oral Investig. 2008;12:109–12. doi: 10.1007/s00784-007-0172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M, Six N, Chaussain C, DenBesten P, Veis A, Poliard A. Dentin extracellular matrix molecules implanted into exposed pulps generate reparative dentin: a novel strategy in regenerative dentistry. J. Dent. Res. 2009;88:396–9. doi: 10.1177/0022034509337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M, Six N, Decup F, Buch D, Soheili Majd E, Lasfargues J-J, Salih E, Stanislawski L. Application of Bioactive Molecules in Pulp-capping Situations. Adv. Dent. Res. 2001;15:91–95. doi: 10.1177/08959374010150012401. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR. Connective Tissue Growth Factor: a Mediator of TGF-β Action on Fibroblasts. Cytokine & Growth Factor Reviews. 1997;8(3):171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Gunatillake P. a, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell. Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. [DOI] [PubMed] [Google Scholar]
- Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J. Cell Commun. Signal. 2008;2:9–17. doi: 10.1007/s12079-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig SM, Greene RR, Dikov MM, Prokop A, Davidson JM. Multifunctional nanoparticulate polyelectrolyte complexes. Pharm. Res. 2007;24:2353–69. doi: 10.1007/s11095-007-9459-1. [DOI] [PubMed] [Google Scholar]
- Healy KE, Thomas CH, Rezania A, Kim JE, McKeown PJ, Lom B, Hockberger PE. Kinetics of bone cell organization and mineralization on materials with patterned surface chemistry. Biomaterials. 1996;17:195–208. doi: 10.1016/0142-9612(96)85764-4. [DOI] [PubMed] [Google Scholar]
- Hench LL. Biomaterials: a forecast for the future. Biomaterials. 1998;19:1419–23. doi: 10.1016/s0142-9612(98)00133-1. [DOI] [PubMed] [Google Scholar]
- Heness G, Ben-Nissan B. Innovative Bioceramics. Mater. Forum. 2004;27:104–114. [Google Scholar]
- Hixson JE, Shimmin LC, Montasser ME, Kim D-K, Zhong Y, Ibarguen H, Follis J, Malcom G, Strong J, Howard T, Langefeld C, Liu Y, Rotter JI, Johnson C, Herrington D. Common variants in the periostin gene influence development of atherosclerosis in young persons. Arterioscler. Thromb. Vasc. Biol. 2011;31:1661–7. doi: 10.1161/ATVBAHA.111.224352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia HC, Schwarzbauer JE. Meet the tenascins: multifunctional and mysterious. J. Biol. Chem. 2005;280:26641–4. doi: 10.1074/jbc.R500005200. [DOI] [PubMed] [Google Scholar]
- Hunter GK, Poitras MS, Underhill TM, Grynpas MD, Goldberg HA. Induction of collagen mineralization by a bone sialoprotein--decorin chimeric protein. J. Biomed. Mater. Res. 2001;55:496–502. doi: 10.1002/1097-4636(20010615)55:4<496::aid-jbm1042>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Jayakumar R, Menon D, Manzoor K, Nair SV, Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010;82:227–232. [Google Scholar]
- Krady MM, Zeng J, Yu J, MacLauchlan S, Skokos E. a, Tian W, Bornstein P, Sessa WC, Kyriakides TR. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am. J. Pathol. 2008;173:879–91. doi: 10.2353/ajpath.2008.080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Hatano H, Ogawa I, Takata T. Periostin: Novel diagnostic and therapeutic target for cancer. Histol. Histopathol. 2007;1:1167–1174. doi: 10.14670/HH-22.1167. [DOI] [PubMed] [Google Scholar]
- Kühn B, del Monte F, Hajjar RJ, Chang Y-S, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat. Med. 2007;13:962–9. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb. Haemost. 2003;90:986–992. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Cheung CY, Murthy N, Bornstein P, Stayton PS, Hoffman AS. pH-sensitive polymers that enhance intracellular drug delivery in vivo. J. Control. Release. 2002;78:295–303. doi: 10.1016/s0168-3659(01)00504-1. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Hartzel T, Huynh G, Bornstein P. Regulation of angiogenesis and matrix remodeling by localized, matrix-mediated antisense gene delivery. Mol. Ther. 2001;3:842–9. doi: 10.1006/mthe.2001.0336. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Leach KJ, Hoffman AS, Ratner BD, Bornstein P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4449–54. doi: 10.1073/pnas.96.8.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J. Cell Commun. Signal. 2009;3:215–25. doi: 10.1007/s12079-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Tam JWY, Bornstein P. Accelerated Wound Healing in Mice With a Disruption of the Thrombospondin 2 Gene. J. Invest. Dermatol. 1999;113:782–787. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J. Cell Biol. 1998;140:419–30. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kzhyshkowska J, Workman G, Cardó-Vila M, Arap W, Pasqualini R, Gratchev A, Krusell L, Goerdt S, Sage EH. Novel function of alternatively activated macrophages: stabilin-1-mediated clearance of SPARC. J. Immunol. 2006;176:5825–32. doi: 10.4049/jimmunol.176.10.5825. [DOI] [PubMed] [Google Scholar]
