Abstract
Background
Obesity is associated with a mild chronic inflammatory response, which has been suggested to be pivotal in the development of cardiometabolic alterations of obesity. However, little is known about the involvement of acute inflammation.
Objective
To evaluate whether circulating neutrophils, markers of acute inflammation, are associated (quantitatively and qualitatively) with adolescent obesity and whether leptin modulates these associations.
Subjects and Methods
We assessed 528 adolescents (16.8 y old, 47% females), without chronic/acute illness. We measured anthropometry and dual energy X-ray absorptiometry and calculated fat mass percent (FM%). Fasting serum glucose, HDL-cholesterol and triglycerides were used with blood pressure and waist circumference to compute a metabolic z-score. Leukocyte and neutrophil counts were obtained, together with levels of serum leptin. In a subsample of 23 males, flow-cytometry was used to assess degranulation (CD66b expression) of neutrophils.
Results
Female sex and obesity were positively related to mean neutrophil counts (p<0.05). When accounting for sex and weight status, leptin was associated with neutrophil counts (p<0.05), partially explaining the association between obesity and neutrophil counts. Neutrophil counts were related to metabolic risk z-scores, controlling for fat mass. Participants with elevated FM% showed more neutrophil degranulation than controls (p<0.05).
Conclusions
Participants with increased adiposity had higher circulating neutrophil counts, suggesting acute inflammation. Furthermore, the neutrophils showed more degranulation, indicating inflammation. Obesity-induced alteration of the adipose secretory pattern (i.e. changes in leptin levels) could be involved in acute inflammation.
Keywords: Obesity, acute inflammation, leukocytes, neutrophils, adolescents
Introduction
Childhood obesity is a worldwide problem with increasing prevalence and severity (1). Obese children are more likely than their non-obese counterparts to have risk factors for later cardiovascular disease (2). As in adults, childhood obesity has been associated with a mild chronic inflammatory state (3,4). Inflammation may be the mediator between excess fat mass and the metabolic/cardiovascular alterations that lead to atheromatosis (3,5,6), the development of plaque on degenerated thickened arterial intima. Atheromatosis is itself an inflammatory process (7).
Both total and differential leukocyte counts have been used to assess obesity-triggered inflammation. Total leukocyte counts are higher in obese subjects (8–10), and have been related to unfavorable metabolic profiles (9,11) and cardiovascular events (12,13), after adjusting for body mass. The association of leukocyte counts with worse metabolic and cardiovascular outcomes could be explained, in part, by increased influx of leukocytes into adipose tissue, intimal atheromatous plaques and key organs including the pancreas (5,12,14). Decreasing specific circulating leukocytes subtypes in murine models reduces their influx into the subendothelial space, thereby reducing the size of atheromatous lesions (15,16).
Much research has focused on the study of circulating monocytes, the macrophage precursors (17), because macrophages are characteristic of chronic inflammation and the main leukocyte subtype that infiltrates tissues in obesity. In humans, the counts of classical subtype of circulating monocyte (i.e. CD14++ CD16−) has been shown to predict ischemic cardiovascular events (18). In addition, inflammatory changes can be seen in neutrophils, pathognomonic of acute inflammation. In obesity, circulating neutrophil counts are elevated (4,10,19,20) and neutrophils are also increased in blood vessels from adipose tissue biopsies (21,22). Moreover, their inflammatory status is exacerbated in obese subjects. The inflammatory status of neutrophils can be measured through the evaluation of phagocytosis, oxidative burst and secretion of pre-formed proteins contained in secretion granules and vesicles. Neutrophils of obese, compared to those of non-obese subjects show greater surface expression of the protein CD66b, which is secreted into specific granules (23), indicating greater degranulation (22). In addition, neutrophils from obese subjects have increased oxygen reactive species, indicating oxidative burst, and greater phagocytosis (24). Additionally, neutrophils have been identified in leukocyte infiltration of key tissues in murine models (e.g. adipose tissue and endothelium) (16,25). In humans, the number of neutrophils in circulation predicts cardiovascular events, even after adjusting for body mass index (13).
The triggers of chronic inflammation in obese individuals are not well understood. The permanent nutritional surplus, associated with overnutrition, results in ongoing intracellular stress, which in turn increases the expression of inflammatory markers in several cell types (26). The involvement of actors of acute inflammation is challenging to understand. As neutrophils have a short lifespan from a few hours to days (27), the existence of active triggers influencing both increased numbers and increased inflammatory status can be assumed. Obesity may result in a change in the secretory profile of adipokines (i.e. leptin) that may have a role in the modulation of the immune response (28).
