Abstract
Objective
This study tested whether the incidence of hospitalization for psychosis was reduced by a community-wide system of early identification and intervention to prevent onset of psychosis.
Method
The Portland Identification and Early Referral (PIER) program was initiated in 2001. Youth and young adults ages 12-35 were identified by professionals in a wide variety of educational, health and mental health settings. PIER staff assessed, confirmed risk for psychosis and provided treatment for 24 months to eligible and consenting young people (N=148). The monthly rate of hospital admissions for first episode psychosis was the outcome measure for efficacy of identification and intervention. Admission rates before and after the program began accepting referrals were compared, both in the experimental area and in aggregated urban areas of Maine. ARIMA models were used to assess the effect.
Results
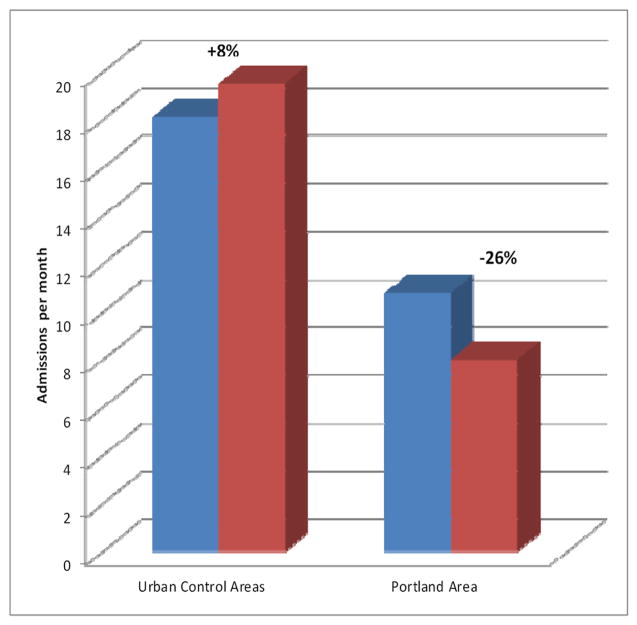
Based on ARIMA models, the rate of hospitalized psychosis decreased significantly by 26% (95% C.I., -64% to -11%) in the Greater Portland area. Conversely, it increased by 8% (95% C.I. -5% to +36%) in the Urban Control area. Including increases in the control area, the actual vs. expected difference (-26% -8%) was -34%. The change was largest for non-affective-non-schizophrenic Disorders.
Conclusions
PIER demonstrated that population-wide early identification is feasible. Preventive intervention can reduce rates of initial hospitalizations for psychosis in a mid-sized city.
Clinical trial registration
The study was reviewed and approved by the Maine Medical Center Institutional Review Board and registered at ClinicalTrials.gov (NCT01597141). All research subjects gave informed consent prior to participating.
INTRODUCTION
Schizophrenia and the psychotic forms of the mood disorders remain a major challenge to public health. Often associated with long-term disability, they rank high among all causes of Disability-Adjusted Life Years 1. In the United States, it has been estimated that the annual costs associated with schizophrenia alone are greater than $61 billion2. While improved treatment of psychotic disorders can ameliorate disability, the prevailing approaches do so to only a limited degree3.
Approaches to several other major causes of disability, such as cardiovascular disease and cancer, increasingly emphasize early intervention, if possible prior to onset of frank disease. A similar trend is emerging for psychotic disorders, where early intervention is increasingly seen as a promising approach for preventing initial episodes as well as for reducing associated disability4-6. Recent research has focused on the “prodromal” period within which it is possible to identify individuals at clinical high risk (CHR) for psychosis7. The preventive treatments tested have included psychoeducational multifamily groups, cognitive therapy, assertive community treatment, antipsychotic medication and omega-3 fatty acids8-16. A recent meta-analysis estimated the risk ratio achieved by preventive intervention to be .34 (95% C.I., .219-.575, p<.001)17. There are legitimate concerns about potential adverse effects of interventions on youth who may not develop psychosis, but these have not yet been documented, with the exception of antipsychotic drugs 18.
No previous large study has examined a central question for public health: can a community-wide effort to enhance early identification and treatment have a meaningful impact on incidence of psychotic disorders at the community level? One small-scale effort in the United Kingdom suggested that such an effect might be possible 19.
