Abstract
Antibiotic use for ocular treatments has been largely limited by poor local bioavailability with conventional eyedrops formulations. Here, we developed a controlled delivery system composed of moxifloxacin-loaded poly(lactic-co-glycolic acid) (PLGA) microparticles encapsulated in a chondroitin sulfate-based, two-component bioadhesive hydrogel. Using a simple and fast electrohydrodynamic spray drying (electrospraying) technique, surfactant-free moxifloxacin-loaded microparticles were fabricated with diameters on the order of 1 μm. A mixed solvent system of methanol/dichloromethane (MeOH/DCM) was employed to prepare the microparticles for the electrospraying processing. Extended release of moxifloxacin using a series of MeOH/DCM mixed solvents was accomplished over 10 days with release concentrations higher than the minimum inhibitory concentration (MIC). In contrast, moxifloxacin loaded directly in hydrogels was released rapidly within 24 h. We observed a decrease of the drug release rate from the microparticles when using an increased percentage of methanol in the mixed solvent from 10% to 30% (v/v), which can be explained by the mixed solvent system providing a driving force to form a gradient of the drug concentrations inside the microparticles. In addition, the delivery system developed in this study, which incorporates a bioadhesive to localize drug release by in situ gelling, may potentially integrate antibiotic prophylaxis and wound healing in the eye.
Keywords: Electrospraying, Moxifloxacin, Microparticle, Bioadhesive, Ophthalmic release
1. Introduction
Moxifloxacin (MXF) is a fourth-generation fluoroquinolone with a wide spectrum of antibacterial activities, which has been used to treat conjunctival infections in ophthalmology [1,2]. Conventional eyedrops show relatively poor ocular bioavailability because of the high drainage rate of tear fluid and limited contact time [3]. Therefore, sustained and localized release of the antibiotics would improve ophthalmic patient compliance. Various delivery vehicles have been developed to control the release of antibiotics, including particles [4,5], gels [6,7], and polymeric inserts [8]. However, the currently available methods still show limited release capability or are inconvenient for the patient, when applied directly to the eye.
Electrohydrodynamic spray drying (electrospraying) is an electrostatic processing technique that has recently attracted increasing attention [9,10]. This technique utilizes high voltage in which a portion of a charged stationary liquid is ejected from the surface because the electrical tension forces overcome the surface tension force [11]. The charged stationary liquid becomes unstable quickly and breaks up into a mist of very fine charged electrosprayed droplets. These droplets exhibit radii from hundreds of micrometers down to a few nanometers, depending upon the electrospraying processing conditions. When a polymer solution is applied, the solvent in the small droplets quickly dries and the droplets, when collected, become polymeric particles. Unlike the double-emulsion technique [12,13], electrospraying requires no surfactant and can produce drug-loaded particles simply and quickly.
Recently, we developed chondroitin sulfate-based bioadhesive systems for medical applications [14–16]. Specifically, a chondroitin sulfate-polyethylene glycol (CS–PEG) two-component bioadhesive system is chosen in this study [15,16]. The first component is chondroitin sulfate (CS) functionalized with N-hydroxysuccinimide (NHS), i.e. chondroitin sulfate succinimidyl succinate (CS–NHS), which is able to form amide bonds by reacting with the primary amines widely available in human tissues. The second component employs six-arm polyethylene glycol amine PEG–(NH2)6 as a crosslinker to provide strong cohesive forces inside the bioadhesive hydrogel. This bioadhesive system (CS–PEG) has demonstrated cytocompatibility with corneal cells and showed great potential as an ophthalmic adhesive [15]. The ability to simultaneously deliver pharmaceuticals from this material would enable more functionality and eliminate the need for eyedrops.
This paper focuses on developing a moxifloxacin delivery system composed of moxifloxacin-loaded microparticles encapsulated in CS–PEG bioadhesives to achieve sustained antibiotic release and improve drug bioavailability for ophthalmic treatments. Microparticles were prepared using the electrospraying technique. The CS–PEG bioadhesives were applied to localize the microparticles by in situ gelling. This unique drug-release system combines the advantages of using microparticles to better control drug release from the hydrogel and using bioadhesives to keep the microparticles from being washed out. Additionally, this system allows convenient application to appropriate ocular infection sites or any other sites of interest [17].
