Summary
Objective
In temporal lobe epilepsy (TLE) the epileptogenic focus is focal and unilateral in the majority of patients. A key characteristic of focal TLE is the presence of sub-clinical epileptiform activity in both the ictal and contralateral "healthy" hemisphere. Such interictal activity is clinically important as it may reflect the spread of pathology, potentially leading to secondary epileptogenesis. The role played by white matter pathways in this process are unknown.
Methods
We compared three interhemispheric white matter tracts (anterior commissure, fornix, and tapetum) to determine the pathway most associated with the presence of contralateral interictal spikes. Forty patients with unilateral left or right TLE were categorized based on the presence or absence of contralateral interictal spikes. ANOVAs were run on diffusion properties from each tract (fractional anisotropy, FA; and mean diffusivity, MD).
Results
The analyses revealed that left TLE patients with bilateral interictal spikes had lower FA and higher MD in the tapetum. RTLE patients did not show this effect. No significant associations with bilateral activity was observed for the other tracts. BOLD functional connectivity data revealed that homotopic lateral, not mesial, temporal areas were reliably correlated in bilateral patients, independent of ictal side.
Significance
Our results indicate that, among the tracts investigated, only the tapetum was associated with contralateral epileptiform activity, implicating this structure in seizures and possible secondary epileptogenesis. We describe two mechanisms that might explain this association (the interruption of inhibitory signals or the toxic effect of carrying epileptiform signals toward the healthy hemisphere), but also acknowledge other rival factors that may be at work. We also report that TLE patients with bilateral spikes had increased lateral bi-temporal lobe connectivity. Our current results can be seen as bringing together important functional and structural data to elucidate the basis of contralateral interictal activity in focal, unilateral epilepsy.
Keywords: epileptogenesis, fornix, anterior commissure, mirror, anisotropy
Introduction
Temporal lobe epilepsy (TLE) is the most common type of focal epilepsy, with about 30% of cases intractable to drug therapy.10 A key characteristic of focal TLE is the eventual presence of sub-clinical epileptiform activity in both the ictal and contralateral "healthy" hemisphere. Such contralateral interictal activity is clinically important as it may represent a form of pathology spread that leads to the birth of independent seizure foci.22; 27 Once the secondary focus is fully capable of generating independent seizures, the clinical value of surgical treatment drops from 78% to 58%.17 Even when a secondary ictal focus is not demonstrated, the simple presence of contralateral interictal spikes pre-surgery and their persistence after surgery is an indicator of poor surgery outcome.14
At a functional level, we recently demonstrated that bilateral spikes are associated with higher resting-state correlation between the two hemispheres.38 In this study, we argued this association represented the breakdown of anti-correlated activity in the healthy hemisphere, activity that was adaptive and potentially responsible for helping to keep seizures unilateral. Despite the clinical relevance of contralateral interictal activity in such processes as secondary (also referred to as mirror) epileptogenesis, no study has looked for structural evidence underlying this process. For instance, the white matter (WM) pathways involved in the emergence of contralateral epileptiform activity and/or secondary epileptogenesis in humans are currently unknown. The identification of the particular tract responsible for such processes in unilateral focal TLE can provide potentially important markers to improve surgical success rates. At the same time, the identification of such tracts could potentially be of therapeutic value by offering surgical disconnection similar to the already used corpus callosotomy3 or by specifying the target for therapeutic brain stimulation.19
This project is a follow-up of our prior work,38 with the current study examining three interhemispheric white matter tracts, seeking to determine if any of them play a role in the formation of interictal activity contralateral to the seizure focus. Such evidence may suggest there is a particular pathway biased toward the spread of epileptiform activity and possible secondary epileptogenesis. We used diffusion tensor imaging (DTI) to extract and quantify the integrity of three pathways known to convey information between homologous areas of the temporal lobes: the anterior commissure, the tapetum, and the body of the fornix. The anterior commissure contains interhemispheric fibers projecting between the respective amygdalae, hippocampi, and anterior temporal neocortices.34 The fornix is the major output pathway from each hippocampus, containing decussating fibers along the body and column of the fornix (hippocampal decussation and ventral hippocampal comissure, 6). The tapetum is part of the callosal bundle that provides connectivity, among other areas, to the homologous lateral and anterior temporal cortices.24; 34 Resting-state functional connectivity was also computed between the temporal regions in each group in order to investigate the relation between structural and functional connectivity in our experimental groups.
Methods
Subjects
Forty TLE patients were recruited from the Thomas Jefferson Comprehensive Epilepsy Center. Patients were excluded from the study for any of the following reasons: medical illness with central nervous system impact other than epilepsy; prior or current alcohol or illicit drug abuse; extratemporal epilepsy; bilateral mesial temporal sclerosis (MTS); any pathology outside the ictal temporal lobe; psychiatric diagnosis for any Axis I disorder listed in the Diagnostic and Statistical Manual of Mental Disorders – IV. Depressive Disorders were allowed in the patient sample, given the high co-morbidity of depression and epilepsy.37 Patients with mental retardation (Full-Scale IQ < 70) who were likely to be unable to cooperate with the MRI examination were also excluded.
