Abstract
There are heterogeneous approaches to cranial irradiation therapy (CRT) for T-lineage acute lymphoblastic leukemia (T-ALL). We performed a systematic review of studies that specified a radiation strategy and reported survival for pediatric T-ALL. Our analysis included 62 publications reporting 78 treatment groups (patient n=5844). The average event-free survival (EFS) was higher by 6% per 5 years (p<0.001). Adjusting for year, EFS differed by radiation strategy. Compared to the reference group (CRT for all) which had a year-adjusted EFS of 65% (95% confidence interval, CI: 61% to 69%) the adjusted EFS was significantly worse (rate difference (RD) = -9%, 95% CI: -15% to -2%) among studies that used a risk-directed approach to CRT (p=0.004). The adjusted EFS for the other strategies were not significantly different compared to the reference group: CRT for central nervous system positive patients only (RD = -3%, 95% CI: -14% to 7%, p=0.49); CRT omitted for all patients (RD = 5%, 95% CI: -4% to 15%, p=0.33). CRT may not be necessary with current chemotherapy for T-ALL. These associations, however, are susceptible to bias and caution should be applied in drawing definitive conclusions on the comparative effectiveness of alternative CRT strategies.
Keywords: acute lymphoblastic leukemia, radiation therapy, t-cell
Introduction
With modern treatment, survival outcomes for children with T-ALL are nearly equivalent to those of all but the lowest risk B-lineage ALL patients [1-4]. These excellent survival outcomes have been documented primarily in single arm cooperative group studies that have evaluated chemotherapy in combination with various cranial radiation therapy (CRT) strategies. There has been a trend to reduce the use of CRT for pediatric T-ALL patients as the intensity of systemic and intrathecal (IT) chemotherapy has increased over the last two decades. Reducing the use of CRT in pediatric T-ALL is desirable in order to limit the late effects of CRT such as secondary malignancies, endocrine abnormalities and cognitive impairment [5]. However, modifications in treatment for pediatric T-ALL patients should be carefully considered given that T-ALL patients have an inferior survival after relapse compared to those with B-ALL [6].
Currently, approaches to the use of CRT for pediatric T-ALL are variable, with some cooperative groups administering CRT to all T-cell patients, some omitting CRT in all patients, and some using a risk-stratified approach [3, 7-12]. We hypothesized that there was limited comparative evidence on the effectiveness and safety of various prophylactic and therapeutic CRT strategies in pediatric T-ALL in the context of current treatment. We sought to explore the evidentiary basis for the movement to reduce the administration of CRT for pediatric T-ALL by means of a methodologically rigorous synthesis of the totality of the available evidence, based on which we draw principled conclusions.
Here, we report a systematic review and meta-analysis of survival data from prospective and retrospective cohort studies in children and adolescents with T-ALL who were treated with one of four prophylactic or therapeutic CRT strategies: (a) CRT for all patients (studies that administered CRT to ≥90% of patients (prophylactic strategy)); (b) risk-directed CRT (studies that administered CRT to a subset of patients based on clinical characteristics such as age and white blood cell count (WBC) at diagnosis (prophylactic strategy for a subset of patients)); (c) CRT for patients with involvement of the CNS with leukemia (CNS positive) at diagnosis only (therapeutic strategy); and (d) CRT omitted for all patients. Our primary aim was to determine if there was an association between a CRT strategy and superior survival in the context of current systemic and intrathecal chemotherapy.
Methods
A protocol was developed prior to the conduct of the systematic review and submitted to PROSPERO [13]. When applicable, we followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement for reporting our results [14].
Literature search
We searched MEDLINE for studies published from inception to December 15, 2013 that reported studies describing central nervous system (CNS) directed therapy comprised of chemotherapy and/or radiation therapy for T-ALL using free-text and MESH terms (e.g., “acute lymphoblastic leukemia,” “drug therapy”, “radiotherapy”) [13]. After three investigators (MK, TAT, SKP) piloted the abstract screening on a subset of 100 studies; one investigator (MK) screened the titles and abstracts of studies returned by the search. We did not set any language restrictions in searches or during abstract screening. We excluded non-English language publications at the full-text screening stage because of resource constraints.
