Abstract
Objective
Considerable breakthroughs in the field of sepsis have been made using animal models. Sepsis exhibits a wide array of derangements that may be evaluated in the blood, including the release of pro- and anti-inflammatory cytokines. The Shock journal adheres to the ARRIVE guidelines regarding reporting in vivo results to allow reproducibility of data findings. It is generally assumed that blood cytokine concentrations collected from typical sampling sites will be similar, but there are no data validating that this is true. The main purpose of the present study was to determine if the location of blood sampling results in cytokine concentration differences following inflammatory insults.
Methods
Two different models of acute inflammation were studied. Adult, female ICR mice were injected with E. coli lipopolysaccharide (LPS, n = 28) or subjected to cecal ligation and puncture (CLP) (n = 16). They were sacrificed at early time points following these inflammatory challenges for the collection of blood from the facial vein, retro-orbital sinus, and heart. Additonal samples were collected in EDTA and heparin. Plasma cytokines from the same mouse were collected from each sampling site and evaluated by ELISA. Clinical chemical parameters including plasma BUN and total protein were also analyzed.
Results
Regardless of model, time of collection, or cytokine measured, cytokine values from heart blood were higher than facial vein values from the same mouse. IL-6 collected from the heart relative to the facial vein demonstrated elevated concentrations following injection of LPS. In a similar manner, higher concentrations of IL-6, MIP-2, IL-10 and IL-1RA were found in cardiac puncture samples compared to other sampling sites 24 hours following sepsis induced by cecal ligation and puncture. Similar differences were not seen when comparing BUN and total protein values from the two different sites. Using plasma IL-6 collected from the heart would incorrectly stratify predicted to live (Live-P) mice into the predicted to die (Die-P) category. Therefore, a simple linear regression model was developed to correctly re-stratify mice to their predicted fate. These data demonstrate that pro- and anti-inflammatory cytokine concentrations are dramatically elevated when drawn centrally from the heart compared to collection from peripheral locations such as the facial vein. It is critical for publications to document the sampling location when evaluating plasma cytokines and attempting to compare studies.
Keywords: Lipopolysaccharide, cecal ligation and puncture, facial vein, cardiac puncture, cytokines, IL-6
Introduction
Unquestionably, sepsis is a complex disease process characterized by a systemic inflammatory response to a microbial infection. Given its significant morbidity and mortality, substantial effort has been invested in gaining an understanding of the pathophysiology and the intricacies that make its inflammatory cascades so difficult to treat (1, 2). While there have been numerous clinical trials studying sepsis in humans, most of these trials were preceded by investigating patterns of sepsis and treatment in animals. Studying sepsis in animals, in particular mice, has been critical to our current understanding about this disease. Animal models are relatively inexpensive, can mimic the clinical setting and recapitulate the immunologic and physiologic sequelae often observed in clinical sepsis. Despite recent controversies in animal model use (3, 4), significant discoveries have been made using animal models of inflammation (5). For example, studies using mice have helped us understand that sepsis consists of a continuum of inflammation represented by pro-inflammatory and anti-inflammatory plasma cytokines rather than the previously thought clear cut transitions of a hyper-inflammatory state followed by an anti-inflammatory phase (6). In addition to gaining a better understanding of the inflammatory response, we have also acquired information regarding therapeutic strategies using mice. Prior to discovering that limiting the effects of TNF-α did not show survival improvement in clinical sepsis (7, 8), a study using the animal model of polymicrobial peritonitis demonstrated similar findings that anti-TNF treatment failed to reduce mortality (9).
A critical component of our understanding of sepsis and its inflammatory and physiologic responses begins with the collection of blood. Many parameters can be measured in the blood including cell counts, adhesion molecules, cytokines, and biomarkers and other markers of organ function and injury. There are many locations from which blood can be collected in mice; the heart, facial vein, retro-orbital sinus and tail vein are some of the more common locations. There are advantages and disadvantages to drawing blood from each site. For example, collecting blood from the retro-orbital sinus and heart provide a significant amount of blood which can then be used for analysis of multiple parameters; however, often times these are terminal procedures and animals need to be sacrificed in order to perform these blood draws (10). Conversely, collecting blood from the facial vein provides the benefit of collecting blood serially and allows for daily measurement of various parameters as is performed in a clinical setting (11, 12). Unfortunately, sampling the facial vein limits the volume of blood that may be collected.
