Abstract
Patients suffering from neuropathic pain have a higher incidence of mood disorders such as depression. Increased expression of tumor necrosis factor (TNF) has been reported in neuropathic pain and depressive-like conditions and most of the pro-inflammatory effects of TNF are mediated by the TNF receptor 1 (TNFR1). Here we sought to investigate: 1) the occurrence of depressive-like behavior in chronic neuropathic pain and the associated forms of hippocampal plasticity, and 2) the involvement of TNFR1-mediated TNF signaling as a possible regulator of such events. Neuropathic pain was induced by chronic constriction injury of the sciatic nerve in wild-type and TNFR1−/− mice. Anhedonia, weight loss and physical state were measured as symptoms of depression. Hippocampal neurogenesis, neuroplasticity, myelin remodeling and TNF/TNFRs expression were analyzed by immunohistochemical analysis and western blot assay.
We found that neuropathic pain resulted in the development of depressive symptoms in a time dependent manner and was associated with profound hippocampal alterations such as impaired neurogenesis, reduced expression of neuroplasticity markers and myelin proteins. The onset of depressive-like behavior also coincided with increased hippocampal levels of TNF, and decreased expression of TNF receptor 2 (TNFR2), which were all fully restored after mice spontaneously recovered from pain. Notably, TNFR1−/− mice did not develop depressive-like symptoms after injury, nor were there changes in hippocampal neurogenesis and plasticity. Our data show that neuropathic pain induces a cluster of depressive-like symptoms and profound hippocampal plasticity that are dependent on TNF signaling through TNFR1.
INTRODUCTION
Over half of all patients who suffer from neuropathic pain develop mood disorders such as depression and anxiety (Maletic and Raison, 2009; McWilliams et al., 2003), but the mechanisms underlying this comorbidity are not fully understood. Accumulating evidence suggests a role for the immune system in the etiology of depression (Eyre and Baune, 2012). Elevated levels of immune mediators such as TNF, have been detected in depressed patients (Mikova et al., 2001; Tuglu et al., 2003), while in rodents high levels of cytokines induce a depressive-like behavior, known as “sickness behavior” (Hart 1988; Kaster et al., 2012). This condition can be reliably reproduced with the administration of cytokines or cytokine-inducers (Harrison et al., 2009; Yirmiya 1996), and blocked by cytokine antagonists, or anti-inflammatory cytokines (Dantzer 2001; Kent et al., 1992; Shamash et al., 2002). Moreover, genetically modified mice that do not express TNF receptors (TNFRs) are more resistant to the development of depressive behavior under stressful conditions, while TNF administration renders mice more susceptible to depression (Simen et al., 2006). It has been shown that antidepressants can reduce plasma TNF concentration (Kubera et al., 2005; Yirmimya et al., 1999), and in clinical trials, in which TNFRs antagonists were used for the treatment of immune pathologies, a significant improvement of depressive symptoms was observed (Bos and Korte, 2006; Ertenli et al., 2012; Tyring et al., 2006).
TNF signals via two distinct receptors which often mediate opposing biological functions: the pro-inflammatory/pro-neurodegenerative/pro-demyelinanting TNF receptor 1 (TNFR1/p55) and the likely neuroprotective TNF receptor 2 (TNFR2/p75) (Baud and Karin, 2001; Brambilla et al., 2011; MacEwan, 2002). Interestingly, TNF has been proven to have a key role in the development of neuropathic pain (George et al., 2004; Martuscello et al., 2012), which has been associated to its action through TNFR1 (Schafers et al., 2002; Vogel et al., 2006).
The hippocampus, a central component of the limbic system, is a crucial mood-regulating region of the brain, also involved in the processing of nociception (Mutso et al., 2012). With the discovery of new neuron formation in this area of the adult brain, significant emphasis has been ascribed to the role of the neurogenic process in mood regulation and impairment of adult hippocampal neurogenesis has been linked to the development of depression (Sahay and Hen, 2007). However, other neuroplastic changes such as reduced spine density and dendritic retraction, were previously shown to occur at this level in animal models of depression or pain (Duman and Charney, 1999; Kodama et al., 2007; Watanabe et at., 1992) and, as with the neurogenic process, these alterations can be reverted by treatment with antidepressants as animals recover from depressive-like symptoms (Reines et al., 2008; Warner-Schmidt and Duman, 2006). It is noteworthy that impairments in brain white matter have been described in psychiatric diseases such as schizophrenia and depression (Cole et al., 2012; Kyriakopoulos et al., 2009; Metternburg et al., 2012), and specifically have been found to be associated with the limbic system in the melancholic subtype of major depressive disorder (Korgaonkar et al., 2011). Interestingly, Zeng et al., (2012) showed that white matter volume is normalized by antidepressant treatment in patients with major depression. So far little is known about the mechanisms implicated in white matter impairments that occurs in depressed patients. Conversely, demyelinating disorders characterized by myelin loss show co-morbidity with depression (Arnett et al., 2008), yet, the possible contribution of myelin remodeling as part of the hippocampal plasticity that occurs in depression has not been addressed.
Notably, it has been shown that TNF can be detrimental for the survival of the new hippocampal neurons (Cacci et al., 2005; Monje et al., 2003) and that TNF receptors have a differential actions in the modulation of hippocampal neurogenesis; TNFR1 acts as a suppressor of adult neurogenesis, whereas the absence TNFR2 results in reduced hippocampal neurogenesis (Chen and Palmer, 2013; Iosif et al., 2006). Interestingly, TNF has been shown to have a role in the pathophysiology of autoimmune demyelinating diseases such as multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) (Finsen et al., 2002) and the role of TNFR1-mediated TNF signaling in causing demyelination has been widely investigated by different authors (Arnett et al., 2001; Eugster et al., 1999; Probert et al., 2000). Finally, we and others have demonstrated that signaling mediated by transmembrane TNF, occurring mainly through TNFR2, has been proven to be essential for axon and myelin preservation and to promote remyelination (Bracchi-Richard et al., 2013; Brambilla et al., 2011; Taoufik et al., 2011). Based upon this evidence we sought to determine whether neuropathic pain-associated depression is related to hippocampal neuroplasticity and myelin alterations, and whether TNF signaling through TNFR1 plays a role in these events. We hypothesized that chronic neuropathic pain would be associated with the occurrence of depressive-like symptomps and that this would be mediated by hippocampal TNF/TNFR1 signaling resulting in impairment of hippocampal neurogenesis and neuroplasticity. Given the role of TNF/TNFR1 in demyelinating pathologies we also tested the hypothesis of myelin remodeling occurring in the hippocampus of mice suffering from neuropathic-pain-induced depression. To this purpose we used wild-type and TNFR1−/− mice to investigate: 1) the occurrence of depressive-like symptoms, such as anhedonia, loss of body weight gain and self-neglect in mice with neuropathic pain; 2) hippocampal neurogenesis, neuronal plasticity and myelin remodeling and 3) hippocampal TNF and TNFRs expression at the onset and recovery from neuropathic pain.
Our data show a temporal relationship between neuropathic pain and the occurrence of depressive-like symptoms as well as structural neuroplastic and white matter impairments of the hippocampus. Moreover, this is the first report to show that neuropathic pain-induced depression is associated with hippocampal TNF activity through TNFR1 and that this correlates with profound hippocampal plasticity.
MATERIALS AND METHODS
Animals
TNFR1 null mice (TNFR1 KO) carrying the Tnfrsf1atm1Mak mutation (TNFR1−/−; Pfeffer et al 1993), were purchased from Jackson Laboratory and backcrossed to C57BL/6 genetic background by breeding with C57BL/6 wild-type (WT) mice (Jackson Laboratory) for 9 generations. Resulting heterozygous mice were then interbred to produce TNFR1−/− mice and wild-type littermate controls for experiments. The genotypes were confirmed by polymerase chain reaction (PCR) from tail DNA (Supplementary 1A). The following primers were used: oIMR0450 5’-ATT CGC CAA TGA CAA GAC GCT GG-3’ for mutant TNFR1 allele, oIMR0448 5’-TGT GAA AAG GGC ACC TTT ACG GC-3’ for wild-type allele, and oIMR0449 5’-GGC TGC AGT CCA CGC ACT GG-3’ common primer.
All animals were housed 4 per cage in a virus/antigen free facility with a 12 h light/dark cycle, under controlled temperature (22 °C ± 1°C), humidity (55% ± 10%), and light (lights on from 06:00 am to 6:00 pm) and unlimited access to water and food (2018 Teklad Global 18% Protein Rodent Diet (Harlan Laboratories)). Experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Miami.
Chronic Constriction Injury (CCI)
Neuropathic pain was induced by chronic constriction injury (CCI) of the sciatic nerve (Bennett and Xie, 1988; Sommer and Schafers, 1998; Caspani et al., 2009; Fu et al., 2010), as previously described (Zhang et al., 2011).
Briefly, wild-type mice were anesthetized with isoflurane (3%), followed by a cocktail of ketamine/xylazine (100 mg/kg, 10 mg/kg, i.p.). The right sciatic nerve was exposed at the level of the mid-thigh, three loose 4.0 USP silk ligatures were placed around the dissected nerve with a 1.0–1.5 mm interval between each ligature. The ligatures were carefully manipulated so that they were loosely tied around the nerve. The wound was closed with 6-0 Ethilon monofilament nylon in layers. Sham operated animals underwent a similar procedure with exposure of the sciatic nerve without ligation. Upon sacrifice an incision was applied at the level of the right thigh and the right sciatic nerve exposed to verify that the ligatures were still intact. Wild-type animals were sacrificed 3 and 12 weeks after surgery, when they experienced pain and after they recovered, respectively (Figure 1A). TNFR1 mice were sacrificed 3 and 5 weeks after surgery, the time points at which wild-type neuropathic mice showed impaired hippocampal neurogenesis/plasticity and experienced depressive-symptoms, respectively (Figure 1B). We performed 3 independent sets of experiments for each time point (3 and 12 weeks) and genotype. Behavioral testing was performed on a total of 1) CCI, n=32; sham, n=26 wild-type mice, sacrificed 3 weeks after injury; 2) CCI, n=34; sham, n=26 wild-type mice sacrificed 12 weeks after injury; 3) TNFR1 knockout mice: CCI, n=22; sham, n=19 and wild-type littermate control mice: CCI, n=22; sham, n=19 sacrificed 3 weeks after injury; 4) TNFR1 knockout mice: CCI, n=22; sham, n=16 and wild-type controls: CCI, n=16; sham, n=16 sacrificed 5 weeks after injury. A subset of mice from each group was used for tissue analysis as follows: (1) 3 weeks after injury: CCI, n=10 and sham, n=9 wild-type mice used for immunohistochemical analysis and CCI, n=10 and sham, n=10 for Western blot assay; (2) 12 weeks after injury: CCI, n=9 and sham, n=9 wild-type mice used for immunohistochemical analysis and CCI, n=10 and sham, n=10 for Western blot assay; (3) TNFR1 knockout mice: CCI, n=12; sham, n=9 and wild-type control mice: CCI, n=12; sham, n=9 sacrificed 3 weeks after injury and used for immunohistochemical analysis; TNFR1 knockout mice: CCI, n=10; sham, n=10 and wild-type control mice: CCI, n=10; sham, n=10 sacrificed 3 weeks after injury for Western blot assay. Serum was collected from CCI, n=11 and sham, n=5 wild-type mice 3 weeks after injury.
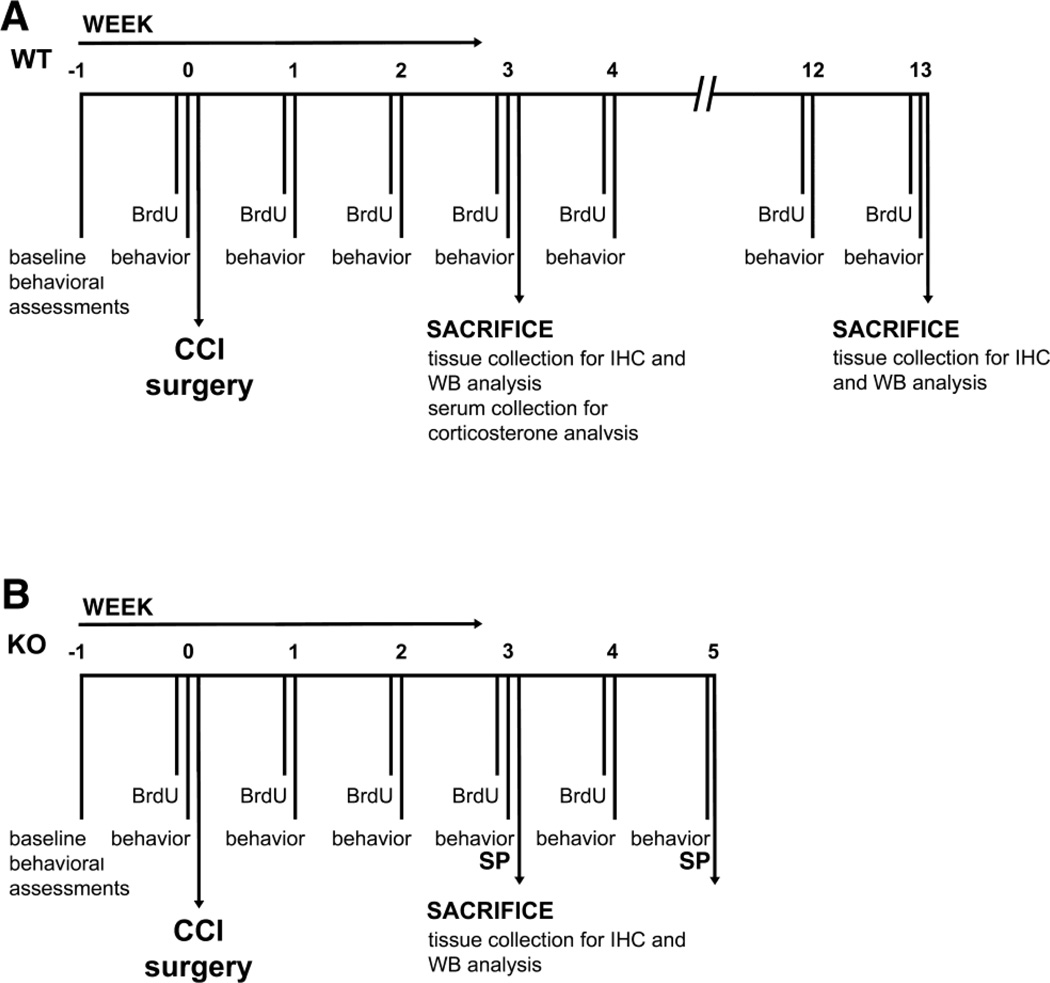
Figure 1.