Patient Classification: Unilateral and Bilateral Interictal Groups
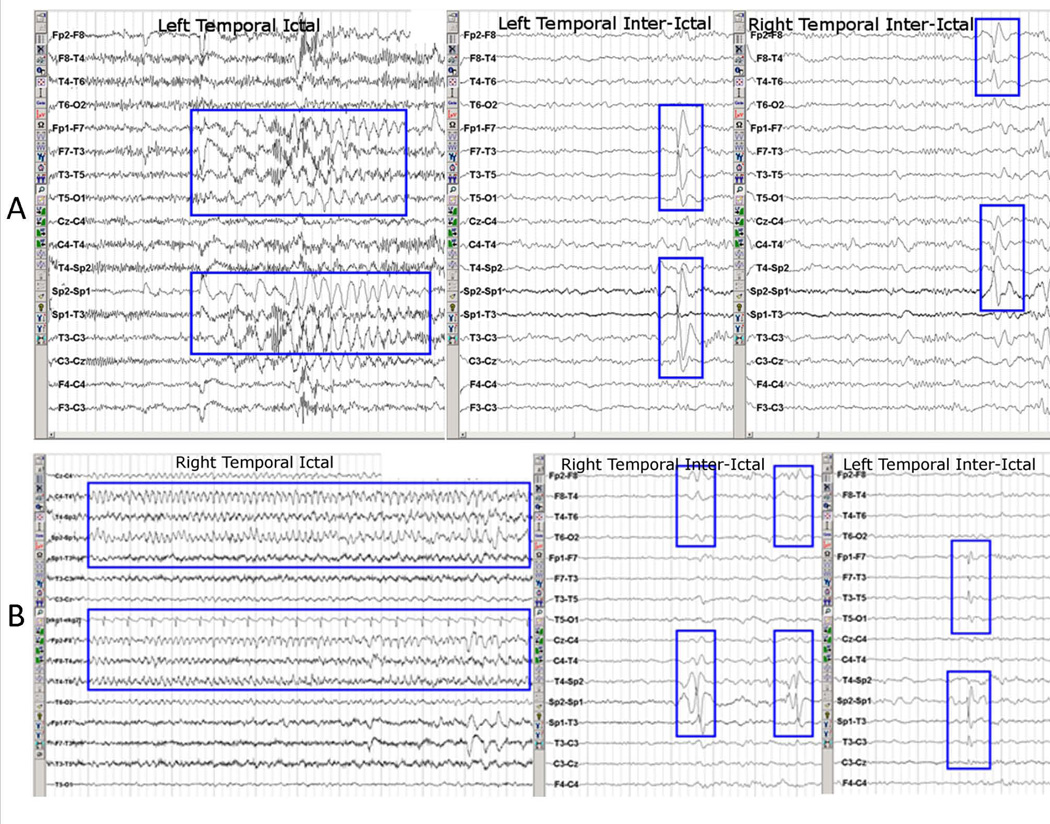
All patients had unilateral temporal epileptogenic focus, and were considered good candidates for unilateral temporal lobe resection. Board certified epileptologists established the side of the epileptogenic focus (20 left TLE, 20 right TLE) based on EEG, MRI, PET, neuropsychological testing, and seizure semiology. EEG monitoring of at least 96 consecutive hours was further inspected by a trained epileptologist (C.S.) to identify interictal spikes and classify patients based on spike laterality. Patients with unilateral interictal spikes reasonably originating in the temporal lobe (ipsilateral to the epileptogenic/ictal pathologic zone, maximal in anterior temporal, mid-temporal or basal temporal electrodes) with no epileptiform activity elsewhere, were defined as the Unilateral Interictal group. Patients with interictal spikes (one or more) reasonably originating in both the ipsilateral (ictal, temporal lobe pathology) and contralateral (non-ictal, no temporal lobe pathology) temporal lobes (spike maximum in anterior temporal, midtemporal or basal temporal electrodes) were defined as the Bilateral Interictal group. Figure 1 shows examples of interictal spikes and ictal episodes from two representative subjects. The final subdivision of the patients produced four groups: 14 left TLE with unilateral interictal (LTLE-Uni), 6 left TLE with bilateral interictal (LTLE-Bil), 12 right TLE with unilateral interictal (RTLE-Uni), and 8 right TLE with bilateral interictal (RTLE-Bil). Table 1 displays the relevant demographic and clinical characteristics of the groups. We acknowledge that we cannot know with 100% certainty where within the temporal lobe the recruited cells causing the interictal activity reside. Nor will we know with certainty that patients with unilateral interictal spikes are truly unilateral. Given the constraints of standard of care and ethical consideration of the data, we must rely on MRI, PET, and surface and sphenoidal EEG in the hands of highly trained epileptologists. Based on this information, we used the lesional/ictal hemisphere as the presumptive epileptogenic focus, keeping in mind that the contralateral interictal activity was not associated with abnormal MRI or ictal activity.
Figure 1.
Example from EEG readings from (A) a left TLE patient with ictal activity on the left and bilateral interictal activity, and (B) a right TLE patients with ictal activity on the right and bilateral interictal activity. Modified with permission from Figure 1 in Tracy JI, Osipowicz K, Spechler P, et al. Functional connectivity evidence of cortico-cortico inhibition in temporal lobe epilepsy. Hum Brain Mapp 2014;35:353–366.
Table 1.
