Abstract
Target-specific oral anticoagulants (TSOACs) provide safe and effective anticoagulation for the prevention and treatment of thrombosis in a variety of clinical settings by interfering with the activity of thrombin (dabigatran) or factor Xa (rivaroxaban, apixaban, edoxaban, betrixaban). Although TSOACs have practical advantages over vitamin K antagonists (VKAs), there are currently no antidotes to reverse their anticoagulant effect. Herein we summarize the available evidence for TSOAC reversal using nonspecific and specific reversal agents. We discuss important limitations of existing evidence, which is derived from studies in human volunteers, animal models and in vitro experiments. Studies evaluating the safety and efficacy of reversal agents on clinical outcomes such as bleeding and mortality in patients with TSOAC-associated bleeding are needed.
Introduction
Vitamin K antagonists (VKAs) have been the mainstay of long-term antithrombotic therapy for prevention and treatment of thromboembolism. VKAs have practical limitations including long half-lives, drug interactions and unpredictable pharmacokinetics necessitating routine monitoring of anticoagulant effect. Unlike VKAs, which impair the production of vitamin-K-dependent coagulation factors II, VII, IX and X, target-specific oral anticoagulants (TSOACs) exert their anticoagulant effect by inhibiting the activity of thrombin (dabigatran) or factor Xa (rivaroxaban, apixaban, edoxaban, betrixaban), coagulation factors that mediate the final stages of coagulation.
TSOACs were developed as alternatives to VKAs owing to practical advantages including rapid onset of action, short half-lives, more-predictable pharmacokinetics, fewer drug interactions and lack of need for routine monitoring. TSOAC drug characteristics are shown in Table 1. Clinical uses of TSOACs include prevention of stroke and systemic embolism in nonvalvular atrial fibrillation, prevention of venous thromboembolism (VTE) following hip and knee arthroplasty and treatment of VTE. Based on large clinical trials and real-world post-marketing surveillance data, TSOACs are at least as effective and safe as VKAs or low molecular weight heparin for approved indications [1–3].
Table 1.
Pharmacologic properties of target-specific oral anticoagulants
| Target-specific oral anticoagulant | Target | Time to peak concentration (h) | Half-life (h) | Renal excretion |
|---|---|---|---|---|
| Dabigatran [28,29] | Thrombin | 1–3 | 7–9a 7–17b |
80–85% |
| Rivaroxaban[30–32] | Factor Xa | 2–4 | 7–17a 12–13c 6–9b |
36% |
| Apixaban [33–35] | Factor Xa | 1–3 | 8–14a | 25% |
| Edoxaban [36] | Factor Xa | 1–2 | 6–11a 9–10b |
36–45% |
| Betrixaban [36,37] | Factor Xa | 3–4 | 19 | <8% |
Healthy adults, single dose.
Healthy adults, multiple doses.
Healthy elderly, single dose.
However, unlike VKAs for which vitamin K and coagulation factor replacement with prothrombin complex concentrate (PCC) or plasma can be used to replace coagulation factors and restore coagulation, there are no antidotes available to reverse the anticoagulant effect of TSOACs in the event of bleeding or need for an emergent procedure. Specific reversal agents are currently undergoing clinical development. In this narrative review, we summarize the current published evidence for TSOAC reversal using nonspecific and specific reversal agents.
Types of reversal agents
Coagulation factor replacement
Plasma
Plasma is the aqueous part of blood that contains dissolved proteins including coagulation factors. Plasma transfusion is associated with health risks including transfusion-associated circulatory overload, transfusion-related acute lung injury, allergy and infection [4].
Prothrombin complex concentrates
PCCs are plasma-derived concentrates of vitamin-K-dependent coagulation factors II, IX and X (3-factor PCC; 3-PCC) or factors II, VII, IX and X (4-factor PCC; 4-PCC) with variable amounts of proteins C and S. There is a low risk of viral transmission owing to viral inactivation during product preparation [5]. Thromboembolism is a potential complication of PCC use occurring at a rate of 1.4% when used to treat VKA-associated bleeding [5].