In the present study, a community-wide intervention, the Portland Identification and Early Referral (PIER) program, was implemented for persons age 12-35 years, in Greater Portland, Maine20. The goal of PIER was to reduce the incidence of psychotic disorders. PIER involved identification and referral for treatment of at-risk youth by primary care and pediatric physicians, and psychiatric, counseling, guidance and nursing personnel in educational, community mental health and health organizations and practices. Assessment of effects on true incidence requires community-ascertained measurement of rates of onset of psychosis; this was not feasible for this study. Instead, we compared the rate of first hospital admission for psychosis in the Greater Portland area, before and during implementation, with the rate in three urban areas of Maine where PIER was not implemented. This is a meaningful measure of impact on mental health policy and economics. The intervention and control areas were well defined communities, and historical data allowed us to account for secular trends. In a community-based quasi-experimental design, a sizable and significant effect on incidence of first-episode admissions requires both accuracy of early identification and efficacy of the preventive treatment, thereby alleviating some of the uncertainty in indicated prevention.
METHODS
Overall study design
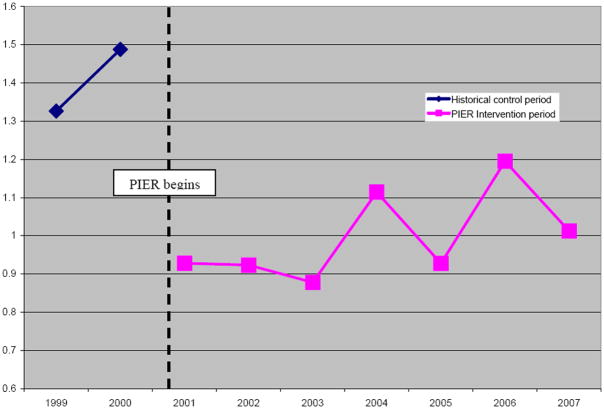
This indicated prevention study attempted to identify and offer treatment to consenting CHR persons ages 12-35 in the Greater Portland, Maine area. Three control areas neither contained nor received comparable community or clinical interventions during the study period. The primary outcome measure was change in rate of first hospital admission for psychosis (RFHAP). We hypothesized that in Greater Portland the RFHAP would be lower in the experimental period (2001-2007) than in the historical control period (1999-2000), and that this reduction would not be observed in the control areas during the same periods. In accepting referrals and computing RFHAP in the intervention and control areas, we adhered rigorously to age and geographic criteria as required for a valid test. Since two control areas were contiguous with Greater Portland, the intervention may have influenced practices in these two areas and attenuated the effect.
Study population
The Greater Portland area comprised 25 towns, including the city of Portland, its suburbs and a few surrounding rural areas. The total population was 313,918 in 2000 and increased to 326,603 by 2010. The control areas (henceforth Urban Control areas) comprised the three other most urban areas in Maine, each having a comparable population in 2000: Bangor (284,166), Augusta (276,450) and Lewiston-Auburn (201,298). Their combined population was 761,914 in 2000, and that increased to 796,484 by 2010. Thus, the total population increased by 4% in both the Greater Portland and Urban Control areas. The 12-35 year age-specific population in 2000 was 92,565 and 223, 585, respectively, and over the subsequent decade increased more in Greater Portland (8% vs. 4%). Greater Portland is almost entirely urban or suburban, whereas substantial parts of the Urban Control areas are rural (population per square mile: 3,493 in Portland, 1,043 in Lewiston, 444 in Bangor and 207 in Augusta; the density ratio, Portland:mean Urban Control area, is 6.2:1. The Greater Portland population is relatively stable, with limited in-and out-migration, and largely homogeneous with respect to race (96% Caucasian), although many diverse immigrant cultures are represented in small numbers. Despite some in-migration by international refugees during the study period, the Urban Control areas are similarly stable and homogeneous (95-98% Caucasian).
Computation of RFHAP
Counts per month for first hospital admissions for psychosis during the historical control (from the 2nd quarter of 1999 through the first quarter of 2001) and experimental (2nd quarter of 2001 through the 3rd quarter of 2007) periods were determined for Greater Portland and the Urban Control areas. The data were derived from that collected by the Maine Health Data Organization (MHDO). The database includes all persons hospitalized in Maine and records their discharge diagnoses, age and residence.
