Abstract
Clinical methods used to assess the electrical activity of excitable cells are often limited by their poor spatial resolution or their invasiveness. One promising solution to this problem is to optically measure membrane potential using a voltage-sensitive dye, but thus far, none of these dyes have been available for human use. Here we report that indocyanine green (ICG), an infrared fluorescent dye with FDA approval as an intravenously administered contrast agent, is voltage-sensitive. The fluorescence of ICG can follow action potentials in artificial neurons and cultured rat neurons and cardiomyocytes. ICG also visualized electrical activity induced in living explants of rat brain. In humans, ICG labels excitable cells and is routinely visualized transdermally with high spatial resolution. As an infrared voltage-sensitive dye with a low toxicity profile that can be readily imaged in deep tissues, ICG may have significant utility for clinical and basic research applications previously intractable for potentiometric dyes.
Voltage-sensitive dyes provide a way to observe cellular electrical activity without the physical limitations imposed by electrodes. Although these dyes can monitor membrane potential with a resolution of a few microns from large populations of cells (1), there are three obstacles that prevent the use of these dyes in many research settings, including clinical research:
-
1.
Most voltage-sensitive dyes use visible wavelengths of light that prevent imaging of tissues beneath the skin.
-
2.
Many of these dyes produce significant toxicity or off-target effects (2).
-
3.
Before this report, to our knowledge, no voltage-sensitive dyes have ever been available for administration in humans, which has limited their value in biomedically focused research.
Here, we show that indocyanine green (ICG), an FDA-approved fluorescent dye routinely used in many clinical tests, is voltage-sensitive. Our initial experimental system used Xenopus laevis oocytes. Changes in the membrane potential of the cell induced by two-electrode voltage-clamp resulted in robust, consistent changes in the fluorescence of ICG (Fig. 1, inset). All data in this work was obtained from single acquisitions with no averaging of multiple images. The voltage-dependent fluorescence changes were roughly linear with respect to membrane potential and had a magnitude of ∼1.9% of the baseline fluorescence per 100 mV of membrane potential change (Fig. 1). Additionally, ICG displayed a rapid response with a primary time constant of 4 ms (see Fig. S1 in the Supporting Material), suggesting that this dye could successfully monitor action potentials.
Figure 1.
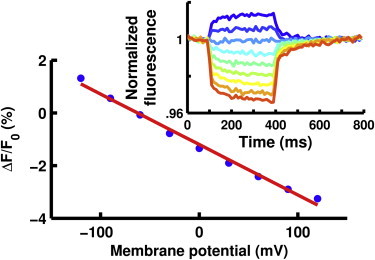
ICG-labeled oocytes showed that ICG’s fluorescence (blue points) is roughly linearly dependent (red line, fit to data) with voltage. (Inset) Oocyte membrane potential was held at −60 mV and then pulsed to potentials ranging from −120 mV (blue) to +120 mV (red). Ex: 780 nm, Em: 818–873 nm.
To test this hypothesis, we transformed our oocytes into synthetic neurons, previously dubbed “excitocytes”, by coinjecting them with cRNA of voltage-gated sodium (Nav) and potassium channel components (3). Under suitable current-clamp conditions, excitocytes fire trains of action potentials similar to those in naturally excitable cells. ICG’s fluorescence clearly recapitulated action potentials firing at speeds above 100 Hz (Fig. 2 A), faster than the physiological firing rates of most neurons (4).
Figure 2.
