Abstract
Objective
The yield of epileptiform abnormalities in serial EEGs has not been addressed in a population-based setting for subjects with incident epilepsy or a single unprovoked seizure, raising the possibility of methodological limitations such as selection bias. Our aim was to address these limitations by assessing the yield and predictors of epileptiform abnormalities for the first and subsequent EEGs in a study of incident epilepsy or single unprovoked seizure in Rochester, Minnesota.
Methods
We used the resources of the Rochester Epidemiology Project to identify all 619 residents of Rochester, Minnesota born in 1920 or later with a diagnosis of incident epilepsy (N=478) or single unprovoked seizure (N=141) between 1960 and 1994, who had at least one EEG. Information on all EEGs and their results was obtained by comprehensive review of medical records.
Results
Among subjects with epilepsy, the cumulative yield of epileptiform abnormalities was 53% after the first EEG and 72% after the third EEG. Among subjects with single unprovoked seizure, the cumulative yield was 39% after the first EEG and 68% after the third EEG. Young age at diagnosis and idiopathic etiology were risk factors for finding epileptiform abnormalities across all EEGs.
Significance
While the cumulative yield of epileptiform abnormalities increases over successive EEGs, there is a decrease in the increment for each additional EEG after the first EEG. This is most evident in incident epilepsy and in younger subjects. Clinically it may be worthwhile to consider that the probability of finding an epileptiform abnormality after the third non-epileptiform EEG is low.
Keywords: Population-based, epidemiology, epileptiform abnormality, epilepsy, EEG
Introduction
The presence of epileptiform abnormalities on electroencephalogram (EEG) augments clinical information about seizures and helps with both diagnosis and classification. Since the first EEG does not always show an epileptiform abnormality and serial EEGs may all be normal 1, multiple EEGs are often recorded in an effort to find an epileptiform abnormality that may guide treatment, particularly if patients continue to experience seizures.
Most previous studies of the yield of epileptiform abnormalities on EEG in new onset seizures have evaluated a single EEG. The reported yield of an epileptiform abnormality on the first EEG in new onset unprovoked seizure or incident epilepsy ranges from 32% to 59% in children 1–5 and from 12% to 44% in adults 3; 6–8. In studies of patients with prevalent epilepsy referred to a specialty center, the reported cumulative yield of epileptiform abnormalities on serial EEGs performed over varying time periods ranges from 60% to 90% 9–11.
Various clinical factors have been reported as predictors of an epileptiform abnormality on the first EEG in subjects with a first unprovoked seizure or incident epilepsy. Epileptiform abnormalities on EEG are reported to be more frequent among younger patients and in subjects with generalized seizures 1; 3; 7. A high frequency of seizures is also reported to be associated with epileptiform abnormalities 11.
The yield of epileptiform abnormalities in serial EEGs has not been addressed in a population-based setting for subjects with incident epilepsy or a single unprovoked seizure. Additionally, there are no studies of the predictors of epileptiform abnormalities on clinical EEGs in an unselected population followed after single unprovoked seizure or incident epilepsy. Such data are critical for evaluating these issues, because all patients, regardless of their later prognosis, are included, and selection bias related to factors other than the presence of epilepsy is minimized. Thus this design is crucial for obtaining valid results that form the basis for planning cost-effective diagnostic work-ups for patients with incident epilepsy or a single unprovoked seizure.
The aims of this study were to assess the yield and predictors of epileptiform abnormalities for the first and the subsequent EEGs in a population-based study of incident epilepsy or incident single unprovoked seizure.
Materials and methods
Subjects
Data were obtained from the “Genetic Epidemiology of Seizure Disorders in Rochester” study (GESDR) 12–14, a population-based investigation using the resources of the Rochester Epidemiology Project (REP). The REP is a unique medical records-linkage system that includes the records of all medical care facilities used by virtually all residents of Rochester, Minnesota 15–17. GESDR includes all individuals born in 1920 or later with incident epilepsy (2 or more unprovoked seizures) or a single unprovoked seizure while residing in Rochester, Minnesota between 1935 and 1994. Reports of incidence and prevalence in this population have been published elsewhere18; 19. Our current analyses were restricted to incident cases from 1960 to 1994, and to EEGs recorded in 1960 or later. Although EEGs were first recorded at the Mayo Clinic in 1938, this restriction was made because EEGs were not routinely used until the late 1940s and the characteristics of the recording system were different and not sufficiently comparable before 1960. The 10–20 electrode system was first used in 1969 at Mayo Clinic facilities, and before that time a specific “Mayo Clinic system” was used that differed primarily in the position of the anterior temporal electrode. The position of this electrode changed slightly in the 10–20 system. Only records with prior authorization for medical record review were included (more than 99% of those identified).
Data Collection
Between 2003 and 2008, study epileptologists (JRB and WAH) comprehensively reviewed the medical records of each proband at the Mayo Clinic and all other local health care providers to confirm study eligibility and update information on clinical diagnosis and classification. The review included all outpatient and inpatient medical visits and test results (including EEG, neuroimaging, seizure descriptions, etc.) from date first seen to last seen by a REP provider, encompassing essentially all medical care delivered while individuals resided locally 15–17. A REP provider refers to any health care provider working at and used by the Rochester and Olmsted County residents, whose records are included in the Rochester Epidemiology Project database. This includes the Mayo Clinic, the Olmsted Medical Center and its local clinics, and some private practices.
An unprovoked seizure was defined as a seizure, occurring in the absence of an identified proximate precipitating factor, thus excluding seizures associated only with an acute insult to the central nervous system (CNS) or a generalized systemic metabolic disturbance (acute symptomatic seizures). Individuals with only febrile seizures or neonatal seizures were classified as acute symptomatic and were excluded 20. Subjects without an EEG were excluded from the analysis.