Since total and differential leukocyte counts are relatively low-cost and available for sampling, the quantitative study of circulating leukocytes could be an attractive method for monitoring obesity-associated cardiometabolic risk. However, there are important barriers to this approach. Inter-individual variability and the wide range of normal values, make it difficult to isolate the influence of obesity. Additionally, other factors influence leukocyte numbers including illness and age (4). There are also sex differences associated with the pro-inflammatory properties of estrogens (29), thus leukocyte counts can vary according to menstrual cycle or oral contraceptive use. In order to overcome these barriers larger sample sizes are needed. Another approach would be to study the qualitative characteristics of leukocytes, using more sophisticated techniques (e.g. flow cytometry or functional assays).
In a sample of healthy adolescents our purpose was to: 1) confirm the presence of obesity-associated increase in neutrophil counts as a marker of acute inflammation, exploring the potential role of leptin levels; 2) examine whether higher neutrophil counts were associated with a composite metabolic risk score and; 3) determine whether inflammatory characteristics of neutrophils were associated with adiposity in a subsample of male participants.
Methods
Participants
This observational cohort study included Chilean adolescents between 16- and 17-years old. Participants were enrolled as infants in a randomized controlled trial of iron supplementation to prevent iron deficiency anemia, in Santiago, Chile from 1991–1996. A detailed description of randomization techniques, sampling, and entrance and exclusion criteria for the original preventive trial is published elsewhere (30). Participants have been studied at 5, 10, and 16 years. For the current analysis, we examined the 16-year follow-up data, which included 679 adolescents assessed between 2009 and 2012. We excluded participants that reported any chronic or acute illnesses (in the last week) and those who had high sensitivity C-reactive protein (hsCRP) levels over 9 mg/L, since these values are suggestive of an acute infectious process. The final analytical sample included 528 participants. The studied subgroup did not differ from the 679 evaluated participants in terms of gender, nutritional status, or metabolic status. Because of the exclusion criteria, the groups differed in their inflammatory parameters (e.g. hsCRP). Parents signed informed consent and adolescents signed informed assent. Ethics Boards at the Institute of Nutrition and Food Technology, University of Chile (INTA), University of California, San Diego, and the University of Michigan approved this study.
Anthropometry, body composition and blood pressure
Two trained physicians performed the anthropometric and blood pressure measurements. Weight and height were assessed in duplicate with a Precision Hispana scale and a stadiometer accurate to 0.1 kg and 0.1 cm, respectively. Participants were measured without shoes, wearing underwear, in the Frankfurt position. Sex- and age-specific body mass index z-scores (BMIz) were calculated using World Health Organization (WHO) growth standards. Weight status was categorized as follows: BMIz < 2 = non-obese; BMIz ≥ 2 = obese. Blood pressure was measured with standard procedures and the average of 2–3 measurements was computed.
Total fat mass was assessed using Lunar Prodigy Dual Energy X-Ray Absorptiometry scan (DXA, Lunar Prodigy instrument; General Electric Medical System, Madison, WI, USA.). All participants were measured according to standard protocols on the same machine calibrated every other day. Fat mass percentage was calculated (fat mass [kg] × 100/body mass [kg]), and categorized as normal (<20%) or elevated (≥20%).
Blood sample processing
Blood samples were obtained after an overnight fast (10–12 hours). ELISA was used for determining serum levels of leptin (DRG International, Inc., USA; 1 ng/mL sensitivity). HsCRP was assessed by immunoturbidimetry (QCA S.A. Amposta, Spain, 0.02 mg/L sensitivity). Serum triglyceride, HDL cholesterol, and glucose levels were determined using an enzymatic-colorimetric test (QCA S.A., Amposta, Spain). Total leukocyte counts were obtained using an automated leukocyte counter (CELL DYN 3200, Abbot Canada) from EDTA-treated blood. Blood smears were analysed for differential leukocyte percentage by an experienced researcher, blinded to participant anthropometric data. Neutrophil counts were further categorized as upper quartile (≥75th percentile) or not (< 75th percentile); percentiles were calculated separately by sex.