Prohemostatic agents
Activated prothrombin complex concentrate
Activated PCC (aPCC) contains plasma-derived activated forms of coagulation factors II, VII, IX and X. aPCC was developed as a prohemostatic agent to treat bleeding in hemophilia patients with inhibitors to factors VIII or IX [6]. There is a low risk of thromboembolism associated with aPCC use (4–8 events per 105 infusions) based on pharmacovigilance data in hemophilia patients [7,8]. However, the majority of these events (81%) occurred in patients with risk factors for thrombosis, which raises concerns regarding aPCC use in patients receiving anticoagulant therapy for prevention or treatment of thrombotic disease.
Recombinant factor VIIa
Recombinant factor VIIa (rVIIa) was also developed as a bypassing agent for bleeding complications in hemophilia patients with inhibitors. Use of rVIIa outside its approved indication is associated with an increased risk of arterial thromboembolism compared with placebo [5.5% vs 3.2%; relative risk 1.68; 95% confidence interval (CI) 1.20–2.36] [9].
Specific reversal agents
A humanized monoclonal antibody fragment against dabigatran (anti-Dabi-Fab; Boehringer Ingelheim, Biberach, Germany) is currently undergoing clinical development as a specific reversal agent [10]. It has ~350-fold greater affinity for dabigatran than it does for thrombin, and does not appear to bind endogenous thrombin substrates, activate coagulation or platelets.
A recombinant factor Xa derivative (PRT064445; Portola Pharmaceuticals, San Francisco, CA, USA) is being developed as a specific factor Xa inhibitor reversal agent [11]. The protein lacks catalytic and membrane-binding activity, but retains the ability to bind factor Xa inhibitors with subnanomolar affinity. Also in development is an inactive zymogen-like factor Xa variant, which has demonstrated reversal of the anticoagulant effect of rivaroxaban in vitro (Thalji, N. et al. abstr. OC 36.4, XXIV Congress of the International Society on Thrombosis and Haemostasis, Amsterdam, Netherlands, July 2013).
PER977 (Perosphere, Bedford, NY, USA) reversed heparin, low molecular weight heparin, fondaparinux, dabigatran, rivaroxaban, apixaban and edoxaban in preclinical studies (Laulicht, B. et al. abstr. AS 47.1, XXIV Congress of the International Society on Thrombosis and Haemostasis, Amsterdam, Netherlands, July 2013). The mechanism by which a single molecule could reverse direct and indirect inhibitors of thrombin and factor Xa is not clear and has not been reported.
Evidence for reversal of target-specific oral anticoagulants
Limitations of evidence
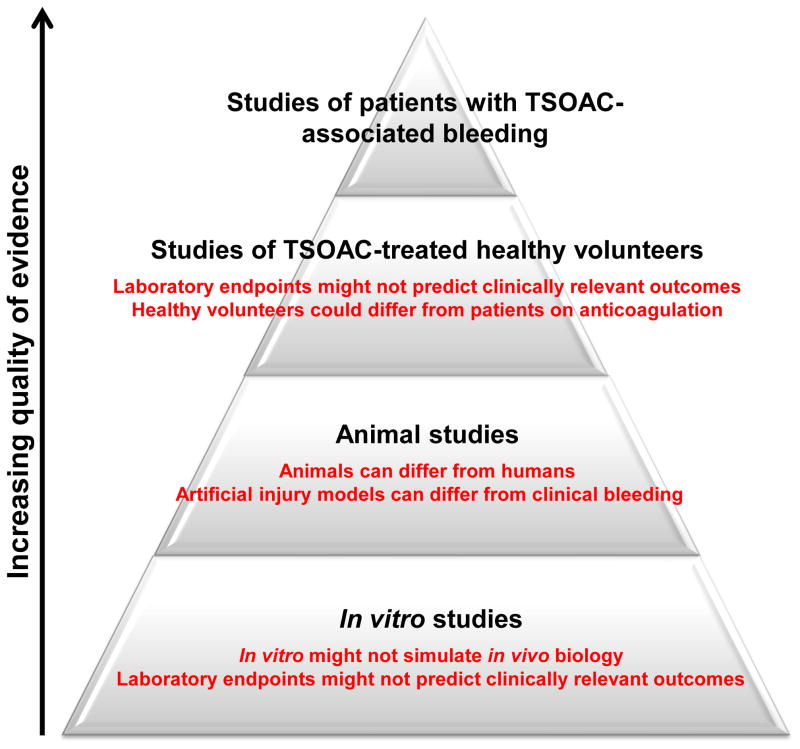
Ideally, evidence for reversal would be based on studies of patients with TSOAC-associated bleeding treated with various reversal strategies. Unfortunately, apart from a small number of case reports, such evidence is nonexistent. Instead, current evidence for reversal is limited to studies in human volunteer subjects, animal models and in vitro experiments, each of which carries important limitations (Figure 1).
Figure 1.
