Abstract
Good executive function has been linked to many positive outcomes in academic performance, health, and social competence. However, some aspects of executive function may interfere with other cognitive processes. Childhood provides a unique test case for investigating such cognitive trade-offs, given the dramatic failures and developments observed during this period. For example, most children categorically switch or perseverate when asked to switch between rules on a card-sorting task. To test potential trade-offs with the development of task switching abilities, we compared 6-year-olds who switched versus perseverated in a card-sorting task on two aspects of inhibitory control: response inhibition (via a stop signal task) and interference control (via a Simon task). Across two studies, switchers showed worse response inhibition than perseverators, consistent with the idea of cognitive trade-offs; however, switchers showed better interference control than perseverators, consistent with prior work documenting benefits associated with the development of executive function. This pattern of positive and negative associations may reflect aspects of working memory (active maintenance of current goals, and clearing of prior goals) that help children focused on a single task-goal but hurt in situations with conflicting goals. Implications for understanding components of executive function and their relationships across development are discussed.
Keywords: executive function, response inhibition, task switching, cognitive development
1. Introduction
Good executive functions, the “cognitive control processes that operate on lower-level processes to regulate and shape behavior” (Friedman et al. 2007), have been linked to many positive outcomes in academics, health, and social functioning. For example, childhood ability to inhibit inappropriate actions predicts kindergarten mathematics and reading skills (Blair & Razza, 2007), and childhood abilities to delay gratification predict higher SAT scores and better social competence in adolescence (Mischel, Shoda, and Rodriguez, 1989) and a lower likelihood of mid-life obesity (Schlam et al., 2013). Broader measures of self-control in childhood also predict less adolescent obesity (Tsukayama, Toomey, Faith, & Duckworth, 2010) and fewer criminal convictions, financial difficulties, and substance abuse problems in middle adulthood (Moffitt et al., 2011). Developmental changes may be as important as initial control levels; for example, slower rates of development in behavioral self-control throughout childhood (in addition to level of preschool control) predict problematic behavior in adolescence (Wong et al., 2006). These findings have led to considerable interest in programs that might improve executive functions (Diamond, 2012).
However, some aspects of executive function may interfere with other cognitive processes (Friedman, Miyake, Robinson, & Hewitt, 2011; Goshke, 2000; Munakata, Snyder, & Chatham, 2013; Thompson-Schill et al., 2009; Chrysikou et al., 2013). Regions of the prefrontal cortex (PFC) are widely recognized as playing a crucial role in supporting executive functions, by biasing neural processing in posterior brain regions in a top-down fashion according to goals being held in mind (Miller & Cohen, 2001). This biasing can have trade-offs, conferring both benefits and costs. On the one hand, developments in the PFC allow better maintenance of goals in working memory, selective attention to task-relevant information, and inhibition of inappropriate responses (e.g., Bunge et al., 2002; Bunge & Zelazo, 2006; Diamond, 2002; Desimone & Duncan, 1995; Durston et al., 2002). On the other hand, such developments may also interfere with cognitive processes such as probabilistic learning and creative thinking, which may be completed more efficiently when neural activity is allowed to spread along posterior cortical connections without any form of top-down bias (Doll, Hutchison, & Frank, 2011; Thompson-Schill, Ramscar, & Chrysikou, 2009; see Newport, 1990 for an early conceptualization of this idea).
In addition to interfering with cognitive processes supported by more posterior cortical brain regions, executive functions may also interfere with each other by virtue of their demands on shared and capacity-limited central resources. For example, random generation, which relies on inhibiting previous responses and updating working memory (Miyake et al., 2000), is impaired by a simultaneous card sorting task, which relies on switching abilities (Baddeley, 1966), suggesting interference between switching and other executive function components (Baddeley, 1996; Baddeley, et al., 1998; see also Abdi, Lemaire & Fayol, 1996; Logie, Gilhooly & Wynn, 1994; Towse, 1998). Switching and inhibition of prepotent responses seem to have an inverse relationship at the level of long-term individual differences, as toddlers who show better compliance with an instruction not to touch an attractive toy went on to demonstrate poorer shifting between tasks as adolescents (Friedman et al., 2011). The source of this interference and of executive limitations more broadly remains a matter of debate. Interference could arise when executive functions are subserved by shared prefrontal neural networks (Dux, Ivanoff, Asplpund & Marois, 2006; Sigman & Dehaene, 2008; Tombu et al, 2011), or due to competitive dynamics between distinct prefrontal regions or networks (for example, as have been proposed to explain functional anticorrelations in the human brain; Fox, Snyder, Vincent, Corbetta, Van Essen & Raichle, 2005; Kelly, Uddin, Biswall, Castellanos & Milham; 2008).
The potential trade-offs between executive functions may be most apparent during childhood, given the dramatic failures and developments observed during this period. Children have notoriously poor executive function, with 3-year-olds struggling at seemingly simple tasks such as the dimensional change card sort (DCCS), which requires them to switch from sorting multi-dimensional cards (e.g., a blue truck) from one rule (e.g., color) to another (e.g., shape; Kirkham & Diamond, 2003; Perner & Lang, 2002; Zelazo & Frye, 1998). Older children continue to struggle with a three dimensional card sort (3D card sort) that introduces a third rule (sorting by size) and no longer provides reminders of the rules. Most 6-year-olds either reliably switch among rules or reliably perseverate on the first rule (Cepeda & Munakata, 2007; Deak, 2003). These categorical groupings allow investigations of cognitive tradeoffs related to children’s success versus failure rather than efficiency (e.g., switch costs in reaction time). Such categorical distinctions have been more effective than continuous performance measures in identifying contributors to individual differences (V. J. Molfese, D. L. Molfese, & Modgline, 2001), and in highlighting lifelong cognitive processes that are masked by support from other cognitive abilities in adults (e.g., Hermer-Vazquez, Spelke, & Katsnelson, 1999; Hermer & Spelke, 1996).
Children’s successful switching on card sorting tasks is associated with better performance in a variety of cognitive domains, but also with at least one trade-off. Children who successfully switch between rules (“switchers”) have advantages relative to children who continue to sort by the first rule (“perseverators”) in working memory (Blackwell, Cepeda, & Munakata, 2009; Blackwell & Munakata, 2014; Marcovitch et al., 2010), theory of mind (Muller, Zelazo, & Imrisek, 2005; Kloo & Perner, 2003), delay of gratification (Hongwanishkul et al., 2005), and abstract rule use (Kharitonova et al., 2009; Kharitonova & Munakata, 2011; Snyder & Munakata, 2010). For example, switchers show better maintenance of information in working memory, as indexed by faster reaction times when asked to remember simple shapes over a long (16 second) delay in a delayed-match-to sample task, even controlling for age and processing speed (Blackwell & Munakata, 2014). However, 6-year-old switchers also have at least one disadvantage, in being more susceptible to distraction than perseverators. Switchers’ working-memory advantage reverses when children are distracted: if children are required to count backwards and tap on the table during the delay period in a working memory task, switchers become slower to respond than perseverators.