(a) Sample demographic and clinical characteristics. Several patients on each group were on multiple medications, therefore the medication count exceeds the group size; (b) ANOVAs on FA and MD values for the three tracts; (c) ANOVAs on functional connectivity data (Pearson correlations) between lateral and mesial temporal lobe regions. Abbreviations: TL=temporal lobe, TBI=traumatic brain injury, LTLE-Uni=left TLE with unilateral interictal spikes; LTLE-Bil=left TLE with bilateral interictal spikes; RTLE-Uni=right TLE with unilateral interictal spikes; RTLE-Bil=right TLE with bilateral interictal spikes; n.s.=non-significant; MTS=mesial temporal sclerosis; CPS=complex partial seizures; GTCS=generalized tonic clonic seizures; SPS=simple partial seizures.
| LTLE-Uni | LTLE-Bil | RTLE-Uni | RTLE-Bil | Result | |
|---|---|---|---|---|---|
| N | 14 | 6 | 12 | 8 | |
| (A) | Demographic/Clinical Characteristics | ||||
| Age | 42±12.5 | 45±6 | 38±17.3 | 38±13.8 | n.s. |
| Gender M/F | 3/11 | 1/5 | 6/6 | 2/6 | n.s. |
| Full Scale IQ | 96±11.8 | 92±16 | 101±11.4 | 96±14 | n.s. |
| Intracranial Volume (Liters) | 1.27±0.24 | 1.24±0.21 | 1.38±0.25 | 1.32±0.26 | n.s. |
| Age at epilepsy onset | 21±14.1 | 26.5±14.1 | 18±11.5 | 26±11.1 | n.s. |
| Duration of Epilepsy | 21±13.7 | 18±14.1 | 21±14.4 | 12±9.8 | n.s. |
| Seizure Types (no. of patients) |
10 – CPS only 2 – CPS + commonGTCS 1 – SPS only 1 – CPS + SPS |
3 – CPS only 2 – CPS + rareGTCS 1 – CPS w/2° GTCS |
7 – CPS only 1 – CPS + rareGTCS 1 – SPS only 2 – CPS + SPS 1 – CPS w/2° GTCS + SPS |
5 – CPS only 1 – CPS w/2° GTCS 2 – CPS + SPS |
|
| MRI/PET abnormality/neuropathology | 7 MTS (50%)[no lateral lesions] 7 Non MTS (50%)[1 – L. Anter. TL encephalomalacia 1 – L. Hippo. Sign. Abnormality (implanted) 1 – L. Poster. TL dysplasia 4 – L. TL hypometabol. (1 implanted)] |
2 MTS (33%)[no lateral lesions, 1 implanted] 4 Non MTS (67%)[1 – L. fusiform hemangioma 1 – ischemic disease+L. TL hypometabolism 2 – L. TL hypometabol (1 implanted)] |
5 MTS (42%)[no lateral lesions] 7 Non MTS (58%)[1 – R. TL horn enlarged+ R. TL hypometabolism 1 – TBI+R. TL hypometabolism 5 – R. TL hypometabol.] |
2 MTS (25%)[no lateral lesions] 6 Non MTS (75%)[2 – R. TL dysplasia 1 – R. Hippo. Sign. Abnormality 1 – R. choroidal enlargement (implanted) 2 – R. TL hypometabol. (1 implanted)] |
|
| Medication (no. of patients) |
5 – Carbamaze. 5 – Lamotrigine 5 – Levetiracet. 2 – Topiramate 1 – Trileptal 5 – Other |
2 – Depakote 2 – Lamotrigine 4 – Levetiracet. 1 – Topiramate 1 – Trileptal 3 – Other |
2 – Carbamaze. 3 – Lamotrigine 6 – Levetiracet. 3 – Topiramate 1 – Trileptal 3 – Other |
1 – Depakote 2 – Levetiracet. 2 – Topiramate 2 – Trileptal 3 – Other |
|
| (B) | Tract Properties | ||||
| FA Ant. Commiss. | 0.395±0.12 | 0.365±0.09 | 0.39±0.09 | 0.39±0.10 | n.s. |
| FA Fornix | 0.398±0.06 | 0.422±0.04 | 0.399±0.06 | 0.403±0.04 | n.s. |
| FA Tapetum | 0.599±0.04 | 0.526±0.05 | 0.547±0.03 | 0.587±0.04 | TLE group × Spikes F[1,36]=10.01, p=.003 |
| MD Ant. Commiss. (× 10−3) |
0.95±0.13 | 1.00±0.26 | 0.93±0.08 | 0.9±0.06 | n.s. |
| MD Fornix (× 10−3) |
2.10±0.94 | 1.53±0.07 | 1.79±0.27 | 1.74±0.48 | n.s. |
| MD Tapetum (× 10−3) |
1.01±0.08 | 1.23±0.18 | 1.12±0.16 | 1.02±0.13 | TLE group × Spikes F[1,36]=13.51, p=.001 |
| (C) | Functional Connectivity | ||||
| Lateral-Lateral | 0.58±0.17 | 0.75±0.07 | 0.62±0.19 | 0.72±0.11 | Spikes F[1,33]=5.47, p=.026 |
| Mesial-Mesial | 0.58±0.16 | 0.66±0.13 | 0.59±0.20 | 0.62±0.17 | n.s. |
Imaging Protocol and Data Analysis
All scans were performed on a Philips Achieva 3T scanner (Amsterdam, the Netherlands) using an 8-channel SENSE head coil.
T1 MP-RAGE
A T1-weighted anatomical MP-RAGE volume was collected in sagittal orientation with in-plane resolution of 256 × 256 and 1mm slice thickness (1mm3 voxels; TR= 650ms, TE= 3.2ms, FOV 256mm, flip angle 8°). The estimated total intracranial volume was obtained from this volume by running segmentation in Freesurfer (v.5.3.0).