While we attempt to decipher the patterns of inflammatory mediators and the phenomenon that is SIRS/CARS, it is important to determine if plasma cytokine concentrations are similar when they are collected from different sampling sites. We therefore aimed to evaluate if collecting blood from multiple sampling sites would yield comparable cytokine values focusing primarily on two specific locations in models of inflammation. These two sites include central collection from the heart and peripheral sampling from the facial vein. The Shock journal complies with the ARRIVE guidelines (13) which “Promotes reproducible, transparent … manuscripts” to improve reporting of research involving animals. However, if plasma cytokine concentrations from the same animal vary by the sample collection site, and the site is not specified, an important aspect of the experimental design will be missing. The current study was specifically designed to test whether blood sample site collection is a critical factor that should be reported to allow comparison of animal studies.
Materials and Methods
Animals
Female outbred Institute of Cancer Research (ICR) mice (Harlan-Sprague Laboratories, Indianapolis, IN) with an average weight of 26 grams were studied. They were allowed to acclimate to the lab environment for at least 1 week prior to any intervention in a temperature and humidity controlled housing with a 12 hour light:dark diurnal cycle. Food and water was provided ad libitum for the entire duration of the experiment. All studies were done following NIH guidelines and approved by the Institutional Care and Use Committee at Boston University School of Medicine.
Models of Inflammation
The first model used for this study was a common model of inflammation in animals and consisted of administration of lipopolysaccharide A endotoxin (14, 15). Twentyeight mice were administered a one time intra-peritoneal dose of LPS (10 μg/mouse) dissolved in 100 μL of 0.9% normal saline solution as previously described (14).
The second model of inflammation consisted of cecal ligation and puncture (CLP) and represents the gold standard of sepsis research in animal models (16) performed as first published (17) with minor modifications (18). Briefly, using 16 mice, general anesthesia was induced with 5% isoflurane and then reduced to 3% for maintenance. The abdominal wall was prepped with Chloroprep antibacterial solution (Bimeda, Le Sueur, MN) and a 1 cm abdominal incision was made followed by cecal ligation and double puncture with a 16 gauge needle. Immediately following a two layer closure of the abdominal wall, anesthesia was discontinued and mice were resuscitated with 1 mL of warm (37°C) D5 lactated ringers solution injected subcutaneously. Antibiotic treatment in the form of imipenem 25 mg/kg (Merck, West Point, PA) was started two hours after surgery and administered every 12 hours until sacrifice. Postoperative pain control was achieved with buprenorphine 0.5 mg/kg and administered every 12 hours.
Sample Collection
All mice were anesthetized with a solution of ketamine 87 μg/gm and xylazine 13 μg/gm mixed in normal saline administered intraperitoneally just prior to collecting blood from all sampling sites. For the endotoxin model of inflammation, mice underwent blood sampling at 2 or 6 hours following LPS administration. Blood was sampled at the 24 hour time point for the mice that underwent CLP.
In both models, blood was first obtained by puncturing the facial vein using a 23 gauge needle. Two, 20 μL specimens of blood from this site were collected into a pipette tip rinsed with EDTA (169 mM tripotassium salt). This blood was immediately diluted in 180 μL of 1:50 PBS:EDTA.
Facial vein sampling was then followed by percutaneous cardiac puncture in a blinded manner so it is not known if the blood was from the right or left ventricle. 700 μL of blood was collected using this technique and immediately mixed with 70 μL of Heparin 1000 units/mL (Sagent Pharmaceuticals, Schaumburg, IL). The influence of of anticoagulants is controversial with some publications reporting that different anticoagulants may affect cytokine concentrations (19, 20) although other studies did not observe this effect (21-23). To evaluate a potential anti-coagulant influence, samples were collected in EDTA or heparin 2 hours after LPS injection in a separate group of mice.
A subset of mice that were subjected to CLP underwent facial vein sampling, followed by retro-orbital venous plexus sampling and lastly cardiac puncture. Blood collected from the retro-orbital plexus and cardiac puncture were each mixed with heparin at the same ratio as described above. Following collection, all blood specimens were centrifuged at 1000g at 4°C for 5 minutes. Plasma was then separated and used for the evaluation of multiple cytokines as well as other clinical chemical parameters including blood urea nitrogen (BUN) and total protein.
Enzyme-Linked Immunosorbent Assay
ELISA was performed on individual plasma samples using matched antibody pairs for capture and detection (R&D Systems Inc., Minneapolis, MN). An aliquot of plasma was collected at the specified time points and used to evaluate IL-6 by ELISA for both inflammatory models as described previously (24). Other plasma cytokines including IL-1B, MIP-2, KC, IL-10, and IL1-RA were also measured by ELISA.