- Lan M. a, Gersbach CA, Michael KE, Keselowsky BG, García AJ. Myoblast proliferation and differentiation on fibronectin-coated self assembled monolayers presenting different surface chemistries. Biomaterials. 2005;26:4523–31. doi: 10.1016/j.biomaterials.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Lawler J. The functions of thrombospondin-1 and-2. Curr. Opin. Cell Biol. 2000;12:634–40. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- Lawler J, Hynes RO. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J. Cell Biol. 1986;103:1635–48. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy DJ, Hendrickson WA, Aukhil I, Erickson HP. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992;258:987–91. doi: 10.1126/science.1279805. [DOI] [PubMed] [Google Scholar]
- Levert HS. Experiments on the use of Metallic Ligatures, as applied to Arteries. Am. J. Med. Sci. 1829;7:17–23. [Google Scholar]
- Liao S, Ngiam M, Chan CK, Ramakrishna S. Fabrication of nano-hydroxyapatite/collagen/osteonectin composites for bone graft applications. Biomed. Mater. 2009;4:1–8. doi: 10.1088/1748-6041/4/2/025019. [DOI] [PubMed] [Google Scholar]
- Lindsley A, Snider P, Zhou H, Rogers R, Wang J, Olaopa M, Kruzynska-Frejtag A, Koushik SV, Lilly B, Burch JBE, Firulli AB, Conway SJ. Identification and characterization of a novel Schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer. Dev. Biol. 2007;307:340–55. doi: 10.1016/j.ydbio.2007.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chen G, Chao T, Ratner BD, Sage EH, Jiang S. Reduced foreign body reaction to implanted biomaterials by surface treatment with oriented osteopontin. J. Biomater. Sci. Polym. Ed. 2008;19:821–35. doi: 10.1163/156856208784522083. [DOI] [PubMed] [Google Scholar]
- Liu S, Thompson K, Leask A. CCN2 expression by fibroblasts is not required for cutaneous tissue repair. Wound Repair Regen. 2014;22:119–24. doi: 10.1111/wrr.12131. [DOI] [PubMed] [Google Scholar]
- Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, Molkentin JD. Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ. Res. 2009;104:e1–7. doi: 10.1161/CIRCRESAHA.108.188649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv S, Bu T, Kayser J, Bausch A, Li H. Towards constructing extracellular matrix-mimetic hydrogels: an elastic hydrogel constructed from tandem modular proteins containing tenascin FnIII domains. Acta Biomater. 2013;9:6481–91. doi: 10.1016/j.actbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Morimoto J, Uede T. Role of matricellular proteins in cardiac tissue remodeling after myocardial infarction. World J. Biol. Chem. 2010;1:69–80. doi: 10.4331/wjbc.v1.i5.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JD, Lin C-C, Anseth KS. Affinity peptides protect transforming growth factor beta during encapsulation in poly(ethylene glycol) hydrogels. Biomacromolecules. 2011;12:1051–7. doi: 10.1021/bm101379v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metters AT, Anseth KS, Bowman CN. Fundamental studies of a novel , biodegradable PEG- b -PLA hydrogel. Polymer. 2000;41:3993–4004. [Google Scholar]
- Mieszawska AJ, Kaplan DL. Smart biomaterials - regulating cell behavior through signaling molecules. BMC Biol. 2010;8:59. doi: 10.1186/1741-7007-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EA, Chaffey BT, McCaskie AW, Lakey JH, Birch MA. Controlled spatial and conformational display of immobilised bone morphogenetic protein-2 and osteopontin signalling motifs regulates osteoblast adhesion and differentiation in vitro. BMC Biol. 2010;8:57. doi: 10.1186/1741-7007-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J. Exp. Med. 2008;205:43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Schultz-Cherry S, Höök M. Transforming growth factor-beta complexes with thrombospondin. Mol. Biol. Cell. 1992;3:181–8. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J. Clin. Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR. The many facets of the matricelluar protein periostin during cardiac development, remodeling, and pathophysiology. J. Cell Commun. Signal. 