Seizures and epilepsies characterized by unique sensitivity to external stimuli such as photoconvulsive seizures, sensory stimulus-sensitive epilepsy, auditory-induced epilepsy, or reading epilepsy were considered unprovoked18. Subjects were classified as having a single unprovoked seizure if they had only one unprovoked seizure between 1960 and 1994, without recurrence while residing locally during the follow-up period extending to 2008. To ensure that subjects in this category had not had additional seizures before diagnosis, only those who had been seen by a REP provider within seven days of the first seizure were included. Incident epilepsy was defined as a history of >2 unprovoked seizures, separated by more than 24 hours and diagnosed by a physician. Subjects were classified as having incident epilepsy if they were initially diagnosed with epilepsy while residents of Rochester from 1960–1994, or had a first unprovoked seizure from 1960–1994 and a recurrence at any time while residing locally during the follow-up period ending in 2008. Thus, subjects who had a first unprovoked seizure while residing in Rochester from 1960–1994 were classified as epilepsy if they had a recurrence during follow-up, but as single unprovoked seizure if they never had a recurrence during follow-up. Subjects were excluded if they had not resided in Rochester, Minnesota for at least one year before the diagnosis date. For each patient with incident single unprovoked seizure or incident epilepsy in Rochester, Minnesota, we examined number of EEGs, age at diagnosis, gender, seizure type, etiology, presence of status epilepticus, and number of seizures per year. For each EEG, detailed information was abstracted on date, type (routine or with video monitoring, etc.) and findings (including specific epileptiform and nonepileptiform abnormalities).
Staff neurologists at the Mayo Clinic assessed 96% of the patients. Patients were classified as having “unknown” seizure type when information was sufficient to confirm the occurrence of a seizure but inadequate to allow further classification.
Electroencephalographic records
We included EEGs with and without activation during the study period and excluded prolonged (longer than 24 hours) and intra-operative EEGs. The first EEG was the first related to the diagnosis of single unprovoked seizure or incident epilepsy, within one year of the date of a first single unprovoked seizure or new diagnosis of epilepsy. Earlier EEGs were excluded, assuming that they could have been recorded for another reason (i.e., febrile seizure, acute symptomatic seizure), unrelated to the seizures under study. All EEGs were requested by the treating physicians in the clinical context and the reasons for performing the number of EEGs are unknown.
Definitions
Epileptiform abnormalities
For each EEG, findings were classified as epileptiform or non-epileptiform.
Interictal and ictal epileptiform abnormalities were defined by the presence of generalized epileptiform abnormalities (typical generalized spike-wave 3 Hz, atypical spike-wave, slow spike-wave, generalized epileptiform fast, hypsarrhythmia, electro-decremental), focal epileptiform abnormalities (spike, spike-wave, sharp wave, periodic lateralized epileptiform discharges (PLEDS), temporal interictal rhythmic delta activity (TIRDA), multifocal, bilateral, independent, or synchronous) or epileptiform abnormality but not determined whether generalized or focal. Seizures recorded during the EEG were classified by seizure type and the nature of the ictal epileptiform discharges.
Any epileptiform abnormality was defined by the presence of interictal or ictal epileptiform abnormalities and dichotomized as present or absent.
Age at EEG recording
The age of the subject at the time of each EEG was recorded.
Age at diagnosis
Age at single unprovoked seizure or incident epilepsy was grouped as <1 year, 1 to 19 years, and 20 years or older (referent).
Seizure classification
Study epileptologists (JRB and WAH) classified unprovoked seizures by etiology, seizure type, and epilepsy syndrome using the 1989 recommendations of the International League Against Epilepsy (ILAE) 21, as these were the standard classification systems at the time of data review.
Patients were classified as having generalized epilepsy syndromes if they had generalized ictal or interictal epileptiform EEG abnormalities or seizure semiology clearly consistent with absence, myoclonic, or atonic seizures, and were subdivided into idiopathic generalized epilepsies, other generalized epilepsies (denoted "cryptogenic" or "symptomatic" in the 1989 ILAE classification) 21. Patients were classified as having focal epilepsy if they had focal epileptiform EEG abnormalities or focal seizure semiology, and were also subclassified into syndromes according to the 1989 ILAE criteria.
When the broad epilepsy syndrome (generalized or focal) could not be determined, the reasons were recorded (nocturnal seizures only, limited semiology information, or lack of EEG findings) and patients were placed in a category of “unclassified” seizure type and of “unknown” syndrome. Classification of etiology, seizure type and epilepsy syndrome were based upon clinical information, EEG and MRI from the diagnosis to six months after the diagnosis. Findings on CT or MRI were used to support the diagnosis (especially if known to be associated with focal epilepsy, e.g. tumor, focal cortical dysplasia) but negative findings were not required for exclusion of structural/metabolic epilepsy. Seizure types and etiologies were classified independently, allowing classification of generalized seizures in some individuals with identified brain injuries.
Presumed cause was assigned based on the history of structural or metabolic central nervous system (CNS) insults occurring before the first unprovoked seizure. Patients with structural or metabolic causes22 were further subdivided into prenatal/developmental (i.e., neurological deficit presumed present at birth, as reflected by intellectual or motor deficits or CNS congenital malformations), identified genetic disorder (e.g., tuberous sclerosis or Down syndrome), or postnatal cause (e.g., stroke or traumatic brain injury). For analysis, we combined subjects with prenatal/developmental and genetic causes.
Status Epilepticus (SE)
The length of seizures was recorded only when it was greater than 5 minutes. SE was defined as a single seizure with duration of 30 minutes or more or repeated seizures lasting 30 minutes or more without recovery in between. SE was considered to be absent when seizures were shorter than 30 minutes or when there was no length recorded for that seizure, under the assumption that the duration was less than 5 minutes. We considered SE occurring before the first EEG, with no SE as the referent.
Number of seizures per year
The number of seizures per year was classified during the first five years following diagnosis and categorized as none, one, two or more, or unknown (including those lost to follow up - those who moved out of the area less than five years after diagnosis). Syndromes characterized by an elevated frequency of seizures were classified as having 2 or more seizures/year.
Statistical analysis
Descriptive statistical analyses were performed to analyze demographic and clinical characteristics, using frequencies and percentages to summarize categorical variables, medians and interquartile ranges for continuous variables. Statistical significance was determined using the χ2 statistic (or Fisher's exact test) and Wilcoxon-Rank sum test for continuous variables. The 0.05 level of significance and two-sided tests were used for all analyses. All analyses were conducted using SAS® software, v 9.2.