Blood Urea Nitrogen (BUN) Assay
Plasma specimens collected using both facial vein sampling and cardiac puncture techniques from the same mice used to evaluate cytokine values were used to determine BUN concentration. BUN was measured using a standard chemistry kit from Pointe Scientific, Inc (Canton, MI) as previously described (25).
Total Protein Assay
Total protein in plasma collected from mice 24 hours after CLP by facial vein and cardiac sampling was evaluated with the Coomassie protein assay using the Bradford Reagent (Sigma-Aldrich, St. Louis, MO) for detection and bovine serum albumin for the standard. Absorbance was read at 590 nm as performed previously (26).
Statistical Analysis
Analysis of the data was performed using Prism 5 (GraphPad Software, San Diego, CA). Initial studies used a paired Student 2 tail t-test to evaluate differences between plasma cytokine, BUN, and total protein values among the same mice. When it became apparent that the cardiac cytokine values were always higher than the peripheral blood, a paired, one tailed t-test was used. Results were reported as mean ± SEM. P value < 0.05 was used to indicate statistical significance. For evaluation of the effect of anticoagulants, t-tests with the Bonferroni for multiple comparisions was employed. Coefficient of variance of plasma IL-6 values was determined using Microsoft Office Excel calculating the mean of five aliquots divided by the standard deviation for each individual mouse.
Results
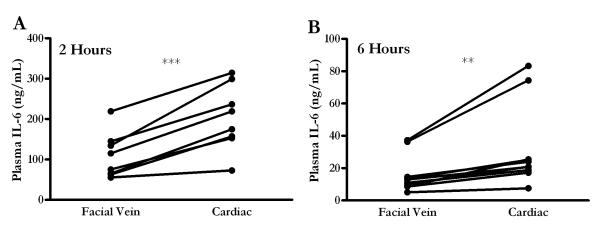
An LPS endotoxin model of inflammation was first used to determine if differences in cytokine values based on the sample site were present. Figure 1 demonstrates plasma IL-6 values at either two or six hours following intraperitoneal LPS administration. The kinetics in this model are similar to previous reports (27); plasma IL-6 peaks at 2 hours and begins to approach baseline values by 6 hours following endotoxin administration. The essential point in this figure is that blood collected centrally by means of cardiac puncture has higher levels of IL-6 compared to the blood drawn peripherally from the facial vein in the same mouse. To highlight this finding, the data from each individual mouse is presented with a connecting line, showing that the cardiac values were always higher. The average plasma IL-6 value at 2 hours was 109.1 ng/mL from the facial vein and 203.4 ng/mL from the heart (Figure 1A). Likewise, a similar statistically significant difference was apparent at 6 hours despite the near resolution of the inflammatory response, where facial vein IL-6 mean was 15.7 ng/mL and cardiac mean value was 31.7 ng/mL (Figure 1B).
FIGURE 1.
Elevated plasma IL-6 after endotoxin injection when sampled by cardiac puncture relative to facial vein. (A) Mice (n = 8) underwent blood sampling from facial vein followed by cardiac puncture two hours following LPS, while (B) a separate group of mice (n = 10) underwent blood sampling from the same two sites 6 hours following injection. Plasma IL-6 collected by cardiac puncture was significantly elevated compared to IL-6 collected by facial vein in the same mouse. ** = p < 0.001 and *** = p < 0.0005.
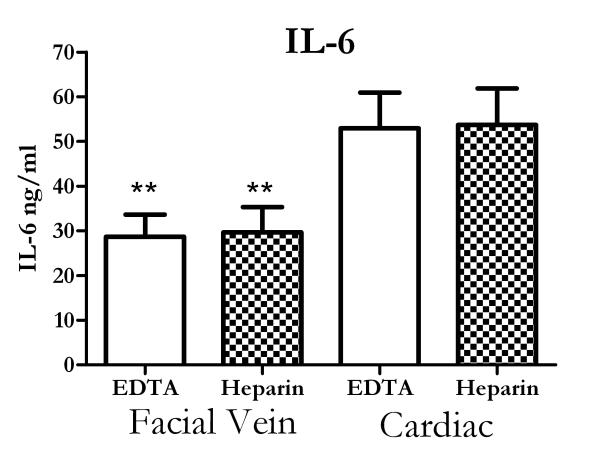
A recent report showed that different anticoagulants could impact the cytokine concentrations in normal human donors (19), although other reports have not shown such an effect (21-23). We examined whether the two anticoagulant used in this study, EDTA or heparin, would impact the cytokine measurements. Figure 2 shows that the anticoagulants did not change the IL-6 concentrations (Figure 2A). It should be noted that the IL-6 levels are lower in figure 2 compared to figure 1, but the experiments were separated by nearly two years. While the actual values were different, the major observation, i.e. cardiac values are higher than facial vein samples, was still found to be true. Anticoagulation with heparin did result in higher KC measurements and this observation will be explored in future studies.
