Abstract
Retrovirus-mediated gene transfer into hepatocytes in vivo results in long-term gene expression. Limitations include the need to remove two-thirds of the liver and the relatively low frequency of gene transfer. To increase gene transfer without surgical hepatectomy, mouse hepatocytes were transduced in vivo with a recombinant adenovirus that transiently expressed urokinase, resulting in high rates of asynchronous liver regeneration. During the regenerative phase, in vivo retroviral-mediated gene transfer in hepatocytes resulted in 5- to 10-fold greater transduction efficiencies than that obtained by conventional partial hepatectomy. In 3-4 weeks, the architecture and microscopic structure of the recipient livers were normal. The two-viral system of achieving permanent transgene expression from hepatocytes in vivo offers an alternative approach to current ex vivo and in vivo gene-transfer models.
Full text
PDF
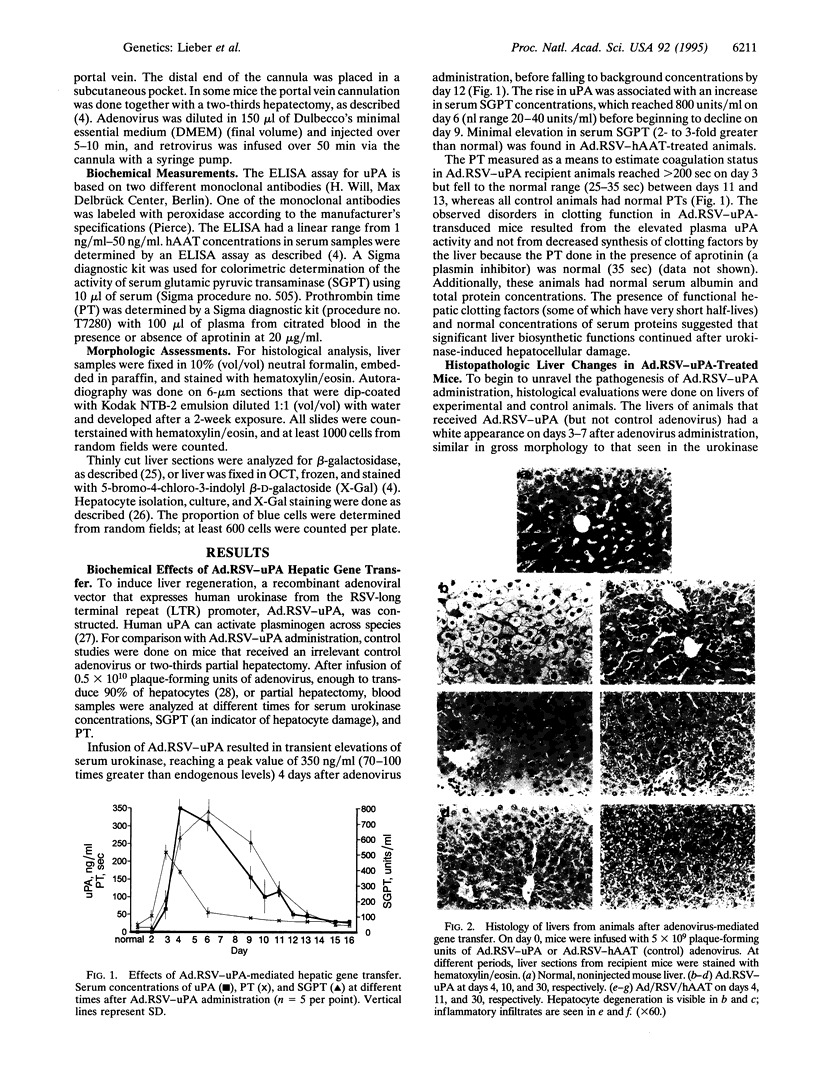
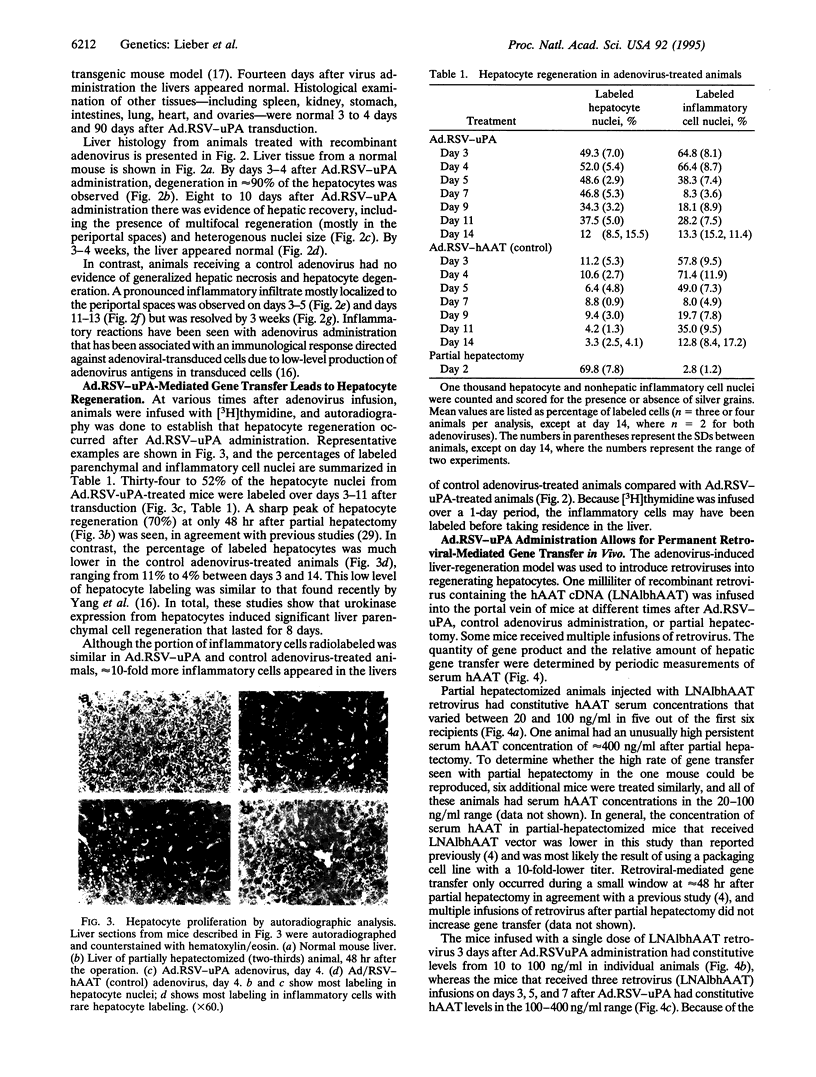
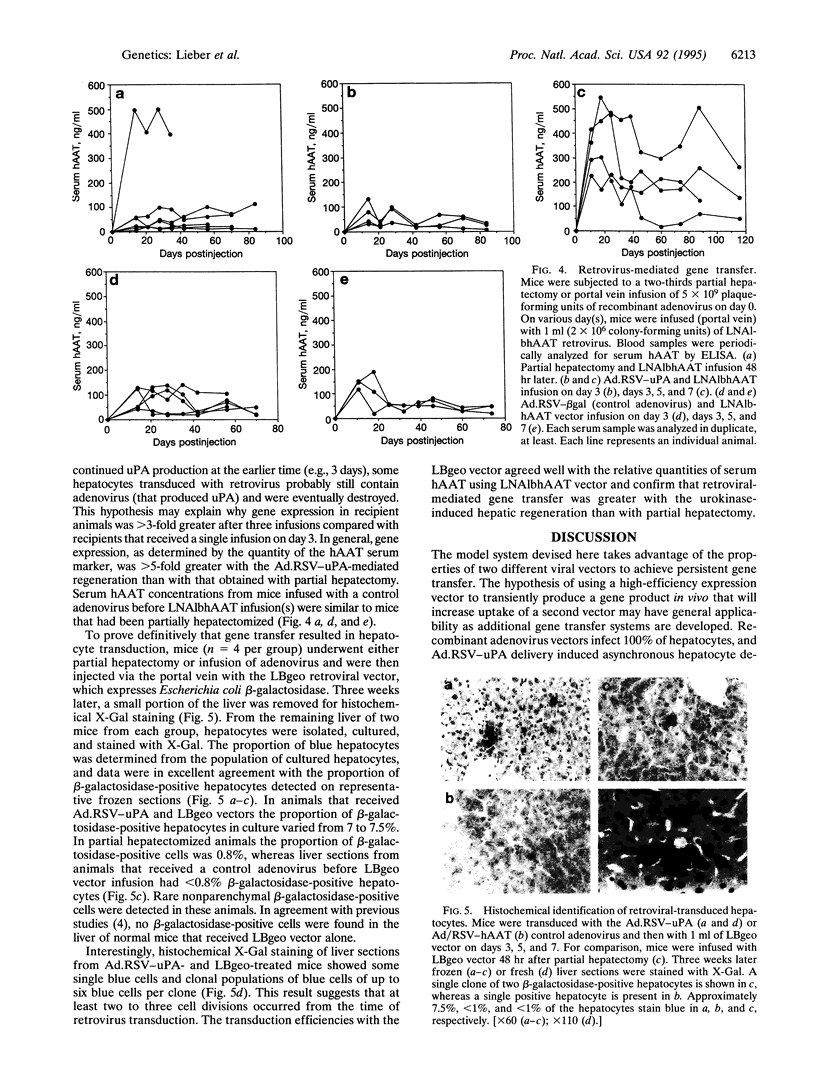

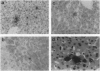
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr D., Tubb J., Ferguson D., Scaria A., Lieber A., Wilson C., Perkins J., Kay M. A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995 Mar;2(2):151–155. [PubMed] [Google Scholar]
- Branchereau S., Calise D., Ferry N. Factors influencing retroviral-mediated gene transfer into hepatocytes in vivo. Hum Gene Ther. 1994 Jul;5(7):803–808. doi: 10.1089/hum.1994.5.7-803. [DOI] [PubMed] [Google Scholar]
- Burns J. C., Friedmann T., Driever W., Burrascano M., Yee J. K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso J. E., Branchereau S., Jeyaraj P. R., Houssin D., Danos O., Heard J. M. In situ retrovirus-mediated gene transfer into dog liver. Hum Gene Ther. 1993 Aug;4(4):411–418. doi: 10.1089/hum.1993.4.4-411. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Grossman M., Gupta S., Chowdhury N. R., Baker J. R., Jr, Wilson J. M. Long-term improvement of hypercholesterolemia after ex vivo gene therapy in LDLR-deficient rabbits. Science. 1991 Dec 20;254(5039):1802–1805. doi: 10.1126/science.1722351. [DOI] [PubMed] [Google Scholar]
