Abstract
Objective
To compare short- and long-term neurological outcomes in comatose survivors of out-of-hospital cardiac arrest (OHCA) treated with mild therapeutic hypothermia (MTH) presenting with non-shockable (nSR) versus shockable (SR) initial rhythms.
Design
Retrospective cohort study.
Setting
ED and ICU of an academic hospital.
Patients
One hundred twenty-three consecutive post-OHCA adults (57 nSR, 67 SR) treated with therapeutic hypothermia between 2006 and 2012.
Measurements and Main Results
Data were collected from electronic health records. Neurological outcomes were dichotomized by Cerebral Performance Category at discharge and 6-12 month follow-up and analyzed via multivariable logistic regressions. Groups were similar, except nSR patients were more likely to have a history of diabetes mellitus (p = 0.01), be dialysis-dependent (p = 0.01), and not have bystander CPR (p = 0.05). At discharge, 3/57 (5%) patients with nSR versus 28/66 (42%) with SR had a favorable outcome (unadjusted OR 0.08, 95% CI 0.02-0.3; adjusted OR 0.1, 95% CI 0.03-0. 4). At follow-up, 4/55 (7%) versus 29/60 (48%) of patients with nSR and SR respectively had a favorable CPC (OR 0.08, 95% CI 0.03-0.3; adjusted OR 0.09, 95% CI 0.09-0.3). Among those surviving hospitalization, neurological outcome was more likely at long-term follow-up than at hospital discharge for both groups (OR 2.5, 95% CI 1.3-4.7; adjusted 2.9, 1.4-6.2). No significant interaction between changes in neurological status over time and presenting rhythm was seen (p=0.93).
Conclusions
These data indicate an association between initial nSR and significantly worse short- and long-term outcomes in patients treated with MTH. Among survivors, neurological status significantly improved over time for all patients and SR patients, and tended to improve over time for the small number of nSR patients who survived beyond hospitalization. No significant interaction between changes in neurological status over time and presenting rhythm was seen.
Introduction
Over 420,000 out of hospital cardiac arrests (OHCA) occur each year in the US, with an estimated overall survival to hospital discharge of 10.4% for EMS-treated non-traumatic arrests.(1) Neurological morbidity and mortality is considerable in patients resuscitated from cardiac arrest.(2) Mild therapeutic hypothermia (MTH) has been shown in randomized clinical trials to benefit patients presenting with shockable rhythms (SR), i.e. ventricular fibrillation and pulseless ventricular tachycardia (VF/VT). (3-5) However, understanding the impact of presenting rhythm on prognosis is becoming increasingly valuable from a clinical practice perspective given that nSRs have been comprising a larger proportion of OHCA over the past several decades. (6, 7) Furthermore, current guidelines indicate that MTH may be considered for comatose patients resuscitated from initially non-shockable rhythms (nSR)(8) based on conflicting observational data despite lack of randomized data in this population.(9-14)
An important limitation of many available observational data is that they often do not assess neurological outcome beyond hospital discharge (10, 11, 14). Studies that have followed patients beyond hospital discharge are largely non-informative regarding the impact of presenting rhythm on recovery due to either recording only one time point or not studying patients of both shockable and non-shockable rhythms. (4, 12, 15-19). Consequently, the long-term impact of rhythm including the likelihood that survivors of both rhythms will recover neurological function from their post-discharge status remains unclear. Since neurological recovery may not plateau for weeks to months after a cardiac arrest, both hospital discharge evaluation and long-term follow-up are needed to determine the effect of presenting rhythm on ultimate functional outcome. Patients with nSR versus SR arrests may differ in either the rate or magnitude of recovery (or both), and these may be identified by examining change between short- and long-term outcomes. Furthermore, comparing outcomes of nSR arrests with those of SR arrests may inform clinical practice of whether to cool nSR arrests by suggesting whether benefits of MTH are sustained over time.
In this study, we sought to compare short- and long-term neurological outcomes in patients presenting with non-shockable versus shockable initial rhythms after implementation of MTH. We hypothesize that patients presenting with nSR will have a worse prognosis than those with SR, but that survivors of both rhythms will remain stable or improve over time.
Methods
Study Design
This was an observational retrospective cohort study.(20) Patients were classified by their initial presenting rhythm by EMS or Emergency Department records and followed in time via electronic health records. Being classified as ‘shockable’ means that an automatic external defibrillator was applied which advised a shock, or the ED or EMS flowsheets/runsheets mark the presenting arrest rhythm as either VF or pulseless VT. Being classified as ‘non-shockable’ means that either it was document that an AED did not advise a shock, ED or EMS flowsheets/runsheets specify PEA or asystole as the presenting cardiac rhythm, or else presenting rhythm and defibrillation is not specifically mentioned but there exists sufficient medical documentation detailing resuscitative efforts such that it would be reasonable to assume that a defibrillation would have been documented if it had been delivered.
Patient Selection
Electronic health records were screened for all patients presenting to the University of Michigan Emergency Department with a presentation or diagnosis coded as OHCA following implementation of MTH program between 7/14/2006-9/14/2012. To ensure that we captured all eligible subjects, we also screened patients included on an independent log of therapeutic temperature modulation (TTM) equipment use. Patients were excluded if they were less than 18 years old at the time of arrest, received temperature management therapy for a diagnosis other than cardiac arrest (i.e. rewarming after environmental hypothermia), initial rhythm could not be ascertained, or if cooling protocol was initiated but aborted before actually receiving cooling. All patients meeting the above criteria with either SR or nSR were included.
