Abstract
The mechanisms which allow cancer cells to adapt to the typical tumor microenvironment of low oxygen and glucose and high lactate are not well understood. GPR81 is a lactate receptor recently identified in adipose and muscle cells that has not been investigated in cancer. In the current study, we examined GPR81 expression and function in cancer cells. We found that GPR81 was present in colon, breast, lung, hepatocellular, salivary gland, cervical and pancreatic carcinoma cell lines. Examination of tumors resected from pancreatic cancer patients indicated that 94% (148/158) expressed high levels of GPR81. Functionally, we observed that the reduction of GPR81 levels using shRNA mediated silencing had little effect on pancreatic cancer cells cultured in high glucose, but led to the rapid death of cancer cells cultured in conditions of low glucose supplemented with lactate. We also observed that lactate addition to culture media induced the expression of genes involved in lactate metabolism including monocarboxylase transporters in control, but not in GPR81 silenced cells. In vivo, GPR81 expression levels correlated with the rate of pancreatic cancer tumor growth and metastasis. Cells in which GPR81 was silenced showed a dramatic decrease in growth and metastasis. Implantation of cancer cells in vivo was also observed to lead to greatly elevate levels of GPR81. These data support that GPR81 is important for cancer cell regulation of lactate transport mechanisms. Furthermore, lactate transport is important for the survival of cancer cells in the tumor microenvironment.
Keywords: lactate, GPR81, monocarboxylate transporters, pancreas cancer, metabolism
Introduction
The increased energy demands required for the chronic and uncontrolled proliferation of malignant cells within the hypoxic and glucose-poor environment of a solid tumor requires alterations in cellular metabolism (1, 2). In tumors, where oxygen availability is limited and fluctuating, cells undergo metabolic adaptations including a switch to aerobic glycolysis, termed the “Warburg effect” (3), which results in higher rates of glycolysis, reduced pyruvate oxidation and increased lactate production (4-6). Elevated lactate concentrations in the tumor microenvironment, ranging from 5-20mM (7-9), may under some circumstances provide an alternate metabolic fuel (5, 6, 10, 11). Elevated levels of lactate within cancer cells have also been shown to disable glycolysis and glutathione synthesis (12). Thus, lactate levels are carefully maintained by way of specific transporters, termed monocarboxylase transporters (MCTs) (6, 13, 14). MCT1 has a high-affinity for lactate and is primarily responsible for lactate influx. Its expression is regulated, in part by peroxisome proliferator-activated receptor (PPAR)-γ co-activator (PGC)-1α (15). PGC-1α has a broad range of biologic activities including mitochondrial biogenesis and glucose/fatty acid metabolism (16). Conversely, MCT4, a hypoxia-inducible MCT, has a low-affinity for lactate and is adapted to release lactate from cells (13, 14). Proper targeting of MCT1 and MCT4 to the plasma membrane requires association with the chaperone, CD147 (17). In tumors, PGC-1α, MCT1, MCT4 and CD147 expression is common, and preclinical studies of MCT1 and CD147 inhibition have validated their use as potential therapeutic targets (10, 18).
Previously, lactate has generally been regarded as a waste product of metabolism. However, in skeletal muscle, lactate has been shown to act as a signaling molecule (19). Skeletal muscle cells stimulated with increasing concentrations of lactate have increased mRNA levels of MCT1 and PGC1α (19). However, the mechanism of lactate regulation of MCTs in skeletal muscle is still unknown. To our knowledge, a similar regulation of the lactate transporters by lactate has not been reported in cancer cells. However, lactate has been shown to indirectly impact several biologically-significant activities in tumors (20), such as hypoxia-independent regulation of HIF-1α (21-24), and lactate-uptake dependent NF-κB/IL-8 activity in endothelial cells (25).
Recently, a receptor for lactate has been described, termed GPR81 (26, 27). This Gi-coupled receptor is expressed mainly in adipocytes (28-30) but has also been found in skeletal muscle (27, 31) and in brain (32). In adipocytes, high glucose levels result in increased insulin-dependent glucose uptake and increased conversion of glucose to lactate. Lactate was found to activate GPR81, which reduced the conversion of ATP to cAMP and reduced lipolysis (27-30). However, the expression of GPR81 and its role in lactate regulation of MCTs have not previously been reported in cancer.
In this study, we demonstrate that GPR81 is highly expressed in multiple cancer cell types including pancreatic ductal adenocarcinoma. Silencing of GPR81 rendered cells insensitive to lactate levels that increased MCTs and PGC-1α in control cells. Reduction of GPR81 levels in xenografted cancer cells reduced tumor growth and metastasis in vivo. Silencing of GPR81 also decreased tumor cell mitochondrial activity and decreased tumor cell proliferation when lactate was the only available energy source. These data confirm the importance of lactate metabolism in cancer and identify GPR81 as an important regulator.
Materials & Methods
Cell Culture
MiaPaca-2, HPAF II, HPAC, Capan-I, Capan-II, BxPc3, ASPC1, CFPAC-1, Panc-3, SU 86.86, SW48, HCT116, LoVo, SK-Hep-1, Hep G2, A549, H3118, A253, NCI-H292, and MCF7 were from ATCC. The immortalized normal human pancreatic ductal epithelial (HPDE) cell line was kindly provided by Dr. M. S. Tsao (University of Toronto, Ontario, Canada). L36.pl cells were provided by Dr. Isaiah Fidler (MD Anderson Cancer Center, Houston TX) and Panc-28 and Panc-48 cells were provided by Dr. Paul Chiao (MD Anderson Cancer Center, Houston TX). MOH cells were provided by Dr. R. Mohammad (Wayne State University, MI) and SiHa cervical cancer cell line provided by Dr. Anil Sood (MD Anderson Cancer Center, Houston, TX). MD Anderson pancreatic adenocarcinoma tumor cells 1 and 3 (MDA-PATC1 and MDA-PATC3) cells were derived from primary tumorgrafts. Cell line authenticated using DNA fingerprinting upon receipt and passaged for fewer than 6 months prior to experiments (data not shown). Cells were routinely cultured in recommended media. All cells were maintained at 37° C in a humidified atmosphere of 5% CO2
Tissue specimens
The primary tumors analyzed in this study were derived from the University of Texas MD Anderson Cancer Center and conformed to the policies and practices of the MD Anderson Cancer Center Internal Review Board. The tissue microarrays used in this study were constructed using formalin-fixed, paraffin embedded archival tissue blocks from the pancreatectomy specimens from 133 patients with stage II PDAC. The matched hematoxylin & eosin (H & E) stained slides were reviewed to identify the representative areas for tumor and benign pancreas. For each patient, two cores of tumor and one core of paired benign pancreatic tissue were sampled from representative areas using a 1.0-mm punch. The tissue microarrays were constructed using a tissue microarrayer (Beecher Instruments, Sun Prairie, WI) as described previously (33).