2. Materials and methods
2.1. Materials
Moxifloxacin HCl was purchased from Bayer (Leverkusen, Germany) and used as received. Poly(lactic-co-glycolic acid) (PLGA, 50:50, MW 40,000–75,000), dichloromethane (DCM), methanol (MeOH), triethylamine, phosphoric acid (50%), and acetonitrile (HPLC grade) were purchased from Sigma-Aldrich and used as received. Chondroitin sulfate succinimidyl succinate (CS–NHS) was synthesized as described previously [15,16] by reacting CS (25 kDa, New Zealand Pharmaceuticals Ltd., Palmerston North, New Zealand) with N-(3-dimethylamino propyl)-N′-ethyl carbodiimide hydrochloride (EDC, Sigma) and N-hydroxysuccinimide (NHS, Pierce). PEG–(NH2)6 (15.0 kDa) was purchased from Sunbio and used as received. Dulbecco's phosphate buffered saline (PBS, 1×) and (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer solution (HEPES, 1 M) were purchased from Invitrogen and used as received.
2.2. Methods
2.2.1. Microparticle preparation
Moxifloxacin HCl-loaded PLGA microparticles were fabricated using an electrospraying technique. Drug-loaded polymer solutions were prepared containing 1.0 wt% PLGA and 0.05 wt% moxifloxacin HCl dissolved in one of the three different mixed solvents of MeOH/DCM = 10:90, 20:80, and 30:70 (v/v), respectively. Each polymer solution was fed into a syringe (30 mL, Norm-Ject) and controlled by a syringe pump (NE-1000, New Era Pump Systems, Inc., Farmingdale, NY) at a flow rate of 2 mL/h. A needle (flat tip, 22G) was connected with the positive electrode of a high voltage supply (Gamma High Voltage Research, Inc., Ormond Beach, FL). A stainless steel tray (32 cm L × 26 cm W × 6 cm H) was placed at an angle of 35° beside the needle with the needle pointing to the center of the tray at a distance of 15 cm. This tray was grounded and used as the particle collector. A high voltage of 11–13 kV was used and the particles were collected for 10 h for each polymer solution. The microparticles on the plate were further dried in air at room temperature for at least 12 h. Distilled water (40 mL) was then used to collect the particles gently using a small brush. The collected particle solutions were loaded into a centrifuge tube (50 mL) and centrifuged at 3500 rpm for 10 min. After pouring out most of the water, the precipitated microparticles were vortexed, stored in the freezer at −80 °C overnight, and then dried completely using lyophilization for three days.
2.2.2. Scanning electron microscopy (SEM)
The morphology of the moxifloxacin-loaded PLGA microparticles was examined using scanning electron microscopy (ESEM, Quanta 200, FEI, Hillsboro, OR) at an accelerating voltage of 5 kV after being coated with a 10 nm layer of Pd. The average size and size distribution of the microparticles were determined from 300–400 microparticles in each condition using ImageJ (NIH 7.0.0) software.
2.2.3. Drug release
Moxifloxacin-loaded PLGA microparticles were embedded in CS–PEG hydrogels for in vitro drug release testing. CS–NHS and PEG–(NH2)6 were dissolved in PBS buffer and PBS/HEPES = 1:1 (v/v) buffer to form 20% (w/v) solutions, respectively. PLGA microparticles of 2–5 mg were dissolved in 50 μL PEG–(NH2)6 solution, and then mixed with 50 μL CS–NHS solution. The mixed solution was set quickly in a cylinder cap with a diameter of 6 mm for 10 min to form a CS–PEG hydrogel. The PLGA microparticle-embedded CS–PEG gel was placed in 2 mL PBS buffer (pH = 7.4) at 37 °C and 5% CO2. The release solutions were sampled and refreshed at predetermined time points. The sampling efficiencies were used to maintain the release solutions in a sink condition. The sampled release solutions were analyzed by high performance liquid chromatography (HPLC, Waters, Milford, MA). Three types of moxifloxacin-loaded PLGA microparticles prepared using different mixed solvents were studied. CS–PEG hydrogels with 5–10 μg moxifloxacin HCl incorporated directly were also tested as controls. Drug release experiments were run in duplicate for each condition and average values were reported for each elution time point. Moxifloxacin loading in PLGA particles was tested by dissolving 1–2 mg particles in 1 mL 0.1 M NaOH at 60 °C for 6 h. The dissolved solution was filtered and examined using HPLC.