Therapeutic Hypothermia
MTH at our institution consisted of endovascular cooling (Innercool-Phillips, San Diego, CA) for 24 hours at a target of 33°C followed by controlled rewarming over 24 hours back to normothermia. Placement of the endovascular temperature control catheter typically occurred in the Emergency Department, but sometimes occurred in the interventional cardiology suite or the intensive care unit depending on clinical situation. Cold intravenous crystalloid bolus was allowed to initiate cooling if placement of the catheter may be delayed. This hypothermia protocol is applied to all rhythms consistently, and protocol at our institution is to cool patients of both rhythms.
Data Collection and Outcome Assessment
This project was reviewed and determined exempt by the University of Michigan Institutional Review Board. Data were collected modeled after Utstein recommendations(21) and reported according to accepted standards in chart review research.(22)
All data were abstracted from University of Michigan electronic medical health records by the primary author (S.T). The author was not blinded to the study purpose or patients' presenting rhythm. Details regarding demographics, past medical history, the event and resuscitation, hypothermia protocol, and discharge vital status were determined. ROSC was defined for purposes of the patient flow diagram is interpreted as more than transient return of spontaneous circulation, i.e. pulse on two consecutive pulse checks and no immediate re-arrest.
Data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools(23, 24) hosted by the University of Michigan.
Neurological outcomes were characterized by Cerebral Performance Category (CPC).(25-27) Outcomes were dichotomized as favorable (CPC 1-2, i.e. no symptoms and/or independence) or unfavorable (CPC 3-5, i.e. dependent, comatose, or dead) at hospital discharge and 6-12 months post-discharge. Outcomes were determined by reviewing inpatient and outpatient physician and Physical/Occupational Therapy evaluations. Determination of CPC from chart review has been previously determined to have moderately good correlation with that determined by patient interview.(28) CPCs were determined by two independent reviewers (S.T. and R.S.). Initial inter-rater agreement was 85% and 73% for non-deceased CPC categories at discharge and follow-up, respectively, and when discordant, resolved by consensus. We used the first note after 6 months that provided sufficient information relevant to neurological symptoms on which to base our assessment. If no informative note existed in the relevant time interval but medical records indicated a consistent CPC before and after the interval of interest without mention of significant intervening event, such records were incorporated. If no such note existed or the patient was lost to follow-up from the University of Michigan electronic records, they were recorded as having a missing CPC. If 6-month vital status was unavailable from medical records, we searched the Social Security Death Index Master File (accessed 5/14/2013).
Statistical Analysis
Descriptive statistics are used to characterize the patient cohort. Continuous variables are described using medians and interquartile ranges. Categorical variables are described as frequencies and percentages within each group. Baseline comparisons between SR and nSR groups were analyzed parametrically or non-parametrically as appropriate using Student's t-tests or Wilcoxon rank sum tests for continuous variables, and Pearson chi-squared or Fisher's exact tests for categorical variables.
Logistic regression models were used to compare neurological outcome for the two types of arrests at discharge and at follow-up. For these models, the 5-category CPC score was dichotomized (CPC 1-2: favorable; CPC 3-5: unfavorable). Favorable neurological category was modeled as the outcome in these models. Separate logistic regression models were fit to compare available CPCs for nSR vs. SR patients at hospital discharge, and then again at follow-up. Multivariable logistic regressions were conducted when analyzing categorical outcomes data first unadjusted, and then adjusted for variables which differed between the two groups including past history of diabetes mellitus, whether the event was witnessed, and whether the patient received bystander CPR. Although history of dialysis-dependence was significantly different between the SR and nSR groups, regressions were not adjusted for dialysis-dependence because all dialysis patients (n=9) had an unfavorable outcome. Adjusting for such a covariate results in a quasi-complete separation in the logistic regression model, which produces an infinite bound for the confidence interval, so dialysis was not included as a covariate.
To test for trend in CPC over time, first we include a McNemar's test comparing CPC at discharge to CPC at follow-up. We then conduct a logistic regression model with generalized estimating equations (GEE) in order to adjust for the same covariates used in the aforementioned adjusted logistic regressions. The GEE analysis takes into account the within-subject correlation by adding a repeated measures structure to the logistic regression. Individuals with missing data points were excluded from relevant analyses. All data were analyzed using SAS 9.3 (SAS Institute, Cary, NC).
A power calculation was performed based on previous studies that captured outcomes for both nSR versus SR treated with hypothermia.(11, 16) These prior data suggest an unadjusted OR of approximately 0.25 for a good CPC at either discharge or follow-up for a nSR compared with SR, and proportion of SR with good outcome was approximately 40-60%. Based on these estimates, we would need a total sample size of at least 92 to detect such differences with 80% power at a 0.05 level of significance.