Hierachy of evidence for reversal of the target-specific oral anticoagulants (TSOACs). Studies of TSOAC reversal can be classified as one of four types. From lowest to highest quality of evidence, these include in vitro studies, animal studies, studies of TSOAC-treated healthy volunteers and studies of patients with TSOAC-associated bleeding. Systematic studies of patients with TSOAC-associated bleeding have not been completed. Major limitations of the other study types are shown in red.
In vitro investigations comprise the weakest form of evidence for reversal. In these studies, anticoagulated human plasma is spiked with a reversal agent and its effect on a laboratory assay is measured. The major limitation of in vitro studies is the use of laboratory test results as a surrogate marker of efficacy as opposed to clinically relevant outcomes such as cessation of bleeding or mortality. Furthermore, widely used tests of coagulation such as the prothrombin time (PT) and activated partial thromboplastin time (aPTT) have variable sensitivity and accuracy for measuring the anticoagulant effect of the TSOACs [12].
Evidence for reversal is also derived from animal models in which bleeding is assessed following an induced injury (e.g. tail vein clipping) in the presence or absence of TSOAC treatment. These studies are limited by the need to extrapolate from animals to humans and inherent differences in artificial injury compared with clinical bleeding in patients.
Finally, several studies have been carried out in which healthy volunteers are given a TSOAC and then a reversal agent to measure the effect of the reversal agent on hemostatic laboratory assays. As with in vitro experiments, a major shortcoming of these ex vivo studies is use of a surrogate laboratory endpoint rather than a clinically relevant outcome. In addition, it is likely that the young healthy subjects recruited for these studies differ from patients on anticoagulation.
Dabigatran
Nonspecific reversal agents
Nonspecific reversal agents have variable effects on the anticoagulant effect of dabigatran as measured by laboratory tests. Administration of 4-PCC (50 U/kg) failed to correct the aPTT, ecarin clotting time (ECT) or thrombin time (TT) in healthy volunteers receiving dabigatran (150 mg twice daily for 2.5 days) in a randomized placebo-controlled crossover study [13]. aPCC, 4-PCC and rVIIa each corrected some, but not all, abnormal thrombin generation indices when added to the plasma of healthy volunteers who had received a single oral dose of dabigatran (150 mg) [14]. When added to the plasma of dabigatran-treated patients, aPCC corrected thrombin generation parameters [15]. In another study, PCC and aPCC, but not rVIIa, corrected the PT, aPTT and some thrombin generation indices when added to plasma from dabigatran-treated patients [16]. Only PCC corrected TT prolongation. None of the reversal agents affected results of the Hemoclot® assay (HYPHEN BioMed, Neuville Sur Oise, France), a commercially available dilute TT that provides an accurate, reproducible measure of dabigatran anticoagulant activity [16,17].