Real-time PCR
RNA was prepared using TRIzol (Invitrogen, Grand Island, NY) according to the manufacturer's instructions. The quality of RNA was evaluated using spectrophotometry. The cDNA used for subsequent PCR was made using iScript (Bio-Rad Laborato- ries, Hercules, CA) and SensiMix real-time PCR kit was used for real-time PCR (Bioline, Taunton, MA). 18s rRNA was used as an internal reference gene to normalize input cDNA. Primer sequences used are listed in Supplemental Table 1. We used the comparative cycle threshold method to compute relative expression values (34).
Immunocytochemistry
Cells were plated on chamber slides and maintained overnight at 37°C in a mixture of 5% CO2 and 95% air in DMEM supplemented with 10% fetal bovine serum (FBS). Cells were fixed in acetone and blocked with 4% fish gelatin for 20 min. Rabbit anti-GPR81 (Abnova, Walnut, CA) was used at 1:120 dilution and incubated overnight at 4°C. Negative controls were done using isotype control antibodies (Jackson ImmunoResearch, West Grove, PA). Following washes, the appropriate fluorophore-conjugated secondary antibody was added (Jackson Immunoresearch, West Grove, PA), nuclei stained with Hoescht (1μg/ml), and slides covered using VECTASHIELD mounting medium (Vector laboratories, Burlingame, CA). Sections were examined on a Zeiss Axioplan2 microscope and images captured with a Hamamatsu ORCA-ER camera with Image-Pro Plus software (Media Cybernetics, Rockville, MD) and analyzed using Simple PCI software (Hamamatsu Corporation, Sewickley, PA).
Immunohistochemistry
Tissues were either fixed in 4% formalin then embedded in paraffin or snap frozen in liquid nitrogen and embedded in ornithine carbamyl transferase medium and sectioned. Paraffin embedded sections were deparaffinized with xylene and rehydrated with ethanol. Antigen retrieval was performed with diva decloaker (Biocare Medical, Concord, CA) in a steamer for 20 minutes. Endogenous peroxidase was blocked with 4% H2O2 and protein-blocked with 4% fish gelatin. Frozen sections were fixed in acetone, briefly air dried, and blocked with 4% fish gelatin for 30 minutes. Primary antibodies were incubated overnight at 4°C and included: rabbit anti-GPR81 (Abnova, Walnut, CA), 1:50 dilution; rabbit anti-MCT1 (Santa Cruz Biotechnology, Santa Cruz, CA), 1:50 dilution; rabbit anti-PGC1α (Novus Biologicals, Littleton, CO), 1:50 dilution; anti-Ki67 (Thermo Fischer Scientific, Waltham, MA). Negative controls were done using isotype control antibodies (Jackson ImmunoResearch, West Grove, PA). Following washes, VECTASTAIN® ABC systems (Vector laboratories, Burlingame, CA) was added to paraffin sections per manufacturer protocol, developed with 3,3-diaminobenzidine substrate, counterstained with hematoxylin and mounted with water soluble mounting media. Frozen sections were developed with fluorophore-conjugated secondary antibody, as described above.
Transient transfection of small interfering RNA
BxPC3 cells were plated on 100-mm dishes and transiently transfected with prevalidated FlexiTube GeneSolution siRNAs (siControl and siGPR81) at a final concentration of 5 nmol/L (Qiagen, Inc., Valencia, CA) with Hiperfect transfection reagent (Qiagen, Inc., Valencia, CA), and lysates were prepared for RT-PCR after 24h as described above.
Stable knockdown of short hairpin RNA and overexpression plasmid
Capan-II cells were infected with recombinant non-replicative lentiviral plasmid from Sigma (St. Louis, MO) containing human shGPR81 (transfected with 2 different shRNA constructs for GPR81 (shGPR81) or with a control plasmid (pLKO.1-puro)) obtained from Sigma. Each construct was co-transfected with packaging constructs PMD.2 and psPAX2 from Addgene (Cambridge, MA). Lentivirus was produced in 293FT cells using LipofectAMINE 2000 reagent (Invitrogen, Carlsbad, CA). Cells were infected with lentivirus (500 μl supernatant/ml medium) mixed with polybrene (4 μg/ml). Stable expression of shControl, shGPR81-1, and shGPR81-2 were established in Capan-II cells by selecting for puromycin resistance (1 mg/mL). For GPR81 gain of function studies, control pcDNA and pcDNA expressing GPR81 (Origene, Rockville, MD) were stably transfected into ASPC1 cells. Silencing or overexpression of GPR81 were confirmed using QPCR and immunocytochemistry as described above (Supp. Fig 1).
Lactate-Stimulation Assays
Capan-II shControl & shGPR81 cells or ASPC1-low &ASPC1-high cells were grown in DMEM without glucose, glutamine and pyruvate (Gibco, Grand Island, NY) with 20mM lactate and 2% FBS were added to a 6 well plate. Cells were maintained at 37°C in a humidified, 5% CO2 atmosphere. At 1-hour and 6-hours after lactate-stimulation, culture media was aspirated and RNA harvested using TRIzol as above.