Chronological overview of the experimental paradigms conducted in this study. A, Wild-type mice were sacrificed 3 and 12 weeks after CCI injury, the time points at which they suffer neuropathic pain and were spontaneously recovered from it. Behavior (allodynia, hyperalgesia, sucrose preference, body weight, physical state measurements, open field test) was performed once a week 24 hours apart in the following order: BW and PS assessment, sucrose preference test, open filed test, von Frey filament test, hot plate. B, TNFR1 knockout mice were sacrificed 3 weeks after injury. Behavior = allodynia, body weight and physical state measurements). Sucrose preference (SP) was measured on week −1, 3 and 5. BrdU was administered weekly.
Behavioral studies
Mice were handled daily for at least 1 week prior to any surgical procedure. Then they were habituated to each behavioral testing apparatus for 30 minutes on 7 consecutive days before initiating data collection. Mice were tested on each behavioral test once a week by a blind observer until complete spontaneous recovery from pain occurred. All behavioral procedures were conducted during the light phase. Investigations were performed by experimenters blinded to the surgery and the genotype. The experimental plan is described in Figure 1.
Mechanical allodynia
To evaluate tactile sensitivity each mouse was placed individually on a wire mesh beneath a transparent plastic jar and habituated for 30 min before starting the test. Von Frey filaments (Touch-Test Sensory Evaluator) were applied to the plantar surface of each hindpaw in a series of ascending forces (ranging between 0.04 - 2 g) starting from 0.04 g filament. A single trial of stimuli consisted of five applications of Von Frey filaments every minute perpendicularly to the plantar surface of the paw for about 4–5 s. Paw withdrawals (at least three times out of five consecutive applications) in response to the stimuli were considered positive, no response was considered negative. Depending on the positive or negative response, subsequent filaments were applied in order of descending or ascending intensity, respectively. If paw withdrawal occurred in response to the minimum 0.04 g filament, the next trial was performed with the same 0.04 g, cut-off filament. In case the maximum 2 g filament was reached with no response, the next trail was performed with the same 2 g, cut-off filament. Each test consisted of 6 trials. Paw movements associated with locomotion or weight-shifting were not counted as a response. The withdrawal threshold was expressed as the tolerance level in grams.
Thermal hyperalgesia
Thermal sensitivity was assessed using a plantar test apparatus (UGOBASILE). Each animal was placed beneath an inverted transparent plastic cage on a thin glass platform and habituated for at least 30 minutes before starting the test. The hind paws were in contact with a 1/4 in thick glass maintained at room temperature. A thermal radiant stimulus was applied to the plantar surface of the hind paws and the latency of the paw withdrawal response was measured automatically with a photoelectric-sensitive device. The heat intensity was set at 50°C (corresponding to 196mW/cm2), which produced a baseline paw withdrawal latency of about 8 sec in naïve animals. The mean latency of withdrawal response of each hind paw was determined by the average of 6 measurements per paw at each time point. A cut off of 20 sec was used. The paw withdrawal latency was determined alternating between the left and right hind paw, with a 5-min inter-trial interval. Only quick hind paw withdrawal movements from the heat light were considered as a response.
Sucrose Preference Training and Sucrose Preference Test
Anhedonia was monitored using a procedure modified from Willner et al., (1996). Sucrose preferring mice were selected using the sucrose preference training that determines which mice prefer sucrose over water (Strekalova et al., 2006; Strekalova and Steinbusch, 2010) and only mice that showed a sucrose preference ≥ 65% were included in this study (Strekalova et al., 2005; Strekalova et al., 2004). Animals were housed individually for 24 hours and presented with two pre-weighed bottles containing either 1% sucrose solution or water. The sucrose preference was evaluated every 2 h by weighing the pre-weighed bottles containing the respective solutions. For each animal, the baseline sucrose preference was evaluated after the conclusion of the training procedure over a 2-hours period as follows: sucrose preference (SP)=[SInt x 100/(SInt +WInt)], where SInt = [(weight of the bottle containing the sucrose solution before the testing)-(weight of the bottle containing the sucrose solution after 2h testing)] and WInt (water intake, g) =[(weight of the bottle containing water before the testing)-(weight of the bottle containing water after 2 h testing)]. Subsequently, the sucrose preference was monitored as described over a 2-hours period, after 14 hours of water deprivation. Water intake, sucrose intake and total fluid intake (sucrose intake + water intake) were measured by weighing the pre-weighed bottles containing the respective solutions before and after the test (Strekalova et al., 2006). For readers’ convenience we used the word “anhedonia” and “depressive-like behavior” interchangeably throughout the text.
Body weight and physical state assessment
Mice were weighted weekly starting on the week before surgery (-1) until week 12, and the measures was recorded in the morning before any other testing. Physical state (PS) was evaluated weekly using a scale from 1 to 3: a healthy state was noted 1 and a damaged state with piloerection and/or dirty fur was noted 3. Intermediate state was noted 2 (Ducottet et al., 2003).
Open Field Test
A subset of mice were tested in the open filed test and sacrificed 12 weeks after surgery (CCI, n = 22, sham, n = 16). To evaluate the effects of CCI injury on anxiety, mice were tested in the open field (Walsh and Cummings, 1976). The open field test was performed in a circular, 85 cm diameter arena with 30 cm high wall, having the floor divided in 2 concentric circles and 4 radiating lines crossing in the center of the arena, resulting in a total of 24 sections. Each mouse was placed individually in the center of the arena. The time spent in the periphery, the number of sections entered with all four feet and the time spent moving were recorded over 5 minutes.
Blood sampling and corticosterone assay
Corticosterone assay was performed on a subgroup of mice (wild-type, 3 weeks, CCI, n = 11; sham, n = 5). Blood samples were collected 1 week before CCI surgery and 3 weeks later when mice experienced pain. Blood was collected using the cheek pouch or submandibular bleeding method via puncture of the vascular bundle located at the rear of the jaw bone. Blood was collected from the puncture into 1.5 ml heparinized tubes at 4°C. Upon centrifugation (12,000 X g, 5 min, 4°C) plasma was collected and stored at −80°C. Samples were dry-ice shipped to Vanderbilt Hormone Assay & Analytical Services Core (Vanderbilt University) for ELISA assay of plasma corticosterone.
Bromodeoxyuridine treatment and immunohistochemistry
Hippocampal neurogenesis was evaluated by immunohistochemical analysis of markers expressed by highly proliferating cells (Ki67), newly born immature neurons (DCX), and newly generated mature neurons (BrdU/NeuN) (Brown et al., 2003; Kee et al., 2002; Kempermann et al., 2004; Scholzen and Gerdes, 2000). Bromodeoxyuridine (BrdU, 150 mg/kg in 0.1M PBS) was injected intra-peritoneally once a week starting from the day of surgery until sacrifice. Animals were transcardially perfused with 4% paraformaldehyde (PFA)/0.1M PBS. Brains were removed, post-fixed and cryoprotected in 15% sucrose/PBS followed by 30% sucrose/PBS. Fifty µm-thick tissues sections were cut in the coronal plan, collected serially and stored at −20°C. For Ki67 immunostaining, brain sections were mounted on Superfrost slides, dried for 2 h, and antigen retrived with the Target Retrieval solution (Dako Cytomation) 30 min at 98°C. After blocking peroxidase activity with 0.3% H2O2, sections were incubated in 5% serum, 0.3% Triton X-100/PBS (1 h, 4°C), and then with rabbit anti- Ki67 antibody (1:150; Dako Cytomation) overnight at 4°C, followed by biotinylated secondary anti-rabbit antibody (1:200, 1.5 h at 4°C; Vector Laboratories). Labeled cells were visualized using the ABC system (Vectastain Elite; Vector Laboratories) using 3,3’-diaminobenzidine as chromogen and counterstained with hematoxylin (Vector Laboratories). Free-floating sections were utilized for doublecortin (DCX) and NeuN/BrdU immunostaining. Sections were blocked with 10% serum in 0.3% Triton X-100/PBS and then incubated with rabbit anti-DCX antibody (1:3000, Abcam) overnight at 4°C. Alexa 488-conjugated anti-rabbit secondary antibody (1:750, Invitrogen) was applied to detect positivity. For NeuN and BrdU double-labeling, sections were incubated with mouse anti-NeuN primary antibody (1:100; Millipore), followed by anti-mouse Alexa 594-conjugated IgG (1:750, Invitrogen). After washing, sections were pretreated with 2N HCl for 30 min at 37°C, and then incubated with rat anti-BrdU (Serotec, 1:200) followed by anti-rat Alexa 488-conjugated IgG (1:750, Invitrogen). For each animal, three adjacent series of one-in-eight sections through the dentate gyrus or one-in-ten sections through the olfactory bulbs were immunostained and analyzed using unbiased stereological estimation of cell number (Stereo Investigator; MicroBright Field). Positive cells were separately counted in the subgranular zone (SGZ) and in the granular cell layer (GCL) of the left and right dentate gyrus, and then summed to obtain the total number in each side and in the entire dentate gyrus. The SGZ was defined as two cell bodies wide of the hilus along the border of the GCL, and cells were qualified as being in the SGZ if they were touching or were within the SGZ.
Western blot
Mice were transcardially perfused with cold 0.1 M PBS pH 7.4, the hippocampi dissected out and homogenized in RIPA buffer (0.01 M Sodium Phosphate pH 7.2, 0.15 M NaCl, 1% Nonidet-40, 1% Sodium Deoxycholate, 0.1% SDS, 2 mM EDTA, containing protease (Roche Diagnostics, Germany) and phosphatase inhibitors (Sigma, USA). Protein samples were electrophoresed onto 6–15% sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose membranes, which were blocked with 5% milk and incubated with antibodies against the following proteins: growth associated protein 43 (GAP43) 1:1000 (Genetex), microtubule-associated protein 2 (MAP2) 1:800, myelin basic protein (MBP) 1:1000 (Millipore), myelin proteolipid protein (PLP) 1:400 (Pierce Biotechnology), synaptotagmin 1:1000 (Synaptic Systems), TNF 1:100, TNFR1 1:300 and TNFR2 1:300 (Santa Cruz). Peroxidase-conjugated anti-rabbit, or anti-mouse IgG (1:5,000, Bio-Rad), or anti-rat IgG (1:2,000, Sigma) were used as secondary antibody. Signal was detected by SuperSignal WestPico Chemiluminescent Substrate (Pierce) according to the manufacturer’s instructions. Membranes were reprobed with mouse anti-β-tubulin (1:10,000, 1 h, RT, Sigma). Quantification of western blots was performed by densitometry using Quantity One, 1-D Analysis software (Bio-Rad), normalizing each band to the β-tubulin signal.
Statistical analysis
Data were analyzed with GraphPad Prism version 5.0 for Windows (San Diego, CA) using KS normality test, D'Agostino & Pearson omnibus normality test and Shapiro-Wilk normality test. After confirming the normality of the data (alpha=0.05), we performed parametric tests. Data were analyzed using the one- or repeated measures two-way ANOVA test, with a level of confidence set at 95% (p<0.05), followed by Bonferroni post-test for comparisons between the experimental groups at each time point. The two time points (12 vs 3 weeks) in each condition (sham or CCI) were additionally compared by paired or unpaired Student t-test. Data are expressed as mean ± SEM. Additionally, correlation analysis was performed using the Pearson test. A value of p<0.05 was considered statistically significant. For correlations between hyperalgesia, allodynia and anhedonia, the mean value of each week for each group was used. For correlations between behavioral tests and hippocampal changes, the individual data of each mouse was used.