DTI
Diffusion data were obtained using a single-shot spin-echo EPI pulse sequence to acquire 32 diffusion weighted (b-factor=850 s/mm2) and 3 non-diffusion volumes (TE=90ms, TR=8609ms, FOV=256mm, 66 axial slices, 2mm thickness, 0mm gap, 128×128 acquisition & reconstruction matrix, 2mm×2mm×2mm voxel size). Fat suppression was achieved using a standard SPIR (spectral pre-saturation with inversion recovery) technique.
Prior to processing, diffusion data were visually inspected and subjects showing excessive motion or artifacts were excluded from the study. Using ExploreDTI,20 data were corrected for subject motion, eddy currents, and EPI distortions, and finally resampled using a cubic-spline interpolation. The RESTORE algorithm (non-linear) was used to estimate tensors. This method is robust to outlier data points and weights these points accordingly during tensor calculation.5 The following DTI derivate measures were exported: fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AX), radial diffusivity (RD), and mode of anisotropy (MO); for an explanation of these measures, see 40. Fiber tractography was performed in FSL (Diffusion Toolbox, www.fmrib.ox.ac.uk/fsl) after a multi-tensor estimation of 2-fibers per voxel.4 Probabilistic tractography was performed by seeding 5,000 fiber samples/voxel. ROI masks were drawn on native space for each participant to capture the three tracts of interest (tapetum, anterior commissure, fornix). Details of this procedure are described in Supplementary Information. For each tract weighted averages of diffusion properties (FA, MD, AX, RD, and MO) were computed based on probabilistic tractography maps; voxels with higher tractography probability were weighted proportionally more. Regarding the tapetum, tractography was performed on all fibers connecting the temporal lobes, but only the superior section of the pathway, containing the callosal fibers, was retained (Figure 2). A fourth WM tract, the dorsal hippocampal commissure, could not be reconstructed successfully with our DTI data; therefore, this structure was not included in our analyses. We were able to reconstruct this tract using spherical deconvolution methods 36 on one patient who had High Angular Diffusion Imaging (HARDI) data available, demonstrating that the tract is present. However, HARDI data were not available on our full sample.
Figure 2.
Depiction of three tracts used for FA and MD analysis. Examples from three temporal lobe epilepsy patients. (A) Patient one (Right TLE, Unilateral group); (B) Patient two (Left TLE, Bialteral group); (C) Patient Three (Right TLE, Bilateral Group).
Resting-State
Resting-state BOLD activity was obtained using a single shot echoplanar gradient echo imaging sequence to acquire 120 T2* volumes over a period of five minutes (34 axial slices, TR=2.5s, TE=35ms, FOV=256mm, flip angle=90°, 2×2mm voxels, 4mm thickness). After excluding three patients without resting state data, the LTLE-Uni group was composed of 12 patients (instead of 14) and the RTLE-Uni group was composed of 11 patients (instead of 12). All -Bil patients had resting-state data.
Using SPM8 (Wellcome Trust Centre for Neuroimaging), resting-state BOLD data were initially corrected for slice timing to adjust for variable acquisition time, followed by motion correction using an affine registration to realign all images to the first volume. Data were spatially normalized to the MNI305 EPI template using a standard method in SPM, consisting in an affine transformation followed by finer non-linear deformations to minimize the sum-of-square differences. Normalized data were smoothed with a full-width at half maximum Gaussian kernel of 8 mm. Interpolation was of 7th B-spline order for all preprocessing steps. Masks of WM and cerebrospinal fluid (CSF) were obtained by segmenting the normalized MP-RAGE volume. The time-courses of both WM and CSF were estimated in the relevant brain tissue classes defined at the segmentation step. A total of eight sources of spurious variance (six motion parameters, the CSF, and WM signals) were then removed from the data through linear regression. Finally, fMRI data were temporally filtered using the REST Toolbox (bandpass [0.008 0.1]Hz).35 The anatomical automatic labeling atlas39 was used to obtain functional connectivity (FC) correlation between homotopic areas of the two hemispheres. Lateral-lateral connectivity was obtained from the collapsed superior and middle temporal gyri in each hemisphere (the inferior temporal gyri are subject to loss of signal and were not included). Mesial-mesial connectivity was obtained from the collapsed hippocampus and parahippocampal areas in each hemisphere.
Results
Demographics
The patient groups were statistically similar in age, age of epilepsy onset, epilepsy duration, patients with/without MTS, IQ, gender distribution, and intracranial volume (assessed by Analyses of Variance (ANOVAs) or Chi-Squared tests, as appropriate; see Table 1). Hemispheric dominance was established based on handedness scores and fMRI evaluations (procedure described in Supplementary Information). Thirty-seven patients were left hemispheric dominant, two patients were right hemispheric dominant (one confirmed with fMRI, in the RTLE-Uni group; one inferred from handedness, in the LTLE-Uni group), and one patient had bilateral dominance (inferred from handedness, in the RTLE-Bil group).
