Abstract
People with incomplete spinal cord injury (SCI) frequently suffer motor disabilities due to spasticity and poor muscle control, even after conventional therapy. Abnormal spinal reflex activity often contributes to these problems. Operant conditioning of spinal reflexes, which can target plasticity to specific reflex pathways, can enhance recovery. In rats in which a right lateral column lesion had weakened right stance and produced an asymmetrical gait, up-conditioning of the right soleus H-reflex, which increased muscle spindle afferent excitation of soleus, strengthened right stance and eliminated the asymmetry. In people with hyperreflexia due to incomplete SCI, down-conditioning of the soleus H-reflex improved walking speed and symmetry. Furthermore, modulation of EMG activity during walking improved bilaterally, indicating that a protocol that targets plasticity to a specific pathway can trigger widespread plasticity that improves recovery far beyond that attributable to the change in the targeted pathway. These improvements were apparent to people in their daily lives. They reported walking faster and farther, and noted less spasticity and better balance. Operant conditioning protocols could be developed to modify other spinal reflexes or corticospinal connections; and could be combined with other therapies to enhance recovery in people with SCI or other neuromuscular disorders.
INTRODUCTION
People with incomplete spinal cord injury (SCI) often have substantial disabilities, even after completing conventional therapy. Common movement problems in individuals with chronic SCI, such as spasticity and weak voluntary muscle contraction, are thought to result from a combination of disrupted supraspinal connections, altered activity of spinal reflex pathways, and altered muscle properties (including disuse muscle atrophy and contracture) (Dietz and Sinkjaer 2007; Hultborn 2003). Thus, reducing abnormalities in one or more of these components may improve functional recovery. In this paper, we focus on spinal reflex abnormalities and on evidence that operant conditioning protocols that target spinal reflex pathways may enhance restoration of motor function after SCI. We first review the spinal reflex abnormalities that contribute to movement problems. We then introduce the operant conditioning methodology that can induce targeted plasticity in a selected reflex pathway and summarize current understanding of the substrates of conditioning-induced reflex change. Finally, we highlight the remarkably broad beneficial effects of changing a specific reflex pathway; and we explicate these effects in terms of a new concept of the role of the spinal cord in motor function.
SPINAL REFLEX ABNORMALITIES AND IMPAIRED LOCOMOTION AFTER INCOMPLETE SCI
Incomplete SCI disrupts supraspinal connections and often alters spinal reflex activity (Crone and others 2003; Stein and others 1993; Thompson and others 2009b; Yang and others 1991). Changes in spinal reflex pathways contribute to the most common and disabling locomotor problems, such as clonus, foot drop, and limited joint motion. For instance, ankle dorsiflexion during walking is often weakened after SCI due to the loss of normal corticospinal activation (Barthelemy and others 2010; Davey and others 1999). Exaggerated stretch reflexes in extensor muscles may counteract the remaining dorsiflexion and exacerbate foot drop (Knutsson 1981; Yang and others 1991). Furthermore, hyperactive stretch reflex pathways may limit joint motion (Hornby and others 2006) and may also lead to clonus (Corcos and others 1986; Hidler and Rymer 1999; Wallace and others 2012; Yang and others 1991).
Normally, spinal reflexes are appropriately modulated according to the current motor task. For instance, soleus H-reflex gain decreases greatly from standing to walking (see Figure 1A) to running (Capaday and Stein 1986; Capaday and Stein 1987; Stein and Capaday 1988), which prevents saturation of motor output and the reflex feedback loop (Capaday and Stein 1987). However, in people with SCI, Ia excitation is heightened (Knutsson and others 1973; Mailis and Ashby 1990) and task-dependent H-reflex modulation is greatly diminished or absent (Boorman and others 1996; Thompson and others 2009b). Furthermore, group Ib inhibition of the soleus by medial gastrocnemius nerve stimulation is impaired (Morita and others 2006), reciprocal and presynaptic inhibition are reduced (Ashby and Wiens 1989; Crone and others 2003; Knikou and Mummidisetty 2011; Morita and others 2001), and recurrent inhibition of the soleus is increased (Shefner and others 1992). Reflex abnormalities also exist in other muscles (Faist and others 1999). Alterations in motoneuron and interneuron properties also contribute to spastic reflex behaviors after SCI (Gorassini and others 2004; Heckman and others 2008; Hornby and others 2006; Hultborn 2003; Onushko and Schmit 2007). Altogether, these studies indicate that abnormal activity in spinal pathways contributes in multiple ways to motor impairments after SCI (Dietz and Sinkjaer 2007; Hultborn 2003; Stein and others 1993; Yang and others 1991).
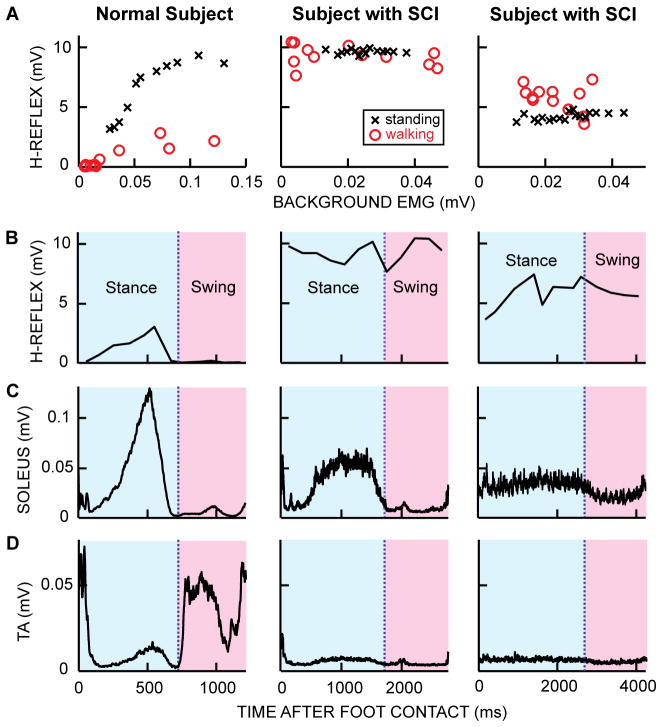
Figure 1.
Soleus H-reflex during standing and walking, soleus and tibialis anterior (TA) EMG activity and soleus H-reflex size over the step-cycle in a neurologically normal subject and in two subjects with chronic incomplete SCI. A: Soleus H-reflex sizes during standing and walking in each of 12 equally spaced step-cycle bins versus soleus EMG activity. Each standing H-reflex symbol (x) is the average of 4 responses and each walking H-reflex symbol (o) is the average of 5–10 responses. M-wave sizes are maintained the same within and between tasks. In the normal subjects, the H-reflex at a specific EMG level is clearly smaller during walking than during standing (Capaday and Stein 1986), whereas in subjects with chronic SCI, this task-dependent modulation of H-reflex size between standing and walking is impaired; and H-reflex size does not increase with background EMG level in standing or walking. B: Soleus H-reflex modulation during walking. In the normal subject, the pattern is similar to the soleus EMG pattern (C). In the subjects with SCI (middle and right), H-reflex modulation across the step-cycle is minimal, and hyperactivity of the H-reflex pathway probably contributes to the abnormally low TA activity during the swing phase (D) and to the resulting foot drop. C: Soleus EMG during walking. In the normal subject, soleus EMG activity gradually increases from heel contact to push off, then falls to near zero and remains low for the entire swing phase. In figure subjects with SCI, soleus EMG activity may (middle) or may not (right) be normally modulated. In the subject on the right column, the soleus remains active during the swing phase. D: TA EMG activity during walking. In the normal subject, TA activity typically shows two distinct peaks: one in the early swing phase and another during the swing-stance transition. In subjects with SCI (middle and right), TA activity is often minimal throughout the step-cycle.