Mitochondrial Metabolism and Cell Death Assay
shControl & shGPR81 cells or ASPC1-low &ASPC1-high cells in DMEM supplemented with 5% FBS ± lactate or DMEM without glucose or glutamine with 20mM lactate and 2% FBS were added to the wells of a 96 well plate. Metabolic activity of mitochondria was assayed using a modified MTT assay kit, (Promega, Madison, WI). MTS solution was added and the absorbance at 490 nm was recorded after incubation for 1h. Cell death was determined using the Hoescht uptake method. shControl or shGPR81 cells were plated in 24-well plates. Media was changed twice daily × 48 hours to maintain low lactate levels. Media was replaced with DMEM without glucose, glutamine or pyruvate with 20mM lactate and 2% FBS and cell death was determined after 24 hours incubation. Stained nuclei (dead cells) were visualized under a fluorescence microscope. The total dead cell area/total cell area was calculated using Simple PCI software.
Quantitation of Lactate
Cell culture supernatants were harvested for lactate quantitation, according to the experimental conditions described above. Lactate concentration was determined using the YSI 2900 Biochemistry Analyzer (YSI Incorporated, Yellow Springs, OH). All assays were performed in triplicate with results normalized to the L-lactate Calibrator Standard (YSI Incorporated). % change lactate uptake was calculated as follows: [lactate-1 hour] / [lactate-6 hour] and graphed.
In vivo studies
Capan-II stably-silenced GPR81 cells or ASPC1 cells stably-overexpressing GPR81 were also transfected stably with luciferase gene by lentivirus transfection (35, 36). 1.8 × 105/50 μl cells were injected into the tail of the pancreas. Tumor growth was assessed every week by bioluminescence imaging for 6 weeks using a cryogenically cooled imaging system coupled to a data acquisition computer running LivingImage software (Xenogen Corp, Alameda, CA). Before imaging, animals were injected subcutaneously with 40 mg/mL of luciferin potassium salt in PBS at a dose of 150 mg/kg body weight. Signal intensity was quantified as the sum of all detected photons within the region of interest per second. Tumor volume, peritoneal dissemination, and metastasis were assessed. Tissues were also fixed with formaldehyde or snap frozen in liquid nitrogen and examined histologically. For the survival study, assessment of animals was done by an experienced observer blinded to the treatment group. Animals were sacrificed for humane purposes if weight loss was >15% of body weight or if they had ascites and weight gain of >10%.
Statistics and Study Approval
Data were analyzed using GraphPad software (GraphPad Prism version 6.0 for Windows, San Diego, CA). Results are expressed as mean ± SEM and analyzed by t-test or ANOVA and results are considered significant at p<0.05. Human cell lines were utilized without access to identifiers and approved by the MD Anderson Institutional Review Board. Animal studies were conducted with the approval and following the recommendations of the MD Anderson Institutional Animal Use Committee.
Results
GPR81 is expressed in cancer
To determine if GPR81 was present in cancer cells of solid tumors, we analyzed its mRNA levels in various cancer cell lines. GPR81 was expressed in colon, breast, lung, hepatocellular, cervical and salivary gland carcinoma cells growing in vitro (Fig. 1A). Pancreatic tumors exist within a microenvironment known to be particularly high in lactic acid and low in oxygen (37), so this tumor type was selected for further study. GPR81 mRNA was detected in 10 of 17 (59%) PDAC cell lines (Fig. 1B). These mRNA levels corresponded with levels of GPR81 protein as indicated by immunofluorescence (Fig 1C). Higher levels of GPR81 protein were noted in BxPC3 cells (higher levels of GPR81 mRNA) and lower levels were found in MDA-PATC3 primary PDAC cells (lower levels of mRNA; Fig. 1C). When tumors resected from PDAC patients were examined using immunohistochemistry, nearly all (148/158, 94%) highly expressed GPR81, whereas normal pancreas had only low levels of expression (Fig. 1D). These results demonstrate that GPR81 is expressed in many cancer cell types and in nearly all human PDAC tumors.
Figure 1. GPR81 is highly expressed in cancer.
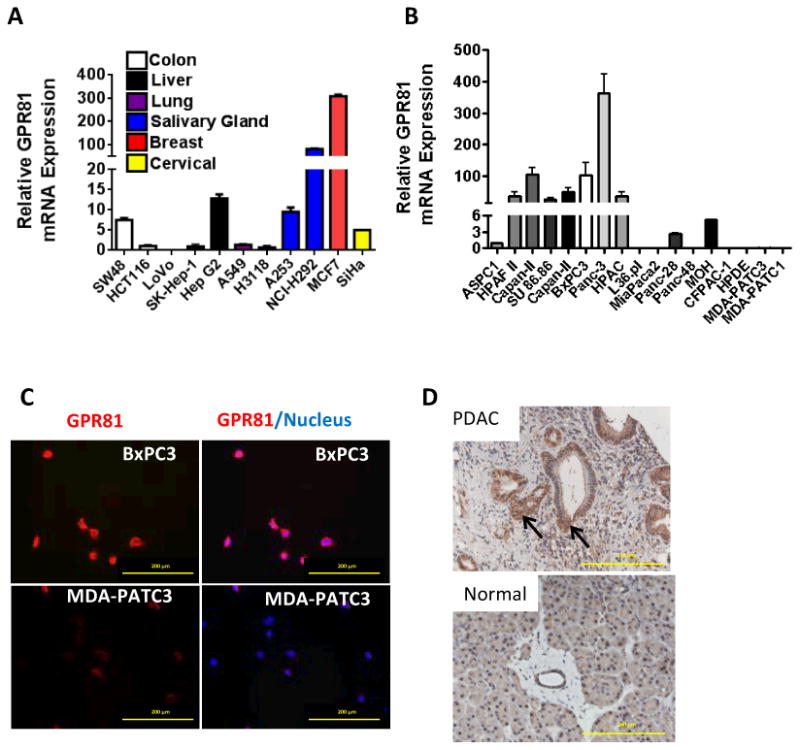
(A) Relative mRNA expression of GPR81 in several different cancer cell lines. Data are mean ± SEM. n ≥ 3.