DTI
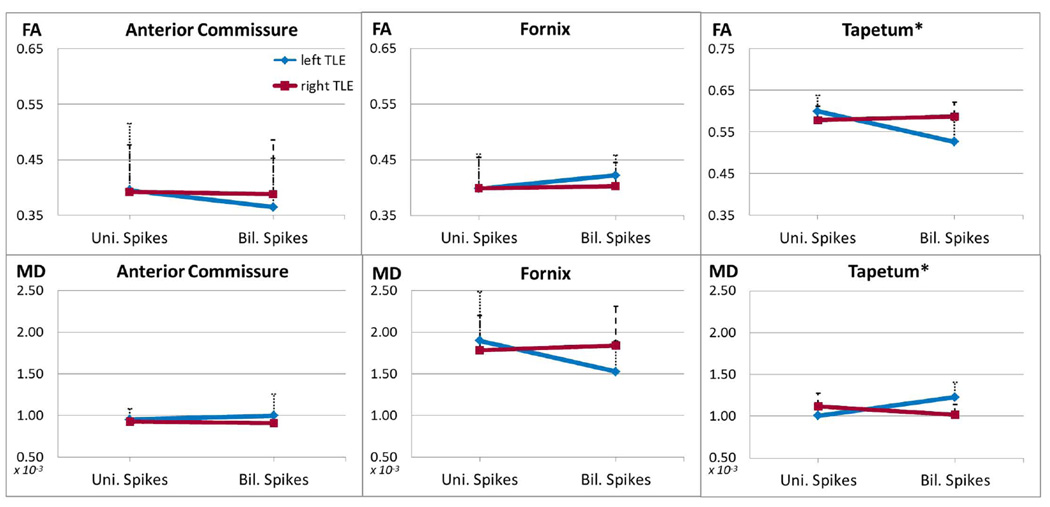
Diffusion properties were compared among groups with a 2×2 ANOVA for factors “TLE group” (left/right) and “Spikes” (unilateral/bilateral). Six ANOVAs were performed and a threshold of p<.0083 was chosen to correct for multiple comparisons. Results are presented in Table 1 and plotted in Figure 3.
Figure 3.
FA and MD values in each tract for patients with unilateral and bilateral spikes (blue for left TLE patients, red for right TLE patients). Significant ANOVAs are marked with an asterisk. Error bars indicate standard deviation.
Both DTI measures were examined for outliers, none were found, and all three measures showed adequate homogeneity of variance among groups (Levene’s Test). The anterior commissure and the fornix showed no significant difference in FA or MD with respect to TLE groups, “Spikes”, or their interaction (all p>.1). FA of the tapetum showed a significant interaction between TLE groups and “Spikes” (F[1,36]=10.01, p=.003, effect size=0.22), with no significant main effects. Post-hoc contrasts revealed lower FA in left TLE patients with bilateral spikes compared to left TLE with unilateral spikes (95% CI [−.11 −.04], p<.001), while right TLE patients with unilateral and bilateral spikes were similar. A similar interaction was observed for MD values (TLE group × “Spikes”: F[1,36]=13.51, p=.001, effect size=0.27), with left TLE showing higher MD for patients with bilateral spikes (95% CI [+.00009 +.00034]), while no difference was present among right TLE with unilateral and bilateral spikes.
As an aid to clarifying underlying neurobiologic processes, and as a check on confounds related to crossing fibers, we investigated axial and radial diffusivities, as well as the mode of anisotropy. AX showed a significant interaction TLE group × “Spikes” (F[1,36]=7.68, p=.009, effect size=0.17) driven by larger values in the bilateral compared to unilateral left TLE patients (95% CI [+.00002 +.00031], p=.027). No difference was observed between the right TLE groups for AX. Similarly, RD showed a significant interaction between TLE group × “Spikes” (F[1,36]=15.77, p<.001, effect size=0.31), driven by larger values in the left bilateral TLE compared to unilateral patients (95% CI [+.00019 +.00037], p<.001). Again, no such difference was observed between the “Spikes” groups in right TLE. The ANOVA on mode of anisotropy in the tapetum was not significant, though a statistical trend was observed for the main effect of “Spikes” with bilateral spikes showing slightly lower mode of anisotropy in the tapetum (F[1,36]=3.72, p=.062, effect size=0.09).
Analysis of covariance on properties the tapetum
We checked whether the interaction between TLE group and “Spikes” for the FA and MD values of the tapetum remained significant after accounting for the variance related to key demographic and clinical variables (age, age of epilepsy onset, epilepsy duration, and intracranial volume). To accomplish this, the original ANOVAs on FA and MD were re-run with the covariate included in the model (separate analysis for each variable). In each ANCOVA, the interaction between TLE group and “Spikes” remained significant (all p<.005). As a final check, ANCOVAs were run with all four covariates included in the model. Again, for both FA and MD, the TLE group and “Spikes” interaction remained significant.
Analysis of the shorter tapetum segment
As differences in the tapetum may arise from partial volume effects and crossing fibers in the most lateral portion (i.e., section that descends to the temporal lobe), we investigated only the mesial tapetum, the portion solely containing homogenous callosal fibers. This portion was extracted as a 15mm ROI centered around the midline of the tapetum for each subject, and did not include the lateral arcuation (see Figure 2). The ANOVAs for this segment again revealed an interaction between TLE group and “Spikes”, albeit with smaller effect sizes (for FA: F[1,36]=4.59, p=.039, effect size=0.11; for MD: F[1,36]=5.17, p=.029, effect size=0.13). Similar to the original findings, the left TLE patients with bilateral spikes showed lower FA and higher MD than the left unilateral group (for FA: 95% CI [−.12 −.02], p<.001; for MD: 95% CI [+.00002 +.00042], p=.029). Again, no difference between the unilateral and bilateral groups was observed within the right TLE patients.
Functional Connectivity
ANOVAs with factors TLE group and “Spikes” were used to investigate the left-right medial and left-right lateral FC. After correction for multiple comparisons (p<.025, effective alpha, p<.05), no significant effects were observed for mesial-mesial connectivity. Lateral-lateral connectivity showed a statistical trend for the main effect of “Spikes” (p=.026), which reached significance when the TLE group factor was excluded (F[1,35]=5.67, p=.023, effect size=0.14).