Switchers’ disadvantage when distracted may stem from their still-developing ability to maintain information in working memory. Switchers’ usual advantage in working memory tasks (e.g., Marcovitch et al., 2010) suggests that they attempt to proactively maintain to-be-remembered information (consistent with the benefits of preparation in reducing adult switch costs; Rogers & Monsell, 1995), while perseverators may rely on a reactive strategy of encoding information and only retrieving it when prompted (which would lead to failure in the 3D card sort due to the lack of distinct cues to indicate which rule should be retrieved at any given time; Blackwell & Munakata, 2014). However, switchers’ proactive maintenance strategy may fail in working memory tasks when there are high demands, such as simultaneously maintaining a second goal of counting backwards; attempting to maintain both goals may overload switchers’ working memory capacity, to the extent that they are unable to successfully maintain the to-be-remembered information, and revert to a different strategy. Although switchers and perseverators may both ultimately succeed using a reactive strategy to recall the to-be-remembered information after the distraction is over, switchers may be slower than perseverators either because they only revert to reactive control after proactive attempts fail, or because their reactive control is less practiced and therefore inefficient.
Alternatively (or in addition), switchers’ disadvantage when distracted may stem from a particular mechanism that supports task switching: “clearing” a goal out of working memory, in order to overcome task set inertia (cf. Allport et al., 1994). Specifically, successful task switching may depend on clearing the contents of working memory when a task changes (Friedman et al., 2011; Herd et al., submitted), so that prior goals are not held in mind when they are no longer relevant (see also Chatham & Badre, 2013). While this would help switchers to clear previously remembered items in the delayed-match-to-sample task, reducing competition from the other potential answers, it could also lead to switchers “clearing” the to-be-remembered shape from working memory when they focus on the goal of counting backward and tapping on the table. Switchers could then have more difficulty than perseverators in retrieving the to-be-remembered information, either because the clearing process leads to an even weaker memory trace than that supported by the weak maintenance abilities in perseverators, or again due to both switchers and perseverators employing a reactive strategy to recall the to-be-remembered information, with switchers’ reactive control being less practiced and therefore less efficient.
The relative advantages and disadvantages in working memory associated with developments in children’s task switching abilities suggest that children may show parallel advantages and disadvantages in inhibitory control. Inhibitory control can be divided into at least two forms (as in Dillon & Pizzagalli, 2007; and Nigg, 2000; see also Huizinga et al. 2006; van der Sluis et al., 2008): response inhibition, defined as “deliberate control of a primary motor response” (Nigg, 2000, p. 223), and interference control, defined as the ability to “ignore certain information that is strongly linked to another, yet inappropriate, response” (Stins, Polderman, Boomsma, & de Geus, 2005, p. 191). In addition to any differences in inhibitory mechanisms involved, the tasks that tap these constructs differ in the number of goals that must be maintained. Response inhibition tasks typically require the maintenance of two distinct goals; one standard measure is the “stop signal” task, in which participants are asked to respond as quickly as possible to a primary task, such as indicating whether a shape is a square or circle (first goal) unless a stop signal is presented, in which case they are to make no response (second goal). In contrast, interference control is typically measured with tasks that require maintaining only one goal, such as focusing on one aspect of a multidimensional, incongruent stimulus (e.g., in the Stroop task, focusing on the color of ink a word is printed in), or one stimulus in the face of misleading distractors (e.g., in the flanker task, responding to the center arrow when surrounding arrows point in the opposite direction).
We predict that switchers will show an advantage on interference control tasks, with a single goal to maintain, but will show a disadvantage on response inhibition tasks, with multiple goals to maintain. This would be consistent with both the overloaded working memory and clearing working memory accounts, but would contrast with the more common pattern of executive functions being correlated at the individual difference level (Friedman & Miyake, 2004; Miyake et al. 2000), and with specific claims that response inhibition supports task switching by suppressing responses based on prior tasks (Aron, Monsell, Sahakian, & Robbins, 2004; Diamond, Kirkham, & Amso, 2002). Specifically, switchers’ proactive strategies and stronger working memories should give them an advantage over perseverators in an interference control task with only a single goal to maintain; however, we predict that switchers will lose that advantage in a response inhibition task, because their ability to maintain a secondary goal (e.g., of stopping after a stop signal) will be compromised by attempts to maintain the primary goal (to “go” on most trials), either due to overloaded working memory or clearing of the secondary goal. Switchers will then need to revert to alternative strategies such as reactive control (which would be helpful in a task with an unambiguous cue to stop), but they may not be as effective at engaging this control as perseverators, who should be more practiced at reactive control or who may have an advantage due to less efficient clearing of the secondary goal of stopping.
To test our prediction of a trade-off between task switching and response inhibition, in contrast with the possibility that these executive functions would correlate with one another, we conducted two experiments with 6-year-olds. The first experiment tested for the unique trade-off between task switching and response inhibition predicted by the overloaded working memory and clearing working memory accounts; the second experiment tested the replicability of this trade-off, and also tested our prediction of an advantage in interference control.
2. Experiment 1
To test the relationship between task switching and response inhibition, we assessed 6- year-olds’ performance on the 3D card sort and a stop signal task. We predicted that switchers on the 3D card sort would have worse response inhibition, consistent with the clearing working memory and/or overloaded working memory accounts above, demonstrating a trade-off in developing executive function and contrasting with previous work showing relationships between these executive functions in adulthood (e.g., Miyake et al., 2000).
2.1. Method
2.1.1. Participants
Thirty-five 5- and 6-year-olds (M = 6.5-years-old, range = 5.8 to 7.0, 20 female) participated in this study. An additional 14 participants were excluded: six did not have sufficient stop signal accuracy to allow an estimation of their response inhibition (as detailed in 2.1.3 Data Trimming), three failed to sort according to the rule during the preswitch phase so that switching could not be assessed, three had mixed switching (switching on one postswitch block, but perseverating on the other)1, one took a bathroom break during the card-sorting task, and data for one participant were lost. Children were recruited from a departmental database of families interested in participating in psychology studies; children whose parents self-reported clinical diagnoses (e.g., ADHD) did not participate. Parents provided informed consent at the beginning of the session, and received $5 for travel expenses; children were rewarded with a small toy at the end of the session.
2.1.2. Materials, Procedure, and Measures
All participants completed the 3D card sort and stop signal tasks, in that order, in keeping with standard individual difference methods (e.g., Friedman et al., 2008). Children were instructed to keep their hands on the computer desk until they were ready to respond, so all participants started with their hands in the same position on all trials of both tasks.