In a subsample of 23 male participants, the inflammatory characteristics of neutrophils (CD16b positive cells) were assessed by looking at the surface expression of the degranulation marker CD66b through flow cytometry. EDTA-treated whole blood was stained with anti CD16b-PE (BD Becton Dickinson; New Jersey, USA) together with CD66b-FITC or isotype control (Biolegend; California, USA) within 2–3 hours of the blood draw. Red blood cells were lysed (BD Becton Dickinson; New Jersey, USA), washed with phosphate buffered saline (PBS), and centrifuged. Stained cells were stored at 4°C in formalin fixative and protected from light until analysis in a flow cytometer (CyAn™ ADP; Beckman Coulter Inc., USA), within 48 hours of staining. Analyses were conducted independently by two researchers using Summit V4.3 software (Dako, Colorado Inc. Colorado, USA). Mean fluorescent intensity (MFI) was calculated in positive cells as the mean fluorescence of CD66b divided by the MFI of cells from the same donor stained with the isotype control.
Statistical analysis
A metabolic syndrome risk z-score was constructed for the whole sample adapted from the work of Brage (31) as previously reported (32), averaging the z-scores of: the reciprocal of HDL cholesterol, mean of systolic and diastolic blood pressure measurements, waist circumference, triglycerides and glucose.
We used non-parametric statistics for skewed variables, and for analyses of the subsample of 23 participants. Comparisons of continuous variables between groups were evaluated using T-tests or Mann-Witney tests, as appropriate. Comparisons were adjusted for fat mass using ANCOVA when appropriate: 1. comparisons between males and females in leukocytes and neutrophils counts, given the sex-related body composition differences and since fat mass content influences blood cell count; 2. comparisons in metabolic z-score between groups based on sex-specific quartiles for neutrophils count. Categorical variables were compared using Chi-square tests. Bivariate correlations (Spearman) were also used. Finally, in order to explore factors associated to the circulatory number of neutrophils, we made multivariable associations (linear models) assessing the influences of sex and obesity, further exploring whether leptin modified the associations. All analyses were performed using SPSS for Windows (version 20.0, Chicago, IL, USA). P-values < 0.05 were considered statistically significant.
Results
Participants were 16.8 years old (range 16.4–18.1), and 47% female. Fifteen percent of participants were obese. Comparisons between gender and weight status are shown in Table 1. Mean BMIz did not differ by sex; nor did the proportion of obese participants (chi square; p=0.52). As expected, fat mass and leptin were significantly higher among females, compared to males. WBC count, neutrophil count and percentage were also significantly higher among females, compared to males. Neither hsCRP nor metabolic z-score differed significantly between males and females.
Table 1.
| Males | Females | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Total (n=278) | Not obese (n=235) | Obese (n=43) | Total (n=250) | Not obese (n=212) | Obese (n=38) | |
| BMI z-score ¥ | 0.6 (0.4–0.7) | 0.2 (0.1–0.4) | 2.6 (2.4–2.7) a | 0.8 (0.7–0.9) | 0.5 (0.4–0.6) | 2.6 (2.4–2.7) a |
| FM % ¥ | 22.4 (21.3–23.4) | 19.8 (18.9–20.8) | 36.0 (34.7–37.3) a | 36.6 (35.7–37.5) b | 34.9 (34.1–35.8) | 45.7 (44.6–46.8) a |
| Waist circumference, cm ¥ | 81.4 (80.0–82.7) | 77.7 (76.8–78.6) | 101.6 (98.8–104.5) a | 81.6 (80.2–83.1) | 78.3 (77.2–79.5) | 100.0 (96.6–103.3) a |
| WBC, 103/μL ¥ | 6016.5 (5843.6–6189.5) | 5865.9 (5684.9–6046.8) | 6832.8 (6367.6–7298.0) a | 6902.8 (6687.5–7118.2) b | 6795.0 (6565.4–7024.7) | 7537.5 (6942.3–8132.7) a |
| Neutrophils, 103/μL ¥ | 3393.5 (3255.5–3531.4) | 3315.2 (3166.3–3464.0) | 3819.4 (3567.3–4171.6) a | 4230.3 (4039.5–4421.0) b | 4209.1 (4010.9–4407.3) | 4348.3 (3729.3–4967.3) |
| Neutrophils, % WBC ¥ | 55.9 (55.0–56.9) | 56.0 (55.0–57.1) | 55.4 (53.0–57.8) | 61.3 (60.3–62.3) b | 61.1 (60.0–62.2) | 62.6 (60.0–65.2) |