In animal models, the ability of nonspecific reversal agents to ameliorate dabigatran-related bleeding is inconsistent. PCC, aPCC and rVIIa did not reduce blood loss following tail transaction in mice receiving dabigatran [18]. In another study, PCC, but not rVIIa or plasma, reduced intracranial hematoma expansion and 24-hour mortality in dabigatran-treated mice [19]. PCC also reduced blood loss following kidney incision in dabigatran-treated rabbits [20].
Specific reversal agent
Administration of anti-Dabi-Fab resulted in a rapid, dose-dependent decrease in blood loss following tail transection in rats receiving supratherapeutic doses of dabigatran (van Ryn, J. et al. abstr. 3418, 54th American Society of Hematology Annual Meeting, Atlanta, GA, December 2012). In monkeys, anti-Dabi-Fab dose-dependently reversed dabigatran anticoagulant activity as measured by the dilute PT assay (Toth, J. et al. abstr. 22, 54th American Society of Hematology Annual Meeting, Atlanta, GA, December 2012). A Phase I clinical trial of this agent is currently enrolling (ClinicalTrials.gov identifier: NCT01955720). Table 2 summarizes evidence for reversal of dabigatran anticoagulant effect.
Table 2.
Summary of ex vivo and in vivo evidence for reversal of dabigatran anticoagulant effect
| Reversal strategy | Animal studies (dabigatran-treated animals) | Ex vivo studies (dabigatran-treated healthy volunteers or patients) | Ex vivo studies (dabigatran-and reversal-agent-treated healthy volunteers) |
|---|---|---|---|
| Nonspecific reversal agents | |||
| PCC |
|
||
| aPCC |
|
||
| rVIIa | |||
| Specific reversal agent | |||
| Anti-Dabi-Fab [10] |
|
||
Abbreviations: Anti-Dabi-Fab, humanized monoclonal antibody fragment against dabigatran; aPCC, activated prothrombin complex concentrate; aPTT, activated partial thromboplastin time; ECT, ecarin clotting time; PCC, prothrombin complex concentrate; PT, prothrombin time; rVIIa, recombinant activated factor VII; TG, thrombin generation; TT, thrombin time.
Factor Xa inhibitors
Nonspecific reversal agents
Unlike dabigatran, administration of 4-PCC (50 U/kg) to healthy volunteers receiving rivaroxaban (20 mg twice daily for 2.5 days) corrected PT prolongation [13]. The addition of aPCC to plasma from rivaroxaban-treated volunteers corrected all thrombin generation parameters, whereas PCC and rVIIA modified only some parameters [14]. Another study showed that PCC, aPCC and rVIIa all corrected PT prolongation when added to plasma from patients receiving rivaroxaban, but only PCC and aPCC modified all abnormal thrombin generation indices [16]. None of the reversal agents normalized anti-Xa activity. In separate studies, the addition of PCC to rivaroxaban-spiked plasma in vitro did not change abnormal coagulation tests, thromboelastometry or thrombin generation tests [21,22]. However, rVIIa corrected PT prolongation at a low rivaroxaban concentration (80 ng/μl) and thromboelastometry results at a high rivaroxaban concentration (200 ng/μl) [22]. When added to apixaban-spiked plasma, PCC, aPCC and rVIIa variably corrected some abnormal thrombin generation and thromboelastometry results [23]. PCC, aPCC and rVIIa corrected PT prolongation induced by edoxaban in vitro [24].