Figure 2.
Effect of anticoagulants on cytokine concentrations. Mice were injected with LPS and facial vein and cardiac blood collected two hours later in either EDTA or heparin. The anticoagulant did not alter IL-6 concentrations. However, cardiac values were still higher than facial vein samples. ** = p<.01 compared to cardiac samples for the same anticoagulant. N= 8-10 mice for each group.
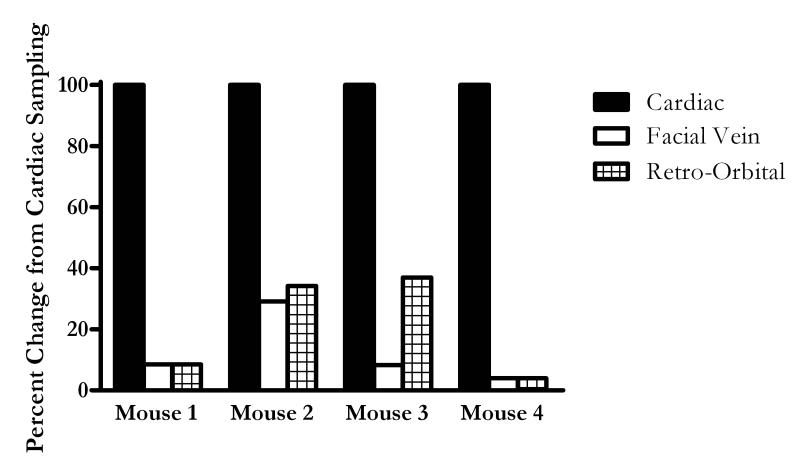
We next evaluated if similar findings were found in other models of inflammation and other cytokines. The CLP model of sepsis was used to validate that there are differences in plasma cytokine values collected from different sites. It should be emphasized that this study was not designed to catalog the inflammatory response to sepsis since these studies already been reported (6). The study tested whether cytokine concentrations from the same animal vary depending on the sample site. Mice underwent plasma sampling twenty-four hours following CLP. Knowing that there are multiple plasma sampling sites in mice, a subset of mice underwent blood collection from three different locations including the facial vein followed by the retro-orbital sinus, and concluding with the terminal cardiac puncture. Figure 3 compares plasma IL-6 results when these three commonly utilized plasma collecting sites are compared up to each other. The values from the different sites were normalized to the cardiac values which were had the highest concentrations of IL-6. Plasma IL-6 values were comparable when drawn from the facial vein or retro-orbital sinus. However, these values collected from peripheral sites were always less than 40% of concentrations of IL-6 centrally collected by cardiac puncture. Therefore, it is reasonable to compare cytokine values collected from the facial vein and the retro-orbital sinus; however, there is a significant deviation when comparing cytokines from distal sites of collection to the values obtained from the heart. It is also important to note that variability in cytokine measurements did not account for the differences observed in the different sample sites for plasma collected by cardiac puncture. To determine if the differences between the sampling sites could be due to assay variability, an intra-assay evaluation was performed. The intra-assay variability of analytical assays for plasma IL-6 expressed as coefficients of variation were less than 11% for each individual mouse. This held true for the entire range of IL-6 values, from a minimum of 0.490 ng/mL to a maximum of 695 ng/mL when collected by cardiac puncture (data not shown).
FIGURE 3.
IL-6 concentrations were similar when comparing plasma collected from facial vein and retro-orbital sinus, both of which are significantly diminished compared to cardiac puncture sampling. Blood was drawn 24 hours post CLP from common sites published in literature, including cardiac puncture, facial vein, and retro-orbital sinus in a subset of mice (n = 4). Plasma IL-6 collected by facial vein and retro-orbital sinus were calculated as a percent of IL-6 collected by cardiac puncture. It is clear that cytokine values following CLP collected by facial vein and retro-orbital sinus sampling were comparable to each other; while cytokines collected by cardiac puncture were elevated compared to other sampling sites.
Having established a clear difference in plasma IL-6 in two different inflammatory models based on sampling location, a more extensive evaluation of a profile of plasma cytokines was performed 24 hours following CLP, illustrated in Table 1. The discovery that plasma cytokines are elevated when collected from the heart appears to apply to not just IL-6, but also to other pro- and anti-inflammatory cytokines and chemokines. Statistically significant differences were readily apparent in the pro-inflammatory cytokine IL-1B, chemokines MIP-2 and KC, as well as anti-inflammatory IL-1RA. Of the six cytokines that were measured, all were statistically significantly elevated in cardiac blood compared to facial vein.