| hsCRP, mg/L † | 0.3 (0.7–1.0) | 0.3 (0.5–0.9) | 0.9 (0.9–1.8) a | 0.4 (0.9–1.2) | 0.3 (0.6–1.0) | 1.3 (1.6–2.9) a |
| Leptin, ng/mL † | 2.2 (5.3–7.7) | 1.4 (4.2–6.9) | 11.3 (9.0–13.7) a | 15.8 (17.2–20.5) b | 13.2 (14.7–17.5) | 31.5 (28.7–40.0) a |
| MetS z-score † | 0.1 (0.1–0.2) | 0.0 (0.0–0.1) | 0.6 (0.5–0.8) a | 0.1 (0.0–0.1) | 0.0 (0.0–0.0) | 0.4 (0.2–0.6) a |
Values represent average (¥) or median (†) and (95% CI)
Not obese=BMIz < 2; Obese=BMIz ≥ 2
p<0.05 comparison between weight status within the same sex using t-test (¥) or ann-Whitney (†)
p<0.05 comparison between sex using t-test (¥) or Mann-Whitney (†)
Abbreviations= BMI: body mass index; FM: fat mass; WBC: white blood cells; hsCRP: high-sensitive C-reactive protein; MetS: z-score for metabolic variables computed as averaging the z-scores of: the reciprocal of HDL cholesterol, mean of systolic and diastolic blood pressure measurements, waist circumference, triglycerides and glucose
Stratified by sex, obese, compared to non-obese, participants showed greater hsCRP, WBC and neutrophil counts (the latter only among males). As expected, leptin levels were also affected by weight status, with an increase in leptin levels among obese participants of both sexes. In females and males, wide ranges of leptin levels were observed: 1.0 to 73.1 ng/dL in non-obese subjects and 1.0 to 76.9 ng/dL in obese participants. Obese males and females had a significantly higher metabolic z-score, compared to their non-obese counterparts (Table 1). Differences in WBC and neutrophil counts between males and females were not explained by body composition since differences associated with sex remained significant after adjusting for fat mass percentage (ANCOVA; both p<0.05).
Table 2 shows multivariable linear regression models predicting neutrophil count. Adjusting for obesity and sex, females had 837.9 × 103/μL more neutrophils compared to males, and obese participants had 332.6 × 103/μL more neutrophils compared to non-obese (Model 1). When leptin was added to the model as a covariate (Model 2), the relationship between neutrophil counts and obesity was no longer significant. As shown in figure 1, participants with a neutrophil count in the highest quartile (sex-specific) had a significantly greater metabolic z-score, after adjusting for fat mass.
Table 2.
Modeling neutrophil count [103/μL]
| Model 1 | Model 2 | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||||||||||||
| Beta | p-value | Beta | p-value | |||||||||||||||||||||||||||||||
| Female (ref=male) | 837.9 | < 0.001 | 697.9 | < 0.001 | ||||||||||||||||||||||||||||||
| Obesity (ref=not obese) | 332.6 | 0.04 | 201.2 | 0.24 | ||||||||||||||||||||||||||||||
| Leptin | - | - | 11.3 | 0.03 | ||||||||||||||||||||||||||||||
Figure 1. Metabolic risk z-score‡ according to neutrophil count†.
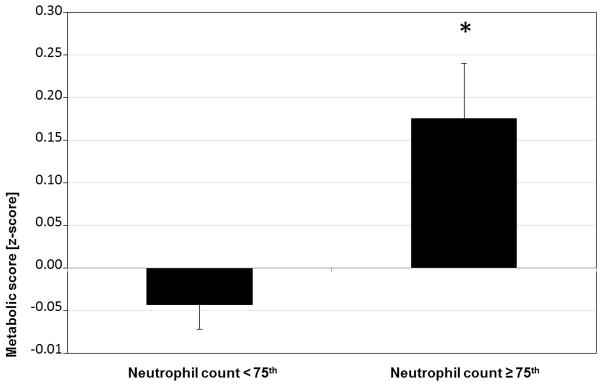
† Percentiles calculated separately for males (≥ 4054.7 × 103/μL) and females (≥ 5045.6 × 103/μL). ‡ Metabolic risk z-score for computed as averaging the z-score of: the reciprocal of HDL cholesterol, mean of systolic and diastolic blood pressure measurements, waist circumference, triglycerides and glucose.
* ANCOVA, adjusted for fat mass percentage, p=<0.01
Descriptive statistics of the male subsample are shown in Table 3. Inflammatory characteristics did not differ according to fat mass status (normal FM < 20% vs elevated FM ≥ 20%). Leptin levels and metabolic z-scores were significantly higher among participants with elevated fat mass. Most neutrophils expressed the degranulation marker CD66b (99.2%), without differences between groups (Mann Whitney; p=0.40); however, the fluorescence intensity (i.e. the amount of CD66b that was expressed on neutrophil surfaces) was significantly greater among adolescents with elevated fat mass (MFI= 8.9 vs 22.9) compared to the normal fat mass group (Figure 2). Moreover, fat mass and CD66b expression were significantly correlated (Spearman; r=0.4; p=0.03).