2.2.4. High performance liquid chromatography (HPLC)
Drug release solutions were characterized using a Waters HPLC system equipped with a pump, an autosampler, a UV/VIS detector set at 293 nm, and an analytical column (Waters, XTerra, RP18, 4.6 mm × 50 mm, 5 μm). The mobile phase consisted of 80:20 (v/v) HPLC grade water (with 2% triethylamine and 2% phosphoric acid (50%))–HPLC grade acetonitrile (with 10% water). The flow rate was set at 1 mL/min. The total run time of the HPLC analysis was 6 min and the retention time of moxifloxacin HCl was 3.7 min. The calibration curve of the area-under-the-curve (AUC) vs. concentration of the drug was determined using four standard solutions with concentrations of 0.0 μg/mL, 2.0 μg/mL, 10.0 μg/mL and 50.0 μg/mL to reveal linear relationships ( R2 = 0.9999).
2.2.5. Statistical fitting
All drug release profiles were fitted to Eq. (1) using SigmaPlot 11.0 software. Nonlinear regression was used with iterations of 100, step size of 100, and tolerance of 0.0001. The coefficient of determination, R2, was determined for each fitting curve.
3. Results and discussion
3.1. Microparticles fabrication
Moxifloxacin-loaded PLGA microparticles were prepared using an electrospraying technique. The electrospraying technique produces small droplets from a polymer solution under high voltage. The small droplets dry quickly in the air and become polymer particles before being collected. The electrospraying processing conditions were carefully optimized in terms of polymer solution preparation, voltage and flow rate applied, and particle collection method.
Three factors play an important role in preparation of the polymer solution: the polymer selection, the solvent system, and the polymer and drug concentrations. As shown in Fig. 1, moxifloxacin has a low molecular weight of 401 g/mol, and a relatively high aqueous solubility compared to its minimum inhibitory concentration (MIC) of 0.03 μg/mL [2]. Hence, the well-studied biocompatible polymer, PLGA (50/50), was selected in this study to extend the release of moxifloxacin by restricting the diffusion of the drug incorporated in the polymer networks due to its hydrophobicity, biocompatibility, and relatively high glass transition temperature [18,19].
Fig. 1.
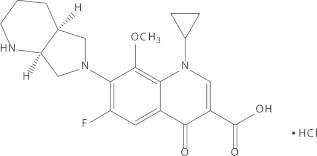
Molecular structure of moxifloxacin.
The selection of the solvent for microparticle fabrication is critical. As noted above, the drug is hydrophilic, but the polymer chosen is hydrophobic. The solvent requires (1) low boiling temperature, (2) hydrophilicity to dissolve antibiotics, and (3) hydrophobicity to dissolve the polymer. Therefore, a two-component mixed solvent system of MeOH/DCM was employed in this study. The moxifloxacin demonstrates good solubility in methanol, but not in dichloromethane. In contrast, the PLGA can dissolve easily in dichloromethane, but not in methanol. Both moxifloxacin and PLGA were able to dissolve in the MeOH/DCM at various ratios of 10:90, 20:80, and 30:70 (v/v). Finally, a low PLGA concentration of 1 wt% in the mixed solvent solution was applied to avoid particle tail formation during the electrospraying process.
The voltage and flow rate applied in this study were also carefully designed. During the electrospraying process (Fig. 2a), the polymer solution ejected from the charged needle under high voltage can demonstrate different geometrical forms [11]. When electric stresses are balanced with the other forces existing in the liquid meniscus, including surface tension force, gravity and viscosity force, the liquid meniscus assumes Taylor cone geometry and breaks up into droplets with a monodispersed size distribution [20,21]. This electrospraying mode is called the cone-jet mode. If the forces on the liquid meniscus cannot maintain a balance under electrospraying conditions, the liquid meniscus becomes unstable and transforms to different shapes other than the Taylor cone geometry [9,11]. In this work, we used a flow rate of 2 mL/h to produce particles at a rate of approximately 20 mg/h. At this flow rate, the high voltage was adjusted carefully so that the polymer solution meniscus ejected from the needle was stable under the cone-jet mode. Specifically, for the three polymer solutions using MeOH/DCM of 10:90, 20:80, and 30:70, we observed a cone-jet mode at 11 kV, 12 kV, and 13 kV, respectively. To avoid any drug release burst, these electrosprayed microparticles were finally collected and rinsed with water, and then lyophilized before use (Fig. 2b).
Fig. 2.