During walking, soleus muscle activity is dynamically modulated; it increases from heel-contact to push-off, at which time the EMG level peaks, and then drops down to almost no activity and remains quiet for the entire swing phase (Figure 1C). Different reflex pathways are thought to contribute to different parts of the soleus EMG burst that occurs in the stance phase of the step cycle. During stance, the ankle undergoes a natural dorsiflexion that stretches the soleus muscle, and input from group Ia and II afferents contributes to the soleus burst (Mazzaro and others 2006; Mazzaro and others 2005; Sinkjaer and others 1996). In addition, input from group Ib afferents enhances the latter part of the burst (Grey and others 2007; Mazzaro and others 2006). The fact that these spinal pathways contribute to normal locomotor EMG activity helps to explain why the reflex abnormalities produced by SCI impair locomotion.
After SCI, excitation of motoneurons by muscle spindle afferents is enhanced and task-dependent H-reflex modulation is often impaired (Knutsson and others 1973; Mailis and Ashby 1990; Thompson and others 2009b). Figure 1 illustrates these abnormalities and the associated impairments in locomotor EMG activity. The normal increase in H-reflex size with increasing background EMG activity during standing is diminished; and the task-dependent modulation of reflex gain from standing to walking that normally prevents saturation of motor output and the reflex feedback loop is lost (Boorman and others 1996; Thompson and others 2009b)(Figure 1A: normal subject data vs. data from subjects with SCI). Reflex modulation over the step cycle may also be lost (Figure 1B), probably due to saturation of the reflex loop (Stein and others 1993; Yang and others 1991). Normally, the soleus stretch reflex (and H-reflex) pathways are active during the stance phase to assist force production, and they are suppressed during the swing phase to prevent foot drop (Capaday and Stein 1986; Schneider and others 2000; Sinkjaer and others 1999). Both overall increase in reflex size and lack of phase-dependent reflex modulation characterize the reflex abnormalities in people with SCI (Boorman and others 1996; Thompson and others 2009b; Yang and others 1991).
In subjects with SCI, the hyperactive reflex pathways and the loss of modulation over the step cycle are associated with abnormal patterns of soleus locomotor EMG activity, such as activity during the swing phase (e.g., Figure 1C, right panel). Furthermore, the unsuppressed soleus reflex activity from the swing to early stance may suppress tibialis anterior (i.e., ankle dorsiflexor) activation and thereby contribute to footdrop (e.g., Figure 1D). The hyperactive soleus stretch reflex pathways also contribute to the clonic activity in soleus that is commonly observed during the early stance phase of the step cycle (Yang and others 1991). These problems may be further exaggerated by abnormalities in other spinal pathways, including those of reciprocal inhibition (Ashby and Wiens 1989; Boorman and others 1996; Crone and others 2003; Knikou and Mummidisetty 2011; Thompson and others 2009b), presynaptic inhibition (Morita and others 2001), and/or cutaneous reflexes (Frigon and others 2009; Knikou 2007).
TREATING REFLEX ABNORMALITIES AFTER SCI
Because the spinal reflex abnormalities associated with incomplete SCI impair locomotion and other important functions, their amelioration continues to be a major therapeutic goal. Current therapies are mainly pharmacological and they seek to weaken muscles or reflexes (e.g., botulinum toxin or baclofen). They are nonspecific in that they affect muscle activity and/or reflex pathways in general and they may have undesirable side effects (Dario and others 2004; Dario and Tomei 2004; Sheean 2006; Ward 2008). Repetitive stimulation of peripheral nerve (i.e., transcutaneous electrical nerve stimulation or functional electrical stimulation) or cortex (repetitive transcranial magnetic stimulation) may be effective in reducing spasticity (Goulet and others 1996; Mori and others 2009; Thompson and others 2011). The responsible mechanisms and the extent of the therapeutic effects are not yet clear.
Novel therapies that can target specific reflex pathways and can strengthen or weaken them as appropriate could substantially improve functional recovery after SCI. Recent studies suggest that operant conditioning of spinal reflexes, which was first developed as a basic science model 30 years ago, can provide a specific, flexible, and clinically practical new approach to addressing the spinal reflex abnormalities associate with incomplete SCI. In rats in which a right lateral column lesion had weakened right stance and produced an asymmetrical gait (i.e., the rats limped), up-conditioning of the right soleus H-reflex, which increased the muscle spindle afferent contribution to the soleus stance burst, strengthened right stance and eliminated the asymmetry (Chen and others 2006d). And in people in whom an incomplete SCI had produced spasticity that impaired locomotion, operantly conditioned decrease in the soleus H-reflex reduced spasticity and improved locomotor speed and symmetry (Thompson and others 2013c). The next sections review spinal reflex conditioning and the complex plasticity that underlies it, describe its impact on locomotion after SCI, consider the mechanisms of its surprisingly broad beneficial effects, and discuss its future therapeutic possibilities.
OPERANT CONDITIONING OF SPINAL REFLEXES
The first reflex operant conditioning protocol was designed to change the spinal stretch reflex (SSR) in the monkey biceps muscle (Wolpaw and others 1983). This protocol had three key features: (1) it required maintaining a certain level of background (pre-stimulus) EMG in the target muscle; (2) it based reward on the size of the reflex as measured by EMG; and (3) the reward contingency (i.e., whether larger or smaller reflexes were rewarded) remained the same over days and weeks. The protocol sought to induce and maintain a long-term change in descending influence over the spinal reflex pathway, and thus to produce plasticity in the pathway ((Wolpaw 1997) for review). These three features have been preserved in all subsequent versions of the protocol, across different species (i.e., monkey, rat, human, mouse), muscles (i.e., biceps brachii, triceps surae, soleus), and reflexes (i.e., SSR, H-reflex, reciprocal inhibition) (Thompson and Wolpaw 2014a).
Spinal Reflex Conditioning in Rats
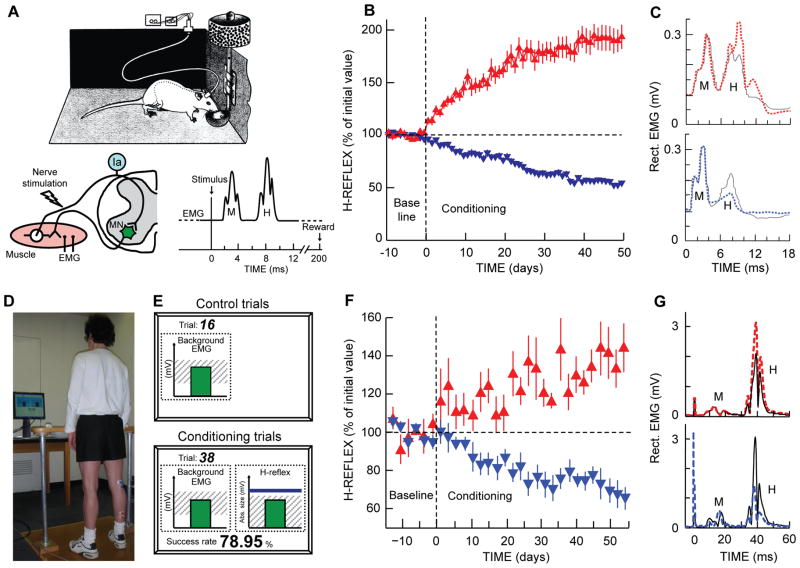
At present, the standard laboratory protocol is operant conditioning of the soleus H-reflex in the rat (Chen and Wolpaw 1995). This protocol is illustrated in Figure 2A–C. A Sprague-Dawley rat is chronically implanted with fine-wire EMG electrodes in the soleus muscle and a stimulating cuff on the posterior tibial nerve. The implanted wires connect through a head-mount, a flexible tether, and a commutator to an EMG amplifier and an electrical simulator. Soleus EMG is monitored continuously (24h/day) in the freely moving animal. Whenever the absolute value of soleus EMG remains within a specified range for a random varying 2.3–2.7 s period, a stimulus through the nerve cuff elicits the M-wave just above threshold and the H-reflex. In the course of its normal activity, the rat usually provides 2500–8000 H-reflex trials per day. For the first 10 days, the rat is exposed to the control mode, in which no reward occurs and the H-reflex is simply measured to determine its baseline (i.e., control) value. For the next 50 days, the rat is exposed to the up-conditioning (HRup) or down-conditioning (HRdown) mode, in which a food reward occurs if the H-reflex is above (HRup) or below (HRdown) a criterion value. Background EMG level and M-wave size remain constant throughout. As shown in Figure 2B and C, chronic exposure to the up- or down-conditioning mode gradually changes the size of the reflex in the correct direction. Successful conditioning (i.e., >20% change in the correct direction (Chen and Wolpaw 1995; Wolpaw and others 1993)) occurs in 75–80% of the animals. The other 20–25% of the rats, in which the H-reflex remains within 20% of its initial value, constitute a useful control population for mechanistic studies.