2009;3:275–86. doi: 10.1007/s12079-009-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Damon B, Mironov V, Kasyanov V, Moreno-rodriguez R, Trusk T, Potts JD, Richard L, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR, Carolina S. Periostin Regulates Collagen Fibrillogenesis and the Biomechanical Properties of Connective Tissues. J. Cell. Biochem. 2012;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole GC, Salih E, Gallagher C, FitzPatrick D, O'Higgins N, O'Rourke SK. Bone sialoprotein-coated femoral implants are osteoinductive but mechanically compromised. J. Orthop. Res. 2004;22:641–6. doi: 10.1016/j.orthres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Imanaka-Yoshida K. Matricellular proteins: new molecular targets to prevent heart failure. Cardiovasc. Ther. 2012;30:e198–209. doi: 10.1111/j.1755-5922.2011.00276.x. [DOI] [PubMed] [Google Scholar]
- Pallero MA, Talbert Roden M, Chen Y-F, Anderson PG, Lemons J, Brott BC, Murphy-Ullrich JE. Stainless steel ions stimulate increased thrombospondin-1-dependent TGF-beta activation by vascular smooth muscle cells: implications for in-stent restenosis. J. Vasc. Res. 2010;47:309–22. doi: 10.1159/000265565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Jiang H, Chen W. The biodegradability of electrospun Dextran/PLGA scaffold in a fibroblast/macrophage co-culture. Biomaterials. 2008;29:1583–92. doi: 10.1016/j.biomaterials.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J, Hubbell JA. SPARC-derived protease substrates to enhance the plasmin sensitivity of molecularly engineered PEG hydrogels. Biomaterials. 2011;32:1301–10. doi: 10.1016/j.biomaterials.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Perbal B. Rapid review CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizzotti BD, Arab S, Kühn B. Intrapericardial delivery of gelfoam enables the targeted delivery of Periostin peptide after myocardial infarction by inducing fibrin clot formation. PLoS One. 2012;7:1–11. doi: 10.1371/journal.pone.0036788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20:2311–21. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- Puolakkainen PA, Bradshaw AD, Brekken RA, Reed MJ, Kyriakides T, Funk SE, Gooden MD, Vernon RB, Wight TN, Bornstein P, Sage EH. SPARC-thrombospondin-2-double-null mice exhibit enhanced cutaneous wound healing and increased fibrovascular invasion of subcutaneous polyvinyl alcohol sponges. J. Histochem. Cytochem. 2005;53:571–81. doi: 10.1369/jhc.4A6425.2005. [DOI] [PubMed] [Google Scholar]
- Puolakkainen P, Bradshaw AD, Kyriakides TR, Reed M, Brekken R, Wight T, Bornstein P, Ratner B, Sage EH. Compromised production of extracellular matrix in mice lacking secreted protein, acidic and rich in cysteine (SPARC) leads to a reduced foreign body reaction to implanted biomaterials. Am. J. Pathol. 2003;162:627–35. doi: 10.1016/S0002-9440(10)63856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner BD. Surface Structure of Polymers for Biomedical Applications. Makromol. Chem. 1988;19:163–178. [Google Scholar]
- Ratner BD. Replacing and renewing: synthetic materials, biomimetics, and tissue engineering in implant dentistry. J. Dent. Educ. 2001;65:1340–7. [PubMed] [Google Scholar]
- Ratner BD. Reducing capsular thickness and enhancing angiogenesis around implant drug release systems. J. Control. Release. 2002;78:211–8. doi: 10.1016/s0168-3659(01)00502-8. [DOI] [PubMed] [Google Scholar]
- Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- Ravi S, Haller CA, Sallach RE, Chaikof EL. Cell behavior on a CCN1 functionalized elastin-mimetic protein polymer. Biomaterials. 2012;33:2431–8. doi: 10.1016/j.biomaterials.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke H, Robey TE, Mignone JL, Muskheli V, Bornstein P, Murry CE. Lack of thrombospondin-2 reduces fibrosis and increases vascularity around cardiac cell grafts. Cardiovasc. Pathol. 2013;22:91–5. doi: 10.1016/j.carpath.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reing JE, Brown BN, Daly KA, Hsiong S, Huber A, Kullas KE, Tottey S, Badylak SF. The Effects of Processing Methods upon Mechanical and Biologic Properties of Porcine Dermal Extracellular Matrix Scaffolds. Biomaterials. 2010;31:8626–8633. doi: 10.1016/j.biomaterials.2010.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A, Thomas CH, Branger AB, Waters CM, Healy KE. The detachment strength and morphology of bone cells contacting materials modified with a peptide sequence found within bone sialoprotein. J. Biomed. Mater. Res. 1997;37:9–19. doi: 10.1002/(sici)1097-4636(199710)37:1<9::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Roach P, Eglin D, Rohde K, Perry CC. Modern biomaterials: a review - bulk properties and implications of surface modifications. J. Mater. Sci. Mater. Med. 2007;18:1263–77. doi: 10.1007/s10856-006-0064-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Yates CC, Nuschke A, Griffith L, Wells A. The Matrikine Tenascin-C Protects Multipotential Stromal Cells / Mesenchymal Stem Cells from Death Cytokines Such as FasL. Tissue Eng. Part A. 2013;19:1972–1983. doi: 10.1089/ten.tea.2012.