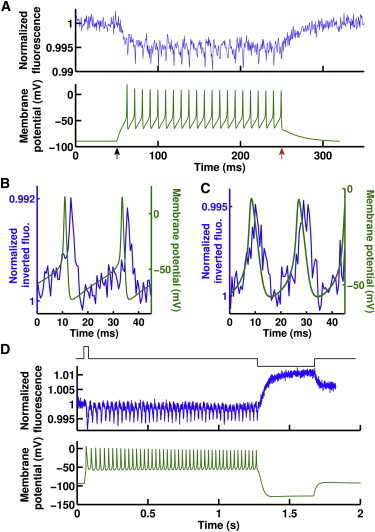
ICG can monitor action potentials. (A) Oocytes coinjected with voltage-gated sodium and potassium channel cRNA fired action potentials (bottom, green) when held under current clamp. ICG fluorescence changes (top, blue) detected these action potentials at a rate of 107 Hz. Stimulus start (black arrow) and end (red arrow) are shown. (B and C) ICG fluorescence (blue, inverted) distinguished between healthy action potentials from wild-type sodium channels (B, green) and diseased action potentials from sodium channels with a myotonic substitution (C, green). Cells are stimulated for the entire time course of these panels. The delay between action potentials and the ICG signal is due to a low-pass filtering effect caused by the dye response time and the camera integration time. (D) In cells with myotonic sodium channels, a brief stimulus (top, black) was sufficient to elicit a train of action potentials (bottom, green) that only ceased upon significant hyperpolarization, as expected in a myotonia. ICG fluorescence (middle, blue) successfully followed each one of these action potentials.
We extended the excitocyte technique from wild-type channels to evaluation of channelopathies and their effects on excitability to determine whether ICG could discriminate between normal and diseased action potentials based on shape. We compared excitocytes injected with wild-type Nav channel cRNA to those injected with cRNA coding for a version of Nav channel containing a point mutation, G1306E, which produces episodic myotonia (5). This disease is characterized by continued action potential firing in skeletal muscles after cessation of voluntary stimuli; the resulting prolonged muscle contractions are the hallmark of myotonia. Compared to the wild-type Nav channel, the G1306E mutation causes a slowing of the fast inactivation of the Nav channels, which in turn results in broadened action potentials (5). The electrical recordings and the ICG fluorescence response clearly distinguished the sharp action potentials produced by the healthy sodium channel (Fig. 2 B, and see Fig. S2) from the wider peaks produced by the myotonic sodium channel (Fig. 2 C, and see Fig. S2). Furthermore, a brief injection of current led to repetitive firing and hyperexcitability that persisted after the stimulus was stopped. ICG fluorescence clearly resolved every action potential of this myotonia-like behavior (Fig. 2 D). The successful recreation of disease-like action potentials validates the excitocyte system as a convenient method for investigating the electrophysiological effects of channelopathies.
We next investigated whether ICG’s voltage sensitivity extended to excitable mammalian tissue. This validation was critical, inasmuch as other voltage-sensitive dyes have shown promise in invertebrate preparations but had much smaller signals in mammalian cells (6). We first measured ICG fluorescence from cultured rat dorsal root ganglion neurons. Under whole-cell current clamp, we observed neurons firing in the stereotypical fashion of the nociceptive C-type fiber, and these action potentials were clearly visible in the ICG fluorescence (Fig. 3 A, and see Fig. S3). We also examined syncytia of cultured cardiomyocytes from neonatal rats (7) to further validate ICG’s utility; these cells beat spontaneously and showed changes in ICG fluorescence indicative of changes in membrane potential (Fig. 3 B). Although we cannot formally exclude the possibility that the cardiomyocytes’ physical motion produced fluorescence changes, several observations suggested that these effects were minimal (see Fig. S4). Taken together, our results in frog and rat cells confirmed that ICG voltage sensitivity was broadly applicable across a range of tissues and not confined to a particular animal or cell lineage.
Figure 3.
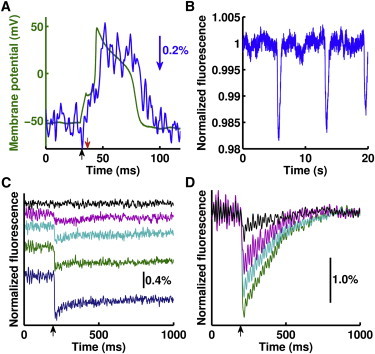
ICG follows electrical activity in living mammalian tissue. (A) Rat cultured dorsal root ganglion cells under current-clamp (black arrow, pulse start; red arrow, pulse end) fired action potentials (green), that ICG fluorescence tracked (blue, inverted, low-pass-filtered at 225 Hz; blue arrow, relative fluorescence change). (B) ICG fluorescence sensed spontaneous membrane potential changes in cardiomyocyte syncytia. (C) In rat brain slices, ICG responds differently to no stimulus (black) and stimuli of increasing intensity (magenta, cyan, green, and blue, increasing amplitude; scale bar shows relative fluorescence change). Weaker stimuli traces (e.g., magenta) show complete fluorescence recovery whereas larger stimuli (e.g., blue) do not fully recover within this time course; traces are vertically offset for clarity. (D) Tetrodotoxin (TTX) reduced the ICG response to a stimulus over 12 min (green, pre-TTX; cyan, magenta, and black, increasing time post-TTX; low-pass-filtered at 40 Hz; black arrow, stimulus).