Figure 2.
H-reflex operant conditioning in rats (A–C) and humans (D–G). A: The soleus H-reflex is elicited in a rat with chronically implanted EMG electrodes and a tibial nerve cuff. Whenever the absolute value of soleus EMG activity stays in a specified range for a 2.3- to 2.7-s period, tibial nerve stimulation through the cuff elicits an M-wave just above threshold and an H-reflex. For the first 10 days (from day −10 to day 0), the rat is exposed to the control mode, in which no reward occurs and the H-reflex is simply measured to determine its initial size. For the next 50 days, the rat is exposed to the up-conditioning (HRup) or down-conditioning (HRdown) mode, in which a food-pellet reward occurs 200 ms after the stimulation whenever the H-reflex is above (HRup) or below (HRdown) a criterion value. B: Average (±SEM) daily H-reflex sizes for 59 successful HRup rats (red upward triangles) and 81 successful HRdown rats (blue downward triangles). C: Average absolute value of post-stimulus EMG activity for representative days from an HRup rat (top) and an HRdown rat (bottom) in the control mode (solid) and near the end of HRup or HRdown conditioning (dashed). (Updated from (Wolpaw 1997). D–G: The subject maintains a natural standing posture and a correct level of soleus EMG activity with the aid of a visual feedback screen (D and E) that shows the current absolute value of soleus EMG activity in relation to a specified range. Whenever the absolute value of soleus EMG activity stays in this range for several sec, tibial nerve stimulation elicits an M-wave just above threshold and an H-reflex. For the first 6 sessions (i.e., baseline sessions, day −14 to day 0), the subject is exposed to the control mode, in which the H-reflex is simply measured to determine its initial size. For the next 24 sessions (i.e., conditioning sessions, days 0–56, 3 sessions/week), the subject is exposed to the HRup or HRdown conditioning mode, in which, after each conditioning trial, the screen provides immediate feedback as to whether the H-reflex was above (HRup) or below (HRdown (shown on screen)) a criterion value (E). The person completes 225 conditioning trials per session. F: Average (±SEM) daily H-reflex sizes for 6 successful HRup people (red upward triangles) and 8 successful HRdown people (blue downward triangles). G: Average peri-stimulus EMG activity from an HRup subject (top) and an HRdown subject (bottom) for a baseline session (i.e., control mode) (solid) and for the last HRup or HRdown conditioning session (dashed). (From (Thompson and others 2009a).)
Spinal Reflex Conditioning in Humans
Figure 2D–G shows the soleus H-reflex conditioning protocol for humans (Thompson and others 2009a). It has the three key features described above. At the same time, it differs from the animal protocols in several ways: (1) conditioning occurs in discrete 1-hr sessions of 225 conditioning trials each at a rate of 3/week over 8–10 weeks (thus people complete only 3–5% as many trials as the rats that are continuously exposed to conditioning over 50 days); (2) the EMG recording and nerve stimulating electrodes are superficial rather than implanted; (3) the reward is visual feedback rather than a food pellet; and (4) each session begins with 20 control trials in which the subject is not asked to change the reflex and receives no feedback as to reflex size. The standard format comprises 6 baseline and then 24 (or 30) conditioning sessions at a rate of three per week. In each trial, the soleus H-reflex is elicited while the subject maintains a natural standing posture and a pre-determined level of soleus background EMG (Figure 2D). M-wave size is kept constant within and across the sessions. In each baseline session, three blocks of 75 control H-reflexes each (i.e., 225 total) are elicited (Figure 2E). In each conditioning or follow-up session, 20 control H-reflexes are elicited as in the baseline sessions and then three blocks of 75 conditioned H-reflexes (i.e., 225 total) are elicited. In these conditioned H-reflex trials, the subject is asked to increase (up-conditioning (HRup)) or decrease (down-conditioning (HRdown)) the H-reflex and is given immediate visual feedback as to whether the H-reflex was larger (HRup) or smaller (HRdown) than a criterion value. Satisfying the criterion on more than a specific percent of the trials earns an additional monetary reward. Background EMG and M-wave size are kept stable throughout data collection.
In the first H-reflex conditioning study in normal humans (Thompson and others 2009a), H-reflex size gradually increased to 140% of baseline in 6 of 8 HRup subjects and decreased to 69% of baseline in 8 of 9 HRdown subjects (Figure 2F and 3B). In these subjects, the conditioned H-reflex change was the sum of within-session change (i.e., task-dependent adaptation, measured as the within-session difference in size between the control and conditioned H-reflexes) and across-session change (i.e., long-term change, measured as the across-session change in control H-reflex size) (Figure 3B). Task-dependent adaptation appeared within 4–6 sessions and then persisted unchanged, while long-term change began after 10–12 sessions and increased gradually thereafter. (See (Thompson and others 2009a) for full discussion of task-dependent adaptation and long-term change.) This study showed that people performing only 3–5% as many trials as the rats can change reflex size nearly as much. The success rate of 82% (14 of 17 subjects changed H-reflex size significantly in the correct direction) was also similar to that of animals (Thompson and others 2009a; Wolpaw 1997). In addition, this study showed that exposure to the reflex operant conditioning paradigm over several months induced both short-term adaptation and long-term plasticity in spinal reflex pathways. This long-term plasticity, a lasting change in the reflex pathway that persists outside of the conditioning paradigm, suggests that reflex conditioning could be of use therapeutically (Thompson and others 2009a; Thompson and others 2013a). Can reflex conditioning protocols induce and guide such long-term plasticity so as to reeducate abnormally functioning spinal reflex pathways and thereby alleviate motor disabilities associated with incomplete spinal cord injuries?
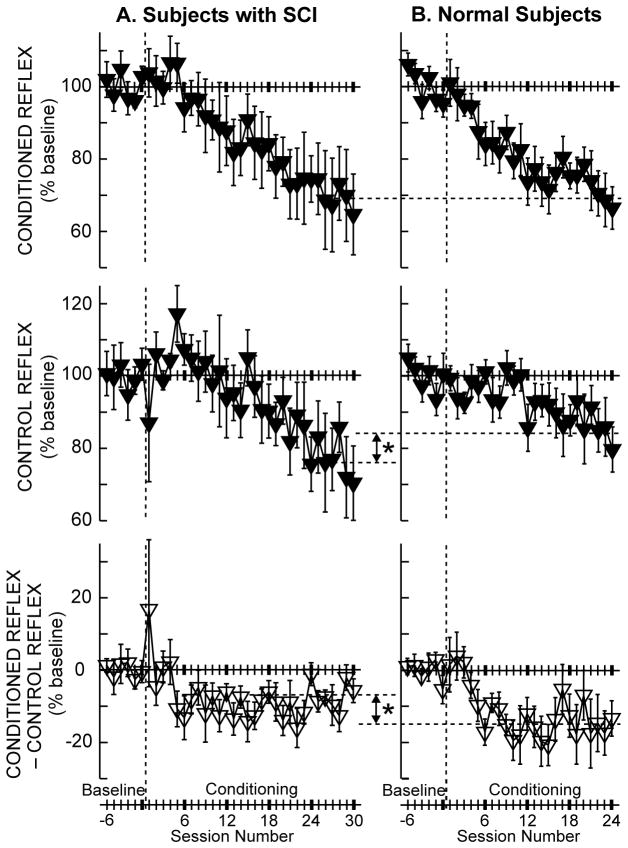
Figure 3.