Eligibility criteria and study selection
One investigator (MK) reviewed full-text articles to determine if studies met eligibility criteria. To be eligible for inclusion studies needed to have included at least 10 participants with T-ALL who were younger than 22 years of age at presentation and had to have reported the primary outcome for this review, 5-year event-free survival (EFS) specifically for those with T-ALL. However given that relapse is infrequent after three years in T-ALL patients, we also included one study that reported 3-year EFS for three cohorts [15] and explored the effect of including these few additional studies with shorter follow-up in a sensitivity analysis. When reviewing studies for eligibility, we considered randomized studies that either: (a) compared CNS-directed therapies while treating with an identical systemic chemotherapy “backbone” or (b) compared different systemic chemotherapy strategies while treating with an identical CNS prophylactic strategy and considered prospective and retrospective cohort studies (comparative or single-group) [16]. Overall survival (OS) and reports of the site of relapse (CNS only, bone marrow only, or combined CNS and bone marrow) were secondary outcomes of interest. We accepted the outcome definitions employed in the included studies.
Studies were categorized on the basis of their CRT strategy into the following a priori defined categories that included both prophylactic and therapeutic CRT strategies: (a) CRT for all patients (studies that administered CRT to ≥90% of patients (prophylactic strategy)); (b) risk-directed CRT (studies that administered CRT to a subset of patients based on clinical characteristics such as age and white blood cell count (WBC) at diagnosis (prophylactic strategy for a subset of patients)); (c) CRT for patients with involvement of the CNS with leukemia (CNS positive) at diagnosis only (therapeutic strategy); and (d) CRT omitted for all patients.
When results for a cohort of subjects were reported in multiple publications the “primary” publication from the study was identified as the first publication reporting EFS for the cohort and was used as the primary source for extraction of data. When details regarding the treatments received or EFS statistics were incomplete in the “primary” publication, review articles or subsequent follow-up articles were used to obtain the missing data. For 20 cohorts we identified multiple publications reporting results on the same patient population (some publications reported on multiple cohorts) [4, 17-20]. In all but two cases the reported data relevant to our analysis were identical. In the two cases, we used data from the primary publication in main analyses, and conducted sensitivity analyses with data from follow-up publications [17, 21, 22].
Data collection and extraction
Two investigators (MK and MG) extracted data and verified the other's extracted information; discrepancies were resolved by consensus involving a third investigator (IJD). We extracted the following information from each eligible study: eligibility criteria; number of patients; CRT strategy (including dose and timing); intrathecal (IT) chemotherapy administered (methotrexate alone vs. double or triple IT therapy) and number of doses; steroids administered; cumulative dose of high-dose methotrexate (sum of all doses ≥1 gram/m2), asparaginase, and anthracyclines; definition of EFS (as we expected heterogeneity in the definition of EFS among the various studies); median follow-up; and outcomes (5-year EFS, 5-year OS, and sites of relapse, with their corresponding standard errors). Baseline demographic and clinical characteristics such as median age, proportion of males, and WBC count at baseline were recorded. However, since these characteristics were rarely provided specifically for the subset of T-ALL patients (as opposed to the entire cohort of ALL patients) these characteristics were not included in the analysis.
Statistical analysis
We obtained summary 5-year EFS and OS probabilities using an inverse variance random effects model for the corresponding Kaplan-Meier estimates [23]. We assessed between study heterogeneity using Cochran's Q statistic [24] and the I2 index [25]. The p-value of the Q-statistic was considered statistically significant at PQ<0.1. I2 represents the proportion of between-study heterogeneity that is beyond chance and takes values from 0 to 100%. We conducted subgroup analyses and univariable random effects meta-regressions to explore associations between EFS and the following a priori selected study-level factors: CRT strategy; IT chemotherapy; maximum number of IT chemotherapy doses; use of high-dose methotrexate (dose ≥ 1 gram/m2), intensive asparaginase (categorically defined as ≥ 400,000 IU/m2 or administration of PEG-asparaginase), high cumulative dose of anthracyclines (daunorubicin plus doxorubicin total ≥300 mg/m2); induction steroid; EFS definition; the year enrollment started for the study; and cumulative dose of asparaginase, high dose methotrexate, and anthracyclines. The meta-regressions generated rate differences in EFS for different levels of categorical variables and for changes in continuous variables. All meta-regression analyses were repeated after adding “year enrollment began” as a covariate, to account for trends over time. A multivariable meta-regression was performed for the primary analysis, the association of CRT strategy with EFS. We performed several sensitivity analyses to assess the robustness of our findings (Methods and Results Supplement).
Assessment of study validity / quality assessment
In lieu of a scale to assign quality scores to the studies, [26] we assessed the following study-level characteristics, which could help us understand the association between CRT and EFS: (a) prospective or retrospective study design; (b) whether the definition of EFS was reported; (c) whether EFS estimates include failures before attainment of remission as outcome events; (d) whether the median follow-up was reported; and (e) whether relapses were categorized by site.