RESULTS
CCI-induced neuropathic pain symptoms precede and parallel the development and resolution of depressive like-behavior
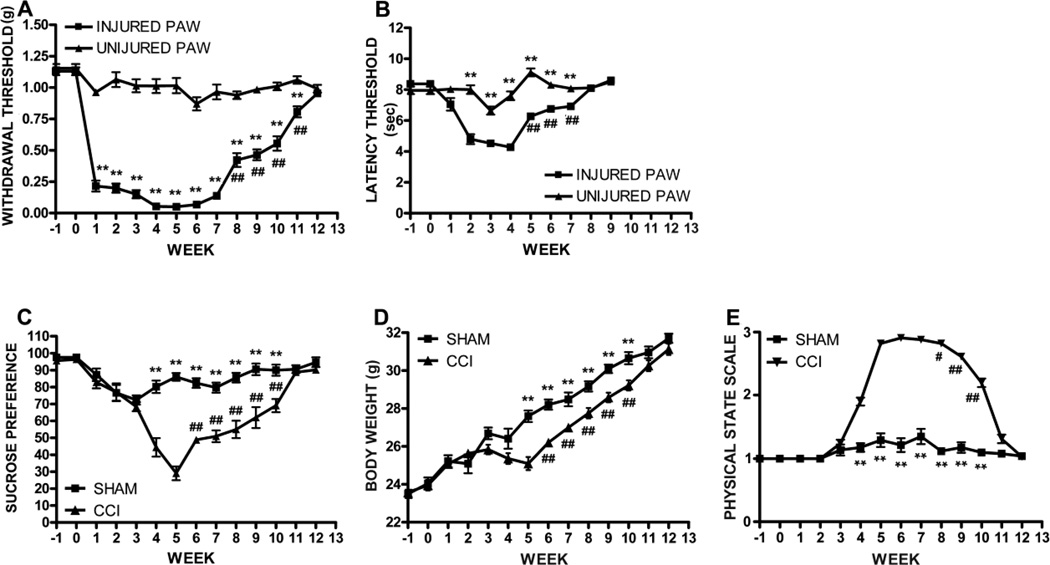
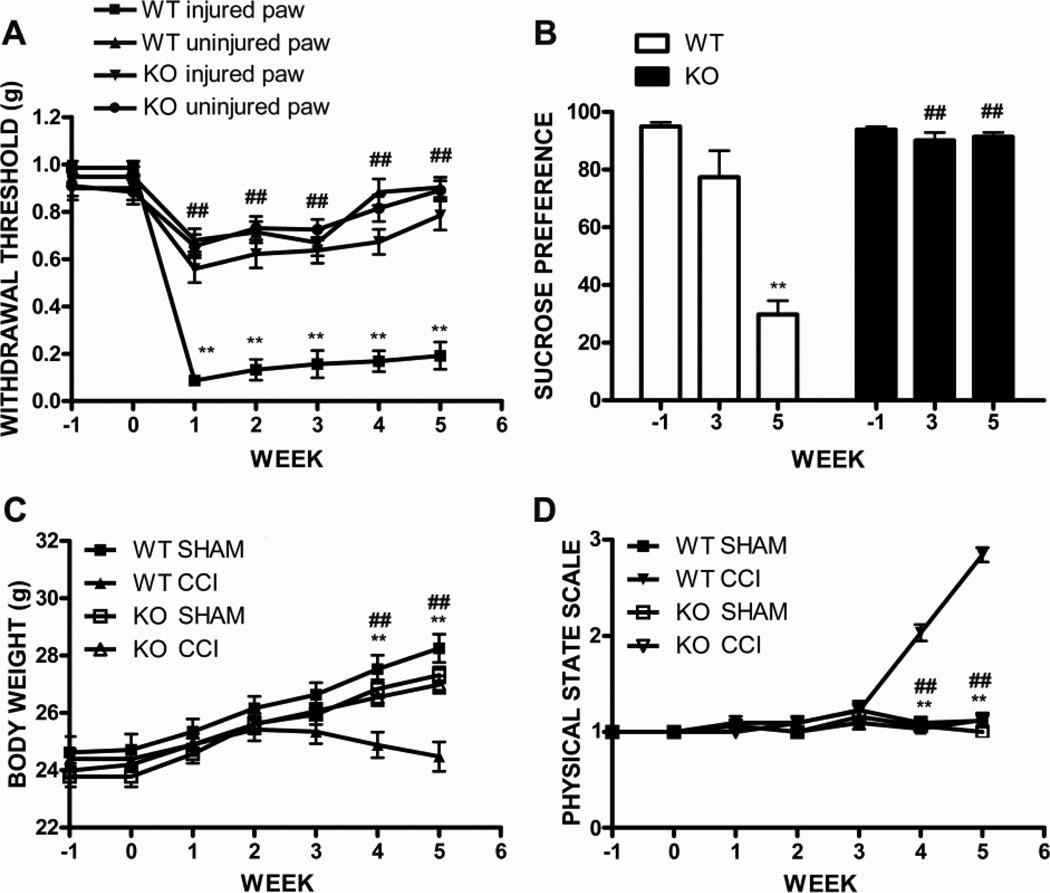
Mice subjected to CCI of the right sciatic nerve developed mechanical allodynia and thermal hyperalgesia on the ipsilateral hind paw, but not on the controlateral paw (two-way ANOVA, injured side vs uninjured side, allodynia: F1,924 = 1415.61, p<0.0001; hyperalgesia: F1,726 = 205.89, p<0.0001; CCI, n = 34, sham, n = 26). In particular mice developed mechanical allodynia one week after surgery (0.2 ± 0.04 g injured side vs 1.0 ± 0.02 g uninjured side, p<0.001), and were completely recovered 12 weeks later (1.0 ± 0.02 g injured side vs 1.0 ± 0.04 g uninjured side, week 12, p>0.05) (Figure 2A). The onset of thermal hyperalgesia was observed 2 weeks after CCI (4.8 ± 0.31 sec injured side vs 8.0 ± 0.27 sec uninjured side, p<0.001; CCI, n = 34, sham, n = 26) (Figure 2B) from which mice were fully recovered by week 8, 4 weeks earlier than they recovered from allodynia (8.1 ± 0.11 sec injured side vs 8.1 ± 0.12 sec uninjured side, p>0.05). We found a significant correlation between hyperalgesia and allodynia (R = 0.73, p<0.01). No significant difference was measured between the shams’ hind paws or between the uninjured paw of CCI mice and the sham’s hind paws, by using the Von Frey filament and the Plantar tests (data not shown).
Figure 2.
Behavioral assessment of allodynia, hyperalgesia and depressive symptomps. A, Mice developed allodynia 1 week after CCI surgery and were completely recovered at week 12. B, Hyperalgesia was evident 2 weeks after surgery until week 7. Mean ± SEM of paw withdrawal threshold (g) and paw latency threshold (sec), respectively; **p<0.01, right paw vs left paw; ##p<0.01, week 8–11 vs 7 (allodynia) and week 5–7 vs 4 (hyperalgesia). C, Anhedonia paralleled the development and resolution of allodynia and hyperalgesia: CCI mice showed reduced sucrose preference 4 weeks after surgery until week 10 compared to sham animals, and were completely recovered at week 11. Mean ± SEM of sucrose preference; **p<0.01, CCI vs sham; ##p<0.01, week 6–10 vs week 5. D-E, Similarly, CCI mice showed a significant reduction of body weight gain (D) and degradation in the physical state of the coat (E), 5 and 4 weeks after injury, respectively, which were restored to the levels measured in sham mice 11 weeks later. **p<0.01, CCI vs sham; #p<0.05, ##p<0.01, weeks 6–10 vs week 5, and week 8–10 vs 7. (CCI, n = 34, sham, n = 26).
In order to investigate the effect of neuropathic pain on depressive-like behavior we used the sucrose preference test (SPT) to measure anhedonia, one of the main symptoms of depression, body weight measurement and the physical state assessment. The SPT showed that 90% of the mice had a baseline sucrose preference > 65% and were included in the study (Supplementary 2). Afterwards, sucrose preference was significantly reduced in CCI animals compared to shams (two-way ANOVA, F1,812 = 184.26, p<0.0001; CCI, n = 34, sham, n = 26). Neuropathic mice showed anhedonia starting at week 4 after injury (sucrose preference: 44 ± 5% CCI vs 80 ± 4 % sham, p<0.001), reached the anhedonic peak at week 5 (29 ± 4% CCI vs 86 ± 2% sham, p<0.001), and were completely recovered 11 weeks after surgery (89 ± 1% CCI vs 90 ± 2% sham, p>0.05) (Figure 2C). Interestingly, we found a significant correlation between anhedonia and allodynia (R = 0.75, p<0.001). When performing the sucrose preference test we also measured total fluid intake (sucrose intake + water intake) before and after the test and found a significant reduction of sucrose intake and total fluid intake in CCI mice compared to shams starting at week 4 after injury until week 9. On the contrary water intake was similar between sham and CCI mice at any time point after injury (data not shown). Since water intake was not affected by injury, the decreased sucrose intake per se accounted for the reduction of total fluid intake in CCI mice. These results indicate that reduction of sucrose preference in CCI mice was not affected by fluid intake but instead was a true manifestation of the anhedonic state of mice after injury, as being a consequence of decreased intake of sucrose only.
Depressive symptoms associated with increased levels of cytokines have been found to often correlate with reduced fluid and food intake in rodents. We did not measure food intake, however we found a significant reduction of body weight gain in CCI wild-type mice starting at 4 weeks after surgery compared to sham mice until week 10, most likely due to a decrease in food intake (Figure 2D). Interestingly, weight loss coincided with the onset of anehdonia as measured by the sucrose preference test.
Parameters such as body weight (BW) and physical state (PS) variations have been previously described by others as reliable tools to evaluate the development of depressive-like symptoms/behavior in rodents and have been proven to correlate with behavioral performances in the sucrose preference test (Ducottet et al., 2003; Griebel et al., 2002). Therefore, since decreased BW or a higher score at the PS assessments are indicative of anhedonic state, we measured BW and assessed PS in our mice after CCI. In line with previous data, we found a reduction of BW gain and increased score at the PS evaluation in mice that developed anhedonia after injury but not in shams (two-way ANOVA, CCI vs sham, BW: F1,812 =70.93, p<0.0001; PS: F1,812 = 1127.44, p<0.0001; CCI, n = 34, sham, n = 26). Moreover, similarly to the sucrose preference, BW and PS were restored to the levels measured in sham mice 11 weeks after injury (BW, 30.3 ± 0.3% CCI vs 31.0 ± 0.3 % sham, p>0.05; PS, 3.0 ± 0.12% CCI vs 3.0 ± 0.04 % sham, p>0.05) (Figure 2D and E). Interestingly, we found a significant correlation between anhedonia and BW and PS score (R = 0.73, p<0.001 and R = 0.75, p<0.0001).
We also assessed the effects of CCI injury on anxiety-like behavior using the open field test (Supplementary 3). As an innate behavior, rodents protect themselves by avoiding open spaces where they can be easily seen and caught by a predator, so that when a mouse is placed in an open space such as the arena utilized for the open field test it tends to spend more time near the edges (Walsh and Cunnings, 1976). Therefore, we measured the time spent in the peripheral part of the arena as a sign of anxiety. Since this parameter could be affected by motor impairment possibly caused by the constriction of the sciatic nerve we also analyzed the number of sections entered by mice in the open field arena and the time spent moving as indications of the overall spontaneous locomotor activity. Our results show no difference between sham and CCI mice in the time spent in the periphery at any time after injury (Supplementary figure 3A) or in the number of sections crossed (Supplementary figure 3B) or in the time spent moving (data not shown). This data indicates that CCI injury did not affect the overall locomotor activity and did not interfere with the measurement of the anxiety-phenotype.
Finally, in order to evaluate the effects of chronic pain on the activation of the hypothalamic-pituitary-adrenal (HPA) axis, we measured corticosterone plasma levels in CCI and sham mice 3 weeks after injury, and we did not find any significant difference between the two groups or before and after surgery consistent with previous reports (Duric and McCarson, 2006) (Supplementary 4).
CCI induces a significant reduction in neurogenesis that returns to baseline levels when animals spontaneously recover from neuropathic pain
Since depressive-like behavior has been associated with impaired hippocampal neurogenesis (Sahay and Hen, 2007), we sought to investigate whether this process was affected during CCI, potentially contributing to CCI-induced anhedonia.
We found a similar number of Ki67+ cycling cells and DCX+ immature neurons in the dentate gyrus of sham and CCI mice three weeks after injury and no significant difference between the ipsilateral and contralateral side, indicating that CCI injury did not affect the proliferation of progenitor cells (Ki67+ cells) or the neuronal commitment of the precursor cells (DCX+ cells) located in the dentate gyrus at this time point (Supplementary 5).
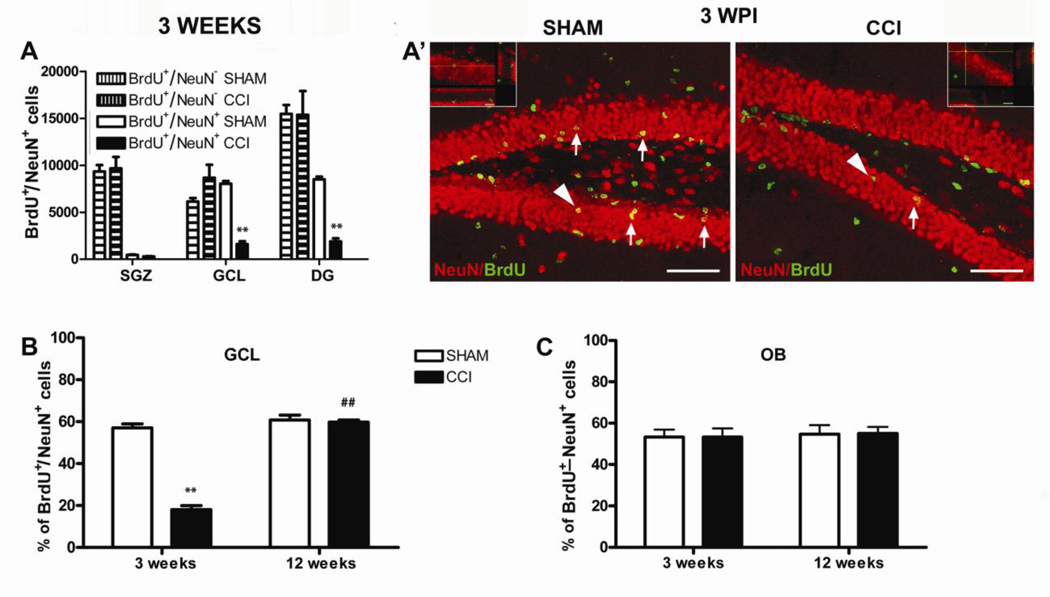
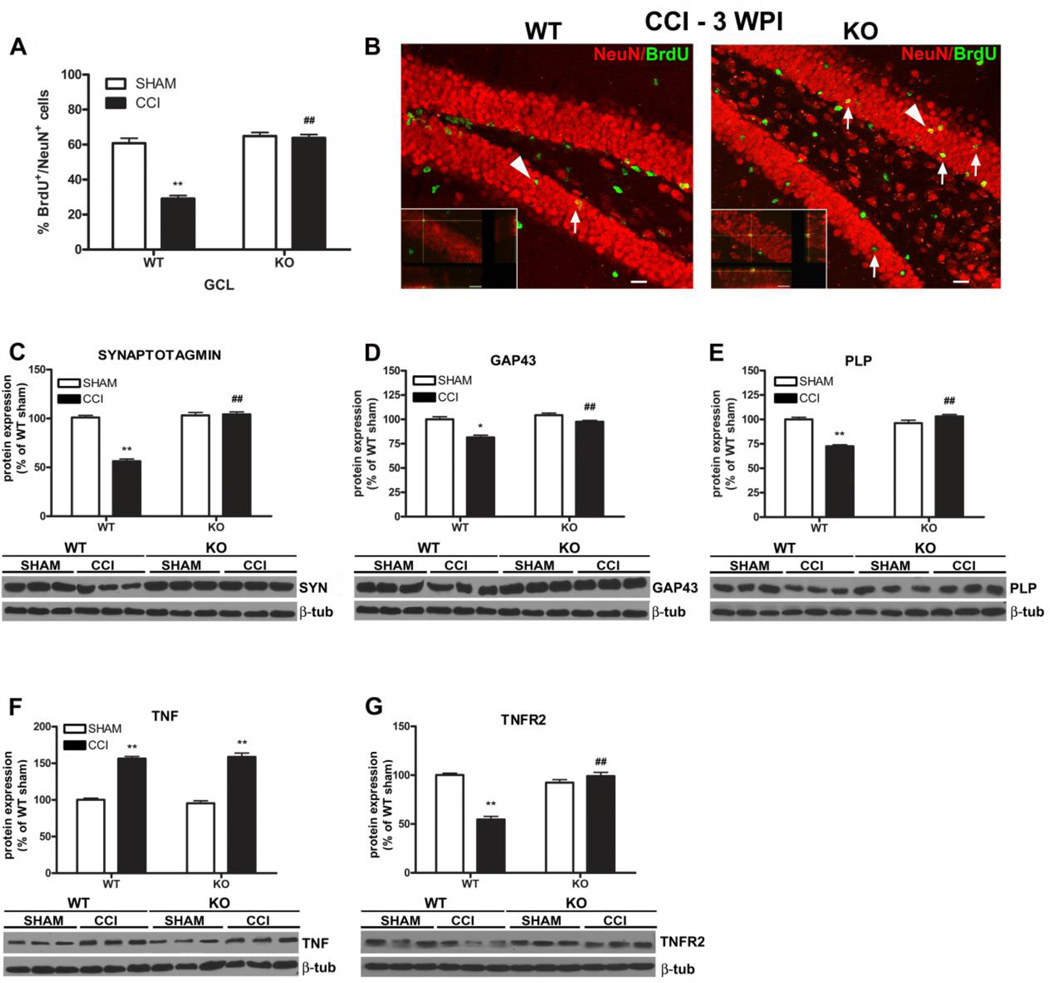
During adult hippocampal neurogenesis, like during embryogenesis, new immature DCX-expressing neurons are generated in surplus and only a few are selected into functional circuits and mature into new fully differentiated NeuN positive neurons while losing DCX expression (Blaschke et al., 1996; Biebl et al., 2000; Kempermann and Gage, 2002). As a consequence it is not sufficient to use immature neuronal markers such as DCX as indicators of net neurogenesis (Gleeson et al., 1999; Cooper-Kuhn and Kuhn, 2002; Kempermann et al., 2003). For this reason, we further analyzed the differentiation of the newly generated mature neurons by BrdU/NeuN double immunostaining (Kempermann et al., 2004). We found that 3 weeks after injury the number of BrdU+/NeuN+ newly generated neurons was significantly lower in the dentate gyrus of the CCI mice compared to the sham mice, while the number of total BrdU+ cells [(BrdU+/NeuN+) + (BrdU+/NeuN−)] was similar between the 2 groups (Figure 3A, A’). In particular we observed a significant reduction of BrdU+/NeuN+ cells in the GCL of the dentate gyrus, the area where the new born neurons migrate and complete their maturation to become new, fully differentiated NeuN+ granular neurons, so that only 18% of the BrdU+ cells were double-stained with NeuN in the GCL of CCI mice compared to the 57% of the sham animals (18 ± 2 % CCI vs 57 ± 2 % sham, unpaired, two-tailed Student’s t test, F9,8= 1.098, p = 0.0001, CCI, n = 10; sham, n = 9) (Figure 3B). Moreover, we did not find any significant difference in the number of BrdU+/NeuN+ cells or in the number of total BrdU+ cells between the ipsilateral and contralateral dentate gyrus, showing that chronic neuropathic pain affects adult hippocampal neurogenesis in both sides of the dentate gyrus (data not shown). Notably, there was a significant correlation between allodynia and the reduction of BrdU+/NeuN+ cells both in the DG (R = 0.90; p<0.001) and in the GCL (R = 0.92; p<0.001) and between anhedonia and the reduction of BrdU+/NeuN+ cells in the DG (R = 0.97; p<0.001) and in the GCL (R = 0.99; p<0.001).