Average (±SE) H-reflexes for baseline and conditioning sessions for down-conditioning subjects with SCI (A, N=6, (Thompson and others 2013c)) and for normal subjects (B, N=8, (Thompson and others 2009a)) in whom the H-reflex decreased significantly. Top: Average conditioned H-reflex size. Middle: Average control H-reflex size (i.e., long-term plasticity (see (Thompson and others 2009a) for details). Bottom: Average of conditioned H-reflex size minus control H-reflex size (i.e., task-dependent adaptation (see (Thompson and others 2009a) for details)). In the subjects with SCI (A), the conditioned H-reflex decreases to 69% of the baseline value over the 30 conditioning sessions. This decrease consists of a relatively small task-dependent adaptation (−7%) and a relatively large across-session control reflex decrease (−24%). In neurologically normal subjects (B), the conditioned H-reflex also decreases to 69% of the baseline value over 24 Conditioning sessions. This decrease is the sum of a relatively large task-dependent adaptation (−15%) and a relatively small across-session control reflex decrease (−16%). The asterisks between A and B indicate significant differences (p<0.01) between the groups in final control H-reflex value (middle) and in task-dependent adaptation (bottom).
The Plasticity Associated with H-Reflex Conditioning
Studies in monkeys and rats show that H-reflex conditioning is associated with plasticity at multiple sites in the spinal cord and brain ((Thompson and Wolpaw 2014b; Wolpaw 2010) for review). Figure 7 summarizes current knowledge of this plasticity. In the spinal cord, successful conditioning is accompanied by changes in motoneuron properties (e.g., firing threshold and axonal conduction velocity), in GABAergic terminals and several other terminal populations on the motoneuron, and in spinal interneurons. Plasticity occurs in the brain as well, most probably in sensorimotor cortex and cerebellum. H-reflex conditioning requires the corticospinal tract (CST); other major descending and ascending pathways are not essential. The picture that is emerging is of a hierarchy of plasticity in which the reward contingency produces plasticity in the brain that guides and maintains the plasticity in the spinal cord that is directly responsible for the conditioned H-reflex change (Thompson and Wolpaw 2014b; Wolpaw 2010).
Figure 7.
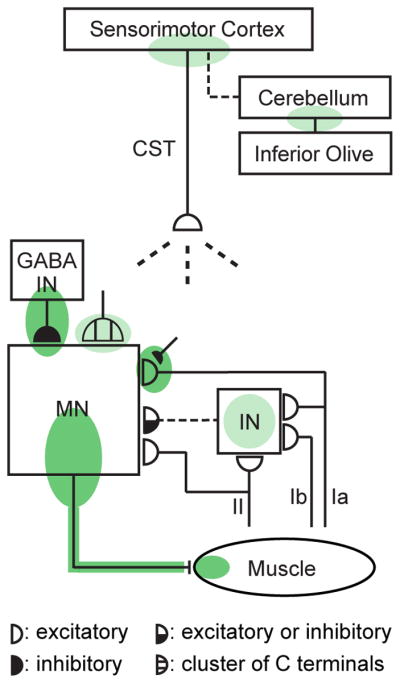
Spinal and supraspinal plasticity underlies H-reflex conditioning. The shaded ovals indicate the sites of plasticity associated with operant conditioning of the H-reflex. “MN” is the motoneuron, “CST” is the main corticospinal tract, “IN” is a spinal interneuron, and “GABA IN” is a GABAergic spinal interneuron. Dashed pathways imply the possibility of intervening spinal interneurons. The monosynaptic and probably oligosynaptic H-reflex pathway from groups Ia, II, and Ib afferents to the motoneuron is shown. Definite (dark green shade) or probable (light green shade) sites of plasticity include: the motoneuron membrane (i.e., firing threshold and axonal conduction velocity); motor unit properties; GABAergic interneurons; GABAergic terminals and C terminals on the motoneuron; the Ia afferent synaptic connection; terminals conveying oligosynaptic groups I and II inhibition or excitation to the motoneuron; sensorimotor cortex; and cerebellum. The latest data suggest that the reward contingency acts through the inferior olive to guide and maintain plasticity in the cerebellum that guides and maintains plasticity in sensorimotor cortex that (via the CST) guides and maintains plasticity in the spinal cord that is directly responsible for the H-reflex change. (From (Thompson and Wolpaw 2014b).)
OPERANT CONDITIONING OF THE H-REFLEX AFTER SCI
Spinal Reflex Conditioning in Rats with SCI
The hypothesis that appropriate operant conditioning of a spinal reflex can ameliorate the motor disability after incomplete SCI was first tested in rats in which a right lateral column lesion had weakened right stance and produced an asymmetrical gait (Chen and others 2006d). The right soleus H-reflex was up-conditioned in order to increase the muscle spindle afferent contribution to the soleus stance burst (Bennett and others 1996; Stein and others 2000). The results were clear and impressive. In successfully up-conditioned rats the H-reflex increased to 212% of its initial value (comparable to the increase in normal rats (Chen and others 2005)), the stance-phase H-reflex increased to 280%, and the right soleus stance burst amplitude increased to 158%. As a result, right stance was strengthened and the right/left asymmetry was eliminated; locomotion was improved significantly in these rats with partial SCI (Chen and others 2006d). Locomotion did not improve in rats in which the H-reflex was simply measured but not conditioned (Chen and others 2006d).
Spinal Reflex Conditioning in Humans with SCI
This evidence for therapeutic efficacy in rats (Chen and others 2006d), together with the successful development of the human H-reflex conditioning protocol (Thompson and others 2009a) and the evidence that it induced long-term plasticity in the human H-reflex pathway (Thompson and others 2009a; Thompson and others 2013a), encouraged an effort to determine whether H-reflex conditioning could improve locomotion in people. We studied individuals in whom an incomplete spinal cord injury had produced spasticity that impaired locomotion. Given that each person displayed a hyperactive soleus H-reflex, we evaluated the therapeutic efficacy of down-conditioning the soleus H-reflex (Thompson and others 2013c).
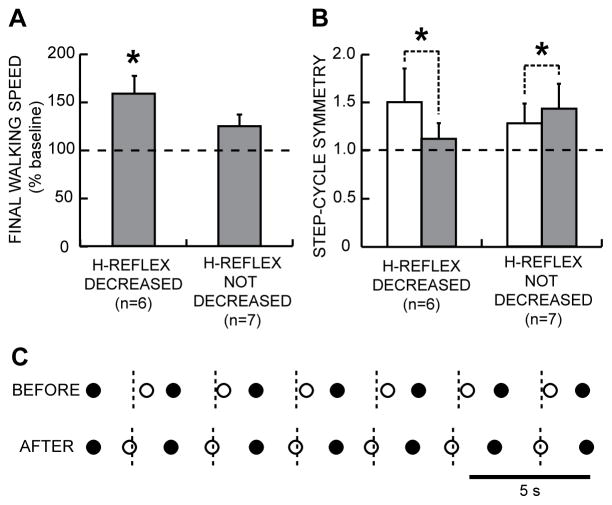
Over 30 down-conditioning sessions, the soleus H-reflex decreased in two-thirds of the subjects and remained smaller several months later. In the subjects in whom H-reflex decreased significantly, locomotion improved; walking speed increased and right-left symmetry improved (Figure 4), with better EMG modulation across the step-cycle in the muscles of both legs. In these subjects, the H-reflex decrease was also evident during locomotion (i.e., 59% of initial value), which helped to explain the improved gait (e.g., Figure 5). Furthermore, beginning about 5 weeks into the conditioning sessions, these subjects commented spontaneously that they were walking faster and farther in their daily lives, and several noted less clonus, easier stepping, less arm weight-bearing, and/or other improvements. Locomotion did not improve in the subjects in whom H-reflex down-conditioning was not successful or in subjects in whom the H-reflex was simply measured over 30 sessions without conditioning. These first results in people with SCI, together with the rat study, suggest that operant conditioning of spinal reflexes can improve gait recovery after chronic incomplete SCI, and possibly in other disorders as well (e.g., (Chen and others 2010)).
Figure 4.