- Ferry N., Duplessis O., Houssin D., Danos O., Heard J. M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M., Raper S. E., Kozarsky K., Stein E. A., Engelhardt J. F., Muller D., Lupien P. J., Wilson J. M. Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolaemia. Nat Genet. 1994 Apr;6(4):335–341. doi: 10.1038/ng0494-335. [DOI] [PubMed] [Google Scholar]
- Hafenrichter D. G., Ponder K. P., Rettinger S. D., Kennedy S. C., Wu X., Saylors R. S., Flye M. W. Liver-directed gene therapy: evaluation of liver specific promoter elements. J Surg Res. 1994 Jun;56(6):510–517. doi: 10.1006/jsre.1994.1082. [DOI] [PubMed] [Google Scholar]
- Hatzoglou M., Lamers W., Bosch F., Wynshaw-Boris A., Clapp D. W., Hanson R. W. Hepatic gene transfer in animals using retroviruses containing the promoter from the gene for phosphoenolpyruvate carboxykinase. J Biol Chem. 1990 Oct 5;265(28):17285–17293. [PubMed] [Google Scholar]
- Heckel J. L., Sandgren E. P., Degen J. L., Palmiter R. D., Brinster R. L. Neonatal bleeding in transgenic mice expressing urokinase-type plasminogen activator. Cell. 1990 Aug 10;62(3):447–456. doi: 10.1016/0092-8674(90)90010-c. [DOI] [PubMed] [Google Scholar]
- Jespersen J., Astrup T. A study of the fibrin plate assay of fibrinolytic agents. Optimal conditions, reproducibility and precision. Haemostasis. 1983;13(5):301–315. doi: 10.1159/000214769. [DOI] [PubMed] [Google Scholar]
- Kaleko M., Garcia J. V., Miller A. D. Persistent gene expression after retroviral gene transfer into liver cells in vivo. Hum Gene Ther. 1991 Spring;2(1):27–32. doi: 10.1089/hum.1991.2.1-27. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Baley P., Rothenberg S., Leland F., Fleming L., Ponder K. P., Liu T., Finegold M., Darlington G., Pokorny W. Expression of human alpha 1-antitrypsin in dogs after autologous transplantation of retroviral transduced hepatocytes. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):89–93. doi: 10.1073/pnas.89.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. A., Graham F., Leland F., Woo S. L. Therapeutic serum concentrations of human alpha-1-antitrypsin after adenoviral-mediated gene transfer into mouse hepatocytes. Hepatology. 1995 Mar;21(3):815–819. [PubMed] [Google Scholar]
- Kay M. A., Landen C. N., Rothenberg S. R., Taylor L. A., Leland F., Wiehle S., Fang B., Bellinger D., Finegold M., Thompson A. R. In vivo hepatic gene therapy: complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2353–2357. doi: 10.1073/pnas.91.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. A., Li Q., Liu T. J., Leland F., Toman C., Finegold M., Woo S. L. Hepatic gene therapy: persistent expression of human alpha 1-antitrypsin in mice after direct gene delivery in vivo. Hum Gene Ther. 1992 Dec;3(6):641–647. doi: 10.1089/hum.1992.3.6-641. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Rothenberg S., Landen C. N., Bellinger D. A., Leland F., Toman C., Finegold M., Thompson A. R., Read M. S., Brinkhous K. M. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993 Oct 1;262(5130):117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- Kolodka T. M., Finegold M., Kay M. A., Woo S. L. Hepatic gene therapy: efficient retroviral-mediated gene transfer into rat hepatocytes in vivo. Somat Cell Mol Genet. 1993 Sep;19(5):491–497. doi: 10.1007/BF01233254. [DOI] [PubMed] [Google Scholar]
- Li Q., Kay M. A., Finegold M., Stratford-Perricaudet L. D., Woo S. L. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther. 1993 Aug;4(4):403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- Lieber A., Vrancken Peeters M. J., Kay M. A. Adenovirus-mediated transfer of the amphotropic retrovirus receptor cDNA increases retroviral transduction in cultured cells. Hum Gene Ther. 1995 Jan;6(1):5–11. doi: 10.1089/hum.1995.6.1-5. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Collen D. Mechanisms of plasminogen activation by mammalian plasminogen activators. Enzyme. 1988;40(2-3):90–96. doi: 10.1159/000469150. [DOI] [PubMed] [Google Scholar]