Table 3.
| Overall | Normal FM (n=5) | Elevated FM (n=18) | p-valuea | |
|---|---|---|---|---|
| BMI z-score | 1.4 (0.8–1.9) | −0.4 (−1.2–0.6) | 2.0 (1.4–2.3) | < 0.001 |
| FM % | 27.4 (23.6–30.4) | 15.8 (13.6–19.2) | 31.1 (27.0–32.9) | < 0.001 |
| Waist circumference, cm | 85.3 (80.5–90.0) | 73.7 (71.4–75.9) | 88.5 (83.4–93.6) | < 0.01 |
| WBC, 103/μL | 5890.0 (5550–6516.1) | 6140.0 (4474.6–7365.4) | 5835.0 (5499.4–6629.5) | 0.85 |
| Neutrophils, 103/μL | 3250.0 (3013.7–3739.6) | 3585.0 (2158.3–4410.4) | 3249.0 (2981.8–3822.8) | 0.91 |
| Neutrophils, % WBC | 55.0 (52.0–60.1) | 55.0 (43.1–67.3) | 56.0 (51.6–61.0) | 0.69 |
| hsCRP, mg/L | 0.5 (0.6–1.6) | 0.38 (−0.0–1.1) | 0.78 (0.6–1.9) | 0.25 |
| Leptin, ng/mL | 2.2 (2.6–7.5) | 1.0 (0.6–1,9) | 3.2 (3.1–9.1) | 0.01 |
| MetS z-score | 0.0 (0.0–0.5) | −0.1 (−0.6–0.34) | 0.5 (0.1–0.7) | 0.03 |
Values represent median and (95% CI)
Normal FM=<20%, Elevated FM= ≥ 20%
Mann-Whitney
Abbreviation= BMI: body mass index; FM: fat mass; WBC: white blood cells; hsCRP: high-sensitive C-reactive protein; MetS: z-score for metabolic variables computed as averaging the z-scores of: the reciprocal of HDL cholesterol, mean of systolic and diastolic blood pressure measurements, waist circumference, triglycerides and glucose
Figure 2. Basal neutrophil degranulation‡ according to fat mass category† in a subsample of male participants (n=23)¥.
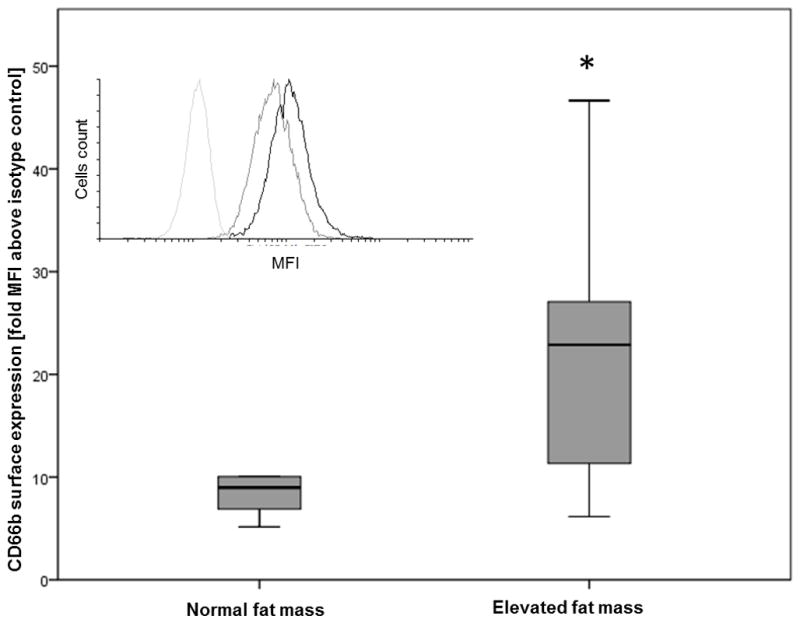
Main figure:
‡ Surface expression of CD66b in granulocytes (CD16b+ cells) by flow cytometry performed in fresh whole blood samples (ratio of CD66b-FITC median fluorescence intensity (MFI) and isotype control MFI)
† Normal fat mass <20%, elevated fat mass ≥ 20%
¥ Boxes represents median (black line) and 25th and 75th percentile and error bars represents range for CD66b surface expression.