A: 10-m walking speeds after the 30 conditioning or control sessions (mean±SE % of baseline speed) for subjects with SCI in whom the H-reflex did or did not decrease significantly. B: Step-cycle symmetry before (open bars) and after (shaded bars) after the 30 conditioning or control sessions for subjects with SCI in whom the H-reflex did or did not decrease significantly. Symmetry is measured as the ratio of the time between the nonconditioned leg’s foot contact (nFC) and the conditioned (or simply stimulated in the case of control subjects) and initially more impaired leg’s foot contact (cFC), to the time between cFC and nFC. A ratio of 1 indicates a symmetrical gait. Initially, the ratio is >1. After the 30 conditioning or control sessions, the ratio has decreased toward 1 in the subjects in whom the H-reflex decreased, while it has increased slightly in the subjects in whom the H-reflex did not decrease. C: Successive step cycles before and after conditioning from a subject in whom the H-reflex decreased. Each nFC (●) and cFC (○) is shown. Short vertical dashed lines mark the midpoints between nFCs (i.e., midpoints of the step-cycle), which is when cFC should occur. Before H-reflex conditioning, cFC is too late; after successful conditioning, it is on time.
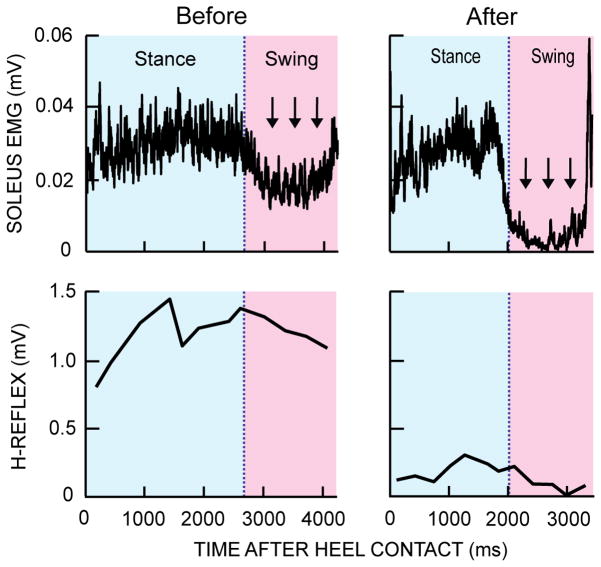
Figure 5.
Rectified soleus EMG activity and locomotor H-reflex size over the step cycle before and after successful H-reflex down-conditioning in a subject with SCI. Successful conditioning results in better EMG and H-reflex modulation. The abnormal tonic EMG activity during the swing phase almost completely disappears after conditioning. The locomotor H-reflex becomes smaller and better modulated after conditioning (i.e., it is lowest during the swing phase). Note also that the step cycle is shorter after conditioning, which translated to an increase in walking speed.
Current understanding of the spinal cord plasticity associated with H-reflex down-conditioning provides some insight into the mechanisms that may underlie the locomotor improvement in people with SCI. In these individuals with spasticity due to SCI, motoneuron excitation from muscle spindle afferents is exaggerated (Knutsson and others 1973; Mailis and Ashby 1990), motoneuron and interneuron properties are altered (Gorassini and others 2004; Heckman and others 2008; Hornby and others 2006; Hultborn 2003; Onushko and Schmit 2007), and inhibitory interneuron activity is abnormal (Ashby and Wiens 1989; Boorman and others 1996; Crone and others 2003; Knikou and Mummidisetty 2011; Morita and others 2001; Thompson and others 2009b). H-reflex down-conditioning raises motoneuron firing threshold, slightly decreases the primary afferent EPSP, and markedly increases the number of identifiable GABAergic terminals on the motoneuron and the number of identifiable GABAergic interneurons in the ventral horn (reviewed in (Wolpaw 2010)). By counteracting the abnormalities associated with SCI, these effects may underlie the locomotor improvement produced by H-reflex down-conditioning.
Spinal Reflex Conditioning in Humans with SCI Differs from That in Normal Humans
In data to date, the probability of successful conditioning and the magnitude of reflex change are comparable in people with or without incomplete SCI (Chen and others 2006d; Chen and others 2005; Thompson and others 2009a; Thompson and others 2013c). However, as Figure 3 shows, these two populations differ markedly in the proportions of task-dependent adaptation and long-term change in the final conditioned H-reflex (Thompson and others 2013c). Task-dependent adaptation (i.e., within-session change), which is thought to reflect immediate change in cortical influence (e.g., on presynaptic inhibition), is significantly greater in neurologically normal subjects than in subjects with SCI (−15% vs. −7%; Figure 3, bottom). This difference may be due to SCI-related reduction in cortical influence due to damage to the corticospinal tract (CST), the supraspinal connection principally responsible for H-reflex conditioning (reviewed in (Thompson and Wolpaw 2014b; Wolpaw 2010)). Such damage may also account for the slightly slower course of H-reflex decrease (i.e., over 30 sessions versus 24 sessions in normal subjects). In contrast, long-term H-reflex change (i.e., across-session change), which is thought to reflect spinal cord plasticity, is significantly greater in subjects with SCI than in normal subjects (−24% vs. −16%; Figure 3, middle)(Thompson and others 2013c).
This difference between people with and without SCI in the magnitude of long-term plasticity is reflected in the difference between them in the locomotor effects of H-reflex conditioning. As described above, appropriate H-reflex conditioning markedly improved locomotion in people with SCI (Thompson and others 2013c); while H-reflex conditioning had no detectable effect on locomotion in people without SCI (Makihara and others 2013). The next section considers the etiology of the greater long-term H-reflex change in people with SCI, and its encouraging implications for the therapeutic use of reflex conditioning protocols.
The Impact of H-Reflex Conditioning on Other Behaviors
The spinal cord is a multi-user system; it is the final common pathway for all the behaviors in an individual’s repertoire. When a behavior uses the spinal cord, it adjusts spinal reflex pathways to serve its needs. For example, by producing descending influence that modifies presynaptic inhibition at primary afferent terminals on motoneurons, the brain controls the amount of motoneuron excitation produced by the pathway that produces the H-reflex. Figure 6A shows the adjustments associated with a variety of different behaviors, ranging from sitting to standing to walking to running, and in some people, to specific athletic skills, such as playing soccer and ballet dancing (Nielsen and others 1993). These behaviors require different activity levels (or gains) in proprioceptive pathways such as that responsible for the soleus H-reflex. For example, H-reflex gain is decreased from sitting to standing (Kawashima and others 2003) and from standing to walking (Capaday and Stein 1986; Stein and Capaday 1988). Such task-dependent adaptation of reflex pathways is important in ensuring satisfactory production of each behavior.
Figure 6.
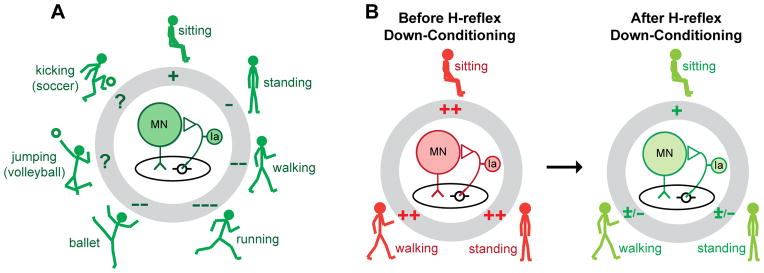
A: In a neurologically normal person, the spinal reflex pathway (center) responsible for the soleus H-reflex participates in many motor behaviors, ranging from standing to walking to running to athletic skills such as ballet, volleyball, and soccer. Each behavior is accompanied by task-dependent adaptation in the gain of the reflex pathway (pluses or minuses in the gray circle), which ensures that input from muscle spindle afferents contributes appropriately to soleus muscle activation during the behavior (Stein and Capaday 1988). B: In a person with spasticity due to SCI, task-dependent adaptation is impaired, and the pathway is hyperactive during sitting, standing, and walking. Down-conditioning of the soleus H-reflex reduces the gain of the reflex pathway for all three tasks, and thereby improves standing and walking.