Figure 3.
Analysis of adult neurogenesis in the hippocampus and olfactory bulbs 3 and 12 weeks after injury. A, The number of BrdU+ cells was similar between CCI and sham mice but the number of BrdU+/NeuN+ cells were dramatically reduced in the GCL of CCI mice compared to shams. Mean ± SEM of: BrdU+ (BrdU+/NeuN + BrdU+/NeuN+ cells) and BrdU+/NeuN+ cells; **p<0.01. A’, Representative images of BrdU (green)/NeuN (red) immunostaining in the DG of sham and CCI animals 3 weeks after surgery. Arrows indicate some BrdU+/NeuN+ cells (yellow). Arrowheads identify the cells in the higher magnification micrographs (scale bar = 60 micron) (CCI, n = 10, sham, n = 9). B, The percentage of BrdU+/NeuN+ neurons was dramatically reduced in the GCL of neuropathic mice 3 weeks after CCI, and then completely restored 12 weeks later (3 weeks: CCI, n = 10, sham, n = 9; 12 weeks: CCI, n = 9, sham, n = 9). C, The percentage of BrdU+ cells that differentiated into new adult NeuN+ neurons in the OB of CCI mice was similar to shams both 3 and 12 weeks after injury. Mean ± SEM of BrdU+/NeuN+ cells % (BrdU+/NeuN+vs total BrdU+ cells); **p<0.001, CCI vs sham; ##p<0.01, CCI weeks 12 vs week 3 (CCI, n = 9, sham, n = 9). DG=dentate gyrus, GCL=granular cell layer, OBs=olfactory bulbs.
To further investigate the correlation between the impairment in hippocampal neurogenesis and the development of anhedonia that occurs in neuropathic pain, we counted the BrdU+/NeuN+ cells in the GCL of mice that had recovered spontaneously from anhedonia and pain, 12 weeks after surgery. We found that at this time point the rate of formation of new BrdU+/NeuN+ neurons in the GCL was similar between injured and sham mice (58 ± 0.7 % of BrdU+/NeuN+ cells CCI vs 61 ± 3% sham, unpaired, two-tailed Student’s t test, F8,8= 15.00, p>0.05; CCI, n = 9; sham, n = 9) and similar to shams at 3 weeks (two-way ANOVA F1,33 = 108.10, p<0.0001; 18 ± 2% CCI vs 57 ± 2% sham, 3 weeks, p<0.001 and 58 ± 0.7 %, CCI vs 61 ± 3%, sham, 12 weeks p>0.05 by Bonferroni post-test) (Figure 3B).
In order to assess whether the impairment of neurogenesis in neuropathic mice was specific to the hippocampus, we evaluated the generation of new neurons in the olfactory bulbs (OB), the other area of the brain where new neurons are added throughout adult life (Gross, 2000). The percentage of BrdU+/NeuN+ cells was similar between CCI and sham mice in the OB both 3 and 12 weeks after injury, indicating that the formation of new neurons was selectively affected only in the hippocampus of neuropathic mice (53 ± 4 % CCI vs 54 ± 3% sham, 3 weeks, and 55 ± 3% CCI vs 55 ± 4% sham, 12 weeks; two-way ANOVA F1,9 = 0.00, p>0.05, , CCI, n = 9; sham, n = 9) (Figure 3C). Data are the results of 3 independent experiments.
CCI-induced neuropathic pain and depressive-like behavior correlate with changes in neuronal and myelin plasticity in the hippocampus
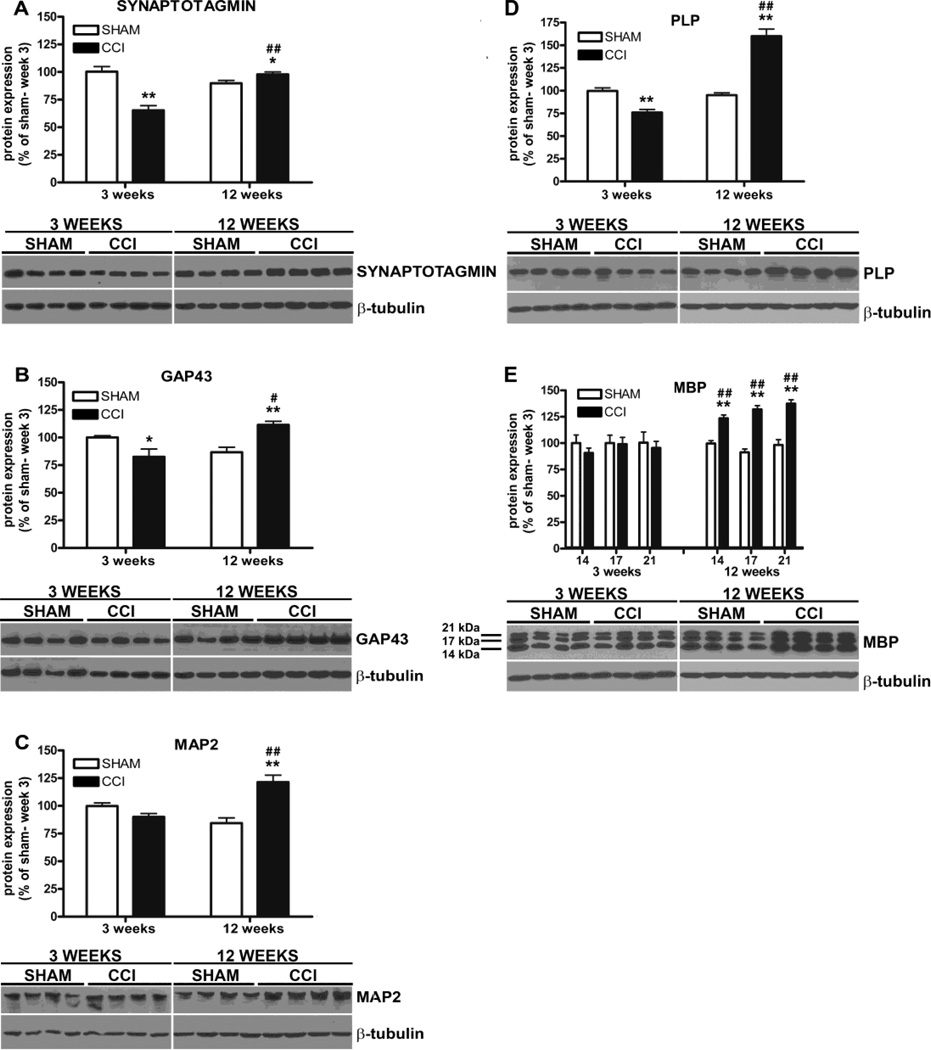
We analyzed the expression of neuronal proteins synaptotagmin, GAP43, and MAP2 in the hippocampus of CCI and sham mice. Three weeks after injury, synaptotagmin and GAP43 were significantly reduced in the CCI group compared to sham mice (two-way ANOVA F1,36 = 42.15, p<0.001; −36 ± 6% CCI vs sham, p<0.001 Bonferroni post-test; two-way ANOVA F1,36 = 21.81, p<0.001; −17 ± 7% CCI vs sham, p<0.05 Bonferroni post-test, respectively; CCI, n = 10; sham, n = 10) (Figure 4A, B). 12 weeks after injury the levels of GAP43 and MAP2 were significantly increased in the CCI mice compared to the shams (+25 ± 6% and +37 ± 7%, respectively, CCI vs sham, Bonferroni post-test, p<0.001) and the markers analyzed were all significantly increased in the CCI group compared to the levels measured at week 3 (+33 ± 5% (synaptotagmin), +29 ± 8% (GAP43), +31 ± 7% (MAP2), paired two-tailed Student’s t test, F9,9 = 3.728, p < 0.0001; F9,9 = 4.584, p < 0.05; F9,9 = 4.524, p < 0.001, respectively) (Figure 4A–C). Finally, the occurrence of synaptic, axonal and dendritic remodeling in the hippocampus of CCI mice correlated significantly both with development and recovery from pain and with anhedonia (allodynia and GAP43 (R = 0.56; p<0.05), allodynia and MAP2 (R = 0.61; p<0.01), allodynia and synaptotagmin (R = 0.88; p<0.001); hyperalgesia and GAP43 (R = 0.65; p<0.001), hyperalgesia and MAP2 (R = 0.55; p<0.05), hyperalgesia and synaptotagmin (R = 0.81; p<0.001); anhedonia and GAP43 (R = 0.71; p<0.001), anhedonia and synaptotagmin (R = 0.76; p<0.001)).
Figure 4.
Western blot analysis of hallmarks of neuronal plasticity and myelin protein in the hippocampus of CCI and sham mice 3 and 12 weeks after injury. A, Synaptotagmin (A) and GAP43 (B) expression were significantly reduced 3 weeks after injury in CCI mice. (A–C) The levels of synaptotagmin, GAP43 and MAP2 were significantly increased in CCI mice 12 weeks after injury compared to 3 weeks. D, PLP levels were significantly decreased in CCI mice compared to shams 3 weeks after surgery. (D-E), Both PLP and MBP levels were significantly increased in CCI mice 12 weeks after surgery compared to shams and to the 3 weeks levels. Mean ± SEM of optical density of each protein normalized to beta-tubulin, percentage of sham at 3 weeks; *p<0.05, **p<0.001, CCI vs sham; ##p<0.001 12 weeks vs 3 weeks (3 weeks: CCI, n=10, sham, n=10; 12 weeks: CCI, n=10, sham, n=10). Representative experiments are shown.
PLP and MBP represent the largest components of myelin proteins and since their expression is altered in demyelinating and re-myelinating conditions of the nervous system, they serve as reliable tools to assess ongoing myelin content modifications and plasticity in pathological conditions (Merkler et al., 2006; Lucchinetti et al., 2011; Mangiardi et al., 2011). We found that PLP expression was significantly reduced in the hippocampus of CCI mice 3 weeks post-injury, just before the onset of anhedonia, and then significantly increased in CCI mice 12 weeks later, concurrently with the recovery from anhedonia and neuropathic pain (two-way ANOVA F1,36 = 83.35, p<0.0001; −24 ± 5%, p<0.01 and +65 ± 8%, p<0.001 respectively, CCI vs sham, Bonferroni post-test; CCI, n = 10; sham, n = 10) (Figure 4D). In parallel MBP expression was also significantly increased in CCI mice 12 weeks after injury (two-way ANOVA: F1,36 = 11.54, p<0.05; +24 ± 4% CCI vs sham, Bonferroni post-test, p<0.01, 14 kDa band; two-way ANOVA: F1,36 = 14.83, p<0.001; +41 ± 5% CCI vs sham, Bonferroni post-test, p<0.001, 17 kDa band; two-way ANOVA: F1,36 = 10.68, p<0.05; + 39 ± 6% CCI vs sham, Bonferroni post-test, p<0.001; 21kDa band). The two-tailed paired t-test also revealed a significant difference in PLP and MBP levels between the two time points for the CCI group (+84 ± 9%, PLP, F9,9= 5.351, p<0.0001; +33 ± 6% MBP (14 kDa), F9,9 = 2.257, p < 0.0001; +33 ± 7% MBP (17kDa), F9,9 = 3.453, p < 0.001; + 31 ± 6% MBP (21kDa), F9,9 = 1.850, p < 0.001, CCI week 12 vs week 3; CCI, n = 10; sham, n = 10) (Figure 4E). Notably, there was a positive correlation between 1) allodynia and PLP (R = 0.77; p<0.001); 2) hyperalgesia and PLP (R = 0.74; p<0.001), hyperalgesia and MBP 14kDa (R = 0.75; p<0.001), MBP 17kDa (R = 0.64; p<0.001), MBP 21kDa (R = 0.72; p<0.001); 3) anhedonia and PLP (R = 0.63; p<0.01), anhedonia and MBP 14kDa (R = 0.56; p<0.01).