Correlation between structural and functional connectivity
Pearson correlations were run between the diffusion properties of the tapetum (FA and MD) and the two FC scores (mesial, lateral). These correlations were run separately within each of our four experimental groups. After correction for multiple comparisons (p<.002, effective alpha, p<.05), no significant correlation emerged in any of the groups.
Discussion
We investigated three WM tracts (fornix, anterior commissure, and tapetum) to identify the structural effects of contralateral epileptiform activity, and processes potentially reflective of seizure spread and secondary epileptogenesis (e.g., development of a mirror focus). To our knowledge, this is the first study to investigate the WM correlates of these epileptiform processes in humans. Our analyses suggested that among the tracts tested, the tapetum emerges as the one most likely to have born the burden of epileptogenic activity outside the epileptogenic temporal lobe involving contralateral temporal structures. To ensure that this effect did not arise from partial voluming or structural alteration in the part of the tapetum not strictly containing callosal fibers (e.g., the lateral tapetum arcuation fibers), we re-examined our data restricting the analysis to the mesial portion of the tapetum. The results were the same, indicating specifically that callosal fibers of the tapetum are more damaged in left TLE patients with bilateral spikes than in those with unilateral spikes, while right TLE patients produced no differences in this regard. We also found no compelling evidence that this effect in the tapetum was contaminated by crossing fibers, a known artifact of DTI (i.e., no difference in mode of anisotropy; 7). Lastly, we found that diffusion changes in the tapetum could not be accounted for by demographic and clinical factors such as intracranial volume, chronological age, epilepsy duration, and age at epilepsy onset.
The change in diffusion properties of the tapetum involved solely left TLE patients with bilateral interictal spikes. A decrease in FA and increase in MD was observed in these patients compared to left TLE patients with unilateral spikes. The increases we observed in axial and radial diffusivity suggest that processes of neuroinflamation, e.g., edema, 2 or chronic WM damage13 may be at work. Ultimately, with the limits of current DTI technology, it is not possible to determine which of these neurobiologic processes are responsible for our findings. Moreover, we should note that in the absence of longitudinal data it is not possible to determine if the WM status of the tapetum is transient or stable.
Early animal models of mirror epileptogenesis showed that callosal transmission is crucial for developing a mirror focus.25; 26 Importantly, our findings provide evidence that a specific section of the corpus callosum (tapetum), may constitute a pathway that in humans is selectively associated with the presence of epileptiform signals in the “healthy” contralateral cortex. Our analysis is based on examination of three potential interhemispheric pathways; however, we must acknowledge that other tracts not investigated here may be playing a role, including indirect pathways via the brainstem, thalamus, and the frontal lobes. The dorsal hippocampal commissure has been previously proposed as an important pathway in seizure spread.12; 23 However, we failed to detect this tract using standard DTI procedures as described in methods. Because of these technical limitations, the dorsal hippocampal commissure could not be included in the current study, but nonetheless remains a potential pathway for the spread of epileptiform processes in the contralateral hemisphere.
The involvement of a callosal tract in the spread of epileptiform activity is not surprising given the findings in animals.26 However, it is surprising with respect to the nature of the involvement. Different from animal studies, where dissection of the callosum was beneficial in the prevention of mirror epileptogenesis, we find that damage to the tapetum is associated, not with the absence, but the presence of bilateral interictal spikes. While the tapetum might be an important pathway bearing signals related to the presence of contralateral interictal spikes, we cannot exclude other rival factors, explaining both the tapetum damage and the presence of contralateral interictal spikes. Contralateral spikes and tapetum damage may be related to the nature of the pathology (e.g., a mesial versus lateral temporal seizure focus), or the impact of particular medication regimens. Our data is strictly correlational in nature, and leaves open the question of direct causality. It is worthwhile, however, to note that our data is consistent with at least two possible causal mechanisms. First, tapetum damage may interrupt inhibitory signals coming from the pathologic hemisphere, allowing for hyper-synchronization of contralateral gray matter regions (i.e., loss of a inhibitory surround, 38). Alternatively, tapetum damage may reflect the burden of carrying excitatory epileptiform signals (or a specific molecule as proposed by 27) in the contralateral hemisphere. The distinction between these mechanisms is important from a therapeutic perspective, as the first hypothesis implies that stimulation of this tract would be beneficial, while the second hypothesis implies, on the contrary, that resection of the tract would be beneficial. Our data remains silent on the comparative validity of these two causal processes.
Previous literature have reported lower functional interhemispheric connectivity in TLE patients compared to healthy controls.21 The pattern of FC we observed, that is, higher correlation between temporal lobes in patients with bilateral spikes, suggest that lower interhemispheric connectivity is beneficial to prevent contralateral epileptiform activity spread. This result is also consistent with our prior work showing that the absence of anti-correlated activity reflects the loss of an important protective mechanism that guards against seizure spread and/or secondary epileptogenesis.38 The present study indicated that in the absence of such anti-correlated activity, lower FC between temporal lobes is beneficial. Though no correlation was found between tapetum properties and functional connectivity, the tapetum is a tract that connects lateral/anterior temporal lobes,34 and, thus, both structural and functional connectivities converge to suggest an effect related to the lateral temporal lobes.