3D card sort
The 3D card sort was adapted from Deák (2003; building on Zelazo et al., 1996) and was identical to that used in Blackwell et al. (2009). Three targets varying along three dimensions were presented on the top half of the screen and remained throughout the task: a large blue cat, small yellow fish, and medium red bird (Figure 1). Children were instructed to sort 12 pictures first by shape, then by color, then by size. For each dimension, children were asked to identify the targets according to the dimension (e.g., “Can you press the cat?”), informed about the rules for the game (e.g., “In the color game, when you see a blue one, press the blue one.”), and asked simple questions about the rules (e.g., “What do you press when you see a small one?”). They were then presented with 12 individual pictures that matched each target on one dimension (e.g., a small red cat) and asked “Which do you press for this one?” Accuracy and reaction time were recorded upon target press. Accuracy had to be at least 75% on the first (shape) block for all children; accuracy on the post-switch color and size blocks had to be 75% for children to be categorized as “switchers”. No reminders and no feedback were provided.
Figure 1.
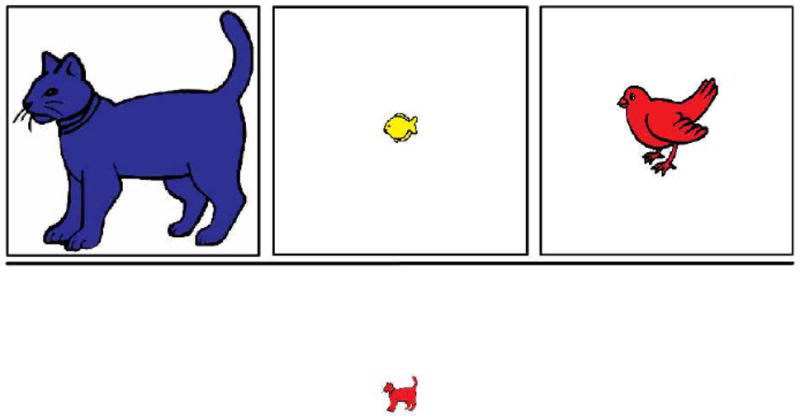
3D card sort measure of task switching. Children were instructed to sort stimuli that matched each target on one dimension: first by shape, then by color, then by size.
Stop signal
The stop signal task (cf. Bedard et al., 2002, 2003) was introduced as a game in which children earned points to help a dog get a ball. Children were instructed to press circles that appeared on the screen as quickly as possible unless the circles said, “stop!” They needed to press most of the circles, which would either say nothing or say “go” to remind children to press them, but they were instructed to not press any circles that said “stop” after they appeared. The task included 7 blocks: one simple reaction time block, in which children were just instructed to press the circles, to calibrate baseline reaction time; one experimenter demonstration block; one practice block, to calibrate the initial stop signal delay; and 5 test blocks. Each test block contained 48 trials, distributed among no signal (50%), go signal (25%), and stop signal (25%)2.
The delay between the circle’s appearance and the “stop” signal (stop signal delay) adapted using a staircase mechanism so that children would be able to stop on approximately 50% of the trials. If a child successfully stopped, the stop signal delay was increased by 50 ms, so that stopping would be more difficult; if the child failed to stop, the stop signal delay was decreased by 50 ms, so that stopping would be easier. If children slowed down in an attempt to decrease their errors (i.e., if their reaction time was 2.3 times slower than in the simple RT block) the program instructed them to go faster. Reminders not to press the circles that said stop and encouragement to continue were provided throughout the task.
Inhibition was measured by the stop signal reaction time (SSRT), a measure of how long it takes to inhibit a response. Intuitively, SSRT is calculated by determining how close to the time of a response subjects can be instructed to stop that response and do so successfully around 50% of the time. An estimate of this time can be derived from the distribution of reaction times, the rate at which stopping is unsuccessful, and the time at which the stop signals are delivered. More specifically, SSRT was calculated as the difference between the estimated finishing time of the stop process (i.e., the estimated reaction time to press the circle if the response had not been stopped) and the stop signal delay. This estimated finishing time was determined via the integration method (Ridderinkhof, Band & Logan, 1999) by taking the ith percentile of the “no signal” trial distribution (or the “go signal” trials, when that was the basis for comparison), where i corresponds to the percentage of failed inhibition on stop trials, and subtracting the average stop signal delay from that reaction time. For example, if a child had failed to stop on 40% of stop trials in a block, their SSRT was the 40th percentile reaction time on the “go” trials, minus the average stop signal delay for that block. A smaller SSRT indicates that the child can stop the response more quickly, and has relatively good response inhibition.
2.1.3. Data Trimming
Each child’s stop signal data was first analyzed individually. Blocks of data were excluded from analysis if: 1) the child missed fewer than 25% or more than 75% of stop trials (SSRT estimates are more reliable when error rates are approximately 50%; Logan, Schachar, & Tannock, 1997); 2) the SSRT estimate for that block was negative (reflecting a dramatic increase in reaction time, suggesting that children had been following but abandoned a strategy of slowing down in anticipation of the stop signal); or 3) the child missed more than 15% of “no signal” trials (again, likely reflecting a strategy of slowing down in anticipation of the stop signal). The individual SSRT estimates for each block were averaged to calculate children’s final SSRT scores, if the child had at least 3 blocks of valid data. Estimates of SSRT were consistent across blocks (tested in 30 children with four blocks of data; Cronbach’s alpha across four blocks was .64, and the Spearman-Brown coefficient for split-half reliability comparing the first two blocks to the final two blocks was .62). Finally, children’s individual SSRT scores were trimmed following Cepeda and Munakata’s (2007) modification of Friedman and Miyake’s (2004) trimming procedure: SSRTs were checked to see if they fell more than 3 SDs from the mean separately for switchers and perseverators; no outliers were identified.
2.2. Results
2.2.1. Characteristics of Switchers and Perseverators
Children were categorized by performance on the color and size blocks. Switchers answered with 75% to 100% accuracy on all blocks (M=98% of shape, 93% of color and 95% of size trials correct), while perseverators answered with high accuracy on the shape block (M = 99%) but low accuracy on the post-switch blocks (0% to 25% correct, M = 1% of color and < 1% of size trials correct). Twenty-one children (60%, 11 female) were categorized as switchers, and 14 children (40%, 9 female) were categorized as perseverators1. Switchers were older than perseverators (6.6 vs. 6.3-years-old, t(33) = 3.1, p = .004), so age was controlled for in all analyses.
2.2.2. Relationship of Task Switching and Response Inhibition
Perseverators on the card-sorting task were better than switchers at inhibiting responses on the stop signal task. Perseverators had significantly faster SSRTs (Figure 2a; 435 vs. 503 ms, F(1,32) = 5.6, p < .03, η2 = .15) calculated from all go trials (i.e., no signal and go signal trials combined, which is consistent with Bedard et al.’s [2002 i.e., no signal and go signal trials combined, which is consistent with Bedard et al.’s [2003] analysis technique). Moreover, SSRT predicted whether children switched or perseverated, controlling for age (Table 1).
