Abstract
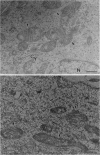
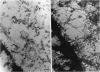
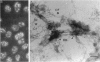
Cell structure, emerging from behind the veil of conventional electron microscopy, appears far more complex than formerly realized. The standard plastic-embedded, ultrathin section can image only what is on the section surface and masks the elaborate networks of the cytoplasm and nucleus. Embedment-free electron microscopy gives clear, high-contrast micrographs of cell structure when combined with removal of obscuring material such as soluble proteins. The resinless ultrathin section is the technique of choice; it is simple and inexpensive, and it uses ordinary electron microscopes. The resulting pictures reveal a world of complex cell structure and function. These images necessarily change our conception of the cytoskeleton, nuclear matrix, mitosis, and the relation of membranes to cytostructure.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggeler J., Ward J., Blackie L. M., Barcellos-Hoff M. H., Streuli C. H., Bissell M. J. Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo. J Cell Sci. 1991 Jun;99(Pt 2):407–417. doi: 10.1242/jcs.99.2.407. [DOI] [PubMed] [Google Scholar]
- Barrack E. R., Coffey D. S. The specific binding of estrogens and androgens to the nuclear matrix of sex hormone responsive tissues. J Biol Chem. 1980 Aug 10;255(15):7265–7275. [PubMed] [Google Scholar]
- Ben-Ze'ev A., Aloni Y. Processing of SV40 RNA is associated with the nuclear matrix and is not followed by the accumulation of low-molecular-weight RNA products. Virology. 1983 Mar;125(2):475–479. doi: 10.1016/0042-6822(83)90218-0. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear protein matrix: association with newly synthesized DNA. Science. 1975 Jul 25;189(4199):291–293. doi: 10.1126/science.1145202. [DOI] [PubMed] [Google Scholar]
- Bidwell J. P., Van Wijnen A. J., Fey E. G., Dworetzky S., Penman S., Stein J. L., Lian J. B., Stein G. S. Osteocalcin gene promoter-binding factors are tissue-specific nuclear matrix components. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3162–3166. doi: 10.1073/pnas.90.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Sheard B., Kallos J. Steroid receptor-nuclear matrix interactions. The role of DNA. J Biol Chem. 1983 Dec 10;258(23):14366–14370. [PubMed] [Google Scholar]
- Capco D. G., Krochmalnic G., Penman S. A new method of preparing embeddment-free sections for transmission electron microscopy: applications to the cytoskeletal framework and other three-dimensional networks. J Cell Biol. 1984 May;98(5):1878–1885. doi: 10.1083/jcb.98.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. C., Bowman D., Carrington W., Fogarty K., McNeil J. A., Fay F. S., Lawrence J. B. A three-dimensional view of precursor messenger RNA metabolism within the mammalian nucleus. Science. 1993 Feb 26;259(5099):1330–1335. doi: 10.1126/science.8446902. [DOI] [PubMed] [Google Scholar]
- Ciejek E. M., Tsai M. J., O'Malley B. W. Actively transcribed genes are associated with the nuclear matrix. Nature. 1983 Dec 8;306(5943):607–609. doi: 10.1038/306607a0. [DOI] [PubMed] [Google Scholar]
- Dworetzky S. I., Wright K. L., Fey E. G., Penman S., Lian J. B., Stein J. L., Stein G. S. Sequence-specific DNA-binding proteins are components of a nuclear matrix-attachment site. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4178–4182. doi: 10.1073/pnas.89.9.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey E. G., Penman S. Nuclear matrix proteins reflect cell type of origin in cultured human cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):121–125. doi: 10.1073/pnas.85.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Getzenberg R. H., Coffey D. S. Tissue specificity of the hormonal response in sex accessory tissues is associated with nuclear matrix protein patterns. Mol Endocrinol. 1990 Sep;4(9):1336–1342. doi: 10.1210/mend-4-9-1336. [DOI] [PubMed] [Google Scholar]
- Getzenberg R. H., Pienta K. J., Huang E. Y., Coffey D. S. Identification of nuclear matrix proteins in the cancer and normal rat prostate. Cancer Res. 1991 Dec 15;51(24):6514–6520. [PubMed] [Google Scholar]
- Glowacki J., Trepman E., Folkman J. Cell shape and phenotypic expression in chondrocytes. Proc Soc Exp Biol Med. 1983 Jan;172(1):93–98. doi: 10.3181/00379727-172-41533. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Yerna M. J., Schloss J. A. Localization and organization of microfilaments and related proteins in normal and virus-transformed cells. J Supramol Struct. 1976;5(2):155–183. doi: 10.1002/jss.400050206. [DOI] [PubMed] [Google Scholar]
- Herman R., Weymouth L., Penman S. Heterogeneous nuclear RNA-protein fibers in chromatin-depleted nuclei. J Cell Biol. 1978 Sep;78(3):663–674. doi: 10.1083/jcb.78.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993 Mar;104(Pt 3):613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Dike L., Hansen L., Karp S., Liley H., Maniotis A., McNamee H., Mooney D., Plopper G., Sims J. Cellular tensegrity: exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int Rev Cytol. 1994;150:173–224. doi: 10.1016/s0074-7696(08)61542-9. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. Transcription occurs at a nucleoskeleton. EMBO J. 1985 Apr;4(4):919–925. doi: 10.1002/j.1460-2075.1985.tb03719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman A. P., Higgs D. R. Nuclear scaffold attachment sites in the human globin gene complexes. EMBO J. 1988 Nov;7(11):3337–3344. doi: 10.1002/j.1460-2075.1988.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanuja P. S., Lehr J. E., Soule H. D., Gehani S. K., Noto A. C., Choudhury S., Chen R., Pienta K. J. Nuclear matrix proteins in normal and breast cancer cells. Cancer Res. 1993 Jul 15;53(14):3394–3398. [PubMed] [Google Scholar]
- Kirsch T. M., Miller-Diener A., Litwack G. The nuclear matrix is the site of glucocorticoid receptor complex action in the nucleus. Biochem Biophys Res Commun. 1986 Jun 13;137(2):640–648. doi: 10.1016/0006-291x(86)91126-5. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Krockmalnic G., Penman S. Imaging cytoskeleton--mitochondrial membrane attachments by embedment-free electron microscopy of saponin-extracted cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8565–8569. doi: 10.1073/pnas.87.21.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M., Vogelstein B., Coffey D. S. A fixed site of DNA replication in eucaryotic cells. Cell. 1980 Feb;19(2):527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Partin A. W., Getzenberg R. H., CarMichael M. J., Vindivich D., Yoo J., Epstein J. I., Coffey D. S. Nuclear matrix protein patterns in human benign prostatic hyperplasia and prostate cancer. Cancer Res. 1993 Feb 15;53(4):744–746. [PubMed] [Google Scholar]
- Porter K. R. The cytomatrix: a short history of its study. J Cell Biol. 1984 Jul;99(1 Pt 2):3s–12s. doi: 10.1083/jcb.99.1.3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuurman N., Van Driel R., De Jong L., Meijne A. M., Van Renswoude J. The protein composition of the nuclear matrix of murine P19 embryonal carcinoma cells is differentiation-stage dependent. Exp Cell Res. 1989 Feb;180(2):460–466. doi: 10.1016/0014-4827(89)90072-4. [DOI] [PubMed] [Google Scholar]
- Wolosewick J. J. The application of polyethylene glycol (PEG) to electron microscopy. J Cell Biol. 1980 Aug;86(2):675–661. doi: 10.1083/jcb.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]