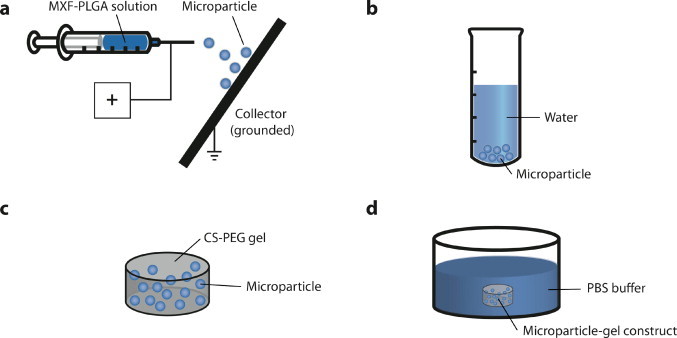
In vitro antibiotic release procedures: (a) moxifloxacin (MXF)-loaded microparticles were prepared from MXF–PLGA solution using electrospraying technique; (b) these moxifloxacin-loaded microparticles were collected by water-rinsing, vortexing, centrifugation, and lyophilization; (c) moxifloxacin particles were encapsulated into a CS–PEG bioadhesive hydrogel; and (d) in vitro release of moxifloxacin was performed in PBS buffer (pH = 7.4).
3.2. Microparticle characterization
The ratio of the MeOH/DCM solvent plays an important role in the morphology of the particles collected. SEM images of moxifloxacin-loaded PLGA microparticles are shown in Fig. 3. These particles have an average diameter of about 1 μm with decreasing particle size observed with increasing methanol content (Table 1). For each solvent system, the PLGA microparticles were formed in two sets of particle sizes, with the smaller particles having diameters of less than 1 μm. These smaller microparticles probably resulted from the break up of larger microparticles because of coulombic fission. Coulombic fission is a unique phenomenon of charged polymer droplets. It occurs when the electrostatic repulsion force resulting from surface charges increases beyond the surface tension force of droplets as a result of evaporation [22]. As methanol content in the solvent system of MeOH/DCM was increased from 10%, to 20%, and to 30% (v/v), the percentage of microparticles with diameters less than 1 μm increased from 29%, to 61%, and to 77%, respectively. Methanol (b.p. 65 °C) and dichloromethane (b.p. 40 °C) have different evaporation rates, so the mixed solvent with the higher methanol content dried more slowly while the particles were flying in the air before collected, and those particles tended to break up more easily before collection. This may also explain why the PLGA microparticles obtained at MeOH/DCM = 30:70 had rougher morphologies compared to those in the other two solvent systems.
Fig. 3.
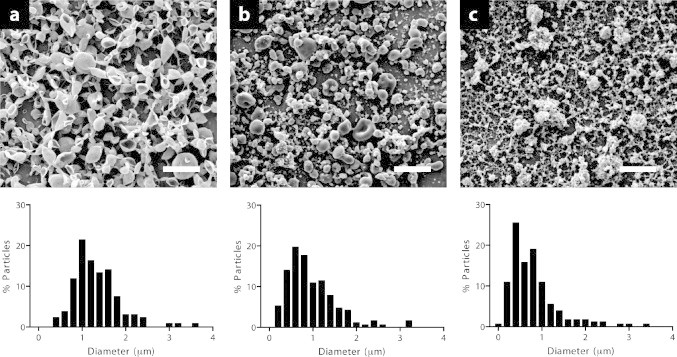
SEM images (top) and particle size distributions (bottom) of moxifloxacin-loaded PLGA microparticles prepared using a mixed solvent system of MeOH/DCM with ratios of (a) 10:90, (b) 20:80, and (c) 30:70. Scale: 5μm.
Table 1.
Characteristic parameters of moxifloxacin-loaded microparticles
| MeOH/DCM (%, v/v) | MXF content (%, w/w) | Voltage (kV) | Particle Dia. (μm) | t50 (d) | t90 (d) | β (d−1) | R2 (%) |
|---|---|---|---|---|---|---|---|
| 10:90 | 0.21 | 11 | 1.3±0.5 | 0.17 | 3.86 | 3.39 | 99.0 |
| 20:80 | 0.16 | 12 | 1.0±0.6 | 0.09 | 2.96 | 5.27 | 97.4 |
| 30:70 | 0.28 | 13 | 0.8±0.6 | 0.66 | 8.31 | 0.88 | 99.5 |
| Ctrl | N/A | N/A | N/A | 0.03 | 0.14 | 43.27 | 99.2 |
3.3. Moxifloxacin release
In vitro release of moxifloxacin from PLGA microparticles embedded in CS–PEG bioadhesive hydrogels was investigated. These particles were collected and rinsed with water (Fig. 2b) to avoid any drug burst release from the moxifloxacin-loaded PLGA microparticles. The remaining drug concentration in the microparticles was 0.21 wt%, 0.16 wt%, and 0.28 wt% for MeOH/DCM of 10:90, 20:80, and 30:70, respectively (Table 1).