Figure 2.
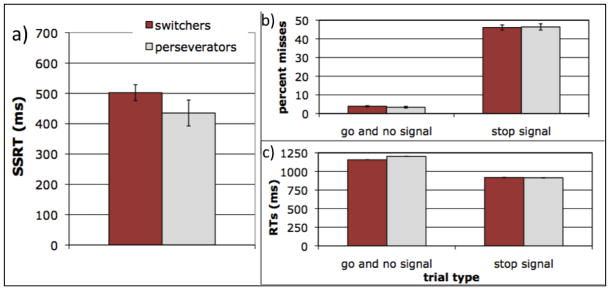
Tradeoff between switching and response inhibition in Experiment 1. a) Perseverators on the task switching measure showed better response inhibition (i.e., faster stop signal reaction times) than switchers. b) Perseverators’ advantage in response inhibition cannot be explained by a speed-accuracy trade-off, as perseverators and switchers were equally accurate in responses to all trial types. c) Perseverators’ advantage in response inhibition cannot be explained by switchers being more primed to go, as perseverators and switchers were equally fast. In addition, both perseverators and switchers showed faster RTs for incorrect stop signal trials than for “no signal” or “go signal” trials, an indicator that serves to validate the stop signal task. Error bars represent 1 SEM.
Table 1.
Hierarchical Regression of Switch Status (Switcher or Perseverator) on Stop Signal Reaction Time in Experiment 1
| Independent Variable | B | Exp(B) | Wald | p | R2 Change |
|---|---|---|---|---|---|
| age | 3.83 | 46.2 | 8.19 | .004 | .289 |
| stop signal reaction time | .008 | 1.0 | 4.57 | .041 | .150 |
Note - Stop signal reaction time predicted switching even after controlling for age.
Several potential explanations for this finding can be ruled out. First, perseverators’ response-inhibition advantage was not due to a speed-accuracy trade-off, as perseverators and switchers did not differ in percent misses on stop signal (46.4% vs. 46.1%), go signal (3.7% vs. 3.4%), or no signal trials (3.2% vs. 4.2%; all Fs < 1; Figure 2b). Second, perseverators’ response-inhibition advantage was not due to switchers being better at maintaining the primary goal of the task (Blackwell et al., 2009; Morton & Munakata, 2002), and thus having more difficulty interrupting their response after a stop signal. Switchers did not have faster no-signal RTs (1130 vs. 1176 ms, F < 1) or go-signal RTs (1183 vs. 1227 ms, F < 1; Figure 2c), indicating that switchers were not more primed to go. Third, perseverators’ response-inhibition advantage was not due to better auditory processing, as perseverators also showed an advantage when SSRT was calculated from go signal trials alone (487 ms vs. 542 ms, F(1,32) = 6.7, p = .01, η2 = .17). Finally, perseverators’ response-inhibition advantage did not seem to reflect differences in the way that either switchers or perseverators (compared to adults) complete the stop signal task, as the predictions of the model underlying the calculation of SSRT (Logan & Cowan, 1984; Band, van der Molen, & Logan, 2003) were met: RTs on failed stop trials (917 ms) were faster than on go trials (1158 ms, F(1,34) = 133.6, p < .001, η2 = .80; Figure 2c), and SSRT and go RTs were not correlated (r = −.02, p > .9). (Verifying these predictions is an important check of the stochastic independence assumption that underlies the race model and allows SSRT to be calculated using the methods described above; for further details, see Logan, 1984). In addition, our accuracy cutoffs yielded close to 50% stop signal errors, which is the value yielding the most efficient estimates of SSRT (Band et al., 2003).
2.3. Discussion
Consistent with the idea that there is a developmental trade-off between task switching and response inhibition, switchers did not outperform perseverators on a stop signal task as they do on other tasks; in fact, switchers showed less efficient response inhibition. This trade-off is consistent with both possible explanations raised in the Introduction. First, switchers may preferentially use a proactive maintenance strategy, attempting to maintain both the primary task (to press the circle as fast as they can) and the secondary task (to not press the circle when the game says “stop”), which overloads working memory and interferes with prompt processing of the stop signal. Second, switchers may be better at clearing information out of working memory, which could be detrimental if they clear the secondary goal of stopping in favor of the primary goal of responding quickly. The failure of either of these strategies (in terms of maintaining the secondary goal of stopping) may lead switchers to revert to the reactive control strategy preferred by perseverators, namely reactively retrieving the instructions to stop when the stop signal is presented; however, switchers may not be as practiced and efficient at this strategy, giving perseverators the advantage at a response inhibition task.
3. Experiment 2
To test the replicability and nature of switchers’ disadvantage at response inhibition, we ran a second study to investigate whether switchers would show an advantage in interference control, and again show a disadvantage in response inhibition. We predicted that switchers would show an advantage at interference control but a disadvantage at response inhibition, consistent with either the clearing working memory or overloading working memory accounts, and contrasting with accounts that posit a shared underlying mechanism for inhibiting actions (e.g., Friedman & Miyake, 2004). Six-year-old children completed three tasks: the 3D card sort (to measure task switching), stop signal (to measure response inhibition), and a Simon interference task (to measure interference control), in that order, again in keeping with standard individual difference methods (e.g., Friedman et al., 2008).
3.1. Method
3.1.1. Participants
Forty-four six-year-olds (M = 6.6-years-old, range = 6.1 to 7.0, 23 female) participated in this study. An additional 22 participants were excluded: 11 did not have sufficient stop signal accuracy to allow an estimation of their response inhibition, four failed to sort according to the rule during the preswitch phase, and seven had mixed switching. Participant recruitment and compensation procedures were identical to Experiment 1.
3.1.2. Materials, Procedure, and Measures
All participants completed the Simon interference task, 3D card sort and stop signal tasks, in that order.
Simon interference task
The Simon arrows task was adapted from Davidson et al. (2006). Children were instructed to press one of two keys to identify the direction an arrow was pointing. The arrows appeared on either the left or right hand side of the screen, and key presses were made with either the left or right hand (the “s” and “l” keys, respectively). On congruent trials, the arrows pointed down, so the key pressed was on the same as the arrow (Figure 3). On incongruent trials, the arrows pointed at a 45-degree angle to the opposite side of the screen, creating conflict between the automatically coded spatial information (e.g., arrow on the left) and the correct response to press the key corresponding to where the arrow was pointing (e.g., button on the right). After an initial practice block of 8 trials, children completed 80 trials of mixed congruent and incongruent trials presented in a random order. Accuracy and reaction time were recorded on all trials.