* Mann Whitney: p=0.015.
Inset figure: Representative histogram of fluorescence intensity in FL1 for granulocytes. Isotype control (light gray line), CD66b-FITC from a low fat mass donor (dark gray line), CD66b-FITC from an increased fat mass donor (black line).
Discussion
In our sample of Chilean adolescents, obese participants showed not only increased levels of classical inflammatory markers (i.e. hsCRP in the subclinical inflammation range and elevated WBCs), indicating chronic inflammation, but also higher numbers of circulating neutrophils, suggesting acute inflammation. We found that leptin levels partially explained the association between obesity and neutrophil counts. Greater neutrophil counts were associated with worse metabolic z-scores, even after controlling for fat mass. Moreover, participants with high fat mass percentage had circulating neutrophils in a greater inflammatory state compared to those with normal fat mass.
While it is well accepted that childhood obesity is a chronic inflammatory condition (3,4,8), little attention has been paid to the involvement of neutrophils, characteristic of acute inflammation. The recent implication of this leukocyte subtype in the pathogenesis of atherosclerosis (16) suggests that increases in circulating numbers could be related to the development of future morbidity.
Our data showed higher neutrophil counts in female compared to male participants, an association not explained by fat mass. The increased inflammatory state in females might be explained by the pro-inflammatory influence of estrogen (29), a hormone that should be accounted for (either by direct assessment in serum or by asking specific questions) in future studies. Skinner et al. also found higher neutrophil counts in girls and adolescent females compared to boys and adolescent males (4). To our knowledge, no other studies have stratified similar data by gender (11,20). The well-reported cardio-protective effect of estrogens seems to contradict the pro-inflammatory effects, highlighting that our understanding of cardiovascular risk factors is only emerging.
There is evidence in humans linking obesity-triggered inflammation with cardiometabolic alterations, after accounting for adiposity. As not all obese individuals develop cardiovascular disease or insulin resistance, it is possible that the development of the pathology depends on the involvement of the immune system. Markers of this involvement include macrophages infiltrating visceral adipose tissue (5), circulating leukocytes (11,19) and hsCRP (6). In our sample, participants whose neutrophil counts were in the highest quartile had worse metabolic scores, after adjusting for fat mass. Neutrophil counts have been previously associated with cardiometabolic outcomes among adults, independent of fat mass or weight status. Reports show that neutrophil counts are positively associated with insulin levels (33), are increased among patients with metabolic syndrome (34) and predict cardiovascular events (13). We are not aware of other reports in a pediatric population.
In our study, obesity was associated with neutrophil counts, and leptin explained some of the variance in this association. The ranges of leptin values among non-obese and obese participants were large and overlapping. Our results suggest that variance in neutrophil counts is more associated with leptin values than with weight status when sex is taken into account. Other reports have shown that total leukocyte counts are positively associated with leptin after controlling for weight status (35). However, to our knowledge, this is the first study showing an influence of leptin on neutrophil counts, even though this adipokine has been previously suggested as a granulopoiesis stimulator in animals (36). Additionally, in vitro leptin stimulation of neutrophils has been shown to be a trigger of chemotaxis and oxidative burst (37), and an inhibitor of apoptosis (38), with at least one report of no effect at physiological doses (39).
In addition to assessing the quantitative effect of obesity on neutrophils, we wanted to explore whether these acute inflammatory cells were in a greater inflammatory state in those with obesity. We did this by assessing the surface expression of the degranulation marker CD66b. Given the influences of sex on neutrophil counts, we decided to study this in male participants only. The subsample studied showed a significant association between fat mass and neutrophil degranulation. Neutrophils from obese patients are expected to have increased adherence (40), phagocytosis and oxidative bursts (24). In line with our results, Nijhuis et al. showed that severely obese adults have greater expression of CD66b (22). However, other authors have reported no change or even a decrease in the expression of different adhesion molecules in neutrophil surface (41). Thus, the effect of obesity may differ depending on the marker analysed. Given the size of the subsample, we were not able to evaluate whether leptin mediated degranulation of neutrophils.