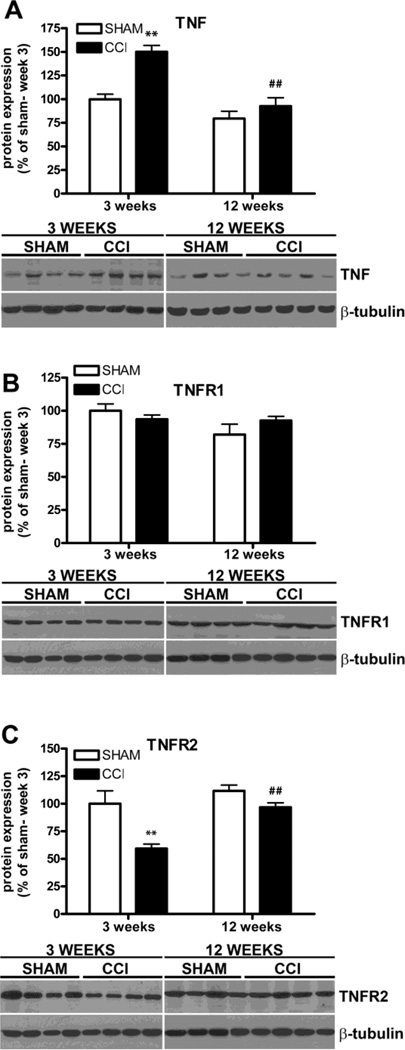
TNF signaling is disrupted in the hippocampus of CCI mice 3 weeks after injury
In an effort to identify signaling pathways associated with the modulation of depressive behavior in neuropathic CCI mice, we explored the involvement of TNF, which has been reported to be up-regulated in depression and depressive-like conditions (Dantzer, 2001; Tuglu et al., 2003). We analyzed TNF, TNFR1 and TNFR2 expression in the hippocampus of CCI mice by western blot and found that TNF was significantly increased in CCI mice compared to shams 3 weeks after injury (two-way ANOVA: F1,36 = 19.02 p<0.001; +50 ± 9%, CCI vs sham, Bonferroni post-test, p<0.001) (Figure 5A). At 12 weeks, however, TNF levels did not differ from those in sham mice and were significantly reduced compared to the levels at 3 weeks (−58 ± 11% CCI week 12 vs CCI week 3, F9,9 = 1.742, p<0.0001, two-tailed paired Student t-test; CCI, n = 10; sham, n = 10). As for TNF receptors, the expression of the likely neuroprotective/pro-myelinating TNFR2 was significantly reduced in the CCI group 3 weeks after injury (two-way ANOVA: F1,36 = 15.78, p<0.001; −41 ± 12%, CCI vs sham, Bonferroni post-test, p<0.001) and restored to baseline control levels at 12 weeks (97 ± 4% CCI 12 weeks vs 100 ± 12% sham 3 weeks, p>0.05 two-tailed unpaired Student t-test, and 97 ± 4% CCI 12 weeks vs 112 ± 5% sham 12 weeks, p>0.05 two-tailed paired Student t-test). The two-tailed paired t-test also revealed that TNFR2 levels were significantly higher in the CCI mice 12 weeks after injury compared to the 3 week (+37 ± 6%, CCI week 12 vs week 3, paired two-tailed Student’s t test, F9,9 = 1.110, p<0.0001), while TNFR1 did not show any change at either time point (two-way ANOVA: F1,36 = 0.17; p>0.05) (Figure 5B, C). The specificity of anti-TNFR1 and anti-TNFR2 antibodies was tested by Western blot assay on hippocampal protein extracts obtained from TNFR1−/− and TNFR2−/− mice, respectively (Supplementary 1B and C). The statistical association analysis showed a significant correlation between: 1) allodynia and TNF (R = −0.73; p<0.001), TNFR2 (R = 0.61; p<0.01; 2) hyperalgesia and TNF (R = −0.64; p<0.01), TNFR2 (R = 0.70; p<0.001); 3) anhedonia and the TNF levels in the hippocampus of the injured mice (R = −0.61; p<0.051. Results are summarized in supplementary, table 1.
Figure 5.
Western blot quantification of TNF and TNFRs in the hippocampus of CCI and sham mice 3 and 12 weeks after injury. A, TNF expression was significantly increased in CCI mice 3 weeks after surgery compared to shams. 12 weeks later TNF levels were restored to normal. B, TNFR1 levels were similar between sham and CCI mice at both 3 and 12 weeks. C, TNFR2 levels were significantly decreased in CCI mice 3 weeks after surgery compared to the shams. 12 weeks later TNFR2 levels were significantly higher in CCI mice compared to 3 weeks. Mean ± SEM of optical density of each protein normalized to beta-tubulin, percentage of sham at 3 weeks; *p<0.05, **p<0.001 CCI vs sham; ##p<0.001 12 weeks vs 3 weeks; (3 weeks: CCI, n=10, sham, n=10; 12 weeks: CCI, n=10, sham, n=10). Representative experiments are shown.
TNFR1 knockout mice do not develop anhedonia after CCI injury
In order to understand the role of TNFR1-mediated TNF signaling in the development of anhedonia and the related hippocampal alterations that followed CCI injury, we performed CCI surgery on TNFR1 knockout mice and investigated whether the absence of TNFR1 could affect the development of depressive symptoms and the hippocampal changes that we found in the wild-type mice. To this purpose we chose to perform our analysis on TNFR1−/− mice at the time points at which we observed major changes in wild-type mice after CCI. Therefore TNFR1−/−animals were sacrificed 3 and 5 weeks after surgery, when wild-type neuropathic mice showed impaired hippocampal plasticity (neurogenesis, neuronal and myelin plasticity, Figure 3 and 4) and experienced the highest level of depressive symptoms, respectively (Figure 2C).
TNFR1 knockout mice showed a lower withdrawal threshold of the ipsilateral hind paw compared to the contralateral side at the Von Frey filament test (two-way ANOVA, injured side vs uninjured side, F1,294 = 7.69, p<0.05, CCI, n = 22). However, the increase in mechanical sensitivity was significantly smaller when compared to the wild-type mice (two-way ANOVA, injured side TNFR1−/− vs wild-type, F1,252 = 144.90, p<0.0001, knockout, n = 22, wt, n = 16) (Figure 6A).
Figure 6.
Behavioral assessment of allodynia, anhedonia, body weight and physical state in TNFR1−/− mice after CCI injury. A, Mechanical allodynia was significantly reduced in TNFR1 knockout mice compared to wild-type littermates starting at 1 after surgery. Mean ± SEM of paw withdrawal threshold (g); ##p<0.001 injured paw, knockout vs wild-type; **p<0.001, wild-type, injured paw vs uninjured paw. B, Sucrose preference was significantly higher in TNFR1−/− injured mice compared to wild-type 5 weeks after injury and similar to the baseline levels; ##p<0.0001, knockout vs wt; **p<0.0001, wild-type, week 5 vs week −1. C, Body weight was similar between TNFR1 knockout CCI mice compared to shams at any time after injury, and significantly higher than injured wild-type mice. D, Physical state was not affected in TNFR1 knockout mice at any time after injury compared to wild-type. ##p<0.0001, CCI, knockout vs wt; **p<0.0001, wild-type, CCI vs sham (KO: CCI n =22 wt, sham n =16; wt: CCI n =22 sham n =16).
Similarly to wild-type mice the majority of TNFR1−/− mice (94%) showed a baseline sucrose preference >65% and were included in the study. No significant difference was observed in the baseline sucrose preference between wild-type and knockout mice (two-way ANOVA, F6,476 = 0.83, p>0.05; 96 ± 0.9% knockout, n=38 vs 97 ± 0.8% wild-type, n = 32, p>0.05 Bonferroni post-test; supplementary 2). As in the previous experiments we found that the sucrose preference was significantly reduced in wild-type animals at 5 weeks after injury compare to the baseline value (29 ± 5% week 5 vs 95 ± 1.4% week −1, F15,15 = 11.27, p<0.0001, two-tailed, paired Student t-test, CCI = 16) (Figure 6B). However, TNFR1−/− mice did not show signs of anhedonia either at 3 or 5 weeks after injury as their sucrose preference was significantly higher when compared to wild-type injured mice and similar to the baseline levels (two-way ANOVA, +61 ± 4% knockout vs wt, 5 weeks, p<0.001, Bonferroni post-test, knockout n = 22, wt n = 16) (TNFR1−/− : 90 ± 3% week 3 and 91 ± 1% week 5 vs 94 ± 1% week −1, two-tailed, paired Student t-test, F21,21 = 8.54 and F21,21 = 2.48 p> 0.05, respectively, knockout n = 22) (Figure 6B). Importantly, sucrose intake and water intake were not affected in TNFR1 knockout mice after injury so that total fluid intake was similar compared to sham animals and higher than wild-type CCI mice (data not shown).
Interestingly, similarly to the sucrose preference, BW and PS were not affected in TNFR1−/− mice compared to wild-type mice after injury (two-way ANOVA, BW, F1,252 = 13.00, p<0.05; PS, F1,252 = 156.03, p<0.001; knockout, n= 22, wt n = 16) (Figure 6C and D). These data support the findings of the sucrose preference test and confirm the development of depressive-like symptoms in mice that underwent CCI surgery, suggesting a role for TNFR1 in this process.
Hippocampal neurogenesis and plasticity are not affected in TNFR1 knockout mice after CCI injury
Since the development of anhedonia in wild-type mice correlated with reduced hippocampal neurogenesis and plasticity, we investigated if similar alterations still occurred in TNFR1−/− mice, which, contrary to wild-type animals, manifested reduced allodynia and did not developed anhedonia after CCI. Interestingly, the analysis of hippocampal neurogenesis revealed that the rate of neuronal differentiation was significantly higher in the dentate gyrus of TNFR1−/− mice compared to wilt-type mice that showed a significant reduction of BrdU/NeuN double-positive cells 3 weeks after injury as previously described (two-way ANOVA F1,38 = 49.90, p<0.0001; 29 ± 2% wt CCI vs 61 ± 3% wt sham, p<0.001 Bonferroni post-test; CCI TNFR1−/− 64 ± 2% vs CCI wild-type 29 ± 2%, F11,11 = 1.398, unpaired two-tailed Student’s t test, p<0.0001; knockout: CCI, n = 12, sham, n = 9; wt: CCI, n = 12, sham, n = 9) (Figure 7A, B).
Figure 7.
A-B Analysis of hippocampal neurogenesis in TNFR1−/− mice 3 weeks after CCI. A, The % of BrdU/NeuN double-positive cells was significantly higher in the GCL of TNFR1−/− than wilt-type mice and similar to shams. Mean ± SEM of BrdU+/NeuN+ cells % (BrdU+/NeuN+vs total BrdU+ cells); ##p<0.001, knockout vs wild-type; **p<0.001, wild-type, CCI vs sham (KO: CCI, n = 12, sham, n = 9; wt: n = 12, sham, n = 9). B, Representative confocal images of BrdU (green)/NeuN (red) immunostaining in the DG of TNFR1−/− and wild-type animals 3 weeks after injury. Arrows indicate BrdU+/NeuN+ cells (yellow). Arrowheads identify the cells in the higher magnification micrographs (scale bar = 60 micron). C-E, Western blot analysis of hallmarks of neuronal plasticity and myelin proteins in the hippocampus of TNFR1 knockout and wild-type mice 3 weeks after injury. The levels of synaptotagmin (C) and GAP43 (D) were similar between CCI and sham TNFR1−/− mice and significantly higher in TNFR1−/− than in wild-type mice. E, Similarly, PLP expression was not affected in TNFR1−/−F-G, Western blot quantification of TNF and TNFR2 in the hippocampus of TNFR1−/− and wild-type mice 3 weeks after injury. F, The levels of TNF were significantly increased in both genotypes after injury. G, TNFR2 expression was similar between TNFR1−/− injured and sham mice and significantly decreased in wild-type CCI compared to TNFR1−/− mice. Data are the mean ± SEM of optical density of each protein normalized to the beta-tubulin and are expressed as percentage of sham at 3 weeks; ##p<0.001, knockout vs wild-type; *p<0.05, **p<0.001, CCI vs sham (KO: CCI, n = 10, sham, n = 10; wt: n = 10, sham, n = 10). Representative experiments are shown.
We then analyzed the expression of plasticity markers and myelin protein in the hippocampus of TNFR1−/− and wild-type mice after CCI injury by western blot assay. Our data show that 3 weeks after injury the levels of synaptotagmin and GAP43 were significantly higher in the TNFR1−/−than in the wild-type mice and similar to the levels measured in the shams (synaptotagmin: two-way ANOVA, F1,36 = 84.63, p<0.001; +1 ± 4% TNFR1−/− CCI vs TNFR1−/− sham, p>0.05 and −45 ± 3% wt CCI vs sham, p<0.001 Bonferroni post-test; 104 ± 2% TNFR1−/− vs 56 ± 2% wt, F9,9 = 1.190, p<0.0001, unpaired two-tailed Student’s t test; GAP43: two-way ANOVA, F1,36 = 7.71, p<0.01; −7 ± 2% TNFR1−/− CCI vs TNFR1−/− sham, p>0.05 and −19 ± 6% wt CCI vs sham, p<0.001 Bonferroni post-test; 98 ± 1% TNFR1−/− vs 79 ± 2% wt, F9,9 = 2.613, p<0.0001, unpaired two-tailed Student’s t test; n = 10/group) (Figure 7C and D). Finally we did not find any significant difference in the levels of MAP2 either between the wild-type and knockout mice or compared to the sham levels (data not shown).