Each task-dependent adaptation affects only its specific behavior; it does not affect other behaviors. For example, after a person acquires the new behavior of a larger or smaller H-reflex through operant conditioning, task-dependent increase or decrease affects only the H-reflexes elicited in the context of the conditioning protocol. However, over the past several decades it has become clear that the impact of individual behaviors on the spinal cord is not limited to task-dependent adaptations. The acquisition of a new behavior can change the spinal cord itself, it can produce long-term plasticity that affects all the behaviors that use the spinal cord (Thompson and Wolpaw 2014b; Wolpaw 2010; Zehr 2006). That is, it can change the background or default state of the spinal cord on which individual behaviors impose their task-dependent changes. For example, when H-reflex conditioning produces long-term plasticity, it changes the central element in Figure 6A, the baseline strength of the H-reflex pathway. It can thereby affect previously acquired behaviors, which must now use a spinal cord that may not respond to descending commands and sensory inputs in exactly the same way that it did prior to H-reflex conditioning. The functional consequences of this impact on other behaviors differ substantially between people with incomplete SCI and people who are neurologically normal. This difference is likely to account for the fact that long-term plasticity is substantially greater in people with SCI than in neurologically normal people.
In normal people, the long-term plasticity produced by H-reflex conditioning may disturb older behaviors and trigger additional compensatory plasticity that preserves their key features. Indeed, in normal rats, up- or down-conditioning of the soleus H-reflex is associated with other changes that prevent the change in the H-reflex pathway from impairing locomotor symmetry (Chen and others 2011). These compensatory changes appear to be initiated and guided by the brain (Chen and others 2006a; Chen and others 2006c). In contrast, in rats or people with SCI, the long-term plasticity produced by appropriate H-reflex conditioning (up-conditioning in rats with weak stance, down-conditioning in people with spasticity) improves locomotion. Thus, in normal individuals, conditioned change in the H-reflex would ideally consist largely of task-dependent adaptation, with very little long-term plasticity to disturb other behaviors; while in people with SCI, conditioned change in the H-reflex would ideally consist largely of long-term plasticity that improves locomotion. As Figure 6B indicates, this appears to be what occurs. How it occurs may be best understood in terms of the recently proposed negotiated equilibrium hypothesis (Wolpaw 2010).
According to this hypothesis, spinal neurons and pathways are continually maintained in a state of “negotiated equilibrium,” a balance that ensures the satisfactory performance of all the behaviors in an individual’s repertoire (Wolpaw 2010). This balance is the result of an ongoing interaction, or negotiation, among the behaviors. Each behavior continually induces plasticity that tends to preserve its key features (e.g., locomotor symmetry) despite the plasticity induced by other behaviors. When the acquisition of a new behavior, such as a larger or smaller H-reflex, changes the spinal cord, it normally triggers the generation of a new equilibrium that accommodates the new behavior as well as all the old behaviors. Thus, in normal individuals, the long-term spinal cord plasticity produced by H-reflex down-conditioning triggers the negotiation of a new equilibrium that produces a smaller H-reflex and still continues to serve other behaviors satisfactorily. In this negotiation, behaviors such as locomotion that are disturbed by the conditioning-induced long-term plasticity in the H-reflex pathway are likely to oppose this plasticity and limit its magnitude (Thompson and others 2013c). As a result, much of the final change in the conditioned H-reflex consists of task-dependent adaptation, which does not disturb other behaviors.
In contrast, for people with spasticity due to SCI, the long-term spinal cord plasticity produced by H-reflex down-conditioning improves locomotion (Thompson and others 2013c). Similarly, in rats in which SCI has weakened stance, the long-term plasticity produced by H-reflex up-conditioning restores step-cycle symmetry (Chen and others 2006d). In both humans and rats with SCI, the long-term plasticity underlying H-reflex change is doubly adaptive: it increases reward probability in the conditioning protocol, and it also improves locomotion. It triggers the creation of a new spinal cord equilibrium superior to the one that prevailed before H-reflex conditioning. Thus, it is probable that long-term H-reflex change is greater in people with SCI than in normal people because it serves more than just a new behavior; it also benefits locomotion. This interpretation is further supported by a recent study that compared the locomotor impact of H-reflex up-conditioning to that of down-conditioning in rats with weak stance due to SCI (Chen and others 2014).
OPERANT CONDITIONING AS A NEW THERAPEUTIC INTERVENTION
Investigation of operant conditioning as a new approach to rehabilitation has just begun. In addition to the fact that it is non-invasive and non-pharmacological, the key features of this method are that it is both specific and flexible. By basing reward on the output of a specific CNS pathway (e.g., the soleus H-reflex pathway), it can target plasticity to that pathway; and by selecting the reward contingency, it can strengthen or weaken that pathway as needed. An appropriate operant conditioning protocol could produce targeted plasticity that addresses the specific motor deficits of each person.
Conditioning protocols might target other proprioceptive or cutaneous reflex pathways (e.g., reciprocal inhibition (Chen and others 2006b). Protocols might also target corticospinal connections. The motor evoked potential (MEP) to transcranial magnetic stimulation (TMS) is often reduced after SCI (Barthelemy and others 2010; Davey and others 1999). Initial investigations suggest that the tibialis anterior MEP can be up-conditioned in people with SCI, and that this can improve ankle joint movement during locomotion (Manella and others 2013).
It should also be possible to design conditioning protocols that complement existing therapeutic methods, such as partial body-weight supported treadmill training (Barbeau 2003; Edgerton and others 2008; Harkema and others 2012; Wernig and Muller 1992) and constraint-induced movement therapy (Taub and others 1999; Wolf and others 2006), in order to maximize functional recovery. For example, H-reflex down-conditioning might be performed during the swing phase of the step cycle, when the H-reflex is very small or absent in normal subjects but abnormally large in people with spastic hyperreflexia due to chronic SCI (Stein and others 1993; Yang and others 1991) (e.g., Fig. 1). By targeting the exact time when reflex activity is abnormal, such a protocol might help restore appropriate phase-dependent reflex modulation during walking. Initial results are promising (Thompson and others 2013b). Conditioning protocols are likely to be particularly useful once significant regeneration becomes possible, and new methods are needed to shape the regenerated spinal cord to provide useful function. Finally, conditioning protocols might improve treatment of other peripheral or central neuromuscular disorders (e.g., (Chen and others 2010)).
While an operant conditioning protocol can target plasticity to a specific pathway, the beneficial impact of appropriate conditioning appears to extend far beyond the effects that can be directly attributed to that plasticity. In people with SCI, unilateral soleus H-reflex down-conditioning improved the locomotor behaviors of knee and ankle extensor and flexor muscles in both legs, which presumably contributed to the improvement in walking speed and symmetry (Thompson and others 2013c). The plasticity underlying a smaller soleus H-reflex in one leg cannot itself account for such broad beneficial impact. Together with animal data (Chen and others 2011; Chen and others 2006d), this impact implies that H-reflex conditioning triggered additional plasticity in other pathways important for locomotion, and thereby improved the entire behavior. It appears that the acquisition of a down-conditioned soleus H-reflex, by disturbing the post-injury equilibrium that the injured spinal cord had reached, triggered widespread adaptive plasticity that produced a new equilibrium that both decreased the H-reflex and improved locomotion. If comparable processes are triggered by other appropriately selected operant conditioning protocols, the therapeutic value of these protocols may be greatly enhanced.
CONCLUSIONS
In people with incomplete SCI, abnormal spinal reflex activity often contributes to chronic motor disabilities. Current therapies are seldom fully effective. Operant conditioning protocols, which can target plasticity to specific reflex pathways, might enhance functional recovery. Indeed, initial studies in animals and humans with incomplete SCI indicate that appropriate operant conditioning of the soleus H-reflex can improve walking. Furthermore, the surprisingly broad beneficial effects imply that a conditioning protocol can trigger widespread plasticity that improves recovery far beyond that attributable to the change in the targeted pathway. Conditioning protocols could be developed to modify other spinal reflexes or corticospinal connections; and might be combined with other rehabilitation methods to enhance recovery in people with SCI or other neurological disorders.
Acknowledgments
This work was supported in part by the New York State Spinal Cord Injury Research Trust [C023685 to AKT]; the National Institutes of Health [NS069551 to AKT, NS22189 to JRW, and NS061823 to JRW and Xiang Yang Chen]; and the Helen Hayes Hospital Foundation [to AKT].