Table I.
Plasma Cytokines 24 Hours Following CLP Sampled by Facial Vein and Cardiac Puncture. Blood was collected 24 hours after CLP and the indicated cytokines were measured. For all of the cytokines the values from cardiac puncture were higher than the facial vein, and these differences were statistically significant for all of the six measured inflammatory mediators.
| Cytokine | Facial Vein (ng/mL) | Cardiac Puncture (ng/mL) | p value |
|---|---|---|---|
| Proinflammatory Cytokines | |||
| IL-6 | 70.9 | 211.1 | < 0.01 |
| IL-1B | 1.46 | 2.59 | < 0.05 |
| Chemokines | |||
| MIP-2 | 23.9 | 50.8 | < 0.05 |
| KC | 88.2 | 132.4 | < 0.01 |
| Anti-inflammatory Cytokines | |||
| IL-1RA | 13.7 | 33.8 | < 0.01 |
| IL-10 | 0.99 | 2.10 | <0.05 |
Discovering that plasma cytokine concentrations vary depending on sampling location following various inflammatory challenges, we sought out to determine if similar findings were present for other traditional clinical chemical parameters. Plasma blood urea nitrogen (BUN) is one clinical chemical parameter that has been measured and found to be consistent regardless of sampling site (28). We therefore evaluated plasma BUN from the same mice that underwent CLP. Similar to published studies, there was no statistically significant difference when comparing BUN collected by facial vein sampling and plasma collected by cardiac puncture (Figure 4A). Total protein in the plasma was also assessed. There was no difference in total protein values between the different sampling sites as values averaged 4.03 gm/dL when collected from the facial vein and 4.22 gm/dL (Figure 4B) from the heart. As it is well known, cytokines make up an almost negligible fraction of total protein concentration; therefore total protein in the plasma would not be expected to change dramatically despite large changes in cytokine values.
FIGURE 4.
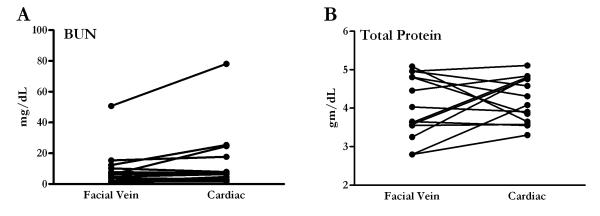
Clinical parameters of other plasma measurements are similar regardless of sampling site. (A) Blood urea nitrogen (BUN) concentrations were measured in plasma collected 24 hours following CLP from the same group of mice that underwent CLP (n = 16). (B) There was no difference in total protein when comparing total protein collected from the two different sites. Unlike the differences seen in cytokine values from two different sampling sites, there was no significant difference in BUN or total protein levels in the samples collected by facial vein or cardiac puncture.
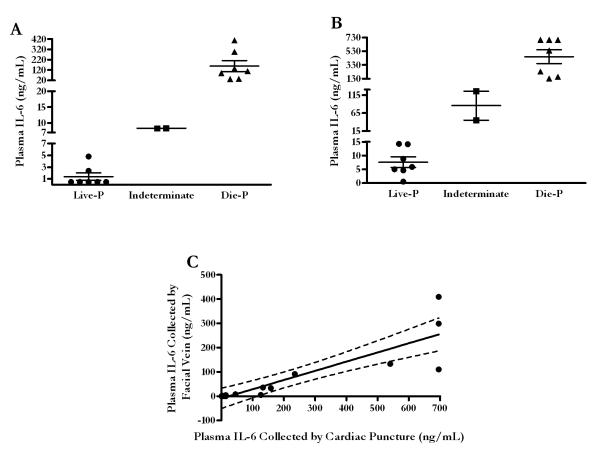
While it is essential to note the sampling location between various studies, it is also crucial to be aware of the impact of these findings beyond a laboratory finding. Plasma levels of IL-6 have been shown to be a very accurate predictor of mortality following a septic insult such as CLP in the murine model (29-31) and can be used to prospectively stratify mice into those predicted to survive the acute phase of sepsis and those predicted to die. However, as described above, stratifying mice with plasma collected from the heart could result in a misclassification of mice if using a scale based on plasma IL-6 values collected from peripheral sites. Mice that should be stratified in predicted to live group could be misclassified into an incorrect group (ie. indeterminate or predicted to die group). Figure 5A illustrates the discrimination values used to predict survival in mice following CLP based on IL-6 values. In our CLP model, which produces approximately 50% mortality in the acute phase of sepsis, the plasma values to predict survival were IL-6 < 6.3 ng/mL and the values to predict mortality were IL-6 > 19.6 ng/mL with a sensitivity of 93% and specificity of 100% when blood is collected from the facial vein. Figure 5B illustrates the dramatic difference in how the scale would change when blood is collected from the heart. Without correcting for the differences between the facial vein and cardiac puncture IL-6 values, nearly 40% of the mice in this sample size would be misclassified if the cardiac puncture values were assessed using the facial vein discrimination values (Figure 5B). A simple linear regression analysis was performed to demonstrate the relationship (Figure 5C). The Spearman r correlation coefficient was 0.9342 with p < 0.0001 for the 16 matched pairs that underwent CLP. Using a correction factor of 0.38 and a y-intercept of −9, 5 out of the 6 mice that had been misclassified into the predicted to live, indeterminate, and predicted to die categories based on the cardiac puncture IL-6 values were subsequently re-stratified into their correct group based on the facial vein IL-6 stratification. Ultimately, this model demonstrates that cytokines can be corrected to accurately predict survival when plasma is collected by cardiac puncture if the appropriate discrimination values are used.