Microparticles were encapsulated in a CS–PEG two-component bioadhesive by in situ gelling (Fig. 2c). The structures of CS–NHS and PEG–(NH2)6 are shown in Fig. 4a and b. Since the NHS functional group is able to form amide bonds by reacting with any primary amines, this bioadhesive can also integrate with human tissues, such as those in the eye (Fig. 4c). The drug release tests of the microparticle–bioadhesive composite were performed in 2 mL PBS buffer solution (pH = 7.4) at 37 °C (Fig. 2d).
Fig. 4.
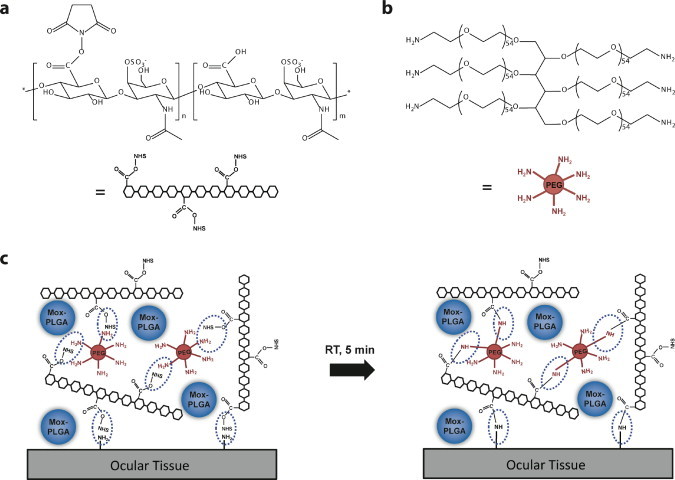
Schematic demonstration of molecular structures of (a) chondroitin sulfate succinimidyl succinate (CS–NHS), (b) six arm polyethylene glycol amine (PEG–(NH2)6), and (c) in situ gelling process of CS–PEG bioadhesive for encapsulating moxifloxacin-loaded PLGA microparticles at room temperature (RT).
We observed slow release of moxifloxacin using the mixed MeOH/DCM solvent. Fig. 5 illustrates the moxifloxacin release profiles from PLGA particles using MeOH/DCM = 30:70 embedded in CS–PEG hydrogel. The two hydrogels were loaded with moxifloxacin of 4.5 μg and 9.3 μg, respectively. Despite the large difference in loaded drug amounts, the release profiles show a consistent fractional release (Fig. 5b).
Fig. 5.
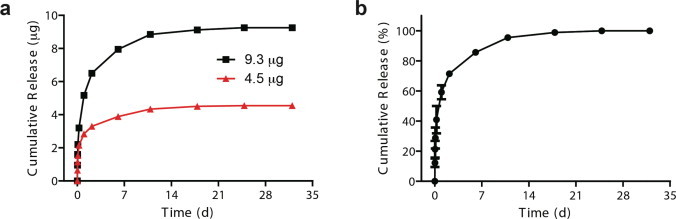
(a) Cumulative mass release and (b) fractional release of moxifloxacin from PLGA microparticles prepared using MeOH/DCM of 30:70 (average of two release experiments).
The solvent system used to prepare the microparticles strongly influenced the drug release rate (Fig. 6). The release speed of moxifloxacin using the mixed solvent of MeOH/DCM = 10:90 was slightly slower than that using MeOH/DCM = 20:80. The time required for 50% drug release, called t50, was decreased from 4.1 h for MeOH/DCM = 10:90 to 2.2 h for MeOH/DCM = 20:80 (Table 1). Similarly, the time required for 90% drug release, t90, was reduced from 3.9 days for MeOH/DCM = 10:90 to 3.0 days for MeOH/DCM = 20:80. The increased drug release rate with increasing content of methanol in the solvent system is probably due to the decrease in microparticle sizes when using MeOH/DCM = 20:80 as compared with MeOH/DCM = 10:90. The microparticles obtained from the MeOH/DCM = 30:70 solvent system had a much slower drug release rate than the other two solvent concentrations (t50 = 15.8 h or 0.66 days, and t90 = 8.31 days), although these particles obtained were even smaller than those in the other two conditions. This will be discussed later. Release of moxifloxacin was much more rapid when the drug molecules were incorporated directly into the hydrogel (t50 = 0.7 h or 0.03 days, and t90 = 3.4 h or 0.14 days).