- Mars W. M., Zarnegar R., Michalopoulos G. K. Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol. 1993 Sep;143(3):949–958. [PMC free article] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- Moscioni A. D., Rozga J., Neuzil D. F., Overell R. W., Holt J. T., Demetriou A. A. In vivo regional delivery of retrovirally mediated foreign genes to rat liver cells: need for partial hepatectomy for successful foreign gene expression. Surgery. 1993 Mar;113(3):304–311. [PubMed] [Google Scholar]
- Naldini L., Tamagnone L., Vigna E., Sachs M., Hartmann G., Birchmeier W., Daikuhara Y., Tsubouchi H., Blasi F., Comoglio P. M. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992 Dec;11(13):4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettinger S. D., Kennedy S. C., Wu X., Saylors R. L., Hafenrichter D. G., Flye M. W., Ponder K. P. Liver-directed gene therapy: quantitative evaluation of promoter elements by using in vivo retroviral transduction. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1460–1464. doi: 10.1073/pnas.91.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- Sandgren E. P., Palmiter R. D., Heckel J. L., Daugherty C. C., Brinster R. L., Degen J. L. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell. 1991 Jul 26;66(2):245–256. doi: 10.1016/0092-8674(91)90615-6. [DOI] [PubMed] [Google Scholar]
- Spessot R., Inchley K., Hupel T. M., Bacchetti S. Cloning of the herpes simplex virus ICP4 gene in an adenovirus vector: effects on adenovirus gene expression and replication. Virology. 1989 Feb;168(2):378–387. doi: 10.1016/0042-6822(89)90279-1. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl R. C., Sinio L., Summaria L., Robbins K. C. Comparative activation kinetics of mammalian plasminogens. Biochim Biophys Acta. 1983 May 30;745(1):20–31. doi: 10.1016/0167-4838(83)90165-6. [DOI] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]