The analysis of myelin protein revealed that the levels of PLP were similar between the TNFR1−/−and sham mice and significantly higher in the knockout mice compared to wild-type mice (two-way ANOVA F1,36 = 62.22, p<0.0001; +7 ± 3% TNFR1−/− CCI vs sham, p>0.05 and −28 ± 3% wt CCI vs sham, p<0.0001 Bonferroni post-test; 103 ± 2% CCI TNFR1−/− vs 73 ± 1% CCI wt, unpaired two-tailed Student’s t test, F9,9 = 1.838, p<0.0001) (Figure 7E). No significant difference was found in the expression of MBP between the two genotypes (data not shown). We previously showed that hippocampal TNF was significantly increased in CCI mice 3 weeks after injury, while TNFR2 levels were decreased and TNFR1 unaffected. We then analysed the expression of TNF and TNFR2 in the TNFR1−/− mice 3 weeks after injury. We found that TNF expression was significantly increased in TNFR1−/− mice compared to shams and similar to the levels measured in wild-type injured animals (two-way ANOVA, F1,36 = 276.26, p<0.0001; +63 ± 6% TNFR1−/− CCI vs sham p<0.001 and, +56 ± 4% wt CCI vs sham p<0.001, Bonferroni post-test; 159 ± 5% TNFR1−/− CCI vs 156 ± 3% wt CCI, unpaired two-tailed Student’s t test, F9,9 = 2.815, p>0.05; n = 10/group) (Figure 7F). Interestingly, the expression of TNFR2 was unchanged in TNFR1−/− and significantly higher than the levels we found in wild-type mice after CCI (two-way ANOVA, F1,36 = 36.83; p<0.0001; +7 ± 5% TNFR1−/− CCI vs sham p>0.05 and −45 ± 4% wt CCI vs sham, p<0.001, Bonferroni post-test; 99 ± 4% CCI TNFR1−/− vs 44 ± 5% CCI wt, unpaired two-tailed Student’s t test, F9,9 = 1.565, p<0.0001) (Figure 7G) (Supplementary 1B, C). Results are summarized in supplementary data, table 2.
DISCUSSION
Our data show a temporal relationship between CCI-induced neuropathic pain and the occurrence of depressive behavior, as well as decreased neurogenesis, neuroplasticity and myelin remodeling in the hippocampus, one of the main mood-regulating regions of the brain. Moreover we report that these changes occurred in a context in which hippocampal TNF signaling was unbalanced towards TNFR1 due to increased expression of TNF and decreased levels of TNFR2. Finally, we showed that TNFR1−/− mice did not develop depressive symptoms after injury, nor changes in hippocampal neurogenesis and plasticity.
This is the first study to investigate TNFRs expression in the hippocampus of mice that underwent peripheral nerve injury and consequently developed neuropathic pain and depressive-like symptoms and to show that neuropathic pain induced depression and the associated hippocampal alterations are dependent on TNF/TNFR1 signaling.
Previous reports investigated either the occurrence of depressive-like symptoms in neuropathic pain, without assessing hippocampal plasticity (Yalcin et al., 2011) or analyzed whether hippocampal neurogenesis is affected in neuropathic pain but did not assess the development of depressive-like behavior (Mutso et al., 2012). In this study we analyzed both the behavioral affective responses and the possible hippocampal plastic changes that may occur in neuropathic pain and found them to be associated with each other. Moreover, we extended our analysis to the pain recovery phase and observed that, as pain resolved, mice recovered from depressive symptoms, while neurogenesis, neuronal and myelin plasticity were restored within the hippocampus. In addition, we showed that the formation of new neurons was not affected in the olfactory bulbs, another area of the brain where new neurons are added during adult life, showing that the impairment of neurogenesis that occurs in parallel with the development of neuropathic pain-induced depression is specific to the hippocampus.
The correlation between hippocampal neurogenesis and depression has been extensively documented (Castrén, 2005), yet, conclusive evidence that decreased hippocampal neurogenesis is the cause of depression is still under debate (Sapolsky, 2004). While some studies support a direct role for impairment of adult neurogenesis in depression (Snyder et al., 2011), other authors demonstrated that re-establishment of neuronal plasticity, rather than neurogenesis, is the basis for the restoration of behavioral homeostasis by antidepressants (Bessa et al., 2009). Therefore, in parallel with neurogenesis, we also investigated hippocampal neuroplasticity by quantifying changes in synaptic, axonal, and dendritic proteins. In this study, both neurogenesis and neuroplasticity were affected and then restored in mice that developed and recovered from neuropathic pain-induced depressive symptoms, respectively, suggesting that these two events are not necessarily mutually exclusive, but may both play a role in the development of depression and in the behavioral improvement observed in response to antidepressant treatments. We evaluated depressive-like behavior by investigating the development of anhedonia, one of the major symptops of depression, as well as changes in body weight and physical appearance. Changes in body weight and physical state have been proven to be reliable indicators of depressive-like behavior and to correlate with the sucrose preference test and other common tests used for the evaluation of depression in rodents such as the forced swimming test (FST) and the tail suspention test (TST) (Ducottet et al., 2003; Griebel et al., 2002). The reason why we did not use the FST and the TST is due to the fact that these tests are stressfull (Dayas et al., 2001; Liu et al., 2003) and could have affected the development of anhedonia in neuropathic pain and altered the results of the sucrose preference test. Instead, the evaluation of body weight and physical state is purely observational and does not cause stress to mice. Analogously to the sucrose preference test, body weight and physical appearance were affected in neuropathic mice and restored to the levels measured in sham mice 11 weeks after injury demostrating that neuropathic pain induces depressive-like behavior.
Stress negatively affects hippocampal neurogenesis and plasticity through the activation of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in the increased production of corticosterone and in the development of depressive-like symptoms (Gould et al., 1998). Since pain can activate the HPA axis, (Aloisi et al.,1995; Harbuz and Lightman,1992) we assessed if our results could be a consequence of the activation of the HPA axis in response to pain as a stressful stimulus. We measured plasma corticosterone levels in mice affected by chronic pain and we did not find any significant increase compared to naïve mice in agreement with other studies. This suggests that the hippocampal neurogenesis and plasticity and the parallel development of depressive behavior are specific to the pain experience (Duric and McCarson, 2006).
Using the open field test we also evaluated the occurrence of anxiety-related symptoms and did not find increased signs of anxiety in neuropathic mice, similarly to previous reports (Goncalves et al., 2008). In this regard, though, the scientific literature is still contradictory, as other authors reported the occurrence of anxiety-related behavior in neuropathic pain (Kontinen et al., 1999; Matsuzawa-Yanagida et al., 2008; Mutso et al., 2012). It is possible that different animal models of neuropathic pain may result in the development of different mood disorders, or that a wider panel of behavioral tests is needed to reveal subtle differences in the anxiety symptoms that may occur in neuropathic pain.
Numerous studies have shown increased levels of pro-inflammatory cytokines, such as TNF, in patients affected by major depression (Dantzer et al., 2008; Miller et al., 2009), while in rodents administration of cytokines such as TNF induces a mood disturbance known as “sickness behavior”, which shares many symptoms of major depression (Anisman et al., 2002; O'Connor et al., 2009a; O'Connor et al., 2009b). As sickness behavior can be blocked by cytokine antagonists (Kent et al., 1992; Shamash et al., 2002), clinical trials showed that TNF inhibitors administered for the treatment of immune pathologies also significantly improve the depressive symptoms that accompany these diseases (Tyring et al., 2006; Ertenli et al., 2012). Based on these findings, we investigated TNF signaling in the hippocampus of CCI mice and found that TNF expression was significantly increased 3 weeks after injury, and returned to normal levels when mice recovered from depressive-like behavior and pain 12 weeks post injury. In parallel, TNFR2 expression was significantly decreased and then restored to basal levels 3 and 12 weeks after injury, respectively, while no difference was seen in TNFR1 expression. TNF exists as soluble TNF (sTNF) and transmembrane TNF (tmTNF) forms. sTNF primarily binds to TNFR1, which mediates pro-inflammatory/pro-apoptotic/pro-demyelinanting functions (Holtmann and Neurath, 2004), while tmTNF binds preferentially to TNFR2, which primarily plays a trophic/protective role in wound healing and neuronal survival (Arnett et al., 2001; MacEwan, 2002).
TNF has a major role in neuropathic pain development and maintenance, which has been linked to its signaling through TNFR1 (Zhang et al., 2013); TNFR1 knockout mice do not develop hyperalgesia and show reduced allodynia after peripheral nerve injury compared to wild-type mice (Ishikawa et al., 2013), moreover, selective block of TNFR1 decreases neuropathic pain symptoms, whereas the block of TNFR2 with selective antibodies has no effect on pain (Sommer and Schafers, 1998; Lindenlaub et al., 2000).
Based on these findings and on our results we hypothesize that hippocampal plasticity and the associated mood disorders we described during chronic pain, could be mediated by TNFR1 signaling as a consequence of decreased expression of hippocampal TNFR2 in presence of higher levels of TNF. In order to understand the role of TNF/TNFR1 signaling in neuropathic pain-induced depression and the associated hippocampal changes we found in CCI mice, we performed CCI injury on TNFR1 null mice and investigated the development of depressive-like symptoms, hippocampal neurogenesis and plasticity vs wild-type littermates. Consistent with a previous report (Vogel et al., 2006), we found that while the baseline threshold did not differ between genotypes, allodynia was significantly reduced in the absence of TNFR1 and that TNFR1−/− mice did not develop depressive behavior after injury. Finally, although TNF levels were increased in the hippocampus of knockout mice 3 weeks after CCI similarly to wild-type, hippocampal neurogenesis and plasticity were not affected in mice that lacked TNFR1. These data suggest that the development of anhedonia and the associated hippocampal alterations that occur in mice that suffer from neuropathic pain after CCI injury are dependent on TNFR1 signaling. In support to our hypothesis it has been shown that TNF receptors may play different roles in the modulation of hippocampal neurogenesis; TNFR1 acts as a suppressor of neurogenesis in the hippocampus, whereas absence of TNFR2 decreases neurogenesis (Iosif et al., 2006).
Interestingly, we also found that the expression of TNFR2 in TNFR1 knockout mice was significantly higher than the levels we found in wild-type mice after CCI injury and similar to shams. Although this data is difficult to interpret, it is possible that signalling pathways downstream of TNFR1 activation could act as negative regulars of TNFR2 expression. A possible mechanism underlying the increased expression of TNFR2 in TNFR1−/− mice following injury could be represented by NF-κB activation through TNFR1. In a recent study we determined that in transgenic mice in which NF-κB activation is selectively inhibited in astrocytes, TNFR2 expression was significantly upregulated in the chronically injured spinal cords compared to the levels measured in the spinal cords of injured wild-type mice (Bracchi-Ricard et al., 2013). These data suggest that NF-κB activation acts as a negative regulator of TNFR2 expression. This is a reasonable supposition when considering that soluble TNF signaling through TNFR1 is a potent activator of NF-κB signaling (Wajant and Scheurich, 2011) and that TNFR1 and TNFR2 signaling have often been considered to be diametrically opposed to one another (Baud and Karin, 2001). Finally, we have recently determined that inhibiting soluble TNF signaling using a selective antagonist, Xpro1595, significantly improved functional recovery following spinal cord injury and upregulated TNFR2 expression in the injured spinal cord relative to injured controls (Bracchi-Ricard et al., unpublished results).
We previously showed that blocking sTNF activity with Xpro1595 reduced demyelination and promoted axonal preservation in a mouse model of multiple sclerosis, while etanercept, a nonselective TNF inhibitor, did not (Brambilla et al., 2011). Reduction in myelination in some areas of the brain has been documented in patients suffering from depression (Garver et al., 2008; Bartzokis, 2011; Zhang et al., 2012), and conversely, demyelinating disorders characterized by myelin loss show co-morbidity with depression (Arnett et al., 2008), but nothing has been reported about the mechanism by which demyelination progresses and so far, very little is known about the contribution of myelin plasticity as part of the hippocampal structural plasticity that occurs in depression (Merkler et al., 2009). Notably, the role of TNFR1-mediated TNF signaling in causing demyelination has been widely investigated by different authors (Arnett et al., 2001; Eugster et al., 1999; Probert et al., 2000). We and others have demonstrated that signaling mediated by tmTNF/TNFR2, is neuroprotective and promotes remyelination in EAE and spinal cord injury models (Bracchi-Richard et al., 2013; Brambilla et al., 2011; Taoufik et al., 2011). Finally, a recent study shows that increased levels of hippocampal TNF and depressive behavior could be prevented by the treatment with Xpro1595, supporting the role of sTNF and TNFR1 signaling in the development of depression (Bedrosian et al., 2012). Based upon this evidence we sought to determine whether myelin alterations occurred in the hippocampus of mice affected by neuropathic pain-iduced depression and the role of TNF signaling through TNFR1 in these events. We found that 3 weeks after injury, the level of PLP, the most abundant myelin protein in the central nervous system, was significantly decreased in the hippocampus of CCI mice, while both PLP and MBP levels were surprisingly higher in mice that recovered from anhedonia and pain as compared to the sham animals. Interestingly, no changes in myelin protein expression were found in TNFR1 knockout mice. These data indicate that the development and recovery from depressive-like behavior after CCI is associated with hippocampal myelin remodeling. Moreover we report that these events coincided with increased expression of hippocampal TNF and were dependent upon TNFR1 signaling.
In this study we have not investigated pathways downstream of TNR1 signaling. Signaling through TNFR1 and TNFR2 mediates pathological and protective effects, respectively (Baud and Karin, 2001). In the CNS TNFR1 elicits signaling through numerous downstream effectors, including NF-κB, p38, JNK, MAPK, and ceramide (MacEwan, 2002b), which have been shown to cause. Specifically, ceramide has been implicated in TNFR1 mediated neurotoxixity (Kolesnick and Golde 1994; Martinez et al., 2012; Obeid et al., 1993). We speculate that ceramide production could represent a potential pathway downstream of TNFR1 activation in hippocampal neurons which may be mediating the behavioral and hippocampal effects seen after CCI surgery. We do not exclude that other factors may be involved as well; the identification of the specific pathways required for TNF-induced structural impairments in the hippocampus will be an essential matter of our future studies.