FIGURE 5.
Misclassification of mortality prediction based on plasma IL-6. (A) All mice subjected to CLP were classified into Live-P, indeterminate, and Die-P based on specific plasma IL-6 values obtained by facial vein. (B) Mice stratified into the three predicted fates based on facial vein plasma IL-6 scale. Appreciating the scale on the y-axis would be vastly different and would result in misclassification if the much lower plasma cut off values used for the facial vein collected plasma were to be used. (C) A simple linear regression model was developed to help reclassify mice into their appropriate category for a more accurate prediction of mortality in acute phase of sepsis following CLP.
Discussion
There are multiple well established animal models that have examined the acute inflammatory response such as the infusion of endotoxin or inducing polymicrobial peritonitis by creating an injury to a protective barrier such as the gastrointestinal tract in the case of CLP. Plasma parameters such as cytokines and chemokines are often measured in these models to better characterize the inflammatory response. The techniques and methods used to induce and evaluate inflammation in both of these models are generally similar among labs. However, one important variable in these studies that may easily be overlooked is the location from which plasma is collected. Plasma may be collected from facial vein sampling, retro-orbital bleeding, cardiac puncture, tail vein sampling, etc; however, plasma from each of these sites may yield variable cytokine values depending on the location. This variability exists even if samples were collected from the same experimental animal at the same time. Determining if there is a difference in cytokine values between the variable locations is important when comparing studies that utilize similar models and measure similar inflammatory mediators and be consistent with the ARRIVE guidelines (13).
This is the first known study that compares plasma cytokine values from various sampling sites following an inflammatory insult. Our results demonstrate that sampling site has a significant effect when analyzing plasma cytokines in at least two of the most commonly used animal models of inflammation—an endotoxin model and CLP. We have demonstrated that pro-inflammatory and anti-inflammatory cytokines and chemokines collected from the heart were dramatically elevated compared to those collected from peripheral sites including the facial vein and retro-orbital sinus. Moreover, BUN, a commonly sampled clinical parameter of renal function, as well as total protein, demonstrated no statistically significant difference within the same animal irrespective of sampling location. This suggests that cytokines have unique characteristics that would cause them to be elevated centrally as opposed to peripheral sites.
While discovering that plasma cytokine concentrations are different depending on sampling site is novel, this is not the first time that discrepancies in plasma parameters have been demonstrated among sampling sites within the same animal. Prior reports illustrated decades ago that the location of plasma sampling has an effect on leukocyte counts. These studies sampled blood from the heart and tail tips, noting increased leukocyte counts the farther away blood was drawn from the heart (32, 33). These findings were confirmed more recently by Nemzek et al who demonstrated that regardless of sampling order, total WBC, neutrophils and lymphocytes were highest when blood was collected from the tail, followed by retro-orbital sinus, and least from the heart (34). While it is not clear why such differences are seen, it has been suggested that the greatest surface area in the vascular system is found peripherally at the capillary beds where blood stasis is greatest. This leads to a decrease in the flow of leukocytes and thus an accumulation of cells at the distal sampling sites.