Results
Included studies
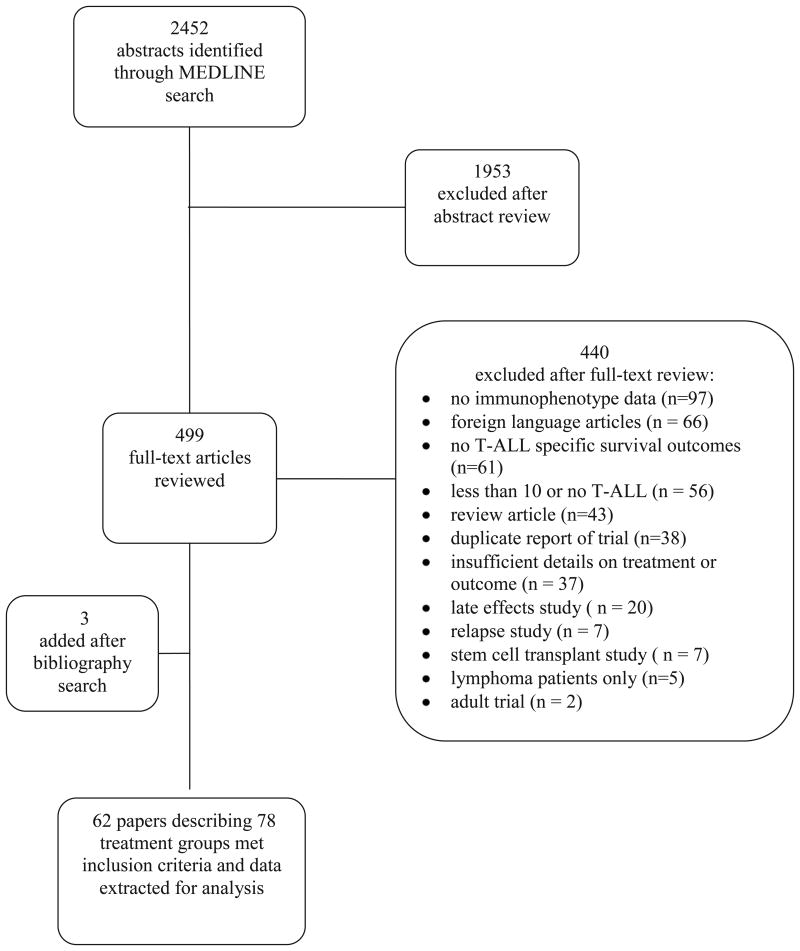
The search returned 2452 abstracts, of which 499 were considered potentially relevant and were reviewed in full text. We found 62 eligible articles (5844 patients with T-ALL enrolled between 1973 and 2011) describing 78 treatment groups (7 studies reported on more than 1 group; Fig 1, Supplemental Fig 1, Supplemental Table I, & Supplemental references #1-59). The search did not return any RCTs that specifically reported EFS among T-ALL patients randomized to treatment with or without CRT. Of the 78 treatment groups, 75 were included in prospective single-group studies and three were included in retrospective single-group studies. The Results Supplement and Supplemental Table II provide a summary of our assessment of study quality.
Figure 1.
Search strategy flowchart.
Event-free survival
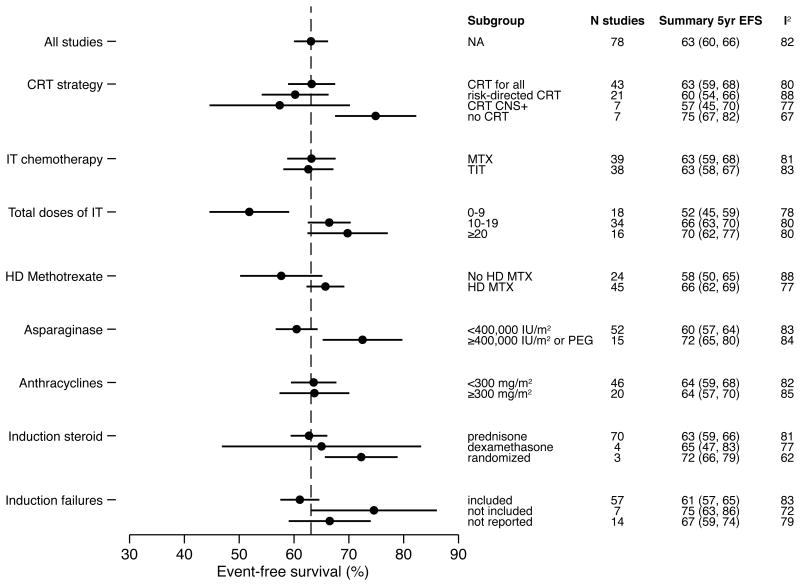
The overall 5-year EFS rate was 63% (95% CI: 60% to 66%; Fig 2 & Supplemental Fig 1). There was extensive heterogeneity among the treatment studies (I2=82%, PQ<0.001). Slightly more than half of the groups (n=43, 55%) administered CRT to all T-ALL patients, (total subject n=2581 (44%)). Twenty-one groups (27%) used a risk-directed approach (total subject n=2207 (38%)) and seven groups (9%) administered CRT to CNS positive patients only (total subject n=315 (5%)). Seven groups (9%) omitted CRT completely (total subject n=741 (13%)). Subgroup and meta-regression analyses demonstrated that studies in the four CRT strategy groups had similar mean EFS (omnibus p-value for comparison across all categories= 0.08): CRT for all patients, 63% (95% CI: 59% to 66%); risk-directed CRT, 60% (95% CI: 54% to 66%); CRT for CNS positive patients only, 57% (95% CI: 45% to 70%); and CRT omitted 75% (95% CI: 67% to 82%), (Fig 2 and Table I). Five-year EFS was higher (absolute rate difference, RD =12%; 95% CI: 1% to 24%; p=0.03) among studies that omitted CRT for all patients compared to the studies that administered CRT to all patients (reference group).