Our current findings also imply that the structural pathways that work to broaden epileptiform activity may vary as a function of hemispheric dominance. This is not surprising given the extensive literature on the differential effects of left versus right hemisphere seizures. For example, left TLE patients have been shown to exhibit greater WM damage than right TLE patients.1; 18 Moreover, through work in our lab and others, task-based and resting-state fMRI show distinct abnormalities in left versus right TLE patients.8; 9; 32 All this suggests that different neuroplastic mechanisms related to seizure spread or mirror epileptogenesis may arise depending on the side of epileptic focus. Our data also show that maladaptive WM changes in the tapetum are only evident in the setting of dominant hemisphere TLE.
The demographic properties of our sample also offer important clues related to the epileptiform processes we have isolated. First, the prevalence of patients with bilateral spikes in our sample (35%) is similar to other reports of patients with potential mirror epileptogenesis.15; 16; 22; 29; 33 It is likely that not all the patients in our sample will develop a secondary epileptogenic focus, but it is re-assuring that the ratio of such patients is similar to other reports. Second, it is worth noting that contrary to the proposals of Morrell, we did not see an increased likelihood for seizure spread or secondary epileptogenesis among patients with longer epilepsy duration (i.e., longer exposure to an ictal generator). Left TLE patients with bilateral spikes had epilepsy duration values similar to left TLE patients with unilateral spikes. Third, we did not observe an increased risk for processes of spread or epileptogenesis in our patients with younger28 as opposed to older16 age of epilepsy onset. In this regard, our findings are similar to those of Niediek et al. 30 and Gilmore et al. 11, who did not find age of epilepsy onset to be a relevant factor.
Several limitations in the current project must be noted. First, our sample is relatively small, a factor which may have limited our sensitivity to subtler WM changes. The incidence of bilateral versus unilateral spikes (35% and 65%, respectively) may suggest that larger cohorts are needed to detect small WM changes from seizure spread or epileptogenesis. Second, we lacked a control group, thus we cannot comment on the status of the tapetum with respect to healthy populations. It is worth noting that all studies to date comparing TLE patients to healthy controls have shown reduced WM integrity in the former.31 Thus, it is likely that the status of the tapetum in our left TLE patients with bilateral spikes is compromised relative to healthy populations. Third, as anticonvulsant medication regimen varied within our sample, we were not able to analyze the effects of medication. Thus, the role of medication remains unknown, although it should be stated that all patients were on anti-convulsants, typically for many years, balancing this factor across statistical comparisons. Fourth, the majority of fibres in the anterior fornix do not form a clear interhemispheric connection. Therefore, the sensitivity of detecting changes involving fibers crossing to the other hemisphere may be more limited in this region compared to the other two pathways, which have a clear inter-hemispheric orientation.
In summary, our findings provide evidence that a specific section of the corpus callosum (tapetum) constitutes a pathway in humans that is selectively associated with the presence of epileptiform signals in the “healthy” contralateral cortex, perhaps by permitting seizure spread and/or fostering development of an independent seizure focus. Moreover, patients with bilateral spikes had increased lateral bi-temporal lobe connectivity. Our current results can be seen as bringing together important functional and structural data to elucidate the basis of contralateral interictal activity in focal, unilateral epilepsy.
Supplementary Material
Acknowledgements
The research presented in this paper was made possible, in part, by an NINDS R21 Grant Award NS056071.
Footnotes
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Ahmadi ME, Hagler DJ, Jr, McDonald CR, et al. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR Am J Neuroradiol. 2009;30:1740–1747. doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asadi-Pooya AA, Sharan A, Nei M, et al. Corpus callosotomy. Epilepsy Behav. 2008;13:271–278. doi: 10.1016/j.yebeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang LC, Walker L, Pierpaoli C. Informed RESTORE: A method for robust estimation of diffusion tensor from low redundancy datasets in the presence of physiological noise artifacts. Magn Reson Med. 2012;68:1654–1663. doi: 10.1002/mrm.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demeter S, Rosene DL, Van Hoesen GW. Interhemispheric pathways of the hippocampal formation, presubiculum, and entorhinal and posterior parahippocampal cortices in the rhesus monkey: the structure and organization of the hippocampal commissures. J Comp Neurol. 1985;233:30–47. doi: 10.1002/cne.902330104. [DOI] [PubMed] [Google Scholar]
- 7.Douaud G, Jbabdi S, Behrens TE, et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. Neuroimage. 2011;55:880–890. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doucet G, Osipowicz K, Sharan A, et al. Extratemporal functional connectivity impairments at rest are related to memory performance in mesial temporal epilepsy. Hum Brain Mapp. 2013;34:2202–2216. doi: 10.1002/hbm.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doucet G, Osipowicz K, Sharan A, et al. Hippocampal functional connectivity patterns during spatial working memory differ in right versus left temporal lobe epilepsy. Brain Connect. 2013;3:398–406. doi: 10.1089/brain.2013.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel J., Jr Mesial temporal lobe epilepsy: what have we learned? Neuroscientist. 2001;7:340–352. doi: 10.1177/107385840100700410. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore R, Morris H, 3rd, Van Ness PC, et al. Mirror focus: function of seizure frequency and influence on outcome after surgery. Epilepsia. 1994;35:258–263. doi: 10.1111/j.1528-1157.1994.tb02429.x. [DOI] [PubMed] [Google Scholar]