Independent analysts at the Maine Health Information Center selected inpatient discharges in the MHDO database meeting the following inclusion criteria: residing in the study catchment areas at the time of admission, at least 12 and less than 36 years of age, and a principal discharge ICD-9 diagnosis code of: schizophrenic disorder (295.xx), or mood disorder with psychotic features (296.x4), or non-affective non-schizophrenic psychosis (Brief Psychotic Episode or Psychosis NOS; 297.x or 298.x). The principal measure for analysis was the aggregate of these categories, i.e., all non-organic psychoses. This corresponded with the psychotic diagnoses identified in the prodromal stage and those used to define psychosis in a recent survey of incidence of psychosis21. Each admission was assigned an ordinal designation within each individual’s record. Counts for RFHAP were of all the admissions that were not preceded by another for a clearance period of at least 9 years preceding the starting date for the historical control period. Counts of admissions were then categorized by 28-day month (n=110 months) and area. Geographic assignment was by residential zip codes, not location of hospital.
Computation of rates
Although ARIMA was appropriate for our primary analysis in this study, this approach can be vulnerable to changes in population denominators. We computed incidence rates of first hospitalizations for psychoses. This enabled us to verify that we obtained consistent results when the denominators were taken into account, and to compare our results with other studies reporting incidence of hospitalized psychoses in well-defined populations. The computation of rates (cases/person-years observation) requires person-year denominators, here derived from 2000 and 2010 U.S. Census data for each area. Age-specific (12-35 years) and total population data were estimated for each year based on an assumption of linear change between the censuses. The population denominator for each period was the mean for the years within that period. We refer to the rate using this denominator as the annual incidence rate of hospitalized psychosis for the respective periods.
Outreach and case-finding operations
The PIER clinical team had two functions: outreach to, and education for, referring professionals, and assessment and treatment. These functions have been described previously20,22 To summarize, a key strategy in this community-wide effort was widespread education outside, as well as within, the mental health system. PIER team staff educated over 7200 physicians, school and college counselors, community mental health practitioners, community agency staff and others who had ongoing contact with potentially at-risk youth and young adults, as well as their parents. These training meetings provided information on the prodromal signs of psychosis, promoted the benefits of early treatment, and encouraged rapid referral of appropriate cases20. All those not meeting criteria were promptly referred to other clinical services.
Initial and conversion assessment
In the experimental area and period, assessment and treatment were recommended and offered to all referred, eligible and consenting youth. Inclusion criteria were: meeting CHR criteria, residing in the catchment area at the time of referral, being 12 to 35 years of age and giving informed consent or assent to research. Exclusion criteria included: a prior psychotic episode greater than 30 days in duration, IQ by history of less than 70, and evidence that psychotic symptoms were solely due to medical or toxic causes. Otherwise, those with substance abuse disorders were included. After a screening telephone interview, the PIER clinical team assessed for CHR of psychosis using the Structured Interview for Prodromal Syndromes (SIPS 23). Only those meeting the scale’s criteria for the prodromal syndrome were admitted to the study for treatment; if the individual met Presence of Psychotic Syndrome (POPS) criteria on any of 5 positive symptoms, he or she was excluded. Conversion to psychosis after intake was rated against the same POPS criteria 23. In this study, agreement between a criterion rater of SIPS ratings and the interviewers was 88% (Kappa = .778) 20, 23.
Intervention
The interventions were designed to prevent the onset of psychosis. The interventions offered to eligible patients were a specially-adapted version of Family-aided Assertive Community Treatment (FACT) or an attenuated version. FACT is an evidence-based combination of psychoeducational multifamily group treatment, assertive community treatment and supported employment and education 10, 20, 24. The attenuated version comprised education and crisis intervention for the family, psychotropic medication administered by the same criteria as in the FACT condition and, if needed, quarterly outreach to prevent drop-out. Although 50 (34%) cases were randomly assigned to the attenuated version, 24 month conversion rates, at 10% vs. 14%, were low, not significantly different and therefore contributed equally to RFHAP. All cases were monitored monthly by clinicians and assessed longitudinally by independent research interviewers for 24 months.