References
- Ashby P, Wiens M. Reciprocal inhibition following lesions of the spinal cord in man. J Physiol. 1989;414:145–57. doi: 10.1113/jphysiol.1989.sp017681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil Neural Repair. 2003;17(1):3–11. doi: 10.1177/0888439002250442. [DOI] [PubMed] [Google Scholar]
- Barthelemy D, Willerslev-Olsen M, Lundell H, Conway BA, Knudsen H, Biering-Sorensen F, et al. Impaired transmission in the corticospinal tract and gait disability in spinal cord injured persons. J Neurophysiol. 2010;104(2):1167–76. doi: 10.1152/jn.00382.2010. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, De Serres SJ, Stein RB. Gain of the triceps surae stretch reflex in decerebrate and spinal cats during postural and locomotor activities. J Physiol. 1996;496 (Pt 3):837–50. doi: 10.1113/jphysiol.1996.sp021731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman GI, Lee RG, Becker WJ, Windhorst UR. Impaired “natural reciprocal inhibition” in patients with spasticity due to incomplete spinal cord injury. Electroencephalogr Clin Neurophysiol. 1996;101(2):84–92. doi: 10.1016/0924-980x(95)00262-j. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. Journal of Neuroscience. 1986;6(5):1308–13. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Difference in the amplitude of the human soleus H reflex during walking and running. Journal of Physiology. 1987;392:513–22. doi: 10.1113/jphysiol.1987.sp016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Carp JS, Chen L, Wolpaw JR. Sensorimotor cortex ablation prevents H-reflex up-conditioning and causes a paradoxical response to down-conditioning in rats. J Neurophysiol. 2006a;96(1):119–27. doi: 10.1152/jn.01271.2005. [DOI] [PubMed] [Google Scholar]
- Chen XY, Chen L, Chen Y, Wolpaw JR. Operant conditioning of reciprocal inhibition in rat soleus muscle. J Neurophysiol. 2006b;96(4):2144–50. doi: 10.1152/jn.00253.2006. [DOI] [PubMed] [Google Scholar]
- Chen XY, Chen Y, Chen L, Tennissen AM, Wolpaw JR. Corticospinal tract transection permanently abolishes H-reflex down-conditioning in rats. J Neurotrauma. 2006c;23(11):1705–12. doi: 10.1089/neu.2006.23.1705. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol. 1995;73(1):411–5. doi: 10.1152/jn.1995.73.1.411. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen L, Liu RL, Wang Y, Chen XY, Wolpaw JR. Locomotor impact of beneficial or non-beneficial H-reflex conditioning after spinal cord injury. J Neurophysiol. 2014 doi: 10.1152/jn.00756.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen L, Wang Y, Wolpaw JR, Chen XY. Operant conditioning of rat soleus H-reflex oppositely affects another H-reflex and changes locomotor kinematics. J Neurosci. 2011;31(31):11370–5. doi: 10.1523/JNEUROSCI.1526-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci. 2006d;26(48):12537–43. doi: 10.1523/JNEUROSCI.2198-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Schalk G, Stokes BT, Wolpaw JR. The interaction of a new motor skill and an old one: H-reflex conditioning and locomotion in rats. J Neurosci. 2005;25(29):6898–906. doi: 10.1523/JNEUROSCI.1684-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang Y, Chen L, Sun C, English AW, Wolpaw JR, et al. H-reflex up-conditioning encourages recovery of EMG activity and H-reflexes after sciatic nerve transection and repair in rats. J Neurosci. 2010;30(48):16128–36. doi: 10.1523/JNEUROSCI.4578-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcos DM, Gottlieb GL, Penn RD, Myklebust B, Agarwal GC. Movement deficits caused by hyperexcitable stretch reflexes in spastic humans. Brain. 1986;109:1043–58. doi: 10.1093/brain/109.5.1043. [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- Dario A, Scamoni C, Picano M, Casagrande F, Tomei G. Pharmacological complications of the chronic baclofen infusion in the severe spinal spasticity. Personal experience and review of the literature. J Neurosurg Sci. 2004;48(4):177–81. [PubMed] [Google Scholar]
- Dario A, Tomei G. A benefit-risk assessment of baclofen in severe spinal spasticity. Drug Saf. 2004;27(11):799–818. doi: 10.2165/00002018-200427110-00004. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Smith HC, Savic G, Maskill DW, Ellaway PH, Frankel HL. Comparison of input-output patterns in the corticospinal system of normal subjects and incomplete spinal cord injured patients. Exp Brain Res. 1999;127(4):382–90. doi: 10.1007/s002210050806. [DOI] [PubMed] [Google Scholar]
- Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007;6(8):725–33. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, et al. Training locomotor networks. Brain Res Rev. 2008;57(1):241–54. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faist M, Ertel M, Berger W, Dietz V. Impaired modulation of quadriceps tendon jerk reflex during spastic gait: differences between spinal and cerebral lesions. Brain. 1999;122:567–79. doi: 10.1093/brain/122.3.567. [DOI] [PubMed] [Google Scholar]
- Frigon A, Barriere G, Leblond H, Rossignol S. Asymmetric changes in cutaneous reflexes after a partial spinal lesion and retention following spinalization during locomotion in the cat. J Neurophysiol. 2009;102(5):2667–80. doi: 10.1152/jn.00572.2009. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127(Pt 10):2247–58. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Goulet C, Arsenault AB, Bourbonnais D, Laramee MT, Lepage Y. Effects of transcutaneous electrical nerve stimulation on H-reflex and spinal spasticity. Scand J Rehabil Med. 1996;28(3):169–76. [PubMed] [Google Scholar]
- Grey MJ, Nielsen JB, Mazzaro N, Sinkjaer T. Positive force feedback in human walking. J Physiol. 2007;581(Pt 1):99–105. doi: 10.1113/jphysiol.2007.130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema SJ, Hillyer J, Schmidt-Read M, Ardolino E, Sisto SA, Behrman AL. Locomotor training: as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch Phys Med Rehabil. 2012;93(9):1588–97. doi: 10.1016/j.apmr.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist. 2008;14(3):264–75. doi: 10.1177/1073858408314986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidler JM, Rymer WZ. A simulation study of reflex instability in spasticity: origins of clonus. IEEE Trans Rehabil Eng. 1999;7(3):327–40. doi: 10.1109/86.788469. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Kahn JH, Wu M, Schmit BD. Temporal facilitation of spastic stretch reflexes following human spinal cord injury. J Physiol. 2006;571(Pt 3):593–604. doi: 10.1113/jphysiol.2005.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. Changes in neuronal properties and spinal reflexes during development of spasticity following spinal cord lesions and stroke: studies in animal models and patients. J Rehabil Med. 2003;(41 Suppl):46–55. doi: 10.1080/16501960310010142. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Sekiguchi H, Miyoshi T, Nakazawa K, Akai M. Inhibition of the human soleus Hoffman reflex during standing without descending commands. Neurosci Lett. 2003;345(1):41–4. doi: 10.1016/s0304-3940(03)00485-3. [DOI] [PubMed] [Google Scholar]
- Knikou M. Plantar cutaneous input modulates differently spinal reflexes in subjects with intact and injured spinal cord. Spinal Cord. 2007;45(1):69–77. doi: 10.1038/sj.sc.3101917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knikou M, Mummidisetty CK. Reduced reciprocal inhibition during assisted stepping in human spinal cord injury. Exp Neurol. 2011;231(1):104–12. doi: 10.1016/j.expneurol.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Knutsson E. Gait control in hemiparesis. Scand J Rehabil Med. 1981;13(2–3):101–8. [PubMed] [Google Scholar]
- Knutsson E, Lindblom U, Martensson A. Differences in effects in gamma and alpha spasticity induced by the GABA derivative baclofen (Lioresal) Brain. 1973;96(1):29–46. doi: 10.1093/brain/96.1.29. [DOI] [PubMed] [Google Scholar]