Fig. 6.
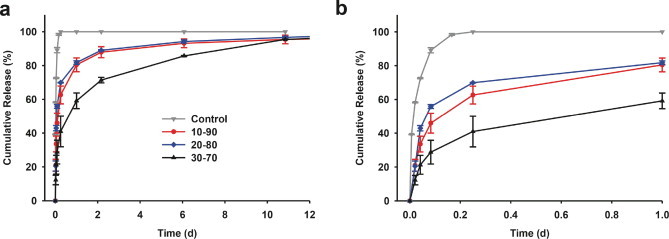
Moxifloxacin release profiles for 12 days (a) from PLGA microparticles embedded in CS–PEG hydrogel prepared using MeOH/DCM of 10:90, 20:80, and 30:70, as well as a control with the antibiotics loaded directly into the CS–PEG hydrogel. (b) Details of the drug release profiles for Day 1.
All of the release curves in Fig. 6 were well fitted by drug release approximation equation based on Fickian diffusional transport [19],
| (1) |
where f(t) is defined as the ratio of the absolute cumulative mass of drug released at time, t, to that at infinite time, and β is the initial diffusion rate constant. As shown in Table 1, the initial diffusion rate constant was decreased from 5.27 d−1 for MeOH/DCM = 20:80, to 3.39 d−1 for MeOH/DCM = 10:90, and to 0.88 d−1 for MeOH/DCM = 30:70. All initial diffusion rate constants were greatly reduced compared to the control with the drug incorporated in the hydrogel directly (i.e. 43.27 d−1). As expected, the initial diffusion rate constant showed an opposite relationship as t50.
The daily release rate of moxifloxacin decreased exponentially with time (Fig. 7). As expected, the slope of the daily release rate vs. time in log–-log scale was smaller for MeOH/DCM = 30:70 than those for MeOH/DCM of 10:90 and 20:80. The two daily release rate slopes were close for two cases of MeOH/DCM = 30:70 solvent loaded with different amounts of moxifloxacin, which indicates that the daily release rate at each time point can be easily scaled up by adding more drug-loaded microparticles. All of these conditions show an effective release over 10 days with the release concentration higher than the minimum inhibitory concentration (MIC, 0.03 μg/mL) [2]. As little as 4.5 μg of moxifloxacin loaded in the microparticles made with MeOH/DCM = 30:70 was sufficient to achieve a drug release that was higher than the MIC for more than 10 days. This amount of moxifloxacin in the microparticles is only 1.8% of the drug in one drop of moxifloxacin eyedrops (VIGAMOX® Ophthalmic Solution, moxifloxacin HCl 0.5%, 5 mg/mL, Alcon Laboratories, Inc., Fort Worth, TX). Currently, moxifloxacin eyedrops are applied 3 times a day for 7 days to the surface of the affected eye. The controlled drug system using moxifloxacin-loaded PLGA particles applied in situ by CS–PEG bioadhesive is promising for a one-time application with much higher efficacy than an eye drop formulation.
Fig. 7.
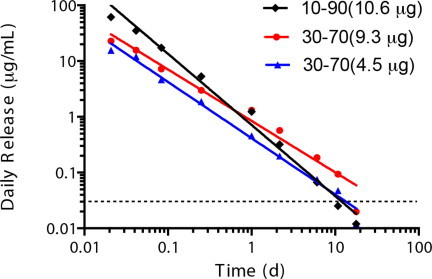
Moxifloxacin release rates from PLGA microparticles prepared using MeOH/DCM of 10:90 and 30:70. The total drug loading mass is indicated. The minimum inhibitory concentration (MIC) of moxifloxacin was 0.03μg/mL, as shown by the dashed reference line.
3.4. Microparticle formation and release mechanism
The unique solvent system applied in the electrospraying solution strongly influenced the drug release behavior. As noted above, moxifloxacin is comparatively hydrophilic and has good solubility in methanol, but not in dichloromethane. In contrast, the PLGA polymer is hydrophobic and can dissolve easily in dichloromethane, but not in methanol. Since methanol has a much higher boiling temperature than dichloromethane, methanol dries more slowly than dichloromethane and shows greater accumulation in the center of the polymer particle. This solvent distribution likely provides a driving force for the drug molecules to diffuse into the center of the particles, leading to a gradient of the drug concentration, and thus a decrease in the drug release rate from the particles (Fig. 8). This may explain why, in the three tested solvent systems, the MeOH/DCM = 30:70 solvent, with the highest content of methanol, produced particles with longest duration drug release, despite having the smallest particle size.