Statistical analysis
In both the Greater Portland and Urban Control areas, first hospitalizations for psychosis (as defined above) were divided into the historical control and experimental periods, i.e. before and after the beginning of the PIER intervention on May 6, 2001; the time series ended on September 30, 2007. To account for strong weekly hospitalization cycles, the study discharges were aggregated into 28-day totals for both the Greater Portland and Urban Control areas. Each time series of 110 observations was then fitted to Auto Regressive Integrated Moving Average (ARIMA) intervention models 25, 26. Where Yt is the number of cases observed in the tth of 110 28-day periods and where It is a (0,1) binary variable coded for the onset of the PIER program, these models can be written as
L(νt) is a lagged ARIMA polynomial constructed empirically to satisfy the “white noise” criterion,
Since L(νt) has no substantive interpretation, its structure can be ignored. Parameters α and β, interpreted as the pre-PIER time series mean and the post-PIER change in mean, were estimated with SCA27 for the Greater Portland and Urban Control areas. Maximum likelihood estimates were calculated for the two time series. The change for each area was expressed as the post-PIER change in mean as a % of the historical control period mean, while the net difference was expressed as the % change in the Greater Portland area minus the % change in the Urban Control area.
As noted earlier, we also conducted a secondary analysis, in which we computed average annual incidence rates of first hospital admissions for psychoses. We report these as “mean annual rates of N per 100,000 persons”. This is essentially equivalent to reporting rates in terms of N per 100,000 person-years, but takes into account that annual rates were derived by averaging across years. As a means of confirming that the intervention was the primary cause of changes in RFHAP, admissions per quarter were tested for correlation with quarterly counts of PIER program intakes from 2003 through 2007, after the majority of community education was completed and intake rates had stabilized.
RESULTS
Individuals referred and treated
The cases treated by the PIER clinical team in the experimental area were drawn from 404 referrals that had been screened for likelihood of meeting CHR criteria from May 6, 2001 to September 30, 2007. Of those, 285 (71%) were interviewed and assessed using the SIPS, 148 (37%) met its associated criteria and, of those, 139 (94%) accepted assignment to treatment and 9 (2%) withdrew. Thus, 56% of youth identified by those trained either met CHR criteria (on average, 23 of 42 cases per year) and offered treatment or were found to be in an early stage of psychosis (n =79, 20%, 13 per year) and were referred elsewhere for treatment. The mean age was 16.6±3.2 years; 53% (n = 78 of 148) were male. As determined by POPS criteria, the overall conversion rate was 8% (n=11 of 148) during the first 12 months.
Admissions meeting criteria
In the MHDO database, there were 13,936 admissions that met the age and diagnostic criteria and living in the control or experimental areas, from which were drawn the cases that were admitted during the study period and that met the first hospitalization criterion. That subset comprised 779 first admissions for psychosis during the historical control period and 2,283 during the intervention period, a total of 3,062.
Effects on rate of first hospital admissions for psychosis: analysis by ARIMA
First hospitalizations in the Greater Portland area decreased significantly during the intervention period. Conversely, first hospitalizations in the Urban Control areas increased (see Table 1). Hospitalizations dropped by 2.82 (95% C.I., -4.01 to -1.63) per 28-day month in the Greater Portland area following the PIER intervention. This 26% reduction translates into 189 fewer hospitalizations during the 332 weeks of the PIER intervention, or 29.7 admissions per year. The reduction is statistically significant by the most conservative criteria. In the Urban Control area, on the other hand, hospitalizations rose by 1.41 (95% C.I., -2.28 to +5.12) per 28-day month, an increase of 8% (see Figure 2). Including increases in the control area, the actual vs. expected difference (-26% -8%) was -34%. The 36 CHR and early first episode cases identified per year approximated the 29.7 calculated to have had initial hospitalizations avoided. The changes were largest for Non-affective-Non-schizophrenic Disorders (-30%) and, in decreasing order, for the Schizophrenic Disorder (-26%) and Mood Disorder with Psychosis subgroups (-19%; see Table 2). To reduce the possibility of a spurious effect, we examined correlation between PIER intake rates and RFHAP. From 2003-2007, quarterly RFHAP and PIER intakes were significantly and inversely correlated (r = -.75, p<0.001) 20.