- 12.Gloor P, Salanova V, Olivier A, et al. The human dorsal hippocampal commissure: An anatomically identifiable and functional pathway. Brain. 1993;116:1249–1273. doi: 10.1093/brain/116.5.1249. [DOI] [PubMed] [Google Scholar]
- 13.Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–1363. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 14.Halasz P, Janszky J, Rasonyi GY, et al. Postoperative interictal spikes during sleep contralateral to the operated side is associated with unfavourable surgical outcome in patients with preoperative bitemporal spikes. Seizure. 2004;13:460–466. doi: 10.1016/j.seizure.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Hughes JR. Long-term clinical and EEG changes in patients with epilepsy. Arch Neurol. 1985;42:213–223. doi: 10.1001/archneur.1985.04060030027006. [DOI] [PubMed] [Google Scholar]
- 16.Janszky J, Rasonyi G, Clemens Z, et al. Clinical differences in patients with unilateral hippocampal sclerosis and unitemporal or bitemporal epileptiform discharges. Seizure. 2003;12:550–554. doi: 10.1016/s1059-1311(03)00069-4. [DOI] [PubMed] [Google Scholar]
- 17.Jehi LE, Silveira DC, Bingaman W, et al. Temporal lobe epilepsy surgery failures: predictors of seizure recurrence, yield of reevaluation, and outcome following reoperation. J Neurosurg. 2010;113:1186–1194. doi: 10.3171/2010.8.JNS10180. [DOI] [PubMed] [Google Scholar]
- 18.Kemmotsu N, Girard HM, Bernhardt BC, et al. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia. 2011;52:2257–2266. doi: 10.1111/j.1528-1167.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koubeissi MZ, Kahriman E, Syed TU, et al. Low-frequency electrical stimulation of a fiber tract in temporal lobe epilepsy. Ann Neurol. 2013 doi: 10.1002/ana.23915. [DOI] [PubMed] [Google Scholar]
- 20.Leemans A, Jeurissen B, Sijbers J, et al. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. 17th Annual Meeting of Intl Soc Mag Reson Med. 2009:3537. [Google Scholar]
- 21.Maccotta L, He BJ, Snyder AZ, et al. Impaired and facilitated functional networks in temporal lobe epilepsy. Neuroimage Clin. 2013;2:862–872. doi: 10.1016/j.nicl.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy RJ, O'Connor MJ, Sperling MR. The mirror focus phenomenon and secondary epileptogenesis in human epilepsy. Journal of Epilepsy. 1997;10:78–85. [Google Scholar]
- 23.Mintzer S, Cendes F, Soss J, et al. Unilateral Hippocampal Sclerosis with Contralateral Temporal Scalp Ictal Onset. Epilepsia. 2004;45:792–802. doi: 10.1111/j.0013-9580.2004.35703.x. [DOI] [PubMed] [Google Scholar]
- 24.Mori S, van Zijl PCM, Oishi K, et al. MRI Atlas of Human White Matter 2nd Edition. Elsevier Science. 2010 [Google Scholar]
- 25.Morrell F. Secondary epileptogenic lesions. Epilepsia. 1960;1:538–560. doi: 10.1111/j.1528-1157.1959.tb04288.x. [DOI] [PubMed] [Google Scholar]
- 26.Morrell F. Callosal Mechanisms in Epileptogenesis. In: Reeves A, editor. Epilepsy and the Corpus Callosum. Springer US; 1985. pp. 99–130. [Google Scholar]
- 27.Morrell F, deToledo-Morrell L. From mirror focus to secondary epileptogenesis in man: an historical review. Adv Neurol. 1999;81:11–23. [PubMed] [Google Scholar]
- 28.Morrell F, Rasmussen T, DeToledo-Morrell L, et al. Frontal lobe epilepsy of neoplastic etiology: incidence of secondary epileptogenesis. Epilepsia. 1984;25:654–655. [Google Scholar]
- 29.Morrell F, Rasmussen T, Gloor P, et al. Secondary epileptogenic foci in patients with verified temporal lobe tumor. Electroencephalogr Clin Neurophysiol. 1983;54 [Google Scholar]
- 30.Niediek T, Franke HG, Degen R, et al. The development of independent foci in epileptic patients. Arch Neurol. 1990;47:406–411. doi: 10.1001/archneur.1990.00530040048018. [DOI] [PubMed] [Google Scholar]
- 31.Otte WM, van Eijsden P, Sander JW, et al. A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia. 2012;53:659–667. doi: 10.1111/j.1528-1167.2012.03426.x. [DOI] [PubMed] [Google Scholar]
- 32.Pereira FR, Alessio A, Sercheli MS, et al. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci. 2010;11:66. doi: 10.1186/1471-2202-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampaio L, Yacubian EM, Manreza ML. The role of mirror focus in the surgical outcome of patients with indolent temporal lobe tumors. Arq Neuropsiquiatr. 2004;62:9–14. doi: 10.1590/s0004-282x2004000100002. [DOI] [PubMed] [Google Scholar]
- 34.Schmahmann JD, Pandya D. Fiber Pathways of the Brain. Oxford University Press, USA; 2009. [Google Scholar]
- 35.Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tournier JD, Calamante F, Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology. 2012;22:53–66. [Google Scholar]
- 37.Tracy JI, Lippincott C, Mahmood T, et al. Are depression and cognitive performance related in temporal lobe epilepsy? Epilepsia. 2007;48:2327–2335. doi: 10.1111/j.1528-1167.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 38.Tracy JI, Osipowicz K, Spechler P, et al. Functional connectivity evidence of cortico-cortico inhibition in temporal lobe epilepsy. Hum Brain Mapp. 2014;35:353–366. doi: 10.1002/hbm.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 40.Winston GP. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg. 2012;2:254–265. doi: 10.3978/j.issn.2223-4292.2012.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.