(B) Relative mRNA expression of GPR81 in 16 PDAC cell lines and immortalized normal human ductal epithelial cells (HPDE). Data are mean ± SEM. n ≥ 3.
(C) BxPC3 (top) or MDA-PATC3 cells (bottom) were co-immunostained for GPR81 (red) and DAPI (blue). Scale bar, 200μm. n ≥ 3.
(D) Representative immunohistochemical staining GPR81 (arrow) in resected human samples of normal pancreas and PDAC. Scale bar, 200μm.
GPR81 regulates expression of genes involved in lactate uptake and metabolism
Lactate regulates expression of MCTs in skeletal muscle cells through as yet unknown mechanisms (19). To determine the relationship between GPR81 and genes involved in lactate metabolism, GPR81 levels were manipulated in both directions and the effects of lactate on MCTs, mitochondrial activity and cell viability were analyzed. Capan-II and BxPC3 PDAC cells, which have high basal levels of GPR81 in vitro, were stably silenced using siRNA (BxPC3) or shRNA (Capan-II, Supp. Fig. 1A-D). ASPC1 cells, which express low levels of GPR81 in vitro (Fig. 1A) were transfected with lentiviral control plasmid (ASPC1-low) or a GPR81-expressing plasmid (ASPC1-high; Supp. Fig. 1E-F). Under standard culture conditions, GPR81-silenced Capan-II and BxPC3 cells had reduced levels of MCTs and PGC-1α compared to the control cells (Fig. 2A-B; Supp Fig. 2). In addition, GPR81-silenced cells higher levels of lactate in the culture media, indicating reduced lactate uptake or increased lactate production compared to shControl cells (Fig. 2C). In conditions simulating the tumor microenvironment (low glucose, glutamine and pyruvate), lactate treatment of parental Capan-II cells expressing GPR81 led to increased levels of MCT1, MCT4, CD147 and PGC1α mRNA after 6 hours (Fig. 2D). In contrast, after GPR81 silencing, lactate treatment had no effect on the mRNA levels of these molecules (Fig. 2D). In addition, GPR81-silenced cells had reduced levels of lactate uptake at 6-hours compared to control cells at 6-hours (Fig 2E). Increased levels of GPR81 expression in transfected ASPC1 cells was associated with increased elevation of MCT1 and PGC1α mRNA following lactate stimulation as well as increased lactate uptake (Fig. 2F & G). Taken together, these data support a role for GPR81 in the regulation of genes involved in lactate uptake and metabolism.
Figure 2. GPR81 regulates expression of genes involved in lactate metabolism and lactate uptake and is required for cancer cell survival when lactate is the primary fuel source.
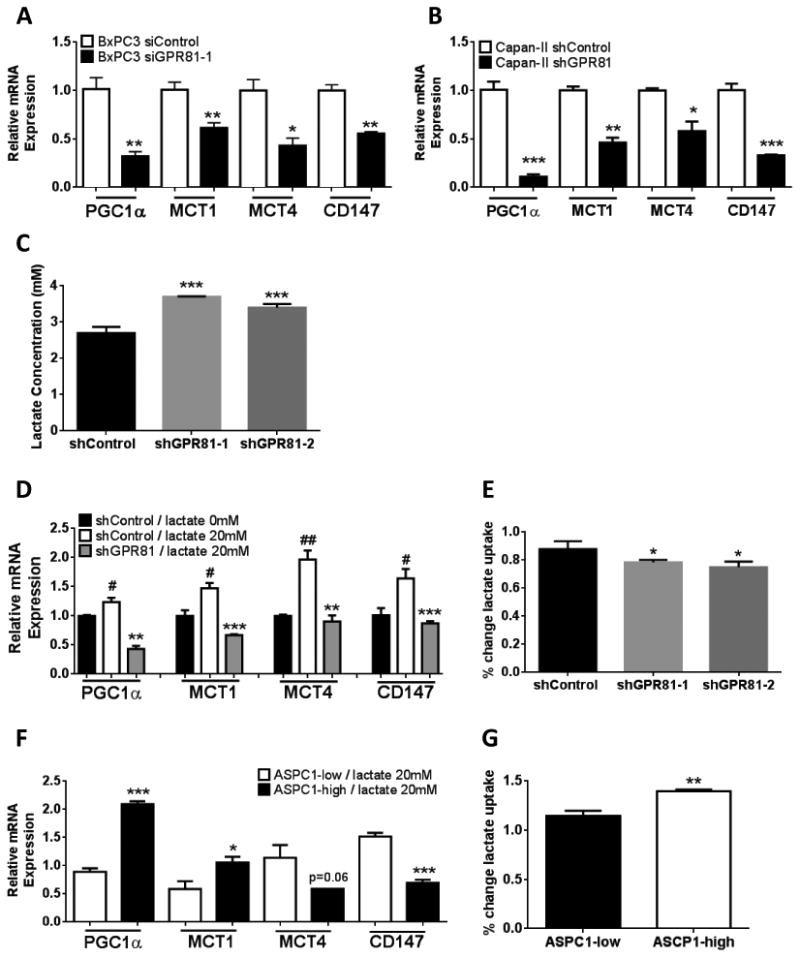
(A-B) Relative mRNA expression of PGC1α, MCT1, MCT4 and CD147 mRNA in BxPC3 (A) or Capan-II (B) si- or shcontrol and si- or shGPR81, grown in DMEM + 10% FBS.