Table 1.
Rates of hospital admission for first episode psychosis
| Historical control period (admissions per 28-day month) | Change in intervention period (admissions per 28-day month) | |||||
|---|---|---|---|---|---|---|
| α | Standard error | β | Standard error | % | 95% C.I. | |
| Greater Portland Area | 10.77 | 0.52 | -2.82 | 0.60 | -26% | -4.01 to -1.63 |
| Urban Control Area | 18.05 | 1.67 | +1.41 | 1.86 | + 8% | -2.28 to +5.12 |
Figure 2.
Rates of hospital admission for first episode psychosis per 100,000 per year: Ratio of Greater Portland area to Urban Control area, 1999-2007
The ratio is the rate for FEP hospitalizations per 100,000 persons age 12-35 per calendar year in Greater Portland divided by the same rate for the Urban Control area in the same year.
Table 2.
Rates of first hospital admission for psychosis per month: historical control vs. intervention periods, by diagnostic group and catchment area
| Control Period | Intervention Period | Change | Net Change (Intervention - Control) | ||||
|---|---|---|---|---|---|---|---|
| Diagnostic Group | Mean | 95% C.I.s | Mean | 95% C.I.s | % | % | |
| Greater Portland | Mood Disorders with Psychosis | 3.63 | 2.96, 4.30 | 2.95 | 2.58, 3.33 | -19% | -20% |
| Non- affective, non-schizophrenic Psychoses | 2.37 | 1.61, 3.13 | 1.67 | 1.39, 1.96 | -30% | -64% | |
| Schizophrenic Disorders | 4.56 | 3.68, 5.44 | 3.36 | 2.92, 3.81 | -26% | -28% | |
| Urban Control Areas | Mood Disorders with Psychosis | 8.22 | 6.96, 9.49 | 8.30 | 7.61, 8.99 | +1% | |
| Non- affective, non-schizophrenic Psychoses | 2.89 | 2.14, 3.64 | 3.88 | 3.45, 4.31 | +34% | ||
| Schizophrenic Disorders | 7.15 | 6.11, 8.19 | 7.30 | 6.59, 8.01 | +2% | ||
Analysis of incidence rates
The mean annual admission rates for age 12-35 years in the Greater Portland area were 148.1/100,000 (44.7/100,000 total population) during the historical control period vs. 107.9/100,000 (33.0/100,000 total population) in the experimental period (see Table 3). The comparable rates in the control area were 106.3/100,000 (30.9/100,000 total population) vs. 110.5/100,000 (33.3/100,000 total population), respectively. In Greater Portland, the annual difference between historical control and experimental periods was -40.2/100,000 (-11.7/100,000 total population).
Table 3.
Annual rates of incidence of hospitalizations for psychosis per 100,000 population
| Historical Control Period 1999-2000 | Intervention Period 2001-2007 | Intervention vs. Control % Change | Net Change in Rate (Intervention – Control area) | |||||
|---|---|---|---|---|---|---|---|---|
| Per 100,000 age-specific population | Per 100,000 total population | Per 100,000 age-specific population | Per 100,000 total population | Per 100,000 age-specific population | Per 100,000 total population | Per 100,000 age-specific population | Per 100,000 total population | |
| Greater Portland Area | 148.1 | 44.7 | 107.9 | 33.0 | -27% | -26% | -31% | -34% |
| Urban Control Areas | 106.3 | 30.9 | 110.5 | 33.3 | +4% | +8% | ||
DISCUSSION
This study is the first to report reduced incidence of hospitalizations for psychotic episodes using an indicated prevention strategy and methods on a large, systematic and community-wide scale. In an attempt to reduce the inherent ambiguity of predictive methods in psychiatric populations, the approach was intended to confirm the accuracy of the methods being used for identifying and treating preventively. RFHAP reduction requires both accurate identification and timely, effective preventive treatment, population-wide.