Based on our current data we cannot establish whether the TNF-effects observed in our model are peripherally or centrally mediated. However, it has become increasingly clear that there is abundant communication between the central nervous system and the peripheral immune system even in the absence of direct injury to the blood-brain barrier (BBB). This crosstalk is mediated largely by TNF and other cytokines produced both peripherally and centrally. TNF is transported across the BBB and into the brain (Pan and Kastin, 2001; Pan et al., 2006) by TNFR1 and TNFR2 dependent mechanisms (Pan and Kastin, 2002; Qin et al., 2007). It is well know that TNF levels are rapidly upregulated at the site of injury and increase rapidly in the blood and CSF after peripheral nerve injury (George et al., 2004; Ren et al., 2011). It is possible that after CCI injury peripherally produced TNF enters the brain inducing its local production, finally resulting in increased hippocampal TNF levels. Since both receptors have a role in mediating TNF transport through the BBB, this could explain the similar increase of TNF expression in the hippocampus of TNFR1 knockout mice compared to wild-type.
Previous studies have shown that centrally administered TNF (i.c.v. - intracerebroventricular) attenuates norepinephrine production and produces depressive-like behavior in mice, which is completely prevented by thalidomide, an inhibitor of TNF synthesis or by i.c.v. administration of anti-TNF antibody or by different classes of peripherally administered antidepressants (Reynolds et al., 2004; Kaster et al., 2012). These results support the central effects of TNF in inducing depressive-like symptoms.
Ren et al., (2011) found that TNF was increased in the cerebrospinal fluid, in the hippocampus and in the plasma after spared nerve injury (SNI). Moreover, they showed that i.c.v. or intrahippocampal injection of recombinant TNF mimicked the effects of SNI in naive rats, whereas inhibition of TNF or genetic deletion of TNFR1 prevented both hippocampal synaptic dysfunction (disruption of LTP and frequency facilitation at CA3-CA1 synapses, reduction of presynaptic boutons in hippocampal CA1 region) and memory deficits that they observed after SNI. These data suggest that central TNF following peripheral nerve injury is critical for development of neuropathic pain and memory/hippocampal deficits.
Our data suggest a crucial role for hippocampal TNF/TNFR1 in the induction of depressive-like symptoms and hippocampal plasticity; however the only way to prove whether central production of TNF is sufficient to cause these alterations or peripheral TNF is leading central TNF production would be by using genetically modified animals lacking either TNF or TNFR1 expression selectively within the hippocampus. Alternativley we could target central or systemic TNFR1 signaling by intracranial or systemic administration of selective sTNF inhibitors such as the XPro1595 that we previously used in EAE and spinal cord injury models (Brambilla et al., 2011; Bracchi-Richard et al., unpublished results). We can speculate that by blocking central TNF activity through TNFR1 we should be able to observe protection in response to peripheral nerve injury and animals would not develop pain or signs of depression and hippocampal myelin remodeling, reduced plasticity and neurogenesis. The results of such a study could give insights to the specific central and peripheral mechanisms involved in brain production of TNF. Cytokines other than TNF, such IL-1β and IL-6, have been found to be have a role in neuropathic pain after peripheral nerve injury (Arruda et al., 2000; Balschun et al., 2004). Like TNF, IL-1β is a pro-inflammatory cytokine that has been implicated in the induction and maintenance of neuropathic pain (Honore et al., 2006; Ren et al., 2009; Bianchi et al., 1998; Hori et al., 1998) and has been found to be upregulated in the hippocampus after spared nerve injury or CCI (Al-Amin et al., 2011; del Rey et al. 2011). Further, interfering with IL-1β signals in the brain reduces depressive-like symptoms observed during spared nerve injury (Norman et al., 2010), indicating that over-expression of IL-1β in the hippocampus may be relevant for these symptoms. Since increased IL-1β has been suggested to lead to decreased neurogenesis in the elderly (Kuzumaki et al., 2010) we cannot exclude that it could play a similar role in our model of neuropathic pain.
Here, we investigated the effects of chronic neuropathic pain on depressive-like behavior and different forms of hippocampal plasticity. Our study expands and complements some aspects of previous works that revealed hippocampal impairments in neuropathic pain (Mutso et al., 2012; Ren et al., 2011) and is the first investigation to show that neuropathic pain-induced depression is dependent on hippocampal TNF through TNFR1 and is associated with different forms of hippocampal changes such as reduced neurogenesis, neuronal plasticity (Ren et al., 2011) and for the first time myelin remodeling. Moreover we extended our observations to a late time point at which mice were spontaneously recovered from pain, and found that all the behavioral and structural abnormalities found in neuropathic mice did not manifest after spontaneous recover from pain had occurred.
Our results suggest a possible new mechanism through which myelin proteins are modulated in the hippocampus during depressive-like conditions and may be part of a broader neuroplastic process, leading to or resulting from neurogenesis and neuronal remodeling and that these changes are dependent upon signaling through TNFR1. We hypothesize that alterations in white matter might disrupt the neural circuits that are involved in emotional functions and thus contribute to the pathophysiology of depression.Whether myelin protein alterations are the original cause of a neuronal transmission dysfunction that led to the structural plasticity changes we observed in the hippocampus of neuropathic mice or are instead a consequence of these neuroplastic modifications remains to be understood and will be the focus of future studies.
CONCLUSIONS
Altogether, our data propose a novel molecular mechanism that regulates the development of depressive behavior associated with impaired hippocampal neurogenesis, neuronal and myelin plasticity and, that relies on hippocampal TNF signaling through TNFR1. We propose that therapeutic interventions based on the use of TNFR1-selective inhibitors may be beneficial not only to the treatment of neuropathic pain but also to the prevention of comorbid diseases such as depression and that this could occur through TNF/TNFR1 modulating the plastic events that take place at the hippocampal level such as myelin remodeling, neuronal plasticity, survival and complete maturation of adult newborn neurons.
Supplementary Material
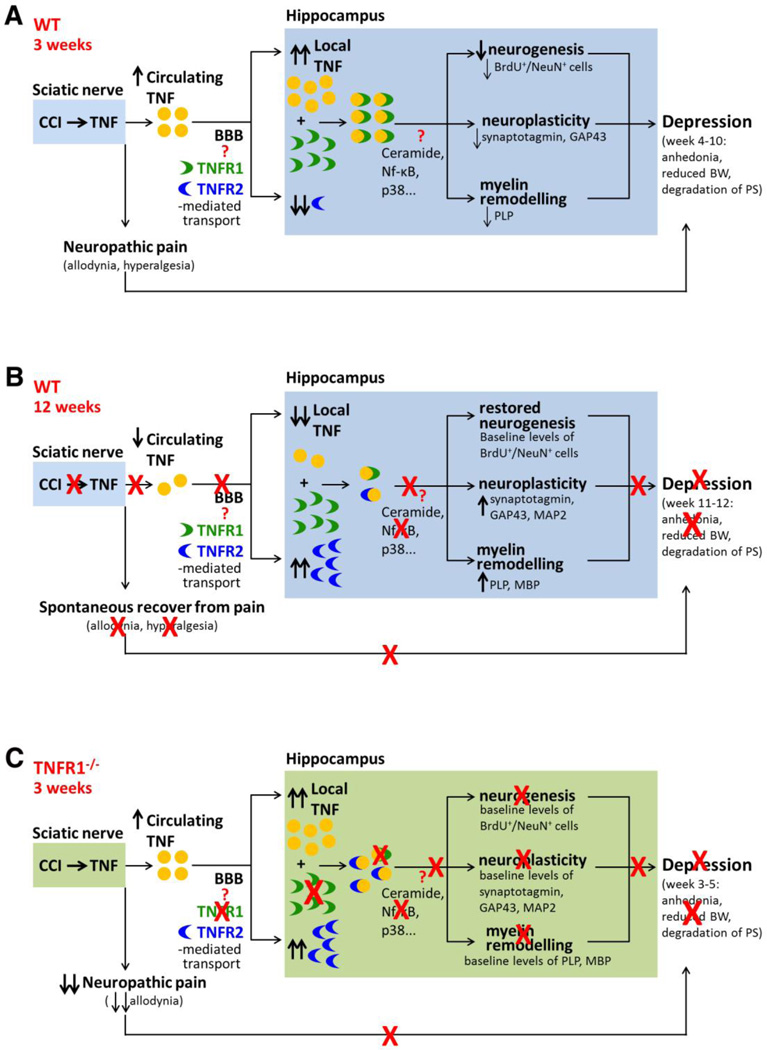
Figure 8.
Model of TNF/TNFR1 signaling-driven hippocampal plasticity possibly responsible for the development of depression following CCI injury in mice. A, Chronic neuropathic pain results in depressive-like behavior, increased hippocampal TNF, and reduced TNFR2 levels 3 weeks after injury. Hippocampal neurogenesis was decreased at this time point, neuroplasticity and myelin remodeling occurred, likely promoting depression. B, Depressive symptoms were no more present in mice that spontaneously recovered from pain 12 weeks after injury as well as hippocampal neurogenesis that was restored to baseline levels. Profound neuronal and myelin plasticity occurred at this time point possibly as a compensatory mechanism to support axonal preservation and functionig after loss of myelin and neuronal plasticity proteins that occured in the hippocampus of animals suffering from pain (A). C, TNFR1 null mice were protected from developing depressive symptoms and hippocampal plastic changes, such as myelin and neuronal plasticity and neurogenesis impairment, indicating a role for TNFR1 signaling in the delopment of depression after CCI.
Highlights (for review).
The development of depressive behavior in neuropathic pain is dependent on TNF signaling through TNFR1 and is associated with hippocampal plasticity and myelin remodeling.
ACKNOWLEDGEMENTS
This work was supported by grants from the Miami Project to Cure Paralysis, Miller School of Medicine, University of Miami, from Ministero della Istruzione Università Ricerca (MIUR), under the Progetti di Interesse Nazionale (PRIN 2007) framework and from Fondazione Cariplo to Mariagrazia Grilli.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
“All authors declare that there are no conflicts of interest.”
REFERENCES
- Al-Amin H, Sarkis R, Atweh S, Jabbur S, Saadé N. Chronic dizocilpine or apomorphine and development of neuropathy in two animal models II: effects on brain cytokines and neurotrophins. Exp Neurol. 2011;228:30–40. doi: 10.1016/j.expneurol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Albonetti ME, Muscettola M, Facchinetti F, Tanganelli C, Carli G. Effects of formalin-induced pain on ACTH, beta-endorphin, corticosterone and interleukin-6 plasma levels in rats. Neuroendocrinology. 1995;62:13–18. doi: 10.1159/000126983. [DOI] [PubMed] [Google Scholar]
- Anisman H, Kokkinidis L, Merali Z. Further evidence for the depressive effects of cytokines: anhedonia and neurochemical changes. Brain Behav Immun. 2002;16:544–556. doi: 10.1016/s0889-1591(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Neuroglialpharmacology: white matter pathophysiologies and psychiatric treatments. Front Biosci (Landmark Ed) 2011;16:2695–2733. doi: 10.2741/3881. [DOI] [PubMed] [Google Scholar]
- Arnett PA, Barwick FH, Beeney JE. Depression in multiple sclerosis: review and theoretical proposal. J Int Neuropsychol Soc. 2008;14(5):691–724. doi: 10.1017/S1355617708081174. [DOI] [PubMed] [Google Scholar]
- Arruda JL, Sweitzer S, Rutkowski MD, DeLeo JA. Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain. Brain Res. 2000;879:216–225. doi: 10.1016/s0006-8993(00)02807-9. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. Faseb J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11(9):372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Weil ZM, Nelson RJ. Chronic dim light at night provokes reversible depression-like phenotype: possible role for TNF. Mol Psychiatry. 2012;18:930–936. doi: 10.1038/mp.2012.96. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bennett DLH, Michael GJ, Ramachandran N, Muson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–773. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Di B, Panerai AE. Interleukin-1 and nociception in the rat. J Neurosci Res. 1998;53:645–650. doi: 10.1002/(SICI)1097-4547(19980915)53:6<645::AID-JNR2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Del Rey A, Yau HJ, Randolf A, Centeno MV, Wildmann J, Martina M, Besedovsky HO, Apkarian AV. Chronic neuropathic pain-like behavior correlates with IL-1beta expression and disrupts cytokine interactions in the hippocampus. Pain. 2011;152:2827–2835. doi: 10.1016/j.pain.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- Bos JD, de Korte J. Effects of etanercept on quality of life, fatigue, and depression in psoriasis. Lancet. 2006;367(9504):6–7. doi: 10.1016/S0140-6736(05)67818-X. [DOI] [PubMed] [Google Scholar]
- Bracchi-Ricard V, Lambertsen KL, Ricard J, Nathanson L, Karmally S, Johnstone J, Ellman DG, Frydel B, McTigue DM, Bethea JR. Inhibition of astroglial NF-kappaB enhances oligodendrogenesis following spinal cord injury. J Neuroinflammation 23. 2013;10(1):92. doi: 10.1186/1742-2094-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Ashbaugh JJ, Magliozzi R, Dellarole A, Karmally S, Szymkowski DE, Bethea JR. Inhibition of soluble tumour necrosis factor is therapeutic in experimental autoimmune encephalomyelitis and promotes axon preservation and remyelination. Brain. 2011;134:2736–2754. doi: 10.1093/brain/awr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cacci E, Claasen JH, Kokaia Z. Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res. 2005;80:789–797. doi: 10.1002/jnr.20531. [DOI] [PubMed] [Google Scholar]
- Caspani O, Zurborg S, Labuz D, Heppenstall PA. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS One. 2009;4:e7383. doi: 10.1371/journal.pone.0007383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castren E. Is mood chemistry? Nat Rev Neurosci. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- Chen Z, Palmer TD. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain, Behavior, and Immunity. 2013;30:45–53. doi: 10.1016/j.bbi.2013.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res Dev Brain Res. 2002;134:13–21. doi: 10.1016/s0165-3806(01)00243-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: Acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Del Rey A, Yau HJ, Randolf A, Centeno MV, Wildmann J, Martina M, Besedovsky HO, Apkarian AV. Chronic neuropathic pain-like behavior correlates with IL-1beta expression and disrupts cytokine interactions in the hippocampus. Pain. 2011;152:2827–2835. doi: 10.1016/j.pain.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducottet C, Griebel G, Belzung C. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:625–631. doi: 10.1016/S0278-5846(03)00051-4. [DOI] [PubMed] [Google Scholar]
- Duman RS, Charney DS. Cell atrophy and loss in major depression. Biol Psychiatry. 1999;45:1083–1084. doi: 10.1016/s0006-3223(99)00057-8. [DOI] [PubMed] [Google Scholar]
- Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006;7:544–555. doi: 10.1016/j.jpain.2006.01.458. [DOI] [PubMed] [Google Scholar]
- Ertenli I, Ozer S, Kiraz S, Apras SB, Akdogan A, Karadag O, Calguneri M, Kalyoncu U. Infliximab, a TNF-alpha antagonist treatment in patients with ankylosing spondylitis: the impact on depression, anxiety and quality of life level. Rheumatol Int. 2012;32:323–330. doi: 10.1007/s00296-010-1616-x. [DOI] [PubMed] [Google Scholar]
- Eugster HP, Frei K, Bachmann R, Bluethmann H, Lassmann H, Fontana A. Severity of symptoms and demyelination in MOG-induced EAE depends on TNFR1. Eur J Immunol. 1999;29(2):626–632. doi: 10.1002/(SICI)1521-4141(199902)29:02<626::AID-IMMU626>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Eyre H, Baune BT. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology. 2012;37:1397–1416. doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Finsen B, Antel J, Owens T. TNFalpha: kill or cure for demyelinating disease? Mol Psychiatry. 2002;7:820–821. doi: 10.1038/sj.mp.4001120. [DOI] [PubMed] [Google Scholar]