Cumulative yield of any epileptiform abnormality
We estimated the cumulative yield of any epileptiform EEG abnormality according to the number of EEGs using a life table approach for clustered observations, with clusters defined by individual subjects and observations defined by EEGs. Since few subjects had more than four EEGs, we considered one, two and three or more EEGs for single unprovoked seizure and one, two, three and four or more EEGs for epilepsy, in order to avoid a distortion of the results due to a small denominator. The log-rank statistic was used to compare the estimate of cumulative proportion of any epileptiform abnormality in single unprovoked seizure versus incident epilepsy. Subjects were followed until the date an epileptiform abnormality was recorded, the date of last visit to a REP provider, the date of death or the end of the study period (1 January 2008), whichever came first.
In subjects with epilepsy, we also compared the yield of any epileptiform abnormality by age and by seizure type (excluding subjects classified as having both focal and generalized seizure types). Among children aged 1–19 years, we compared the yield by syndrome. Analysis of the yield of EEG abnormalities according to seizure type and syndrome was circular since classification of seizure type and syndrome was based in part on EEG findings during the first 6 months. This problem was most apparent for primary generalized seizures, which by definition require the presence of generalized EEG abnormalities. Due to this problem, we conducted an additional analysis that excluded EEGs done in the first 6 months of diagnosis for primary generalized seizures only.
Cox proportional hazard regression analysis
In subjects with epilepsy, a Cox proportional hazard regression model was used to evaluate the hazard of recording any first epileptiform abnormality, accounting for the number of EEGs performed until the first epileptiform abnormality and the number of seizures during the first five years of follow-up. The model included age group at diagnosis and etiology. Subjects were censored after their last EEG, date of death or at last date of follow up in Rochester, Minnesota, whichever came first. The same model was used to evaluate the hazard of recording any epileptiform abnormality, accounting for the time from the diagnosis of epilepsy to the first epileptiform EEG abnormality recorded.
To account for the clustering of EEGs within subjects, we used the marginal Cox model approach for clustered data, applying the Wei, Lin and Weissfel method 23.
The proportional hazard assumption was tested graphically.
Proportion of epileptiform abnormalities on EEGs
We evaluated the proportion of epileptiform abnormalities recorded after the first abnormality was found. To compare our findings with the literature, we reproduced a “prevalent subset” of subjects, consisting of subjects with EEGs performed >5 years after onset.
Results
We studied 677 Rochester, Minnesota residents aged one month or older with incident single unprovoked seizure (N=159, 23.5%) or epilepsy (N=518, 76.5%) from 1960 through 1994. Among the 501 subjects with incident epilepsy whose date of first unprovoked seizure was known, the median latency between first unprovoked seizure and the diagnosis of epilepsy was 204 days (Interquartile Range-IQR=33–764).
Among the 677 subjects evaluated, 619 subjects had one or more EEGs and they differed from the 58 subjects without EEGs. Among subjects with a single unprovoked seizure, those with an EEG were more likely to be male (49.6% vs. 22.2%; p=0.04). In subjects with epilepsy, those with an EEG were more likely to have focal seizures (58.6% vs. 32.5%), and less likely to have generalized (23.4% vs. 27.5%) or unclassifiable seizures (17.2% vs. 37.5%; p=0.003). Individuals with an EEG were also more likely to have had >2 seizures during the first five years of follow-up, and less likely to have had an unknown number of seizures (p<0.05 for each year of follow up).
Demographics by diagnosis among subjects with one or more EEGs
Single Unprovoked Seizure
The median time to the first EEG was three days (IQR=1–6). The median age at diagnosis was 19.6 years (IQR=6.3–38.0); the majority of subjects had an unknown etiology (76.6%) (Table 1).
Table 1.
Clinical and demographic features of subjects with single unprovoked seizure and incident epilepsy, for subjects with at least one EEG
| Single Unprovoked Seizure | Epilepsy | |
|---|---|---|
| 141 N (%) |
478 N (%) |
|
| Age at diagnosis (years) median (IQR) | ||
| N (%) | 19.6 (6.3–38.0) | 17.3 (6.5–33.7) |
| <1 | 6 (4.3) | 29 (6.1) |
| 1–19 | 65 (46.1) | 234 (49.0) |
| >20 | 70 (49.7) | 215 (45) |
| Gender | ||
| Male | 70 (49.7) | 250 (52.3) |
| Female | 71 (50.3) | 228 (47.7) |
| Seizure type | ||
| Generalized | 0 (0) | 112 (23.4) |
| Focal | 0 (0) | 280 (58.6) |
| Both | 0 (0) | 4 (0.8) |
| Unclassifiable | 0 (0) | 82 (17.2) |
| Isolated unprovoked | 141 (100) | 0 (0) |
| History of status epilepticus | ||
| No | 134 (95.0) | 467 (97.7) |
| Yes | 7 (5.0) | 11 (2.3) |
| Etiology: | ||
| Idiopathic | 0 (0) | 86 (18) |
| Prenatal/developmental/genetic/metabolic | 6 (4.3) | 71 (14.8) |
| Postnatal symptomatic | 27 (19.1) | 107 (22.4) |
| Unknown | 108 (76.6) | 214 (44.8) |
| Number of EEGs | ||
| 1 | 78 (55.3) | 118 (24.7) |
| 2 | 40 (28.4) | 115 (24.1) |
| 3 | 7 (5.0) | 81 (17.0) |
| >4 | 16 (11.3) | 164 (34.3) |
| Number of seizures during 1st year | ||
| None | 141 (100) | 115 (24.1) |
| 1 | 0 (0) | 59 (12.3) |
| ≥ 2 | 0 (0) | 257 (53.8) |
| Unknown | 0 (0) | 47 (9.8) |
| Number of seizures during 2nd year | ||
| None | 141 (100) | 209 (43.7) |
| 1 | 0 (0) | 46 (9.6) |
| ≥ 2 | 0 (0) | 150 (31.4) |
| Unknown | 0 (0) | 73 (15.3) |
| Number of seizures during 3rd year | ||
| None | 141 (100) | 218 (45.6) |
| 1 | 0 (0) | 46 (9.6) |
| ≥ 2 | 0 (0) | 116 (24.3) |
| Unknown | 0 (0) | 98 (20.5) |
| Number of seizures during 4th year | ||
| None | 141 (100) | 239 (50.0) |
| 1 | 0 (0) | 24 (5.0) |
| ≥ 2 | 0 (0) | 100 (20.9) |
| Unknown | 0 (0) | 115 (24.1) |
| Number of seizures during 5th year | ||
| None | 141 (100) | 245 (51.3) |
| 1 | 0 (0) | 24 (5.0) |
| ≥ 2 | 0 (0) | 78 (16.3) |
| Unknown | 0 (0) | 131 (27.4) |
Epilepsy
The median time to the first EEG was four days (IQR=1–16). The median age at diagnosis was 17.3 years (IQR: 6.5–33.7); unknown etiology was most common (44.8%). Seizure type was classified as focal in 58.6% (Table1).