Figure 2.
Results of subgroup meta-analyses.
Table I. Association of treatment characteristics with EFS.
| Treatment characteristic | Subgroup | N studies | 5-yr EFS of reference group (95% CI) and absolute rate differences of comparison subgroups (95% CI) | Omnibus p-value | Adjusteda 5-yr EFS of reference group (95% CI) and absolute rate differences of comparison subgroups (95% CI) | Omnibus p-value |
|---|---|---|---|---|---|---|
| CRT | CRT for all (reference) | 43 | 63 (59, 68) | 0.08 | 65 (61,69) | 0.02 |
| Risk-directed CRT | 21 | -3 (-11, 5) | -9 (-15, -2) | |||
| CNS + only | 7 | -6 (-18, 7) | -3 (-14, 7) | |||
| No CRT | 7 | 12 (1, 24) | 5 (-4, 15) | |||
| IT chemotherapy | MTX (reference) | 39 | 63 (58, 68) | 0.89 | 65 (61, 69) | 0.11 |
| TIT | 38 | 0 (-1, 1) | -5(-11, 1) | |||
| Total doses of IT | 0-9 (reference) | 18 | 52 (45, 59) | <0.001 | 56 (49, 64) | 0.16 |
| 10-19 | 34 | 14 (6, 22) | 8 (-1, 17) | |||
| >=20 | 16 | 18 (8, 27) | 10 (-1, 21) | |||
| HD Methotrexate | No HD MTX (reference) | 24 | 58 (52,64) | 0.045 | 61 (55, 66) | 0.40 |
| HD MTX | 45 | 8 (0, 15) | 3 (-4, 10) | |||
| Asparaginase | <400,000 IU (reference) | 52 | 60 (56, 65) | 0.006 | 60 (57, 64) | 0.007 |
| >=400,000 IU or PEG | 15 | 12 (4, 20) | 10 (3, 17) | |||
| Anthracycline | <300 mg/m2(reference) | 46 | 64 (59, 68) | 0.97 | 62 (58, 65) | 0.14 |
| >=300 mg/m2 | 20 | 0 (-8, 8) | 5 (-2, 12) | |||
| Induction steroid | prednisone (reference) | 70 | 63 (59, 66) | 0.54 | 63 (60, 66) | 0.49 |
| dexamethasone | 4 | 2 (-14, 19) | -9 (-24, 6) | |||
| randomized | 3 | 9 (-7, 25) | -2 (-16, 12) | |||
| EFS definition | includes induction failures (reference) | 57 | 61 (57, 65) | 0.06 | 61 (58, 65) | 0.07 |
| excludes induction failures | 7 | 14 (1, 26) | 12 (2,23) | |||
| definition not reported | 14 | 5 (-3, 14) | 1 (-6, 9) | |||
| Enrollment year | per 5 years | 6 (4, 9) | <0.001 | N/A | N/A | |
| Asparaginase | per 100,000 IU/m2 | 3 (1, 5) | <0.001 | 2 (1, 4) | 0.004 | |
| HD Methotrexate | per 5 grams/m2 | 1 (-1, 3) | 0.20 | 1 (-1, 2) | 0.33 | |
| Anthracycline | per 100 mg/m2 | 0 (-4, 3) | 0.77 | 2 (-1, 4) | 0.27 |
Adjusted for enrollment start year.
EFS= event-free survival;CRT=cranial irradiation therapy; IT = intrathecal; HD = high dose; PEG = polyethylene glycosylated asparaginase.