Fig. 8.
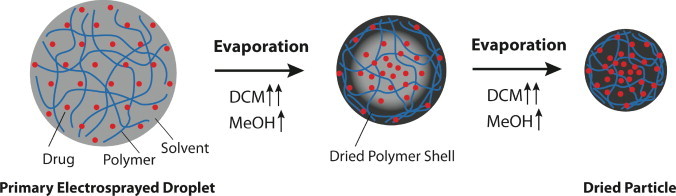
Microparticle formation mechanism during electrospraying processing. During evaporation of a primary electrosprayed droplet, the mixed solvent system of MeOH/DCM provides a driving force for the moxifloxacin molecules to diffuse into the center of the polymeric particles.
This phenomenon may also explain why a bowl-like morphology was obtained when using the MeOH/DCM = 10:90 and 20:80 mixed solvents. During the drying process, the solvent at the edge of the electrosprayed droplets evaporated first and formed a polymer shell. If the particles are collected before they are completely dry, the high impact of the particles when reaching the collector could force the particles to form in a bowl shape.
4. Conclusion
We investigated controlled delivery of moxifloxacin from polymer microparticles encapsulated in a CS–PEG two-component bioadhesive hydrogel for ocular treatments. Moxifloxacin-loaded PLGA microparticles were successfully prepared using an electrospraying technique under optimized conditions for polymer solution preparation, voltage and flow rate applied, and particle collection method. We achieved extended release of moxifloxacin using a series of mixed MeOH/DCM solvents. All release curves follow a Fickian diffusional release pattern. We found that the mixed solvent system may provide a driving force for the moxifloxacin molecules to diffuse into the center of the polymer particles when prepared by electrospraying processing. This would likely lead to a gradient of drug concentrations in the particles and, thus, a decrease in the drug release rate from the particles. This electrospraying technique using the unique solvent systems may be applicable for achieving controlled release of other types of small molecule hydrophilic drugs, which, using conventional techniques, has been a challenge.
Currently, ophthalmic release of the antibiotics between 7 and 14 days is suggested to increase bacterial eradication and to avoid possible adverse events [23]. The controlled release of moxifloxacin for 10 days, as achieved in this study, may lead to development of a moxifloxacin in situ gelling microparticles–bioadhesive delivery system that may be applied in one dose and will have a much higher efficacy than conventional eyedrop formulations have. Furthermore, the microparticle–bioadhesive delivery system in this study also provides a template for controlled release of drugs other than moxifloxacin and for localized release in human tissues other than those in the eye. Additionally, this delivery system may be particularly helpful for farm, lab and pet animals when confronting with dosage difficulties.
Acknowledgments
We acknowledge the Arthritis Foundation Postdoctoral Fellowship Award (QG) and the Congressionally Directed Medical Research Program under the U.S. Army Medical Research and Materiel Command (Contract no. W81XWH-09-2-0173, Program Manager Dr. Dwayne Taliaferro).
References
- 1.Culley C.M., Lacy M.K., Klutman N., Edwards B. Moxifloxacin: clinical efficacy and safety. American Journal of Health-System Pharmacy. 2001;58:379–388. [PubMed] [Google Scholar]
- 2.Speciale A., Musumeci R., Blandino G., Milazzo I., Caccamo F., Nicoletti G. Minimal inhibitory concentrations and time-kill determination of moxifloxacin against aerobic and anaerobic isolates. International Journal of Antimicrobial Agents. 2002;19:111–118. doi: 10.1016/s0924-8579(01)00486-1. [DOI] [PubMed] [Google Scholar]
- 3.Miller D. Review of moxifloxacin hydrochloride ophthalmic solution in the treatment of bacterial eye infections. Clinical Ophthalmology. 2008;2:77–91. doi: 10.2147/opth.s1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kisich K.O., Gelperina S., Higgins M.P., Wilson S., Shipulo E., Oganesyan E., Heifets L. Encapsulation of moxifloxacin within poly(butyl cyanoacrylate) nanoparticles enhances efficacy against intracellular Mycobacterium tuberculosis. International Journal of Pharmaceutics. 2007;345:154–162. doi: 10.1016/j.ijpharm.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 5.Ventura C.A., Tommasini S., Crupi E., Giannone I., Cardile V., Musumeci T., Puglisi G. Chitosan microspheres for intrapulmonary administration of moxifloxacin: interaction with biomembrane models and in vitro permeation studies. European Journal of Pharmaceutics and Biopharmaceutics. 2008;68:235–244. doi: 10.1016/j.ejpb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S., Lobel E., Trevgoda A., Peled Y. A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. Journal of Controlled Release. 1997;44:201–208. [Google Scholar]