Principal findings
There was a large and significant decrease in first admissions for psychosis from historical control to intervention periods in the Greater Portland area, while that measure increased in the Urban Control catchment area. The largest difference was for Psychosis NOS/Brief Psychotic Episode, the diagnosis group with the least precision but that corresponded to the type of cases that were identified. The second largest effect was for Schizophrenic Disorders, the original target of the early intervention paradigm28. In addition, in the Greater Portland area the RFHAP rate was inversely correlated with treatment intakes, suggesting that changes in RFHAP were largely associated with the intervention program.
Comparison to other studies
The computation of annual incidence rates makes it possible to compare our results with previous studies. The results based on annual incidence rates were concordant with the ARIMA analysis. In two recent studies that reported national age-specific incidence rates of first hospitalizations for psychosis, the annual incidence rates were somewhat lower than in our study29, 30. This might be partly due to the selection of urban vs. semi-urban areas for our study. There may also be other unknown reasons. The relatively high rates for this study suggest that reduced hospitalization did not result from restrictions on access to hospital admission.
Support and threats to validity
The strength of this study lies in its simultaneous evaluating whether the community-wide process led to identification, accuracy of the identifying criteria and efficacy of the treatment in preventing hospitalization among at-risk youth and young adults. Failure of any one of those components would obviate a measurable effect. The database was comprehensive and of sufficient duration, statistical power and diagnostic detail to support the analysis.
Although first hospital admission rates do not capture non-hospitalized persons with psychoses, they are highly relevant to public health and mental health policy. The data presented here suggest that the reduction in the proportion of initial episodes that required hospitalization was primarily due to mitigation of symptoms that lead to acute episodes and other manifestations of psychiatric disorder. Further, because they were rarely hospitalized, cases of early psychosis also contributed to a reduced RFHAP, even though they were referred to but not treated by the PIER team and, in the technical sense, were not prevented from psychosis. Prevention of the initial episode of psychosis, regardless of diagnostic distinctions, makes possible a longer-term reduction in prevalence, even if only delaying the initial episode allows those at risk to develop additional resistance to later episodes. Given the enormous costs of providing inpatient treatment2, early intervention may reduce costs of care for a population that ultimately represents 2-3% of the adult population.
A legitimate concern about pre-psychotic intervention is that a majority of high risk youth (approximately 60-80%) will not develop psychosis within 1-2 years in the absence of treatment31. Recent reports indicate, however, that those who do not develop psychosis already have or will develop another psychiatric disorder and are therefore likely to benefit from early intervention 32, 33. Many will develop psychosis years later 34, 35. Because they were very early in the onset phase and of lower severity, many first-episode cases referred to PIER avoided hospitalization altogether. These factors mitigate such concerns, although they need further examination. Finally, it is possible that intervention has some adverse effects 29.
While the reduction of RFHAP was on the order of 26-34%, 66-74% of the first-admission rate was unaffected. A significant reduction to the burden of disease is in itself important, but further research on pathways to psychosis is needed. Some youth who did not meet criteria were at risk for a later psychosis or were experiencing non-psychotic disorders. Few referrals of adults in the late-20 to 35-year age range were received. Population-based public education, as occurs in medical disorders, might increase self-referrals in population subgroups missed by PIER.
Hospitalization for psychiatric disorders can be influenced by a variety of secular trends that can lead to spurious findings regarding incidence rates. The change in hospitalization rate or differences between areas could have been due to factors other than the intervention alone rates, e.g., outpatient service availability. However, the time series statistic tests the difference at the precise point of initiation of the PIER intervention, greatly reducing the chance that the effect was due to other unknown causes. Though the areas are potentially non-equivalent, the control areas in large part abut Greater Portland geographically and are similar epidemiologically and socioeconomically. Thus, the differences in admission rates corresponded precisely to the beginning of the PIER intervention and the boundaries of the catchment areas. The inherent incidence inaccuracy in this study is balanced by the fact that this dataset includes virtually all admissions of clinically incident cases among the population of Maine. The most plausible and parsimonious explanation is that the change observed was due to the intervention itself.
This study in a mid-sized U.S. city suggests that combined early identification and treatment can be effective as a public health approach for reducing rates of hospitalized first psychotic episodes, by about 1/3. It offers promising possibilities for reducing the tremendous personal, social and economic burdens imposed by the psychotic disorders. We are currently testing the same system in six cities with more diverse populations33. We hope that this study will promote wider testing and implementation of the indicated prevention approach.