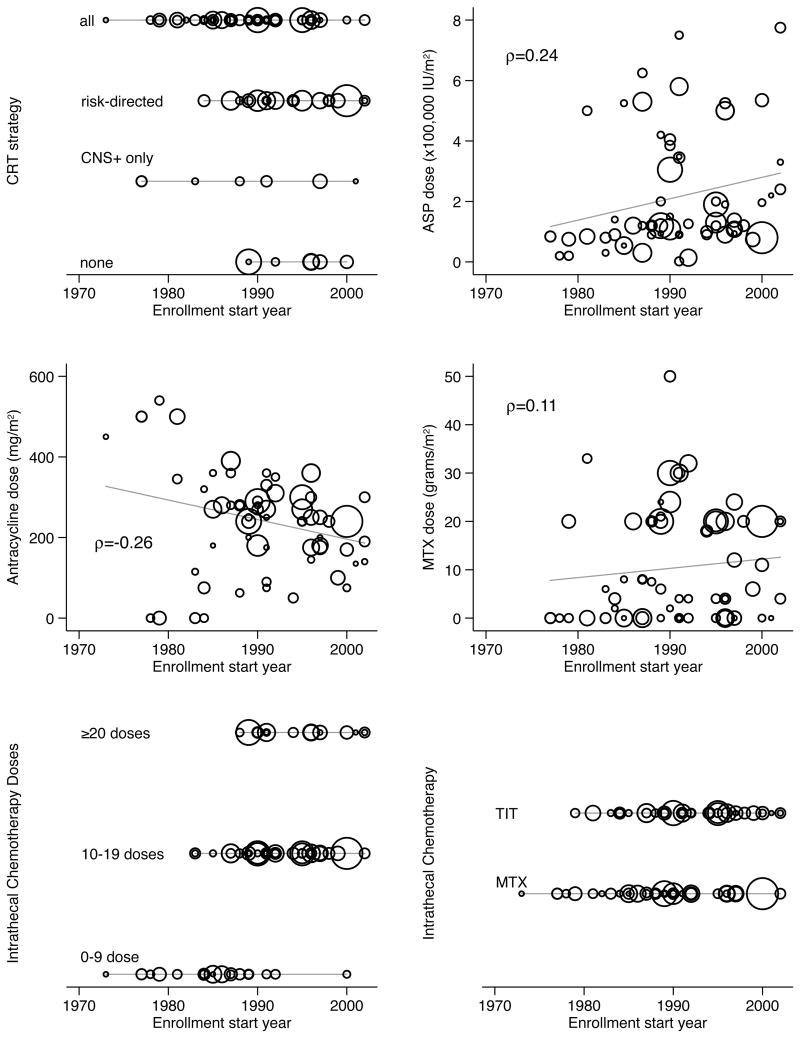
This difference in EFS should not be uncritically attributed to the CRT strategies. Figure 3 plots each CRT strategy over enrollment start year; more current studies were more likely to omit CRT. More recent studies also reported the use of higher cumulative doses of asparaginase and high-dose methotrexate and the administration of more IT chemotherapy.
Figure 3.
Evolution of treatment strategies over time.
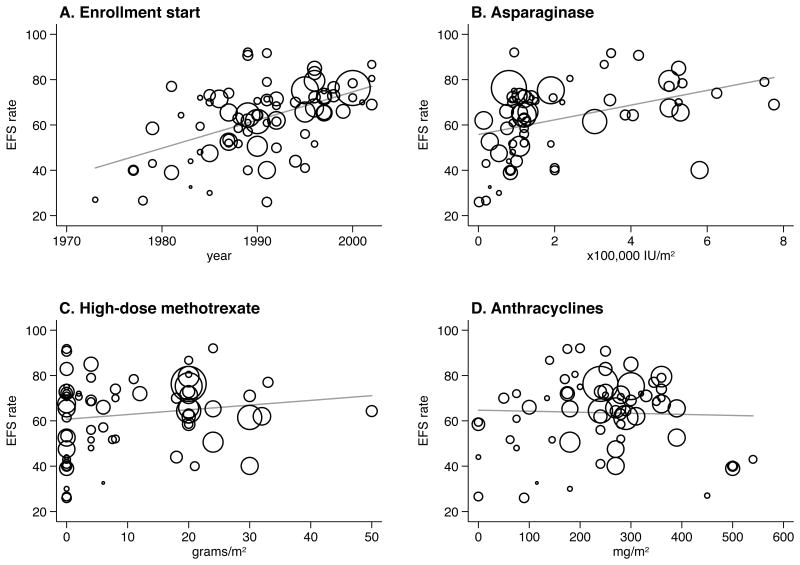
EFS was significantly associated with the year study enrollment began (p<0.001); in random effects meta-regression average EFS was higher by 6% (95% CI: 4% to 9%) per 5 calendar years (Table I, Fig 4). The following factors were also associated with higher EFS on univariable analysis: the administration of 10-19 or ≥20 doses of IT (as compared to <10 doses), the administration of high-dose methotrexate, and intensive asparaginase administration (Table I, Figs 2 & 4).