Studies in animal models also show variable results for rivaroxaban reversal using nonspecific reversal agents. In a rabbit bleeding model, PCC and aPCC normalized the aPTT and partially corrected the PT, but neither agent reduced blood loss in rivaroxaban-treated rabbits [25]. Conversely, PCC (50 U/kg, but not 25 U/kg), aPCC and rVIIa corrected PT prolongation and reduced bleeding time in rivaroxaban-treated rats [26]. In the same study, aPCC and rVIIa corrected PT prolongation, but only aPCC reduced bleeding time in primates treated with rivaroxaban; aPCC and rVIIa also significantly shortened the bleeding time in edoxaban-treated rats[24].
Specific reversal agent
PRT064445 dose-dependently reversed the anticoagulant activity of rivaroxban, apixaban and betrixaban in vitro as measured by anti-Xa activity assays [11]. PT prolongation induced by rivaroxaban was also corrected with the addition of PRT064445. In the same study, PRT064445 administered to rats in vivo following rivaroxaban, apixaban and betrixaban infusions rapidly reduced the international normalized ratio (INR). In rivaroxaban-treated rats, PRT064445 decreased plasma concentrations of free rivaroxaban, the fraction responsible for mediating anticoagulant activity. Abnormal PT, aPTT and anti-Xa assays were corrected and blood loss reduced when PRT064445 was given to rabbits receiving rivaroxaban in a liver laceration model.
Preliminary results of an ongoing Phase II, randomized, double-blind trial in healthy volunteers (apixaban 5 mg twice daily, 11 doses) showed that PRT064445 decreased anti-Xa activity and reduced plasma concentrations of free apixaban compared with placebo (ClinicalTrials.gov identifier: NCT01758432) (Crowther, M. et al. abstract in J Thromb Haemost 2013;11:30). Table 3 summarizes evidence for reversal of factor Xa inhibitor anticoagulant effect.
Table 3.
Summary of ex vivo and in vivo evidence for reversal of factor Xa inhibitor anticoagulant effect
| Reversal strategy | Animal studies (factor-Xa-inhibitor-treated animals) | Ex vivo studies (factor-Xa-inhibitor-treated healthy volunteers or patients) | Ex vivo studies (factor-Xa-inhibito- and reversal-agent-treated healthy volunteers) |
|---|---|---|---|
| Nonspecific reversal agents | |||
| PCC | Rivaroxaban | Rivaroxaban | Rivaroxaban
|
| aPCC | Rivaroxaban
Edoxaban
|
Rivaroxaban | |
| rVIIa | Rivaroxaban
Edoxaban
|
Rivaroxaban | |
| Specific reversal agent | |||
| PRT064445 [11] | Rivaroxaban
|
Apixaban
|
|
Abbreviations: aPTT, activated partial thromboplastin time; aPCC, activated prothrombin complex concentrate; INR, international normalized ratio; PCC, prothrombin complex concentrate; PT, prothrombin time; rVIIa, recombinant activated factor VII; TEM, thromboelastometry; TG, thrombin generation.
Concluding remarks
Although TSOAC-associated bleeding occurs with similar or reduced frequency compared to VKAs for approved indications, effective TSOAC reversal agents are likely to improve the morbidity and mortality of bleeding events when they occur. Although specific reversal agents are currently undergoing clinical development and not yet available, nonspecific reversal therapies such as PCC, aPCC and rVIIa have variable efficacy and show conflicting results when used to reverse TSOAC anticoagulant effect in human volunteers, animal models and in vitro experiments. In addition, nonspecific therapies are known to increase the risk of thrombosis, which must be balanced against potential efficacy before administration for TSOAC-associated bleeding.
In the absence of high-quality studies that measure clinically relevant outcomes in anticoagulated patients with bleeding, provision of evidence-based recommendations regarding the use of nonspecific reversal therapies is problematic. Further studies that incorporate clinical and patient-important outcomes such as blood loss and mortality are needed to establish the efficacy and safety of putative reversal agents. In the interim, the mainstay of management of TSOAC-associated bleeding is supportive measures and urgent referral for definitive intervention [27].
Highlights.