Figure 1.
Rates of hospital admission for first episode psychosis: Greater Portland Area vs. Urban Control Area
Acknowledgments
This research was supported by the Center for Mental Health Services and the National Institute of Mental Health (grant number R01MH065367) and the Robert Wood Johnson, Bingham and Unum Foundations and the Betterment Fund.
Footnotes
This study was reported at the International Early Psychosis Conference, October 11-13, 2012, San Francisco, CA and the 166th Annual Meeting of the American Psychiatric Association, May 22, 2013, also in San Francisco, CA.
Contributor Information
William R. McFarlane, Maine Medical Center, Center for Psychiatric Research, 22 Bramhall Street, Portland, Maine, 04102, mcfarw@mmc.org
EZRA SUSSER, NYSPI, 722 W 168TH ST, BOX 24, NEW YORK, New York 10032.
Richard McCleary, University of California at Irvine, Irvine, California.
Mary Verdi, Maine Medical Center - Maine Medical Center Researrch Institute, Portland, Maine.
Sarah Lynch, Maine Medical Center - Maine Medical Center Research Institute, Portland, Maine.
Deanna Williams, Maine Medical Center - Maine Medical Center Research Institute, Portland, Maine.
Ian W McKeague, Columbia University - Biostatistics, New York, New York.
References
- 1.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Wu EQ. The economic burden of schizophrenia in the United States. Journal of Clinical Psychiatry. 2005;66:1122–1129. doi: 10.4088/jcp.v66n0906. [DOI] [PubMed] [Google Scholar]
- 3.Lehman AF, Steinwachs DM, Buchanan R, et al. Translating research into practice: The Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophrenia Bulletin. 1998;24:1–10. doi: 10.1093/oxfordjournals.schbul.a033302. [DOI] [PubMed] [Google Scholar]
- 4.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Archives of General Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, Meneghelli A, Valmaggia L, et al. Duration of untreated prodromal symptoms and 12-month functional outcome of individuals at risk of psychosis. The British Journal of Psychiatry. 2009;194:181–182. doi: 10.1192/bjp.bp.107.047951. [DOI] [PubMed] [Google Scholar]
- 6.O’Connell ME, Boat T, Warner KE, editors. Preventing Mental, Emotional and Behavioral Disorders Among Young People. Washington, D.C.: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 7.Ruhrmann S, Schultze-Lutter F, Salokangas RK, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Archives of General Psychiatry. 2010;67:241–251. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- 8.McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. American Journal of Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- 9.Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: Randomised controlled trial. British Journal of Psychiatry. 2004;185:291–297. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]
- 10.Nordentoft M, Thorup A, Petersen L, et al. Transition rates from schizotypal disorder to psychotic disorder for first-contact patients included in the OPUS trial. A randomized clinical trial of integrated treatment and standard treatment. Schizophrenia Research. 2006;83:9–40. doi: 10.1016/j.schres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Amminger GP, Schafer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Archives of General Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 12.Bechdolf A, Wagner M, Ruhrmann S, et al. Preventing progression to first-episode psychosis in early initial prodromal states. The British Journal of Psychiatry. 2012;200:22–29. doi: 10.1192/bjp.bp.109.066357. [DOI] [PubMed] [Google Scholar]
- 13.van der Gaag M, Nieman H, Rietdijk J, et al. Cogntive behavioral therapy for subjects at ultrahigh risk for developing psychosis. Schizophrenia Bulletin. 2012;38:1180–1188. doi: 10.1093/schbul/sbs105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGorry P, Yung A, Phillips L, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Archives of General Psychiatry. 2002;59:921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- 15.Addington J, Epstein I, Liu L, et al. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophrenia Research. 2011;125:54–61. doi: 10.1016/j.schres.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 16.McGorry PD, Nelson B, Phillips LJ, et al. Randomized controlled trial of interventions for young people at ultra-high risk of psychosis. Twelve-month outcome Journal of Clinical Psychiatry. 2013;74:349–356. doi: 10.4088/JCP.12m07785. [DOI] [PubMed] [Google Scholar]