Figure 3.
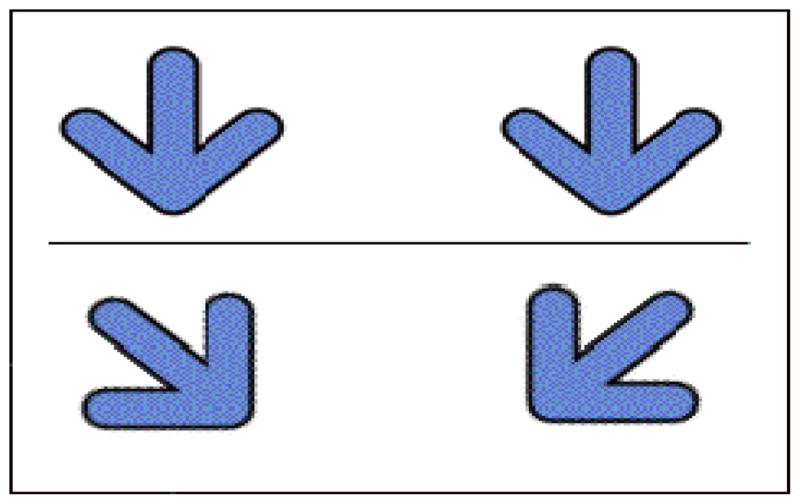
Simon interference task (Experiment 2). Congruent arrows (top) are presented on the same side as the button to which they point. Incongruent arrows (bottom) are presented on the opposite side, and the spatial information must be ignored to make a speedy response.
3D card sort
The 3D card sort was the same as in Experiment 1.
Stop signal
The stop signal task was the same as in Experiment 1, except that children completed no more than 4 test blocks (instead of 5), to reduce the possibility of fatigue.
3.1.3. Data Trimming
Data were trimmed as in Experiment 1. One switcher and one perseverator had SSRTs greater than 3 SDs from the mean of their group and were excluded from further analyses. Eleven children (6 switchers) were excluded from Simon interference analysis because of because of poor performance on the congruent trials, failing to respond correctly in the time limit on more than 20% of the congruent trials; of the remaining data, no individual means were more than 3 SDs from the average of the remaining means.
3.2. Results
3.2.1. Characteristics of Switchers and Perseverators
Twenty-nine children (66%, 14 female) were categorized as switchers (M = 93% of color and size trials correct), and 15 children (34%, 9 female) were categorized as perseverators (M = 4% of color and 0% of size trials correct). Switchers were not older than perseverators (both 6.6-years-old, t(42) = .38, p > .7), but age was controlled as in Experiment 1.
3.2.2. Relationship of Task Switching and Response Inhibition
Switchers once again showed a disadvantage in response inhibition. Perseverators had significantly faster SSRTs (Figure 4a; 428 vs. 501 ms, F(1,39) = 4.4, p = .04, η2 = .10) calculated from all go trials. Moreover, SSRT predicted whether children switched or perseverated, controlling for age (Table 2). As in Experiment 1, perseverators’ advantage was not due to a speed-accuracy trade-off, as perseverators and switchers had equal percent misses on stop signal (47% vs. 48%, F < 1), go signal (3.4% vs. 5.3%; F(1,39) =2.6, p > .1) and no signal trials (3.8% vs. 4.1%; F < 1; Figure 4b). Perseverators’ advantage was also not due to weaker maintenance of the primary goal, as perseverators did not have slower no-signal RTs (1100 ms vs. 1085 ms, F < 1), or go-signal RTs (1186 ms vs. 1146 ms, F < 1; Figure 4c). The desired features of stop signal performance were again observed, as RTs on failed stop trials (884 ms) were faster than on go trials (1109 ms, F(1, 41) = 125.1, p < .001, η2 = .75; Figure 4c), SSRT and go RTs were not correlated (r(42) = .19, p > .2), and stop signal errors were close to 50%.3
Figure 4.
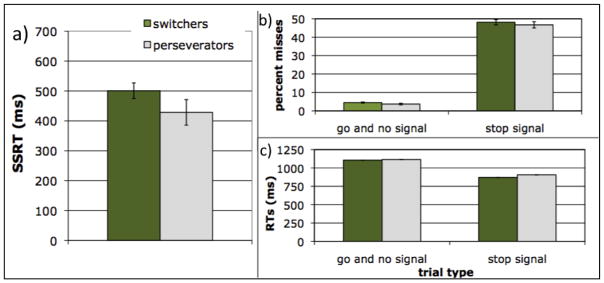
Tradeoff between switching and response inhibition in Experiment 2. a) Perseverators on the task switching measure showed better response inhibition (i.e., faster stop signal reaction times) than switchers. b) Perseverators’ advantage in response inhibition cannot be explained by a speed-accuracy trade-off, as perseverators and switchers were equally accurate in responses to all trial types. c) Perseverators’ advantage in response inhibition cannot be explained by switchers being more primed to go, as perseverators and switchers were equally fast. In addition, both perseverators and switchers showed faster RTs for incorrect stop signal trials than for “no signal” or “go signal” trials, an indicator that serves to validate the stop signal task. Error bars represent 1 SEM.
Table 2.
Hierarchical Regression of Switch Status (Switcher or Perseverator) on Stop Signal Reaction Time and Incongruency Cost in Experiment 2
| Independent Variable | B | Exp(B) | Wald | p | R2 Change |
|---|---|---|---|---|---|
| age | .61 | 1.9 | 0.1 | .754 | .001 |
| stop signal reaction time | .01 | 1.0 | 4.8 | .029 | .239 |
| incongruency cost | −.04 | 1.0 | 5.5 | .019 | .319 |
Note - Stop signal reaction time and incongruency cost independently predicted switching even after controlling for age.
3.2.2. Relationship of Task Switching and Interference Control
In contrast to switchers’ disadvantage on the stop signal task, switchers had a clear advantage on the Simon interference task. Although all children’s reaction times were slower for incongruent trials than congruent trials (842 ms vs. 781 ms, F(1,32) = 54.0, p < .001, η2 = .63), switchers were slowed less (45 ms) than perseverators (93 ms; F(1,30) = 8.4, p = .007, η2 = .22). This is again not a result of a speed-accuracy trade-off, as switchers and perseverators were equally accurate on congruent trials (95% and 96%) and on incongruent trials (both groups M = 86%; all Fs < 1). Although the stop signal and Simon arrows tasks are both typical measures of inhibition, performance on these tasks was not related (r(31) = .01, p > .9), and both SSRT and incongruency cost independently predict switch status (Table 2).