Finally, we tested whether ICG voltage sensitivity could be detected in a complex tissue. Rat hippocampal slice cultures comprise a well-described organotypic preparation in which the three-dimensional architecture, neuronal connections, and glial interactions are maintained (8,9). Using these rat brain explants, we found that brain excitation produced by field electrode stimulation was clearly accompanied by ICG fluorescence changes (see Fig. S5). Additionally, ICG discriminated between electrical responses caused by differing excitation intensities and durations (Fig. 3 C, and see Fig. S5). To confirm that the fluorescence changes originated from changes in excitable cell activity, we used the Nav channel blocker tetrodotoxin (TTX). When applied to brain slices, electrical excitability was clearly inhibited (Fig. 3 D, and see Fig. S5) and partial recovery was observed upon subsequent TTX removal (see Fig. S5). These signals measuring brain slice activity were similar in shape and magnitude to those reported using other voltage-sensitive dyes (10,11). This demonstrates that ICG can report on electrical activity even in a physiological architecture with many nonexcitable cells.
To our knowledge, this is the first report that a clinically approved fluorescent dye is voltage-sensitive. Our results demonstrate that indocyanine green can accurately detect action potentials at firing rates common in mammalian neurons, and that it is sensitive enough to distinguish between healthy and diseased action potentials in a model system. ICG can measure electrical activity in mammalian neurons, cardiomyocytes, and explanted brain tissue. This voltage sensitivity was observed with both monochromatic and broad-band illumination sources (data not shown), under labeling conditions that differed in solution composition, duration, and dye concentration (see Methods in the Supporting Material), and at temperatures ranging from 19°C to 30°C. ICG’s water solubility further extends its potential utility. This robustness suggests that ICG can be used to measure voltage in many environments and tissues.
ICG has been FDA-approved for use in ophthalmic angiography, as well as in tests of cardiac output and hepatic function (12) and is additionally used off-label in a number of surgical applications (13). Interestingly, ICG has been shown to clearly label retinal ganglion cells in human patients (see Fig. S6) (14). This provides immediate motivation for biomedical investigations, because laboratory findings with ICG can potentially be translated to humans. Although many other voltage-sensitive dyes have been described, some with similar structures to ICG (15) and others with faster or larger signals (15,16), as of this writing none of these are FDA-approved. Additionally, ICG utilizes wavelengths further into the infrared spectrum than other available fast potentiometric dyes (17) and can thus be imaged in tissues up to 2 cm deep (18). This presents the possibility of optically imaging electrical activity deeper inside tissues than is feasible today. Although two-photon excitation with voltage-sensitive dyes can improve imaging depth, it remains intrinsically limited by the unaffected emission wavelength (19). Finally, ICG has been used in patients for more than 50 years and is known to have low toxicity (18,20). These properties suggest that ICG voltage sensitivity could extend the capabilities of modern electrophysiological techniques for disease diagnosis and monitoring in the clinic, and allow for the investigation of previously inaccessible experimental systems in basic research.
Acknowledgements
We thank Aya Pusic, Lisa Won, and Dr. Richard Kraig for brain slices and cardiomyocyte culture rats, Dr. Maria Traka for neuron culture rats, and Dr. Joao de Souza for patch-clamp assistance.
Support was provided by NIH grant GM030376. NIH GM07281 provided extra support to J.S.T, NS081954 to M.F.P and the Mayo Foundation for Medical Research and Research to Prevent Blindness to R.I.
Footnotes
Jeremy S. Treger and Michael F. Priest contributed equally to this article.