(C) Lactate concentration in the supernatant of Capan-II shControl and shGPR81 were measured using YSI 2900 Biochemistry Analyzer after 24-h of culture in fresh medium; n = 3.
(D & F) Relative mRNA expression of PGC1α, MCT1, MCT4 and CD147 mRNA in (D) Capan-II shControl or shGPR81 cells or (F) ASPC1-low and ASPC1-high cells after lactate stimulation (+) in DMEM without glucose, glutamine or pyruvate + 20mM lactate and 2% FBS for 6 hours normalized to shControl /lactate 0mM. vs. shControl / 20mM lactate. Data are mean ± SEM. n ≥ 3. # vs. lactate 0mM;
(E & G) Lactate concentration in the supernatant of (E) Capan-II shControl or shGPR81 cells or (G) Lactate uptake was measured in ASPC1-low and ASPC1-high cells after 1-h & 6-h of culture DMEM without glucose, glutamine or pyruvate + 20mM lactate and 2% FBS; n = 3.% change lactate uptake was calculated as follows: [lactate-1 hour] / [lactate-6 hour]. **p <0.01 *p<0.05, **p<0.01,***p<0.001 by t-test.
Loss of GPR81 alters mitochondrial activity when lactate is the primary fuel source
Lactate has been previously suggested as an alternative energy source for cancer cells. To determine whether GPR81 was required for cancer cells to utilize lactate as an energy source, we measured mitochondrial activity in cells with manipulated levels of GPR81 expression. When Capan-II cells were cultured in the presence of glucose, minimal differences were observed in mitochondrial activity between cells with high or low levels of GPR81 cells (Fig. 3A). However, when the cells were grown in media lacking glucose, glutamine and pyruvate and with 20mM lactate as the main available energy source, silencing of GPR81 led to an ∼50% reduction in mitochondrial activity within 24 hours (Fig. 3B). In concert, there was no difference in mitochondrial activity of ASPC1 cells with high or low levels of GPR81 when cultured in the presence of glucose (Fig. 3C). In addition, elevated expression of GRP81 rescued ASPC1 cells from the decrease in mitochondrial activity observed in media where lactate was the primary fuel source (Fig. 3D). Cell death was also greatly elevated (500% increase) in Capan-II cells lacking GPR81 at 24 hours (Fig. 3E). These data indicated that GPR81 was required for tumor cell survival when lactate was the major available fuel source.
Figure 3. GPR81 is required for cancer cell survival when lactate is the primary fuel source.
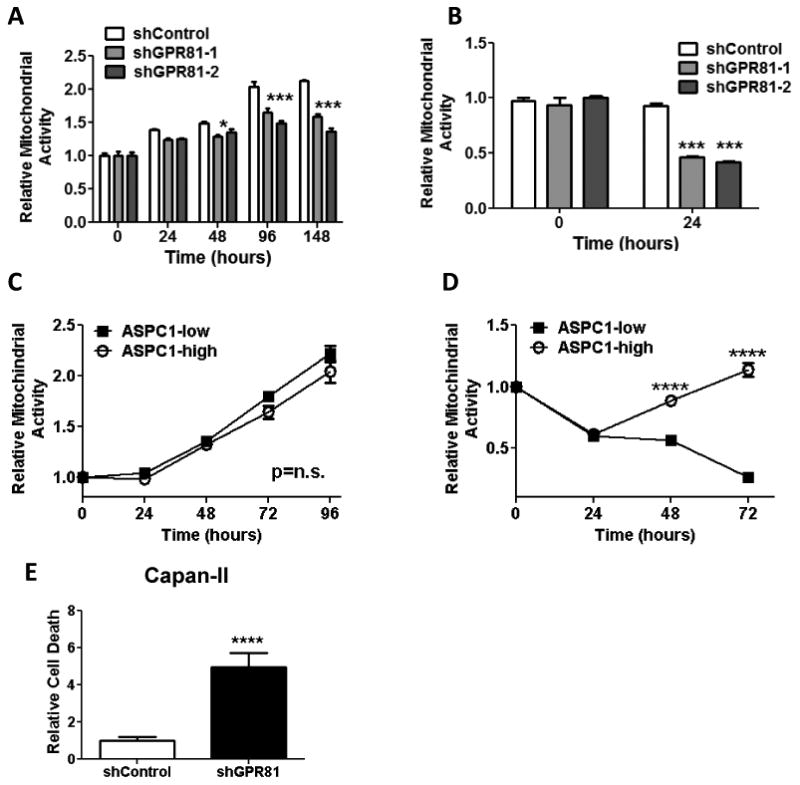
(A-D) Mitochondrial activity of Capan-II shControl and shGPR81 cells (A & B) or ASPC1-low and ASPC1-high cells (C & D) measured by MTS assay in DMEM + 5% FBS (A & C) or DMEM without glucose, glutamine or pyruvate + 20mM lactate and 2%FBS (B & D). Data are mean ± SEM, normalized to day 0. n ≥ 3, . *p<0.05, ***p<0.001; ****p<0.0001 by ANOVA.
(E) Relative cellular death, as measured by Hoescht uptake at 24 hours of shControl and shGPR81 Capan-II cells. Total dead cell area (DAPI stained nuclei)/total cell area (brightfield) was calculated using Simple PCI software and normalized to shControl cells. Data are mean ± SEM, normalized to day 0. n ≥ 3; ****p<0.0001 by t-test.