3.3. Discussion
Switchers again showed worse response inhibition than perseverators, replicating Experiment 1, but switchers also showed better interference control than perseverators. Switchers’ advantage on the Simon task is consistent with the overloaded-working-memory and clearing-working-memory accounts, both of which posit that the dual-goal task demands of the stop signal task (which are not present in the Simon task) cause switchers’ disadvantage at response inhibition. Future studies could try to distinguish these two accounts, and could also test individual differences in attempting to maintain multiple goals versus clearing previous or secondary goals from working memory. Children may develop these skills at different rates, with some children more effectively clearing irrelevant goals from working memory, while others are more likely to attempt to proactively maintain current task goals in working memory; use of either strategy could help children switch between rules but could create difficulties with the stop signal task. With either possible explanation, the selective trade-off between task switching and response inhibition demonstrates the potential costs associated with some executive functions, and the insights that developmental research can provide.
4. General Discussion
Children experience a trade-off between two aspects of executive function, task switching and inhibitory control, for one type of inhibitory control but not another. Specifically, 6-year-old children who successfully switched between tasks on the 3D card sort had worse response inhibition on a stop signal task (replicated across two studies), but better interference control. This pattern complements previous findings demonstrating that developing executive functions can be associated with both positive and negative outcomes (e.g., Blackwell & Munakata, 2014; Friedman et al., 2011; Thompson-Schill et al., 2009), and prior work demonstrating interference among executive functions that share prefrontal networks (Dux et al., 2006; Sigman & Dehaene, 2008; Tombu et al., 2011) or use distinct prefrontal networks that have competitive dynamics (Fox et al., 2005; Kelly et al., 2008). Such findings highlight the importance of considering both the advantages and disadvantages associated with the development of executive functions, and suggest caution before committing to programs that might improve them.
The trade-off observed in these studies may be inherently developmental in nature, given that in adults, positive correlations are observed among response inhibition, interference control, and task switching (e.g., Aron et al., 2004; Friedman and Miyake, 2004; Miyake et al., 2000). Such developmental trade-offs could be indicative of steps in the development of executive functions. For example, the trade-off may result from inappropriately clearing the secondary goal from working memory, before children develop more adaptive strategies for clearing goals from working memory when it is beneficial (e.g., when switching from one task to another) but not when clearing is not beneficial (e.g., when multiple goals are all relevant).
Alternatively, the trade-off observed in these studies may be most evident during development, but may reflect processes that continue into adulthood. For example, in childhood, good task switchers may rely on proactive maintenance of goals and attempt but fail to maintain two distinct goals for stopping and going on a response inhibition task. Further development of working memory might allow successful maintenance of multiple simultaneous goals, which could promote faster response inhibition and explain adult correlations between task switching and response inhibition (e.g., Miyake et al., 2000; Aron et al., 2004) and between response inhibition and interference control (e.g., Friedman et al., 2004), as well as benefits to response inhibition that occur following training of proactive control in older children (Chevalier, Chatham, & Munakata, 2014). From this perspective, under more demanding response-inhibition conditions that tax working memory, adults might revert to showing a trade-off between task-switching and response inhibition like that observed in children. Such findings would suggest that the same underlying mechanisms and trade-offs are at work in adults, but are masked by greater capacities and so are revealed only in more subtle ways or under more demanding conditions, as in other domains (e.g., Hermer-Vazquez, Spelke, & Katnelson, 1999; Diamond & Kirkham, 2005).
At a neural level, these trade-offs could reflect developmental changes in the substrates that children (versus adults) recruit for executive functions such as response inhibition (e.g., Bunge, 2002; Casey, Tottenham, Liston, & Durston, 2005; Hwang, Velanova, & Luna, 2010; Rubia, Smith, Taylor & Brammer, 2007). For example, as children increasingly engage prefrontal regions for response inhibition across development, they may ultimately show fewer trade-offs with other executive functions. In the midst of this transition, children who recruit prefrontal regions may actually show worse response inhibition than children who recruit primarily posterior cortical brain regions (Bunge et al., 2002), which may indicate transitional trade-offs as prefrontal control is engaged. These trade-offs could also reflect developmental changes in the temporal dynamics of processing in common neural substrates (e.g., Wendelken, Munakata, Baym, Souza, & Bunge, 2012 with fewer tradeoffs observed as neural processing becoming increasingly anticipatory and less sluggish with development. For example, initial developments in the efficiency of prefrontal processing may support proactively maintaining one goal, with further development necessary to support proactively maintaining two goals simultaneously. In keeping with this, when children are further along in a transition from reactive to proactive control, they show greater consistency across measures of executive function (Chatham, Provan, & Munakata, submitted).
At a functional level, the trade-off we have observed may be adaptive in some way during development. For example, failures associated with children’s use of developing proactive control may ensure children’s continued practice of reactive control. Such practice could serve to prevent the atrophying of reactive processes, which would be adaptive given that reactive processes can be helpful for learning and other cognitive processes during childhood (Munakata, Snyder, & Chatham, 2013; see also Newport, 1990, and Thompson-Schill et al., 2009), as well as in certain situations during adulthood (e.g., Braver, Paxton, Loch & Barch, 2009; Cohen, Lewis-Peacock, & Norman, 2012). The extended use of reactive control across development due to trade-offs associated with proactive control could also support the development of prefrontal-hippocampal networks (e.g., Chatham, Frank, & Munakata, 2009; Finn, Sheridan, Kam, Hinshaw & D’Esposito, 2010).
Additional studies should shed light on the trade-off observed here between task-switching and response inhibition. Such research should ideally be done across a variety of ages (e.g., from 6-year-olds who show the trade-off through 18-year-olds who do not), under a variety of conditions (e.g., with varying demands on working memory), testing multiple aspects of inhibitory control, and including neuroimaging approaches, to further test the specificity of tradeoffs between executive functions, their underlying mechanisms, and how they might be resolved in the transition to adulthood. Such research should help to inform an understanding of the relationships among executive functions, and discussions on whether or when to attempt to speed developments in children’s executive functions.
Research Highlights.
Two executive functions, task switching and inhibitory control, were assessed in six-year-olds.
Switchers showed worse response inhibition than perseverators, but better interference control.
Trade-off may reflect working memory processes, such as goal maintenance and clearing prior goals.
Such processes may help children with a single task-goal, but hurt in the face of conflicting goals.
Trade-off shows advantages and disadvantages associated with development of executive functions.
Acknowledgments
This work was supported by grants from the National Institutes of Health (RO1 HD37163 and P50-MH079485). We thank Kelly Reid, Sara McQuiston, Joedy Hulings, and other members of the Cognitive Development Center for useful discussions and assistance with this study.
Footnotes
Children’s performance, including rates of insufficient preswitch accuracy and mixed switching, and the final distribution of switchers and perseverators were similar in both experiments to those in similar task-switching paradigms (Blackwell et al., 2009; Kharitonova et al, 2009; Kharitonova & Munakata, 2011). Although mixed switching performance may result from intermediate switching abilities, it may also result from children’s distraction on one post-switch block, so these children are not included in the analysis.