Results
Patient Characteristics
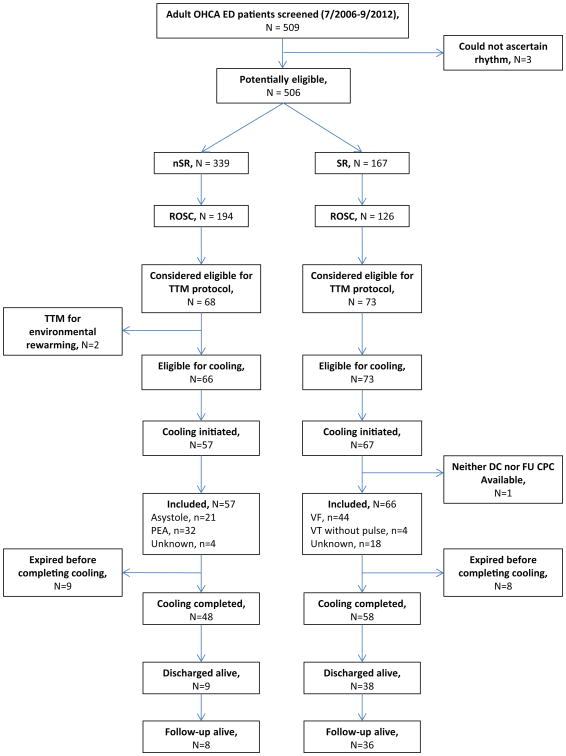
The population screened included 509 adult patients presenting to the ED or diagnosed with OHCA in the ED within the given dates (Figure 1). We were able to ascertain whether the initial rhythm was shockable in 506. Of these 506, there were 339 nSR, 194 of whom had any ROSC, and 57 had cooling initiated. Of the 506, there were 167 SR, 126 of whom had any ROSC, and 67 had cooling initiated. One SR patient who would have been eligible did not have a CPC available at either discharge or follow-up, so 66 SR patients were included. Of adult OHCAs with ROSC who were admitted, cooling was initiated in 59/150 (39%) of nSR arrests vs. 67/114 (59%) of SR arrests (p=0.002). Among included nSR, 9/57 (16%) terminated the cooling protocol prematurely due to deteriorating clinical condition; among included SR, 8/66 (12%) did so (p=0.55).
Figure 1. Case Selection.
Patient baseline characteristics for included patients are displayed in Table 1. The two groups had similar distributions of demographic variables, but those with nSR were more likely to have a history of diabetes mellitus (p = 0.006), be dialysis-dependent (p = 0.01), not have bystander CPR (p=0.047), and tended to have more unwitnessed arrests (p = 0.052). Etiology of arrests were also significantly different (p<0.0001) with cardiac causes most prevalent in the SR group and respiratory causes more prevalent in those with nSR.
Table 1. Baseline Characteristics.
| Non-shockable (n = 57) | Shockable (n = 66) | p | ||
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Median (IQR) Age (years) | 59 (52-71) | 62 (52-72) | 0.5 | |
| Median (IQR) BMI | 28 (23-33) | 27 (24-30) | 0.23 | |
| Sex (male) | 32 (56%) | 47 (71%) | 0.08 | |
| PMH | ||||
| MI | 8 (14%) | 15 (23%) | 0.22 | |
| CHF | 8 (14%) | 11 (17%) | 0.69 | |
| Cerebrovascular | ||||
| Diseasea | 7 (12%) | 8 (12%) | 0.98 | |
| Dementia | 0 (0%) | 2 (4%) | 0.21 | |
| DM | 21 (37%) | 10 (15%) | 0.006 | |
| Dialysis | 8 (14%) | 1 (2%) | 0.01 | |
| Witnessed | 41 (72%) | 56 (86%) | 0.052 | |
| Bystander CPR | 34 (61%) | 51 (77%) | 0.047 | |
| Etiology | <0.0001 | |||
| CV | 3 (5%) | 28 (42%) | ||
| Respiratory | 13 (23%) | 1 (2%) | ||
| Unknown | 30 (53%) | 31 (47%) | ||
| Otherb | 11 (19%) | 6 (9%) | ||
| Time to target (hours)c | 5.9 (3.8-8.5) | 5 (3.5-8.2) | 0.3 |
IQR: Interquartile range; BMI: body mass index (kg/m2); PMH: past medical history; MI: myocardial infarction; CHF: congestive heart failure; DM: diabetes mellitus; CPR: cardiopulmonary resuscitation; CV: cardiovascular
Includes ischemic or hemorrhagic stroke, or TIA
Includes electrolyte abnormality (1 SR, 1 NSR), drowning (1 NSR), allergic reaction (1 NSR), hanging (2 NSR), drug overdose (4 NSR), sepsis (1 NSR), trauma (1 NSR), electrocution (4 SR), coitus (1 SR)
Duration between Emergency Department arrival and consecutive recorded core body temperatures < 33.5°C
Among those who expired, life support was withdrawn prior to expiration in 38/48 (79%) of patients with nSR and 24/28 (86%) of patients with SR (p=0.48).
Comparison of nSR versus SR Outcomes
No CPCs were missing at discharge. Two of 57 (4%) nSR vs. 6/66 (9%) SR patients had a CPC missing at follow-up (p=0.28).