Among 246 subjects without an epileptiform abnormality on the first EEG, those with only one EEG were less likely than those with >2 EEGs to have a focal seizure type (44% vs. 63.2%, p=0.006) and more likely to have unclassifiable seizures (44% vs. 22.8%, p=0.006). Those with only one EEG also experienced fewer seizures during the second (p=0.0004), third (p=0.003) and fourth year of follow up (p=0.01) than those who had a second EEG. Among the 232 subjects with an epileptiform abnormality on the first EEG, those without a second EEG were older than those who had additional EEGs (p=0.0004, data not shown).
Any epileptiform abnormality on EEGs over time
Overall, 619 subjects (91.4%) underwent at least one EEG between the diagnosis, or within a year prior to diagnosis, and the end of the study period (1 January 2008). At least one EEG was performed in 141 (88.7%) subjects with a single unprovoked seizure and 478 (92.3%) subjects with epilepsy. Among all subjects with a first EEG, 89.5% had photic stimulation and 69.6% had sleep. These percentages were essentially unchanged over the 2nd and 3rd EEG.
Single unprovoked seizure
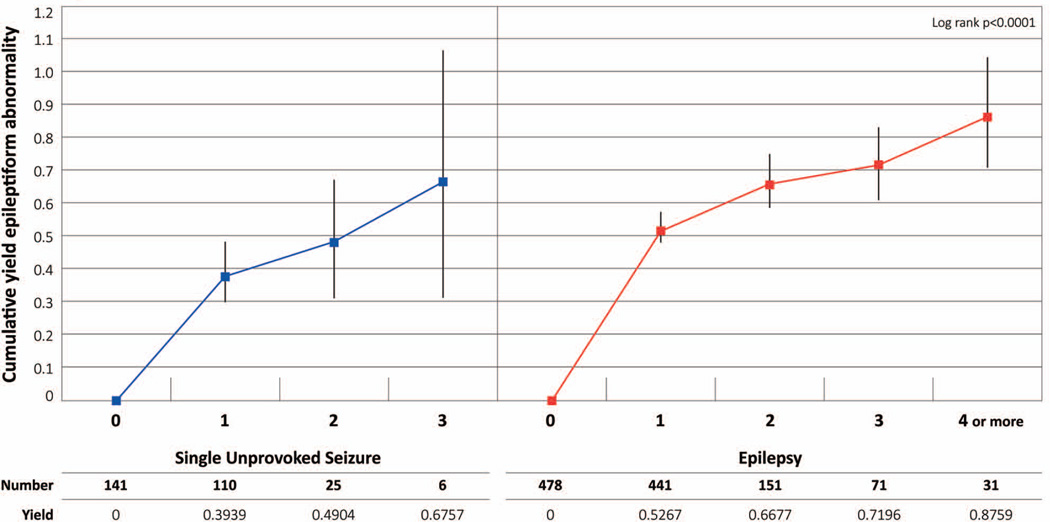
Cumulative percentage of any epileptiform abnormality on EEGs (Fig. 1)
Figure 1. Epileptiform abnormality in single unprovoked seizure and incident epilepsy. Cumulative yield and 95% C.I.
The Ns for each EEG represent the effective sample size, which consider the censored data and the probability at midpoint in actuarial lifetable.
An epileptiform abnormality was identified in the first EEG in 39.1%. A second EEG identified epileptiform abnormalities in an additional 10% and an additional 18.5% were identified on the third EEG (although numbers were small). By the third EEG, 67.6% of subjects had an epileptiform abnormality. Among subjects that had more than one EEG etiology was either symptomatic or unknown.