Typically, stop signal paradigms compare performance on “no signal” and “stop signal” trials. The “go signal” trials were included to provide a baseline RT that would include any auditory processing of the “go” or “stop” signals.
Although perseverators’ advantage was no longer significant when calculating SSRT from go-signal trials alone (500 ms vs. 540 ms, F < 1), this likely reflects the noise of calculating from fewer trials (only 25% of trials, vs. 75%); there were no main effects or interactions as a function of experiment (all Fs < 1), and perseverators’ advantage on SSRT calculated from go-signal trials was significant with both studies combined (F(1,72) = 4.2, p < .05, η2 = .06). Thus, perseverators’ response-inhibition advantage was not due to better auditory processing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allport DA, Styles EA, Hsieh S. Shifting intentional set: Exploring the dynamic control of tasks. In: Umilta C, Moscovitch M, editors. Attention and performance: Conscious and nonconscious information processing. Vol. 15. Cambridge: MIT Press; 1994. pp. 421–452. [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The capacity for generating information by randomization. Quarterly Journal of Experimental Psychology. 1966;18:119–129. doi: 10.1080/14640746608400019. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Exploring the central executive. Quarterly Journal of Experimental Psychology. 1996;49A(1):5–28. [Google Scholar]
- Baddeley A, Emslie H, Kolodny J, Duncan J. Random generation and the executive control of working memory. Quarterly Journal of Experimental Psychology. 1998;51:819–852. doi: 10.1080/713755788. [DOI] [PubMed] [Google Scholar]
- Band GPH, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychologia. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the lifespan. Developmental Neuropsychology. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. Journal of Abnormal Child Psychology. 2003;31:315–327. doi: 10.1023/a:1023285614844. [DOI] [PubMed] [Google Scholar]
- Blackwell KA, Cepeda NJ, Munakata Y. When simple things are meaningful: Working memory strength predicts children’s cognitive flexibility. Journal of Experimental Child Psychology. 2009;103:241–249. doi: 10.1016/j.jecp.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell KA, Munakata Y. Costs and benefits linked to developments in cognitive control. Developmental Science. 2014;17:203–211. doi: 10.1111/desc.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S, Zelazo P. A brain-based account of the development of rule use in childhood. Psychological Science. 2006;15:118–121. [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Munakata Y. Why do children perseverate when they seem to know better: Graded working memory, or directed inhibition? Psychonomic Bulletin and Review. 2007;14:1058–1065. doi: 10.3758/bf03193091. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Gould IC, English T, Garavan H, McNaught E, Kamke M, et al. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. Journal of Neurophysiology. 2007;98:3638–3647. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- Chatham CH, Frank MJ, Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proceedings of the National Academy of Sciences. 2009;106:5529–5533. doi: 10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Badre D. Working memory management and predicted utility. Frontiers in Behavioral Neuroscience. 2013;7:83. doi: 10.3389/fnbeh.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Provan L, Munakata Y. Dissociable sources of individual differences in the developmental shift from reactive to proactive control in 5- and 6-year-olds. 2014. Manuscript submitted for publication. [Google Scholar]
- Chevalier N, Chatham CH, Munakata Y. The practice of going helps children to stop: The importance of context monitoring. Journal of Experimental Psychology General. 2014 doi: 10.1037/a0035868. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG, Hamilton RH, Coslett HB, Datta A, Bikson M, Thompson-Schill SL. Noninvasive transcranial direct current stimulation over the left prefrontal cortex facilitates cognitive flexibility in tool use. Cognitive Neuroscience. 2013:1–9. doi: 10.1080/17588928.2013.768221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Lewis-Peacock JA, Norman KA. Neural evidence for the flexible use of working memory and episodic memory in prospective remembering. Poster presented at the Society for Neuroscience Annual Meeting; New Orleans, LA. Oct, 2012. [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák GO. The development of cognitive flexibility and language abilities. In: Kail R, editor. Advances in child development and behavior. Vol. 31. San Diego: Academic Press; 2003. pp. 271–327. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Reviews of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Experimental Brain Research. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. [Google Scholar]
- Diamond A, Kirkham N. Not quite as grown-up as we like to think. Psychological Science. 2005;16:291–297. doi: 10.1111/j.0956-7976.2005.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Kirkham N, Amso D. Conditions under which young children can hold two rules in mind and inhibit a prepotent response. Developmental Psychology. 2002;38:352–362. [PubMed] [Google Scholar]
- Diamond A. Activities and programs that improve children’s executive functions. Current Directions in Psychological Science. 2012;21:335–341. doi: 10.1177/0963721412453722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Pizzagalli DA. Inhibition of action, thought, and emotion: A selective neurobiological review. Applied and Preventive Psychology. 2007;12:99–114. doi: 10.1016/j.appsy.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Hutchison KE, Frank MJ. Dopaminergic genes predict individual differences in susceptibility to confirmation bias. Journal of Neuroscience. 2011;31:6188–6198. doi: 10.1523/JNEUROSCI.6486-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Ivanoff J, Asplund CL, Marois R. Isolation of a central bottleneck of information processing with time-resolved fMRI. Neuron. 2006;52:1109–1120. doi: 10.1016/j.neuron.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:F9–F16. [Google Scholar]
- Finn AS, Sheridan MA, Kam CLH, Hinshaw S, D’Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. Journal of Neuroscience. 2010;30:11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology: General. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Haberstick BC, Wilcutt EG, Miyake A, Young SE, Corley RP, Hewitt JK. Greater attention problems during childhood predict poorer executive functioning in late adolescence. Psychological Science. 2007;18:893–900. doi: 10.1111/j.1467-9280.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Robinson JL, Hewitt JK. Developmental trajectories in toddlers’ self-restraint predict individual differences in executive functions 14 years later: A behavioral genetic analysis. Developmental Psychology. 2011;47:1410–1430. doi: 10.1037/a0023750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goschke T. Intentional reconfiguration and involuntary persistence in task set switching. In: Mosell S, Driver J, editors. Attention and Performance XVIII: Control of cognitive processes in attention, memory and language. Cambridge, MA: MIT Press; 2000. pp. 331–335. [Google Scholar]
- Hamilton AC, Martin RC. Dissociations among tasks involving inhibition: A single-case study. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:1–13. doi: 10.3758/cabn.5.1.1. [DOI] [PubMed] [Google Scholar]
- Herd SA, Hazy T, Chatham CH, O’Reilly RC, Friedman N. Neural mechanisms underlying the switching component of executive function. (submitted) [Google Scholar]