It is unclear why cytokine values collected from different locations would vary. One theory is that the vascular response in acute inflammation, which leads to vasodilatation with an increase in microvasculature permeability and stasis, is greatest in the periphery. This promotes an outpouring of relatively small sized plasma proteins such as cytokines that would result in a decrease of its concentration within the intravascular space. Total protein remained unchanged suggesting that the majority of proteins were too large to extravasate into the interstitial space. Therefore, when peripheral blood is sampled, at the facial vein for example, the cytokine concentration may be lowest given that the vascular response is greatest in the capillary beds found in the periphery. This is also supported by the fact that plasma IL-6 values collected from the facial vein and heart in naïve mice who have not been subjected to either LPS administration or CLP were below detection levels (data not shown) when sampled from both sites; there is little to no micro-vascular permeability when the systemic inflammatory response has not been stimulated.
Several studies have evaluated cytokine measurements in the arterial blood supply and venous drainage of an organ, to determine if the cytokine was produced by that organ. It has been documented that following CLP there are differences in cytokine production in different organs (35). The intent of the current study was to document that the site of sampling may yield different cytokine concentrations, not to determine the anatomic location of cytokine production. A brief review of 20 published papers in the past 2 years shows that the sampling site was not stated in 40% of the manuscripts while cardiac puncture provided blood samples in 25% of the studies. As our manuscript demonstrates, knowing the source of the blood is necessary to compare cytokine values from different studies.
In conclusion, this study demonstrates that the location from which plasma is collected is critical when analyzing cytokine concentrations collected centrally from the heart and those collected from the periphery such as the facial vein and retro-orbital sinus. This is critical for two reasons. First, it makes us aware of disparities between cytokine values when comparing values collected from a site in one study relative to values collected from a different site in another study. Second and potentially most importantly, it highlights that if one is performing a terminal procedure on a mouse, such as a cardiac puncture, and an investigator needs to predict mortality in the acute phase of sepsis, the data presented here could be used to help differentiate and stratify mice into predicted to live and die categories. We have formulated a correction factor that will assist in properly assigning mice to the correctly predicted fate. While the correction factor used in this study is based on a small sample size and the data presented is not intended to serve as a normogram, a similar model could easily be developed that pertains to other labs. This information will help to standardize methods and allow investigators to directly compare the results from different laboratories, but collected from different sites.
Acknowledgements
We would like to thank Elizabeth Duffy for her laboratory expertise and contributions.
This work was performed in the Department of Pathology and Laboratory Medicine, Boston University School of Medicine, 670 Albany Street, Room 441, Boston, MA 02118, USA.
This work was supported by NIH grant RO1 GM 082962 and T32 GM 86308.
Footnotes
Disclosures The authors have no financial conflict of interests.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remick D. Use of animal models for the study of human disease-a shock society debate. Shock. 2013;40(4):345–6. doi: 10.1097/SHK.0b013e3182a2aee0. [DOI] [PubMed] [Google Scholar]
- 5.Osuchowski MF, Remick DG, Lederer JA, Lang CH, Aasen AO, Aibiki M, Azevedo LC, Bahrami S, Boros M, Cooney R, Cuzzocrea S, Jiang Y, Junger WG, Hirasawa H, Hotchkiss RS, Li XA, Radermacher P, Redl H, Salomao R, Soebandrio A, Thiemermann C, Vincent JL, Ward P, Yao YM, Yu HP, Zingarelli B, Chaudry IH. Abandon the Mouse Research Ship? Not Just Yet! Shock. 2014 doi: 10.1097/SHK.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177(3):1967–74. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 7.Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S, Cooney R, Levy H, Baughman R, Rumbak M, Light RB, Poole L, Allred R, Constant J, Pennington J, Porter S. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351(9107):929–33. [PubMed] [Google Scholar]
- 8.Fisher CJ, Jr., Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, Abraham E, Schein RM, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334(26):1697–702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 9.Remick D, Manohar P, Bolgos G, Rodriguez J, Moldawer L, Wollenberg G. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock. 1995;4(2):89–95. doi: 10.1097/00024382-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1(2):87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weixelbaumer KM, Raeven P, Redl H, van Griensven M, Bahrami S, Osuchowski MF. Repetitive low-volume blood sampling method as a feasible monitoring tool in a mouse model of sepsis. Shock. 2010;34(4):420–6. doi: 10.1097/SHK.0b013e3181dc0918. [DOI] [PubMed] [Google Scholar]