Incident epilepsy
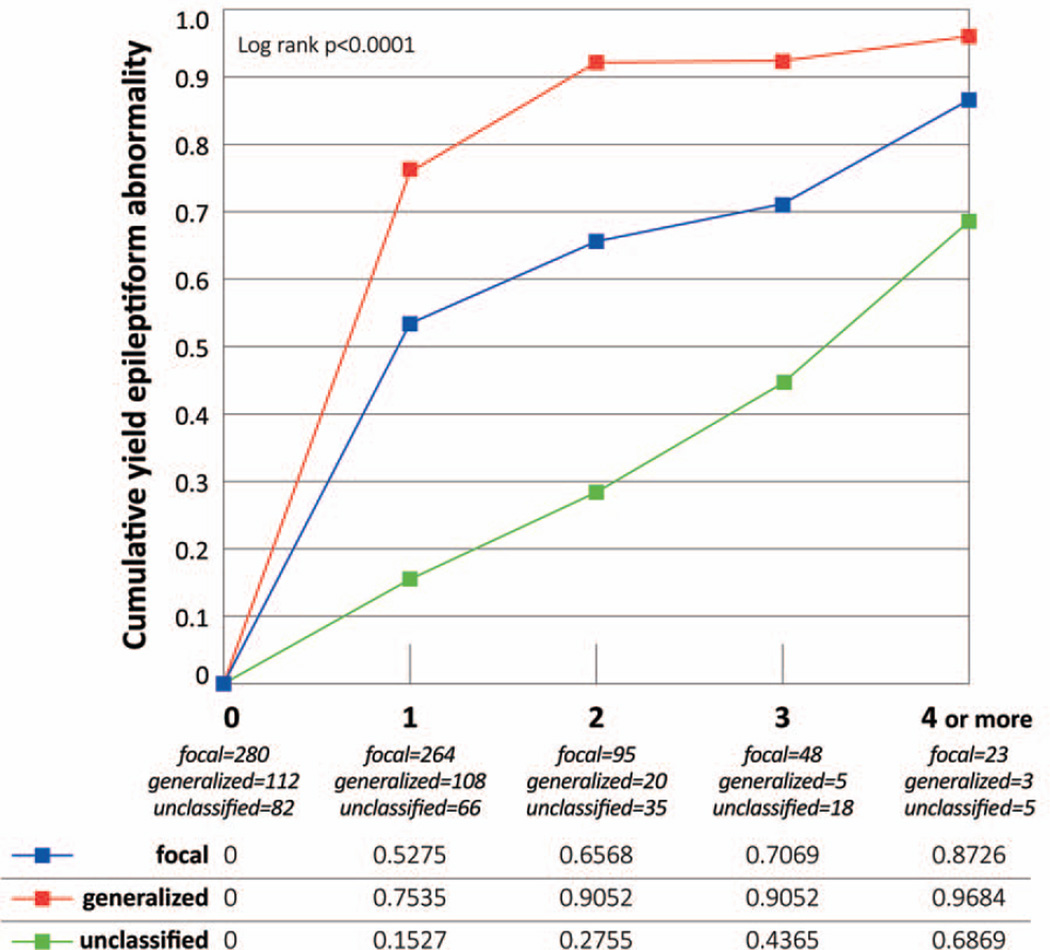
Cumulative percentage of any epileptiform abnormality on EEGs (Fig. 1–3)
Figure 3. Epileptiform abnormality in 474 subjects with epilepsy, by seizure type (excluded subjects with seizures classified as both focal and generalized).
The Ns for each EEG represent the effective sample size, which consider the censored data and the probability at midpoint in actuarial lifetable.
Epileptiform abnormalities were identified on the first EEG in 52.7%. A second EEG identified an additional 14.1%, and a third EEG added 5.2%. By the fourth EEG, the cumulative yield was 87.6% (Fig. 1).
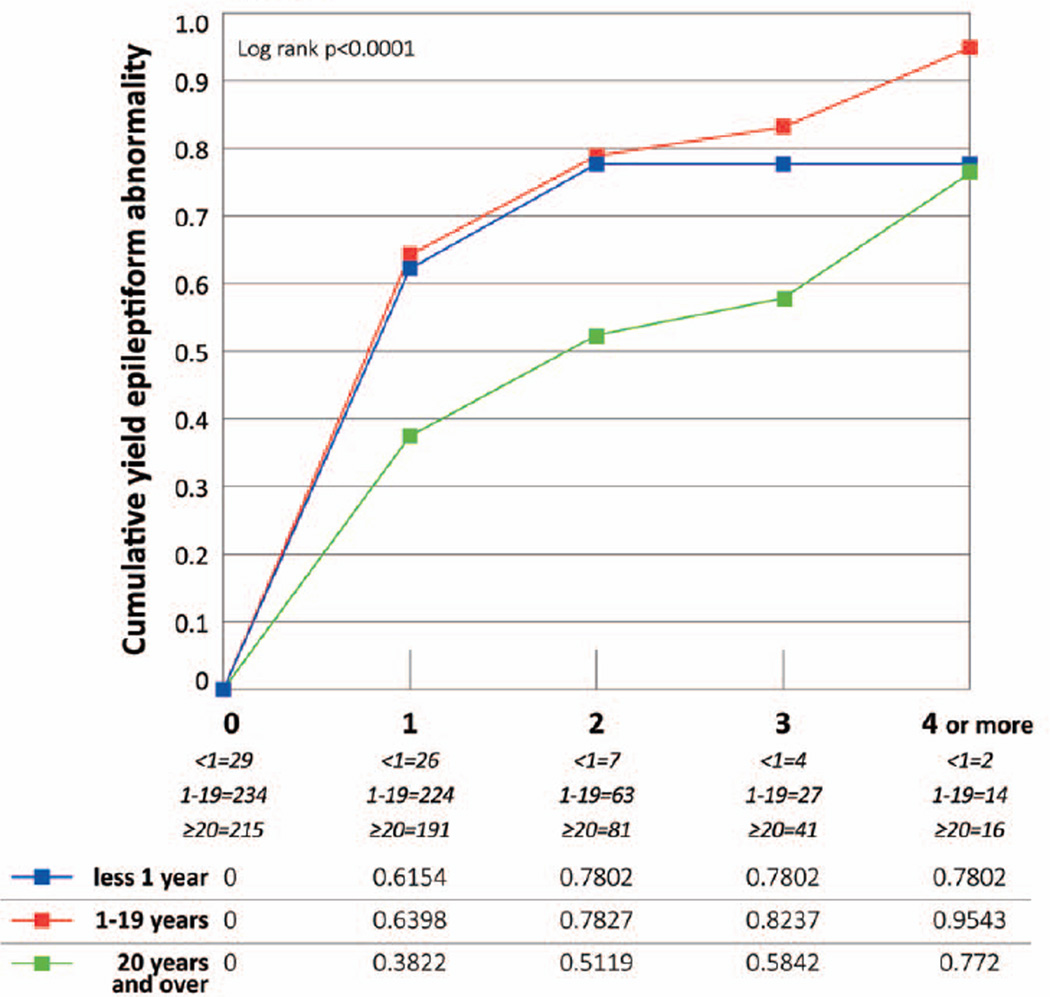
Subjects aged 20 years or older were less likely than younger subjects to have an epileptiform abnormality (Fig. 2). By the third EEG, the cumulative yield was 82.4% among subjects aged 1–19 years and 78.0% among those younger than one year compared to 58.4% for those 20 years or older (p<0.0001).
Figure 2. Epileptiform abnormality in 478 subjects with epilepsy, by age group.
The Ns for each EEG represent the effective sample size, which consider the censored data and the probability at midpoint in actuarial lifetable.
The cumulative yield was significantly greater for subjects with generalized seizures than focal or unclassified seizures (Fig. 3). The same pattern was seen when EEGs performed during the first six months were excluded for generalized seizures (data not shown).