- Hermer-Vazquez L, Spelke ES, Katsnelson AS. Sources of flexibility in human cognition: Dual-task studies of space and language. Cognitive Psychology. 1999;39:3–36. doi: 10.1006/cogp.1998.0713. [DOI] [PubMed] [Google Scholar]
- Hermer L, Spelke E. Modularity and development: The case of spatial reorientation. Cognition. 1996;61:195–232. doi: 10.1016/s0010-0277(96)00714-7. [DOI] [PubMed] [Google Scholar]
- Hongwanishkul D, Happaney KR, Lee WSC, Zelazo PD. Assessment of hot and cool executive function in young children: Age-related changes and individual differences. Developmental Neuropsychology. 2005;28:617–644. doi: 10.1207/s15326942dn2802_4. [DOI] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: A functional magnetic resonance imaging effective connectivity study. The Journal of Neuroscience. 2010;30:15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswall BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kharitonova M, Chien S, Colunga E, Munakata Y. More than a matter of getting “unstuck”: Flexible thinkers use more abstract representations than perseverators. Developmental Science. 2009;12:662–669. doi: 10.1111/j.1467-7687.2008.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonova M, Munakata Y. The role of representations in executive function: Investigating a developmental link between flexibility and abstraction. Frontiers in Psychology. 2011;2:347. doi: 10.3389/fpsyg.2011.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham NZ, Diamond A. Sorting between theories of perseveration: Performance in conflict tasks requires memory, attention and inhibition. Developmental Science. 2003;6:474–476. [Google Scholar]
- Kloo D, Perner J. Training transfer between card sorting and false belief understanding: Helping children apply conflicting descriptions. Child Development. 2003;74:1823–1839. doi: 10.1046/j.1467-8624.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- Lemaire P, Abdi H, Fayol M. The role of working memory resources in simple cognitive arithmetic. European Journal of Cognitive Psychology. 1996;8:73–10. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 2006;8:60–64. [Google Scholar]
- Marcovitch S, Boseovski JJ, Knapp RJ. Use it or lose it: Examining preschoolers’ difficulty in maintaining and executing a goal. Developmental Science. 2007;10:559–564. doi: 10.1111/j.1467-7687.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H-L, Houts R, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfese VJ, Molfese DL, Modgline AA. Newborn and preschool predictors of second-grade reading scores: An evaluation of categorical and continuous scores. Journal of Learning Disabilities. 2001;34:545–554. doi: 10.1177/002221940103400607. [DOI] [PubMed] [Google Scholar]
- Morton JB, Munakata Y. Active versus latent representations: A neural network model of perseveration, dissociation, and decalage. Developmental Psychobiology. 2002;40:255–265. doi: 10.1002/dev.10033. [DOI] [PubMed] [Google Scholar]
- Müller U, Zelazo PD, Imrisek S. Understanding false belief and executive function: How specific is the relation? Cognitive Development. 2005;20:173–189. [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC. A unified framework for inhibitory control. Trends in Cognitive Sciences. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, Snyder HR, Chatham CH. Developing cognitive control: The costs and benefits of active, abstract representations. In: Zelazo P, Sera M, editors. Developing Cognitive Control Processes: Mechanisms, Implications, and Interventions. Vol. 37. Erlbaum; 2013. pp. 55–90. [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective, and Behavioral Neuroscience. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Newport EL. Maturational constraints on language learning. Cognitive Science. 1990;14:11–28. [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Pennington BF. Dimensions of executive functions in normal and abnormal development. In: Krasnegor NA, Lyon GR, Goldman-Rakic PS, editors. Development of the prefrontal cortex: Evolution, neurobiology, and behavior. Baltimore, MD: P. H. Brookes; 1997. pp. 265–281. [Google Scholar]
- Perner J, Lang B. What causes 3-year-olds’ difficulty on the dimensional change card sorting task? Infant and Child Development. 2002;11:93–105. [Google Scholar]
- Ridderinkhof KR, Band GPH, Logan GD. A study of adaptive behavior: Effects of age and irrelevant information on the ability to inhibit one’s actions. Acta Psychologia. 1999;101:315–337. [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–231. [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior front-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Geurts H, Morein-Zamir S, Meiran N, Schut H, Vlasveld L, et al. Executive functioning in boys with ADHD: Primarily an inhibition deficit? Archives of Clinical Neuropsychology. 2004;19:569–594. doi: 10.1016/j.acn.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sigman M, Dehaene S. Brain mechanisms of serial and parallel processing during dual-task performance. Journal of Neuroscience. 2008;28:7585–7598. doi: 10.1523/JNEUROSCI.0948-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Munakata Y. Becoming self-directed: Abstract representations support endogenous flexibility in children. Cognition. 2010;116:155–167. doi: 10.1016/j.cognition.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair-Thompson HL, Gathercole S. Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Quarterly Journal of Experimental Psychology. 2006;59:745–759. doi: 10.1080/17470210500162854. [DOI] [PubMed] [Google Scholar]
- Stins J, Polderman JC, Boomsma D, de Geus E. Response interference and working memory in 12-year-old children. Child Neuropsychology. 2005;11:191–201. doi: 10.1080/092970490911351. [DOI] [PubMed] [Google Scholar]
- Thomspon-Schill SL, Ramscar M, Chrysikou EG. Cognition without control: When a little frontal lobe goes a long way. Current Directions in Psychological Science. 2009;18:259–263. doi: 10.1111/j.1467-8721.2009.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombu MN, Asplund CL, Dux PE, Godwin D, Martin JW, Marois R. A unified attentional bottleneck in the human brain. Proceedings of the National Academy of Sciences. 2011;108:13426–13431. doi: 10.1073/pnas.1103583108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukayama E, Toomey SL, Faith M, Duckworth AL. Self-control protects against overweight status in the transition from childhood to adolescence. Archives of Pediatric and Adolescent Medicine. 2010;164:631–635. doi: 10.1001/archpediatrics.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Liefooghe B, Szmalec A, Vandierendonck A. Inhibiting responses when switching: Does it matter? Experimental Psychology. 2005;52:125–130. doi: 10.1027/1618-3169.52.2.125. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. NeuroImage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Munakata Y, Baym C, Souza M, Bunge SA. Flexible rule use: Common neural substrates in children and adults. Developmental Cognitive Neuroscience. 2012;2:329–339. doi: 10.1016/j.dcn.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, Glass JM, Adams K. Behavioral control and resiliency in the onset of alcohol and illicit drug use: A prospective study from preschool to adolescence. Child Development. 2006;77:1016–1033. doi: 10.1111/j.1467-8624.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Frye D. Cognitive complexity and control: II. The development of executive function in childhood. Current Directions in Psychological Science. 1998;7:121–126. [Google Scholar]
- Zelazo PD, Frye D, Rapus T. An age-related dissociation between knowing rules and using them. Cognitive Development. 1996;11:37–63. [Google Scholar]