Our study has several limitations. We did not assess whether female participants were using hormonal contraception, which could have influenced inflammatory status. The small subsample, in which we studied surface expression of the degranulation marker CD66b using flow cytometry, was composed of only males. This was necessary due to the potential effects of the timing of the menstrual cycle or the use of hormonal contraceptives. We used an adaptation of the work of Brage et al. (31), which includes waist circumference, but not skinfold thickness or insulin. Comparisons with other published work should take this into consideration. Another limitation is the cross-sectional nature or our study. The strengths of this study include that the analyses were based on a large sample of 528 adolescents with data on body composition. As participants belong to a cohort that is being followed from adolescence into adulthood to assess the development of cardiometabolic alterations, next steps could include studying how baseline neutrophil levels and functioning influence the incidence and severity of the comorbidities of obesity.
In conclusion, overweight and obese adolescents had a higher number of circulating neutrophils, suggesting that obesity triggers acute inflammatory changes. A higher neutrophil count was associated with worse metabolic condition after controlling for fat mass. Moreover, in a subsample of male adolescents, participants with elevated fat mass percentage had increased inflammatory status in their circulating neutrophils, providing more evidence that overnutrition is associated with acute inflammation. Obesity was no longer significantly associated with neutrophil counts when leptin was included in the model, suggesting that alterations in the secretory profile of adipose tissue may be involved in the acute inflammatory changes observed. Neutrophils appear to be an attractive target for future studies of the role of obesity in the development of inflammation.
Acknowledgments
We thank all participants and their families for their trust and ongoing participation; we also thank the research and health professionals involved in the assessments and laboratory analysis. This study was supported by grants from the National Institutes of Health, Heart, Lung, And Blood Institute (HL088530, PI: Gahagan), and the Nevin Scrimshaw International Nutrition Foundation (Re-entry fellowship, PI: Reyes).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the founding Institutions.
Competing interests: the authors have no competing interests.
References
- 1.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–64. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 2.Burrows RA, Leiva LB, Weisstaub G, et al. High HOMA-IR, adjusted for puberty, relates to the metabolic syndrome in overweight and obese Chilean youths. Pediatr Diabetes. 2011;12:212–8. doi: 10.1111/j.1399-5448.2010.00685.x. [DOI] [PubMed] [Google Scholar]
- 3.de Ferranti SD, Gauvreau K, Ludwig DS, Newburger JW, Rifai N. Inflammation and changes in metabolic syndrome abnormalities in US adolescents: findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clin Chem. 2006;52:1325–30. doi: 10.1373/clinchem.2006.067181. [DOI] [PubMed] [Google Scholar]
- 4.Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: cross-sectional analyses throughout childhood. Pediatrics. 2010;125:e801–9. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kloting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–15. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 6.Reyes M, Gahagan S, Diaz E, et al. Relationship of adiposity and insulin resistance mediated by inflammation in a group of overweight and obese Chilean adolescents. Nutr J. 2011;10:4. doi: 10.1186/1475-2891-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–60S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 8.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107:E13. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- 9.Pratley RE, Wilson C, Bogardus C. Relation of the white blood cell count to obesity and insulin resistance: effect of race and gender. Obes Res. 1995;3:563–71. doi: 10.1002/j.1550-8528.1995.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 10.Oliver SR, Rosa JS, Milne GL, et al. Increased oxidative stress and altered substrate metabolism in obese children. Int J Pediatr Obes. 2010;5:436–44. doi: 10.3109/17477160903545163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facchini F, Hollenbeck CB, Chen YN, Chen YD, Reaven GM. Demonstration of a relationship between white blood cell count, insulin resistance, and several risk factors for coronary heart disease in women. J Intern Med. 1992;232:267–72. doi: 10.1111/j.1365-2796.1992.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 12.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44:1945–56. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 13.Sweetnam PM, Thomas HF, Yarnell JW, Baker IA, Elwood PC. Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. Am J Epidemiol. 1997;145:416–21. doi: 10.1093/oxfordjournals.aje.a009123. [DOI] [PubMed] [Google Scholar]