To consider the impact of specific epileptic syndromes, we analyzed the yield in children aged 1–19 years (Supplementary Fig. 1). Subjects with generalized epilepsy syndromes (i.e. IGE and generalized not IGE) showed a higher yield (96.1% and 95.8% respectively) compared to focal non-idiopathic syndromes (76.6%) (p<0.0001).
Factors associated with any epileptiform abnormality across all EEGs (Table 2)
Table 2.
Cox proportional hazard regression analysis for any new ictal or interictal epileptiform abnormality in 478 people with epilepsy and at least one EEG
| Factors | Crude HR (95% CI) |
Adjusted HR* (95% CI) |
|---|---|---|
| Age at diagnosis (years) | ||
| <1 | 1.7 (1.1–2.8) | 1.3 (0.8–2.2) |
| 1–19 | 2.1 (1.7–2.7) | 1.8 (1.3–2.3) |
| >20 | 1.0 (referent) | 1.0 (referent) |
| Gender | ||
| Male | 1.0 (referent) | 1 |
| Female | 1.0 (0.8–1.3) | |
| Etiology | ||
| Idiopathic | 1.0 (referent) | 1.0 (referent) |
| Prenatal/developmental/genetic/metabolic | 0.8 (0.5–1.1) | 0.9 (0.7–1.2) |
| Postnatal symptomatic | 0.4 (0.3–0.6) | 0.9 (0.6–1.3) |
| Unknown | 0.5 (0.4–0.6) | 0.7 (0.6–0.97) |
| Status epilepticus | ||
| No | 1.0 (referent) | 1 |
| Yes | 0.5 (0.2–1.3) |
accounting for number of EEGs done before finding abnormality (continuous) and for the number of seizures during each of the first five years of follow up (categorized as none, one, two or more, or unknown).
HR= Hazard Ratio; 95% CI= 95% Confidence Interval
Factor not included in the model.
The hazard of finding any epileptiform abnormality was increased in subjects 1–19 years old at diagnosis compared to those 20 years or older. The hazard of finding an epileptiform abnormality was also greater in idiopathic epilepsies than in epilepsies of unknown etiology (Table 2). In a similar model adjusted for the time from diagnosis to first epileptiform abnormality and the number of seizures, the same factors were associated with a greater hazard of having an epileptiform abnormality (data not shown).
Proportion of epileptiform abnormality on EEGs
There were 232 subjects with an epileptiform abnormality on the first EEG. Among these, 72% had an abnormality on the second EEG, 59.7% on the third EEG, and 65.2% on the fourth EEG. The same pattern of increasing then decreasing propensity to have an abnormality was evident when the first epileptiform abnormality was recorded on the second EEG or on the third EEG (data not shown).
Results were comparable with those of the overall sample when a “prevalent subset” of subjects was considered (i.e., EEGs in incident epilepsy after five years of follow-up). Among the 157 prevalent subjects, 73 (46.5%) had an epileptiform abnormality on the first EEG. Of these, an epileptiform abnormality was recorded in 70.6% on the second EEG, in 64.9% on the third and in 64.3% on the fourth EEG.
Discussion
This is the first population-based study of the risk of finding epileptiform abnormalities over multiple EEGs in people with incident epilepsy or single unprovoked seizure. The yield of any epileptiform abnormality increased with increasing number of EEGs, although the incremental yield after the first EEG decreased with each additional EEG. The cumulative yield was greatest for incident epilepsy in which the cumulative yield of any epileptiform abnormality was 72% by the third EEG.
We compared our results to previous studies in clinic-based select populations.
Yield after the first EEG
Previous findings of the yield of epileptiform abnormalities in a first EEG with or without sleep were similar to ours. Unlike our study, most other studies combined subjects with incident epilepsy and single unprovoked seizure. A similar yield was reported in prevalent epilepsy in adults 10; 11, and in subjects of all ages 9.
Cumulative yield over multiple EEGs
In prevalent epilepsy, a few prior studies have considered the yield of epileptiform abnormalities over multiple EEGs. In these studies, the yield of epileptiform abnormalities increased with increasing number of EEGs. After the third EEG, the cumulative yield ranged from 60% to 77% 10; 11 and was >90% after more than six EEGs 9; 10 (Table 3). We found a similar cumulative yield (58.4%) after the third EEG in adults with incident epilepsy.
Table 3.
Studies of yield of epileptiform abnormalities
| Author | Age range | Source population | Type of population |
Yield at 1st EEG |
Cumulative Yield at 2nd EEG |
Cumulative Yield at 3rd EEG |
|---|---|---|---|---|---|---|
| Current study | All ages (≥1 month) |
Population based. All subjects with incident epilepsy or single unprovoked seizure in Rochester within study period (1960–1994) |
Incident | SUS:39.1% EP:52.7% |
SUS:50.3% EP:66.8% |
SUS:68.4% EP:72% |
| Salinsky et al, 198710 | Male Adults (≥18 years) | Veterans with epilepsy referred to an Epilepsy Center (1979–1986) | Prevalent | 28.7% | 46.1% | 59.5% |
| Ajmone Marsan et al, 19709 | All ages (1–64 years) | Epilepsy patients retrospectively and non- randomly selected from EEG laboratory files | Prevalent | 55.5% | – | 93.9 % after an average of 6 EEGs |
| Sundaram et al, 199011 | Adults (≥ 16 years) | Subjects with history of seizure(s) referred to an EEG laboratory within a 6-months period (1988) | Prevalent | 44.8% | - | 77%* |
| Camfield et al, 19855 | Children | Retrospective, children with first unprovoked seizure, residents of Nova Scotia between Jan 1978–Dec 1982 Excluded seizure types: absence, myoclonic, akinetic, infantile spasm |
Incident | 43.5% | - | - |
| Shinnar et al, 19944 | Children (1 month-19 years) | Prospective, first unprovoked afebrile seizure examined at centers in the Bronx between 1983–1990 | Incident | 31.8% | - | - |
| Carpay et al, 19971 | Children (1 month-16 years) | Prospective, idiopathic or remote symptomatic single unprovoked seizure or epilepsy seen at a center between 1988–1992 | Incident | US: 46% EP:59% |
US: 78.8% EP: 94.3% |
- |
| King et al, 19983 | ≥5 years | Prospective, first unprovoked seizure referred to a medical center in Melbourne, Australia within study period | Incident | Children (≤16): 59.3% Adults: 39% | 61% sleep deprived all ages | - |
| Hamiwka et al, 20082 | Children (1 month-17 years) | Prospective, first seizure between Jan 2004 and Aug 2005 | Incident | 46.8% | - | - |
| van Donselaar et al, 19928 | Adult (≥15 years) | Consecutive patients with clinically presumed first seizure referred to four hospitals between Mar 1986 and Mar 1988 | Incident | 11.5% | 25.7% | - |
| Kim et al, 20066 | All ages | Subjects enrolled in RCT with single unprovoked seizure or epilepsy. | Incident | 43.5% | - | - |
| Neufeld et al, 20007 | Adults (≥15 years) | Retrospective, first seizure admitted to hospital through ER (1991–1995) | Incident | 20.9% | - | - |
percentage of interictal epileptiform abnormality referred to the initial number of subjects included that underwent first EEG.