No antidotes are currently available for reversal of the target-specific oral anticoagulants (TSOACs)
Nonspecific and specific agents have been put forward as potential reversal strategies
Evidence for the efficacy and safety of these strategies is of low quality
The evidence is derived from in vitro investigations, animal bleeding models and human volunteer studies
Studies of patients with TSOAC-associated bleeding that use clinically relevant outcomes are urgently needed
Footnotes
Conflicts of interest
A.C. has served as a consultant for Baxter, Bayer and Genzyme; has served on an advisory board for CSL Behring, Daiichi Sankyo and Genzyme; and has received research support from Diagnostica Stago. D.M.S. has no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dentali F, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381–2391. doi: 10.1161/CIRCULATIONAHA.112.115410. [DOI] [PubMed] [Google Scholar]
- 2.Fox BD, et al. Efficacy and safety of novel oral anticoagulants for treatment of acute venous thromboembolism: direct and adjusted indirect meta-analysis of randomised controlled trials. BMJ. 2012;345:e7498. doi: 10.1136/bmj.e7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann I, et al. Oral direct factor Xa inhibitors versus low-molecular-weight heparin to prevent venous thromboembolism in patients undergoing total hip or knee replacement: a systematic review and meta-analysis. Ann Intern Med. 2012;156:710–719. doi: 10.7326/0003-4819-156-10-201205150-00421. [DOI] [PubMed] [Google Scholar]
- 4.Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52 (Suppl 1):65–79. doi: 10.1111/j.1537-2995.2012.03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dentali F, et al. Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists. A meta-analysis. Thromb Haemost. 2011;106:429–438. doi: 10.1160/TH11-01-0052. [DOI] [PubMed] [Google Scholar]
- 6.Key NS, Negrier C. Coagulation factor concentrates: past, present, and future. Lancet. 2007;370:439–448. doi: 10.1016/S0140-6736(07)61199-4. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich HJ, et al. Safety of factor VIII inhibitor bypass activity (FEIBA): 10-year compilation of thrombotic adverse events. Haemophilia. 2002;8:83–90. doi: 10.1046/j.1365-2516.2002.00532.x. [DOI] [PubMed] [Google Scholar]
- 8.Aledort LM. Comparative thrombotic event incidence after infusion of recombinant factor VIIa versus factor VIII inhibitor bypass activity. J Thromb Haemost. 2004;2:1700–1708. doi: 10.1111/j.1538-7836.2004.00944.x. [DOI] [PubMed] [Google Scholar]
- 9.Levi M, et al. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–1800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 10.Schiele F, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554–3562. doi: 10.1182/blood-2012-11-468207. [DOI] [PubMed] [Google Scholar]
- 11.Lu G, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19:446–451. doi: 10.1038/nm.3102. [DOI] [PubMed] [Google Scholar]
- 12.Garcia D, et al. Laboratory assessment of the anticoagulant effects of the next generation of oral anticoagulants. J Thromb Haemost. 2013;11:245–252. doi: 10.1111/jth.12096. [DOI] [PubMed] [Google Scholar]
- 13.Eerenberg ES, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 14.Marlu R, et al. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217–224. doi: 10.1160/TH12-03-0179. [DOI] [PubMed] [Google Scholar]
- 15.Khoo TL, et al. The use of FEIBA(R) in the correction of coagulation abnormalities induced by dabigatran. Int J Lab Hematol. 2013;35:222–224. doi: 10.1111/ijlh.12005. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann R, et al. Thrombin generation using the calibrated automated thrombinoscope to assess reversibility of dabigatran and rivaroxaban. Thromb Haemost. 2014;111:989–995. doi: 10.1160/TH13-07-0607. [DOI] [PubMed] [Google Scholar]
- 17.Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis. 2012;23:138–143. doi: 10.1097/MBC.0b013e32834f1b0c. [DOI] [PubMed] [Google Scholar]