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Supporting Material
Supporting Citations
Ref. (21) appears in the Supporting Material.
References and Footnotes
- 1.Cohen L.B., Salzberg B.M. Optical measurement of membrane potential. Rev. Physiol. Biochem. Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- 2.Peterka D.S., Takahashi H., Yuste R. Imaging voltage in neurons. Neuron. 2011;69:9–21. doi: 10.1016/j.neuron.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro M.G., Priest M.F., Bezanilla F. Thermal mechanisms of millimeter wave stimulation of excitable cells. Biophys. J. 2013;104:2622–2628. doi: 10.1016/j.bpj.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzsáki G., Mizuseki K. The log-dynamic brain: how skewed distributions affect network operations. Nat. Rev. Neurosci. 2014;15:264–278. doi: 10.1038/nrn3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerche H., Heine R., Lehmann-Horn F. Human sodium channel myotonia: slowed channel inactivation due to substitutions for a glycine within the III-IV linker. J. Physiol. 1993;470:13–22. doi: 10.1113/jphysiol.1993.sp019843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross W.N., Reichardt L.F. Species-specific effects on the optical signals of voltage-sensitive dyes. J. Membr. Biol. 1979;48:343–356. doi: 10.1007/BF01869445. [DOI] [PubMed] [Google Scholar]
- 7.Fu J., Gao J., Liu P. An optimized protocol for culture of cardiomyocyte from neonatal rat. Cytotechnology. 2005;49:109–116. [Google Scholar]
- 8.Pusic A.D., Pusic K.M., Kraig R.P. IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J. Neuroimmunol. 2014;266:12–23. doi: 10.1016/j.jneuroim.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pusic A.D., Grinberg Y.Y., Kraig R.P. Modeling neural immune signaling of episodic and chronic migraine using spreading depression in vitro. J. Vis. Exp. 2011;52:2910. doi: 10.3791/2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson G.C., Coulter D.A. In vitro functional imaging in brain slices using fast voltage-sensitive dye imaging combined with whole-cell patch recording. Nat. Protoc. 2008;3:249–255. doi: 10.1038/nprot.2007.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contreras D., Llinas R. Voltage-sensitive dye imaging of neocortical spatiotemporal dynamics to afferent activation frequency. J. Neurosci. 2001;21:9403–9413. doi: 10.1523/JNEUROSCI.21-23-09403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akorn, Inc. 2012. IC-GREEN [package insert]. Akorn, Lake Forest, IL.
- 13.Alander J.T., Kaartinen I., Välisuo P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging. 2012;2012:940585. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashikari M., Ozeki H., Ogura Y. Long-term retention of dye after indocyanine green-assisted internal limiting membrane peeling. Jpn. J. Ophthalmol. 2006;50:349–353. doi: 10.1007/s10384-006-0325-1. [DOI] [PubMed] [Google Scholar]
- 15.Cohen L.B., Salzberg B.M., Wang C.H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J. Membr. Biol. 1974;19:1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- 16.Loew L.M. Design and use of organic voltage sensitive dyes. In: Canepari M., Zecevic D., editors. Membrane potential imaging in the nervous system: methods and applications. Springer; New York: 2011. pp. 13–23. [Google Scholar]
- 17.Matiukas A., Mitrea B.G., Loew L.M. New near-infrared optical probes of cardiac electrical activity. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2633–H2643. doi: 10.1152/ajpheart.00884.2005. [DOI] [PubMed] [Google Scholar]
- 18.Marshall M.V., Rasmussen J.C., Sevick-Muraca E.M. Near-infrared fluorescence imaging in humans with indocyanine green: a review and update. Open Surg. Oncol. J. 2010;2:12–25. doi: 10.2174/1876504101002010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher J.A.N., Barchi J.R., Salzberg B.M. Two-photon excitation of potentiometric probes enables optical recording of action potentials from mammalian nerve terminals in situ. J. Neurophysiol. 2008;99:1545–1553. doi: 10.1152/jn.00929.2007. [DOI] [PubMed] [Google Scholar]
- 20.Frangioni J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Narahashi T., Moore J.W., Scott W.R. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J. Gen. Physiol. 1964;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