GPR81 is required for rapid tumor growth and metastasis and silencing leads to reduced survival
We next performed in vivo studies to evaluate the role of GPR81 in tumor growth. For these studies, GPR81 levels were manipulated in both directions and the effects on tumor growth were compared with control cells. Capan-II shControl and shGPR81 cells were implanted orthotopically in nude mice and tumor growth was monitored using non-invasive bioluminescence imaging. Tumors from Capan-II shGPR81 cells with reduced GPR81 levels grew at a significantly slower rate than those in shControl animals (Fig. 4A). Animals possessing Capan-II shGPR81 tumors also had significantly longer median survival (70 days) than animals with tumors formed with shControl cells (42 days; Fig. 4B). Low levels of GPR81 were also associated with decreased metastatic burden (Fig. 4C) and decreased cancer cell proliferation, as evidenced by Ki67 localization (Fig. 4D). MCT1 levels were found to be greatly reduced in tumors formed from cells lacking GPR81 (Fig. 4E-F). Of interest, GPR81 protein levels were found to be variable and to correlate with tumor size in the Capan-II shGPR81 xenografts. Small shGPR81 tumors had minimal levels of GPR81 expression while larger tumors expressed levels similar to shControl tumors (Fig. 4G). As the cell population utilized in this experiment was not clonal, this observation suggests that cells with lower levels of GPR81 expression were selected against in vivo.
Figure 4. GPR81 is required for rapid tumor growth and metastasis and leads to reduced survival.
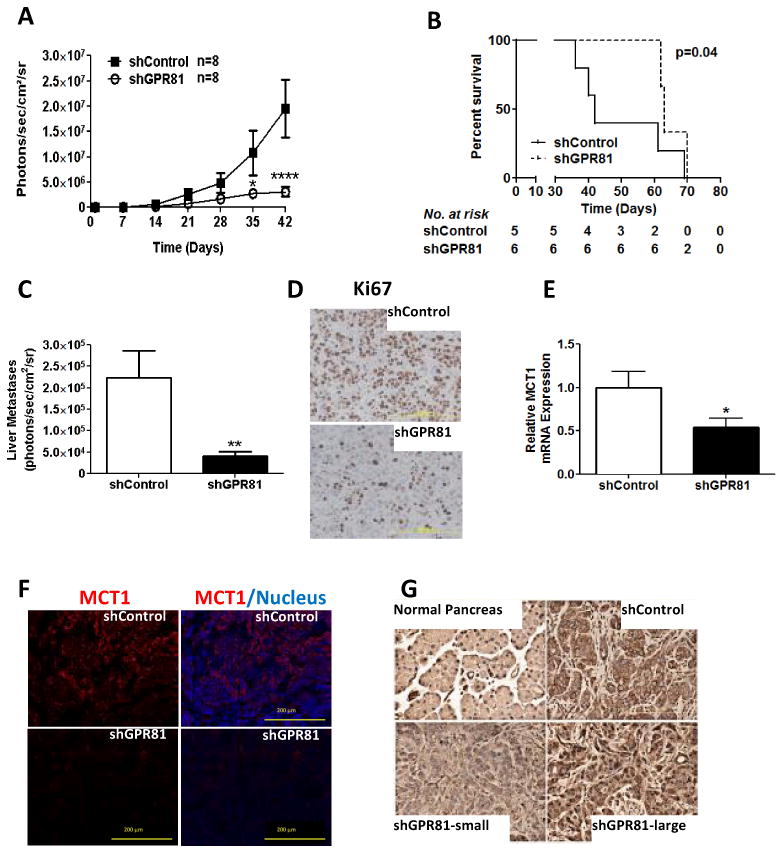
(A) Tumor growth estimated using bioluminescent imaging for Capan-II shControl or shGPR81 cells stably transfected with luciferase and injected into the tail of the pancreas of athymic nude mice (n=8/group).
(B) Kaplan-Meier survival graph demonstrating overall survival of animal injected with Capan-II shControl or shGPR81 cells. Median survival: shControl 42 days; shGPR81 70 days.
(C) Liver metastatic disease (estimated with bioluminescent imaging) at 6 weeks in Capan-II shControl or shGPR81 tumors. Data are mean photons/sec/cm2 ± SEM. n=8/group.
(D) Representative images of immunohistochemistry of Ki67 in Capan-II shControl and shGPR81. Scale bar, 200μm. n ≥ 5/group.
(E) Representative images of immunofluorescence for MCT1 (red) and DAPI in Capan-II shControl (top) and shGPR81 (bottom) tumors (Scale bar, 200μm).
(F) Quantification of MCT1 mRNA levels, relative to shControl. Data are mean ± SEM. n ≥ 4. *p<0.05 by t-test.
(G) Representative images of immunohistochemistry for GPR81 in Capan-II shControl or shGPR81 large and small tumors. Scale bar, 200μm. n ≥ 3/group.
In contrast to the diminished tumor growth observed after reducing GPR81 levels in Capan II cells, we found no difference in tumor growth (Fig. 5A) or metastatic disease (Fig. 5B) between ASPC1-low GPR81 and ASPC1-high GPR81 cells. However, examination of tumors taken from these animals at the end of the experiment surprisingly showed no difference in GPR81 levels between ASPC1-low and ASPC1-high tumors in vivo, both of which had elevated expression of GPR81 (Fig. 5C). These data support the hypothesis that higher levels of GPR81 expression are favored in vivo.
Figure 5. GPR81 is up-regulated in the tumor microenvironment.
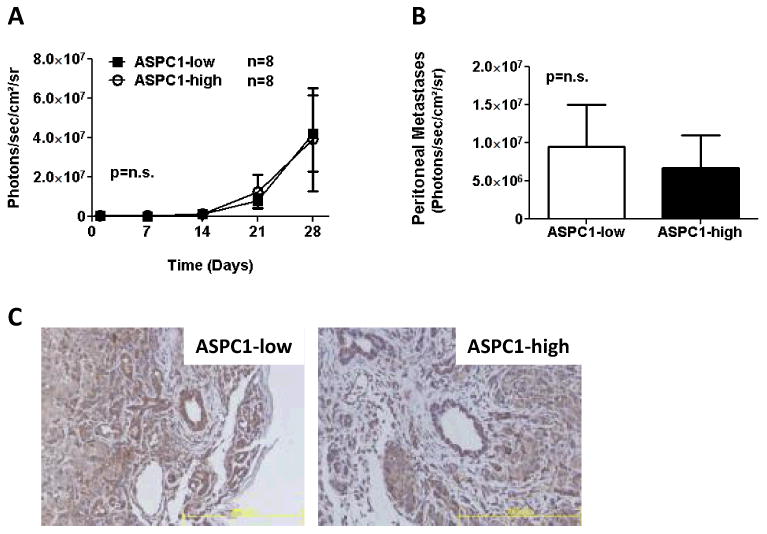
(A) Tumor growth estimated using bioluminescent imaging of ASPC1-low or ASPC1-high cells stably transfected with luciferase, injected into the tail of the pancreas of athymic nude mice (n=8/group).