- 17.Fusar-Poli P, Borgwardt SJ, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francey SM, N B, Thompson A, Parker AG, Kerr M, Macneil C, Fraser R, Hughes F, C K, Harrigan S, Wood SJ, Berk M, McGorry PD. Who needs antipsychotic medication in the earliest stages of psychosis? A reconsideration of benefits, risks, neurobiology and ethics in the era of early intervention. Schizophrenia Research. 2010;119:1–10. doi: 10.1016/j.schres.2010.02.1071. [DOI] [PubMed] [Google Scholar]
- 19.Falloon IR. Early intervention for first episodes of schizophrenia: a preliminary exploration. Psychiatry. 1992;55:4–15. doi: 10.1080/00332747.1992.11024572. [DOI] [PubMed] [Google Scholar]
- 20.McFarlane WR, Cook WL, Downing D, et al. Portland Identification and Early Referral: a community-based system for identifying and treating youths at high risk of psychosis. Psychiatric Services. 2010;61:512–515. doi: 10.1176/appi.ps.61.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkbride JB, Fearon P, Morgan C, et al. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center AeSOP study. Archives of General Psychiatry. 2006;63:250–258. doi: 10.1001/archpsyc.63.3.250. [DOI] [PubMed] [Google Scholar]
- 22.Joly BM, Pukstas BK, Elbaum WM, et al. Promoting Early Detection of Psychosis: The Role of Community Outreach. Journal of Public Mental Health. 2012;11:195–208. [Google Scholar]
- 23.McGlashan TH, Miller T, Woods S, et al. Structured Interview for Prodromal Syndromes. New Haven, Connecticut: Yale School of Medicine; 2003. Jul 1, Report No. [Google Scholar]
- 24.McFarlane WR. Family-based treatment in prodromal and first-episode psychosis. In: Miller T, editor. Early Intervention in Psychotic Disorders. Amsterdam: Kluwer Academic Publishers; 2001. pp. 197–230. [Google Scholar]
- 25.McCleary R, McDowall D. Time series designs. In: Cooper H, editor. Handbook of Research Methods in Psychology. Washington, DC: American Psychological Association; 2012. [Google Scholar]
- 26.Box GEP, Tiao GC. Intervention analysis with applications to economic and environmental problems. Journal of the American Statistical Association. 1975;349:70–79. [Google Scholar]
- 27.Liu L-M, Hudak GB, Box GEP, et al. Forecasting and Time Series Analysis Using the SCA Statistical System: Scientific Computing Associates Corporation. 1997 [Google Scholar]
- 28.McGlashan TH. Early detection and intervention in schizophrenia: Research. Schizophrenia Bulletin. 1996;22:327–345. doi: 10.1093/schbul/22.2.327. [DOI] [PubMed] [Google Scholar]
- 29.Becker LA. Effect size calculators. [April 11, 2010];1998 updated 2000, Available from: http://www.uccs.edu/~faculty/lbecker/index.html.
- 30.United States Census [database on the Internet] [April 1, 2011];2007 Available from: http://www.census.gov/compedia/statab/2011/tables/11s00006.xls.
- 31.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk. Archives of General Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornblatt B, Lencz T, Obuchowski M. The schizophrenia prodrome: Treatment and high-risk perspectives. Schizophrenia Research. 2002;54:177–186. doi: 10.1016/s0920-9964(01)00365-6. [DOI] [PubMed] [Google Scholar]
- 33.McFarlane WR, Cook WL, Downing D, et al. Early Detection, Intervention and Prevention of Psychosis Program: rationale, design, and sample description. Adolescent Psychiatry. 2012;2:112–124. [Google Scholar]
- 34.Nelson B, Yuen HP, Wood SJ. Long-term Follow-up of a Group at Ultra High Risk (“Prodromal”) for Psychosis. The PACE 400 Study. JAMA Psychiatry. 2013;70:793–802. doi: 10.1001/jamapsychiatry.2013.1270. [DOI] [PubMed] [Google Scholar]
- 35.Klosterkotter J, Hellmich M, Steinmeyer EM, et al. Diagnosing schizophrenia in the initial prodromal phase. Archives of General Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]