- 7.Shastri D.H., Prajapati S.T., L.D.Patel Design and development of thermoreversible ophthalmic in situ hydrogel of moxifloxacin HCl. Current Drug Delivery. 2010;7:238-243 doi: 10.2174/156720110791560928. [DOI] [PubMed] [Google Scholar]
- 8.Chee S.P. Moxifloxacin punctum plug for sustained drug delivery. Journal of Ocular Pharmacology and Therapeutics. 2012;28:340–349. doi: 10.1089/jop.2011.0162. [DOI] [PubMed] [Google Scholar]
- 9.Jaworek A., Sobczyk A.T. Electrospraying route to nanotechnology: an overview. Journal of Electrostatics. 2008;66:197–219. [Google Scholar]
- 10.Chakraborty S., Liao I.C., Adler A., Leong K.W. Electrohydrodynamics:a facile technique to fabricate drug delivery systems. Advanced Drug Delivery Reviews. 2009;61:1043–1054. doi: 10.1016/j.addr.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaworek A., Krupa A. Classification of the modes of EHD spraying. Journal of Aerosol Science. 1999;30:873–893. [Google Scholar]
- 12.Rosca I.D., Watari F., Uo M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. Journal of Controlled Release. 2004;99:271–280. doi: 10.1016/j.jconrel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Khoee S., Yaghoobian M. An investigation into the role of surfactants in controlling particle size of polymeric nanocapsules containing penicillin-G in double emulsion. European Journal of Medicinal Chemistry. 2009;44:2392–2399. doi: 10.1016/j.ejmech.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 14.Wang D.A., Varghese S., Sharma B., Strehin I., Fermanian S., Gorham J., Fairbrother D.H., Cascio B., Elisseeff J.H. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nature Materials. 2007;6:385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strehin I., Ambrose W.M., Schein O., Salahuddin A., Elisseeff J. Synthesis and characterization of a chondroitin sulfate-polyethylene glycol corneal adhesive. Journal of Cataractand Refractive Surgery. 2009;35:567–576. doi: 10.1016/j.jcrs.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Strehin I., Nahas Z., Arora K., Nguyen T., Elisseeff J. A versatile pH sensitive chondroitin sulfate-PEG tissue adhesive and hydrogel. Biomaterials. 2010;31:2788–2797. doi: 10.1016/j.biomaterials.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peppas N.A., Sahlin J.J. Hydrogels as mucoadhesive and bioadhesive materials:a review. Biomaterials. 1996;17:1553–1561. doi: 10.1016/0142-9612(95)00307-x. [DOI] [PubMed] [Google Scholar]
- 18.Shive M.S., Anderson J.M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced Drug Delivery Reviews. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 19.Guo Q., Knight P.T., Mather P.T. Tailored drug release from biodegradable stent coatings based on hybrid polyurethanes. Journal of Controlled Release. 2009;137:224–233. doi: 10.1016/j.jconrel.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Taylor G. Disintegration of water drops in an electric field. Proceedings of the Royal Society of London, Series A. 1964;280:383–397. [Google Scholar]
- 21.Meesters G.M.H., Vercoulen P.H.W., Marijnissen J.C.M., Scarlett B. Generation of micron-sized droplets from the Taylor cone. Journal of Aerosol Science. 1992;23:37–49. [Google Scholar]
- 22.Duft D., Achtzehn T., Muller R., Huber B.A., Leisner T. Coulomb fission: Rayleigh jets from levitated microdroplets. Nature. 2003;421:128. doi: 10.1038/421128a. [DOI] [PubMed] [Google Scholar]
- 23.Miehlke S., Krasz S., Schneider-Brachert W., Kuhlisch E., Berning M., Madisch A., Laass M.W., Neumeyer M., Jebens C., Zekorn C., Knoth H., Vieth M., Stolte M., Lehn N., Morgner A. Randomized trial on 14 versus 7 days of esomeprazole, moxifloxacin, and amoxicillin for second-line or rescue treatment of Helicobacter pylori infection. Helicobacter. 2011;16:420–426. doi: 10.1111/j.1523-5378.2011.00867.x. [DOI] [PubMed] [Google Scholar]