Figure 4.
Event-free survival meta-regression plots. The area of the circles represents the weight of each study in the meta-analysis.
After adjusting for enrollment year there remained differences in the same direction in EFS by CRT strategy (Table I); however, the EFS differences across the four groups became statistically significant (omnibus p= 0.02). Compared to the reference group (CRT for all) the adjusted EFS was significantly worse (RD = -9%, 95% CI: -15% to -2%) among studies that used a risk-directed approach to CRT (p=0.004). The adjusted EFS for the other CRT strategies were not significantly different when compared to the reference group: CRT for CNS positive patients only (RD = -3%, 95% CI: -14% to 7%, p=0.49); CRT omitted for all patients (RD = 5%, 95% CI: -4% to 15%, p=0.33). Similarly, intensive asparaginase dosing also remained significantly associated with higher EFS after adjustment for enrollment year when analyzed as a continuous or categorical variable (p=0.004 and p=0.007, respectively; Table I). A multivariable meta-regression, which analyzed the association of CRT strategy with EFS while adjusting for enrollment year and continuous asparaginase dose, showed qualitatively similar results to the year-adjusted regression; however the difference across CRT strategies was no longer statistically significant (omnibus p=0.10; Table II).
Table II. Multivariable meta-regression analysis for 5-year EFS.
| CRT strategy | N studiesa | Adjustedb 5-yr EFS of reference group (95% CI) and absolute rate differences of comparison subgroups (95% CI) | Omnibus p-value |
|---|---|---|---|
| CRT for all (reference) | 33 | 64 (59, 68) | 0.10 |
| Risk-directed CRT | 20 | -6 (-14, 1) | |
| CNS + only | 7 | -2(-13, 10) | |
| No CRT | 5 | 7 (-5, 19) |
Some studies had missing data for asparaginase dose and were excluded from this analysis.
Adjusted for enrollment start year and asparaginase dose (continuous variable).
CI = confidence interval; CRT = cranial irradiation; EFS = event-free survival.
Our findings were robust to extensive sensitivity analyses (Results Supplement). Results were also qualitatively similar for the outcome of OS (both in meta-analyses and meta-regression analyses; Supplemental Figs 2-4 and Supplemental Table III).
Discussion
Prospective cohort studies of childhood T-ALL have demonstrated excellent outcomes with varied CRT approaches [3, 7, 8, 11] leading to calls to restrict or completely omit CRT for all pediatric ALL patients [5]. Two individual patient data meta-analyses have demonstrated that the administration of CRT does not improve survival for B-lineage ALL patients in the context of current therapy, however, these meta-analyses provide limited data for which to draw conclusions on the role of CRT specifically for T-lineage patients [27, 28]. Meanwhile over the past two decades while there has been a reduction in the frequency of CRT administration and the dose of CRT administered for T-ALL patients there have been simultaneous intensifications of systemic chemotherapy and intrathecal chemotherapy. The intensive administration of high-dose methotrexate[12], asparaginase,[3, 29] and dexamethasone, [29, 30] have sought to prevent both marrow and CNS relapses making it difficult to clarify the role of CRT within the context of current systemic and intrathecal chemotherapy for T-ALL.
We sought to summarize the available evidence for this important and evolving aspect of T-ALL treatment. Our synthesis of studies spanning almost 30 years found that the evidence base is comprised entirely of single group noncomparative cohort studies. We found on average that EFS for T-ALL improved over time. There were also indications that intensive chemotherapy with asparaginase was associated with improvement in EFS. In multivariable meta-regression analyses, adjusted for year of treatment and continuous dose of asparaginase, we found similar EFS among studies that used any of three approaches: CRT for all patients, CRT for CNS positive patients, or CRT omitted. However, the evidentiary basis for this conclusion is weak and susceptible to bias. It is well understood that drawing casual inferences from noncomparative studies is precarious, even if there is a “clear signal” of improved outcomes [16, 31]. We used state-of-the-science methods (random effects meta-regression methods) to understand how clear a signal the single arm trials provide, under the best-case (but implausible) scenario that the comparison is unbiased. We demonstrate that, even if one were willing to use this body of evidence for making causal claims, no clear signals exist favoring any particular CRT strategy.