EP-epilepsy; SUS- single unprovoked seizure; US- unprovoked seizure
Cumulative yield by age
In previous studies of subjects with incident unprovoked seizures, the yield of an epileptiform abnormality on a first EEG only was 32%-59% in children 1–5 and 12%-44% in adults 3; 6–8 (Table 3). Similarly, we found that the cumulative yield was higher for younger subjects compared to those aged 20 years or older over multiple EEGs.
Risk factors for epileptiform abnormality
We found that the major risk factor for a greater yield of epileptiform abnormalities was younger age. This was true for age group 1–19 years compared to people of older ages after adjusting for seizure frequency, suggesting that the EEG examination is most useful in children and adolescents. Others have also found a greater yield of epileptiform abnormalities in younger subjects 3; 7, and in subjects with a high seizure frequency 9; 11.
The incidence/prevalence paradox
The yield of epileptiform abnormalities over successive EEGs does not differ between clinical series of prevalent epilepsy 9–11 and our population-based incidence cohort. We observed this paradox even when we restricted our analysis to a prevalent subsample at follow-up that was selected from the incident cohort. The proportion of epileptiform abnormality on the first EEG was 48.5% in our full cohort and 46.5% in the prevalent subsample. This unexpected similar pattern in incident and prevalent cohorts may reflect the remission and relapse pattern of epilepsy 24; 25 or an intrinsic frequency of epileptiform abnormalities in each person.
Strengths and limitations
Strengths of our study include its population-based design, the expertise of clinicians in and around Rochester and the excellent quality of the Mayo Clinic medical records and EEGs. The demographics of Rochester, MN differ from the rest of the US, because most of the Rochester population is white, of northern European origin, and higher socioeconomic status 16; 26. However, these differences are unlikely to impact the yield of EEGs. Thus, our findings can be generalized and considered of practical value for health care providers in optimizing the care of and resources for patients with epilepsy.
Over the study period the number of available diagnostic procedures in epilepsy has increased (i.e., MRI examination was only available for part of the time period of our study) and this might have affected the etiologic classification of the cases identified in the earlier phase.
A small proportion of individuals were seen by a REP provider after moving out of Rochester, MN. It was impossible to determine whether EEGs were performed elsewhere in these subjects. While this might lead to underestimation of number of EEGs, the clinical characteristics of these subjects did not differ from subjects residing in the county for the entire follow-up period. Also, because of the way our data were collected (through retrospective medical record review), inter-rater reliability of EEG findings could not be assessed.
We analyzed EEG epileptiform abnormalities by seizure type and syndrome. However, in keeping with usual clinical practice, the presence and type of EEG abnormalities in the first 6 months after diagnosis was taken into account in the classification of seizure type and epilepsy syndrome. This diagnostic issue creates a circularity of reasoning, especially with regard to primary generalized seizures and generalized syndromes, where classification is greatly dependent on the identification of generalized epileptiform EEG abnormalities. In order to account for the effect of this circularity, we performed an analysis of EEGs in individuals with primary generalized seizures after excluding EEGs done within the first 6 months after diagnosis and found no difference in the results.
Data were not collected on use of antiepileptic drugs in the follow-up period. Antiepileptic drugs could affect the detection of an epileptiform abnormality on the EEG either increasing or decreasing their presence, depending on the specific drugs used, although this has not been shown consistently 9; 27; 28.
A substantial proportion of seizure types were unclassifiable. This is a weakness of our study, but is typical of population-based studies of incident epilepsy and single unprovoked seizure 20; 29; 30. Additionally, we did not examine specific epileptiform abnormalities, but only the abnormalities overall.
Conclusion
The cumulative yield of any epileptiform abnormality through the third EEG is about 70% for both incident epilepsy and single unprovoked seizure. The incremental yield decreases for each additional EEG after the first EEG. Clinically it may be worthwhile to consider the low probability of finding an epileptiform abnormality after the third non-epileptiform EEG. This is most evident in incident epilepsy, and specifically, in younger subjects. The utility of ordering multiple EEGs over time should be considered carefully, to avoid potentially unnecessary testing.
Supplementary Material
Acknowledgements
We thank Jane Emerson, RN, Melissa Petersen, RN, Diane Carlson, RN, Ann Van Oosten, and Thomas Bitz for assistance with data collection. This research was supported by U.S. NIH grant R01 NS043472 from the NINDS. Study data were obtained from the REP, which is supported by the NIA of the NIH under R01 AG034676. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosure
Dr. Baldin has no disclosure.
Dr. Hauser was a consultant to Eisai Pharmaceuticals and the Federal Aviation Administration; a member of the Data Safety Monitoring Boards of Teva Pharma and NINDS; received funding for travel from the International League Against Epilepsy and received research support from the University of Kansas, from the CDC, and from NINDS.
Dr. Buchhalter served as a consultant for Lundbeck, Inc., Eisai, Ltd, and Upsher-Smith; is on the editorial advisory board for Epilepsy Currents; received research support from the Alberta Children’s Hospital Foundation and from NINDS. He also provides clinical evaluations and neurophysiology services that will not be benefitted by this paper.