(B) Metastatic disease to the peritoneum estimated using bioluminescent imaging at 4 weeks at the time of sacrifice. Data are mean photons/sec/cm2/sr ± SEM.
(C) Representative images of immunohistochemistry of GPR81 expression in ASPC1-low or ASPC1-high tumor sections. Scale bar, 200μm. n ≥ 5/group.
Discussion
In this study, we sought to determine the presence and function of GPR81 in cancer. GPR81 is a Gi-coupled receptor, which is expressed mainly in adipocytes, but is also present at low levels in a variety of normal cells (30). Altered cellular metabolism is a hallmark of cancer and lactate metabolism has increasingly been recognized to have a critical role for tumor cell survival (1, 2, 6, 10). In this study, we demonstrated that several cancer cell types, including colon, breast, lung, cervical and pancreatic express GPR81. In contrast, normal pancreatic duct cells express very low levels of GPR81. This aberrant expression pattern of GPR81 suggests a potential broad role of GPR81 in tumorigenesis. Functional studies indicated that GPR81 is important for lactate regulation of genes involved in lactate uptake and metabolism. GPR81 was not critical for cancer cell survival when glucose was abundant, as is found in typical tissue culture conditions. However, in the absence of glucose and the presence of lactate, GPR81 was critical for cancer cell survival. Moreover, GPR81 levels correlated with rates of cancer cell proliferation and metastasis in vivo. GPR81 levels were also elevated in xenografted cells. These data suggest that expression of GPR81 is fundamental for cancer cells within in the microenvironment of tumors.
Tumors contain both oxygenated and hypoxic regions. Therefore, it is not surprising that there is a heterogeneous population of tumor cells with different metabolic profiles, depending on the availability of oxygen, glucose and lactate (10). The expression of MCTs involved in lactate transport has previously been shown to be regulated by a variety of factors, including oxygen levels via HIF-1, cytokines, p53 and peroxisome proliferator-activated receptor (PPAR)-γ co-activator (PGC)-1α (6, 38). Hypoxic tumor cells depend on anaerobic glycolysis to produce ATP, which leads to the production of lactate. This lactate is exported primarily by MCT4. Oxidative tumor cells can import lactate through MCT1, where it is oxidized to pyruvate by LDH-1, incorporated into the TCA cycle to yield up to 18 ATP per molecule of lactate (5, 6, 10, 11, 13, 14). Thus, a symbiotic relationship between hypoxic and oxidative cancer cells has been proposed that relies on the activity of the MCTs (10). Further, MCT1 and MCT4 require expression of the plasma membrane glycoprotein CD147 for proper cell membrane insertion (17). Support for the importance of these processes has recently come from preclinical data demonstrating that inhibition or reduction of MCTs holds promise for cancer therapeutics (10, 18, 25, 39, 40). For example, MCT1 inhibition with α-cyano-4-hydroxycinnamate delays tumor growth, induces tumor core necrosis, and decreases tumor hypoxia in Lewis Lung carcinoma and WiDr human colorectal adenocarcinoma xenographs. Further, inhibition of CD147, the MCT chaperone, by RNA interference inhibits PDAC cell growth and MCT1 and MCT4 expression (31). These data support the idea that inhibition of lactate metabolism represents a potential therapeutic approach to cancer.
Our data indicate that GPR81 acts as a lactate sensor and signaling molecule in cancer. The addition of lactate resulted in increased levels of PGC1α, MCT1 & MCT4 and CD147 in PDAC cells expressing GPR81, but not in those in which it was silenced. GPR81-silenced cells had reduced levels of lactate uptake, while there was increased lactate uptake in cells expressing higher levels of GPR81. These data demonstrate for the first time the presence of lactate-sensitive regulation of MCT expression in cancer cells and indicate that it is dependent on the presence of GPR81. GPR81-knockdown cells displayed a greatly exaggerated reduction in mitochondrial activity when lactate was the only available energy source. Thus, GPR81 mediated induction of MCTs is necessary for lactate uptake as an alternative energy source. Whether GPR81 mediated regulation of MCTs is necessary for the efflux of lactate under circumstances where cellular levels were above those of the environment is unknown. We observed that the loss of GPR81 reduced MCT levels and diminished the ability of the cancer cells to grow as tumors and to undergo metastasis in vivo. Whether this was primarily due to increased uptake or efflux of lactate is not yet understood. We also observed that levels of GPR81 were increased in cells when they were placed within the environment of orthotopic xenograft tumors. The specific mechanisms which regulate GPR81 expression in vivo have not yet been determined. However, the induction of GPR81 expression by factors within the tumor microenvironment offers an explanation for the observation that although >90% of human PDAC tumor samples expressed high levels of GPR81, only ∼60% of PDAC cells lines expressed GPR81 in vitro.
In conclusion, we have shown that GPR81 is highly expressed in cancer and is critical for sensing extracellular lactate. Activation of GPR81 by lactate leads to increased expression of MCTs, CD147 and PGC1α, which are critical for lactate transport and metabolism. These data demonstrate for the first time the presence of GPR81 and its role in the lactate-sensitive regulation of cancer cell metabolism.