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell. Mol. Life Sci. 2009;66:2219–30. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka OM, Bozkir A. Formulation and in vitro characterization of PEGylated chitosan and polyethylene imine polymers with thrombospondin-I gene bearing pDNA. J. Biomed. Mater. Res. B. Appl. Biomater. 2012;100:984–92. doi: 10.1002/jbm.b.32661. [DOI] [PubMed] [Google Scholar]
- Sarvestani AS, He X, Jabbari E. Osteonectin-derived peptide increases the modulus of a bone-mimetic nanocomposite. Eur. Biophys. J. 2008;37:229–34. doi: 10.1007/s00249-007-0198-3. [DOI] [PubMed] [Google Scholar]
- Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–62. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J. Cell Biol. 1993;122:923–32. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman CE, Seidman JG. Identifying sarcomere gene mutations in hypertrophic cardiomyopathy: a personal history. Circ. Res. 2011;108:743–50. doi: 10.1161/CIRCRESAHA.110.223834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- Shubina AN, Egorova AA, Baranov VS, Kiselev AV. Recent Advances in Gene Therapy of Endometriosis. Recent Pat. DNA Gene Seq. 2013;7:1–10. doi: 10.2174/18722156113079990021. [DOI] [PubMed] [Google Scholar]
- Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med. 2011;17:424–32. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Spengler C, Jonigk D, Klingenberg M, Schrimpf C, Lu S, Harder M, Kreipe H, Haverich A, Wilhelmi M. Coating Decellularized Equine Carotid Arteries with CCN1 Improves Cellular Repopulation , Local Biocompatibility , and Immune Response in Sheep. Tissue Eng. Part A. 2013;19:1829–42. doi: 10.1089/ten.TEA.2012.0558. [DOI] [PubMed] [Google Scholar]
- Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135–8. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M. Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 1999;96:14888–93. doi: 10.1073/pnas.96.26.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M, Stephen AE, Hawighorst T, Matsuda K, Lange-asschenfeldt B, Brown LF, Vacanti JP, Detmar M. Systemic Inhibition of Tumor Growth and Angiogenesis by Thrombospondin-2 Using Cell-based Antiangiogenic Gene Therapy Systemic Inhibition of Tumor Growth and Angiogenesis by Thrombospondin-2. 20022004–2012 [PubMed] [Google Scholar]
- Sullivan MM, Sage EH. Hevin/SC1, a matricellular glycoprotein and potential tumor-suppressor of the SPARC/BM-40/Osteonectin family. Int. J. Biochem. Cell Biol. 2004;36:991–6. doi: 10.1016/j.biocel.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Sweetwyne MT, Pallero M. a, Lu A, Van Duyn Graham L, Murphy-Ullrich JE. The calreticulin-binding sequence of thrombospondin 1 regulates collagen expression and organization during tissue remodeling. Am. J. Pathol. 2010;177:1710–24. doi: 10.2353/ajpath.2010.090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AT, Rice J, Scatena M, Liaw L, Ratner BD, Giachelli CM. The role of osteopontin in foreign body giant cell formation. Biomaterials. 2005;26:5835–43. doi: 10.1016/j.biomaterials.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–18. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- Wei H, Wu S, Feng Z, Zhou W, Dong Y, Wu G, Bai S, Zhao Y. Increased fibroblast functionality on CNN2-loaded titania nanotubes. Int. J. Nanomedicine. 2012;7:1091–100. doi: 10.2147/IJN.S28694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed. Mater. 2006;1:R1–9. doi: 10.1088/1748-6041/1/1/R01. [DOI] [PubMed] [Google Scholar]
- Xu L, Anderson AL, Lu Q, Wang J. Role of fibrillar structure of collagenous carrier in bone sialoprotein-mediated matrix mineralization and osteoblast differentiation. Biomaterials. 2007;28:750–61. doi: 10.1016/j.biomaterials.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Yang Z, Strickland DK, Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J. Biol. Chem. 2001;276:8403–8. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Altalhi WA, Ng JJ, Courtman DW. Derivation of human peripheral blood derived endothelial progenitor cells and the role of osteopontin surface modification and eNOS transfection. Biomaterials. 2013;34:7292–301. doi: 10.1016/j.biomaterials.2013.06.003. [DOI] [PubMed] [Google Scholar]