- 14.Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–70. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 15.Combadiere C, Potteaux S, Rodero M, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–57. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 16.Zernecke A, Bot I, Djalali-Talab Y, et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–17. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 17.Schipper HS, Nuboer R, Prop S, et al. Systemic inflammation in childhood obesity: circulating inflammatory mediators and activated CD14++ monocytes. Diabetologia. 2012;55:2800–10. doi: 10.1007/s00125-012-2641-y. [DOI] [PubMed] [Google Scholar]
- 18.Berg KE, Ljungcrantz I, Andersson L, et al. Elevated CD14++CD16− Monocytes Predict Cardiovascular Events. Circ Cardiovasc Genet. doi: 10.1161/CIRCGENETICS.111.960385. [DOI] [PubMed] [Google Scholar]
- 19.Kim JA, Park HS. White blood cell count and abdominal fat distribution in female obese adolescents. Metabolism. 2008;57:1375–9. doi: 10.1016/j.metabol.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Herishanu Y, Rogowski O, Polliack A, Marilus R. Leukocytosis in obese individuals: possible link in patients with unexplained persistent neutrophilia. European journal of haematology. 2006;76:516–20. doi: 10.1111/j.1600-0609.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 21.Shah TJ, Leik CE, Walsh SW. Neutrophil infiltration and systemic vascular inflammation in obese women. Reprod Sci. 2010;17:116–24. doi: 10.1177/1933719109348252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nijhuis J, Rensen SS, Slaats Y, van Dielen FM, Buurman WA, Greve JW. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity (Silver Spring) 2009;17:2014–8. doi: 10.1038/oby.2009.113. [DOI] [PubMed] [Google Scholar]
- 23.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes and infection/Institut Pasteur. 2003;5:1317–27. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Nieman DC, Henson DA, Nehlsen-Cannarella SL, et al. Influence of obesity on immune function. J Am Diet Assoc. 1999;99:294–9. doi: 10.1016/S0002-8223(99)00077-2. [DOI] [PubMed] [Google Scholar]
- 25.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbas ALA, Pillai S, editors. Cellular and Molecular Immunology. Elsevier Inc; Place: 2012. [Google Scholar]
- 28.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. The British journal of nutrition. 2004;92:347–55. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt M, Naumann H, Weidler C, Schellenberg M, Anders S, Straub RH. Inflammation and sex hormone metabolism. Ann N Y Acad Sci. 2006;1069:236–46. doi: 10.1196/annals.1351.021. [DOI] [PubMed] [Google Scholar]
- 30.Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–54. [PubMed] [Google Scholar]
- 31.Brage S, Wedderkopp N, Ekelund U, et al. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: the European Youth Heart Study (EYHS) Diabetes Care. 2004;27:2141–8. doi: 10.2337/diacare.27.9.2141. [DOI] [PubMed] [Google Scholar]
- 32.Khuc K, Blanco E, Burrows R, et al. Adolescent metabolic syndrome risk is increased with higher infancy weight gain and decreased with longer breast feeding. International journal of pediatrics. 2012;2012:478610. doi: 10.1155/2012/478610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon JB, O’Brien PE. Obesity and the white blood cell count: changes with sustained weight loss. Obesity surgery. 2006;16:251–7. doi: 10.1381/096089206776116453. [DOI] [PubMed] [Google Scholar]
- 34.Kaur H, Adams-Huet B, Smith G, Jialal I. Increased neutrophil count in nascent metabolic syndrome. Metabolic syndrome and related disorders. 2013;11:128–31. doi: 10.1089/met.2012.0179. [DOI] [PubMed] [Google Scholar]
- 35.Mabuchi T, Yatsuya H, Tamakoshi K, et al. Association between serum leptin concentration and white blood cell count in middle-aged Japanese men and women. Diabetes/metabolism research and reviews. 2005;21:441–7. doi: 10.1002/dmrr.540. [DOI] [PubMed] [Google Scholar]
- 36.Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci U S A. 2008;105:2017–21. doi: 10.1073/pnas.0712053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caldefie-Chezet F, Poulin A, Vasson MP. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free radical research. 2003;37:809–14. doi: 10.1080/1071576031000097526. [DOI] [PubMed] [Google Scholar]
- 38.Bruno A, Conus S, Schmid I, Simon HU. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005;174:8090–6. doi: 10.4049/jimmunol.174.12.8090. [DOI] [PubMed] [Google Scholar]
- 39.Kamp VM, Langereis JD, van Aalst CW, van der Linden JA, Ulfman LH, Koenderman L. Physiological concentrations of leptin do not affect human neutrophils. PloS one. 2013;8:e73170. doi: 10.1371/journal.pone.0073170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmblad J, Hallberg D, Engstedt L. Polymorphonuclear (PMN) function after small intestinal shunt operation for morbid obesity. Br J Haematol. 1980;44:101–8. doi: 10.1111/j.1365-2141.1980.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 41.Cottam DR, Schaefer PA, Fahmy D, Shaftan GW, Angus LD. The effect of obesity on neutrophil Fc receptors and adhesion molecules (CD16, CD11b, CD62L) Obesity surgery. 2002;12:230–5. doi: 10.1381/096089202762552674. [DOI] [PubMed] [Google Scholar]