Supplementary Material
Acknowledgments
We gratefully acknowledge members of the Logsdon laboratory for their support and insightful discussion. This work was supported by the Lockton Endowment (CDL), NIH DK052067 (CDL), Cancer Center Support Core grant CA016672, and the NIH T32 CA009599 Ruth L. Kirschstein National Research Service Award (CLR & WRB).
Footnotes
Conflict of Interest: The authors have no conflicts of interest.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–70. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- 4.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy KM, Dewhirst MW. Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 2010;6:127–48. doi: 10.2217/fon.09.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walenta S, Salameh A, Lyng H, Evensen JF, Mitze M, Rofstad EK, et al. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol. 1997;150:409–15. [PMC free article] [PubMed] [Google Scholar]
- 8.Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–21. [PubMed] [Google Scholar]
- 9.Yamagata M, Hasuda K, Stamato T, Tannock IF. The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. Br J Cancer. 1998;77:1726–31. doi: 10.1038/bjc.1998.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–42. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–5. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 12.Doherty JR, Yang C, Scott KE, Cameron MD, Fallahi M, Li W, et al. Blocking Lactate Export by Inhibiting the Myc Target MCT1 Disables Glycolysis and Glutathione Synthesis. Cancer Research. 2014;74:908–20. doi: 10.1158/0008-5472.CAN-13-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draoui N, Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. 2011;4:727–32. doi: 10.1242/dmm.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, et al. Reciprocal Metabolic Reprogramming through Lactate Shuttle Coordinately Influences Tumor-Stroma Interplay. Cancer Res. 2012;72:5130–40. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 15.Benton CR, Yoshida Y, Lally J, Han XX, Hatta H, Bonen A. PGC-1alpha increases skeletal muscle lactate uptake by increasing the expression of MCT1 but not MCT2 or MCT4. Physiol Genomics. 2008;35:45–54. doi: 10.1152/physiolgenomics.90217.2008. [DOI] [PubMed] [Google Scholar]
- 16.Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–51. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 17.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. Embo J. 2000;19:3896–904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneiderhan W, Scheler M, Holzmann KH, Marx M, Gschwend JE, Bucholz M, et al. CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut. 2009;58:1391–8. doi: 10.1136/gut.2009.181412. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. Faseb J. 2007;21:2602–12. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 20.Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des. 2012;18:1319–30. doi: 10.2174/138161212799504902. [DOI] [PubMed] [Google Scholar]
- 21.Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280:41928–39. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 22.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–5. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 23.De Saedeleer CJ, Copetti T, Porporato PE, Verrax J, Feron O, Sonveaux P. Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PLoS One. 2012;7:e46571. doi: 10.1371/journal.pone.0046571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7:e33418. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–60. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 26.Lee DK, Nguyen T, Lynch KR, Cheng R, Vanti WB, Arkhitko O, et al. Discovery and mapping of ten novel G protein-coupled receptor genes. Gene. 2001;275:83–91. doi: 10.1016/s0378-1119(01)00651-5. [DOI] [PubMed] [Google Scholar]
- 27.Ge H, Weiszmann J, Reagan JD, Gupte J, Baribault H, Gyuris T, et al. Elucidation of signaling and functional activities of an orphan GPCR, GPR81. Journal of lipid research. 2008;49:797–803. doi: 10.1194/jlr.M700513-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, et al. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell metabolism. 2010;11:311–9. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Cai TQ, Ren N, Jin L, Cheng K, Kash S, Chen R, et al. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochemical and biophysical research communications. 2008;377:987–91. doi: 10.1016/j.bbrc.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. The Journal of biological chemistry. 2009;284:2811–22. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- 31.Kuei C, Yu J, Zhu J, Wu J, Zhang L, Shih A, et al. Study of GPR81, the lactate receptor, from distant species identifies residues and motifs critical for GPR81 functions. Molecular pharmacology. 2011;80:848–58. doi: 10.1124/mol.111.074500. [DOI] [PubMed] [Google Scholar]
- 32.Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, et al. Lactate Receptor Sites Link Neurotransmission, Neurovascular Coupling, and Brain Energy Metabolism. Cerebral cortex. 2013 doi: 10.1093/cercor/bht136. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Zhang W, Fuller GN. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol. 2002;12:95–107. doi: 10.1111/j.1750-3639.2002.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlen Y, McNair A, Perseguers S, Mazza C, Mermod N. Statistical significance of quantitative PCR. BMC Bioinformatics. 2007;8:131. doi: 10.1186/1471-2105-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arumugam T, Brandt W, Ramachandran V, Moore TT, Wang H, May FE, et al. Trefoil factor 1 stimulates both pancreatic cancer and stellate cells and increases metastasis. Pancreas. 2011;40:815–22. doi: 10.1097/MPA.0b013e31821f6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–18. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iovanna JL, Marks DL, Fernandez-Zapico ME, Urrutia R. Mechanistic Insights into Self-Reinforcing Processes Driving Abnormal Histogenesis During the Development of Pancreatic Cancer. Am J Pathol. 2013;182(4):1078–86. doi: 10.1016/j.ajpath.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boidot R, Vegran F, Meulle A, Le Breton A, Dessy C, Sonveaux P, et al. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res. 2012;72:939–48. doi: 10.1158/0008-5472.CAN-11-2474. [DOI] [PubMed] [Google Scholar]
- 39.Bhalla K, Hwang BJ, Dewi RE, Ou L, Twaddel W, Fang HB, et al. PGC1alpha promotes tumor growth by inducing gene expression programs supporting lipogenesis. Cancer Res. 2011;71:6888–98. doi: 10.1158/0008-5472.CAN-11-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan Y, He B, Song G, Bao Q, Tang Z, Tian F, et al. CD147 silencing via RNA interference reduces tumor cell invasion, metastasis and increases chemosensitivity in pancreatic cancer cells. Oncol Rep. 2012;27:2003–9. doi: 10.3892/or.2012.1729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