- 18.Lambourne MD, et al. Prothrombin complex concentrates reduce blood loss in mice rendered coagulopathic by warfarin but not dabigatran etexilate. Transfusion. 2012;52:56A–57A. doi: 10.1111/j.1538-7836.2012.04863.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke. 2011;42:3594–3599. doi: 10.1161/STROKEAHA.111.624650. [DOI] [PubMed] [Google Scholar]
- 20.Pragst I, et al. Reversal of dabigatran anticoagulation by prothrombin complex concentrate (Beriplex P/N) in a rabbit model. J Thromb Haemost. 2012;10:1841–1848. doi: 10.1111/j.1538-7836.2012.04859.x. [DOI] [PubMed] [Google Scholar]
- 21.Dinkelaar J, et al. In vitro assessment, using thrombin generation, of the applicability of prothrombin complex concentrate as an antidote for rivaroxaban. J Thromb Haemost. 2013;11:1111–1118. doi: 10.1111/jth.12236. [DOI] [PubMed] [Google Scholar]
- 22.Korber MK, et al. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost. 2013 doi: 10.1177/1076029613494468. [DOI] [PubMed] [Google Scholar]
- 23.Escolar G, et al. Reversal of apixaban induced alterations in hemostasis by different coagulation factor concentrates: significance of studies in vitro with circulating human blood. PLoS One. 2013;8:e78696. doi: 10.1371/journal.pone.0078696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda T, et al. Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thrombosis and Haemostasis. 2012;107:253–259. doi: 10.1160/TH11-09-0668. [DOI] [PubMed] [Google Scholar]
- 25.Godier A, et al. Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabbit model. Anesthesiology. 2012;116:94–102. doi: 10.1097/ALN.0b013e318238c036. [DOI] [PubMed] [Google Scholar]
- 26.Perzborn E, et al. Reversal of rivaroxaban anticoagulation by haemostatic agents in rats and primates. Thromb Haemost. 2013;110:162–172. doi: 10.1160/TH12-12-0907. [DOI] [PubMed] [Google Scholar]
- 27.Siegal DM, Cuker A. Reversal of novel oral anticoagulants in patients with major bleeding. J Thromb Thrombolysis. 2013;35:391–398. doi: 10.1007/s11239-013-0885-0. [DOI] [PubMed] [Google Scholar]
- 28.Stangier J, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292–303. doi: 10.1111/j.1365-2125.2007.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blech S, et al. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386–399. doi: 10.1124/dmd.107.019083. [DOI] [PubMed] [Google Scholar]
- 30.Kubitza D, et al. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59 -7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412–421. doi: 10.1016/j.clpt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Kubitza D, et al. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939--an oral, direct factor Xa inhibitor--after multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005;61:873–880. doi: 10.1007/s00228-005-0043-5. [DOI] [PubMed] [Google Scholar]
- 32.Weinz C, et al. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos. 2009;37:1056–1064. doi: 10.1124/dmd.108.025569. [DOI] [PubMed] [Google Scholar]
- 33.Raghavan N, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81. doi: 10.1124/dmd.108.023143. [DOI] [PubMed] [Google Scholar]
- 34.Frost C, et al. Apixaban, an oral, direct factor Xa inhibitor: single-dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75:476–487. doi: 10.1111/j.1365-2125.2012.04369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett YC, et al. A randomised assessment of the pharmacokinetic, pharmacodynamic and safety interaction between apixaban and enoxaparin in healthy subjects. Thromb Haemost. 2012;107:916–924. doi: 10.1160/TH11-09-0634. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson BI, et al. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokinet. 2009;48:1–22. doi: 10.2165/0003088-200948010-00001. [DOI] [PubMed] [Google Scholar]
- 37.Palladino M, et al. Evaluation of the oral direct factor Xa inhibitor - betrixaban. Expert Opin Investig Drugs. 22:1465–1472. doi: 10.1517/13543784.2013.825605. [DOI] [PubMed] [Google Scholar]