Dr. Hesdorffer serves as consultant at the Mount Sinai Medical Center, Injury Prevention Center and at NYU Epilepsy Center; she received research support from AUCD, CDC, NINDS, Epilepsy Foundation of America, PCORI, CURE, Epilepsy Study Consortium; she serves on the advisory boards and received funding for travel from UCB, Esai, Upsher-Smith Laboratories and Cyberonics.
Dr. Ottman serves on the scientific advisory board for and holds stock options in Trigeminal Solutions, Inc., received funding for travel from École des Hautes Etudes en Santé Publique, and received research support from NINDS and NHGRI.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Carpay JA, de Weerd AW, Schimsheimer RJ, et al. The diagnostic yield of a second EEG after partial sleep deprivation: a prospective study in children with newly diagnosed seizures. Epilepsia. 1997;38:595–599. doi: 10.1111/j.1528-1157.1997.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 2.Hamiwka L, Singh N, Kozlik S, et al. Feasibility and clinical utility of early electroencephalogram (EEG) in children with first seizure. J Child Neurol. 2008;23:762–765. doi: 10.1177/0883073808315619. [DOI] [PubMed] [Google Scholar]
- 3.King MA, Newton MR, Jackson GD, et al. Epileptology of the first-seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet. 1998;352:1007–1011. doi: 10.1016/S0140-6736(98)03543-0. [DOI] [PubMed] [Google Scholar]
- 4.Shinnar S, Kang H, Berg AT, et al. EEG abnormalities in children with a first unprovoked seizure. Epilepsia. 1994;35:471–476. doi: 10.1111/j.1528-1157.1994.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 5.Camfield PR, Camfield CS, Dooley JM, et al. Epilepsy after a first unprovoked seizure in childhood. Neurology. 1985;35:1657–1660. doi: 10.1212/wnl.35.11.1657. [DOI] [PubMed] [Google Scholar]
- 6.Kim LG, Johnson TL, Marson AG, et al. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial. Lancet Neurol. 2006;5:317–322. doi: 10.1016/S1474-4422(06)70383-0. [DOI] [PubMed] [Google Scholar]
- 7.Neufeld MY, Chistik V, Vishne TH, et al. The diagnostic aid of routine EEG findings in patients presenting with a presumed first-ever unprovoked seizure. Epilepsy Res. 2000;42:197–202. doi: 10.1016/s0920-1211(00)00183-2. [DOI] [PubMed] [Google Scholar]
- 8.van Donselaar CA, Schimsheimer RJ, Geerts AT, et al. Value of the electroencephalogram in adult patients with untreated idiopathic first seizures. Arch Neurol. 1992;49:231–237. doi: 10.1001/archneur.1992.00530270045017. [DOI] [PubMed] [Google Scholar]
- 9.Ajmone Marsan C, Zivin L. Factors Related to the Occurrence of Typical Paroxysmal Abnormalities in the EEG Records of Epileptic Patients. Epilepsia. 1970;11:361–381. doi: 10.1111/j.1528-1157.1970.tb03903.x. [DOI] [PubMed] [Google Scholar]
- 10.Salinsky M, Kanter R, Dasheiff RM. Effectiveness of multiple EEGs in supporting the diagnosis of epilepsy: an operational curve. Epilepsia. 1987;28:331–334. doi: 10.1111/j.1528-1157.1987.tb03652.x. [DOI] [PubMed] [Google Scholar]
- 11.Sundaram M, Hogan T, Hiscock M, et al. Factors affecting interictal spike discharges in adults with epilepsy. Electroencephalogr Clin Neurophysiol. 1990;75:358–360. doi: 10.1016/0013-4694(90)90114-y. [DOI] [PubMed] [Google Scholar]
- 12.Ottman R, Barker-Cummings C, Leibson CL, et al. Validation of a brief screening instrument for the ascertainment of epilepsy. Epilepsia. 2010;51:191–197. doi: 10.1111/j.1528-1167.2009.02274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ottman R, Barker-Cummings C, Leibson CL, et al. Accuracy of family history information on epilepsy and other seizure disorders. Neurology. 2011;76:390–396. doi: 10.1212/WNL.0b013e3182088286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peljto AL, Barker-Cummings C, Vasoli VM, et al. Familial risk of epilepsy: a population-based study. Brain. 2013 doi: 10.1093/brain/awt368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clinic Proceedings. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 19.Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576–586. doi: 10.4065/71.6.576. [DOI] [PubMed] [Google Scholar]
- 20.ILAE. Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia. 1993;34:592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 21.Commission on Classification Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 22.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 23.Wei L, Lin D, Weissfeld L. Regression-analysis of multivariate incomplete failure time data by modeling marginal distributions. Journal of the American Statistical Association. 1989;84:1065–1073. [Google Scholar]
- 24.Berg AT, Levy SR, Testa FM, et al. Remission of epilepsy after two drug failures in children: a prospective study. Ann Neurol. 2009;65:510–519. doi: 10.1002/ana.21642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodie MJ, Barry SJ, Bamagous GA, et al. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–1554. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan JS. Antiepileptic drugs and the electroencephalogram. Epilepsia. 1987;28:259–266. doi: 10.1111/j.1528-1157.1987.tb04216.x. [DOI] [PubMed] [Google Scholar]
- 28.Pillai J, Sperling MR. Interictal EEG and the diagnosis of epilepsy. Epilepsia. 2006;47(Suppl 1):14–22. doi: 10.1111/j.1528-1167.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 29.Forsgren L, Bucht G, Eriksson S, et al. Incidence and clinical characterization of unprovoked seizures in adults: a prospective population-based study. Epilepsia. 1996;37:224–229. doi: 10.1111/j.1528-1157.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 30.Manford M, Hart YM, Sander JWAS, et al. The National General-Practice Study of Epilepsy - the Syndromic Classification of the International-League-against-Epilepsy Applied to Epilepsy in a General-Population. Archives of Neurology. 1992;49:801–808. doi: 10.1001/archneur.1992.00530320025008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.