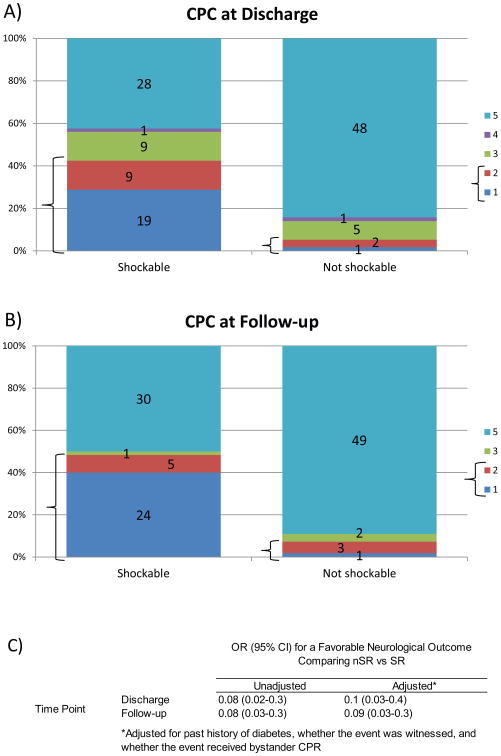
At hospital discharge, 3/57 (5%) patients with nSR versus 28/66 (42%) with SR had a favorable outcome (unadjusted OR 0.08, 95% CI 0.02-0.3, p<0.0001; adjusted OR 0.1, 95% CI 0.03-0. 4, p=0.0005). (Fig 2a,c).
Figure 2.
Comparison of Neurological Outcomes for nSR and SR Arrests and Discharge and Follow-up: (a and b) Bold markers delineate favorable CPC (1 or 2). Numbers inside each category on the graph indicate n-values. c) Results of multivariable logistic regression for a favorable neurological outcome (dichotomized outcome) comparing nSR vs SR arrests at hospital discharge and again at follow-up.
Follow-up occurred at a median 6.8 months (IQR 6.0-8.6). Length of follow-up was similar between nSR and SR groups (p=0.85). At follow-up, 4/55 (7%) versus 29/60 (48%) of patients with nSR and SR respectively had a favorable CPC (OR 0.08, 95% CI 0.03-0.3, p<0.0001; adjusted OR 0.09, 95% CI 0.09-0.3, p=0.0001). (Fig 2b,c).
Comparison of Follow-up versus Discharge Outcomes for All Patients
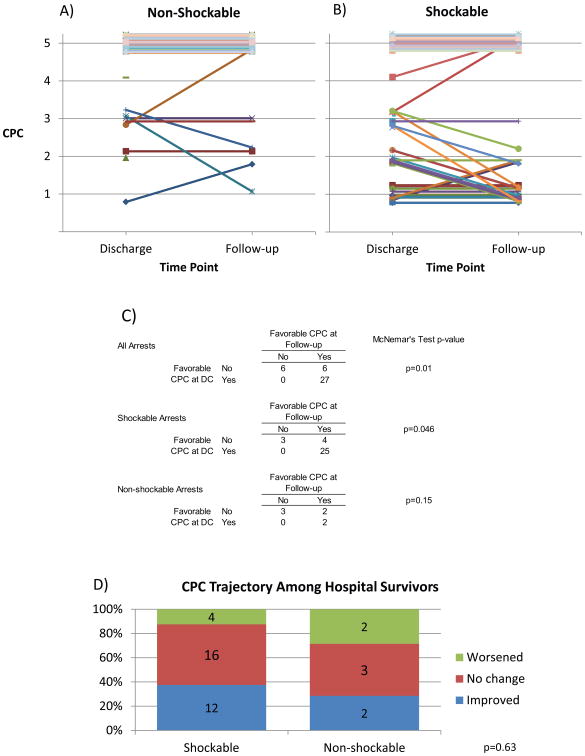
Individual outcomes over time for all patients by rhythm are traced in Fig 3a,b. As displayed in Fig 2, among patients with nSR arrests, 3/57 (5%) had a favorable outcome at discharge vs, 4/55 (7%) at follow-up (McNemar's test p=0.16). Among SR arrests, 28/66 (42%) had a favorable outcome at discharge vs 29/60 (48%) at follow-up, respectively (McNemar's test p=0.046). Overall, 31/123 (25%) of patients had a favorable outcome at hospital discharge vs. 33/115 (29%) at follow-up (McNemar's test p=0.01).
Figure 3.
Comparison of Neurological Outcomes for nSR and SR Arrests and Discharge and Follow-up: (a and b) Each line represents an individual patient. Data appear staggered around CPC integers to assist visualization, though all CPCs are integer values. Plots include all patients, according to rhythm. Individuals with only a single data point available are represented by a point. C) Comparison of dichotomized outcomes at discharge versus follow-up. D) Depiction of whether individuals who were discharged alive worsened, remained stable, or improved in CPC between hospital discharge and follow-up. Numbers inside each category on the graph indicate n-values. P-value is for chi-squared test.
Comparison of Follow-up versus Discharge Outcomes Among Hospital Survivors
Given that CPCs of patients who expired in the hospital will not change over time, we then conducted detailed analyses of the 47/123 (38%) patients who were discharged from the hospital alive in order to characterize neurological recovery overall and according to rhythm among survivors.
Among nSR arrests who survived, 3/9 (33%) had a favorable outcome at discharge vs, 4/7 (57%) at follow-up (McNemar's test p=0.16, Fig 3c). Note that these numbers do not exactly match those in Fig 3c because Fig 3c includes patients only if they have an outcome available at both discharge and follow-up. Among those patients with a CPC at both time points, 2/7 (29%) improved, 2/7 (29%) worsened, and 3/7 (43%) did not change CPC (Fig 3d).