- 12.Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005;34(9):39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds P, Wall P, van Griensven M, McConnell K, Lang C, Buchman T. Shock supports the use of animal research reporting guidelines. Shock. 2012;38(1):1–3. doi: 10.1097/SHK.0b013e31825f396c. [DOI] [PubMed] [Google Scholar]
- 14.Xing L, Remick DG. Mechanisms of oxidant regulation of monocyte chemotactic protein 1 production in human whole blood and isolated mononuclear cells. Shock. 2007;28(2):178–85. doi: 10.1097/shk.0b013e3180311cf4. [DOI] [PubMed] [Google Scholar]
- 15.Remick DG, Garg SJ, Newcomb DE, Wollenberg G, Huie TK, Bolgos GL. Exogenous interleukin-10 fails to decrease the mortality or morbidity of sepsis. Crit Care Med. 1998;26(5):895–904. doi: 10.1097/00003246-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4(10):854–65. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 17.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 18.Ebong SJ, Call DR, Bolgos G, Newcomb DE, Granger JI, O’Reilly M, Remick DG. Immunopathologic responses to non-lethal sepsis. Shock. 1999;12(2):118–26. doi: 10.1097/00024382-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Biancotto A, Feng X, Langweiler M, Young NS, McCoy JP. Effect of anticoagulants on multiplexed measurement of cytokine/chemokines in healthy subjects. Cytokine. 2012;60(2):438–46. doi: 10.1016/j.cyto.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patil R, Shukre S, Paranjape R, Thakar M. Heparin and EDTA anticoagulants differentially affect the plasma cytokine levels in humans. Scand J Clin Lab Invest. 2013;73(5):452–5. doi: 10.3109/00365513.2013.798869. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan VV, Ravindran R, Wun T, Luciw PA, Khan IH, Janatpour K. Multiplexed measurements of immunomodulator levels in peripheral blood of healthy subjects: Effects of analytical variables based on anticoagulants, age, and gender. Cytometry B Clin Cytom. 2014 doi: 10.1002/cyto.b.21147. [DOI] [PubMed] [Google Scholar]
- 22.de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thavasu PW, Longhurst S, Joel SP, Slevin ML, Balkwill FR. Measuring cytokine levels in blood. Importance of anticoagulants, processing, and storage conditions. J Immunol Methods. 1992;153(1-2):115–24. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]
- 24.Nemzek JA, Siddiqui J, Remick DG. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods. 2001;255(1-2):149–57. doi: 10.1016/s0022-1759(01)00419-7. [DOI] [PubMed] [Google Scholar]
- 25.Craciun FL, Iskander KN, Chiswick EL, Stepien DM, Henderson JM, Remick DG. Early murine polymicrobial sepsis predominantly causes renal injury. Shock. 2014;41(2):97–103. doi: 10.1097/SHK.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iskander KN, Craciun FL, Stepien DM, Duffy ER, Kim J, Moitra R, Vaickus LJ, Osuchowski MF, Remick DG. Cecal ligation and puncture-induced murine sepsis does not cause lung injury. Crit Care Med. 2013;41(1):159–70. doi: 10.1097/CCM.0b013e3182676322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12(1):60–7. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnell MA, Hardy C, Hawley M, Propert KJ, Wilson JM. Effect of blood collection technique in mice on clinical pathology parameters. Hum Gene Ther. 2002;13(1):155–61. doi: 10.1089/10430340152712700. [DOI] [PubMed] [Google Scholar]
- 29.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17(6):463–7. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Turnbull IR, Javadi P, Buchman TG, Hotchkiss RS, Karl IE, Coopersmith CM. Antibiotics improve survival in sepsis independent of injury severity but do not change mortality in mice with markedly elevated interleukin 6 levels. Shock. 2004;21(2):121–5. doi: 10.1097/01.shk.0000108399.56565.e7. [DOI] [PubMed] [Google Scholar]
- 31.Moitra R, Beal DR, Belikoff BG, Remick DG. Presence of preexisting antibodies mediates survival in sepsis. Shock. 2012;37(1):56–62. doi: 10.1097/SHK.0b013e3182356f3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quimby FH, Saxon PA, Goff LG. Total White Cell Counts of Peripheral and Heart Blood of the Rat. Science. 1948;107(2783):447. doi: 10.1126/science.107.2783.447. [DOI] [PubMed] [Google Scholar]
- 33.Goldie H, Jones AM, Ryan H, Simpson M. Leukocyte counts in the blood from the tail and the heart of the mouse. Science. 1954;119(3089):353–4. doi: 10.1126/science.119.3089.353. [DOI] [PubMed] [Google Scholar]
- 34.Nemzek JA, Bolgos GL, Williams BA, Remick DG. Differences in normal values for murine white blood cell counts and other hematological parameters based on sampling site. Inflamm Res. 2001;50(10):523–7. doi: 10.1007/PL00000229. [DOI] [PubMed] [Google Scholar]
- 35.Salkowski CA, Detore G, Franks A, Falk MC, Vogel SN. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect Immun. 1998;66(8):3569–78. doi: 10.1128/iai.66.8.3569-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]