- Fu ES, Zhang YP, Sagen J, Candiotti KA, Morton PD, Liebl DJ, Bethea JR, Brambilla R. Transgenic inhibition of glial NF-kappa B reduces pain behavior and inflammation after peripheral nerve injury. Pain. 2010;148:509–518. doi: 10.1016/j.pain.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver DL, Holcomb JA, Christensen JD. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. Int J Neuropsychopharmacol. 2008;11:49–61. doi: 10.1017/S1461145707007730. [DOI] [PubMed] [Google Scholar]
- George A, Buehl A, Sommer C. Wallerian degeneration after crush injury of rat sciatic nerve increases endo- and epineurial tumor necrosis factor-alpha protein. Neurosci Lett. 2004;372:215–219. doi: 10.1016/j.neulet.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gonçalves L, Silva R, Pinto-Ribeiro F, Pêgo JM, Bessa JM, Pertovaara A, Sousa N, Almeida A. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp Neurol. 2008;213(1):48–56. doi: 10.1016/j.expneurol.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95(2):570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steiberg R, Jung M, Gull D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist: II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2009;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL. Stress and the hypothalamopituitary-adrenal axis: Acute, chronic and immunological activation. J Endocrinol. 1992;134:327–339. doi: 10.1677/joe.0.1340327. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12(2):123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Holtmann MH, Neurath MF. Differential TNF-signaling in chronic inflammatory disorders. Curr Mol Med. 2004;4:439–444. doi: 10.2174/1566524043360636. [DOI] [PubMed] [Google Scholar]
- Honore P, Wade CL, Zhong C, Harris RR, Wu C, Ghayur T, Iwakura Y, Decker MW, Faltynek C, Sullivan J, Jarvis MF. Interleukin-1alphabeta gene-deficient mice show reduced nociceptive sensitivity in models of inflammatory and neuropathic pain but not post-operative pain. Behav Brain Res. 2006;167:355–364. doi: 10.1016/j.bbr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Hori T, Oka T, Hosoi M, Aou S. Pain modulatory actions of cytokines and prostaglandin E2 in the brain. Ann N Y Acad Sci. 1998;840:269–281. doi: 10.1111/j.1749-6632.1998.tb09567.x. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Miyagi M, Kamoda H, Orita S, Eguchi Y, Arai G, Suzuki M, Sakuma Y, Oikawa Y, Inoue G, Aoki Y, Toyone T, Takahashi K, Ohtori S. Differences between tumor necrosis factor-alpha receptors types 1 and 2 in the modulation of spinal glial cell activation and mechanical allodynia in a rat sciatic nerve injury model. Spine. 2013;38:11–16. doi: 10.1097/BRS.0b013e3182610fa9. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Gadotti VM, Calixto JB, Santos AR, Rodrigues AL. Depressive-like behavior induced by tumor necrosis factor-alpha in mice. Neuropharmacology. 2012;62:419–426. doi: 10.1016/j.neuropharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Genetic influence on phenotypic differentiation in adult hippocampal neurogenesis. Brain Res Dev Brain Res. 2002;134:1–12. doi: 10.1016/s0165-3806(01)00224-3. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Kodama D, Ono H, Tanabe M. Altered hippocampal long-term potentiation after peripheral nerve injury in mice. Eur J Pharmacol. 2007;574:127–132. doi: 10.1016/j.ejphar.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Kolesnick R, Golde DW. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77(3):325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Kontinen VK, Kauppila T, Paananen S, Pertovaara A, Kalso E. Behavioural measures of depression and anxiety in rats with spinal nerve ligation-induced neuropathy. Pain. 1999;80(1–2):341–346. doi: 10.1016/s0304-3959(98)00230-9. [DOI] [PubMed] [Google Scholar]
- Korgaonkar MS, Grieve SM, Koslow SH, Gabrieli JD, Gordon E, Williams LM. Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum Brain Mapp. 2011;32(12):2161–2171. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M, Maes M, Kenis G, Kim YK, Lason W. Effects of serotonin and serotonergic agonists and antagonists on the production of tumor necrosis factor alpha and interleukin-6. Psychiatry Res. 2005;134:251–258. doi: 10.1016/j.psychres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, Ando T, Ushijima T, Suzuki T, Narita M. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010;64:721–728. doi: 10.1002/syn.20800. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, Devries AC. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry. 2010;15:404–414. doi: 10.1038/mp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos M, Perez-Iglesias R, Woolley JB, Kanaan RA, Vyas NS, Barker GJ, Frangou S, McGuire PK. Effect of age at onset of schizophrenia on white matter abnormalities. Br J Psychiatry. 2009;195:346–353. doi: 10.1192/bjp.bp.108.055376. [DOI] [PubMed] [Google Scholar]
- Lindenlaub T, Teuteberg P, Hartung T, Sommer C. Effects of neutralizing antibodies to TNF-alpha on pain-related behavior and nerve regeneration in mice with chronic constriction injury. Brain Res. 2000;866:15–22. doi: 10.1016/s0006-8993(00)02190-9. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15(12):1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Bruck W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365:2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEwan DJ. TNF ligands and receptors--a matter of life and death. Br J Pharmacol. 2002;135:855–875. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci (Landmark Ed) 2009;14:5291–5338. doi: 10.2741/3598. [DOI] [PubMed] [Google Scholar]
- Mangiardi M, Crawford DK, Xia X, Du S, Simon-Freeman R, Voskuhl RR, Tiwari-Woodruff SK. An animal model of cortical and callosal pathology in multiple sclerosis. Brain Pathol. 2011;21:263–278. doi: 10.1111/j.1750-3639.2010.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez TN, Chen X, Bandyopadhyay S, Merrill AH, Tansey MG. Ceramide sphingolipid signaling mediates Tumor Necrosis Factor (TNF)-dependent toxicity via caspase signaling in dopaminergic neurons. Mol Neurodegener. 2012;7:45. doi: 10.1186/1750-1326-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martuscello RT, Spengler RN, Bonoiu AC, Davidson BA, Helinski J, Ding H, Mahajan S, Kumar R, Bergey EJ, Knight PR, Prasad PN, Ignatowski TA. Increasing TNF levels solely in the rat hippocampus produces persistent pain-like symptoms. Pain. 2012;153:1871–1882. doi: 10.1016/j.pain.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa-Yanagida K, Narita M, Nakajima M, Kuzumaki N, Niikura K, Nozaki H, Takagi T, Tamai E, Hareyama N, Terada M, Yamazaki M, Suzuki T. Usefulness of antidepressants for improving the neuropathic pain-like state and pain-induced anxiety through actions at different brain sites. Neuropsychopharmacology. 2008;33(8):1952–65. doi: 10.1038/sj.npp.1301590. [DOI] [PubMed] [Google Scholar]
- McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- Merkler D, Ernsting T, Kerschensteiner M, Bruck W, Stadelmann C. A new focal EAE model of cortical demyelination: multiple sclerosis-like lesions with rapid resolution of inflammation and extensive remyelination. Brain. 2006;129:1972–1983. doi: 10.1093/brain/awl135. [DOI] [PubMed] [Google Scholar]
- Merkler D, Klinker F, Jurgens T, Glaser R, Paulus W, Brinkmann BG, Sereda MW, Stadelmann-Nessler C, Guedes RC, Bruck W, Liebetanz D. Propagation of spreading depression inversely correlates with cortical myelin content. Ann Neurol. 2009;66:355–365. doi: 10.1002/ana.21746. [DOI] [PubMed] [Google Scholar]
- Mettenburg JM, Benzinger TL, Shimony JS, Snyder AZ, Sheline YI. Diminished performance on neuropsychological testing in late life depression is correlated with microstructural white matter abnormalities. Neuroimage 1. 2012;60(4):2182–2190. doi: 10.1016/j.neuroimage.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikova O, Yakimova R, Bosmans E, Kenis G, Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eurm Neuropsychopharmacol. 2001;11:203–208. doi: 10.1016/s0924-977x(01)00081-5. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, Devries AC. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry. 2010;15:404–414. doi: 10.1038/mp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009a;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009b;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Increase in TNFα transport after SCI is specific for time, region, and type of lesion. Exp Neurol. 2001;170:357–363. doi: 10.1006/exnr.2001.7702. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. TNFα transport across the blood-brain barrier is abolished in receptor knockout mice. Exp Neurol. 2002;174:193–200. doi: 10.1006/exnr.2002.7871. [DOI] [PubMed] [Google Scholar]
- Pan W, Ding Y, Yu Y, Ohtaki H, Nakamachi T, Kastin AJ. Stroke upregulates TNFα transport across the blood-brain barrier. Exp Neurol. 2006;198:222–233. doi: 10.1016/j.expneurol.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Krönke M, Mak TWl. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Probert L, Eugster HP, Akassoglou K, Bauer J, Frei K, Lassmann H, Fontana A. TNFR1 signaling is critical for the development of demyelination and the limitation of T-cell responses during immune-mediated CNS disease. Brain. 2000;123:2005–2019. doi: 10.1093/brain/123.10.2005. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinés A, Cereseto M, Ferrero A, Sifonios L, Podestà MF, Wikinski S. Maintenance treatment with fluoxetine is necessary to sustain normal levels of synaptic markers in an experimental model of depression: correlation with behavioural response. Neuropsycolpharmacol. 2008;33:1896–1908. doi: 10.1038/sj.npp.1301596. [DOI] [PubMed] [Google Scholar]
- Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents. Neuropsychopharmacology. 2011;36:979–992. doi: 10.1038/npp.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JL, Ignatowski TA, Sud R, Spengler RN. Brain-derived tumor necrosis factor-alpha and its involvement in noradrenergic neuron functioning involved in the mechanism of action of an antidepressant. J Pharmacol Exp Ther. 2004;310:1216–1225. doi: 10.1124/jpet.104.067835. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–139. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Schafers M, Schmidt C, Vogel C, Toyka KV, Sommer C. Tumor necrosis factor-alpha (TNF) regulates the expression of ICAM-1 predominantly through TNF receptor 1 after chronic constriction injury of mouse sciatic nerve. Acta Neuropathol. 2002;104:197–205. doi: 10.1007/s00401-002-0541-9. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002;22:3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, Schafers M. Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784:154–162. doi: 10.1016/s0006-8993(97)01327-9. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25(12):3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Dolgov O, Bartsch D. Stress-induced hyperlocomotion as a confounding factor in anxiety and depression models in mice. Behav Pharmacol. 2005;16:171–180. doi: 10.1097/00008877-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol. 2006;17:271–287. doi: 10.1097/00008877-200605000-00008. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Steinbusch HW. Measuring behavior in mice with chronic stress depression paradigm. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:348–361. doi: 10.1016/j.pnpbp.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Taoufik E, Tseveleki V, Chu SY, Tselios T, Karin M, Lassmann H, Szymkowski DE, Probert L. Transmembrane tumour necrosis factor is neuroprotective and regulates experimental autoimmune encephalomyelitis via neuronal nuclear factor-kappaB. Brain. 2011;134:2722–2735. doi: 10.1093/brain/awr203. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Rothe M, Hu YF, Goeddel DV. Tumor necrosis factor's cytotoxic activity is signaled by the p55 TNF receptor. Cell. 1993;73(2):213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology 1. 2003;70:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Vogel C, Stallforth S, Sommer C. Altered pain behavior and regeneration after nerve injury in TNF receptor deficient mice. J Peripher Nerv Syst. 2006;11:294–303. doi: 10.1111/j.1529-8027.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Wajant H, Scheurich P. TNFR1-induced activation of the classical NF-κB pathway. FEBS J. 2011;278(6):862–876. doi: 10.1111/j.1742-4658.2011.08015.x. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummings RA. The open field test: a critical review. Psycol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Willner P, Moreau JL, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, Freund-Mercier MJ, Barrot M. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol Psychiatry. 2011;70:946–953. doi: 10.1016/j.biopsych.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Yang L, Lindholm K, Konishi Y, Li R, Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci. 2002;22(8):3025–3032. doi: 10.1523/JNEUROSCI.22-08-03025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanga Y, Tiana Z, Teng D, Zhang J, Wang J, Wang J. High-level production of a candidacidal peptide lactoferrampin in Escherichia coli by fusion expression. J Biotechnol. 2009;139:326–331. doi: 10.1016/j.jbiotec.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R, Barak O, Reichenberg A, Cohen E, Shavit Y, Ovadia H. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv Exp Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- Zeng LL, Liu L, Liu Y, Shen H, Li Y, Hu D. Antidepressant treatment normalizes white matter volume in patients with major depression. PLoS One. 2012;7(8):44248. doi: 10.1371/journal.pone.0044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Fu ES, Sagen J, Levitt RC, Candiotti KA, Bethea JR, Brambilla R. Glial NF-kappaB inhibition alters neuropeptide expression after sciatic nerve injury in mice. Brain Res. 2011;1385:38–46. doi: 10.1016/j.brainres.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, He J, Zhu S, Zhang H, Wang H, Adilijiang A, Kong L, Wang J, Kong J, Tan Q, Li XM. Myelination deficit in a phencyclidine-induced neurodevelopmental model of schizophrenia. Brain Res. 2012;1469:136–143. doi: 10.1016/j.brainres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang H, Dougherty PM. Dynamic effects of TNF-alpha on synaptic transmission in mice over time following sciatic nerve chronic constriction injury. J Neurophysiol. 2013;110:1663–1671. doi: 10.1152/jn.01088.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.