Among SR arrests who survived, 28/38 (74%) had a favorable outcome at discharge vs 29/32 (91%) at follow-up, respectively (McNemar's test p=0.046, Fig 3c). Among those patients with a CPC at both time points, 12/32 (38%) improved, 4/32 (13%) worsened, and 16/32 (50%) did not change CPC (Fig 3d).
Overall, 31/47 (66%) of survivors had a favorable outcome at hospital discharge vs. 33/39 (85%) at follow-up (McNemar's test p=0.01, Fig 3c). No patient of either rhythm who had a favorable CPC at discharge declined to an unfavorable CPC at follow-up, though patients did improve from an unfavorable to a favorable CPC. There was no difference in distribution of CPC improvement according to rhythm (p=0.63, Fig 3d).
We then conducted regressions using GEEs to determine if neurological recovery of hospital survivors was modified by rhythm, and to adjust for confounders (Table 2). In a model including rhythm, follow-up, and their interaction, patients with nSR arrests non-significantly tended to be more likely to have a favorable CPC at discharge versus follow-up (unadjusted OR 3.0, 95% CI 0.7-13, p=0.13; adjusted OR 1.6, 95% CI 0.4-6.7, p=0.52), and patients with SR arrests were significantly more likely to have a favorable CPC at discharge versus follow-up (unadjusted OR 2.8, 95% CI 1.2-6.3, p=0.0.01; adjusted OR 3.1, 95% CI 1.4-7.1, p=0.007). The interaction between rhythm and follow-up was not significant (unadjusted p=0.93, adjusted p=0.93), which indicates that CPC improvement did not significantly change according to rhythm. We therefore refit the model dropping the interaction term. In such a model, patients had a significantly higher odds of a favorable CPC at follow-up vs. discharge (unadjusted OR 2.5, 95% CI 1.3-4.7, p=0.004; adjusted OR 2.9, 95% CI 1.4-6.2, p=0.005).
Table 2.
Comparison of Neurological Outcomes for nSR and SR Arrests and Discharge and Follow-up: Results are obtained from GEE analysis. The ‘nSR Only’ and ‘SR Only’ rows are obtained from the full models including an interaction term between time and rhythm. The ‘All’ row includes patients of both rhythms, and does not include an interaction term, given that the interaction term was not significant.
| OR (95% CI) for a Favorable Neurological Outcome Comparing FU vs DC Among Hospital Survivors | |||
|---|---|---|---|
|
| |||
| Unadjusted | Adjusted* | ||
|
|
|||
| Rhythm | All (no interaction term) | 2.5 (1.3-4.7) | 2.9 (1.4-6.2) |
| nSR Only | 3.0 (0.7-13) | 1.6 (0.4-6.7) | |
| SR Only | 2.8 (1.2-6.3) | 3.1 (1.4-7.1) | |
Adjusted for past history of diabetes, whether the event was witnessed, and whether the event received bystander CPR. The ‘All’ model is also adjusted for nSR vs SR.
We performed a sensitivity analysis excluding all dialysis-dependent patients, given that dialysis patients did overwhelmingly poorly. All analyses were repeated, and no results significantly changed.
Discussion
MTH has been demonstrated to reduce neurological morbidity and mortality in randomized trials for survivals of OCHA with shockable presenting rhythms.(3-5) Since then, clinicians have been left to struggle with whether MTH should be applied to other comatose survivors of cardiac arrest.(29) With regard to patients presenting with nSR, there are essentially no informative randomized controlled data. One meta-analysis(9) has examined the effect of hypothermia on patients presenting with nSR. They identified only 2 pilot randomized studies including a total 22 patients with nSR treated with MTH(30, 31) from which they estimated a relative risk 0.85 (0.65-1.11) for 6 month mortality compared with a nSR arrest not treated with hypothermia. In addition to being too small to inform clinical decision making, these two studies employed experimental cooling methods that are not generally used in practice, limiting their external validity. Observational studies have been heterogeneous.(11, 13, 14, 32) Ten observational studies included in the meta-analysis suggested a RR of 0.84 (95% CI 0.71-0.92) and 0.93 (95% CI 0.88 – 1.00) for hospital mortality and unfavorable neurological outcomes, respectively, both in favor of hypothermia. Controlled trial data are still needed to determine efficacy of MTH in these expanded patient populations.
The association between presenting rhythm and outcome has been evaluated in the pre-hypothermia era (33-36). One study in the pre-hypothermia era suggested that survivors of both nSR and SR arrests had similar cognitive, physical, and psychosocial function at follow-up regardless of presenting rhythm. (37) Another pre-hypothermia study found that 73% of those surviving 1 year beyond the arrest returned to pre-arrest function, though they did not differentiate between nSR and SR.(38) On the other hand, delayed deaths and disability may also occur in survivors of cardiac arrest (39, 40), so an appreciation of patient outcomes over time is essential to make informed treatment decision and prognoses. Furthermore, it is appreciated that conventional prognostic signs may be altered in the presence of hypothermia and continue to be evaluated (19), so studying such questions specifically in the hypothermia population is essential.