To definitively determine the association of CRT with survival for T-ALL better research is needed. A randomized controlled trial (RCT) of CRT for T-ALL could determine the treatment effect of CRT when applied to a uniform approach to systemic and IT chemotherapy. Data from our analyses may be a good starting point for sample size calculations. We found that studies that omitted CRT had mean EFS higher by 5% compared to those that administered CRT to all participants (this result is from meta-regressions adjusted for the year enrollment started). If the expected EFS probabilities in a hypothetical patient population are 75% and 70% among children not receiving and children receiving CRT, respectively, a RCT would need to enroll 2942 patients (1471 per arm) to attain power of 85% (for a two-sided alpha of 5%) for detecting the EFS difference between the two treatments. It is unlikely that such a trial will be done because of the large number of patients needed, the time and expense required, and because of preferences for treating with and without CRT among international cooperative groups. A more pragmatic approach is to conduct a meta-analysis of individual patient data (MIPD). Such a meta-analysis would allow for better estimating if there is a relapse risk reduction with the administration or omission of CRT because patient and treatment level characteristics (such as age, sex, WBC count at diagnosis, cumulative asparaginase, number of intrathecal chemotherapy doses) could be adjusted for in the analysis. A MIPD could be completed more quickly than an RCT and offers an opportunity to align the major stakeholders in ALL therapy to address this important clinical question.
In conclusion, there is insufficient evidence to determine if survival is improved in pediatric T-ALL by a treatment regimen including or not including CRT. Further, the decision to administer CRT with initial therapy is complicated by the paucity of comparative data on cure rates after relapse, especially stratified by site of relapse and by initial CRT strategy. Treatment strategies for T-ALL should also consider the long-term effects of treatment with systemic and intrathecal chemotherapy alone or a regimen that also includes CRT. Comparisons of neurocognitive outcomes among children with ALL treated with systemic and intrathecal chemotherapy alone compared to those who additionally received 18 Gy of CRT have demonstrated mild differences in neurocognitive outcomes between the two groups [32, 33]. Future research on this topic should integrate health-related quality of life following therapy with or without CRT with survival outcomes, particularly in the context of the lower doses of CRT (12-18 Gy) used in contemporary treatment protocols.[11, 29]. A decision analysis could incorporate information on the short and long-term outcomes of alternative strategies, along with patient/parent preferences.
Supplementary Material
Acknowledgments
The authors thank Doris Hernandez, research assistant in the Institute for Clinical Research and Health Policy Studies at Tufts Medical Center, for assistance with manuscript preparation and Amy Lapidow, research librarian at Tufts University School of Medicine, who assisted with the literature search.
Funding: This work was funded in part by a KM1 award to Dr. Kelly from the National Cancer Institute (KM1 CA 156726, PI: Selker). The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Note: Supplementary information is available at the journal's website.
Author contributions: MK, TAT, and SKP developed the research plan. MK and MG extracted data. MK and IJD performed the statistical analysis, with input from TAT. All authors interpreted the data. MK drafted the manuscript and TAT, IJD, MG, and SKP critically revised it for important intellectual content.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Moricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–84. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 2.Gaynon PS, Angiolillo AL, Carroll WL, Nachman JB, Trigg ME, Sather HN, et al. Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children's Oncology Group Report. Leukemia. 2010;24:285–97. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverman LB, Stevenson KE, O'Brien JE, Asselin BL, Barr RD, Clavell L, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000) Leukemia. 2010;24:320–34. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pui CH, Mullighan CG, Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 2012;120:1165–74. doi: 10.1182/blood-2012-05-378943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008;22:2142–50. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veerman AJ, Kamps WA, van den BH, van den BE, Bokkerink JP, Bruin MC, et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997-2004) Lancet Oncol. 2009;10:957–66. doi: 10.1016/S1470-2045(09)70228-1. [DOI] [PubMed] [Google Scholar]