- Mailis A, Ashby P. Alterations in group Ia projections to motoneurons following spinal lesions in humans. J Neurophysiol. 1990;64(2):637–47. doi: 10.1152/jn.1990.64.2.637. [DOI] [PubMed] [Google Scholar]
- Makihara Y, Segal RL, Wolpaw JR, Thompson AK. Operant down-conditioning of the soleus H-reflex in normal humans does not induce long-term changes in gastrocnemius H-reflexes and does not appear to disturb locomotion. Soc Neurosci 43rd Annual Meeting Abstr; CA. 2013. Program No. 645.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manella KJ, Roach KE, Field-Fote EC. Operant conditioning to increase ankle control or decrease reflex excitability improves reflex modulation and walking function in chronic spinal cord injury. J Neurophysiol. 2013;109(11):2666–79. doi: 10.1152/jn.01039.2011. [DOI] [PubMed] [Google Scholar]
- Mazzaro N, Grey MJ, do Nascimento OF, Sinkjaer T. Afferent-mediated modulation of the soleus muscle activity during the stance phase of human walking. Exp Brain Res. 2006;173(4):713–23. doi: 10.1007/s00221-006-0451-5. [DOI] [PubMed] [Google Scholar]
- Mazzaro N, Grey MJ, Sinkjaer T. Contribution of afferent feedback to the soleus muscle activity during human locomotion. J Neurophysiol. 2005;93(1):167–77. doi: 10.1152/jn.00283.2004. [DOI] [PubMed] [Google Scholar]
- Mori F, Koch G, Foti C, Bernardi G, Centonze D. The use of repetitive transcranial magnetic stimulation (rTMS) for the treatment of spasticity. Prog Brain Res. 2009;175:429–39. doi: 10.1016/S0079-6123(09)17528-3. [DOI] [PubMed] [Google Scholar]
- Morita H, Crone C, Christenhuis D, Petersen NT, Nielsen JB. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain. 2001;124(Pt 4):826–37. doi: 10.1093/brain/124.4.826. [DOI] [PubMed] [Google Scholar]
- Morita H, Shindo M, Momoi H, Yanagawa S, Ikeda S, Yanagisawa N. Lack of modulation of Ib inhibition during antagonist contraction in spasticity. Neurology. 2006;67(1):52–6. doi: 10.1212/01.wnl.0000223399.59212.f4. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Crone C, Hultborn H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol. 1993;66(2):116–21. doi: 10.1007/BF01427051. [DOI] [PubMed] [Google Scholar]
- Onushko T, Schmit BD. Reflex response to imposed bilateral hip oscillations in human spinal cord injury. J Neurophysiol. 2007;98(4):1849–61. doi: 10.1152/jn.00461.2007. [DOI] [PubMed] [Google Scholar]
- Schneider C, Lavoie BA, Capaday C. On the origin of the soleus H-reflex modulation pattern during human walking and its task-dependent differences. J Neurophysiol. 2000;83(5):2881–90. doi: 10.1152/jn.2000.83.5.2881. [DOI] [PubMed] [Google Scholar]
- Sheean G. Botulinum toxin treatment of adult spasticity: a benefit-risk assessment. Drug Saf. 2006;29(1):31–48. doi: 10.2165/00002018-200629010-00003. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Berman SA, Sarkarati M, Young RR. Recurrent inhibition is increased in patients with spinal cord injury. Neurology. 1992;42(11):2162–8. doi: 10.1212/wnl.42.11.2162. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. J Neurophysiol. 1996;76(2):1112–20. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Nielsen JF, Hansen HJ. Soleus long-latency stretch reflexes during walking in healthy and spastic humans. Clin Neurophysiol. 1999;110(5):951–9. doi: 10.1016/s1388-2457(99)00034-6. [DOI] [PubMed] [Google Scholar]
- Stein RB, Capaday C. The modulation of human reflexes during functional motor tasks. Trends Neurosci. 1988;11(7):328–32. doi: 10.1016/0166-2236(88)90097-5. [DOI] [PubMed] [Google Scholar]
- Stein RB, Misiaszek JE, Pearson KG. Functional role of muscle reflexes for force generation in the decerebrate walking cat. J Physiol. 2000;525(Pt 3):781–91. doi: 10.1111/j.1469-7793.2000.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Yang JF, Belanger M, Pearson KG. Modification of reflexes in normal and abnormal movements. Prog Brain Res. 1993;97:189–96. doi: 10.1016/s0079-6123(08)62277-3. [DOI] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Pidikiti R. Constraint-Induced Movement Therapy: a new family of techniques with broad application to physical rehabilitation--a clinical review. J Rehabil Res Dev. 1999;36(3):237–51. [PubMed] [Google Scholar]
- Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci. 2009a;29(18):5784–92. doi: 10.1523/JNEUROSCI.4326-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Chen XY, Wolpaw JR. Soleus H-reflex operant conditioning changes the H-reflex recruitment curve. Muscle Nerve. 2013a;47(4):539–44. doi: 10.1002/mus.23620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Estabrooks KL, Chong S, Stein RB. Spinal reflexes in ankle flexor and extensor muscles after chronic central nervous system lesions and functional electrical stimulation. Neurorehabil Neural Repair. 2009b;23(2):133–42. doi: 10.1177/1545968308321067. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Lapallo B, Duffield M, Abel BM, Pomerantz F. Repetitive common peroneal nerve stimulation increases ankle dorsiflexor motor evoked potentials in incomplete spinal cord lesions. Exp Brain Res. 2011;210(1):143–52. doi: 10.1007/s00221-011-2607-1. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Pomerantz F, Wolpaw JR. Operant down-conditioning of the soleus H-reflex during walking in people with incomplete spinal cord injury: preliminary results. Soc Neurosci 43rd Annual Meeting; 2013b. Abstr. Program No. 645.01. [Google Scholar]
- Thompson AK, Pomerantz FR, Wolpaw JR. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci. 2013c;33(6):2365–75. doi: 10.1523/JNEUROSCI.3968-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Wolpaw JR. Operant conditioning of spinal reflexes: from basic science to clinical therapy. Frontiers in Integrative Neuroscience. 2014a doi: 10.3389/fnint.2014.00025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Wolpaw JR. The simplest motor skill: mechanisms and applications of reflex operant conditioning. Exerc Sport Sci Rev. 2014b doi: 10.1249/JES.0000000000000010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DM, Ross BH, Thomas CK. Characteristics of lower extremity clonus after human cervical spinal cord injury. J Neurotrauma. 2012;29(5):915–24. doi: 10.1089/neu.2010.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AB. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115(4):607–16. doi: 10.1007/s00702-007-0833-2. [DOI] [PubMed] [Google Scholar]
- Wernig A, Muller S. Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia. 1992;30(4):229–38. doi: 10.1038/sc.1992.61. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. Jama. 2006;296(17):2095–104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. The complex structure of a simple memory. Trends Neurosci. 1997;20(12):588–94. doi: 10.1016/s0166-2236(97)01133-8. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. What can the spinal cord teach us about learning and memory? Neuroscientist. 2010;16(5):532–49. doi: 10.1177/1073858410368314. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Braitman DJ, Seegal RF. Adaptive plasticity in primate spinal stretch reflex: initial development. J Neurophysiol. 1983;50(6):1296–311. doi: 10.1152/jn.1983.50.6.1296. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Herchenroder PA, Carp JS. Operant conditioning of the primate H-reflex: factors affecting the magnitude of change. Exp Brain Res. 1993;97(1):31–9. doi: 10.1007/BF00228815. [DOI] [PubMed] [Google Scholar]
- Yang JF, Fung J, Edamura M, Blunt R, Stein RB, Barbeau H. H-reflex modulation during walking in spastic paretic subjects. Can J Neurol Sci. 1991;18(4):443–52. doi: 10.1017/s0317167100032133. [DOI] [PubMed] [Google Scholar]
- Zehr EP. Training-induced adaptive plasticity in human somatosensory reflex pathways. J Appl Physiol. 2006;101(6):1783–94. doi: 10.1152/japplphysiol.00540.2006. [DOI] [PubMed] [Google Scholar]