In the hypothermia era, studies have been conducted with short-term endpoints.(10, 11, 14) For example, Oddo et al (10) sought to define factors associated with favorable neurological outcomes at hospital discharge by reviewing medical records and found that 3/36 (8%) and 21/38 (55%) of patients with nSR and SR arrests, respectively, had a favorable neurological outcome (p<0.001). However, after multivariable analysis adjusting for time from collapse to ROSC, blood lactate, and other covariates, initial rhythm was no longer a significant predictor. This differs from our results possibly because we did not adjust for time to ROSC. Doing so would have excluded all unwitnessed arrests, an important subgroup, from our analysis. Oddo et al's results support the presumption that the effect of rhythm may be a surrogate for ischemic duration, severity of injury, or ability to promptly correct the underlying etiology; i.e., poor prognosis may not be inherent to the nSR itself.
It is also important to understand long-term outcomes of different cooled patient populations to determine if potential treatment effects are sustained. Several prospective studies seeking to determine factors associated with prognosis have evaluated patients at time points several months after discharge and have found nSR to be a significantly, albeit not invariably, poor prognostic factor in the context of multimodal clinical evaluation (17-19). Testori et al(12) recorded the best 6-month outcome in 135 patients with nSR treated with MTH and found a treatment effect as compared to a normothermic cohort. However, they did not evaluate the trajectory of recovery or compare long-term recovery to patients with SR. They also excluded arrests that were unwitnessed or fatal within 24 hours, which may overestimate favorable outcomes and exclude and important patient population. Cronberg et al (16) evaluated victims of cardiac arrest that had been treated with hypothermia and were still alive at 6 months. They found a favorable CPC (1-2) in 31% of those with nSR as compared to 63% of those with SR, but did not examine recovery trajectory and did not consider those that did not survive to 6 months. Bro-Jeppesen et al(15) evaluated patients treated with hypothermia after CA and determined CPC at ICU discharge and then again at hospital discharge, but only reported data for the 52 presenting with SR. They also evaluated survival, cognitive function, and health related quality of life at 6 months in 21 of these SR patients. Changes occurred in CPC between ICU and hospital discharge, but they did not evaluate CPC at 6 months so it is difficult to make conclusions about long-term trajectories from their data. Rossetti et al(17) found a favorable CPC in 9% versus 32% of those with nSR and SR (p=0.005) at 3-6 months . However, as was the case in aforementioned studies, they did not record CPCs at more than one time point for comparison to fully evaluate recovery.
Such studies suggest that rhythm is associated with outcomes, whether or not a patient suffering OHCA is treated with hypothermia. However, an important question unanswered by these studies is the degree to which survivors recover brain function after discharge, and whether recovery back to adequate cerebral function depends upon the presenting rhythm. The post-cardiac arrest syndrome involves a complex physiological cascade of brain and myocardial dysfunction followed by a systemic ischemic reperfusion response which is in rapid flux post-ROSC. (41)The ability to trace individual-level data over time as we have done is extremely useful to explore the interaction between rhythm and time in determining outcomes as patients transition from the dynamic acute to a more stable chronic state post-arrest care.
We evaluated outcomes for each patient at both discharge and at 6-12 months post-discharge. We then evaluated within-subject change in CPC between hospital discharge and 6-12 month follow-up. As expected, nSR patients exhibited poorer outcomes compared with SR patients at both time points. Patients who survived the hospitalization tended to improve over time, i.e. had a significantly higher odds of favorable CPC at follow-up compared with discharge. This improvement was not significantly modified by presenting rhythm, though it is possible that with a larger sample of surviving nSR an interaction would have been seen. SR patients alone had a significantly higher odds of favorable outcome at follow-up versus discharge. The small number of nSR who survived hospitalization had a non-significant trend towards improvement over time, though our available sample size was too small to draw definitive conclusions regarding the trajectory of recovery for nSR survivors, except to say that no survivor who was discharged with a favorable outcome deteriorated to an unfavorable outcome over time and only a small number of initial survivors died within the year post-discharge.
Limitations and Strengths
First, there are several potential sources of selection bias. Chart review studies may suffer from missing data. However, no outcomes data were missing at discharge, and only 4% vs. 9% of nSR vs. SR CPCs were missing at follow-up (p=0.28). Therefore, missingness was not a significant issue in the present study. Selection bias may have also stemmed from who was selected for cooling. A higher proportion of SR than nSR arrests received cooling. This is partly attributable to a higher proportion of SR achieving ROSC, but also attributable to a higher proportion of admitted patients received cooling in the SR group. We have clearly described patient flow in our Table 1 to allow the reader to assess such biases. It should also be noted that the ‘self-fulfilling prophecy’ and differential selection of patients receiving hypothermia is an important limitation of all available prognostic studies in cardiac arrest, surely not limited to the current study. (18)
Second, we conducted a single-center study. This was a disadvantage in terms of sample size. Few nSR patients survived over a 5-6 year period at our institution. This limited our ability to draw conclusions about neurological recovery for nSR survivors. However, our sample size still enabled us to detect plausible statistical differences regarding outcomes by rhythm at both time points and significant improvement over time in the SR and total population. Perhaps if we had had more nSR survivors we would have detected a difference in the effect of time on outcomes by rhythm, or an interaction would have been detected. However, we did have a substantial SR and total patient population sizeable enough to draw a number of statistical conclusions, and a sizeable enough nSR overall population to compare with the overall SR population. Even with a small number of nSR survivors, we can still observe that no patient deteriorated from a favorable to an unfavorable outcome. This might suggest that benefits of cooling may be sustained in both rhythms and that cooling these patients may not be a futile effort, though we do not make claims in this study regarding the efficacy of cooling for nSR patients. Also, being a single-center study may preclude generalizing our results to smaller community institutions without endovascular capability. However, being a single-centered study did increase internal validity by allowing a more consistent application of a single cooling protocol to all patients.