- 8.Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arico M, Valsecchi MG, Rizzari C, Barisone E, Biondi A, Casale F, et al. Long-term results of the AIEOP-ALL-95 Trial for Childhood Acute Lymphoblastic Leukemia: insight on the prognostic value of DNA index in the framework of Berlin-Frankfurt-Muenster based chemotherapy. J Clin Oncol. 2008;26:283–9. doi: 10.1200/JCO.2007.12.3927. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell C, Payne J, Wade R, Vora A, Kinsey S, Richards S, et al. The impact of risk stratification by early bone-marrow response in childhood lymphoblastic leukemia: results from the United Kingdom Medical Research Council trial ALL97 and ALL97/99. Brit J Haematol. 2009;146:424–36. doi: 10.1111/j.1365-2141.2009.07769.x. [DOI] [PubMed] [Google Scholar]
- 11.Schrappe M, Valsecchi MG, Bartram CR, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118:2077–84. doi: 10.1182/blood-2011-03-338707. [DOI] [PubMed] [Google Scholar]
- 12.Asselin BL, Devidas M, Wang C, Pullen J, Borowitz MJ, Hutchison R, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children's Oncology Group (POG 9404) Blood. 2011;118:874–83. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly M, Trikalinos T, Parsons S, Dahabreh I, Gianferante DM. Cranial irradiation for pediatric T-lineage acute lymphoblastic leukemia and lymphoma: a systematic review and meta-analysis. PROSPERO 2012:CRD42012002442. 2012 doi: 10.1002/ajh.23784. Available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42012002442. [DOI] [PMC free article] [PubMed]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falletta JM, Shuster JJ, Crist WM, Pullen DJ, Borowitz MJ, Wharam M, et al. Different patterns of relapse associated with three intensive treatment regimens for pediatric E-rosette positive T-cell leukemia: a Pediatric Oncology Group study. Leukemia. 1992;6:541–6. [PubMed] [Google Scholar]
- 16.Ip S, Paulus JK, Balk EM, Dahabreh IJ, Avendano EE, Lau J. Role of Single Group Studies in Agency for Healthcare Research and Quality Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. Internet. Report No.: 13-EHC007-EF. [PubMed] [Google Scholar]
- 17.Pui CH, Pei D, Sandlund JT, Ribeiro RC, Rubnitz JE, Raimondi SC, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–82. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamps WA, van der Pal-de Bruin KM, Veerman AJ, Fiocco M, Bierings M, Pieters R. Long-term results of Dutch Childhood Oncology Group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia. 2010;24:309–19. doi: 10.1038/leu.2009.258. [DOI] [PubMed] [Google Scholar]
- 19.Conter V, Arico M, Basso G, Biondi A, Barisone E, Messina C, et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:255–64. doi: 10.1038/leu.2009.250. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchida M, Ohara A, Manabe A, Kumagai M, Shimada H, Kikuchi A, et al. Long-term results of Tokyo Children's Cancer Study Group trials for childhood acute lymphoblastic leukemia, 1984-1999. Leukemia. 2010;24:383–96. doi: 10.1038/leu.2009.260. [DOI] [PubMed] [Google Scholar]
- 21.Rivera GK, Raimondi SC, Hancock ML, Behm FG, Pui CH, Abromowitch M, et al. Improved outcome in childhood acute lymphoblastic leukemia with reinforced early treatment and rotational combination chemotherapy. Lancet. 1991;337:61–6. doi: 10.1016/0140-6736(91)90733-6. [DOI] [PubMed] [Google Scholar]
- 22.Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, et al. Improved outcome for children with acute lymphoblastic leukemia: Results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104:2690–6. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–60. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 27.Clarke M, Gaynon P, Hann I, Harrison G, Masera G, Peto R, et al. CNS-directed therapy for childhood acute lymphoblastic leukemia: Childhood ALL Collaborative Group overview of 43 randomized trials. J Clin Oncol. 2003;21:1798–809. doi: 10.1200/JCO.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 28.Richards S, Pui CH, Gayon P. Systematic review and meta-analysis of randomized trials of central nervous system directed therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;60:185–95. doi: 10.1002/pbc.24228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vrooman LM, Stevenson KE, Supko JG, O'Brien J, Dahlberg SE, Asselin BL, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study--Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31:1202–10. doi: 10.1200/JCO.2012.43.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teuffel O, Kuster SP, Hunger SP, Conter V, Hitzler J, Ethier MC, et al. Dexamethasone versus prednisone for induction therapy in childhood acute lymphoblastic leukemia: a systematic review and meta-analysis. Leukemia. 2011;25:1232–8. doi: 10.1038/leu.2011.84. [DOI] [PubMed] [Google Scholar]
- 31.Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. 2nd. Stamford: Cengage Learning; 2001. [Google Scholar]
- 32.Waber DP, Turek J, Catania L, Stevenson K, Robaey P, Romero I, et al. Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 Gy cranial radiation as CNS treatment in acute lymphoblastic leukemia: findings from Dana-Farber Cancer Institute ALL Consortium Protocol 95-01. J Clin Oncol. 2007;25:4914–21. doi: 10.1200/JCO.2007.10.8464. [DOI] [PubMed] [Google Scholar]
- 33.Krull KR, Brinkman TM, Li C, Armstrong GT, Ness KK, Srivastava DK, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the st jude lifetime cohort study. J Clin Oncol. 2013;31:4407–15. doi: 10.1200/JCO.2012.48.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.