Third, initial detected rhythm may misclassify patients given that rhythm is dynamic and may not reflect the patient's true initial rhythm. This non-differential misclassification of the exposure variable would likely bias our results towards the null, so if anything the true association between initial rhythm and neurological outcome would have even been stronger if no misclassification occurred.
Fourth, retrospective evaluation of CPC may be too crude to fully capture neurological outcomes, and large-scale prospective multi-centered data could better characterize outcomes.
Our study also has strengths in terms of our data collection and statistical analysis. To our knowledge, no study has thus far carefully charted individual patient trajectories over time as we have done. Furthermore, we have charted such outcomes according to rhythm in detail. Our statistical approach is especially novel and informative within this literature. Our data structure allowed us to perform GEE regression analysis to evaluate recovery over time both overall and according to rhythm.
Conclusions
These data are consistent with an association between resuscitation from nSR and significantly worse short- and long-term outcomes than resuscitation from SR in patients treated with MTH. Among survivors, neurological status tended to improve between hospital discharge and long-term follow-up. This effect was not modified by rhythm, given that an interaction between time and rhythm was not seen. Future research on patient and process parameters will be needed to elucidate the independent factors underlying this relationship and to better identify the subpopulations of patients surviving cardiac arrest that benefit from therapeutic hypothermia.
Acknowledgments
The study was funded internally. No sponsors had any role in the study design or collection, analysis, or interpretation of data, writing of the manuscript, or decision to submit the manuscript for publication. We thank Breanna Miller from the U-M Center for Statistical Consultation and Research (CSCAR) for advice on the analysis. We thank senior statistician Kathy Welch also of the U-M CSCAR for her thorough statistical review of this manuscript to ensure rigorous methodology.
Financial support for this study was provided entirely by internal institutional funds No funds are available to order paper reprints
Dr. Silbergleit is employed by the University of Michigan. His institution received grant support from NIH. Dr. Terman received support for travel from the University of Michigan (graduate school funded travel to and registration for conference to present results) and received support from the Neurological Emergencies Treatment Trials (hourly wage for role as a research assistant. His institution received grant support from NIH. Dr. Hume received support for article research from NIH. His institution received grant support from NIH.
Footnotes
This work was performed at the University of Michigan.
Copyright form disclosures: Dr. Meurer disclosed that he does not have any potential conflicts of interest.
Conflicts of Interest: No author has any financial or personal relationships with other people or organisations that could inappropriately influence or bias their work.
Ethical Approval The work has been reviewed and determined exempt from review by the University of Michigan IRB (HUM00018775).
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laver S, Farrow C, Turner D, et al. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 5.Arrich J, Holzer M, Havel C, et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2012;9:CD004128. doi: 10.1002/14651858.CD004128.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Cobb LA, Fahrenbruch CE, Olsufka M, et al. Changing incidence of out-of-hospital ventricular fibrillation, 1980-2000. JAMA. 2002;288:3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 7.Herlitz J, Engdahl J, Svensson L, et al. Decrease in the occurrence of ventricular fibrillation as the initially observed arrhythmia after out-of-hospital cardiac arrest during 11 years in Sweden. Resuscitation. 2004;60:283–290. doi: 10.1016/j.resuscitation.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768–786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 9.Kim YM, Yim HW, Jeong SH, et al. Does therapeutic hypothermia benefit adult cardiac arrest patients presenting with non-shockable initial rhythms?: A systematic review and meta-analysis of randomized and non-randomized studies. Resuscitation. 2012;83:188–196. doi: 10.1016/j.resuscitation.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Oddo M, Ribordy V, Feihl F, et al. Early predictors of outcome in comatose survivors of ventricular fibrillation and non-ventricular fibrillation cardiac arrest treated with hypothermia: a prospective study. Crit Care Med. 2008;36:2296–2301. doi: 10.1097/CCM.0b013e3181802599. [DOI] [PubMed] [Google Scholar]
- 11.Dumas F, Grimaldi D, Zuber B, et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients?: insights from a large registry. Circulation. 2011;123:877–886. doi: 10.1161/CIRCULATIONAHA.110.987347. [DOI] [PubMed] [Google Scholar]
- 12.Testori C, Sterz F, Behringer W, et al. Mild therapeutic hypothermia is associated with favourable outcome in patients after cardiac arrest with non-shockable rhythms. Resuscitation. 2011;82:1162–1167. doi: 10.1016/j.resuscitation.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Lundbye JB, Rai M, Ramu B, et al. Therapeutic hypothermia is associated with improved neurologic outcome and survival in cardiac arrest survivors of non-shockable rhythms. Resuscitation. 2012;83:202–207. doi: 10.1016/j.resuscitation.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Don CW, Longstreth WT, Jr, Maynard C, et al. Active surface cooling protocol to induce mild therapeutic hypothermia after out-of-hospital cardiac arrest: a retrospective before-and-after comparison in a single hospital. Crit Care Med. 2009;37:3062–3069. doi: 10.1097/CCM.0b013e3181b7f59c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bro-Jeppesen J, Kjaergaard J, Horsted TI, et al. The impact of therapeutic hypothermia on neurological function and quality of life after cardiac arrest. Resuscitation. 2009;80:171–176. doi: 10.1016/j.resuscitation.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Cronberg T, Lilja G, Rundgren M, et al. Long-term neurological outcome after cardiac arrest and therapeutic hypothermia. Resuscitation. 2009;80:1119–1123. doi: 10.1016/j.resuscitation.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Rossetti AO, Oddo M, Logroscino G, et al. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67:301–307. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
- 18.Bouwes A, Binnekade JM, Kuiper MA, et al. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Ann Neurol. 2012;71:206–212. doi: 10.1002/ana.22632. [DOI] [PubMed] [Google Scholar]
- 19.Oddo M, Rossetti AO. Early Multimodal Outcome Prediction After Cardiac Arrest in Patients Treated With Hypothermia. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 20.The Strengthening the reporting of observational studies in epidemiology (STROBE) checklist for cohort studies. University of Bern; 2009. [Google Scholar]
- 21.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa) Resuscitation. 2004;63:233–249. doi: 10.1016/j.resuscitation.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert EH, Lowenstein SR, Koziol-McLain J, et al. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med. 1996;27:305–308. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.REDCap (Reseasrch Electronic Data Capture) 2013 cited Available from: http://www.project-redcap.org/
- 25.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 26.Brain Resuscitation Clinical Trial II Study Group. A randomized clinical study of a calcium-entry blocker (lidoflazine) in the treatment of comatose survivors of cardiac arrest. N Engl J Med. 1991;324:1225–1231. doi: 10.1056/NEJM199105023241801. [DOI] [PubMed] [Google Scholar]
- 27.Safar P, Bircher NG. Cardiopulmonary cerbral resuscitation: basic and advanced cardiac and trauma life support: an introduction to resuscitation medicine. 3. London: Saunders; 1998. [Google Scholar]
- 28.Raina KD, Callaway C, Rittenberger JC, et al. Neurological and functional status following cardiac arrest: method and tool utility. Resuscitation. 2008;79:249–256. doi: 10.1016/j.resuscitation.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernard S. Hypothermia after cardiac arrest: expanding the therapeutic scope. Crit Care Med. 2009;37:S227–233. doi: 10.1097/CCM.0b013e3181aa5d0c. [DOI] [PubMed] [Google Scholar]
- 30.Hachimi-Idrissi S, Corne L, Ebinger G, et al. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation. 2001;51:275–281. doi: 10.1016/s0300-9572(01)00412-9. [DOI] [PubMed] [Google Scholar]
- 31.Laurent I, Adrie C, Vinsonneau C, et al. High-volume hemofiltration after out-of-hospital cardiac arrest: a randomized study. J Am Coll Cardiol. 2005;46:432–437. doi: 10.1016/j.jacc.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 32.Arrich J. Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35:1041–1047. doi: 10.1097/01.CCM.0000259383.48324.35. [DOI] [PubMed] [Google Scholar]
- 33.Wright D, Bannister J, Ryder M, et al. Resuscitation of patients with cardiac arrest by ambulance staff with extended training in West Yorkshire. BMJ. 1990;301:600–602. doi: 10.1136/bmj.301.6752.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weston CF, Wilson RJ, Jones SD. Predicting survival from out-of-hospital cardiac arrest: a multivariate analysis. Resuscitation. 1997;34:27–34. doi: 10.1016/s0300-9572(96)01031-3. [DOI] [PubMed] [Google Scholar]
- 35.Pepe PE, Levine RL, Fromm RE, Jr, et al. Cardiac arrest presenting with rhythms other than ventricular fibrillation: contribution of resuscitative efforts toward total survivorship. Crit Care Med. 1993;21:1838–1843. doi: 10.1097/00003246-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Engdahl J, Bang A, Lindqvist J, et al. Can we define patients with no and those with some chance of survival when found in asystole out of hospital? Am J Cardiol. 2000;86:610–614. doi: 10.1016/s0002-9149(00)01037-7. [DOI] [PubMed] [Google Scholar]
- 37.van Alem AP, Waalewijn RA, Koster RW, et al. Assessment of quality of life and cognitive function after out-of-hospital cardiac arrest with successful resuscitation. Am J Cardiol. 2004;93:131–135. doi: 10.1016/j.amjcard.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 38.Graves JR, Herlitz J, Bang A, et al. Survivors of out of hospital cardiac arrest: their prognosis, longevity and functional status. Resuscitation. 1997;35:117–121. doi: 10.1016/s0300-9572(97)00035-x. [DOI] [PubMed] [Google Scholar]
- 39.Edgren E, Hedstrand U, Kelsey S, et al. Assessment of neurological prognosis in comatose survivors of cardiac arrest. Lancet. 1994;343:1055–1059. doi: 10.1016/s0140-6736(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 40.Jorgensen EO, Holm S. The natural course of neurological recovery following cardiopulmonary resuscitation. Resuscitation. 1998;36:111–122. doi: 10.1016/s0300-9572(97)00094-4. [DOI] [PubMed] [Google Scholar]
- 41.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]